Abstract
Objectives
To delineate the impact of diabetes mellitus (DM) on the development of cardiovascular diseases in a community population.
Patients & Methods
Cross-sectional survey of residents randomly selected through the Rochester Epidemiology Project (REP), age 45 years or older of Olmsted County as of June 1st 1997. Responders (2,042) underwent assessment of systolic and diastolic function using echocardiography. The current analyses included all subjects with DM and were compared to a group of non DM subjects matched 1:2 for age, gender, hypertension and coronary artery disease. Baseline characteristics, laboratory and echocardiogram findings between groups were compared along with rates of mortality due to various cardiovascular conditions.
Results
We identified 116 subjects with DM and 232 matched non-DM subjects. Subjects with DM had higher BMI, plasma insulin and serum glucose levels. While LV EF was similar, E/e' ratio (9.66 vs. 8.50, P=.001) was higher in DM vs. non-DM. Over a follow-up of 10.8 years (IQR: 7.8–11.7), diabetics had higher incidence of HF; HR 2.13(1.25, 3.62; P=.01), 10 year Kaplan-Meier (KM) rate 21% vs 12% compared to non-diabetics. We also examined the subgroup of subjects without diastolic dysfunction. In this subgroup, DM subjects had an increased risk of HF, HR 2.52 (1.01, 6.27; P=.04).
Conclusions
In this cohort, subjects with DM have increased incidence of HF over a 10-year follow up period even in the absence of underlying diastolic dysfunction. These findings suggest that DM is an independent risk factor for the development of HF and supports the concept of DM cardiomyopathy.
Introduction
Diabetes mellitus (DM) impacts roughly 9.4% of the US population. Despite improvements in medical therapy, DM remains the seventh leading cause of death in the United States of America.1 Cardiovascular complications in DM patients are especially debilitating and account for a large portion of morbidity and mortality in DM. Coronary artery disease, stroke, atherosclerosis and hypertension are all common cardiovascular complications in DM.2,3 DM patients are also at increased risk for the development of HF and mortality from HF.4,5,6 DM patients represent a large portion of HF admissions to hospitals in the United States, around 33%.7
Additionally, in older DM patients, 22% will have a diagnosis of HF.8,9 The development of HF in DM patients can be secondary to concurrent hypertension or coronary heart disease, which are both well-established causes of HF. However, less studied is the development of primary HF from DM itself or diabetic cardiomyopathy (DCM). First described in Rubler et al., diabetic cardiomyopathy is defined as a myocardial disease secondary to diabetic microangiography or abnormal myocardial metabolism in DM leading to cardiomegaly and HF.10 Recently, studies have shown that even without signs of overt structural heart disease or HF, DM patients with preclinical diastolic dysfunction were more likely to develop HF and have higher rates of mortality compared to DM without diastolic dysfunction.11
Due to the concurrent presence of hypertension and coronary heart disease in many DM patients, the actual incidence of HF and DCM due to DM is not well defined.
The primary objective of the current study is to determine the long term impact of DM on the development of HF, both with preserved EF and reduced EF, and mortality in a community population controlling for hypertension, coronary artery disease and diastolic function. We also set out to follow DM patients over an extended period to determine disease progression and to monitor for the development of other cardiovascular complications.
Patients & Methods
Study approval was issued by the Mayo Foundation review board. Informed consent was obtained from all patients. Using the Rochester Epidemiology Project (REP), individuals older than 45 years old were selected for participation in the study. As outlined in previous studies, this database is centered in Olmsted County, MN.12,13 The REP cohort is predominately white, middle class. Further specifics of the population are previously described in past studies.14,15 The REP has been successful in mapping out complex disease associations in community settings.16
The REP identified a random selection of 4,203 residents. From the original random sample, 2,042 (47%) agreed to participate in the study. Between June 1st 1997-September 30th 2000 subjects were recruited and data was actively collected from the REP as previously described.17 For this study, we first identified subjects with DM from the initial cohort of 2,042. To control for potential differences between those with and without DM, subjects with DM were matched 1:2 for age, hypertension, gender, coronary artery disease and diastolic dysfunction to subjects without DM. Comparisons were made between the groups' baseline characteristics, echocardiography studies and lab results along with comparisons regarding rates of mortality due to cardiovascular disease, including HF. The final sample included 116 DM and 232 non DM subjects.
Each of the enrolled subjects underwent a physical exam, baseline blood work which included kidney function, lipid testing and neurohormonal biomarkers of cardiovascular damage/distress along with echocardiography between June 1st 1997 & September 30th 2000.11,15,17 As previously described, measurements of body mass index (BMI), blood pressure, height and weight were taken during the initial data collection period.16,17 Of the patients identified but did not participate in the study, 500 were randomly selected and their medical records were reviewed. This review revealed similar distributions in terms of age, gender, medical issues and comorbidities when compared between the study participants and non study participants.15,16,17 All subjects completed medical surveys and questionnaires following initial data collection.
During the initial data collection between June 1st 1997- September 30th 2000, the medical records of all 2,042 subjects were reviewed by trained nursing staff. Medical records were comprehensively reviewed and diagnoses of myocardial infarction, stroke and hypertension were noted if established criteria were met as outlined in the International Diagnostic Criteria for Acute Myocardial Infarction and Acute Stroke.16,17,18,19 Hypertension was noted if criteria were met as established by the JNC committee guidelines.19 During record review, previous diagnoses of diabetes and coronary artery disease were also noted if there was clinical or historical documentation of these conditions. Metabolic syndrome was defined in this study per the current ATP-NCEP III criteria based on the presence of any of the three following characteristics. 1) abdominal obesity, defined as a waist circumference in men ≥102 cm (40 in) and in women ≥88 cm (35 in); 2) serum triglycerides ≥150 mg/dL (1.7 mmol/L) or drug treatment for elevated triglycerides; 3) Serum HDL cholesterol <40 mg/dL (1 mmol/L) in men and <50 mg/dL (1.3 mmol/L) in women or drug treatment for low HDL-C; 4) blood pressure ≥130/85 mmHg or drug treatment for elevated blood pressure 5) fasting plasma glucose (FPG) ≥100 mg/dL (5.6 mmol/L). The diagnosis of DM in Olmsted County through a review of ICD-9 codes has previously been validated as an accurate way to establish DM with a >98% accuracy.20 As previously outlined, the medical records of the participants were also reviewed for the diagnosis of HF.21 These diagnoses were verified using the Framingham criteria.16,17 In order to fulfill the Framingham criteria for the diagnosis of HF, 2 major criteria or 1 major and 2 minor criteria are needed. Major criteria include elevated JVD, presence of a third heart sound, cardiomegaly on chest x-ray or symptoms of orthopnea and paroxysmal nocturnal dyspnea. Pulmonary rales on physical exam or pulmonary edema on chest x-ray are also major criteria. Minor criteria include, dyspnea on exertion, an elevated HR >120, peripheral edema or night cough.16 For this study, HF diagnoses included both HF with reduced EF and preserved EF.
Following active data collection, the medical records of the subjects were monitored periodically for mortality data, along with subsequent diagnoses of HF, MI or stroke. This was last performed in 2013.
Subjects in this study had 2D and color Doppler echocardiograms performed during data collection to assess for valvular pathology, cardiac structure, systolic and diastolic function, as previously described.17, 22
Statistical Analysis
After identification of DM subjects, a greedy matching algorithm was used to match 2 non-DM subjects to each DM subject. Gender and diastolic dysfunction (yes/no) was required to be an exact match, while age, CAD, and HTN were matched as closely as possible. Baseline characteristics of DM and non-DM subjects were then summarized using frequency and percentage for categorical variables and compared between groups using Pearson chi-square test. Continuous variables with approximate normal distributions were summarized by mean and standard deviation (SD) and compared between groups using two-sample t-tests. Those continuous variables that were not normally distributed are presented using median and quartiles and were compared between groups using the Wilcoxon rank-sum test. Outcomes were analyzed using Kaplan-Meier methods. Subjects without events were censored at last known Mayo follow-up and groups were compared using the log-rank test. Cox proportional hazards regression methods were used to compute hazard ratios (HR) and associated 95% confidence limits. Age and gender were used as adjustment factors in the proportional hazards regression models. SAS version 9.4 (Cary, NC) was used for analyses and two-sided P-values ≤.05 were considered to be statistically significant.
Results
Diabetes Mellitus (DM) was identified in 116 subjects out of 2,042 randomly selected residents of Olmsted County. These subjects were compared to a group of 232 non-DM subjects and matched 1:2 for age, gender, hypertension, coronary artery disease and diastolic dysfunction.
Baseline Characteristics
When comparing baseline properties among the groups, DM was associated with higher patient BMI (29± 5 vs. 31± 6) along with higher percentages of atrial fibrillation (9% vs. 4%) and baseline HF (3% vs. 0%) (Table 1). Metabolic syndrome was also in higher percentages in the DM group (54% vs. 23%). Triglycerides were higher at baseline in DM compared to non-DM patients (183± 122 mg/dL vs. 146± 80 mg/dL). Additionally, median insulin was higher in DM patients (8.5 uU/ml vs. 5.8 uU/ml) and serum glucose levels were also elevated in DM compared to non-DM patients (130 mg/dL vs. 95mg/dL). Of the DM patients, 21% were defined as insulin-dependent diabetics (24 out of 116) and maintained on insulin alone. No DM patients in this study were maintained on the newer oral antidiabetic drugs such as the SGLT2 inhibitors or DPP4 inhibitors. Baseline prevalence of comorbidities such as MI, stroke, CAD, HTN and systolic blood pressure between the two groups were not statistically significant. Creatinine was similar between the two groups (Table 1). Biomarkers NT-proBNP and aldosterone levels were similar between both groups (Table 1). The percentage of subjects using an ACE inhibitor was higher in DM compared to non-DM (32% vs. 10%). However, the usage of antilipemic therapy, beta blockers, calcium channel blockers were similar between the two groups.
Table 1.
Baseline Characteristics of all DM and non-DM Patients
| Variable | Non-DM | DM | P-value |
|---|---|---|---|
| (N=232) | (N=116) | ||
| Age at Exam (years) | 66.6± 9.4 | 66.6± 9.4 | .98 |
| Gender, n (%) | 98 (42%) | 49 (42%) | -- |
| BMI of Patient | 28.8± 5.2 | 30.8± 5.5 | .001 |
| BMI>30, n (%) | 84 (36%) | 58 (50%) | .01 |
| Waist>102M, 88F, n(%) | 92 (40%) | 57 (49%) | .09 |
| Metabolic Syndrome, n (%) | 54 (23%) | 62 (54%) | .001 |
| Verified Hypertension, n(%) | 118 (51%) | 62 (53%) | .65 |
| CAD (%) | 58 (25%) | 38 (33%) | .13 |
| HF (n%) | 1 (0%) | 4 (3%) | .03 |
| MI n,(%) | 20 (9%) | 17 (15%) | .09 |
| Atrial Fib/Flutter n,(%) | 9 (4%) | 11 (9%) | .03 |
| Stroke n,(%) | 5 (2%) | 2 (2%) | .79 |
| Beta Blocker, n(%) | 59(27%) | 33 (29%) | .63 |
| Calcium Channel Blocker, n(%) | 35 (16%) | 19 (17%) | .82 |
| ACE Inhibitor, n(%) | 23 (10%) | 36 (32%) | <.001 |
| Antilipemic Therapy, n(%) | 66 (30%) | 40 (35%) | .30 |
| Total Cholesterol (mg/dL) | 199.9± 36.9 | 138.2± 20.4 | .04 |
| LDL Cholesterol (mg/dL) | 125.9± 32.8 | 115.5± 32 | .005 |
| Triglycerides (mg/dL) | 146.4± 79.6 | 183.2± 121.8 | <.001 |
| HDL (mg/dL) <40M, <50F, n(%) | 122 (53%) | 83 (72%) | <.001 |
| NT-proBNP (pg/mL), median (Q1, Q3) | 76.9 (32.3, 153.6) | 81.9 (31.8, 182.3) | .69 |
| Aldosterone (pg/ml), median (Q1, Q3) | 5.6 (3, 8.7) | 4.9 (2.5, 8.6) | .52 |
| Insulin (uU/mL), median (Q1, Q3) | 5.8 (4.1, 8.7) | 8.5 (5.1, 15.7) | <.001 |
| Creatinine (mg/dL), median (Q1, Q3) | 0.9 (0.8, 1.0) | 0.9 (0.8, 1.0) | .63 |
| SBP (mmHg) | 136.8± 22.2 | 138.2±20.4 | .56 |
| DBP (mmHg) | 74.7± 10.6 | 72.3±8.5 | .04 |
| Serum Glucose (mg/dL), median (Q1, Q3) | 95 (90, 102) | 130 (107,167) | <.001 |
ACE angiotensin-converting enzyme, BMI body mass index, CAD coronary artery disease, HF heart failure, DBP diastolic blood pressure, MI myocardial infarction, HDL high-density lipoprotein, LDL low-density lipoprotein, NT-proBNP n-terminal pro hormone b-type natriuretic peptide, SBP systolic blood pressure
dL deciliter, mg milligram, mmHg millimeters of mercury, Pg picogram, uU international units
Echographic Parameters
E/e’ ratio was significantly elevated in DM compared to non-DM patients (9.7 vs. 8.5, P <0.001). Ejection fraction, Left Atrial (LA) size and diastolic dysfunction were not statistically different between the subjects with DM and the non-DM subjects (Table 2).
Table 2.
Echographic Parameters in All Patients and in Patients with No Diastolic Dysfunction
| All Patients | |||
| Variable | Non-DM | DM | P-value |
| (N=232) | (N=116) | ||
| Ejection Fraction (%) | 63.5 ± 5.7 | 63.8± 5.7 | .73 |
| LVMI> 115M, 95F (g/m2), n(%) | 50 (31%) | 28 (33%) | .76 |
| E/A ratio | 1.0 ± 0.4 | 0.9± 0.3 | .04 |
| E/e’ (medial) | 8.5± 2.9 | 9.7± 2.9 | .001 |
| LA Size cm2 | 4.1± 0.6 | 4.1± 0.5 | .96 |
| PASP (mmHg) | 22.7± 4.4 | 23.7± 5 | .13 |
| Diast_dys_gp, n(%) | .79 | ||
| Normal | 128 (55%) | 64 (55%) | |
| Mild | 79 (34%) | 42 (36%) | |
| Moderate/severe | 25 (11%) | 10 (9%) | |
| Patients with no Diastolic Dysfunction | |||
| (N=128) | (N=64) | ||
| Ejection Fraction (%) | 63.1 ± 5.4 | 63.5 ± 4.8 | .64 |
| LVMI> 115M, 95F (g/m2), n(%) | 20 (19%) | 10 (21%) | .84 |
| E/A ratio | 1.2 ± 0.3 | 1.1 ± 0.3 | .13 |
| E/e’ (medial) | 8.2 ± 2.6 | 9.0 ± 2.3 | .06 |
| LA Size cm2 | 4.0 ± 0.6 | 4.0 ± 0.5 | .53 |
| PASP (mmHg) | 22.3 ± 4.1 | 23.8 ± 4.5 | .09 |
A=late transmitral left ventricular inflow during left atrial contraction; E=passive transmitral left ventricular inflow velocity; e’ = tissue Doppler imaging velocity of the medial mitral annulus during passive filling
Diast_dys_gp diastolic dysfunction; LA left atrium; LVMI left ventricular mass index; PASP pulmonary artery systolic pressure
mmHg millimeters of mercury
Clinical Outcomes
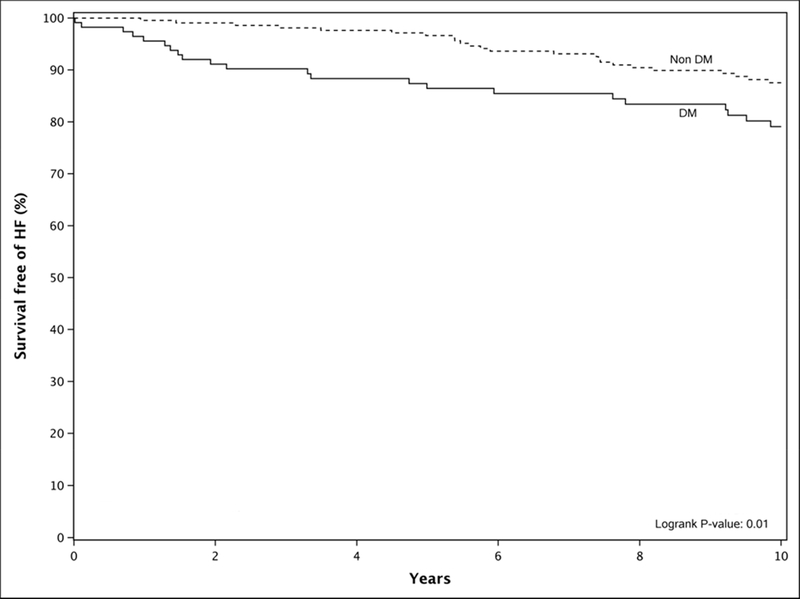
Among subjects with DM, the overall development of HF based on Kaplan-Meier analysis over a follow-up period of 10.8 years (IQR: 7.8–11.7) was statistically higher than non-DM patients with a hazard ratio of 2.1 (1.2, 3.6; P= .01) (Fig.1). Follow up at one year from initial evaluation showed 4% of DM patients had developed HF and at five years, 14% of DM had developed new HF (Fig. 1). At 10 years from initial data collection, 21% of DM patients had developed HF compared to only 12% of non-DM patients. In addition, cardiac death, MI and stroke were not statistically different between DM and non-DM patients. Over the same follow-up period, all-cause mortality was similar in DM and non-DM patients, HR 1.2(0.8, 1.7; P=.85). Age and gender adjusted analysis of these events showed a similar risk between DM and non-DM subjects.
Figure 1: Kaplan Meier Curve Illustrating Survival Free Heart Failure (HF) Development in Diabetic Versus Non-Diabetic Patients.
Diabetic (DM) patients had a higher risk of developing heart failure compared to matched nondiabetics (Non DM) over 10 years.
Subgroup with No Diastolic Dysfunction: DM vs. Non-DM
Subjects were divided further into groups with diastolic dysfunction and without diastolic dysfunction. We specifically examined the subgroups of DM with and without diastolic dysfunction with their matched controls.
Baseline Characteristics
When comparing baseline properties among the groups, DM was associated with higher patient BMI (30± 6 vs. 29± 5, P= .04) and metabolic syndrome (56% vs. 23%, P <0.001). Baseline prevalence of MI, stroke, atrial fibrillation, CAD, HTN and systolic blood pressure between the two groups were not statistically significant. Both the DM and non-DM groups had zero HF cases at baseline. Creatinine was similar between the two groups (Table 3). Biomarkers, NT-proBNP and aldosterone levels were similar between both groups. The percentage of subjects using an ACE inhibitor was higher in DM compared to non-DM (28% vs. 8%). However, the usage of beta blockers and calcium channel blockers were similar between the two groups.
Table 3.
Baseline Characteristics of DM and non DM Groups with no Diastolic Dysfunction
| Variable | Non-DM | DM | P-value |
|---|---|---|---|
| (N=128) | (N=64) | ||
| Age at Exam (years) | 62.6 ± 9.2 | 62.7± 9.2 | .94 |
| Gender, n (%) | 48 (38%) | 24 (38%) | -- |
| BMI of Patient | 28.8± 4.9 | 30.4± 5.9 | .04 |
| BMI>30, n (%) | 48 (38%) | 29 (45%) | .30 |
| Waist>102M, 88F, n(%) | 50 (39%) | 31 (48%) | .21 |
| Metabolic Syndrome, n (%) | 29 (23%) | 35 (56%) | <.001 |
| Verified Hypertension, n(%) | 54 (42%) | 29 (45%) | .68 |
| CAD (%) | 33 (26%) | 21 (33%) | .31 |
| HF (n%) | 0 (0%) | 0 (0%) | -- |
| MI n,(%) | 10 (8%) | 10 (16%) | .09 |
| Atrial Fib/Flutter n,(%) | 4 (3%) | 5 (8%) | .15 |
| Stroke n,(%) | 0 (0%) | 0 (0%) | -- |
| Beta Blocker, n(%) | 26 (22%) | 19 (31%) | .17 |
| Calcium Channel Blocker, n(%) | 17 (14%) | 5 (8%) | .24 |
| ACE Inhibitor, n(%) | 10 (8%) | 17 (28%) | <.001 |
| Antilipemic Therapy, n(%) | 27 (23%) | 25 (41%) | .01 |
| Total Cholesterol (mg/dL) | 201.7 ± 38.1 | 188.9 ± 40.8 | .04 |
| LDL Cholesterol (mg/dL) | 126.8 ± 33.3 | 114.9 ± 34.2 | .02 |
| Triglycerides (mg/dL) | 153.5 ± 87.9 | 180.4 ± 117.3 | .08 |
| HDL (mg/dL) <40M, <50F, n(%) | 64 (51%) | 46 (73%) | .004 |
| NT-proBNP (pg/mL), median (Q1, Q3) | 76.9 (32.3, 153.6) | 61.7 (15.6, 126) | 0.85 |
| Aldosterone (pg/ml), median (Q1, Q3) | 4.8 (2.9, 9.2) | 5.1 (2.5, 8.3) | 0.64 |
| Insulin (uU/mL), median (Q1, Q3) | 5.6 (3.6, 8.1) | 8.3 (5, 15) | <.001 |
| Creatinine (mg/dL), median (Q1, Q3) | 0.9 (0.8, 1) | 0.9 (0.7, 1) | .63 |
| SBP (mmHg) | 131.7 ± 19 | 134 ± 17 | .43 |
| DBP (mmHg) | 73.6 ± 10.2 | 72.8 ± 7.3 | .59 |
| Serum Glucose (mg/dL), median (Q1, Q3) | 94 (89, 100) | 129 (111, 158) | <.001 |
ACE angiotensin-converting enzyme, BMI body mass index, CAD coronary artery disease, HF heart failure, DBP diastolic blood pressure, MI myocardial infarction, HDL high-density lipoprotein, LDL low-density lipoprotein, NT-proBNP n-terminal pro hormone b-type natriuretic peptide, SBP systolic blood pressure
dL deciliter, mg milligram, mmHg millimeters of mercury, Pg picogram, uU international unit
Echographic Parameters
E/e’ ratio was similar in DM compared to non-DM patients (8.96 vs. 8.16, P=.06). Ejection fraction, PASP, LA size and LVMI were not statistically different between the patients with DM and the non-DM patients (table 2).
Clinical Outcomes
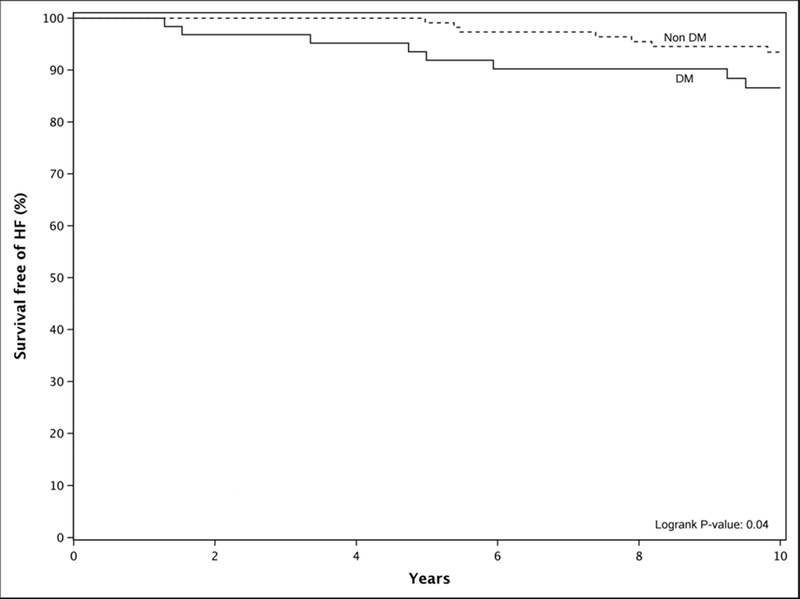
In the subgroup without diastolic dysfunction, DM patients had higher incidences of HF (HR 2.5 [1.0, 6.3], P=.04) over 10 years compared to non-DM patients (Fig.2). Follow up at 10 years from the initial evaluation showed 13% of DM patients had developed HF as compared to only 7% of non-DM subjects. Over the same follow-up period, the incidence of death was similar in DM and non-DM patients, with an age and gender adjusted hazard ratio of 1.1 (0.6, 1.9; P=.73). Cardiac death was also not statistically different between the groups. MI and stroke events happened at similar rates between DM and non-DM patients with an adjusted HR of 1.22 (0.57, 2.61) and 1.00 (0.49, 2.01).
Figure 2: Kaplan Meier Curve Illustrating Survival Free Heart Failure (HF) Development in Patients with no Diastolic Dysfunction (DD): Diabetic Versus Non-Diabetic Patients.
In the subgroup analysis of patients without diastolic dysfunction (DD), diabetic (DM) patients had an increased risk of heart failure compared to nondiabetic (Non DM) patients. This figure illustrates that the absence of diastolic dysfunction is not protective against heart failure in diabetic patients.
Discussion
In this community-based cohort, matched for age, gender, hypertension coronary artery disease and diastolic function, subjects with diabetes have increased incidence of HF over a 10-year follow up period even in the absence of underlying diastolic dysfunction. These findings suggest that DM is an independent risk factor for the development of HF and supports the concept of DM cardiomyopathy.
The baseline characteristics between the DM and non-DM groups are similar except for BMI, insulin levels, glucose levels, metabolic syndrome and cholesterol levels. These differences between DM and non-DM are consistent with previously described mechanisms regarding DM cardiomyopathy development. These differences are also noted epidemiologic risk factors for HF development that have previously been well outlined. It is currently believed that in DM patients obesity, BMI, insulin resistance, elevated glucose levels and hyperlipidemia trigger downstream effects at a cellular level that cause myocardial dysfunction leading to eventual organ level dysfunction, cardiomyopathy physiology and HF.23 Obesity, in particular doubles the rate of HF as shown in Kenchaiah et al.24 Elevated BMI is thought to contribute to the development of HF by augmenting LV dysfunction and causing activation of the neurohormonal cascade, increasing the hemodynamic load and causing increased reactive oxygen species (ROS) formation.24, 25
Higher levels of insulin resistance have also been shown to be associated with higher NYHA classes and associated with the development of HF.26 In our cohort, the DM patients had significantly higher levels of insulin as compared to the non-DM patients. Increases in insulin resistance change the metabolism of myocardial cells leading to increased usage of lipids as a primary energy source and subsequently leads to lipotoxicity and chronic inflammation, ROS formation, myocardial fibrosis, decreased cardiac efficiency, diastolic dysfunction, apoptosis and cellular calcium misbalance which eventually decreases myocardial function.23,27, 28 Elevated fasting glucose levels alone are linked to left ventricular diastolic dysfunction (LVH) and HF.29,30 Elevated levels of glucose and decreased glucose usage by cardiomyocytes in DM patients leads to DNA damage through the creation of reactive oxygen species and the deposition of collagen due to the production of glycation end products, eventually causing myocardial fibrosis and a structurally dysfunctional heart.28,30
Echocardiographic findings in DM patients demonstrate the resultant impact of the above pathophysiology on the heart. DM patients are more likely to have increased left ventricular mass and have increases in left ventricle wall thickness compared to non-DM patients.31,32 Furthermore, DM patients have more signs of myocardial fibrosis and stiffness as evidenced by autopsy results/histopathology information and also measures of diastolic dysfunction, i.e., E/e’ on echocardiogram.33 In the current study, baseline echocardiogram findings such as LV EF, LVMI and LA size were similar between DM and non-DM groups. However, E/e’ was higher in the DM groups suggestive of higher LV filling pressure. These echographic findings suggest that in our cohort, the cardiac structure is similar between the 2 groups, which is supported by similar levels of humoral biomarkers such as NT pro-BNP and Aldosterone. Despite a similar cardiac structure at baseline, the DM group had increased risk for the development of HF during the follow-up period, suggesting that DM is associated with the development of HF. This is further substantiated by the fact that in the subgroup of the cohort without diastolic dysfunction (i.e. normal LV filling pressure), DM patients still developed HF at a higher rate over time than non-DM patients. While previous studies have reported that increased LV filling pressures and diastolic dysfunction are associated with the development of HF in DM, in those studies, subjects with increased filling pressures also had significant comorbidities such as HTN and CAD with structural abnormalities such as LV hypertrophy.11,20 The current study confirms and extends previous findings by demonstrating that despite the absence of cardiac structure abnormality, DM patients are at an increased risk of developing HF. More significantly, we also demonstrate that in the subgroup of the cohort with normal LV filling pressure, DM was still associated with increased risk for the development of HF.
Limitations
This study was performed using a predominately rural Caucasian population located in Olmsted County, MN in the Midwest. Given the homogeneity of the population, it may be difficult to use this study to completely equivocate the results to the United States population as a whole. However, despite the homogeneity, the impact shown in this study in regards to DM and HF development still stands. The current study both confirms and expands on previous population data looking at the impact of DM on HF development. In this specific population it shows that even without cardiac structural abnormalities, the risk of HF development in DM is still present. The overall size of the cohort examined in this paper is relatively small. This leads to the possibility that further research into the findings of this paper would be needed on a large prospective scale. Additionally, the study period was roughly 10 years. Further data retrieval would most likely show a continued trend of HF development as the cohort ages. This limitation of a relatively short follow up period makes it possible the data underestimates the incidence of HF in the DM population. Additionally, due to the lack of echocardiography data during follow up, this study is unable to determine the distribution of HF preserved EF versus HF reduced EF. Ischemia from undiagnosed asymptomatic coronary artery disease leading to cardiac dysfunction and heart failure in the cohort is one that cannot be completely ruled out, and therefore possibly limits some of the study results.
Conclusions
Diabetes is an independent risk factor and is associated with the development of HF over time. Roughly 21% of diabetic patients developed HF over a 10 year period, which is significantly elevated when compared to non-diabetics (12%). In this study, we have also shown that diabetic subjects, even in the absence of diastolic dysfunction are more likely than non-diabetics to develop HF. The findings in this paper support the concept of diabetic cardiomyopathy. Future research should be focused on whether aggressive management of risk factors such as BMI, glucose and cholesterol will decrease the development of HF in DM patients.
Acknowledgments
Grant support: The design and conduct of the study; collection, management, analysis, and interpretation of the data were supported by the grants from National Institutes of Health [P01 HL76611, R01HL84155, and R01-HL136440 to H.H.C] and [HL RO1-55502 to R.J.R].
The design and conduct of the study; collection, management, analysis, and interpretation of the data were supported by the grants from National Institutes of Health [P01 HL76611, R01HL84155, and R01-HL136440 to H.H.C] and [HL RO1-55502 to R.J.R].
Abbreviations
- BMI
body Mass Index
- CAD
coronary artery disease
- DCM
diabetic Cardiomyopathy
- DM
diabetes mellitus
- E
passive transmitral left ventricular inflow velocity
- e’
tissue Doppler imaging velocity of the medial mitral annulus during passive filling
- EF
ejection Fraction
- HF
heart failure
- HTN
hypertension
- LV
left Ventricle
- MI
myocardial infarction
- REP
Rochester epidemiology project
Footnotes
Horng H. Chen is co-founder of Zumbro Discovery. There are no other financial or other relations that could lead to a conflict of interest in the context of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017. [Google Scholar]
- 2.Kannel WB, McGee DL. Diabetes and cardiovascular disease: The Framingham study. JAMA 1979;241(19):2035–2038. [DOI] [PubMed] [Google Scholar]
- 3.Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical Update: Cardiovascular Disease in Diabetes Mellitus: Atherosclerotic Cardiovascular Disease and Heart Failure in Type 2 Diabetes Mellitus - Mechanisms, Management, and Clinical Considerations. Circulation 2016;133(24):2459–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: The Framingham study. Am J Cardiol 1974;34(1):29–34. [DOI] [PubMed] [Google Scholar]
- 5.Nichols GA, Hillier TA, Erbey JR, Brown JB. Congestive Heart Failure in Type 2 Diabetes. Prevalence, incidence, and risk factors. Diabetes care 2001;24(9):1614–1619. [DOI] [PubMed] [Google Scholar]
- 6.Bell DSH. Heart Failure. The frequent, forgotten, and often fatal complication of diabetes. Diabetes care 2003;26(8):2433–2441. [DOI] [PubMed] [Google Scholar]
- 7.Reis SE, Holubkov R, Edmundowicz D et al. Treatment of Patients Admitted to the Hospital With Congestive Heart Failure: Specialty-Related Disparities in Practice Patterns and Outcomes. Am J Cardiol 1997;30(3):733–738. [DOI] [PubMed] [Google Scholar]
- 8.Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL, Goff DC. Heart Failure Prevalence, Incidence, and Mortality in the Elderly With Diabetes. Diabetes Care 2004;27(3):699–703. [DOI] [PubMed] [Google Scholar]
- 9.Aronow WS, Ahn C, Kronzon I. Comparison of incidences of congestive heart failure in older African-Americans, Hispanics, and Whites. Am J Cardiol 1999;84(5):611–612. [DOI] [PubMed] [Google Scholar]
- 10.Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol 1972;30(6):595–602. [DOI] [PubMed] [Google Scholar]
- 11.From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction a population-based study. J Am Coll Cardiol 2010;55(4):300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.St Sauver JL, Grossardt BR, Yawn BP et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. International journal of epidemiology 2012;41(6):1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ, 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc 2012;87(12):1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melton LJ. History of the Rochester Epidemiology Project Mayo Clin Proc 1996;71(3):266–274. [DOI] [PubMed] [Google Scholar]
- 15.Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol 2002;40(5):976–982. [DOI] [PubMed] [Google Scholar]
- 16.Redfield MM, Jacobsen SJ, Burnett et al. Burden of systolic and diastolic ventricular dysfunction in the community: Appreciating the scope of the heart failure epidemic. JAMA 2003;289(2):194–202. [DOI] [PubMed] [Google Scholar]
- 17.Dandamudi S, Slusser J, Mahoney DW, Redfield MM, Rodeheffer RJ, Chen HH. The prevalence of diabetic cardiomyopathy: a population-based study in Olmsted County, Minnesota. Journal of cardiac failure 2014;20(5):304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillum RF, Fortmann SP, Prineas RJ, Kottke TE. International diagnostic criteria for acute myocardial infarction and acute stroke. Am Heart J 1984;108(1):150–158. [DOI] [PubMed] [Google Scholar]
- 19.The sixth report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Archives of Internal Medicine 1997;157(21):2413–2446. [DOI] [PubMed] [Google Scholar]
- 20.From AM, Scott CG, Chen HH. Changes in diastolic dysfunction in diabetes mellitus over time. Am J Cardiol 2009;103(10):1463–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Senni M, Tribouilloy Christophe M, Rodeheffer Richard J et al. Congestive Heart Failure in the Community. Circulation 1998;98(21):2282–2289. [DOI] [PubMed] [Google Scholar]
- 22.Pritchett AM, Jacobsen SJ, Mahoney DW, Rodeheffer RJ, Bailey KR, Redfield MM. Left atrial volume as an index of left atrial size: a population-based study. J Am Coll Cardiol 2003;41(6):1036–1043. [DOI] [PubMed] [Google Scholar]
- 23.Lorenzo-Almorós A, Tuñón J, Orejas M, Cortés M, Egido J, Lorenzo Ó. Diagnostic approaches for diabetic cardiomyopathy. Cardiovascular Diabetology 2017;16(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kenchaiah S, Evans J.C., Levy D et al. Obesity and the Risk of Heart Failure. N Engl J Med 2002;347(5):305–313. [DOI] [PubMed] [Google Scholar]
- 25.Zhou YT, Grayburn P, Karim A et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proceedings of the National Academy of Sciences of the United States of America 2000;97(4):1784–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong AKF, ALZadjali MA, Choy A-MJ, Lang CC. Insulin Resistance: A Potential New Target for Therapy in Patients with Heart Failure. Cardiovascular Therapeutics 2008;26(3):203–213. [DOI] [PubMed] [Google Scholar]
- 27.Aroor AR, Mandavia CH, Sowers JR. Insulin resistance and heart failure: molecular mechanisms. Heart failure clinics 2012;8(4):609–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nature reviews Endocrinology 2016;12(3):144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milwidsky A, Maor E, Kivity S et al. Impaired fasting glucose and left ventricular diastolic dysfunction in middle-age adults: a retrospective cross-sectional analysis of 2971 subjects. Cardiovascular diabetology 2015;14(1):119–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nielson C, Lange T. Blood Glucose and Heart Failure in Nondiabetic Patients. Diabetes Care 2005;28(3):607–611. [DOI] [PubMed] [Google Scholar]
- 31.Ilercil A, Devereux RB, Roman MJ et al. Relationship of impaired glucose tolerance to left ventricular structure and function: The Strong Heart Study. Am Heart J 2001;141(6):992–998. [DOI] [PubMed] [Google Scholar]
- 32.Rutter Martin K, Parise H, Benjamin Emelia J et al. Impact of Glucose Intolerance and Insulin Resistance on Cardiac Structure and Function. Circulation 2003;107(3):448–454. [DOI] [PubMed] [Google Scholar]
- 33.Negishi K Echocardiographic feature of diabetic cardiomyopathy: where are we now? Cardiovascular diagnosis and therapy 2018;8(1):47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]