Abstract
Background
In recent years, numerous bacteria have become resistant to conventional antibiotics. Fortunately, an increasing body of research indicates that through the addition of specific metabolites (like sugars), the antibacterial activity of certain drugs can be enhanced. A new type of self-assembled nano-peptide amphiphile (SANPA) was designed in this study to treat antibiotic-resistant bacterial infections and to reduce the use of antibiotics.
Methods
Here, SANPAs were self-assembled into nanorod structures with a diameter of ca. 10.5 nm at concentrations greater than the critical micelle concentration (CMC) of 44.67 μM. Both Gram-positive and Gram-negative bacteria were treated with SANPAs with fructose supplementation.
Results
After a 30-min fructose pre-incubation, SANPAs reduced bacteria growth relative to non-fructose treatments at all concentrations. Cytotoxicity assays indicated that the presence of fructose seemed to slightly ameliorate the cytotoxic effect of the treatment on model human fetal osteoblasts (or bone-forming cells) and human dermal fibroblasts.
Conclusion
We demonstrated here that SANPAs-like nanomaterials have a promising potential to treat antibiotic-resistant bacteria, especially when added to fructose, potentially limiting their associated infections.
Keywords: MRSA, MDR-E.coli, bacteria, fructose, antibiotic resistance, nano peptide amphiphiles
Introduction
The Centers for Disease Control (CDC) highlighted the unprecedented problem we will have with antibiotic-resistant bacteria in the years to come. Specifically, by 2050, the CDC predicts more death from bacteria than all cancers combined.1 This is because numerous Gram-positive and Gram-negative bacteria have become resistant to conventional antibiotics, presenting significant problems for the treatment of bacterial infectious diseases. To overcome bacterial drug resistance and to avoid or lower the administration of antibiotics, several kinds of nanomaterials have been recently developed, including nanotubes, nanospheres, nanofibers, nanotapes and hydrogels.2
In particular, antimicrobial peptides (AMPs) act as excellent building-blocks for bionanotechnology due to the ease of their synthesis, small size to penetrate biofilms and bacteria, relative stability and their ability to form from different amino acid sequences.2 AMPs are typically composed of 12–50 amino acids with an overall positive charge along with hydrophobic fatty acid components.3 AMPs can be found in many living organisms and have been found to eliminate pathogenic microorganisms, including bacteria, fungi, and viruses.4–7 Since AMPs possess multiple modes of action including targeting the bacterial cell membrane, it is less likely for microbes to develop a resistance towards them; therefore, AMPs are considered as some of the most promising antibiotic candidates.8,9 In particular, the overall positively charged AMPs bind to negatively charged bacteria cell membranes via a non-specific physical process, compared with conventional antibiotics that act on a specific target. Therefore, the bacteria resistance that develops to antibiotics will most likely not occur when treated with AMPs as an alternative. In addition, compared with bacteria, AMPs have low toxicity to mammalian cells due to the presence of membrane cholesterol that either stabilizes the lipid bilayer or interacts with the peptide.10,11
Besides discovering new antimicrobial agents, new therapeutic approaches are also required to solve the challenging antibiotic resistance issue. Indeed, recent studies have shown that supplementing drugs with specific metabolites can greatly reduce bacterial tolerance and enhance efficacy. The proposed mechanism of fructose enhancement includes that the transported metabolites in the cytoplasm enter glycolysis, generating NADH, which is then oxidized by enzymes that subsequently elevate the proton-motive force (PMF). This PMF promotes the uptake of developed drug molecules (such as AMPs)12–14 into bacteria.
Taking advantage of AMPs and the stimulation that metabolites may induce to increase the amount of drug uptake into bacteria, we hypothesized here that AMPs with fructose supplements would exhibit greater antimicrobial efficacy compared to the same compound without metabolite supplements. Here, self-assembled peptide amphiphile nanoparticles were designed, synthesized, and characterized. The peptide was designed with β-sheet forming properties, about 43.75% hydrophobic amino acid content, and 9 positively charged amino acids in each single molecule. The antimicrobial activity of SANPAs with supplemented fructose was examined with Gram-positive bacteria Staphylococcus aureus (S. aureus), methicillin-resistant S. aureus (MRSA), and Gram-negative bacteria Escherichia coli (E. coli) and multidrug-resistant-E. coli (MDR-E. coli). Furthermore, their cytotoxicity towards mammalian cells was tested, and the effects of supplemented fructose are discussed. Although recent studies indicated that passive AMP resistance may occur in certain bacterial species,15 this resistance phenomenon was not observed among the bacteria we tested, including antibiotic resistance species: MRSA and MDR-E. coli. In summary, this work suggests a strategy for improving the antibacterial efficacy of AMPs based on sugar metabolism and highlights the importance of the metabolic environment to treat antibiotic-resistant bacterial infections.
Materials and Methods
Preparation of Peptide Amphiphile Self-Assembled Nanoparticles
The synthesized nano-peptide amphiphiles were purchased from Biomatik (Ontario, Canada) with a purity of 96.6% as certified via high-performance liquid chromatography (HPLC). Lyophilized nano-peptide amphiphile powders (MW = 2423.15 g/mole) were re-suspended in sterile deionized water (DI water, Milli-Q system) followed by vortexing and sonicating (for an additional 60 seconds) at room temperature. The stock solution was then kept away from light at 4 °C for later use. To visualize a direct and detailed view of SANPA molecular structure, and to further simulate AMPs interactions with bacterial membrane models in the future study, a single nanorod model was conducted with Materials Studio® software (Accelrys Inc., San Diego, CA, USA). Firstly, a peptide layer was built with 16 single peptide molecules. Then, a single cylindrical nanorod structure composed of 16 peptide layers was assembled with antiparallel layer arrangement with H-bond between neighboring layers.
Preparation of Fructose Stock Solution
D(-)fructose (36 mg, Sigma-Aldrich, St. Louis, MO) was dissolved in 20 mL of DI water to make a 10-mM concentration and then was separated evenly into two 15-mL tubes for respective cell and bacteria studies.
Transmission Electron Microscopy (TEM) Characterization
The morphologies of SANPAs were visualized using TEM (JEM-1010, Peabody, MA). Briefly, 10 μL of the 100-μM SANPA samples were applied onto a 300-mesh copper-coated carbon grid (Electron Microscopy Sciences, Hatfield, PA), and then negatively stained using a 1.5% uranyl acetate solution thrice and air-dried for 15 min before imaging. After obtaining the TEM images, ImageJ (National Institute of Health, USA; ImageJ) was used to measure the diameters of the individual SANPA nanorods.
Determination of the Critical Micelle Concentration (CMC)
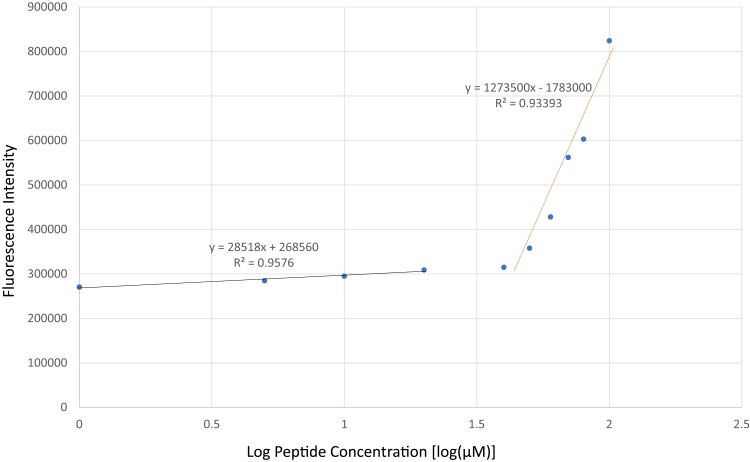
The CMC of SANPAs was determined using a solvatochromic Nile Red dye (9-diethylamino-5-benzo-[a]-phenoxazinone, Thermo Fisher, Waltham, MA, USA).16 The mechanism here is that Nile Red is mostly non-fluorescent in water or other polar solvents but undergoes fluorescence enhancement in nonpolar environments, with a large blue shift for both absorption and emission. In brief, 0.5 μL of a 2.5 mM Nile Red solution was added to 500 μL of the peptide solutions at varying concentrations from 0 mM to 100 mM. One hundred μL of each mixed concentration was transferred in triplicate to a 96-well plate. Next, the emission spectra of the Nile Red were recorded from 600-nm to 750-nm wavelength with 5-nm intervals by using a spectrophotometer (SpectraMax M3, Molecular Devices, Sunnyvale, CA, US) with an excitation wavelength of 550 nm. The corrected fluorescence intensity at 642 nm (λmax) was plotted against the log of peptide concentration, and then the estimated CMC was determined by the greatest slope to intersect with the baseline defined by the lowest concentration. The x-value of this intersection was used as the CMC.
Zeta Potential Measurements
A concentration of 100 μM SANPAs was prepared from a stock solution and diluted with sterile deionized water, followed by sonication for 60 s at room temperature. The measurements of the SANPAs Zeta potential were completed using a ZS90 Nanosizer (Malvern Instruments, Malvern, UK) and software provided by the manufacturer. One milliliter of each sample was measured over 10 runs.
Dynamic Light Scattering (DLS)
Six hundred μL of the 100-μM SANPAs solution was prepared and tested using the same machine for Zeta Potential measurements and particle sizing software provided by the manufacturer. The sample was measured over 10 runs.
Bacteria Culture
In total, four strains of both Gram-positive and Gram-negative bacteria were used: Staphylococcus aureus (S. aureus, ATCC no. 25923), Methicillin-resistant S. aureus (MRSA, ATCC no. 43300), Escherichia coli (E. coli, ATCC no. 25922), and Multidrug-resistant E. coli (MDR E. coli, ATCC no. BAA-2471). Each hydrated strain was seeded onto tryptic soy agar (Sigma-Aldrich, St. Louis, MO). A single colony was isolated and re-suspended in 5-mL tryptic soy broth media (TSB; Sigma-Aldrich, St. Louis, MO) for each trial. Then, the bacteria suspension was placed onto a 200 RPM shaker located in a 37 °C shaking incubator overnight. A spectrophotometer (SpectraMax M3, Molecular Devices, Sunnyvale, CA) was used to perform optical density measurements, and the bacteria culture was then adjusted to an optical density of about 0.52 (109 cells/mL) at 562 nm (OD562). Subsequently, each bacterial sample was diluted to about 106 colony-forming units per milliliter (CFU/mL).
Bacterial Colony Counting
Bacteria (S. aureus, MRSA, E. coli, MDR-E. coli) were seeded in a 96-well plate (Thermo Fisher Scientific, Waltham, MA), and then were pre-incubated with 5 μL of a 10-mM fructose solution for 30 min before being treated with varying amounts of SANPAs to reach a final concentration of 0, 20, 40, 60, 80, and 100 μM. The well plate was then placed in a stationary incubator maintained at 37°C and 5% CO2/95% air for 4 h. After incubation, serial dilutions of the samples were pipetted onto TSB agar plates (3 X 10 μL per dilution). CFU were manually counted after ca. 12 hrs of incubation. For each sample, the results were plotted as the log10 values of the CFU number.
Cell Culture
Human dermal fibroblast cells (HDF, ATCC no. CCL-110, passage No. 5–9) were cultured as recommended by ATCC in 45% Ham’s F12 medium, 45% Dulbecco’s modified Eagles Medium (DMEM; Fisher, Pittsburgh, PA), 10% fetal bovine serum (ATCC SCRR-30-2020), and 1% penicillin/streptomycin (ATCC 30–2300). Cells were grown at 37 °C and 5% CO2. Human fetal osteoblast cells (hFOB, ATCC no. CRL-11,372, passage No. 4–8) were cultured in osteoblast medium (PromoCell) supplemented with OB GM supplemental mix (PromoCell) under 34 °C and 5% CO2. Medium was changed every 2–3 days.
Cytotoxicity
The cytotoxicity of the SANPAs was determined using an MTS cell viability assay kit (Promega, Madison, WI, USA). Cells were seeded onto tissue-culture-treated 96-well plates (Thermo Fisher Scientific, Waltham, MA) at a density of approximately 10,000 cells/cm2. After overnight cell adhesion, half of the samples were pre-incubated by adding 5 μL of a 10-mM fructose solution for 30 min, and the cells were then co-cultured with SANPAs at various concentrations (0, 20, 40, 60, 80, and 100 μM) for 24 h. The treatment media were then removed, and cells were washed with phosphate buffered saline (PBS, pH = 7.4, Fisher, PA, USA). A mixture of 100-μL fresh media and 20-μL MTS reagent was added to each well. After an additional 3.5 h of incubation, the absorbance at 490 nm was measured using a spectrophotometer (SpectraMax M3, Molecular Devices, Sunnyvale, CA). The density of the adherent cells was determined by comparing the resulting absorbance values to a standard curve for each cell line.
Statistical Analysis
All experiments were repeated in triplicate to ensure the reliability of results. Statistical significance was considered at p ≤ 0.05 using a two-tailed Student’s t-test. Results are expressed as the mean ± standard error of the mean (S.E.M.).
Results and Discussion
Self-Assembly of Peptide Amphiphiles
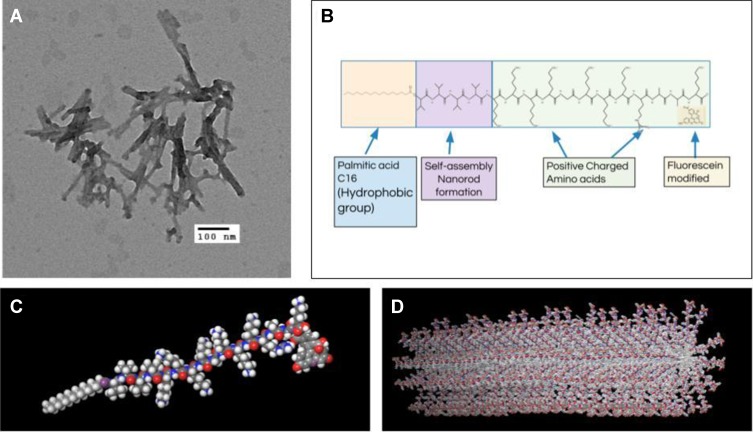
First, Nile Red was used to determine the CMC of SANPAs after the self-assembled peptide amphiphile nanoparticles were prepared. Results demonstrated that the SANPAs self-assembled at a concentration of approximately 44.67 μM [Figure 1]. TEM revealed that the SANPAs were nanorod in shape with an average outer diameter of 9.8 nm [Figure 2A]. DLS validated SANPA nanorods with an 8.5-nm average diameter and length ranging from 45 nm to 73 nm. The difference in diameter between the results from TEM and DLS is because in TEM, SANPAs are negatively stained, and therefore the outer diameter includes the additional thickness from the stain. The SANPAs chemical structure was proposedly designed with a C16 palmitic acid group combined with 4 valine groups to form a self-assembled nanorod suprastructure, and positively charged Lysine and Arginine groups enable the peptides to approach the negatively charged bacterial membranes by electrostatic interaction [Figure 2B]. Zeta potential measurements confirmed that SANPAs were positively charged at 52.17 mv. Figure 2C and D illustrate the SANPA single-molecule chemical structure and self-assembled cylindrical nanorod structure, respectively. The palmitic C16 hydrophobic core stays in the center of the structure, polar head groups expose on the surface, while the β-sheet segment and lysine spacer connect the core and head groups to form a nanorod during the self-assembly process.
Figure 1.
Determination of CMC. The curves from the fluorescence intensity of the Nile Red dye (Ex=550 nm) against log10 values of the peptide concentration. The result indicated that SANPAs induced enhancement of Nile Red uptake.
Figure 2.
Structures of SANPAs. (A) Transmission electron microscopy (TEM) image of SANPAs. Scale bar = 100 nm. (B) Chemical structure of self-assembled C16VVVVKKKKGKKKRAAK(FITC) amphiphilic peptide. (C) SANPA single-molecule structure. (D) Self-assembled SANPAs nanorod structure.
Fructose-Enhanced Antibacterial Effects of Peptides Against Gram-Positive and Gram-Negative Bacteria
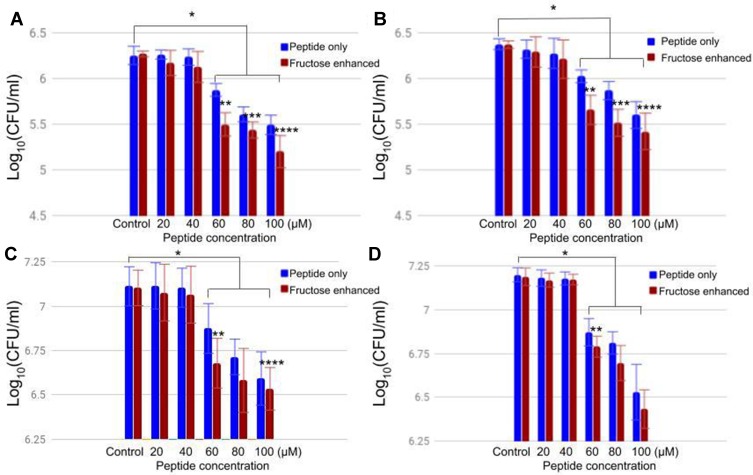
Both SANPAs treatments with and without fructose pre-incubation significantly inhibited the growth of Gram-positive (S. aureus and MRSA) and Gram-negative bacteria (E. coli and MDR E. coli), as determined by colony counting assays [Figure 3]. In this assay, bacteria were treated with peptides for 4 h, and the results are displayed by log10 [CFU/mL]. For both Gram-positive and Gram-negative bacteria, peptide concentrations above CMC indicated significant antibacterial activity; however, at lower concentrations than CMC, the peptides showed no significant reduction in bacterial activity compared with control samples. The decrease in bacterial density was dependent on the concentration of SANPAs. For Gram-positive bacteria, treatments with the fructose pre-incubation showed a significant decrease in bacterial activity compared to non-fructose-treated samples at the same peptide concentration. For Gram-negative bacteria, fructose also enhanced the antimicrobial activity for E. coli at 60 μM and 100 μM, and MDR-E. coli at 60 μM compared with fructose-free treatments. However, for other peptide concentrations, fructose exhibited no significant difference relative to the peptide-TSB only treatments for Gram-negative bacteria. Therefore, a concentration higher than CMC is recommended for antibacterial and cytotoxicity studies.
Figure 3.
Effects of SANPAs treatment with and without 50-μM fructose pre-incubation on Gram-positive (A) S. aureus, (B) MRSA, and Gram-negative (C) E. coli, (D) MDR-E. coli bacteria for 4 h. The seeding density was 106 CFU/mL, and x-axis shows the concentrations of SANPAs. Samples treated with peptide only are in blue, samples treated in addition with 30-min fructose pre-incubation are in red. Data was expressed by standard error of the mean (± S.E.M.), and N=3, *p<0.05 compared with control samples (0-μM peptide and 0μM fructose), **p<0.05 compared with 60-μM peptide with no fructose pre-incubation, ***p<0.05 compared with 80-μM peptide with no fructose pre-incubation, and ****p<0.05 compared with 100-μM peptide with no fructose pre-incubation.
In vitro Cytotoxicity
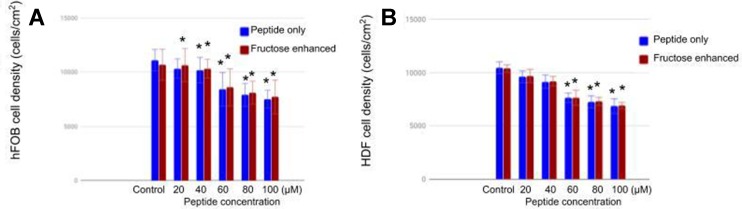
The effect of SANPAs/fructose treatments on mammalian cells was assessed using MTS assays, as described in the Methods section. Human fetal osteoblast (hFOB) and human dermal fibroblast (HDF) cells were incubated under a SANPAs treatment for 24 h. For the fructose-enhanced samples, cells were pre-incubated for 30 min before a 24-h treatment with SANPAs. Results [Figure 4] showed that SANPAs had lower toxicity toward both hFOB and HDF than that tested on bacteria. The SANPAs-only solution at 20 μM did not have a significant toxic effect on hFOB [Figure 4A], as well as on HDF for all of the concentrations below 40 μM [Figure 4B]. Although it had moderate cytotoxicity at higher concentrations, SANPAs still killed more bacteria than hFOB and HDF at the same concentration range. Here, the most cytotoxic treatment was the 100-μM peptide without fructose, where both of the cell viabilities were still above 65%; however, for treatments on bacteria, SANPA caused about a 10-fold decline in bacteria density in general. Moreover, the pre-incubation of fructose seemed to slightly ameliorate the cytotoxic effect of the treatment to a minor degree, although this was not statistically significant and requires more investigation. The mechanism of fructose protective effects might be that fructose slows down adenosine triphosphate (ATP) depletion caused by the drug treatments.17 The relatively high cellular ATP levels compared with non-fructose treatment ameliorated the SANPAs cytotoxicity toward mammalian cells.17 Although the performance for SANPAs was not ideal, it still exhibited higher inhibitory effects on bacteria than hFOB and HDF cells.
Figure 4.
hFOB (A) and HDF (B) cell density after treatment with varying concentrations of SANPAs and fructose supplementation for 24 h. Samples treated with peptide only are in blue, samples treated in addition to 30-min fructose pre-incubation are in red. Data were expressed as a percentage of living cells over the control sample (0-μM peptide and 0-μM fructose), and error bars indicate standard error of the mean (±S.E.M.), and N=3, *p<0.05 compared with the control sample.
Conclusion
The structure design and antibacterial effects of self-assembled peptide amphiphiles have attracted increasing attention in both research and medical applications over the past decade. In this study, we designed and characterized rod-shaped nanoparticles self-assembled from antimicrobial peptide amphiphiles. At concentrations above the CMC, SANPAs self-assembled into nanorods with an average diameter of about 10.5 nm. Four bacteria strains (S. aureus, MRSA, E. coli, and MDR-E. coli) were treated with SANPAs, including half of the samples pre-incubated with fructose prior to the treatment. The results indicated that SANPAs have excellent antimicrobial properties against both Gram-positive and Gram-negative bacteria above the CMC, regardless of whether or not the bacteria are antibiotic-resistant. Furthermore, for the concentration under the CMC, SANPAs showed minor antibacterial activity and cytotoxicity due to the limited amount of self-assembled nanorods in solution. Peptides with fructose supplementation exhibited a significant reduction of bacteria compared with samples at the same peptide concentration without fructose. This study thus implies that using fructose as an adjuvant to SANPAs would be beneficial in the treatment of chronic bacterial infections. Future studies will focus more on the sequence design and therapeutic approach combinations to reduce or replace the use of antibiotics with SANPAs.
Acknowledgment
The authors gratefully acknowledge Northeastern University for funding and William Fowle for assistance with TEM.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Shallcross LJ, Howard SJ, Fowler T, Davies SC. Tackling the threat of antimicrobial resistance: from policy to sustainable action. Philos Trans Royal Soc B. 2015;370(1670). doi: 10.1098/rstb.2014.0082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colombo G, Soto P, Gazit E. Peptide self-assembly at the nanoscale: a challenging target for computational and experimental biotechnology. Trends Biotechnol. 2007;25(5):211–218. doi: 10.1016/j.tibtech.2007.03.004 [DOI] [PubMed] [Google Scholar]
- 3.Hancock REW, Sahl H-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotech. 2006;24:1551–1557. doi: 10.1038/nbt1267 [DOI] [PubMed] [Google Scholar]
- 4.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098 [DOI] [PubMed] [Google Scholar]
- 5.Aerts AM, Francois IE, Cammue BP, Thevissen K. The mode of antifungal action of plant, insect and human defensins. Cell Mol Life Sci. 2008;65:2069–2079. doi: 10.1007/s00018-008-8035-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuwano K, Tanaka N, Shimizu T, Kida Y. Antimicrobial activity of inducible human beta defensin-2 against Mycoplasma pneumoniae. Curr Microbiol. 2006;52:435–438. doi: 10.1007/s00284-005-0215-7 [DOI] [PubMed] [Google Scholar]
- 7.Klotman ME, Chang TL. Defensins in innate antiviral immunity. Nat Rev Immunol. 2006;6:447–456. doi: 10.1038/nri1860 [DOI] [PubMed] [Google Scholar]
- 8.Sarkar S, DeSantis ER, Kuper J. Resurgence of colistin use. Am J Health Syst Pharm. 2007;64:2462–2466. doi: 10.2146/ajhp060501 [DOI] [PubMed] [Google Scholar]
- 9.Domalaon R, Zhanel GG, Schweizer F. Short antimicrobial peptides and peptide scaffolds as promising antibacterial agents. Curr Top Med Chem. 2016;16:1217–1230. doi: 10.2174/1568026615666150915112459 [DOI] [PubMed] [Google Scholar]
- 10.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a [DOI] [PubMed] [Google Scholar]
- 11.Porter EA, Wang X, Lee HS, Weisblum B, Gellman SH. Non-haemolytic b-amino acid oligomers. Nature. 2000;404:565. doi: 10.1038/35007145 [DOI] [PubMed] [Google Scholar]
- 12.Allison KR, Brynildsen MP, Collins JJ. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature. 2011;473(7346):216–220. doi: 10.1038/nature10069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durmus NG, Taylor EN, Inci F, Kummer KM, Tarquinio KM, Webster TJ. Fructose-enhanced reduction of bacterial growth on nanorough surfaces. Int J Nanomed. 2012;7:537–545. doi: 10.2147/IJN.S27957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durmus NG, Taylor EN, Kummer KM, Webster TJ. Enhanced efficacy of superparamagnetic iron oxide nanoparticles against antibiotic-resistant biofilms in the presence of metabolites. Adv Mater. 2013;25:5706–5713. [DOI] [PubMed] [Google Scholar]
- 15.Andersson D, Hughes D, Kubicek-Sutherland J. Mechanisms and consequences of bacterial resistance to antimicrobial peptides. Drug Resist Updat. 2016;26:43–57. doi: 10.1016/j.drup.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 16.Chang R, Subramanian K, Wang M, Webster TJ. Enhanced antibacterial properties of self-assembling peptide amphiphiles functionalized with heparin-binding cardin-motifs. ACS Appl Mater Interfaces. 2017;9(27):22350–22360. doi: 10.1021/acsami.7b07506 [DOI] [PubMed] [Google Scholar]
- 17.Nakagawa Y, Suzuki T, Inomata A. Preventive effects of fructose and N‐acetyl‐L‐cysteine against cytotoxicity induced by the psychoactive compounds N‐methyl‐5‐(2‐aminopropyl)benzofuran and 3,4‐methylenedioxy‐N‐methamphetamine in isolated rat hepatocytes. J Appl Toxicol. 2018;38(2):284–291. doi: 10.1002/jat.3523 [DOI] [PubMed] [Google Scholar]