Abstract
Outcomes related to alcohol use after hepatitis C virus (HCV) treatment are unknown in the direct‐acting antiviral (DAA) era. We assessed levels of alcohol use before and after HCV treatment and their association with long‐term outcomes in a cohort of U.S. veterans. In this retrospective cohort analysis, 29,037 patients who initiated DAA regimens between 2013 and 2015 were followed for a mean of 3.04 years. We categorized alcohol use into three categories (nondrinking, low‐level drinking, and unhealthy drinking) using Alcohol Use Disorders Identification Test‐Consumption questionnaires administered within 1 year before (baseline) and after treatment. Multivariable Cox proportional hazards regression was used to determine the associations between alcohol use and mortality or liver‐related outcomes. Before DAA treatment, 68% of veterans reported nondrinking, 22.9% reported low‐level drinking, and 9.1% reported unhealthy drinking. Compared to patients with baseline non‐drinking, those with unhealthy drinking had a higher risk of mortality (adjusted hazard ratio [HR] 1.53, 95% confidence interval [CI]: 1.34‐1.75) and decompensated cirrhosis (adjusted HR 1.30, 95% CI: 1.06‐1.59) and lower likelihood of liver transplantation (adjusted HR 0.24, 95% CI: 0.06‐0.92). These associations were greater in patients without sustained virologic response than in those with sustained virologic response. When alcohol use before and after treatment was modeled as a time‐varying covariate, similar associations were observed. Survival analysis also found that unhealthy drinking was significantly associated with a lower probability of survival compared with nondrinking. Low‐level alcohol use was not associated with increased risk of adverse outcomes. Conclusion: In this large cohort of U.S. veterans with HCV who received DAAs, unhealthy drinking was common and associated with a higher risk of posttreatment mortality. Interventions to achieve alcohol cessation before and during antiviral treatment should be encouraged.
In a cohort of 29,037 U.S. veterans who received hepatitis C virus treatment with direct‐acting antivirals, we assessed levels of alcohol use and their association with long‐term outcomes. Unhealthy drinking was associated with a higher risk of posttreatment mortality. Interventions to achieve alcohol cessation before and during antiviral treatment should be encouraged.

Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- AUDIT‐C
Alcohol Use Disorders Identification Test–Consumption
- BMI
body mass index
- CI
confidence interval
- DAA
direct‐acting antivirals
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- HR
adjusted hazard ratio
- ICD
International Classification of Diseases
- INR
international normalized ratio
- MELD
Model for End‐Stage Liver Disease
- SVR
sustained virologic response
- VA
Veteran Affairs
Alcohol use in the presence of hepatitis C virus (HCV) infection can have detrimental effects on the progression of liver disease. Alcohol appears to have a synergistic effect, with HCV leading to faster progression of liver fibrosis,1 higher rates of cirrhosis,2 and higher rates of overall and liver‐related mortality.3 Although the interaction between alcohol and HCV remains unclear, some proposed pathways include alcohol increasing oxidative stress and changing the microbiome leading to inflammatory cytokines.4 Both the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America therefore recommend counseling all persons with active HCV infection to abstain from alcohol use to minimize liver disease progression.5
The introduction of direct‐acting antiviral (DAA) therapies has dramatically increased both HCV treatment and cure rates, with sustained virologic response (SVR) rates now over 92%.6 During the interferon‐based antiviral therapy era, heavy alcohol use was associated with lower rates of SVR; therefore, HCV was often not treated until abstinence from alcohol was achieved.7 However, in a previous study among U.S. veterans treated with DAAs, SVR did not significantly differ between those reporting no alcohol use and any alcohol use.8 Thus, although every effort should be made to help HCV‐infected patients reduce or stop alcohol completely, DAA treatment should not be postponed until abstinence is achieved.
Still, much remains unknown about alcohol use among patients with HCV. Specifically, patterns of alcohol consumption before and after DAA therapy have not been described, and it is unknown whether patients treated with DAAs who drink alcohol change their use after treatment. Furthermore, although successful DAA therapy has been associated with lower rates of hepatocellular carcinoma (HCC) and mortality,9, 10 and may also influence regression of liver fibrosis, improve portal hypertension, and reduce extrahepatic manifestations of liver disease,11 a substantial proportion of patients who achieve SVR still develop HCC, cirrhosis, or cirrhosis‐related complications. Because alcohol influences these conditions, studies are needed to understand the extent to which alcohol use—both before and after DAA treatment—increases the risk for these adverse outcomes in patients with and without SVR. Therefore, we aimed to describe patterns of alcohol use before and after DAA treatment in a national cohort of U.S. veterans and evaluate the association between severity of alcohol use and mortality and adverse liver‐related outcomes.
Patients and Methods
Data Source and Patient Population
The U.S. Veterans Health Administration is the largest integrated healthcare system in the U.S. and provides health care to approximately 9 million veterans each year, at 168 Veteran Affairs (VA) Medical Centers and 1,053 outpatient clinics. Laboratory tests, demographics, comorbidities, alcohol use questionnaires, and pharmacy/clinical outcomes data were extracted from the VA’s Corporate Data Warehouse, a data repository derived from the VA’s electronic medical records.
We identified 38,093 veterans with HCV who had received DAA‐only regimens from the VA between 2013 and 2015. Of these, 5,162 patients were excluded, as they did not have Alcohol Use Disorders Identification Test–Consumption (AUDIT‐C) scores (a measure of alcohol use described later) within 1 year before or after DAA treatment. Furthermore, 3 patients died within 180 days of starting antiviral treatment, 1 had less than 180 days of follow‐up, 825 received a liver transplant before treatment, and 1,986 had missing SVR data. The final study cohort therefore included 29,037 patients who initiated 30,116 regimens during the study period, including 26,028 who achieved SVR. For patients who received more than one regimen, only the first was analyzed. The most common DAA regimen prescribed was sofosbuvir/ledipasvir (58.4%) followed by paritaprevir/ritonavir/ombitasvir/dasabuvir (18.8%), sofosbuvir (±daclatasvir) (13.3%), and sofosbuvir/simeprevir (9.6%). This study was approved by the institutional review board of the VA Puget Sound Health Care System.
Assessment of Alcohol Use
Alcohol use was assessed using the AUDIT‐C questionnaire, which is a validated screening tool for identifying unhealthy alcohol use (Supporting Table S1).12, 13 Scores ranged from 0‐12, with 0 indicating nondrinking and higher scores reflecting greater amounts of alcohol consumption.12 Since 2004, AUDIT‐C has been used to screen most VA outpatients for unhealthy alcohol use annually and is therefore available in electronic health record data.14 Baseline alcohol use was defined by AUDIT‐C score reported within 1 year before initiation of antiviral treatment and classified into three categories: nondrinking (score of 0), low‐level drinking (score of 1‐3 in men, 1‐2 in women), and unhealthy drinking (score of 4‐12 in men, 3‐12 in women).8, 15 We also extracted all AUDIT‐C scores recorded at least 3 months after treatment completion and categorized them as previously, in order to model changes in AUDIT‐C scores over time.
Outcome Measures
Patients were followed starting 180 days after initiation of antiviral treatment and up to until February 14, 2019 (mean follow‐up 3.04 years), to assess outcomes. Outcomes included death from any etiology, development of cirrhosis, decompensated cirrhosis (defined as development of variceal bleeding, hepatic encephalopathy, ascites, spontaneous bacterial peritonitis, hepatorenal syndrome and hepatopulmonary syndrome), HCC, and receipt of liver transplantation. These outcomes were defined using appropriate International Classification of Diseases, Ninth Revision (ICD‐9)/ICD, Tenth Revision (ICD‐10) codes (Supporting Table S2) recorded at least twice during follow‐up in inpatient or outpatient medical records, and this method has been used and validated by us and other investigators in multiple studies. Because patients receiving liver transplantation are transplanted at very high Model for End‐Stage Liver Disease (MELD) scores and their survival would have been negligible without transplantation, we also analyzed death or liver transplantation first, as a combined single outcome, and second, as competing risks. As the hazard ratios (HRs) were similar between the two models, we reported death or liver transplantation as a combined outcome.
Other Patient Characteristics
We defined SVR as a nondetectable serum HCV RNA level at least 12 weeks after HCV treatment completion. Age, sex, race/ethnicity, body mass index (BMI), HCV regimen, receipt of prior antiviral treatment, HCV genotype, and comorbidities including diabetes, hepatitis B virus (HBV) co‐infection, human immunodeficiency virus (HIV) co‐infection, nonalcohol substance use disorder, depression, anxiety, and posttraumatic stress disorder were also extracted as covariates. Comorbidities were included if documented at least twice before initiating antiviral treatment based on ICD‐9/1CD‐10 codes. The Charlson comorbidity index (Supporting Table S1) was calculated using appropriate ICD‐9/10 codes to capture and adjust for the overall burden of comorbidities.16, 17
Statistical Analysis
Multivariable Cox proportional hazards regression was used to assess for associations between severity of pretreatment alcohol use (AUDIT‐C score within 1 year before treatment) and overall mortality or each of the liver‐related outcomes listed previously. Patients were censored at time of death, liver transplantation, or last follow‐up visit at the VA.
We also modeled the pretreatment AUDIT‐C score and all available AUDIT‐C scores after treatment completion as a time‐varying covariate, to capture the effect of any changes in alcohol consumption over time on outcomes. In this analysis, each patient is analyzed under a particular AUDIT‐C category only for the time period that they are in that category. For example, a patient who reported unhealthy alcohol use before treatment but then reported no alcohol consumption at time t after treatment is analyzed under unhealthy drinking from time 0 to time t and under nondrinking after time t.
We adjusted for the following potential confounders that may be associated with both alcohol consumption and risk of adverse liver‐related outcomes: SVR, history of cirrhosis or decompensated cirrhosis or HCC, Charlson comorbidity index, age, sex, race/ethnicity, BMI, HCV genotype, HIV, HBV, diabetes, platelet count, serum bilirubin, serum creatinine, serum albumin, serum aspartate aminotransferase (AST)/√alanine aminotransferase (ALT) ratio, blood international normalized ratio (INR), and blood hemoglobin level. Continuous variables were categorized and modeled as dummy categorical variables. Survival analyses were stratified by the VA facility at which the antiviral treatment was administered.
We performed analyses for the entire population and by clinically meaningful subgroups defined by SVR or cirrhosis status. All patients were included in the analysis of mortality. However, patients who already had a specific outcome before treatment were excluded from the analysis of that outcome (e.g., patients who had cirrhosis at baseline were excluded from the analysis of cirrhosis risk).
Results
Characteristics of Study Population
Among the 29,037 DAA‐treated patients, the mean age was 61.1 years (SD 6.4 years) and 97% were male, 52% non‐Hispanic white, 33% non‐Hispanic black, 5% Hispanic, and 2% other race/ethnicity. The mean BMI was 28.2%, and 30% had diabetes, 28%‐48% had a psychiatric comorbidity, and 39% had nonalcohol substance use disorder. Nearly a quarter of veterans had previously received HCV antiviral therapy, and 30.5% had cirrhosis prior to DAA treatment. At baseline, 68% of veterans reported nondrinking, 23% reported low‐level drinking, and 9% reported unhealthy drinking before DAA treatment. Patients with unhealthy drinking tended to be younger (59.5 years vs. 61 years vs. 61.3 years; P < 0.001), non‐Hispanic white (56.9% vs. 50.2% vs. 52.3%; P < 0.001), and have a lower BMI (27.2 vs. 28.1 vs. 28.4; P < 0.001), lower rate of diabetes (18.9% vs. 27.7% vs. 32.3%; P < 0.001), higher rate of other substance use (44.4% vs. 34.4% vs. 39.3%; P < 0.001), and a lower proportion of MELD score of 9 or higher (24% vs. 25.8% vs. 31.2%; P < 0.001) and Charlson comorbidity index score of 2 or higher (25.6% vs. 28.1% vs. 32.2%; P < 0.001) than patients with low‐level drinking or nondrinking. Baseline rates of cirrhosis and HCC were highest among nondrinking veterans followed by unhealthy drinking then low‐level drinking (P < 0.001). There was no difference in SVR rates by alcohol use. Other characteristics by overall cohort and baseline severity of alcohol use are summarized in Table 1.
Table 1.
Baseline Characteristics of HCV‐Infected Veterans According to Alcohol Use Before DAA‐Only Treatment
| Characteristics at Antiviral Treatment Initiation | Total | Baseline Severity of Alcohol Use† | P Value* | ||
|---|---|---|---|---|---|
| Nondrinking | Low‐Level Drinking | Unhealthy Drinking | |||
| n = 29,037 | n = 19,447 | n = 6,804 | n = 2,786 | ||
| Age (years; mean ± SD) | 61.1 ± 6.4 | 61.3 ± 6.3 | 61.0 ± 6.5 | 59.5 ± 6.8 | <0.001 |
| Male (%) | 96.7 | 96.7 | 97 | 96.2 | 0.08 |
| Race/ethnicity (%) | <0.001 | ||||
| White, non‐Hispanic | 52.3 | 52.3 | 50.3 | 56.9 | |
| Black, non‐Hispanic | 33.2 | 32.9 | 35.6 | 28.9 | |
| Hispanic | 5.3 | 5.3 | 5.4 | 4.7 | |
| Other | 1.7 | 1.6 | 1.7 | 2 | |
| Declined/missing | 7.6 | 7.8 | 6.9 | 7.5 | |
| BMI (kg/m2; mean ± SD) | 28.2 ± 5.4 | 28.4 ± 5.5 | 28.1 ± 5.4 | 27.2 ± 5.0 | <0.001 |
| Diabetes (%) | 30 | 32.3 | 27.7 | 18.9 | <0.001 |
| Depression (%) | 47.7 | 49.6 | 42.4 | 47.7 | <0.001 |
| Anxiety (%) | 34.5 | 36.2 | 29.7 | 33.8 | <0.001 |
| PTSD (%) | 27.7 | 29.5 | 23.3 | 26.2 | <0.001 |
| Nonalcohol substance use disorder (%) | 38.6 | 39.3 | 34.4 | 44.4 | <0.001 |
| HBV co‐infection (%) | 1 | 1 | 1 | 1.2 | 0.74 |
| HIV co‐infection (%) | 4.2 | 4.2 | 4.5 | 3.4 | 0.04 |
| Charlson comorbidity index (%) | <0.001 | ||||
| 0 | 18.1 | 18.7 | 17.5 | 15.5 | |
| 1 | 32.5 | 30.6 | 35.4 | 39 | |
| 2 | 18.8 | 18.5 | 18.9 | 19.8 | |
| >2 | 30.6 | 32.2 | 28.1 | 25.6 | |
| Cirrhosis (%) | 30.5 | 33.8 | 23.4 | 25.2 | <0.001 |
| Decompensated cirrhosis (%) | 0.1 | 0.1 | 0 | 0.1 | 0.13 |
| HCC (%) | 2.4 | 2.8 | 1.5 | 1.9 | <0.001 |
| HCV genotype (%) | 0.03 | ||||
| 1 | 83.9 | 83.9 | 84.6 | 82 | |
| 2 | 9.1 | 9.1 | 8.8 | 10.2 | |
| 3 | 5 | 4.9 | 4.6 | 6 | |
| 4 | 0.8 | 0.8 | 0.8 | 0.5 | |
| Missing | 1.3 | 1.3 | 1.2 | 1.3 | |
| Before antiviral treatment (%) | 23.2 | 26 | 19 | 14.1 | <0.001 |
| MELD score ≥ 9 (%) | 29.3 | 31.2 | 25.8 | 24 | <0.001 |
| SVR | 89.6 | 89.5 | 90.2 | 88.9 | 0.10 |
P value considered statistically significant if less than 0.05.
Baseline severity of alcohol use defined by AUDIT‐C score recorded within 1 year before antiviral treatment: nondrinking: AUDIT‐C score 0; low‐level drinking: AUDIT‐C score 1‐3 in men, 1‐2 in women; and unhealthy drinking: AUDIT‐C score 4‐12 in men, 3‐12 in women.
Abbreviation: PTSD, post‐traumatic stress disorder.
Change in Alcohol Use After DAA Treatment
Veterans decreased unhealthy alcohol use after DAA treatment (as measured within 1 year after treatment) regardless of SVR and cirrhosis status. In the overall cohort, there was a 1.4% decline (9.1% to 7.7%) in unhealthy drinking after treatment. Among those with and without SVR, there was a 1.4% and 2.4% decline in unhealthy drinking, respectively. In patients with and without cirrhosis, there was a 1.5% decline in unhealthy drinking. Patterns of alcohol use before and after treatment are summarized in Supporting Table S3. Interestingly, we observed that the shift in alcohol use was more likely to go from unhealthy use to nondrinking in patients who did not achieve SVR and in those with cirrhosis. In patients who did achieve SVR or did not have cirrhosis, we observed the shift in alcohol use go from unhealthy use to both low‐level and nondrinking (Fig. 1).
Figure 1.
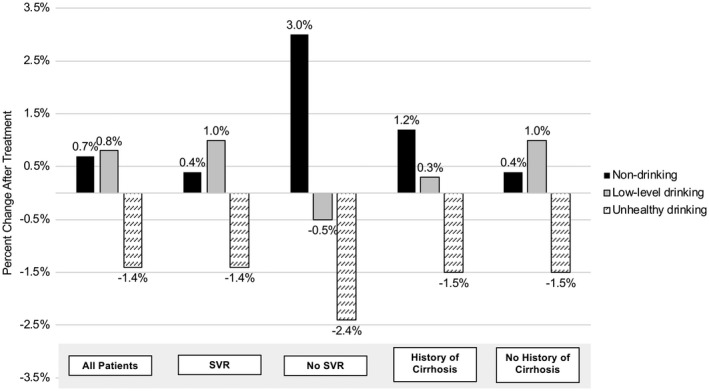
Change in alcohol use before and after receipt of HCV treatment. (Alcohol use defined by AUDIT‐C score recorded within 1 year before treatment and 3‐12 months after treatment completion.)
Associations Between Alcohol Use and Mortality or Adverse Liver‐Related Outcomes
During a mean follow‐up of 3.04 years, there were 2,524 deaths, 1,411 patients who developed cirrhosis, 1,217 patients who developed decompensated cirrhosis, 937 patients who developed HCC, and 100 patients who underwent liver transplantation (Table 2). Figure 2 shows survival curves by severity of alcohol use for mortality and adverse liver‐related outcomes. Patients with baseline unhealthy drinking had a significantly lower probability of survival at 4 years after treatment initiation than patients with nondrinking (P < 0.001). However, there was no statistically significant difference in the presence of cirrhosis, decompensated cirrhosis, and HCC on survival analysis. On multivariable Cox proportional hazards regression, compared with patients with baseline nondrinking, those with unhealthy use had a higher risk of mortality (adjusted HR 1.53, 95% confidence interval [CI]: 1.34‐1.75) and decompensated cirrhosis (HR 1.30, 95% CI: 1.06‐1.59), and a lower likelihood of liver transplantation (HR 0.24, 95% CI: 0.06‐0.92). Unhealthy drinking was also associated with a higher risk of the combined outcome of liver transplantation or death (HR 1.44, 95% CI: 1.26‐1.65). When alcohol use was modeled as a time‐varying covariate, we observed similar associations (Table 3). Compared with patients reporting nondrinking, those reporting unhealthy drinking had a higher risk of mortality (HR 1.43, 95% CI: 1.21‐1.66) and decompensated cirrhosis (HR 1.42, 95% CI: 1.08‐1.87). There was no difference in the risk of cirrhosis or HCC between unhealthy drinking and nondrinking based on baseline alcohol use or time‐varying covariate modelling.
Table 2.
Association of Pretreatment Alcohol Use With Mortality and Liver‐Related Outcomes in All Patients
| Severity of Alcohol Use* | Number of Patients (%) | Patient‐Years (P‐Y) | Number With Outcome (%) | Incidence (per 100 P‐Y) | HR | Adjusted HR† |
|---|---|---|---|---|---|---|
| Mortality | ||||||
| Nondrinking | 19,448 (67.0) | 62,035 | 1,727 (8.9) | 2.97 | 1 | 1 |
| Low‐level drinking | 6,808 (23.4) | 21,084 | 489 (7.2) | 2.39 | 0.81 (0.73‐0.90) | 0.96 (0.86‐1.07) |
| Unhealthy drinking | 2,781 (9.6) | 8,309 | 308 (11.1) | 3.83 | 1.32 (1.17‐1.48) | 1.53 (1.34‐1.75) |
| Cirrhosis | ||||||
| Nondrinking | 12,080 (63.6) | 35,613 | 934 (7.7) | 2.7 | 1 | 1 |
| Low‐level drinking | 4,950 (26.1) | 14,498 | 328 (6.6) | 2.32 | 0.85 (0.75‐0.97) | 0.91 (0.79‐1.04) |
| Unhealthy drinking | 1,958 (10.3) | 5,583 | 149 (7.6) | 2.74 | 0.99 (0.84‐1.18) | 1.04 (0.87‐1.24) |
| Decompensated cirrhosis ‡ | ||||||
| Nondrinking | 19,206 (66.9) | 59,573 | 898 (4.7) | 1.66 | 1 | 1 |
| Low‐level drinking | 6,760 (23.5) | 20,604 | 205 (3.0) | 1.03 | 0.62 (0.53‐0.72) | 0.94 (0.80‐1.11) |
| Unhealthy drinking | 2,743 (9.6) | 8,025 | 114 (4.2) | 1.48 | 0.89 (0.73‐1.08) | 1.30 (1.06‐1.59) |
| HCC | ||||||
| Nondrinking | 18,898 (66.7) | 59,374 | 671 (3.6) | 1.24 | 1 | 1 |
| Low‐level drinking | 6,702 (23.7) | 20,508 | 175 (2.6) | 0.9 | 0.73 (0.62‐0.86) | 0.99 (0.83‐1.19) |
| Unhealthy drinking | 2,726 (9.6) | 8,026 | 91 (3.3) | 1.2 | 0.97 (0.78‐1.21) | 1.25 (0.98‐1.58) |
| Liver transplantation | ||||||
| Nondrinking | 19,420 (67.0) | 61,678 | 89 (0.5) | 0.17 | 1 | 1 |
| Low‐level drinking | 6,804 (23.5) | 21,031 | 9 (0.1) | 0.04 | 0.25 (0.13‐0.50) | 0.48 (0.23‐0.98) |
| Unhealthy drinking | 2,780 (9.6) | 8,289 | 2 (0.1) | 0.02 | 0.14 (0.03‐0.56) | 0.24 (0.06‐0.92) |
| Liver transplantation or death | ||||||
| Nondrinking | 19,420 (67.0) | 61,693 | 1,816 (9.4) | 3.15 | 1 | 1 |
| Low‐level drinking | 6,804 (23.5) | 21,046 | 498 (7.3) | 2.43 | 0.78 (0.71‐0.86) | 0.93 (0.83‐1.03) |
| Unhealthy drinking | 2,780 (9.6) | 8,305 | 310 (11.2) | 3.85 | 1.24 (1.10‐1.40) | 1.44 (1.26‐1.65) |
Baseline severity of alcohol use defined by AUDIT‐C score recorded within 1 year before antiviral treatment: nondrinking: AUDIT‐C score 0; low‐level drinking AUDIT‐C score 1‐3 in men, 1‐2 in women; and unhealthy drinking: AUDIT‐C score 4‐12 in men, 3‐12 in women.
Adjusted for SVR, history of cirrhosis or decompensated cirrhosis or HCC, age, sex, race/ethnicity, BMI, HCV genotype, HIV co‐infection, HBV co‐infection, Charlson comorbidity index, type 2 diabetes mellitus, platelet count, serum bilirubin, serum creatinine, serum albumin, serum AST/√ALT ratio, blood INR, and blood hemoglobin levels.
Decompensated cirrhosis defined by ascites, spontaneous bacterial peritonitis, hepatic encephalopathy, bleeding varices, hepatorenal syndrome, and hepatopulmonary syndrome.
Figure 2.
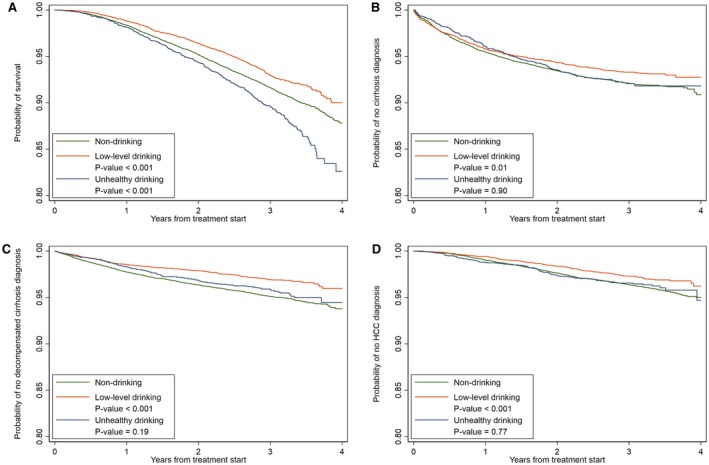
Survival curves for mortality (A), cirrhosis (B), decompensated cirrhosis (C), and HCC (D) by severity of alcohol use. * P value considered statistically significant if less than 0.05.
Table 3.
Association of Pretreatment and Posttreatment Alcohol Use Modeled as a Time‐Varying Covariate With Mortality and Liver‐Related Outcomes
| Severity of Alcohol Use‡ | Mortality* | Cirrhosis | Decompensated Cirrhosis† | HCC * | Liver Transplantation or Death * | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | Adjusted HR§ | HR | Adjusted HR§ | HR | Adjusted HR§ | HR | Adjusted HR§ | HR | Adjusted HR§ | |
| Nondrinking | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Low‐level drinking | 0.67 (0.60‐0.75) | 0.83 (0.73‐0.93) | 0.88 (0.73‐1.06) | 0.95 (0.78‐1.16) | 0.59 (0.49‐0.72) | 0.95 (0.77‐1.17) | 0.76 (0.63‐0.91) | 1.06 (0.87‐1.28) | 0.65 (0.58‐0.73) | 0.81 (0.72‐0.91) |
| Unhealthy drinking | 1.15 (1.00‐1.31) | 1.43 (1.24‐1.66) | 1.09 (0.85‐1.41) | 1.06 (0.80‐1.41) | 0.90 (0.69‐1.16) | 1.42 (1.08‐1.87) | 0.74 (0.56‐0.99) | 0.96 (0.70‐1.32) | 1.10 (0.96‐1.26) | 1.37 (1.18‐1.59) |
Adjusted for characteristics as previously, with death, HCC, and liver transplant as competing risks for each other.
Decompensated cirrhosis is defined by ascites, spontaneous bacterial peritonitis, hepatic encephalopathy, bleeding varices, hepatorenal syndrome, and hepatopulmonary syndrome.
Severity of alcohol use defined by AUDIT‐C score: nondrinking: mean AUDIT‐C score 0 1; low‐level drinking: mean AUDIT‐C score 1‐3 in men, 1‐2 in women; and unhealthy drinking: mean AUDIT‐C score > 3 in men, > 2 in women.
Adjusted for SVR, history of cirrhosis or decompensated cirrhosis or HCC, age, sex, race/ethnicity, BMI, HCV genotype, HIV co‐infection, HBV co‐infection, Charlson comorbidity index, type 2 diabetes mellitus, platelet count, serum bilirubin, serum creatinine, serum albumin, serum AST/√ALT ratio, blood INR, and blood hemoglobin levels.
Compared with baseline nondrinking, low‐level drinking was not associated with an increased risk of any adverse outcomes, but was associated with a lower likelihood of liver transplantation (HR 0.48, 95% CI: 0.23‐0.98). On survival analysis, low‐level drinking was associated with a lower probability of mortality, cirrhosis, decompensated cirrhosis, and HCC than nondrinking at 4 years after treatment initiation.
Associations Between Alcohol Use and Outcomes Among Patients by SVR Status
Among patients who achieved SVR, baseline unhealthy alcohol use was associated with a higher risk of mortality (HR 1.45, 95% CI: 1.25‐1.68) compared with nondrinking (Fig. 3, Supporting Table S4), but no differences in risk of cirrhosis, decompensated cirrhosis, or HCC were observed. Relative to nondrinking, low‐level drinking was not associated with an increased risk of any adverse outcomes.
Figure 3.
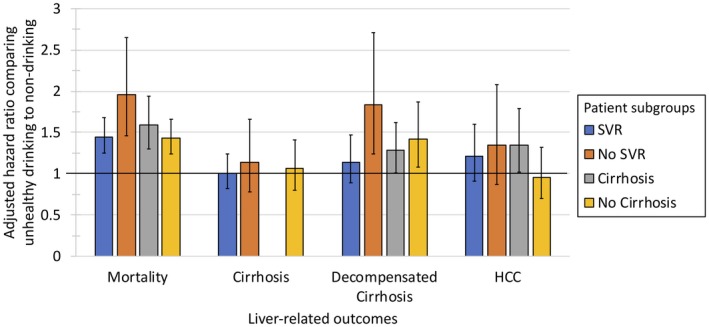
Association of alcohol use (unhealthy vs. nondrinking) with mortality and liver‐related outcomes by SVR and cirrhosis status.
Among patients who did not achieve SVR, baseline unhealthy alcohol use was associated with a higher risk of mortality (HR 1.96, 95% CI: 1.46‐2.65) and decompensated cirrhosis (HR 1.84, 95% CI: 1.24‐2.71) relative to baseline nondrinking, but no differences were observed in the risk of HCC or cirrhosis (Fig. 3, Supporting Table S4). Relative to nondrinking, low‐level drinking was not associated with an increased risk of adverse outcomes.
Associations Between Alcohol Use and Outcomes Among Patients With Cirrhosis
Among patients with cirrhosis, baseline unhealthy alcohol use was associated with a higher risk of mortality (HR 1.59, 95% CI: 1.30‐1.94), HCC (HR 1.35, 95% CI: 1.02‐1.79), and decompensated cirrhosis (HR 1.28, 95% CI: 1.01‐1.62) relative to nondrinking (Fig. 3, Supporting Table S4). Low‐level drinking was not associated with an increased risk of adverse outcomes relative to nondrinking.
Discussion
HCV‐related mortality in the United States has recently decreased partly due to increased access to successful DAA regimens.18 DAA treatment has been associated with a reduction in all‐cause mortality and HCC, and DAAs can be offered to patients even when they use alcohol.19 However, the burden of alcohol use among patients with HCV remains significant, and the long‐term impact of alcohol use on the risk of death and other adverse outcomes after HCV treatment has not been previously studied. In this large cohort of 29,037 U.S. veterans who underwent DAA treatment and were followed for a mean of 3.04 years after treatment, we extend our knowledge of the associations between alcohol use and long‐term outcomes in several ways. First, we showed that unhealthy alcohol use was common before antiviral treatment (9.1%) and decreased only slightly to 7.7% after antiviral treatment. Second, we found that unhealthy alcohol use was associated with an increased risk of mortality and decompensated cirrhosis after DAA therapy, relative to nondrinking. As expected, we found that nondrinking Veterans had slightly higher rates of baseline cirrhosis compared with low‐level‐drinking and unhealthy‐drinking veterans (as they may have stopped drinking alcohol due to their cirrhosis diagnosis), but we found that even after adjusting for potential confounders including cirrhosis status, unhealthy alcohol use was associated with adverse outcomes after therapy. Furthermore, the magnitude of these associations appeared to be greater in patients who did not achieve SVR and in those with underlying cirrhosis. These findings demonstrate increased long‐term risk for patients with unhealthy alcohol use even after HCV treatment with DAA therapy and highlight the need for improved strategies to achieve alcohol cessation before and during antiviral treatment.
We observed that the risk of adverse outcomes was greater among nonresponders to DAA therapies and those already with advanced liver disease. This is particularly concerning, as an unprecedented number of patients with cirrhosis are now receiving HCV treatment in the DAA era. In a recent multicenter study, among patients with HCV diagnosis, the incidence of decompensated cirrhosis was higher among patients with alcohol use disorder.20 Two additional smaller studies also found that alcohol in the presence of HCV and HBV cirrhosis, respectively, increases the risk of HCC.21, 22 We therefore add to the growing international literature that alcohol use may have more severe effects in subgroups of patients, including those who do not respond to treatment or have more advanced liver disease prior to therapy.
We found two additional interesting findings. First, both low‐level and unhealthy alcohol use were associated with a lower likelihood of liver transplantation, relative to those reporting nondrinking. This finding may reflect U.S. transplant programs’ practice of excluding patients from transplant lists if they are actively drinking. In a recent survey of 49 United Network for Organ Sharing–approved transplant programs, 43% of programs required a period of abstinence before transplantation.23 Second, we found a small reduction in unhealthy drinking after DAA treatment in the overall cohort and all subgroups by SVR and cirrhosis status. In the pegylated interferon era, the data have been mixed regarding patterns of alcohol use after therapy.24, 25 In our study, there was a 1.5%‐2.4% absolute reduction in unhealthy alcohol use across subgroups, with the greatest reduction among patients who did not achieve SVR. Although we were unable to assess for predictors or the statistical significance of the changes in alcohol use, we observed that receipt of DAA therapy may influence posttreatment alcohol use.
Our findings have key clinical implications. In the VA health care system, alcohol abstinence is appropriately not required before initiation of DAA regimens, because alcohol use does not substantially affect SVR rates. However, it is remarkable that such a high proportion of HCV‐infected patients reported low‐level drinking and even unhealthy drinking, when all professional guidelines recommend complete abstinence. We therefore believe that the time period before and during antiviral therapy should be considered an “actionable moment,” when providers can engage patients to achieve long‐term alcohol abstinence to minimize the increased mortality and morbidity associated with alcohol use as described in our study. In patients who screen positive for unhealthy alcohol use, the U.S. Preventive Services Task Force already recommends brief intervention for treatment.26 Multiple evidence‐based interventions including psychosocial counseling and pharmacotherapy are also available to address unhealthy drinking.27 However, interventions to address alcohol use have rarely been delivered to patients with HCV28 despite findings that concurrent HCV and addiction treatment can be transformative, and that alcohol use is associated with lower quality of life and functional status.29 HCV treatment—typically delivered over multiple visits—may therefore provide timely opportunities to deliver and better integrate evidence‐based alcohol‐related interventions in HCV treatment clinics. Furthermore, the risks associated with alcohol use that we identified in the present study could be incorporated into feedback provided to patients with concurrent HCV and unhealthy alcohol use during treatment.
Our study has several limitations. First, our findings may not be generalizable to women as most of our cohort was male. Second, our findings are also not generalizable to patients with missing AUDIT‐C and SVR data, as these patients were excluded from our analysis. Although we did conduct a comparison of patients with and without missing AUDIT‐C scores and found that both groups of patients were similar (Supporting Table S5), we recognize that excluded patients may have had other differing factors that could have contributed to their subsequent loss to follow‐up. Third, despite the use of a validated screening tool, AUDIT‐C screening done at a given time point is still self‐reported and may not accurately reflect levels of alcohol consumption in an individual due to recall bias or social desirability bias.30 However, as AUDIT‐C scores were not linked to HCV or treatment in our cohort, our patients were more likely to be truthful in their report. We were also able to improve our assessment of alcohol use by obtaining AUDIT‐C scores over time (1 year before and at least 3 months after treatment completion) and incorporating time‐varying covariate modeling in our analysis. Fourth, AUDIT‐C screening requires patient engagement. Thus, patients who declined or were unable to complete the screening may not be adequately represented by our findings. However, in our study, we were able to account for most U.S. veterans who received DAA therapy, as remarkably only 13.6% (n = 5,162) were excluded from the study due to missing AUDIT‐C data. In addition, AUDIT‐C scores can also be influenced by alcohol cessation or counseling programs, which could not be adjusted for in our study. Finally, our findings are limited to an approximately 3‐year follow‐up period after HCV treatment completion. Additional studies with a longer follow‐up period as we get further away from the introduction of DAAs will help evaluate whether our associations with unhealthy alcohol use persist further out from HCV treatment completion.
In summary, this large retrospective study of veterans who received DAA therapy showed that unhealthy alcohol use is associated with a higher risk of mortality and decompensated cirrhosis, with the mortality risk seemingly greatest among patients who did not achieve SVR after DAA therapy. The increased risk of decompensated cirrhosis and HCC associated with unhealthy alcohol use was also greater among patients who did not achieve SVR and who had cirrhosis. After DAA therapy, unhealthy alcohol use appeared to decline, albeit by a small percentage. We recommend that clinicians providing HCV treatment consider the time period before and during antiviral therapy as an opportunity to engage patients in long‐term abstinence from alcohol use, to minimize posttreatment mortality and morbidity.
Supporting information
Acknowledgment
Other than the authors listed, there are no additional contributors to this manuscript.
Supported by the NIH/NCI grant number R01CA196692 and VA CSR&D grant number I01CX001156 to Dr. George N. Ioannou, and VA HSRD grant number IIR 17‐120 to Dr. Emily C. Williams.
Potential conflict of interest: Nothing to report.
References
Author names in bold designate shared co‐first authorship.
- 1. Serfaty L, Poujol‐Robert A, Carbonell N, Chazouilleres O, Poupon RE, Poupon R. Effect of the interaction between steatosis and alcohol intake on liver fibrosis progression in chronic hepatitis C. Am J Gastroenterol 2002;97:1807‐18 12. [DOI] [PubMed] [Google Scholar]
- 2. Hutchinson SJ, Bird SM, Goldberg DJ. Influence of alcohol on the progression of hepatitis C virus infection: a meta‐analysis. Clin Gastroenterol Hepatol 2005;3:1150‐1159. [DOI] [PubMed] [Google Scholar]
- 3. Younossi ZM, Zheng L, Stepanova M, Venkatesan C, Mir HM. Moderate, excessive or heavy alcohol consumption: each is significantly associated with increased mortality in patients with chronic hepatitis C. Aliment Pharmacol Ther 2013;37:703‐709. [DOI] [PubMed] [Google Scholar]
- 4. Fuster D, Samet JH. Alcohol use in patients with chronic liver disease. N Engl J Med 2018;379:1251‐1261. [DOI] [PubMed] [Google Scholar]
- 5. AASLD‐IDSA . Monitoring patients who are starting HCV treatment, are on treatment, or have completed therapy, December 4, 2017. https://www.hcvguidelines.org/evaluate/monitoring. Accessed January 3, 2019.
- 6. Falade‐Nwulia O, Suarez‐Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct‐acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med 2017;166:637‐648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Okazaki T, Yoshihara H, Suzuki K, Yamada Y, Tsujimura T, Kawano K, et al. Efficacy of interferon therapy in patients with chronic hepatitis C: comparison between non‐drinkers and drinkers. Scand J Gastroenterol 1994;29:1039‐1043. [DOI] [PubMed] [Google Scholar]
- 8. Tsui JI, Williams EC, Green PK, Berry K, Su F, Ioannou GN. Alcohol use and hepatitis C virus treatment outcomes among patients receiving direct antiviral agents. Drug Alcohol Depend 2016;169:101‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ioannou GN, Green PK, Berry K. HCV eradication induced by direct‐acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol 2017;68:25‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Backus LI, Belperio PS, Shahoumian TA, Mole LA. Direct‐acting antiviral sustained virologic response: impact on mortality in patients without advanced liver disease. Hepatology 2018;68:827‐838. [DOI] [PubMed] [Google Scholar]
- 11. Ioannou GN, Feld JJ. What are the benefits of a sustained virologic response to direct‐acting antiviral therapy for HCV infection? Gastroenterology 2019;156:446‐460. [DOI] [PubMed] [Google Scholar]
- 12. Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT‐C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med 1998;158:1789‐1795. [DOI] [PubMed] [Google Scholar]
- 13. Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT‐C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res 2007;31:1208‐1217. [DOI] [PubMed] [Google Scholar]
- 14. Bradley KA, Williams EC, Achtmeyer CE, Volpp B, Collins BJ, Kivlahan DR. Implementation of evidence‐based alcohol screening in the Veterans Health Administration. Am J Manag Care 2006;12:597‐606. [PubMed] [Google Scholar]
- 15. Williams EC, Rubinsky AD, Lapham GT, Chavez LJ, Rittmueller SE, Hawkins EJ, et al. Prevalence of clinically recognized alcohol and other substance use disorders among VA outpatients with unhealthy alcohol use identified by routine alcohol screening. Drug Alcohol Depend 2014;135:95‐103. [DOI] [PubMed] [Google Scholar]
- 16. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373‐383. [DOI] [PubMed] [Google Scholar]
- 17. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care 2005;43:1130‐1139. [DOI] [PubMed] [Google Scholar]
- 18. Kim D, Li AA, Gadiparthi C, Khan MA, Cholankeril G, Glenn JS, et al. Changing trends in etiology‐based annual mortality from chronic liver disease, from 2007 through 2016. Gastroenterology 2018;155:1154‐1163.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carrat F, Fontaine H, Dorival C, Simony M, Diallo A, Hezode C, et al. Clinical outcomes in patients with chronic hepatitis C after direct‐acting antiviral treatment: a prospective cohort study. Lancet 2019;393:1453‐1464. [DOI] [PubMed] [Google Scholar]
- 20. Alavi M, Janjua NZ, Chong M, Grebely J, Aspinall EJ, Innes H, et al. The contribution of alcohol use disorder to decompensated cirrhosis among people with hepatitis C: an international study. J Hepatol 2018;68:393‐401. [DOI] [PubMed] [Google Scholar]
- 21. Vandenbulcke H, Moreno C, Colle I, Knebel JF, Francque S, Serste T, et al. Alcohol intake increases the risk of HCC in hepatitis C virus‐related compensated cirrhosis: a prospective study. J Hepatol 2016;65:543‐551. [DOI] [PubMed] [Google Scholar]
- 22. Lin CW, Lin CC, Mo LR, Chang CY, Perng DS, Hsu CC, et al. Heavy alcohol consumption increases the incidence of hepatocellular carcinoma in hepatitis B virus‐related cirrhosis. J Hepatol 2013;58:730‐735. [DOI] [PubMed] [Google Scholar]
- 23. Zhu J, Chen PY, Frankel M, Selby RR, Fong TL. Contemporary policies regarding alcohol and marijuana use among liver transplant programs in the United States. Transplantation 2018;102:433‐439. [DOI] [PubMed] [Google Scholar]
- 24. Yeung MW, Young J, Moodie E, Rollet‐Kurhajec KC, Schwartzman K, Greenaway C, et al. Changes in quality of life, healthcare use, and substance use in HIV/hepatitis C coinfected patients after hepatitis C therapy: a prospective cohort study. HIV Clin Trials 2015;16:100‐110. [DOI] [PubMed] [Google Scholar]
- 25. Knight R, Roux P, Vilotitch A, Marcellin F, Rosenthal E, Esterle L, et al. Significant reductions in alcohol use after hepatitis C treatment: results from the ANRS CO13‐HEPAVIH cohort. Addiction 2017;112:1669‐1679. [DOI] [PubMed] [Google Scholar]
- 26. U.S. Preventive Services Task Force . Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: recommendation statement. Am Fam Physician 2004;70:353‐358. [PubMed] [Google Scholar]
- 27. Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta‐analysis. JAMA 2014;311:1889‐900. [DOI] [PubMed] [Google Scholar]
- 28. Owens MD, Ioannou GN, Tsui JL, Edelman EJ, Greene PA, Williams EC. Receipt of alcohol‐related care among patients with HCV and unhealthy alcohol use. Drug Alcohol Depend 2018;188:79‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sims OT, Oh H, Pollio DE, Hong BA, Pollio EW, North CS. Quality of life, functioning, and coping in HCV patients continuing versus ceasing alcohol use. Health Promot Pract 2019; 10.1177/1524839919837968. [DOI] [PubMed] [Google Scholar]
- 30. Latkin CA, Edwards C, Davey‐Rothwell MA, Tobin KE. The relationship between social desirability bias and self‐reports of health, substance use, and social network factors among urban substance users in Baltimore, Maryland. Addict Behav 2017;73:133‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


