Graphical abstract
Keywords: Malaria, Epidemiology, Plasmodium vivax, Plasmodium falciparum, Force of infection, Prevalence, Incidence, Thailand
Highlights
-
•
There is a highly heterogenous risk of malaria infection among villagers in western Thailand.
-
•
The molecular force of infection was determined in a low endemic setting.
-
•
There is a strong correlation between malaria prevalence and the force of infection.
Abstract
Over the past decades, the malaria burden in Thailand has substantially declined. Most infections now originate from the national border regions. In these areas, the prevalence of asymptomatic infections is still substantial and poses a challenge for the national malaria elimination program. To determine epidemiological parameters as well as risk factors for malaria infection in western Thailand, we carried out a cohort study in Kanchanaburi and Ratchaburi provinces on the Thailand-Myanmar border. Blood samples from 999 local participants were examined for malaria infection every 4 weeks between May 2013 and Jun 2014. Prevalence of Plasmodium falciparum and Plasmodium vivax was determined by quantitative PCR (qPCR) and showed a seasonal variation with values fluctuating from 1.7% to 4.2% for P. vivax and 0% to 1.3% for P. falciparum. Ninety percent of infections were asymptomatic. The annual molecular force of blood-stage infection (molFOB) was estimated by microsatellite genotyping to be 0.24 new infections per person-year for P. vivax and 0.02 new infections per person-year for P. falciparum. The distribution of infections was heterogenous, that is, the vast majority of infections (>80%) were found in a small number of individuals (<8% of the study population) who tested positive at multiple timepoints. Significant risk factors were detected for P. vivax infections, including previous clinical malaria, occupation in agriculture and travel to Myanmar. In contrast, indoor residual spraying was associated with a protection from infection. These findings provide a recent landscape of malaria epidemiology and emphasize the importance of novel strategies to target asymptomatic and imported infections.
1. Introduction
In recent years, significant progress has been made in controlling malaria worldwide. The malaria incidence and mortality rates of Southeast Asia declined by approximately 50% between 2000 and 2015 (WHO, 2015). In the same period, the number of malaria cases in Thailand was reduced from 150,000 to 24,850 (ThaiDDC, 2016). The incidence rate in most areas is now below 1 case per 1000 person-years at risk. As part of the global malaria elimination effort, the country is aiming to achieve malaria elimination before 2025 (WPRO, 2015), a commendable goal which will require commitment from all parties involved.
Notwithstanding the recent success in reducing the malaria burden, a significant proportion of the population living in endemic areas of Thailand is still at risk of Plasmodium spp. infections. Historically, Plasmodium falciparum was the predominant parasite species in Thailand, but Plasmodium vivax has recently taken over as the primary parasite whereas Plasmodium malariae, Plasmodium ovale and Plasmodium knowlesi are only found sporadically (ThaiMoPH, 2017). Malaria infections in Thailand are seasonal, the peak season lasting from May to September (Phimpraphi et al., 2008). Transmission is concentrated along the western Myanmar border and the eastern Cambodian border. Cross border movements are thought to contribute significantly to maintenance of the disease (Sriwichai et al., 2017). Anopheles dirus, Anopheles minimus and Anopheles maculatus are the most important local malaria vectors in these parts of the country (Tananchai et al., 2012, Sriwichai et al., 2016). Recently, an increasing number of clinical infections were also reported from the southern provinces (ThaiMoPH, 2017) which is likely due to reduced surveillance and control activities as a result of political unrest in the area.
Several studies have reported the presence of a large number of asymptomatic infections in Thailand (Li et al., 2014, Baum et al., 2015, Baum et al., 2016, Parker et al., 2015, Nguitragool et al., 2017). As most asymptomatic carriers do not seek treatment, they are a neglected reservoir that may help sustain disease transmission (Kiattibutr et al., 2017). To accelerate progress towards elimination, it is important for the national malaria program to have an appropriate strategy to manage these infections. A better understanding of which individuals are at the greatest risk of harboring infections, symptomatic or asymptomatic, is thus of fundamental importance, especially when resources for malaria control are limited. At present, the Thai national malaria control program relies mostly on clinical cases detected at health facilities as the main indicator of malaria incidence to coordinate its control efforts. Data on asymptomatic infection rates in Thailand are thus restricted to cross-sectional surveys conducted in a few areas (Kritsiriwuthinan and Ngrenngarmlert, 2011, Li et al., 2014, Baum et al., 2015, Baum et al., 2016, Parker et al., 2015).
In this study, we sought to better understand the dynamics of malaria infection and its risk factors in populations along the Thailand-Myanmar border, an area where malaria infection rates are still substantially higher than in other parts of Thailand. We conducted a longitudinal cohort study in two villages between May 2013 and Jun 2014 in which finger-prick blood samples were obtained from participants every 4 weeks. Malaria parasites were detected by qPCR and parasite genotypes were assessed by amplification of length polymorphic markers. Parasite positivity and molecular force of blood-stage infection (molFOB, the rate of acquisition of new blood-stage clones) (Mueller et al., 2012, Koepfli et al., 2013) were used to quantify the risk of infection as well as the underlying risk factors.
2. Materials and methods
2.1. Study sites
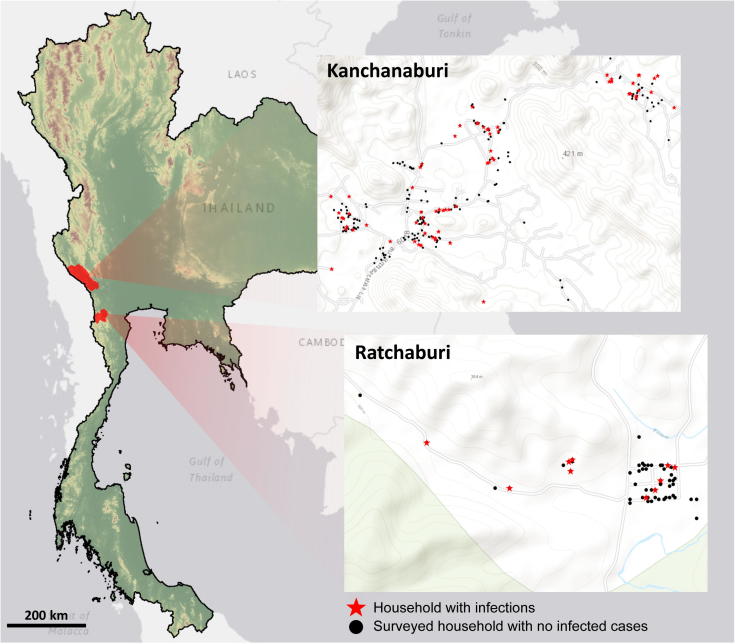
This study was conducted in two villages, one in the Bong Ti subdistrict of Kanchanaburi province and the other in the Suan Phueng subdistrict of Ratchaburi Province of Thailand (Fig. 1). These two sites are located within a few km of the Thailand-Myanmar border. The main ethnic groups in both sites were Thai and Karen. Agriculture and farming were the main occupations. Forest foraging was a frequent activity for residents in these areas. The village of Bong Ti is located approximately 65 km west of the main district of Kanchanaburi province in a hilly terrain. Most houses were accessible by paved roads. A cross-sectional survey of malaria infection by qPCR (Nguitragool et al., 2017) indicated 3.8% Plasmodium vivax and 1.4% P. falciparum prevalence in August-September of 2012. The study village in Suan Phueng subdistrict is located approximately 160 km from Bangkok. The village is located in a mountainous area on the Tanaosri mountain range. Our survey in September-October of 2012 indicated a prevalence of 3.1% for P. vivax and 1.2% for P. falciparum at this site (Nguitragool et al., 2017).
Fig. 1.
Map of the two study sites in Bong Ti, Kanchanaburi and Suan Phueng, Ratchaburi on the Thailand-Myanmar border. Study households are shown (●) as well as households in which infections with Plasmodium falciparum or Plasmodium vivax were detected over the course of the study (★).
2.2. Study design
A total of 999 participants were enrolled for a 1 year prospective cohort study. No treatment was given at the baseline. A total of 14 visits to each study participant were made between May 2013 and June 2014, with time between consecutive visits being approximately 4 weeks. A finger prick blood sample was collected from each participant at each visit for malaria parasite detection, species identification, and genotyping by length polymorphic markers for molFOB determination. Additional finger prick blood samples were also obtained through passive case detection, when participants visited malaria clinics in the study areas during the time between study visits.
At each visit, participants were asked to provide information regarding factors relevant for malaria control including access to and usage of insecticide-treated nets, travel history, clinical malaria history, and treatment taken since the last visit. Before taking the blood sample, the body temperature of each participant was measured using an infrared thermometer. Participants with fever (body temperature ≥37.5 °C) were tested for malaria infection on-site using a rapid diagnostic test (SD BIOLINE Malaria Ag P.f./Pan, Standard Diagnostics, Republic of Korea) and referred to a nearby malaria clinic for treatment if the test was positive. The study was approved by the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University, Thailand (EC approval number TMEC 13-020).
2.3. Molecular analysis
Finger prick whole blood (200 μl) was collected from each participant into an EDTA-containing microtainer. After plasma removal, blood pellets were stored at −20 °C until DNA extraction. DNA was extracted using FavorPrep 96-well Genomic DNA Extraction Kits (Favorgen, Taiwan), following the standard protocol provided by the manufacturer. The final purified DNA was eluted into 100 μl of elution buffer. A volume of 4 μl of purified DNA (equivalent to 8 μl of whole blood) was used as the template for malaria parasite detection by a genus-specific qPCR assay targeting the Plasmodium 18S rRNA genes (Wampfler et al., 2013). For samples positive by the genus-specific assay, P. vivax- and P. falciparum-specific assays (Rosanas-Urgell et al., 2010) were used to determine the parasite species. Plasmodium vivax genotypes were determined using PvMS2 and Pvmsp1F3 markers as previously described (Schoepflin et al., 2009). The combined expected heterozygosity, He, of the two markers was 0.89. Capillary electrophoresis for microsatellite genotyping was performed on an ABI3730XL instrument (Macrogen, Republic of Korea).
2.4. Determination of the molecular force of blood infection (molFOB)
The molecular force of blood infection (molFOB) was determined as described previously (Koepfli et al., 2013). Briefly, the molFOB is the observed number of new blood-stage infections, as identified by individual parasite genotypes, divided by the time-at-risk (i.e., the incidence of new blood-stage infections). An observed infection, as identified by a specific genotype, was considered to be ‘new’ if the same genotype had not been seen in the two previous active or passive case detection visits. As such, the molFOB can be determined for each study participant or as a sum over the entire study population or subpopulations.
2.5. Statistical analysis
Factors influencing i) the overall rate of acquisition of new blood infections (molFOB); ii) being parasite-positive for P. vivax and/or P. falciparum and iii) being subject to a clinical episode, defined by fever + parasitemia detectable by light microscopy (Robinson et al., 2015) were investigated using statistical models. Travel to Myanmar was quantified as ‘trips per year’ based on answers to questions about travel history at each visit. Since the molFOB is a count variable measured per individual over a specific exposure time (time at risk), and is overdispersed, a negative binomial regression model was chosen in which the exposure time at risk is used as tge offset (µ*j = exp(βxj + offsetj + νj), where offsetj = log(exposure time) and eνj follows a gamma distribution. Because using the collapsed data to model the molFOB for each individual does not allow for the analysis of time-changing covariates, factors influencing the frequency of parasite positivity and frequency of clinical episodes within the study period were explored using multiple failure time models allowing for time-changing covariates (Therneau and Grambsch, 2000). In these models, parasite positivity and clinical episodes were equivalent to a ‘failed’ outcome, respectively. In addition to the statistical models presented in the main manuscript, univariate analyses (Supplementary Tables S1, S3, S5, S7) and multivariate analyses with backward selection (Supplementary Tables S2, S4, S6) are provided.
To test whether infections were spatially correlated, the molFOB was tested for spatial autocorrelation using the Moran’s I test statistic, which takes into account the value of the molFOB for each location and the distance between all locations. A Morans I > 0 indicates spatial clustering of high/high and/or low/low molFOB values. This analysis was done once for each individual, accepting that the distance between individuals in the same household is 0, and once for each household by calculating the average molFOB per household (i.e., the total number of new infections divided by the total time at risk per household).
3. Results
3.1. Characteristics of the study population
In total, 999 participants were enrolled into this study in May 2013. Eight hundred and twelve participants were from Bong Ti, Kanchanaburi and 187 participants were from Suan Phueng, Ratchaburi. Fig. 1 shows the maps of these two study sites where the household locations of the study participants were marked.
Of the 999 participants, the large majority (n = 799, 80%) were seen 13 or more times (interquartile range (IQR): 13–14; range: 1–14) during the study period resulting in 12,559 blood samples from active case detection (ACD). In addition, 281 blood samples were obtained through passive case detection (PCD) when participants presented at local malaria clinics due to illness. The median time that the participants remained in the study was 368 days (IQR: 361–379; range: 0–378). An overview of the characteristics of the study population is given in Table 1.
Table 1.
Characteristics of the western Thailand study population.
| Characteristic | n (%) or median (range) |
|---|---|
| Agea (yr) | 23 (1–83) |
| Male/Female | 452 (46.2)/527 (53.8) |
| Kanchanaburi/Ratchaburi | 812 (81.3)/187 (18.7) |
| Exposure (days) | 368 (22–378) |
| Infection status at first visit | |
| Plasmodium vivax | 34 (3.4) |
| Plasmodium falciparum | 7 (0.8) |
| Mixed P. vivax/P. falciparum | 3 (0.3) |
| Insecticide treated net possession/usagea | |
| Never | 62 (6.3) |
| <6 months | 11 (1.1) |
| 6 months–1 year | 12 (1.2) |
| 1 year–2 years | 34 (3.5) |
| >2 years | 860 (87.8) |
| Indoor residual sprayinga | 719 (73%) |
| Window screensa | 20 (0.2%) |
At first visit (May 2013).
3.2. Parasite prevalence and molFOB
From samples collected at both ACD and PCD visits, the genus-specific PCR assay detected 735 infections in the study participants, of which 512 (70.0%) were successfully genotyped for the molFOB (i.e. the incidence of genetically distinct blood-staged infections) analysis. Plasmodium vivax monoinfections constituted 84.6% (n = 433/512), P. falciparum monoinfections 9.2% (n = 47/512) and mixed species infections 6.3% (n = 32/512). As such, mixed species infections were over-represented by a factor of approximately 10 (Fisher’s exact test, P < 0.001) indicating a strong clustering of infection. A proportion of 8.5% (37/433) of P. vivax infections and 21.3% (10/47) of the P. falciparum infections were classified as clinical, i.e., detectable parasitemia was accompanied by a measured or reported fever in either PCD or ACD visits. The difference is statistically significant (analysis of proportions, chi-square test, P = 0.006) and suggests that P. falciparum was more likely to cause clinical symptoms. Notably, none of the 32 mixed infections was classified as a clinical episode.
The prevalence of P. falciparum and P. vivax showed seasonal variation with prevalence varying from 4.2% to 1.7% for P. vivax and from 1.3% to 0% for P. falciparum (Fig. 2). The peak between June and September coincided with the rainy season.
Fig. 2.
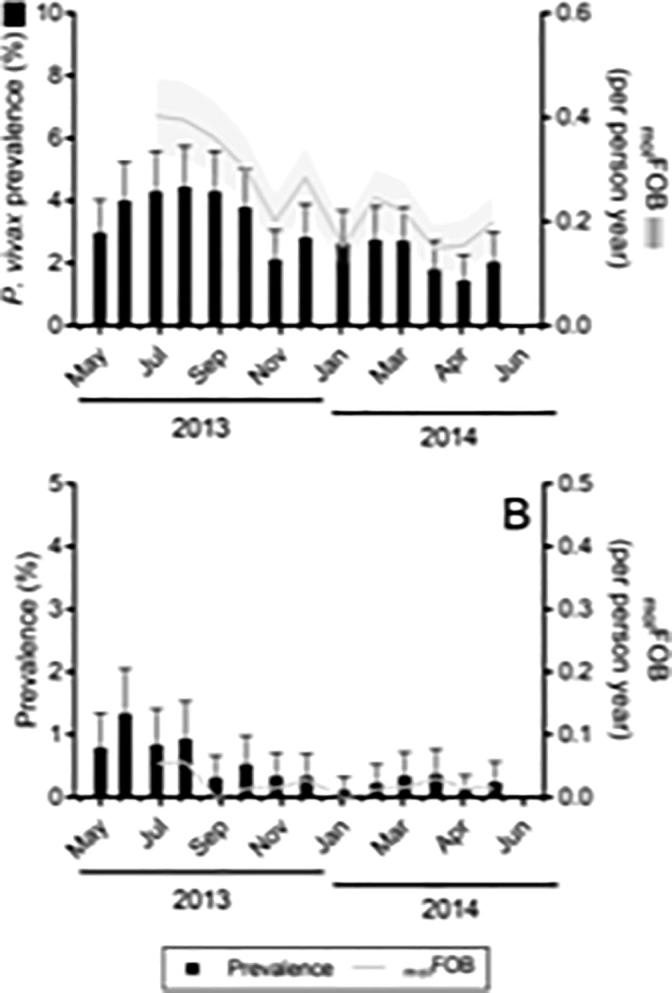
Prevalence and molecular force of blood-stage infection (molFOB) of Plasmodium vivax (A) and Plasmodium falciparum (B) at each study site by month of study visit. Error bars (prevalence) represent the upper limit of the 95% confidence interval; shaded area (molFOB) represents S.E.M. Note that molFOB could not be determined for visits one and two as it depends on the observations from two previous study visits.
During the study period, an estimated total of 226 new (i.e. genetically distinct) P. vivax and 18 new P. falciparum blood-stage infections were detected, resulting in an average molFOB of 0.24 new infections per person-year for P. vivax and 0.02 new infections per person-year for P. falciparum. The seasonal variation of the molFOB resembled that of parasite prevalence for both species, with the peak in the wet season.
3.3. Clustering of new infections
A number of study participants experienced a disproportionately high molFOB (Fig. 3). The excess of high molFOB values compared with the homogeneous distribution (Poisson) is statistically significant for P. vivax (goodness of fit test for Poisson distribution P < 10−6), suggesting either a non-uniform risk of acquiring new infection through mosquito bites or relapses of parasites from the liver. Spatial analysis suggests that the P. vivax molFOB in Bong Ti, Kanchanaburi was clustered at both the individual level (global Moran’s I = 0.12, P < 0.0001) and, more loosely, at the household level (global Moran’s I = 0.03, P = 0.02). The low number of infections precluded an in-depth analysis of heterogeneities in the risk of P. falciparum infection.
Fig. 3.
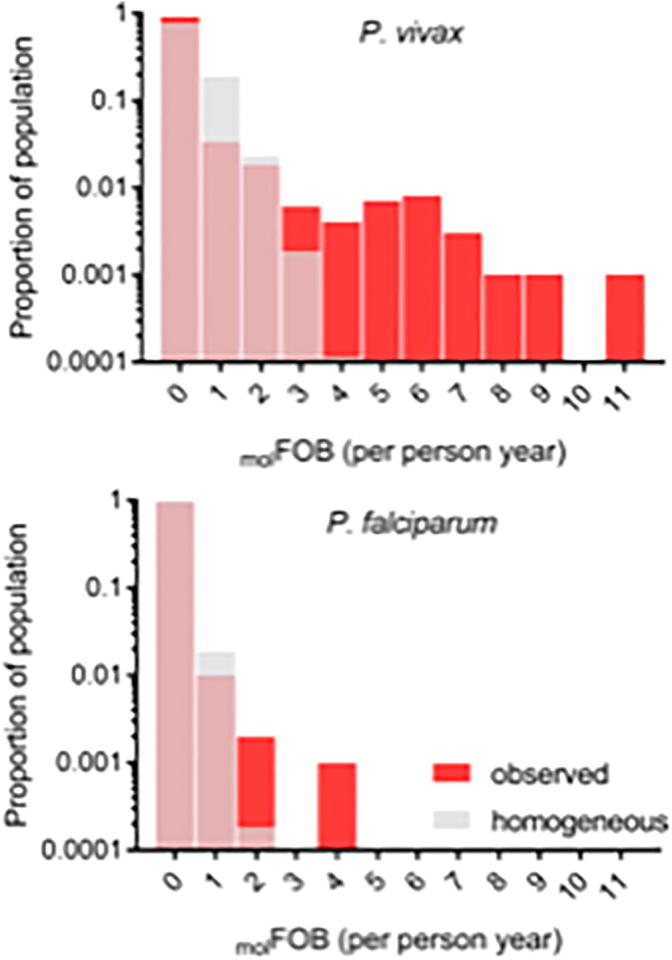
Distributions of the molecular force of blood-stage infection (molFOB). The proportion of study participants at each level of molFOB is shown for Plasmodium vivax (A) and Plasmodium falciparum (B). Red: normalized distributions of observed molFOB in the study participants. Grey: Poisson distributions computed based on the mean molFOB. The Poisson distributions are overlaid on top of the observed distributions.
3.4. Factors associated with P. vivax infection and illness
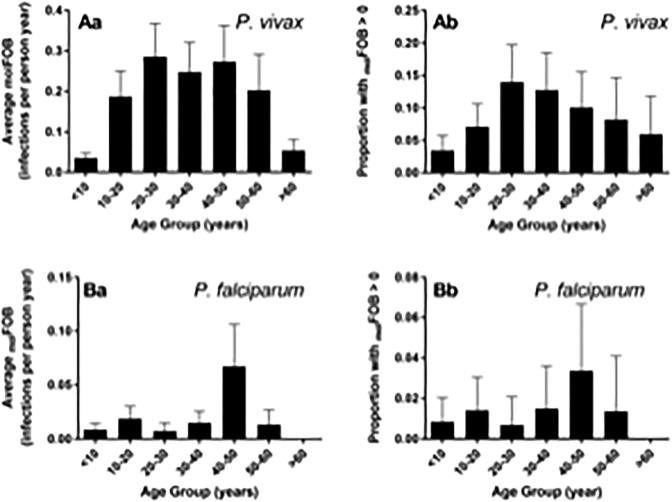
The age profile of the molFOB for P. vivax revealed a higher number of new blood-stage clones in adolescents and adults compared with children (Fig. 4). In addition to age, analysis of risk factors revealed that increased frequency of travel to Myanmar, previous malaria episodes, and employment in agriculture were significantly associated with increased risk of new P. vivax blood-stage infections as measured by the molFOB (Table 2).
Fig. 4.
The molecular force of blood-stage infection (molFOB) as a function of age in the study population. (Aa) The average Plasmodium vivaxmolFOB in each age group. (Ab) The proportion of individuals with P. vivaxmolFOB > 0 in each age group. (Ba) The average Plasmodium falciparummolFOB in each age group. (Bb) The proportion of individuals with P. falciparummolFOB > 0 in each age group. Error bars indicate the S.E.M. (Aa, Ba) or the upper limit of the 95% confidence interval (Ab, Bb).
Table 2.
Factors associated with Plasmodium vivaxmolFOB (negative binomial model) in western Thailand.
| Risk factor | aIRR | 95% CI | P |
|---|---|---|---|
| Kanchanaburi | 1.83 | 0.64–5.23 | 0.257 |
| Age group (years; ref: 0–6) | |||
| 7–12 | 22.79 | 3.34–155.6 | <0.001 |
| 13–17 | 17.10 | 2.15–135.84 | |
| 18–60 | 15.67 | 2.15–114.15 | |
| >60 | 3.85 | 0.44–33.31 | |
| Male | 1.86 | 1.1–3.14 | 0.02 |
| Frequency of travel to Myanmar (per visit/year) | 1.15 | 1.05–1.24 | 0.001 |
| Previous clinical malariaa | 2.43 | 1.2–4.91 | 0.014 |
| House treated with IRSa | 0.56 | 0.3–1.04 | 0.067 |
| Reported bednet possessiona | 0.98 | 0.39–2.43 | 0.957 |
| Work in agriculturea | 2.25 | 1.04–4.86 | 0.039 |
aIRR, adjusted incidence risk ratio; CI, confidence interval; IRS, indoor residual spray
Status at enrolment.
In order to assess time changing covariates, risk factors associated with P. vivax positivity were also determined using a multiple failure time model (Table 3). In general, predictors of P. vivax molFOB were predictors of P. vivax positivity. While similar effect sizes were observed, the analysis of P. vivax positivity additionally detected indoor residual spraying (IRS) as a significant factor associated with reduced risk of infection.
Table 3.
Risk factors associated with western Thailand Plasmodium vivax positivity (multiple failure time model).
| Risk factor | aHR | P |
|---|---|---|
| Kanchanaburi | 2.22 (1.06–4.76) | 0.03 |
| Season (May-September) | 3.41 (2.19–5.31) | <0.001 |
| Age group (years; ref: 0–6) | ||
| 7–12 | 3.64 (0.94–14.02) | 0.02 |
| 13–17 | 4.99 (1.22–20.34) | |
| 18–60 | 3.69 (1.03–13.21) | |
| >60 | 0.99 (0.22–4.48) | |
| Male | 2.32 (1.36–3.96) | <0.001 |
| Work in agriculturea | 2.01 (1.14–3.55) | 0.02 |
| Frequency of travel to Myanmarb | 1.06 (1.01–1.11) | 0.01 |
| Average bednet usageb, c | 1 (0.95–1.05) | 0.92 |
| House treated with IRSa | 0.47 (0.28–0.79) | <0.001 |
| Previous clinical malariaa | 1.87 (1.13–3.1) | 0.02 |
aHR, adjusted hazard ratio; IRS, indoor residual spray.
Status at enrolment.
As a time-changing covariate (average observed at time of outcome).
Average bednet usage was defined as the proportion of times a person had answered ‘yes’ to the question: ‘Did you sleep under a bednet last night?’ during active case detection.
Several predictors of P. vivax infection including season, age, occupation, and reported previous clinical malaria, were also significantly associated with clinical episodes of malaria (Table 4). Notably, while adult age was a predictor of increased risk of infection (Table 2, Table 3), it was associated with a reduced risk of clinical episodes (Table 4). This indicates stronger protective immunity in adults compared with young children. Although travel to Myanmar was a predictor of infection and IRS was associated with a reduced risk of infection, these two factors were not significantly associated with clinical episodes. This is consistent with their expected relationship with parasite exposure but not clinical outcomes. Interestingly, despite the lack of association of bednet usage with P. vivax molFOB and positivity, bednet usage was significantly associated with a small reduction in clinical episodes. The reason for this is unclear and may deserve further investigation. None of the mixed species infections (P. falciparum/P. vivax, n = 32) corresponded to a clinical episode, possibly reflecting parasite-parasite interaction or co-development of host protective immunity against these sympatric species.
Table 4.
Risk factors associated with western Thailand Plasmodium vivax clinical episodes (multiple failure time model).
| Risk factor | aHR | P |
|---|---|---|
| Kanchanaburi | 1.01 (0.3–3.33) | 0.99 |
| Season (May-September) | 19.47 (1.82–208.79) | 0.01 |
| Age group (ref: 0–12)b | ||
| 13–17 | 0.75 (0.19–3.05) | <0.001 |
| 18–60 | 0.24 (0.1–0.6) | |
| >60 | 0.35 (0.09–1.29) | |
| Male | 1.66 (0.63–4.36) | 0.31 |
| Work in agriculturec | 4.64 (2.18–9.86) | <0.001 |
| Travel frequency to Myanmara | 1.04 (0.94–1.15) | 0.50 |
| Average bednet usagea, d | 0.89 (0.83–0.96) | <0.001 |
| IRSc | 1.64 (0.61–4.45) | 0.33 |
| Previous clinical malariac | 2.21 (0.98–4.97) | 0.06 |
aHR, adjusted hazard ratio; IRS, indoor residual spray.
As a time-changing covariate (floating average at time of outcome).
The age groups were further aggregated when compared with Table 3, as some age groups did not contain any positive outcomes.
Status at enrolment.
Average bednet usage was defined as the proportion of times a person had answered ‘yes’ to the question: ‘Did you sleep under a bednet last night?’.
3.5. Factors associated with P. falciparum infection
For P. falciparum, risk factors for increased molFOB were not determined due to the small number of observations (n = 18 new P. falciparum infections out of 12,559 samples). Predictors of infection were determined only from positivity data. In the univariate analysis, only travel frequency to Myanmar was found to be a predictor of parasite positivity (Supplementary Table S7). The small number of detected P. falciparum infections did not allow detection of other risk factors. Based on the results presented in Table 5, however, season, age, male gender and IRS may also show association with a higher infection rate in a larger, more highly powered, study.
Table 5.
Risk factors associated with western Thailand Plasmodium falciparum positivity (multiple failure time model).
| Risk factor | aHR | P |
|---|---|---|
| Kanchanaburi | 1.69 (0.54–5.26) | 0.36 |
| Season (May–September) | 2.12 (1.03–4.36) | 0.04 |
| Age group (years; ref: 0–6) | ||
| 7–12 | 4.15 (0.38–45.23) | 0.12 |
| 13–17 | 4.54 (0.45–46.25) | |
| ≥18 | 10 (1.1–91.05) | |
| Male | 2.9 (0.73–11.52) | 0.13 |
| Work in agriculturea | 0.36 (0.05–2.63) | 0.32 |
| Travel frequency to Myanmarb | 1.08 (0.96–1.22) | 0.18 |
| Average bednet usageb, c | 1.04 (0.83–1.3) | 0.76 |
| IRSa | 2.12 (0.77–5.82) | 0.15 |
| Previous clinical malariaa | 0.96 (0.28–3.24) | 0.95 |
aHR, adjusted hazard ratio; IRS, indoor residual spray.
Status at enrolment.
As a time-changing covariate (floating average at time of outcome).
Average bednet usage was defined as the proportion of times a person had answered ‘yes’ to the question: ‘Did you sleep under a bednet last night?’.
4. Discussion
This study has confirmed the high degree of heterogeneity of malaria risk in Thai populations living along the Thailand – Myanmar boarder and identified important risk factors for P. vivax infection and disease. One key epidemiological parameter determined in this study is the molFOB which reflects the rate of acquiring a new blood-stage clone of the parasite. Two genetic markers (PvMS2 and Pvmsp1F3) were used to genotype P. vivax. Because the combined He of the two loci was 0.89, approximately 10% of all new clones are expected to have been missed. This, together with the limited sensitivity of molecular genotyping (69.5% of samples were PvMS2-positive; 78.9% of samples were Pvmsp1F3-positive), would lead to an underestimation of the true molFOB. These limitations are shared by our previous studies using the same methodology (Mueller et al., 2012, Koepfli et al., 2013, Hofmann et al., 2017). The analyses of risk factors, however, depends only on the relative values of the molFOB.
Overall the prevalences of P. falciparum (0–1.3%) and P. vivax (1.7–4.2%) were similar to the 4.9–5.7% Plasmodium prevalence previously reported in Kanchanaburi and Ratchaburi in 2012 (Patel et al., 2014, Nguitragool et al., 2017). The majority of infections detected at each visit were asymptomatic and resolved naturally without developing into clinical malaria. Using the same dataset, we recently estimated the duration of blood-stage infection detectable by molecular genotyping to be 29 days for P. vivax and 135 days for P. falciparum (White et al., 2018).
The overall prevalence as well as the predominance of P. vivax infections reflect the current trend in Thailand and the Greater Mekong Subregion where P. vivax has become the predominant malaria species (Baum et al., 2015, Imwong et al., 2015, Nguitragool et al., 2017). The P. vivax pre-dominance is less pronounced among the clinical/febrile cases (Pv: 79%) than asymptomatic infections (Pv: 91.5%). The predominance was most striking in the number of genetically distinct blood-stage infections acquired during follow-up (molFOB) with only 7.4% of all newly acquired infections due to P. falciparum, resulting in an average molFOB of 0.02 and 0.24 new infections per person-year for P. falciparum and P. vivax, respectively. The proportion of P. vivax infection relative to P. falciparum was much higher in our cohort than among clinical cases recorded by the Thai health system (ThaiDDC, 2016, WHO, 2016), indicating that the true burden of (mostly asymptomatic) P. vivax infections in Thailand may be substantially higher than reported.
The risk of P. vivax infection was highly heterogeneous in our study sites, which are fairly typical of areas along the Thailand-Myanmar border where the landscape is mountainous, agriculture and forest harvesting are the means of living, and cross-border migrant workers are common. Only 15% of the 999 study participants tested positive during the study, and people who were infected at multiple visits accounted for >80% of all infections. The observed concentration of infections in a small sub-population suggests that appropriate interventions targeted at high-risk individuals may be effective in accelerating malaria elimination.
In order to target malaria interventions more efficiently, it is essential to better understand the geographical, demographic, and behavioral risk factors for Plasmodium spp. infections in these Thai border communities. For P. vivax, the risk factors for higher parasite prevalence and molFOB were very similar and included rainy season, aged 7–60 years, male gender, past experience of clinical malaria, being employed in agriculture, and travel to Myanmar. As areas with perennial transmission, our study sites received deltamethrin IRS twice each year according to the policy of the Thai Ministry of Public Health. Our analysis found that this intervention was associated with reduced infection risk. Thus, even though malaria infection risk was generally related to occupational exposure (as exemplified by male adults working in agriculture or in the forest and people visiting areas of higher transmission in Myanmar), the high risk in children 7–17 years of age and the association of IRS with protection indicate ongoing local P. vivax transmission, including in or near people’s houses. While the high risk in male adults and those regularly crossing the Myanmar border has been well established (Nguitragool et al., 2017), the association between IRS and reduced P. vivax positivity was a surprise in light of the predominantly outdoor biting preference of the malaria vectors in western Thailand (Muenworn et al., 2009, Tananchai et al., 2012, Tisgratog et al., 2012, Sriwichai et al., 2017). It provides a strong argument for continued support for the national IRS program in country.
The risk of suffering a clinical P. vivax episode was highest in children <7 years of age and decreased strongly with age, indicating that residents with higher risks of P. vivax infections did acquire substantial clinical immunity. Although it is well established that immunity to P. vivax is acquired more rapidly than to P. falciparum (Longley et al., 2016), it cannot be ruled out that at least part of the immunity observed in older age groups was acquired during earlier periods when transmission intensity was substantially higher (Phimpraphi et al., 2008). Although they are less infectious to mosquitoes than symptomatic infections (Kiattibutr et al., 2017), asymptomatic P. vivax infections are much more prevalent and may pose a particular challenge to malaria elimination in Thailand. Overall, the observed risk patterns indicate that residual transmission of P. vivax persists in our study areas.
There was only a small number of P. falciparum-positive cases detected during the study period. Due to this, for P. falciparum the risk factor analysis was limited to qPCR positivity. Only frequency of travel to Myanmar was found to be a statistically significant determinant for risk of P. falciparum infection. Although not statistically significant due to the profound lack of power, the multivariate analysis does indicate that the risk of P. falciparum was highest in male (adjusted hazard ratio (aHR) = 2.9) adults (aHR = 10.0) with an excess risk also during the rainy seasons and in areas where IRS had been conducted. The higher risk in people who traveled frequently across the border is a common feature of the residual burden both in this (Nguitragool et al., 2017) and other border regions of Thailand (Kitvatanachai et al., 2003, Bhumiratana et al., 2013). As found in neighboring Tak province, where P. falciparum malaria was four times more likely in recent migrants compared with Thai patients and correlated with Anopheline vector capture rates (Sriwichai et al., 2017), this indicates that importation of P. falciparum to Ratchaburi and Kanchanaburi contributed significantly to prevalence.
To our knowledge, this study is the first from a hypoendemic area to determine the molFOB. Previous studies of Plasmodium molFOB were restricted to Papua New Guinea where transmission intensity was much higher with molFOB in the range of 5–14 new infections per person-year (Mueller et al., 2012, Koepfli et al., 2013, Hofmann et al., 2017). These previous studies demonstrated that in such a setting, acquisition of new P. falciparum clones was a major factor of clinical malaria in children (Mueller et al., 2012) and that high molFOB likely contributed to rapid acquisition of immunity against P. vivax malaria (Koepfli et al., 2013). The molFOB for both P. falciparum and P. vivax were at least 50-fold lower in the current study. This study thus represents a scenario at the opposite end of the malaria transmission spectrum.
Compared with parasite positivity, the molFOB is a more direct measure of P. falciparum transmission. For P. vivax, the molFOB is a combined measure of blood-stage infections arising from both new mosquito bites and relapses. Without the knowledge about the relative contributions of mosquito bites and relapses, it is difficult to estimate how much the molFOB reflects transmission intensity. In this study, we found that the seasonal variation of parasite positivity closely followed that of the molFOB (Fig. 2) for both parasite species. Risk factor analyses of the molFOB and positivity of P. vivax also yielded similar results; all significant risk factors apparent in the molFOB analysis were apparent in the analysis that used positivity. The close association between P. vivax prevalence and the molFOB is consistent with the short duration (29 days) estimated for blood-stage infection in Thailand (White et al., 2018).
In summary, our study highlights the different challenges posed by P. falciparum and P. vivax to Thailand’s declared goal of eliminating local malaria transmission by 2025. In line with our earlier studies in Tak Province (Baum et al., 2015, Baum et al., 2016, Parker et al., 2015, Sriwichai et al., 2017), we have confirmed the continued presence of local P. vivax transmission in Thai villages along the western border. Most of the P. vivax infections were asymptomatic and often of very low density and would not have been detected by the standard surveillance methods based on microscopy and centered around passive case detection. Efficient elimination of P. vivax from Thailand will thus require additional novel strategies targeting this asymptomatic reservoir, including the silent hypnozoite reservoir (Robinson et al., 2015). On the other hand, P. falciparum infections were rare. With a large proportion of infection linked to recent travel to Myanmar, it will be important to strengthen malaria control measures on both sides of the border and to raise awareness of the travel-associated risk.
Acknowledgements
This study was supported by the TransEPI consortium funded by the Bill & Melinda Gates Foundation, USA (www.gatesfoundation.org), a National Health and Medical Research Council (NHMRC) Project Grant, Australia (#1021455, www.nhmrc.gov.au), and Swiss National Science Foundation grant (310030_159580, www.snf.ch). WN was supported by a Wellcome Trust Intermediate Fellowship in Public Health and Tropical Medicine, United Kingdom (101073/Z/13Z). IM was supported by a NHMRC Senior Research Fellowship (#1043345) and JS was supported by the National Institutes of Health, USA (# 5R01 AI 104822). This work was made possible through Victorian State Government (Australia) Operational Infrastructure Support and Australian Government NHMRC IRIISS. The authors also thank Wutthichai Chaimungkun, Supalarp Puangsa-art, Nipon Thanyavanich, Surapon Yimsamran, Wanchai Maneeboonyang, Patiwat Sa-angchai, Prasert Rukmanee, Sutthiporn Prommongkol, Chalermpon Kumpitak, Teerawat Saeseu, and Pornpimol Chobson for blood sample collection and technical assistance with molecular analyses.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpara.2019.01.004.
Contributor Information
Ivo Mueller, Email: mueller@wehi.edu.au.
Jetsumon Sattabongkot, Email: jetsumon.pra@mahidol.ac.th.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Baum E., Sattabongkot J., Sirichaisinthop J., Kiattibutr K., Davies D.H., Jain A., Lo E., Lee M.C., Randall A.Z., Molina D.M., Liang X., Cui L., Felgner P.L., Yan G. Submicroscopic and asymptomatic Plasmodium falciparum and Plasmodium vivax infections are common in western Thailand – molecular and serological evidence. Malar. J. 2015;14:95. doi: 10.1186/s12936-015-0611-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum E., Sattabongkot J., Sirichaisinthop J., Kiattibutr K., Jain A., Taghavian O., Lee M.C., Huw Davies D., Cui L., Felgner P.L., Yan G. Common asymptomatic and submicroscopic malaria infections in Western Thailand revealed in longitudinal molecular and serological studies: a challenge to malaria elimination. Malar. J. 2016;15:333. doi: 10.1186/s12936-016-1393-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhumiratana A., Intarapuk A., Sorosjinda-Nunthawarasilp P., Maneekan P., Koyadun S. Border malaria associated with multidrug resistance on Thailand-Myanmar and Thailand-Cambodia borders: transmission dynamic, vulnerability, and surveillance. Biomed. Res. Int. 2013;2013 doi: 10.1155/2013/363417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann N.E., Karl S., Wampfler R., Kiniboro B., Teliki A., Iga J., Waltmann A., Betuela I., Felger I., Robinson L.J., Mueller I. The complex relationship of exposure to new Plasmodium infections and incidence of clinical malaria in Papua New Guinea. Elife. 2017;6 doi: 10.7554/eLife.23708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imwong M., Nguyen T.N., Tripura R., Peto T.J., Lee S.J., Lwin K.M., Suangkanarat P., Jeeyapant A., Vihokhern B., Wongsaen K., Van Hue D., Dong le T., Nguyen T.U., Lubell Y., von Seidlein L., Dhorda M., Promnarate C., Snounou G., Malleret B., Renia L., Keereecharoen L., Singhasivanon P., Sirithiranont P., Chalk J., Nguon C., Hien T.T., Day N., White N.J., Dondorp A., Nosten F. The epidemiology of subclinical malaria infections in South-East Asia: findings from cross-sectional surveys in Thailand-Myanmar border areas, Cambodia, and Vietnam. Malar. J. 2015;14:381. doi: 10.1186/s12936-015-0906-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiattibutr K., Roobsoong W., Sriwichai P., Saeseu T., Rachaphaew N., Suansomjit C., Buates S., Obadia T., Mueller I., Cui L., Nguitragool W., Sattabongkot J. Infectivity of symptomatic and asymptomatic Plasmodium vivax infections to a Southeast Asian vector, Anopheles dirus. Int. J. Parasitol. 2017;47:163–170. doi: 10.1016/j.ijpara.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitvatanachai S., Janyapoon K., Rhongbutsri P., Thap L.C. A survey on malaria in mobile Cambodians in Aranyaprathet, Sa Kaeo Province, Thailand. Southeast Asian J. Trop. Med. Public Health. 2003;34:48–53. [PubMed] [Google Scholar]
- Koepfli C., Colborn K.L., Kiniboro B., Lin E., Speed T.P., Siba P.M., Felger I., Mueller I. A high force of Plasmodium vivax blood-stage infection drives the rapid acquisition of immunity in papua new guinean children. PLoS Negl. Trop. Dis. 2013;7 doi: 10.1371/journal.pntd.0002403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritsiriwuthinan K., Ngrenngarmlert W. Molecular screening of Plasmodium infections among migrant workers in Thailand. J. Vector Borne Dis. 2011;48:214–218. [PubMed] [Google Scholar]
- Li P., Zhao Z., Wang Y., Xing H., Parker D.M., Yang Z., Baum E., Li W., Sattabongkot J., Sirichaisinthop J., Li S., Yan G., Cui L., Fan Q. Nested PCR detection of malaria directly using blood filter paper samples from epidemiological surveys. Malar. J. 2014;13:175. doi: 10.1186/1475-2875-13-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longley R.J., Sattabongkot J., Mueller I. Insights into the naturally acquired immune response to Plasmodium vivax malaria. Parasitology. 2016;143:154–170. doi: 10.1017/S0031182015000670. [DOI] [PubMed] [Google Scholar]
- Mueller I., Schoepflin S., Smith T.A., Benton K.L., Bretscher M.T., Lin E., Kiniboro B., Zimmerman P.A., Speed T.P., Siba P., Felger I. Force of infection is key to understanding the epidemiology of Plasmodium falciparum malaria in Papua New Guinean children. Proc. Natl. Acad. Sci. USA. 2012;109:10030–10035. doi: 10.1073/pnas.1200841109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muenworn V., Sungvornyothin S., Kongmee M., Polsomboon S., Bangs M.J., Akrathanakul P., Tanasinchayakul S., Prabaripai A., Chareonviriyaphap T. Biting activity and host preference of the malaria vectors Anopheles maculatus and Anopheles sawadwongporni (Diptera: Culicidae) in Thailand. J. Vector Ecol. 2009;34:62–69. doi: 10.1111/j.1948-7134.2009.00008.x. [DOI] [PubMed] [Google Scholar]
- Nguitragool W., Mueller I., Kumpitak C., Saeseu T., Bantuchai S., Yorsaeng R., Yimsamran S., Maneeboonyang W., Sa-Angchai P., Chaimungkun W., Rukmanee P., Puangsa-Art S., Thanyavanich N., Koepfli C., Felger I., Sattabongkot J., Singhasivanon P. Very high carriage of gametocytes in asymptomatic low-density Plasmodium falciparum and P. vivax infections in western Thailand. Parasit. Vectors. 2017;10:512. doi: 10.1186/s13071-017-2407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker D.M., Matthews S.A., Yan G., Zhou G., Lee M.C., Sirichaisinthop J., Kiattibutr K., Fan Q., Li P., Sattabongkot J., Cui L. Microgeography and molecular epidemiology of malaria at the Thailand-Myanmar border in the malaria pre-elimination phase. Malar. J. 2015;14:198. doi: 10.1186/s12936-015-0712-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J.C., Lucchi N.W., Srivastava P., Lin J.T., Sug-Aram R., Aruncharus S., Bharti P.K., Shukla M.M., Congpuong K., Satimai W., Singh N., Udhayakumar V., Meshnick S.R. Field evaluation of a real-time fluorescence loop-mediated isothermal amplification assay, RealAmp, for the diagnosis of malaria in Thailand and India. J. Infect. Dis. 2014;210:1180–1187. doi: 10.1093/infdis/jiu252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phimpraphi W., Paul R.E., Yimsamran S., Puangsa-art S., Thanyavanich N., Maneeboonyang W., Prommongkol S., Sornklom S., Chaimungkun W., Chavez I.F., Blanc H., Looareesuwan S., Sakuntabhai A., Singhasivanon P. Longitudinal study of Plasmodium falciparum and Plasmodium vivax in a Karen population in Thailand. Malar. J. 2008;7:99. doi: 10.1186/1475-2875-7-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson L.J., Wampfler R., Betuela I., Karl S., White M.T., Li Wai Suen C.S., Hofmann N.E., Kinboro B., Waltmann A., Brewster J., Lorry L., Tarongka N., Samol L., Silkey M., Bassat Q., Siba P.M., Schofield L., Felger I., Mueller I. Strategies for understanding and reducing the Plasmodium vivax and Plasmodium ovale hypnozoite reservoir in Papua New Guinean children: a randomised placebo-controlled trial and mathematical model. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosanas-Urgell A., Mueller D., Betuela I., Barnadas C., Iga J., Zimmerman P.A., del Portillo H.A., Siba P., Mueller I., Felger I. Comparison of diagnostic methods for the detection and quantification of the four sympatric Plasmodium species in field samples from Papua New Guinea. Malar. J. 2010;9:361. doi: 10.1186/1475-2875-9-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepflin S., Valsangiacomo F., Lin E., Kiniboro B., Mueller I., Felger I. Comparison of Plasmodium falciparum allelic frequency distribution in different endemic settings by high-resolution genotyping. Malar. J. 2009;8:250. doi: 10.1186/1475-2875-8-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriwichai P., Samung Y., Sumruayphol S., Kiattibutr K., Kumpitak C., Payakkapol A., Kaewkungwal J., Yan G., Cui L., Sattabongkot J. Natural human Plasmodium infections in major Anopheles mosquitoes in western Thailand. Parasit. Vectors. 2016;9:17. doi: 10.1186/s13071-016-1295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriwichai P., Karl S., Samung Y., Kiattibutr K., Sirichaisinthop J., Mueller I., Cui L., Sattabongkot J. Imported Plasmodium falciparum and locally transmitted Plasmodium vivax: cross-border malaria transmission scenario in northwestern Thailand. Malar. J. 2017;16:258. doi: 10.1186/s12936-017-1900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tananchai C., Tisgratog R., Juntarajumnong W., Grieco J.P., Manguin S., Prabaripai A., Chareonviriyaphap T. Species diversity and biting activity of Anopheles dirus and Anopheles baimaii (Diptera: Culicidae) in a malaria prone area of western Thailand. Parasit. Vectors. 2012;5:211. doi: 10.1186/1756-3305-5-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ThaiDDC . Ministry of Public Health; Thailand: 2016. Annual Report 2016. Department of Disease Control. [Google Scholar]
- ThaiMoPH, 2017. Thailand Malaria Elimination Program Ministry of Public Health, Thailand. http://203.157.41.215/malariar10/index_newversion.php.
- Therneau T.M., Grambsch P.M. Springer; New York: 2000. Modeling Survival Data: Extending the Cox Model. [Google Scholar]
- Tisgratog R., Tananchai C., Juntarajumnong W., Tuntakom S., Bangs M.J., Corbel V., Chareonviriyaphap T. Host feeding patterns and preference of Anopheles minimus (Diptera: Culicidae) in a malaria endemic area of western Thailand: baseline site description. Parasit. Vectors. 2012;5:114. doi: 10.1186/1756-3305-5-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wampfler R., Mwingira F., Javati S., Robinson L., Betuela I., Siba P., Beck H.P., Mueller I., Felger I. Strategies for detection of Plasmodium species gametocytes. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0076316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M.T., Karl S., Koepfli C., Longley R.J., Hofmann N.E., Wampfler R., Felger I., Smith T., Nguitragool W., Sattabongkot J., Robinson L., Ghani A., Mueller I. Plasmodium vivax and Plasmodium falciparum infection dynamics: re-infections, recrudescences and relapses. Malar. J. 2018;17:170. doi: 10.1186/s12936-018-2318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2015. World Malaria Report 2015. [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2016. World Malaria Report 2016. [Google Scholar]
- WPRO . World Health Organization Regional Office for the Western Pacific; Manila, Philippines: 2015. Strategy for Malaria Elimination in the Greater Mekong Subregion: 2015–2030. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.