Abstract
Introduction
The availability of potent antiretroviral therapy has transformed HIV infection into a chronic disease such that people living with HIV (PLWH) have a near normal life expectancy. However, there are continuing challenges in managing HIV infection, particularly in older patients, who often experience age‐related comorbidities resulting in complex polypharmacy and an increased risk for drug‐drug interactions. Furthermore, age‐related physiological changes may affect the pharmacokinetics and pharmacodynamics of both antiretrovirals and comedications thereby predisposing elderly to adverse drug reactions. This review provides an overview of the therapeutic challenges when treating elderly PLWH (i.e. >65 years). Particular emphasis is placed on drug‐drug interactions and other common prescribing issues (i.e. inappropriate drug use, prescribing cascade, drug‐disease interaction) encountered in elderly PLWH.
Discussion
Prescribing issues are common in elderly PLWH due to the presence of age‐related comorbidities, organ dysfunction and physiological changes leading to a higher risk for drug‐drug interactions, drugs dosage errors and inappropriate drug use.
Conclusions
The high prevalence of prescribing issues in elderly PLWH highlights the need for ongoing education on prescribing principles and the optimal management of individual patients. The knowledge of adverse health outcomes associated with polypharmacy and inappropriate prescribing should ensure that there are interventions to prevent harm including medication reconciliation, medication review and medication prioritization according to the risks/benefits for each patient.
Keywords: HIV, ageing, comorbidities, polypharmacy, drug‐drug interactions, prescribing issues
1. Introduction
Effective antiretroviral treatments mean that persons living with HIV (PLWH) have a chronic disease with a life expectancy close to the general population 1, 2, 3, although there are differences in estimates when considering parameters such as HIV transmission risk group, lifestyle, race, gender or CD4 cell counts at treatment initiation 4. Older PLWH includes patients infected at an older age as well as patients diagnosed previously and who are ageing with HIV infection 5. Several modelling studies 6, 7 have projected the increase in median age of patients on antiretroviral treatment over the next decade. Forty percent of the HIV population will be constituted of PLWH aged ≥60 years of whom 28% are predicted to have ≥3 comorbidities 6.
However, age‐related comorbidities result in complex polypharmacy and an increased risk for drug‐drug interactions (DDIs). Furthermore, physiological changes related to ageing may affect pharmacokinetics and pharmacodynamics thereby putting elderly PLWH at risk of inappropriate prescribing. It should be noted that according to the World Health Organization 8, the term elderly refers to ≥65 years old.
Ageing leads to physiological, anatomical and biological modifications that can alter drug pharmacokinetics 9, 10. These changes include a reduced gastric acid secretion and a delayed gastric emptying time, although the clinical relevance remains unclear 10. Drug distribution may be impaired by the reduction in total body water and lean body mass with a relative increase in body fat and an increased distribution of lipophilic drugs. In addition, decreased serum albumin leads to an increase in unbound drug and uptake in peripheral tissue. The observed decrease in hepatic clearance (30% to 40%) in older age results from the decline in both liver mass and blood flow rather than changes in the activity of hepatic enzymes 11. Liver mass reduces by 10% to 15% and by 20% per age decade after the age of 65 years in women and men respectively 10. For many drugs, the most apparent effect of ageing is the progressive decrease in renal clearance explained by a lower glomerular filtration rate 10 resulting in a reduced clearance of renally eliminated drugs. Since elderly individuals are often excluded from clinical trials, there is a lack of data on the effect of ageing on the pharmacokinetics of antiretroviral drugs. Available data have shown that the exposure of the non‐nucleoside reverse transcriptase inhibitor (NNRTI) efavirenz and the integrase inhibitor (INI) raltegravir was not significantly changed in PLWH >60 or 45 to 79 years, whereas plasma concentrations of protease inhibitors (PI) were increased 12, 13, 14. Ageing impacted differently nucleoside reverse transcriptase inhibitors (NRTI)/nucleotide reverse transcriptase inhibitors (NtRTI) as tenofovir exposure was reduced by 8% to 13%, whereas conversely emtricitabine was increased by 19% to 73% in PLWH ≥55 years 15. Dolutegravir maximal concentrations were shown to be increased by 25% in PLWH ≥60 years, however, this change did not modify sleep or daytime functioning 16. There is a need for more pharmacokinetic data in elderly PLWH, especially those with comorbidities or frailty.
The pharmacodynamics can also be impacted by age‐associated physiological changes leading in a more or less pronounced drug effect, particularly for cardiovascular or central nervous systems drugs. The modification of the pharmacodynamic effect is driven by changes in the affinity to receptor sites or in their number as well as the alteration of homeostatic processes with advanced age 17. One important example relates to the reduction in cholinergic receptors in the brain which means that elderly PLWH are more likely to experience central anticholinergic adverse reactions (i.e. cognitive impairment, delirium) therefore drugs with anticholinergic properties should be avoided 18.
Altogether, the presence of comorbidities and age‐related physiological changes predispose elderly PLWH not only to the well‐known risk of DDI with antiretroviral drugs but also to other prescribing issues as discussed in the following sections.
2. Discussion
2.1. Comorbidities among HIV‐positive individuals
As summarized in Table 1, commonly observed comorbidities which may contribute to the issue of polypharmacy in ageing PLWH are hypertension, dyslipidaemia, diabetes mellitus, kidney disease, cardiovascular disease, respiratory disorders, bone disorders or cancer. Of interest, several studies have reported a higher prevalence of comorbidities in PLWH compared to age‐matched uninfected individuals 5, 19, 20, 21, 22, 23, 24, 25, 26, 27. Furthermore, multimorbidity defined by the concurrent presence of ≥2 comorbidities has been shown to be significantly higher in PLWH compared to uninfected controls, particularly in those with a long history of HIV infection 5, 19, 25.
Table 1.
Prevalence of comorbidities in ageing PLWH (when available comorbidities in age‐matched uninfected individuals are presented in italic)
| Country data source | Population | Mean age, years | Diabetes, % | Dyslipidaemia, % | Hypertension, % | Renal disease, % | CVD disease, % | Bone disorder, % | Respiratory disorder, % | Cancer, % |
|---|---|---|---|---|---|---|---|---|---|---|
| Brazil 28 | 451 PLWH | 58 | 14.9 | 26.7 | 6.7 | 3.1 | ||||
| 3 HIV centres | ||||||||||
| Brazil 29 | 208 PLWH | 57 | 22.6 | 62.0 | 16.8 | 9.6 | 52.9 | 10.6 | ||
| Brazilian cohort | 208 HIV neg | 57 | 28.4 | 69.7 | 6.7 | 12.5 | 10.1 | 6.3 | ||
| USA 30 | 2359 PLWH | 71 | 25.9 | 35.7 | 47.9 | 20.9 | 20.3 | 31.3 | ||
| Medicare | 2 mio HIV neg | 76 | 24.1 | 46.9 | 59.4 | 19.6 | 21.4 | 26.3 | ||
| Portugal 31 | 401 PLWH | 59 | 13.5 | 60.8 | 39.7 | 8.0 | 5.7 | 9.0 | 8.0 | |
| 7 HIV centres | ||||||||||
| France 32 | 16436 PLWH | 56 | 9.1 | 58.3 | 21.0 | 4.5 | 10.8 | 6.4 | 12.3 | |
| Dat'AIDS cohort | 572 PLWH | 78 | 22.0 | 60.8 | 43.5 | 29.4 | 23.4 | 12.6 | 22.9 | |
| France 19 | 10318 PLWH | 56 | 9.3 | 23.6 | 21.0 | 9.6 | 9.0 | 14.6 | ||
| 11 HIV centres | ||||||||||
| Europe 33 | 3797 | 50 to 60 | 8.0 | 79.5 | 79.0 | 7.0 | 7.0 | |||
| EuroSIDA cohorta |
PLWH 1837 PLWH |
≥60 | 17.0 | 84.0 | 84.0 | 23.0 | 15.5 | |||
| Italy 34 | 965 PLWH | 65 to 74 | 27.5 | 70.0 | 60.8 | 17.1 | 16.9 | 6.6 | ||
| GEPPO cohort | 224 HIV neg | 65 to 74 | 22.3 | 57.8 | 66.5 | 5.0 | 18.3 | 9.1 | ||
|
293 PLWH |
≥75 | 31.2 | 74.6 | 71.8 | 26.0 | 29.2 | 9.8 | |||
| 91 HIV neg | ≥75 | 15.4 | 50.0 | 67.0 | 10.0 | 30.8 | 18.9 | |||
| Switzerland 35 | 2233 PLWH | 50 to 64 | 7.0 | 69.8 | ||||||
| SHCS cohort | 450 PLWH | ≥65 | 16.2 | 78.9 |
CVD, cardiovascular; mio, million.
Study period 2014.
The earlier occurrence of age‐related comorbidities in PLWH compared to uninfected individuals may be explained by factors such as immune senescence or chronic immune activation 36 as well as lifestyle factors (e.g. smoking, alcohol consumption, recreational drug use), viral coinfections (e.g. hepatitis, sexually transmitted diseases) or toxicity of certain antiretroviral drugs 37. Metabolic disorders 38, 39, renal toxicity 40, or CNS side effects 39 are notably observed with the first‐generation antiretroviral drugs.
As expected, the number of comorbidities in PLWH has been shown to increase with age: 18.4% of PLWH aged ≥75 years from the French Dat'AIDS cohort had ≥4 comorbidities versus 4.3% of those aged 50 to 74 years 32. A similar picture is observed in the Swiss HIV Cohort Study (SHCS) as the number of age‐associated comorbidities is significantly higher in PLWH aged 65 years compared to those aged 50 to 64 years 35. Importantly, the study of the SHCS has demonstrated that the higher number of comorbidities with ageing is correlated with a higher use of comedications and consequently a higher risk to have polypharmacy 35.
Older age, obesity, smoking and duration of HIV infection have been associated with an increased risk for multimorbidity in PLWH 5, 34, 41. Of interest, comorbidities have been shown to co‐occur in the same individual in specific patterns. Furthermore, correlations have also been reported between patterns with, for instance a strong association between cardiovascular and metabolic diseases 42. Finally, comorbidities patterns were shown to have different risk factors with older age and a higher body mass index being risk factors for cardiovascular and metabolic disorders. Finally, comorbidities patterns were shown to affect differently health outcomes with, for example cardiovascular disease being associated with poorer physical health, higher risk of functional impairment, hospitalization and a higher number of medical visits 43. These findings could help the development of targeted interventions to prevent, treat and better manage multimorbidity in PLWH.
2.2. Polypharmacy
Polypharmacy is commonly defined as the concurrent administration of ≥5 medications, a cut‐off that has been associated with an increased risk of adverse health outcome 44. In HIV medicine, the term polypharmacy most often refers to non‐HIV medications given in addition to antiretroviral drugs. Polypharmacy has been shown to be common in PLWH aged ≥50 years, ranging from 15% up to 94% as reported by several HIV Cohort analyses summarized in Table 2 34, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57. Of interest, polypharmacy was shown to be less prevalent in the Ugandan cohort possibly due to limited access to care and/or medications 56. This assumption is supported by the observation that access to care, through medication insurance coverage and healthcare use, was shown to be a key driver for polypharmacy in the MACS cohort 57. Large cohort studies comparing the prevalence of polypharmacy in PLWH and age‐matched uninfected individuals have shown higher prevalence in infected individuals across different age categories 52, 58. Of interest, differences in the prevalence of polypharmacy between PLWH and age‐matched uninfected individuals were shown to be less pronounced in older age groups (i.e. 65 to 74 and ≥75 years) 30, 52. This finding may be explained by the natural occurrence of age‐related chronic diseases regardless of HIV infection.
Table 2.
Prevalence of polypharmacy (≥5 non‐HIV drugs) in PLWH aged 50 years and older
| Country | Number PLWH | Age, years | Polypharmacy, % | Reference |
|---|---|---|---|---|
| Switzerland | 111 | ≥75 | 60 | Livio et al. 2018 51 |
| Switzerland | 131 | ≥65 | 46 | Courlet et al. 2019 45 |
| Italy | 1258 | ≥65 | 37 | Guaraldi et al. 2018 34 |
| USA | 1311 | ≥65 | 43 | Justice et al. 2018 49 |
| USA | 89 | ≥60 | 74 | Greene et al. 2014 46 |
| USA | 1715 | ≥50 | 36 | Ware et al. 2019 57 |
| UK/Ireland | 698 | ≥50 | 30 | Halloran et al. 2019 47 |
| Spain | 10073 | ≥50 | 47 | Lopez‐Centeno et al. 2019 52 |
| Spain | 242 | ≥50 | 48 | Nunez‐Nunez et al. 2018 54 |
| USA | 248 | ≥50 | 94 | Mc Nicholl et al. 2017 53 |
| USA | 1312 | ≥50 | 54 | Holtzman et al. 2013 48 |
| Canada | 386 | ≥50 | 43 | Krentz et al. 2016 50 |
| Japan | 526 | ≥50 | 35 | Ruzicka et al. 2018 55 |
| Uganda | 411 | ≥50 | 15 | Ssonko et al. 2018 56 |
Medications implicated in polypharmacy belong mostly to drug classes used in older individuals such as cardiovascular drugs, gastro‐intestinal agents, hormone replacement therapies or antiplatelet/anticoagulant medications 52, 59.
Polypharmacy brings several challenges. The related increase in pill burden can have a negative effect of treatment adherence. Although available studies have shown inconsistent findings 60, 61, 62, 63, this is likely explained by the fact that adherence is a complex behaviour involving drug‐related but also psychological factors. Polypharmacy may increase the risk of adverse drug reactions due to the use of medications with overlapping side effects, which may convert asymptomatic side effects to a reason for hospitalization. Polypharmacy has been associated with several adverse health outcomes including physical decline, cognitive impairment, falls, hospitalization and mortality 64, 65, 66, 67, 68, 69. However, it should be highlighted that the causality between polypharmacy and the aforementioned outcomes is difficult to ascertain as the outcomes could be a direct consequence of the primary conditions. Finally, polypharmacy has been associated with an increased risk of DDIs and other prescribing issues such as inappropriate drug use, prescribing cascade or drug‐disease interactions 46, 48, 49, 51, 53, 59.
Inappropriate drugs for use in elderly PLWH are generally defined as drugs for which the risk of an adverse event outweighs the clinical benefit. Therefore, the use of inappropriate drugs can lead to adverse drug reactions with the subsequent risk of starting a prescribing cascade. The latter occurs when an adverse drug reaction is misinterpreted as a new disease leading to the prescription of an unnecessary medication, which in turn, can cause an adverse drug reaction leading to the prescription of more drugs 70. The risk of starting a prescribing cascade is higher in elderly PLWH because they are often polymedicated and because they are more susceptible to adverse drug reactions due to age‐related physiological changes affecting pharmacokinetics, pharmacodynamics and homeostatic processes. Ageing PLWH could also be more susceptible to adverse drug reactions due to long‐term exposure to antiretroviral drugs leading potentially to cumulative toxicity. Finally, elderly have more comorbidities and therefore are at higher risk for drug‐disease interactions. The latter occurs when the prescription of a medication to treat a given condition may adversely aggravate a coexisting condition.
Very few studies have assessed the extent of inappropriate prescribing in elderly PLWH. To date, only three studies have addressed this question using tools such as the Beers criteria and/or the STOPP/START criteria for the detection of inappropriate dosing, indication, treatment duration, drugs or treatment omission 71, 72. These studies have revealed that inappropriate prescribing was common with 52% up to 69% of elderly PLWH presenting at least one medication problem 46, 51, 53. Importantly, prescribing issues included more often non‐HIV medications and other prescribing issues than DDIs with antiretroviral drugs suggesting the need for education on geriatric medicine principles. In addition, there is a need for more real‐world studies that quantify the risk associated with various forms of polypharmacy and DDIs to help guide treatment management.
Interventions to prevent unnecessary polypharmacy and limit inappropriate prescribing include medication reconciliation, medication review and medication prioritization. The decision to prescribe should take into account the risk/benefit of each medication, the care goals, the remaining life expectancy and the current level of functioning as well as the patient preference (patient‐centred approach) 73. In this context, the concept of deprescribing or the process of dose reduction or stopping medications that may be causing harm or no longer provide benefit has gained increasing attention as a means to reduce unnecessary/inappropriate polypharmacy in elderly individuals 74.
Of interest, markers of disease severity like the VACS index developed for PLWH 75 or the Charlson comorbidity index 76 might be useful indices to consider for medication prioritization. Furthermore, these indices may help identify individuals at greatest risk of harm from polypharmacy as they reflect physiological frailty more precisely than age alone.
2.3. Evaluating the drug‐drug interaction potential of a drug
The potential for DDIs is mainly investigated before the marketing of a drug. It is important to gain an in‐depth understanding of the disposition of the drug from in vitro data, studies in selected animal species (bearing in mind marked species differences in drug handling) and then first in human studies. Some key considerations are metabolic pathways, transporter involvement and protein binding since this will give a framework for understanding the potential of the drug to be a “victim” of DDIs. Similarly, there needs to be early data on the drug as a “perpetrator” of DDIs either by induction or inhibition of metabolic enzymes and/or transporters ‐ these being the key, although not exclusive, pathways of pharmacokinetic interactions. The aim of DDI studies performed on a drug in development is to gain knowledge of how this new chemical entity affects the safety and efficacy of other drugs and vice versa. Specific DDI studies performed in healthy volunteers will be based on plausible interaction mechanisms and key/frequently used medication in the target patient population. Overall, an early understanding of the DDI potential of a drug is critical to ensure safety during clinical phase II and III studies, as well as post approval. Additional studies may be required post‐approval due to emerging science or as a result of case reports of suspected DDIs or population pharmacokinetic data from large phase III real‐world studies.
One important emerging area is physiologically based pharmacokinetic modelling (PBPK) which has been applied with significant impact during drug development and post marketing phases and has achieved regulatory acceptance (e.g. FDA, EMA). In brief, PBPK models represent the body and compartments parameterized based on physiology of tissues and organs. PBPK models integrate this physiological description with compound‐specific data to predict the pharmacokinetics of drugs, allowing simulation of the time course of drug concentrations in plasma and tissues. This approach is being increasingly used to simulate and predict DDIs 77, 78. The various approaches to evaluate the DDI potential of a given drug are depicted in Figure 1.
Figure 1.

Evaluating the drug‐drug interaction potential of a drug.
PBPK, physiologically based pharmacokinetic modelling.
The consequences of an observed or predicted DDI is assessed and treatment recommendations follow. Information about DDIs are presented in the relevant sections of the product label, the purpose of which is to assist the prescriber in the use of a specific medicine. However, a recent assessment of the consistency of DDI information in drug labels in several countries (USA, UK, China, Japan, Korea) showed only a moderate level of agreement among the countries' labelling 79. The study concluded that there is a need for international harmonization of the drug labelling process and regulation to produce standardized information that can ensure safe drug therapy worldwide. To illustrate the point of labelling differences we show the example of the established and other potentially significant DDIs of the integrase inhibitor dolutegravir in the US prescribing information, the European summary of product characteristics and Japan prescribing information 80, 81, 82 (Table 3). It is immediately clear that there is not only different wording used (e.g. for cation‐containing antacids) but different recommendations (e.g. carbamazepine, phenytoin).
Table 3.
| Comedication | US prescribing information | European summary of product characteristics | Japan prescribing information |
|---|---|---|---|
| Dofelitide | Contraindicated | Contraindicated | Pilsicainide ‐ Cautiona |
| Carbamazepine | Same recommendation: DTG 50 mg twice daily in INI naïve patients | ||
| Oxcarbazepine | Should be avoided | DTG 50 mg twice daily in INI naïve patients | –a |
| Phenobarbital | Should be avoided | DTG 50 mg twice daily in INI naïve patients | Cautiona |
| Phenytoin | Should be avoided | DTG 50 mg twice daily in INI naïve patients | Cautiona |
| St John's Wort | Should be avoided | DTG 50 mg twice daily in INI naïve patients | Cautiona |
| Rifampicin | Same recommendation: DTG 50 mg twice daily in INI naïve patients | ||
| Efavirenz | Same recommendation: DTG 50 mg twice daily in INI naïve patients | ||
| Etravirine | Should not be used without ATV/r, DRV/r, or LPV/r | Use 50 mg twice daily without a bPI. Should not be used without bPI in INI‐resistant patients | Use 50 mg twice daily without a bPI. Do not use without either ATV/r, DRV/r, or LPV/r in INI‐resistant patients |
| Fosamprenavir | DTG 50 mg twice daily in INI naïve patients | No dose adjustment in INI naïve patients or in absence of INI resistance | Do not use in INI‐resistant patients |
| Cation‐containing antacids | DTG two hours before or six hours after | Antacid two hours after or six hours before | DTG two hours before or six hours after |
| Iron/calcium supplements | DTG two hours before or six hours after | Antacid two hours after or six hours before | DTG two hours before or six hours after but with food at the same timea |
| Metformin | Close monitoring; limit total daily dose | Dose adjustment should be considered | Administer with care; reduce dose as necessarya |
ATV/r, atazanavir boosted with ritonavir; bPI, boosted protease inhibitor; DRV/r,darunavir boosted with ritonavir; DTG, dolutegravir; INI, integrase inhibitor; LPV/r, lopinavir boosted with ritonavir.
Japan label differs from the US prescribing information and the European summary of product characteristics.
While product labels summarize the essential clinical pharmacology and are vital resources, the difference in interpretation of the same DDI data make other resources essential. One seemingly “grey” area is how physicians should use “new” medications in patients on concomitant medications that are not mentioned in the product label. In this context a DDI checker such as that developed by the University of Liverpool (http://www.hiv-druginteractions.org) 83 is an indispensable resource for management of DDIs.
2.4. Mechanisms of drug‐drug interactions with antiretroviral drugs
Antiretroviral drugs have a high potential for DDIs as these drugs can be affected by comedications (victim of DDIs) and can also impact comedications (perpetrator of DDIs) resulting in either a lower or higher exposure of the HIV drug or the comedication and consequently to reduced efficacy or toxicity.
Pharmacokinetic DDIs with antiretroviral drugs can occur at the level of absorption, metabolism or elimination via the following mechanisms (Figure 2):
Figure 2.

Mechanisms of drug‐drug interactions with antiretroviral drugs.
Victim means that the exposure of the antiretroviral drug can be increased or decreased by a comedication with inhibitory or inducing properties on drug‐metabolizing enzymes or drug transporters. Conversely, perpetrator means that the antiretroviral drug inhibits and/or induces drug‐metabolizing enzymes and/or transporters and therefore can alter the exposure of the coadministered drug. Figure reproduced from reference 84 with permission from the journal Taylor & Francis (https://tandfonline.com). c, cobicistat; PI, protease inhibitor; r, ritonavir; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
-
‐
Gastric pH changes: Antacids markedly decrease the absorption of atazanavir 85 and rilpivirine 86 since a low pH is required for their solubility. There is the potential for efficacy to be compromised.
-
‐
Chelation: Since integrase inhibitors exert their effect by binding to a divalent cation in the active site of the integrase enzyme, the divalent cations aluminium, calcium and magnesium in antacids/supplements, and also iron products are able to form a complex with INIs, thereby impairing their absorption and efficacy 87, 88, 89.
-
‐
Inhibition/induction of intestinal cytochrome P450 3A4 (CYP3A4) and/or intestinal transporters: Rifampicin is a potent enzyme/transporter inducing agent and can affect the oral availability of TAF by increasing P‐gp mediated efflux in the enterocytes. On the other hand, PIs boosted with cobicistat increase the absorption of dabigatran due to the inhibition of intestinal P‐gp. This increases the systemic concentrations of dabigatran and consequently increases the risk of bleeding 90.
-
‐
Inhibition/induction of hepatic CYPs and/or glucuronidation enzymes and/or hepatic transporters: The liver is the major site for DDIs and there are multiple examples of clinically relevant DDIs. An important example is the impact of PIs boosted with ritonavir or cobicistat on the exposure of several statins via inhibition of CYP3A4 and/or hepatic transporters thereby increasing the risk of myopathy or rhabdomyolysis 91. The flip side is induction and there are many clinically relevant inducers of CYP enzymes, glucuronyl transferases and influx (e.g. OATP1B1/3) and efflux (e.g. P‐gp; MRP2) drug transporters.
-
‐
Inhibition of renal tubular transporters: There are some endogenous compounds (e.g. creatinine) and several therapeutic agents (e.g. metformin) which are actively transported in the renal proximal tubule. Dolutegravir and bictegravir inhibit the OCT2 mediated uptake of metformin in the tubular cells, whereas cobicistat and ritonavir inhibit metformin secretion in the urine via the multidrug and toxin extrusion protein MATE1; both mechanisms increase the exposure of the antidiabetic drug 92, 93.
Pharmacodynamic DDIs may be encountered with certain antiretroviral drugs when coadministered with drugs characterized by a similar toxicity profile thereby increasing the risk of additive adverse drug effects. As an example, both acute and chronic renal toxicity have been associated with TDF 40, 94. Thus, coadministration of TDF and nephrotoxic medications can increase the risk of nephrotoxicity, particularly in PLWH with pre‐existing renal impairment or when treatment are administered for a long duration 95. Another example is synergistic QT prolongation when a patient is taking more than one drug with a QT liability. The NNRTI rilpivirine has been associated with prolongation of the QTc interval at supratherapeutic doses and there are other drugs (e.g. escitalopram) which carry warnings of dose‐dependent QT effects.
When possible, antiretroviral drugs with a lower potential for DDIs such as the unboosted INIs (raltegravir, dolutegravir, bictegravir), or the NNRTIs doravirine or rilpvirine should be favoured where there is polypharmacy. Figure 3 shows the DDI profile of antiretroviral drugs based on an evaluation of DDI data with ≥750 comedications taken from the website http://www.hiv-druginteractions.org 83.
Figure 3.
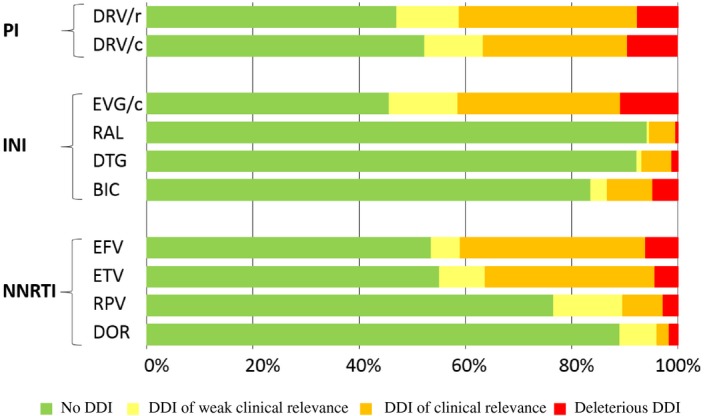
Drug‐drug interaction profiles of selected antiretroviral drugs.
Percentage of green, yellow, amber and red DDIs considering 750 comedications listed in the Liverpool HIV interaction website 83 for selected antiretroviral drugs belonging to the protease inhibitor (PI), integrase inhibitor (INI) and non‐nucleoside reverse transcriptase inhibitor (NNRTI) classes. BIC, bictegravir; DRV/c, darunavir boosted with ritonavir; DRV/r, darunavir boosted with ritonavir; DOR, doravirine; DTG, dolutegravir; EVG/c, elvitegravir boosted with cobicistat; EFV, efavirenz; ETV, etravirine; RAL, raltegravir; RPV, rilpivirine.
Selected clinically important DDIs and their management are presented in Table 4. More information on DDIs can be found in the Liverpool HIV drug interactions website 83.
Table 4.
Selected clinically important drug‐drug interactions and their management
| Drug class | ARV | Comments/recommendations |
|---|---|---|
| Statinsa |
Boosted PI Elvitegravir/c |
Boosted ARVs increase the exposure of several statins. The magnitude of the DDI depends on the metabolic pathway of the statin and its affinity to hepatic drug transporters 91
|
| Calcium channel inhibitorsa |
Boosted PI Elvitegravir/c |
|
| Antidiabeticsa |
Boosted PI Bictegravir Elvitegravir/c Dolutegravir |
|
| Vitamine K antagonistsa |
Boosted PI Elvitegravir/c |
Boosted ARVs have both inhibitory/inducing effects on CYPs and therefore are expected to alter vitamin K antagonists effect. Closely monitor INR 98, 99, 100, 101, 102
|
| Direct‐acting anticoagulantsa |
Boosted PI Elvitegravir/c |
Boosted ARVs cause clinically significant DDIs with direct‐acting anticoagulants due to inhibition of CYPs and/or transporters. Data on management of DDIs are limited 90, 104, 105, 106
|
| Antiplateletsa |
Boosted PI Elvitegravir/c |
|
|
Antacids H2‐receptor blockers Proton pump inhibitors |
Atazanavir Rilpivirine |
Solubility of ARV decreases as pH increases 85, 86. Administration recommendations:
|
|
Antacids Mineral supplements (iron, calcium, magnesium) |
Bictegravir Dolutegravir Elvitegravir/c Raltegravir |
Integrase inhibitors form a complex with divalent cations at the level of the gastro intestinal tract thus reducing their absorption 87, 88, 89, 110. Administration recommendations:
|
| Corticosteroidsa |
Boosted PI Elvitegravir/c |
Boosted ARVs inhibit steroids metabolism thereby increasing the risk of Cushing syndrome. Risk is not limited to oral administration but may also occur after topical, ocular, intra‐articular or intrathecal administration of steroids 111, 112, 113. The risk of Cushing syndrome is not eliminated by reducing the dosage of the corticosteroid. Avoid boosted ARVs when possible or, if unavoidable, use a corticosteroid with a lower propensity to cause Cushing syndrome with periodic control of cortisol
|
|
Antituberculosis drugs Rifampicin, rifabutin Bedaquilinea Delamanid Ethambutol, isoniazid, linezolid, pyrazinamide |
PI/r |
|
| PI/c |
|
|
| Elvitegravir/c |
|
|
| Bictegravir |
|
|
| Dolutegravir |
|
|
| Raltegravir |
|
|
| Doravirine |
|
|
| Etravirine |
|
|
| Rilpivirine |
|
|
| Efavirenz |
|
|
|
Boosted PI Elvitegravir/c |
Boosted ARVs inhibit bedaquiline metabolism resulting in increased exposure and related increased risk of QT interval prolongation. Given bedaquiline's prolonged half‐life, coadministration with boosted ARV should not exceed 14 days. Monitor ECG and transaminases. Coadministration with saquinavir is contraindicated | |
|
Boosted PI Elvitegravir/c |
Boosted ARVs can increase delamanid exposure resulting in an increased risk of QT interval prolongation. Monitor ECG. Coadministration with saquinavir is contraindicated | |
| ARVs | No DDIs |
More information on DDIs can be obtained from the University of Liverpool HIV drug interactions website: http://www.hiv-druginteractions.org 83. ARV, antiretroviral drug; ATV, atazanavir; BIC, bictegravir; c, cobicistat; CYP, cytochromes; DDI, drug‐drug interaction; DTG, dolutegravir; EVG/c, elvitegravir/cobicistat; OATP1B1, organic anion transporting polypeptide 1B1; OCT2, organic cation transporter 2; PI, protease inhibitor; PI/c, protease inhibitor boosted with cobicistat; PI/r, protease inhibitor boosted with ritonavir; RAL, raltegravir; RPV, rilpivirine.
Drug exposure can be lowered when coadministered with the non‐nucleoside reverse transcriptase inhibitors efavirenz, etravirine and nevirapine.
2.5. Current issues when managing drug‐drug interactions
One current issue is that only few drug associations are evaluated in clinical studies, thus guidance on how to manage DDIs is mostly theoretical or is lacking, particularly when associating mutually interacting drugs as often encountered in clinical practice. A good knowledge of the metabolic pathway of drugs and a good understanding of the mechanisms of DDIs as well as the therapeutic index are essential to predict the risk of having a clinically relevant interaction. A strong inhibition or induction of a major metabolic pathway is generally expected to cause a large magnitude DDI that may require dosage adjustment. Conversely, the magnitude of DDIs will be mitigated when drugs have multiple metabolic or elimination pathways as metabolism and elimination can still occur through the unaffected pathways 117. A misunderstanding of these concepts and of the different DDI of each antiretroviral drugs can lead to an overestimation of the risk of DDI leading consequently to sub‐optimal treatment. This issue has been reported for antidepressants since a larger proportion of PLWH were shown to have sub‐therapeutic antidepressants levels compared to uninfected individuals suggestive of a deliberate lower dosing as clinicians fear DDIs with antiretroviral drugs 118. It should be highlighted that most antidepressants are metabolized by several cytochromes and therefore the magnitude of DDIs with boosted regimens tend to be mitigated. In addition, the pharmacokinetic boosters ritonavir and cobicistat inhibit only weakly cytochrome 2D6 103, which is the major contributor of the metabolism of most antidepressants. Underestimating the risk of DDIs can also occur; this is notably exemplified with the DDI between boosted antiretroviral drugs and corticosteroids. The coadministration of boosted antiretroviral drugs and potent corticosteroids is contraindicated due to the risk of developing a Cushing syndrome. Nevertheless, these drugs are being used together in clinical practice as indicated by two large independent European cohort studies 52, 119. The fact that corticosteroids are administered by different routes (oral, inhalation, intra‐articular, topical) may lead to an underestimation of the risk of DDI. In addition, corticosteroids are used across a large variety of medical specialties (dermatology, pneumology or rheumatology) and therefore are likely to be prescribed by non‐HIV specialists who are not aware of DDIs with antiretroviral drugs.
2.6. Drug‐drug interactions and long‐acting antiretroviral drugs
Long‐acting drugs and formulations are an established part of the management of several medical conditions including contraception, schizophrenia and osteoporosis. Long‐acting drug delivery is considered to be a key solution to the problem of poor adherence and since daily oral pills remain a barrier to long‐term suppression of viral replication in PLWH there is understandably much interest in both injectables and implants of antiretrovirals 120, 121. Long‐acting nanocrystal suspensions of the INI cabotegravir and the NNRTI rilpivirine are in advanced clinical development with data from large Phase III studies in maintenance therapy (ATLAS, FLAIR) recently presented at IAS 2019 122. There are other exciting developments using implant technology with non‐degradable subcutaneous implants of two NRTIs ‐ tenofovir alafenamide (TAF) and 4ʹ‐ethynyl‐2‐fluoro‐2ʹ‐deoxyadenosine (EFdA; MK8591) ‐ about to enter clinical testing.
Given that with non‐oral drug administration, the first pass metabolism is bypassed (i.e. the initial metabolism/disposition within the gastrointestinal tract and liver), should it be anticipated that long‐acting regimens will be substantially devoid of DDIs? Clearly, DDIs are drug specific and all aspects involved in the disposition of a given compound have to be considered. In addition, DDIs data from other therapeutic areas have to be reviewed. Considering contraception, the levonorgestrel (LNG) subdermal implants are a highly efficacious and safe form of long‐acting reversible contraception. However, >50% lower LNG exposure was shown in women receiving the LNG subdermal implant with efavirenz‐based antiretroviral treatment compared to antiretroviral treatment‐naïve women in Uganda 123. Despite doubling the dose of the LNG implants, LNG concentrations remained >30% lower when women were on efavirenz‐based antiretroviral treatment 124. Therefore, there is uncertain contraceptive effectiveness even when modifying the dose to overcome the DDI.
Going forward with long‐acting antiretrovirals, it is almost certain that it will be data from short‐term oral DDIs studies that will help inform PBPK modelling. For example rifampicin administration was shown to reduce the exposure of single oral dose cabotegravir by 59% 125. Rajoli et al 77 then designed PBPK models (verified against the observed data for oral cabotegravir, rilpivirine and rifampicin) to predict the DDI of the long‐acting regimen. According to the models, there was a predicted reduction in cabotegravir exposure of 41% and rilpivirine of 82% for the first maintenance dose with 600 mg once daily oral rifampicin. This strongly suggests that coadministration of rifampicin with these long‐acting formulations will be problematic and strategies will need to be considered to overcome the DDI. However, increasing the dose is unlikely due to volume of injection and shortening the dosing interval will present logistical challenges. Therefore, there are some important challenges relating to DDIs when considering the role out of long‐acting regimens.
3. Conclusions
DDIs in HIV really came to the forefront of attention more than 20 years ago with the PI era and the realization that boosting of the PI often also resulted in the boosting of other comedications. Although we have moved into the INI era and have unboosted regimens with a greatly reduced liability to DDIs, there still needs to be an awareness of relevant DDIs both with INI and the earlier generation antiretroviral drugs which are still important in certain settings. This is particularly relevant in the older population who often have multiple comorbidities and therefore polypharmacy. DDIs are still an issue we have to face and manage.
Strategies to prevent prescribing errors are important which must include education on key DDIs with each class of antiretroviral drugs and on prescribing principles for specific groups of patients. Medication reconciliation and regular medication review is essential with de‐prescribing if appropriate.
As we look to the future, there are clearly exciting developments in antiretroviral therapy particularly in relation to long‐acting injectables and implants. The question then is “will DDIs still be an issue”? Despite bypassing gastrointestinal absorption there are still hepatic DDIs to consider (and maybe interactions relating to the injection or implant site) and so we need to be clear on the process of generating key data (likely PBPK modelling) and the strategies to deal with clinically relevant DDIs.
Competing interests
DB has received educational grants for http://www.hiv-druginteractions.org from Gilead, MSD, Janssen, ViiV. Honoraria for speakers' bureau or advisory boards received from Gilead, MSD, ViiV. CM received speaker honoraria for her institution from MSD.
Authors' contributions
David Back and Catia Marzolini designed the review, drafted the manuscript and critically revised it.
Back, D. and Marzolini, C. . The challenge of HIV treatment in an era of polypharmacy. J Intern AIDS Soc. 2020; 23(2):e25449
References
- 1. Antiretroviral Therapy Cohort Collaboration . Survival of HIV‐positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. 2017;4(8):e349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gueler A, Moser A, Calmy A, Gunthard HF, Bernasconi E, Furrer H, et al. Life expectancy in HIV‐positive persons in Switzerland: matched comparison with general population. AIDS. 2017;31(3):427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, et al. Closing the gap: increases in life expectancy among treated HIV‐positive individuals in the United States and Canada. PLoS ONE. 2013;8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wandeler G, Johnson LF, Egger M. Trends in life expectancy of HIV‐positive adults on antiretroviral therapy across the globe: comparisons with general population. Curr Opin HIV AIDS. 2016;11(5):492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guaraldi G, Zona S, Brothers TD, Carli F, Stentarelli C, Dolci G, et al. Aging with HIV vs. HIV seroconversion at older age: a diverse population with distinct comorbidity profiles. PLoS ONE. 2015;10;e0118531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smit M, Brinkman K, Geerlings S, Smit C, Thyagarajan K, Sighem A, et al. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis. 2015;15(7):810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smit M, Cassidy R, Cozzi‐Lepri A, Quiros‐Roldan E, Girardi E, Mammone A, et al. Projections of non‐communicable disease and health care costs among HIV‐positive persons in Italy and the U.S.A.: a modelling study. PLoS ONE. 2017;12;e0186638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization . Definition of an older or elderly person [cited 2019 Sep 1]. Available from: https://www.who.int/healthinfo/survey/ageingdefnolder/en/
- 9. Mangoni AA, Jackson SH. Age‐related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57(1):6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stader F, Siccardi M, Battegay M, Kinvig H, Penny MA, Marzolini C. Repository describing an aging population to inform physiologically based pharmacokinetic models considering anatomical, physiological, and biological age‐dependent changes. Clin Pharmacokinet. 2019;58(4):483–501. [DOI] [PubMed] [Google Scholar]
- 11. Polasek TM, Patel F, Jensen BP, Sorich MJ, Wiese MD, Doogue MP. Predicted metabolic drug clearance with increasing adult age. Br J Clin Pharmacol. 2013;75(4):1019–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Calza L, Colangeli V, Magistrelli E, Bussini L, Conti M, Ramazzotti E, et al. Plasma trough concentrations of darunavir/ritonavir and raltegravir in older patients with HIV‐1 infection. HIV Med. 2017;18(7):474–481. [DOI] [PubMed] [Google Scholar]
- 13. Crawford KW, Spritzler J, Kalayjian RC, Parsons T, Landay A, Pollard R, et al. Age‐related changes in plasma concentrations of the HIV protease inhibitor lopinavir. AIDS Res Hum Retroviruses. 2010;26(6):635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Winston A, Jose S, Gibbons S, Back D, Stohr W, Post F, et al. Effects of age on antiretroviral plasma drug concentration in HIV‐infected subjects undergoing routine therapeutic drug monitoring. J Antimicrob Chemother. 2013;68(6):1354–9. [DOI] [PubMed] [Google Scholar]
- 15. Dumond JB, Adams JL, Prince HM, Kendrick RL, Wang R, Jennings SH, et al. Pharmacokinetics of two common antiretroviral regimens in older HIV‐infected patients: a pilot study. HIV Med. 2013;14(7):401–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Elliot ER, Wang X, Singh S, Simmons B, Vera JH, Miller RF, et al. Increased dolutegravir peak concentrations in people living with human immunodeficiency virus aged 60 and over, and analysis of sleep quality and cognition. Clin Infect Dis. 2019;68(1):87–95. [DOI] [PubMed] [Google Scholar]
- 17. Wooten JM. Pharmacotherapy considerations in elderly adults. South Med J. 2012;105(8):437–45. [DOI] [PubMed] [Google Scholar]
- 18. Ruxton K, Woodman RJ, Mangoni AA. Drugs with anticholinergic effects and cognitive impairment, falls and all‐cause mortality in older adults: A systematic review and meta‐analysis. Br J Clin Pharmacol. 2015;80(2):209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cuzin L, Katlama C, Cotte L, Pugliese P, Cheret A, Bernaud C, et al. Ageing with HIV: do comorbidities and polymedication drive treatment optimization? HIV Med. 2017;18(6):395–401. [DOI] [PubMed] [Google Scholar]
- 20. Kendall CE, Wong J, Taljaard M, Glazier RH, Hogg W, Younger J, et al. A cross‐sectional, population‐based study measuring comorbidity among people living with HIV in Ontario. BMC Public Health. 2014;13(14):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kooij KW, Vogt L, Wit F, van der Valk M, van Zoest RA, Goorhuis A, et al. Higher prevalence and faster progression of chronic kidney disease in human immunodeficiency virus‐infected middle‐aged individuals compared with human immunodeficiency virus‐uninfected controls. J Infect Dis. 2017;216(6):622–31. [DOI] [PubMed] [Google Scholar]
- 22. Mahale P, Engels EA, Coghill AE, Kahn AR, Shiels MS. Cancer risk in older persons living with human immunodeficiency virus infection in the United States. Clin Infect Dis. 2018;67(1):50–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paisible AL, Chang CC, So‐Armah KA, Butt AA, Leaf DA, Budoff M, et al. HIV infection, cardiovascular disease risk factor profile, and risk for acute myocardial infarction. J Acquir Immune Defic Syndr. 2015;68(2):209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rasch MG, Helleberg M, Feldt‐Rasmussen B, Kronborg G, Larsen CS, Pedersen C, et al. Increased risk of dialysis and end‐stage renal disease among HIV patients in Denmark compared with the background population. Nephrol Dial Transplant. 2014;29(6):1232–8. [DOI] [PubMed] [Google Scholar]
- 25. Schouten J, Wit FW, Stolte IG, Kootstra NA, van der Valk M, Geerlings SE, et al. Cross‐sectional comparison of the prevalence of age‐associated comorbidities and their risk factors between HIV‐infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis. 2014;59(12):1787–97. [DOI] [PubMed] [Google Scholar]
- 26. Silverberg MJ, Lau B, Achenbach CJ, Jing Y, Althoff KN, D'Souza G, et al. Cumulative incidence of cancer among persons with hiv in north america: a cohort study. Ann Intern Med. 2015;163(7):507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tripathi A, Liese AD, Winniford MD, Jerrell JM, Albrecht H, Rizvi AA, et al. Impact of clinical and therapeutic factors on incident cardiovascular and cerebrovascular events in a population‐based cohort of HIV‐infected and non‐HIV‐infected adults. Clin Cardiol. 2014;37(9):517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brites C, Schiavon Nogueira R, Gosuen GC, Kalmar EMC, Leme STS, Martins RT, et al. Getting older in HIV: increasing frequency of comorbidities and polypharmacy in Brazilian HIV patients. AIDS Res Hum Retroviruses. 2019;35(11–12):1103–1105. [DOI] [PubMed] [Google Scholar]
- 29. Maciel RA, Moreira Kluck H, Durand M, Sprinz E. Comorbidity is more common and occurs earlier in persons living with HIV than in HIV‐uninfected matched controls, aged 50 years and older: a cross‐sectional study. Int J Infect Dis. 2018;70:30–5. [DOI] [PubMed] [Google Scholar]
- 30. Kong AM, Pozen A, Anastos K, Kelvin EA, Nash D. Non‐HIV comorbid conditions and polypharmacy among people living with HIV age 65 or older compared with HIV‐negative individuals age 65 or older in the United States: a retrospective claims‐based analysis. AIDS Patient Care STDS. 2019;33(3):93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Serrao R, Pinero C, Velez J, Coutinho D, Maltez F, Lino S, et al. Non‐AIDS‐related comorbidities in people living with HIV‐1 aged 50 years and older: the aging positive study. Int J Infect Dis. 2019;79:94–100. [DOI] [PubMed] [Google Scholar]
- 32. Allavena C, Hanf M, Rey D, Duvivier C, BaniSadr F, Poizot‐Martin I, et al. Antiretroviral exposure and comorbidities in an aging HIV‐infected population: The challenge of geriatric patients. PLoS ONE. 2018;13:e0203895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pelchen‐Matthews A, Ryom L, Borges AH, Edwards S, Duvivier C, Stephan C, et al. Aging and the evolution of comorbidities among HIV‐positive individuals in a European cohort. AIDS. 2018;32:2405–16. [DOI] [PubMed] [Google Scholar]
- 34. Guaraldi G, Malagoli A, Calcagno A, Mussi C, Celesia BM, Carli F, et al. The increasing burden and complexity of multi‐morbidity and polypharmacy in geriatric HIV patients: a cross sectional study of people aged 65–74 years and more than 75 years. BMC Geriatr. 2018;18(1):65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hasse B, Ledergerber B, Furrer H, Battegay M, Hirschel B, Cavassini M, et al. Morbidity and aging in HIV‐infected persons: the Swiss HIV cohort study. Clin Infect Dis. 2011;53(11):1130–9. [DOI] [PubMed] [Google Scholar]
- 36. Appay V, Sauce D. Immune activation and inflammation in HIV‐1 infection: causes and consequences. J Pathol. 2008;214(2):231–41. [DOI] [PubMed] [Google Scholar]
- 37. Sabin CA, Reiss P. Epidemiology of ageing with HIV: what can we learn from cohorts? AIDS. 2017;1 31 Suppl 2:S121–8. [DOI] [PubMed] [Google Scholar]
- 38. Calza L, Colangeli V, Manfredi R, Bon I, Re MC, Viale P. Clinical management of dyslipidaemia associated with combination antiretroviral therapy in HIV‐infected patients. J Antimicrob Chemother. 2016;71(6):1451–65. [DOI] [PubMed] [Google Scholar]
- 39. European AIDS . Clinical Society (EACS) guidelines. 2018. [cited 2019 Sep 1]. Available from: https://www.eacs.society.org
- 40. Mocroft A, Lundgren JD, Ross M, Fux CA, Reiss P, Moranne O, et al. Cumulative and current exposure to potentially nephrotoxic antiretrovirals and development of chronic kidney disease in HIV‐positive individuals with a normal baseline estimated glomerular filtration rate: a prospective international cohort study. Lancet HIV. 2016;3(1):e23–32. [DOI] [PubMed] [Google Scholar]
- 41. Hasse B, Tarr PE, Marques‐Vidal P, Waeber G, Preisig M, Mooser V, et al. Strong impact of smoking on multimorbidity and cardiovascular risk among human immunodeficiency virus‐infected individuals in comparison with the general population. Open Forum Infect Dis. 2015;2(3):ofv108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. De Francesco D, Verboeket SO, Underwood J, Bagkeris E, Wit FW, Mallon PWG, et al. Patterns of co‐occurring comorbidities in people living with HIV. Open Forum Infect Dis. 2018;5(11):ofy272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. De Francesco D, Underwood J, Bagkeris E, Anderson JA, Williams I, Vera JH, et al. Risk factors and impact of patterns of co‐occurring comorbidities in people living with HIV. AIDS. 2019;33(12):1871–80. [DOI] [PubMed] [Google Scholar]
- 44. Gnjidic D, Hilmer SN, Blyth FM, Naganathan V, Waite L, Seibel MJ, et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community‐dwelling older men at risk of different adverse outcomes. J Clin Epidemiol. 2012;65(9):989–95. [DOI] [PubMed] [Google Scholar]
- 45. Courlet P, Marzolini C, Cavassini M, Battegay M, Alves Saldanha S, Alves D, et al. Polypharmacy, inappropriate drugs, and drug‐drug interactions in HIV‐infected elderly. Conference on Retroviruses and Opportunistic Infections, Seattle, March 7–9, 2019, abstract 466.
- 46. Greene M, Steinman MA, McNicholl IR, Valcour V. Polypharmacy, drug‐drug interactions, and potentially inappropriate medications in older adults with human immunodeficiency virus infection. J Am Geriatr Soc. 2014;62(3):447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Halloran MO, Boyle C, Kehoe B, Bagkeris E, Mallon P, Post FA, et al. Polypharmacy and drug‐drug interactions in older and younger people living with HIV: the POPPY study. Antivir Ther. 2019;24(3):193–201. [DOI] [PubMed] [Google Scholar]
- 48. Holtzman C, Armon C, Tedaldi E, Chmiel JS, Buchacz K, Wood K, et al. Polypharmacy and risk of antiretroviral drug interactions among the aging HIV‐infected population. J Gen Intern Med. 2013;28(10):1302–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Justice AC, Gordon KS, Skanderson M, Edelman EJ, Akgun KM, Gibert CL, et al. Nonantiretroviral polypharmacy and adverse health outcomes among HIV‐infected and uninfected individuals. AIDS. 2018;32(6):739–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Krentz HB, Gill MJ. The impact of non‐antiretroviral polypharmacy on the continuity of antiretroviral therapy (ART) among HIV patients. AIDS Patient Care STDS. 2016;30(1):11–7. [DOI] [PubMed] [Google Scholar]
- 51. Livio F, Rrustemi F, Moffa G, Elzi L, Stader F, Braun D, et al. Polypharmacy, drug‐drug interactions and potentially inappropriate prescribing in elderly patients of the Swiss HIV Cohort Study. 19th International Workshop on Clinical Pharmacology of Antiviral Therapy, May 22–24, Baltimore, MD: 2018. [Google Scholar]
- 52. Lopez‐Centeno B, Badenes‐Olmedo C, Mataix‐Sanjuan A, McAllister K, Bellon J, Gibbons S, et al. Polypharmacy and drug‐drug interactions in HIV‐infected subjects in the region of Madrid, Spain: a population‐based study. Clin Infect Dis. 2019. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 53. McNicholl IR, Gandhi M, Hare CB, Greene M, Pierluissi E. A pharmacist‐led program to evaluate and reduce polypharmacy and potentially inappropriate prescribing in older HIV‐positive patients. Pharmacotherapy. 2017;37(12):1498–506. [DOI] [PubMed] [Google Scholar]
- 54. Nunez‐Nunez M, Castaneda‐Macias I, Sandoval‐Fernandez Del Castillo S. Potential interactions in a cohort of elderly HIV‐positive patients. Farm Hosp. 2018;42(4):163–167. [DOI] [PubMed] [Google Scholar]
- 55. Ruzicka DJ, Imai K, Takahashi K, Naito T. Comorbidities and the use of comedications in people living with HIV on antiretroviral therapy in Japan: a cross‐sectional study using a hospital claims database. BMJ Open. 2018;8:e019985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ssonko M, Stanaway F, Mayanja HK, Namuleme T, Cumming R, Kyalimpa JL, et al. Polypharmacy among HIV positive older adults on anti‐retroviral therapy attending an urban clinic in Uganda. BMC Geriatr. 2018;18(1):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ware D, Palella FJ, Chew KW, Friedman MR, D'Souza G, Ho Ken, et al. Examination of polypharmacy trajectories among HIV‐positive and HIV‐negative men in an ongoing longitudinal cohort from 2004 to 2016. AIDS Patient Care STDs. 2019;33(8):354–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rasmussen LD, Kronborg G, Larsen CS, Pedersen C, Gerstoft J, Obel N, et al. Use of non‐antiretroviral drugs among individuals with and without HIV‐infection: a Danish nationwide study. Infect Dis (Lond). 2017;49(1):42–54. [DOI] [PubMed] [Google Scholar]
- 59. Marzolini C, Back D, Weber R, Furrer H, Cavassini M, Calmy A, et al. Ageing with HIV: medication use and risk for potential drug‐drug interactions. J Antimicrob Chemother. 2011;66(9):2107–11. [DOI] [PubMed] [Google Scholar]
- 60. Grant RW, Devita NG, Singer DE, Meigs JB. Polypharmacy and medication adherence in patients with type 2 diabetes. Diabetes Care. 2003;26(5):1408–12. [DOI] [PubMed] [Google Scholar]
- 61. Monane M, Bohn RL, Gurwitz JH, Glynn RJ, Levin R, Avorn J. The effects of initial drug choice and comorbidity on antihypertensive therapy compliance: results from a population‐based study in the elderly. Am J Hypertens. 1997;10 7 Pt 1:697–704. [DOI] [PubMed] [Google Scholar]
- 62. Salazar JA, Poon I, Nair M. Clinical consequences of polypharmacy in elderly: expect the unexpected, think the unthinkable. Expert Opin Drug Saf. 2007;6(6):695–704. [DOI] [PubMed] [Google Scholar]
- 63. Shalansky SJ, Levy AR. Effect of number of medications on cardiovascular therapy adherence. Ann Pharmacother. 2002;36(10):1532–9. [DOI] [PubMed] [Google Scholar]
- 64. Fried TR, O'Leary J, Towle V, Goldstein MK, Trentalange M, Martin DK. Health outcomes associated with polypharmacy in community‐dwelling older adults: a systematic review. J Am Geriatr Soc. 2014;62(12):2261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pugh MJ, Palmer RF, Parchman ML, Mortensen E, Markides K, Espino DV. Association of suboptimal prescribing and change in lower extremity physical function over time. Gerontology. 2007;53(6):445–53. [DOI] [PubMed] [Google Scholar]
- 66. Rawle MJ, Cooper R, Kuh D, Richards M. Associations between polypharmacy and cognitive and physical capability: a British birth cohort study. J Am Geriatr Soc. 2018;66(5):916–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28(2):173–86. [DOI] [PubMed] [Google Scholar]
- 68. Thai L, Young M. The impact of number of medications on falls in aging persons living with HIV. 9th International Workshop on HIV & Aging, September 13‐14, New York: 2018. [Google Scholar]
- 69. Womack JA, Murphy TE, Rentsch CT, Tate JP, Bathulapalli H, Smith AC, et al. Polypharmacy, hazardous alcohol and illicit substance use, and serious falls among PLWH and uninfected comparators. J Acquir Immune Defic Syndr. 2019;82(3):305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rochon PA, Gurwitz JH. The prescribing cascade revisited. Lancet. 2017;389(10081):1778–80. [DOI] [PubMed] [Google Scholar]
- 71. By the American Geriatrics Society Beers Criteria Update Expert Panel . American geriatrics society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227–46. [DOI] [PubMed] [Google Scholar]
- 72. O'Mahony D, O'Sullivan D, Byrne S, O'Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Boyd CM, Lucas GM. Patient‐centered care for people living with multimorbidity. Curr Opin HIV AIDS. 2014;9(4):419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Scott IA, Hilmer SN, Reeve E, Potter K, Le Couteur D, Rigby D, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827–34. [DOI] [PubMed] [Google Scholar]
- 75. Justice AC, Modur S, Tate JP, Althoff KN, Jacobson LP, Gebo K, et al. Predictive accuracy of the Veterans Aging Cohort Study (VACS) index for mortaity with HIV infection: a north American cross cohort analysis. J Acquir Immune Defic Syndr. 2013;62(2):149–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Charlson ME, Pompei P, Aes KL, MacKenzie CR. A new method of classifying pronostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 77. Rajoli RKR, Curley P, Chiong J, Back D, Flexner C, Owen A, et al. Predicting drug‐drug interactions between rifampicin and long‐acting cabotegravir and rilpivirine using physiologically based pharmacokinetic modeling. J Infect Dis. 2019;219(11):1735–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Stader F, Penny MA, Siccardi M, Marzolini C. A comprehensive framework for physiologically based pharmacokinetic modelling in Matlab((R)). CPT Pharmacometrics Syst Pharmacol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jeong S, Kam G, Li J, Lee S, Lee H, Noh Y, et al. Assessment of consistency of drug interaction information in drug labels among the United States, the United Kingdom, China, Japna, and Korea. Clin Pharmacol Ther. 2019;105(2):505–14. [DOI] [PubMed] [Google Scholar]
- 80. Food and Drug Administration . Tivicay prescribing information. 2019. [cited 2019 Sep 1]. Available from: http://www.accessdata.fda.gov
- 81. European Agency Medicine . Tivicay summary of prescribing characteristics. 2019. [cited 2019 Sep 1]. Available from: https://www.medicines.org.uk
- 82. Pharmaceutical and Medical devices agency . Tivicay Japan prescribing information (obtained from ViiV healthcare). 2019. [cited 2019 Sep 1]. Available from: https://www.pmda.go.jp
- 83. Liverpool HIV drug interactions website [cited 2019 Sep 1]. Available from: http://www.hiv-druginteractions.org
- 84. Marzolini C, Livio F. Prescribing issues in elderly individuals living with HIV. Expert Rev Clin Pharmacol. 2019;12(7):643–59. [DOI] [PubMed] [Google Scholar]
- 85. Zhu L, Persson A, Mahnke L, Eley T, Li T, Xu X, et al. Effect of low‐dose omeprazole (20 mg daily) on the pharmacokinetics of multiple‐dose atazanavir with ritonavir in healthy subjects. J Clin Pharmacol. 2011;51(3):368–77. [DOI] [PubMed] [Google Scholar]
- 86. Crauwels H, van Heeswijk RP, Stevens M, Buelens A, Vanveggel S, Boven K, et al. Clinical perspective on drug‐drug interactions with the non‐nucleoside reverse transcriptase inhibitor rilpivirine. AIDS Rev. 2013;15(2):87–101. [PubMed] [Google Scholar]
- 87. Kiser JJ, Bumpass JB, Meditz AL, Anderson PL, Bushman L, Ray M, et al. Effect of antacids on the pharmacokinetics of raltegravir in human immunodeficiency virus‐seronegative volunteers. Antimicrob Agents Chemother. 2010;54(12):4999–5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ramanathan S, Mathias A, Wei X, Shen G, Koziara J, Cheng A, et al. Pharmacokinetics of once‐daily boosted elvitegravir when administered in combination with acid‐reducing agents. J Acquir Immune Defic Syndr. 2013;64(1):45–50. [DOI] [PubMed] [Google Scholar]
- 89. Song I, Borland J, Arya N, Wynne B, Piscitelli S. Pharmacokinetics of dolutegravir when administered with mineral supplements in healthy adult subjects. J Clin Pharmacol. 2015;55(5):490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kumar P, Gordon LA, Brooks KM, George JM, Kellogg A, McManus M, et al. Differential influence of the antiretroviral pharmacokinetic enhancers ritonavir and cobicistat on intestinal p‐glycoprotein transport and the pharmacokinetic/ pharmacodynamic disposition of dabigatran. Antimicrob Agents Chemother. 2017;61(11):e01201-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chauvin B, Drouot S, Barrail‐Tran A, Taburet AM. Drug‐drug interactions between HMG‐CoA reductase inhibitors (statins) and antiviral protease inhibitors. Clin Pharmacokinet. 2013;52(10):815–31. [DOI] [PubMed] [Google Scholar]
- 92. Song IH, Zong J, Borland J, Jerva F, Wynne B, Zamek‐Gliszczynski MJ, et al. The effect of dolutegravir on the pharmacokinetics of metformin in healthy subjects. J Acquir Immune Defic Syndr. 2016;72(4):400–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhang HSW, Vu A, Martin H, Graham H, Quirk E, Kearney BP. Lack of clinically relevant effect of bictegravir on metformin pharmacokinetics and pharmacodynamics. 18th International Workshop on Clinical Pharmacology of Antiviral Therapy, June 14–16, Chicago: 2017. [Google Scholar]
- 94. Gupta SK, Anderson AM, Ebrahimi R, Fralich T, Graham H, Scharen‐Guivel V, et al. Fanconi syndrome accompanied by renal function decline with tenofovir disoproxil fumarate: a prospective, case‐control study of predictors and resolution in HIV‐infected patients. PLoS ONE. 2014;9:e92717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Psevdos G Jr, Gonzalez E, Sharp V. Acute renal failure in patients with AIDS on tenofovir while receiving prolonged vancomycin course for osteomyelitis. AIDS Read. 2009;19(6):245–8. [PubMed] [Google Scholar]
- 96. Glesby MJ, Aberg JA, Kendall MA, Fichtenbaum CJ, Hafner R, Hall S, et al. Pharmacokinetic interactions between indinavir plus ritonavir and calcium channel blockers. Clin Pharmacol Ther. 2005;78(2):143–53. [DOI] [PubMed] [Google Scholar]
- 97. Mukherjee D, Zha J, Menon RM, Shebley M. Guiding dose adjustment of amlodipine after co‐administration with ritonavir containing regimens using a physiologically‐based pharmacokinetic/pharmacodynamic model. J Pharmacokinet Pharmacodyn. 2018;45(3):443–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Fulco PP, Zingone MM, Higginson RT. Possible antiretroviral therapy‐warfarin drug interaction. Pharmacotherapy. 2008;28(7):945–9. [DOI] [PubMed] [Google Scholar]
- 99. Good BL, Gomes DC, Fulco PP. An unexpected interaction between warfarin and cobicistat‐boosted elvitegravir. AIDS. 2015;29(8):985–6. [DOI] [PubMed] [Google Scholar]
- 100. Hughes CA, Freitas A, Miedzinski LJ. Interaction between lopinavir/ritonavir and warfarin. CMAJ. 2007;177(4):357–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Tseng AL, Luetkehoelter J, Walmsley SL. Increase in international normalized ratio after switching from atazanavir/ritonavir to darunavir/cobicistat in a patient on warfarin: boosters are not always equal. AIDS. 2017;31(1):175–176. [DOI] [PubMed] [Google Scholar]
- 102. Welzen ME, van den Berk GE, Hamers RL, Burger DM. Interaction between antiretroviral drugs and acenocoumarol. Antivir Ther. 2011;16(2):249–52. [DOI] [PubMed] [Google Scholar]
- 103. Marzolini C, Gibbons S, Khoo S, Back D. Cobicistat versus ritonavir boosting and differences in the drug‐drug interaction profiles with co‐medications. J Antimicrob Chemother. 2016;71(7):1755–8. [DOI] [PubMed] [Google Scholar]
- 104. Corallo CE, Grannell L, Tran H. Postoperative bleeding after administration of a single dose of rivaroxaban to a patient receiving antiretroviral therapy. Drug Saf Case Rep. 2015;2(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lakatos B, Stoeckle M, Elzi L, Battegay M, Marzolini C. Gastrointestinal bleeding associated with rivaroxaban administration in a treated patient infected with human immunodeficiency virus. Swiss Med Wkly. 2014;22(144):w13906. [DOI] [PubMed] [Google Scholar]
- 106. Yoong D, Naccarato M, Gough K. Extensive bruising and elevated rivaroxaban plasma concentration in a patient receiving cobicistat‐boosted elvitegravir. Ann Pharmacother. 2017;51(8):713–4. [DOI] [PubMed] [Google Scholar]
- 107. Bravo I, Alvarez H, Marino A, Clotet B, Molto J. Recurrent coronary disease in HIV‐infected patients: role of drug‐drug interactions. Br J Clin Pharmacol. 2018;84(7):1617–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Itkonen MK, Tornio A, Lapatto‐Reiniluoto O, Neuvonen M, Neuvonen PJ, Niemi M, et al. Clopidogrel increases dasabuvir exposure with or without ritonavir, and ritonavir inhibits the bioactivation of clopidogrel. Clin Pharmacol Ther. 2019;105(1):219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Marsousi N, Daali Y, Fontana P, Reny JL, Ancrenaz‐Sirot V, Calmy A, et al. Impact of boosted antiretroviral therapy on the pharmacokinetics and efficacy of clopidogrel and prasugrel active metabolites. Clin Pharmacokinet. 2018;57(10):1347–54. [DOI] [PubMed] [Google Scholar]
- 110. Krishna R, East L, Larson P, Valiathan C, Butterfield K, Teng Y, et al. Effect of metal‐cation antacids on the pharmacokinetics of 1200 mg raltegravir. J Pharm Pharmacol. 2016;68(11):1359–65. [DOI] [PubMed] [Google Scholar]
- 111. Elliot ER, Theodoraki A, Jain LR, Marshall NJ, Boffito M, Baldeweg SE, et al. Iatrogenic Cushing's syndrome due to drug interaction between glucocorticoids and the ritonavir or cobicistat containing HIV therapies. Clin Med (Lond). 2016;16(5):412–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Peyro‐Saint‐Paul L, Besnier P, Demessine L, Biour M, Hillaire‐Buys D, de Canecaude C, et al. Cushing's syndrome due to interaction between ritonavir or cobicistat and corticosteroids: a case–control study in the French Pharmacovigilance Database. J Chem. 2019;74(11):3291–4. [DOI] [PubMed] [Google Scholar]
- 113. Saberi P, Phengrasamy T, Nguyen DP. Inhaled corticosteroid use in HIV‐positive individuals taking protease inhibitors: a review of pharmacokinetics, case reports and clinical management. HIV Med. 2013;14(9):519–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Dooley KE, Sayre P, Borland J, Purdy E, Chen S, Song I, et al. Safety, tolerability, and pharmacokinetics of the HIV integrase inhibitor dolutegravir given twice daily with rifampin or once daily with rifabutin: results of a phase 1 study among healthy subjects. J Acquir Immune Defic Syndr. 2013;62(1):21–7. [DOI] [PubMed] [Google Scholar]
- 115. Taburet AM, Sauvageon H, Grinsztejn B, Assuied A, Veloso V, Pilotto JH, et al. Pharmacokinetics of raltegravir in hiv‐infected patients on rifampicin‐based antitubercular therapy. Clin Infect Dis. 2015;61(8):1328–35. [DOI] [PubMed] [Google Scholar]
- 116. Atwine D, Bonnet M, Taburet AM. Pharmacokinetics of efavirenz in patients on antituberculosis treatment in high human immunodeficiency virus and tuberculosis burden countries: A systematic review. Br J Clin Pharmacol. 2018;84(8):1641–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Stader F, Kinvig H, Battegay M, Khoo S, Owen A, Siccardi M, et al. Analysis of clinical drug‐drug interaction data to predict magnitudes of uncharacterized interactions between antiretroviral drugs and comedications. Antimicrob Agents Chemother. 2018;62(7):e00717-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Cattaneo D, Baldelli S, Resnati C, Giacomelli A, Meraviglia P, Minisci D, et al. Evaluation of the concentrations of psychotropic drugs in HIV‐infected versus HIV‐negative patients: potential implications for clinical practice. World J Biol Psychiatry. 2018;1–7. [DOI] [PubMed] [Google Scholar]
- 119. Desmessine L, Peyro‐Saint‐Paul L, Gardner EM, Ghosn J, Parienti JJ. Risk and cost associated with drug‐drug interactions among agong HIV patients receiving combined antiretroviral therapy in France. Open Forum Infect Dis. 2019;6(3)ofz051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Flexner C, Thomas DL, Swindells S. Creating demand for long‐acting formulations for the treatment and prevention of HIV, tuberculosis, and viral hepatitis. Curr Opin HIV AIDS. 2019;14(1):13–20. [DOI] [PubMed] [Google Scholar]
- 121. Owen A, Rannard S. Strengths, weaknesses, opportunities and challenges for long acting injectable therapies: Insights for applications in HIV therapy. Adv Drug Deliv Rev. 2016;1(103):144–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Overton ET, Orkin C, Swindells S, Arasteh K, Gorgolas Hernandez‐Mora M, Pokrovsky V, et al. Monthly long‐acting cabotegravir and rilpivirine is non‐inferior to oral ART as maintenance therapy for HIV‐1 infection: week 48 pooled analysis from the phase 3 ATLAS and FLAIR studies. Conference on HIV Science (IAS), 21‐24th July, 2019. Abstract MOPEB257.
- 123. Scarsi KK, Darin KM, Nakalema S, Back DJ, Byakika‐Kibwika P, Else LJ, et al. Unintended pregnancies observed with combined use of the levonorgestrel contraceptive implant and efavirenz‐based antiretroviral therapy: a three‐arm pharmacokinetic evaluation over 48 weeks. Clin Infect Dis. 2016;62(6):675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Scarsi KK, Cirrincione L, Nakalema S, Darin K, Musinguzi I, Kyohairwe I, et al. Double‐dose levonorgestrelimplant does not fully overcome drug‐drug interaction with efavirenz. Conference on Retroviruses and Opportunistic Infections, Seattle, March 7–9, 2019, abstract O51. [Google Scholar]
- 125. Ford SL, Sutton K, Lou Y, Zhang Z, Tenorio A, Trezza C, et al. Effect of rifampin on the single‐dose pharmacokinetics of oral cabotegravir in healthy subjects. Antimicrob Agents Chemother. 2017;61(10):e00487-17. [DOI] [PMC free article] [PubMed] [Google Scholar]


