Abstract
The hepatitis B virus (HBV) is an important human pathogen. Unvaccinated infants infected through mother‐to‐child transmission (MTCT) are at >95% risk of developing serum hepatitis B surface antigen‐positive chronic hepatitis B (CHB). Despite complete passive‐active HBV immunoprophylaxis, approximately 10% of infants born to mothers who are highly viremic develop CHB, and thus maternal treatment with nucleos(t)ide analogs (tenofovir disoproxil fumarate, lamivudine, or telbivudine) is recommended in the third trimester of pregnancy to reduce MTCT risk. Viral rebound usually occurs after stopping treatment and, in the context of maternal immunologic reconstitution postpartum, can also precipitate host immune‐mediated hepatic (biochemical) flares. In this article, we review the epidemiology of HBV MTCT, discuss management and potential mechanisms of HBV vertical transmission, and highlight recent studies on virologic and immunologic aspects of hepatitis B in pregnancy and postpartum.
This manuscript reviews epidemiological, clinical, virological and immunological aspects of chronic hepatitis B infection in pregnancy.
Abbreviations
- “a” determinant
antigenic determinant
- ALT
alanine aminotransferase
- anti‐HBs
antibody to hepatitis B surface antigen
- BCP
basal core promoter
- cccDNA
covalently closed circular DNA
- CD
clusters of differentiation
- CHB
chronic hepatitis B
- FDA
U.S. Food and Drug Administration
- HBeAg
hepatitis B e antigen
- HBIG
hepatitis B immune globulin
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- IFN
interferon
- IL
interleukin
- LMV
lamivudine
- MTCT
mother‐to‐child transmission
- NA
nucleos(t)ide analog
- NK
natural killer
- OBI
occult hepatitis B infection
- PBMC
peripheral blood mononuclear cell
- PC
precore
- qHBsAg
quantitative hepatitis B surface antigen
- TAF
tenofovir alafenamide fumarate
- TDF
tenofovir disoproxil fumarate
- Th
T helper
Chronic hepatitis B (CHB) is a serious public health problem affecting approximately 250 million people worldwide, including 65 million women of childbearing age, leading to 8,00,000 deaths annually.1 Acute hepatitis B virus (HBV) infection in healthy immunocompetent adults results in a self‐limiting disease, with <2% to 3% progressing to serum hepatitis B surface antigen‐positive (HBsAg)+ CHB.2 However, acutely infected infants are at >95% risk of developing CHB, and hence vertical or mother‐to‐child transmission (MTCT) from mothers who are HBsAg+ is responsible for approximately 50% of the global disease burden. The World Health Organization recommends that all infants receive the birth dose HBV vaccine soon after birth, followed by two doses to complete the primary series. The implementation of universal childhood HBV vaccination with or without hepatitis B immune globulin (HBIG) in more than 200 countries since the 1990s has led to a significant decline in MTCT and the global incidence of CHB.1 In this review, we summarize the current literature on HBV immunopathogenesis in pregnancy and proposed mechanisms of HBV MTCT.
Epidemiology of HBV and MTCT
The global prevalence of CHB (serum HBsAg+) is 3.6%, with the highest prevalence in Africa (8.8%) and the Western Pacific (5.2%). More than 75% of individuals with CHB worldwide are found in the Asia Pacific region, and vertical transmission is more common in Asia than Africa.3 The risk of CHB following exposure to HBV varies from approximately 90% in infants to 30% to 50% in toddlers and young children up to 5 years of age. The rates of MTCT also vary depending on maternal hepatitis B e antigen (HBeAg) status, with a 70% to 90% transmission rate for mothers who are HBeAg+ versus 10% to 40% in HBeAg− infection.4
Summary of Current Clinical Guidelines and Recent Approaches for Management of CHB and Pregnancy
All HBV clinical practice guidelines recommend prenatal HBsAg screening in pregnancy and administering postnatal passive‐active immunoprophylaxis with HBIG along with the three‐dose HBV vaccine series to infants born to mothers who are HBsAg+ (Table 1). HBV immunoprophylaxis failures can occur in approximately 10% of infants born to mothers who are HBsAg+ and HBeAg+ with HBV‐DNA 9 log10 copies/mL (1 IU = ~5 virus copies/mL). Current guidelines recommend initiation of oral antiviral (nucleos(t)ide analog [NA]) therapy in the third trimester in mothers who are highly viremic with HBV‐DNA levels >6 log10 copies/mL to reduce the risk of MTCT.5, 6, 7, 8 According to the U.S. Food and Drug Administration (FDA), the three oral NAs considered safe in pregnancy are lamivudine (LMV; category class C) and class B drugs telbivudine and tenofovir disoproxil fumarate (TDF). However, it is important to note that animal reproductive toxicity studies are not always predictive of human response, thus NAs should be used during pregnancy only if potential benefits outweigh the risks. TDF is preferred due to a better resistance profile and safety data for treatment of HBV during pregnancy.7, 9 Although TDF is detected in breast milk, it has low oral bioavailability, and infants are exposed to minimal oral concentrations (<0.03% of recommended neonatal dose).6 In nonpregnant patients with CHB, long‐term TDF therapy is linked to metabolic bone disease and renal dysfunction, but to date no significant maternal safety concerns have been reported with NA therapy during pregnancy.10 A new formulation of TDF (tenofovir alafenamide fumarate [TAF]) was recently approved for treatment of CHB and human immunodeficiency virus (HIV) infection. TAF is a TDF prodrug with lower serum bioavailability due to rapid cellular uptake into hepatocytes and lymphocytes, with associated lower risk of renal and metabolic bone effects in treatment of either CHB or HIV.11, 12 In women of childbearing age with CHB, viral kinetics over a 12‐week treatment regimen with TAF or TDF showed comparable efficacy of both drugs in lowering serum viral loads from approximately 5 log10 IU/mL to <29 IU/mL.13 TAF may be a future therapeutic option for MTCT prevention, although safety data are limited (pending more data in the Antiretroviral Pregnancy Registry). However, it is noted that TAF will not receive FDA classification because this specific ranking system for drug use in pregnancy ended in 2015.14
Table 1.
Summary of Major Guideline Recommendations for HBV Management in Pregnancy
| AASLD 20186 | EASL 20177 | APASL 20168 | |
|---|---|---|---|
| HBV‐DNA threshold for treatment | >2 × 105 IU/mL (106 copies/mL) or HBsAg >4log IU/mL | >2 × 105 IU/mL (106 copies/mL) | 106‐107 IU/mL (5 × 106 copies/mL) |
| Treatment initiation gestational age | 28‐32 weeks | 28‐32 weeks | 28‐32 weeks |
| Preferred drug | TDF (LMV or TBV alternative) | TDF (LMV or TBV alternative) | TDF (LMV or TBV alternative) |
| Therapy discontinuation | At delivery or up to 12 weeks after delivery; postpartum ALT monitoring suggested every 3 months for 6 months | 12 weeks after delivery | At delivery or 4‐12 weeks after delivery |
| Breastfeeding | Not contraindicated. Risk of low‐level antiviral exposure to infants should be discussed with mothers | Not contraindicated in untreated and TDF‐treated women | Discouraged while mothers are on antiviral therapy |
| Mode of delivery | Cesarean section is not indicated | No comment | No comment |
Abbreviations: AASLD, American Association for the Study of Liver Diseases; APASL, Asian Pacific Association for the Study of the Liver; EASL, European Association for the Study of the Liver; TBV, telbivudine.
In a multicenter randomized clinical trial, Jourdain et al.15 demonstrated the successful prevention of MTCT by administration of the standard dose of HBIG and five instead of only three doses of the HBV vaccine (at 0, 1, 2, 4, and 6 months of age) in infants born to mothers who were HBeAg+. The study did not show a significant difference in immunoprophylaxis failure rates between mothers who received placebo (n = 147) versus TDF (n = 147) in the third trimester. Although this study highlights the effectiveness of a more aggressive immunoprophylaxis strategy in preventing perinatal HBV transmission, given the robust data on safety of TDF in pregnancy, TDF remains the drug of choice to treat CHB and prevent MTCT.15, 16 Other studies have explored the potential of antenatal administration of multiple low and high doses of HBIG to reduce MTCT; however, well‐designed clinical trials are necessary to compare HBIG versus antiviral therapy to draw meaningful conclusions.17, 18
Expert guidelines recommend several viral markers (HBV‐DNA, HBeAg) and clinical tests (i.e., alanine aminotransferase [ALT], liver histology, or noninvasive tests, such as liver stiffness measurement [LSM] by transient elastography [TE] or FibroScan) to determine the need for antiviral treatment and assess liver disease progression.7, 19 The key viral marker of HBV persistence is the resilient intrahepatic HBV covalently closed circular DNA (cccDNA) template. Due to the invasive nature of liver biopsy to assess HBV‐cccDNA levels, there is significant interest in surrogate serum biomarkers in CHB.19 Quantitative HBsAg (qHBsAg) levels may be a useful biomarker because it originates from both HBV‐cccDNA (either secreted as subviral particles or from the intact virion) and integrated HBV.20 We and others found that qHBsAg could be a surrogate marker for HBV‐DNA in women who are HBeAg+ during pregnancy. Moreover, a cost‐effectiveness analysis showed that qHBsAg testing was significantly more cost effective compared to HBV‐DNA.21, 22 In other studies, no correlation was observed between HBV‐DNA and qHBsAg.23, 24 These conflicting reports may be attributable to the data being limited in certain genotypes and the presence of integrated virus.25 An interesting study that assessed correlation between qHBsAg levels and vertical transmission risk showed that a maternal HBsAg level >4.5 log10 IU/mL and a maternal viral load cutoff approximately 6 log10 IU/mL is associated with a higher risk of infant infection, with a sensitivity of 100% and specificity of 71%.26 Given the overall lower risk of transmission in patients who are HBeAg−, qHBsAg testing for predicting high maternal viremia could be performed as a surrogate when HBV‐DNA quantitation is not possible in mothers who are HBeAg+ in resource‐limited regions. Additionally, circulating HBV‐RNA has been proposed as a useful biomarker for monitoring response to NA therapy.27 A strong correlation was observed between serum HBV‐RNA and intrahepatic HBV‐cccDNA levels. Our recent study found that in pregnant CHB carriers, serum HBV‐RNA levels correlate with HBeAg, qHBsAg, and HBV‐DNA and hence could be used as a complementary viral marker in assessing liver disease risk in pregnancy.28 Currently, HBV‐DNA is the most important viral marker for predicting HBV MTCT risk, maternal liver disease progression, and need for TDF therapy in pregnancy. Additional studies on novel HBV biomarkers and relevant host immunologic markers are needed to evaluate their prognostic and diagnostic potential in management of patients with CHB in pregnancy and postpartum.
Factors Involved in Infant HBV Infection
The dynamic course of CHB infection depends on age of infection, route of infection, host immune response, and viral factors, including genotypes and variants, as discussed below.
Age‐Dependent Immune Response to HBV Infection
The “immaturity” and/or “immune tolerance” of the immune system during infancy was thought to be responsible for the weak HBV‐specific immune response in neonates resulting in CHB. However, there is now a paradigm shift and reports suggesting that the immune system of newborns appears to be less proinflammatory, which is probably an adaption to avoid aggravated immune responses in utero.29, 30 Koumbi et al.31 found HBV core‐specific and HBsAg‐specific T‐cell responses in vaccinated uninfected infants born to mothers who were HBsAg+/HBeAg−, suggesting an in utero viral encounter that did not result in overt infection but led to priming of HBV‐specific immunity. In preclinical studies, livers from young HBV transgenic mice produced much lower interleukin (IL)‐21 than adult mice livers, resulting in a weaker clusters of differentiation (CD)8+ T‐cell and B‐cell response. The chemokine (C‐X‐C motif) ligand 13 is involved in germinal center activity and was expressed in an age‐dependent manner in both adult mouse and human Kupffer cells.32, 33 In a mouse model of HBV infection, Chou et al. 34 demonstrated the role of gut microbiota and innate immunity in modulating HBV clearance with 12‐week‐old mice (carrying wild‐type toll‐like receptor 4) with stable microbiome clearing HBV within 6 weeks of hydrodynamic injection. In comparison, 6‐week‐old mice in which the gut microbiota was still developing remained HBsAg+ at 26 weeks after injection. Antibiotic treatment of mice from 6 to 12 weeks of age inhibited HBV clearance. Based on preclinical studies, it appears that switching from an anti‐inflammatory to a proinflammatory milieu from infancy to adulthood, along with changes in the microbiome, impacts progression of CHB. Additional studies are needed in HBV‐exposed newborns and children with CHB to delineate the innate and adaptive immune mechanism(s) early on in CHB.
Potential Modes and Risk Factors for HBV MTCT
HBV vertical transmission is the main cause of CHB, particularly in HBV‐endemic countries that lack effective HBV immunization programs. HBV MTCT can occur prenatal or intrauterine, natal or at the time of birth, and postnatal or postpartum (Fig. 1).35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51 Most HBV infections occur perinatally (at birth or soon after) in unvaccinated infants, but reports suggest that approximately 3% to 8% of infections may occur through the intrauterine route.52 It is believed that intrauterine transmission of HBV is the most significant contributor to MTCT and immunoprophylaxis failure.49 The potential mechanisms involved in MTCT and viral/host factors associated with MTCT risk are summarized below.
Figure 1.
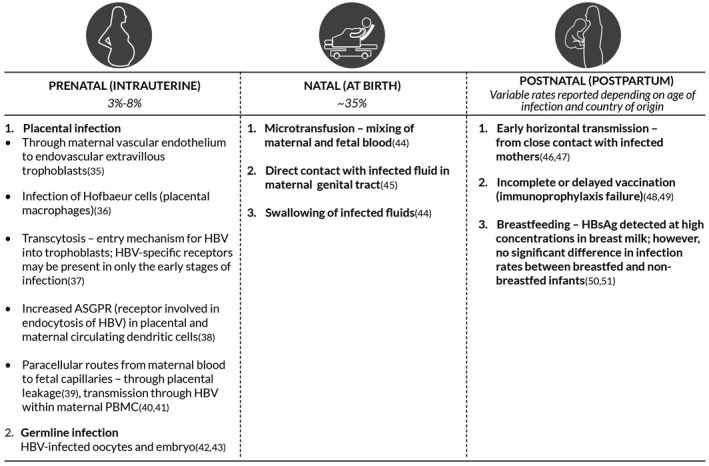
Summary of proposed modes of HBV MTCT and underlying mechanisms. Abbreviation: ASGPR, asialoglycoprotein receptor.
Role of HBV Proteins in MTCT
Maternal HBeAg seropositivity is a significant risk factor for MTCT. It is recognized that the HBeAg has an immunomodulatory role in virus–host interactions and that early in utero exposure may promote chronicity instead of viral clearance.53 In infants infected by MTCT, in utero transfer of maternal HBeAg has been shown to be involved in persistence of HBV due to immunologic tolerance. Tian et al.54 showed in a mouse model that conditioning of hepatic macrophages by maternal HBeAg generates anti‐inflammatory macrophages following subsequent HBeAg exposure, leading to HBV persistence. However, in the absence of HBeAg preconditioning, macrophages acquire a proinflammatory phenotype, leading to HBV clearance by activated CD8+ T cells. In pregnant women with viral loads of >3 log10 copies/mL, functional hepatitis B X protein (HBx) produced in HBV‐infected placenta cells could activate phosphoinositide 3‐kinase in placenta, which signals inhibition of apoptosis in placental cells, allowing for HBV persistence in trophoblasts.55 Overall, these studies shed light on the immunomodulatory role of HBeAg and also how other viral proteins (HBx) can impact the risk of MTCT.
Maternal Viral Load and MTCT
High viral load (>8 log10 copies/mL) is a known significant risk factor for MTCT. In an Australian study of 313 pregnant women who were HBsAg+, it was found that 9% (4/47) of infants of mothers who were HBeAg+ with HBV‐DNA >8 log10 copies/mL were perinatally infected despite appropriate immunoprophylaxis, whereas none of the infants born to mothers with viral loads <8 log10 copies/mL were infected.56 A large study of more than a thousand mother–infant pairs from China stratified maternal HBV‐DNA levels as <6 log10, 7 log10, 7 to 8 log10, and >8 log10 copies/mL and found that the corresponding rates of immunoprophylaxis failure were 0%, 3.2%, 6.7%, and 7.6%, respectively.57 Xu et al.35 found that placental infection occurs progressively through different cellular layers from maternal to the fetal side, with the depth of placental tissue infection in direct proportion to maternal viral load.
Occult Hepatitis B Infection in MTCT
The diagnosis of CHB is confirmed by the presence of HBsAg in serum for >6 months in infected infants. However, a covert form of HBV infection, occult hepatitis B infection (OBI), has been described. OBI is characterized by HBsAg negativity and natural immunity (with HBV anti‐core and/or antibody to HBsAg [anti‐HBs]) antibodies, low‐level viremia within the liver, and serum and extrahepatic reservoirs (peripheral blood mononuclear cells [PBMCs] or lymphoid system).58 OBI is implicated in liver fibrosis progression and the development of hepatocellular carcinoma (HCC).59 Bai et al.40 reported that 16/60 (27%) infants born to mothers with undetectable serum HBV‐DNA but HBV‐DNA positivity in PBMCs became infected with HBV. It should be noted, however, that the neonate blood collection occurred within 24 hours of birth and prior to administration of infant immunoprophylaxis. A prospective longitudinal study found that 42% of infants born to mothers with CHB developed OBI despite HBIG and HBV vaccination.60 Taken together, these data imply that vertical transmission of OBI may occur despite appropriate immunoprophylaxis.
Obstetric Factors in HBV MTCT
HBV vertical transmission in mothers with viral load >7 log10 copies/mL undergoing amniocentesis has been reported.61 In another study, it was found that infants born to mothers who were HBeAg+ and who were delivered vaginally versus by cesarean section had a higher rate of CHB.62 However, because appropriate and timely passive‐active immunoprophylaxis and maternal antiviral therapy will greatly reduce the risk of MTCT, the mode of delivery should only be determined by obstetric indications.
HBV Genome Variability and Impact on HBV Management in Pregnancy
The HBV replicates by reverse transcription of an RNA intermediate. This reverse transcription is catalyzed by a virus‐encoded polymerase that lacks proofreading ability, which leads to sequence heterogeneity.63
HBV Genotypes and Variants
The HBV is classified into eight major HBV genotypes (A‐H) worldwide, with a distinct geographic distribution, which are identified by approximately 8% divergence across the HBV full genome.64, 65 In a Japanese study, genotype C was correlated with increased perinatal transmission rates compared to genotype B. However, the increased MTCT risk may be due to higher viral loads and HBeAg positivity in mothers with HBV genotype C infection.66 Other studies did not find an association between MTCT risk and the HBV genotype.67
HBV Precore/Basal Core Promoter Mutants
HBV variants are selected under host and antiviral pressure, producing immune escape and drug resistance variants. HBV precore (PC) or basal core promoter (BCP) mutations introduce a stop codon that interferes with HBeAg protein translation without affecting viral replication.68 The HBV PC mutant guanine (G) to adenine (A) at nucleotide position 1896 (G1896A) mutation is implicated in acute liver failure, and the dual BCP mutations adenine (A) to thymine (T) at nucleotide position 1762/G to A at nucleotide position 1764 (A1762T/G1764A) were strongly associated with cirrhosis and HCC regardless of the HBV genotype.69, 70, 71 Papadakis et al.72 found that in 32 pregnant women who were HBeAg− and carrying PC mutant G1896A and HBV‐DNA levels of 4 to 5 log10 copies/mL, HBV transplacental transmission did not occur, as evidenced by HBV‐DNA negativity in the analyzed placenta tissues. Additionally, passive‐active immunoprophylaxis failure was not observed in the infants at 1 year of age. A retrospective study from China of 332 mothers with CHB compared HBV mutants in mothers with and without in utero transmission of HBV. Dual BCP mutants were found in mothers who did not transmit HBV to their neonates, suggesting a protective role in MTCT but also likely related to lower maternal viral loads.73 Our recent study suggests that the presence of BCP/PC mutants in PBMCs of TDF‐treated and untreated mothers did not cause an overt HBV infection in infants who received complete immunoprophylaxis and also did not increase the risk of maternal liver disease at 4 years of follow‐up.74 In summary, although combined maternal HBeAg positivity and high viral loads is an established risk factor for MTCT, HBV genotype, PC/BCP mutations, and risk of immunoprophylaxis failure are not well understood and may be an area of future studies.
Host and Viral Factors in HBV Immunoprophylaxis Failure
Hepatitis B Vaccine‐Specific Responses in Infants Born to Mothers Who Were HBsAg+
There are few reports on the long‐term efficacy of postnatal passive‐active HBV vaccination. In a study from Taiwan, approximately 16% of the subjects who had received the HBV vaccine as infants were found to have anti‐HBs at 15 years of age.75 However, the study did not assess memory B‐ or T‐cell response to the vaccine. Transplacental passage of HBsAg and transient HBsAg positivity in infants was associated with blunted immune response in infants born to mothers who were HBsAg+/HBeAg+.76 In infants born to mothers with HBeAg positivity, increasing the vaccine dose from 10 μg to 20 μg improved vaccine immunogenicity.77 Studies have also shown that the HBV vaccine alone is effective in preventing HBV infection or reactivation in infants born to mothers who were HBeAg− compared to passive‐active prophylaxis with HBIG+ vaccine.78 These studies highlight the importance of administering HBV vaccine to all newborns of mothers with HBsAg positivity regardless of their HBeAg status, especially in resource‐limited countries. Most studies assessing long‐term response to postnatal vaccination have focused on anti‐HBs response as a measure of vaccine immunity. There are few studies analyzing T‐cell responses in vaccinated uninfected infants born to mothers with CHB. In infants who received complete immunoprophylaxis, lower amounts of IL‐2 (T helper 1 [Th1] cytokine) secretion in an in vitro stimulation assay were associated with vaccine failure.79 Interestingly, Koumbi et al.31 found HBV‐specific T‐cell responses in uninfected vaccinated infants born to mothers who were HBsAg+/HBeAg−, suggesting in utero encounter to HBV antigens. Further, they found that this exposure did not impair the neonatal B‐cell and T‐cell vaccine response.
A large multicenter study by Chen et al.80 of 1,063 mothers who were HBsAg−/anti‐HBs+ and their infants showed a strong negative correlation between maternal anti‐HBs and infant anti‐HBs titers in vaccinated infants. Further, up to 23% of infants born to mothers with protective anti‐HBs titers >10 IU/L did not respond to the standard vaccination series. These findings may have implications regarding the age of infancy/childhood vaccination. Some studies support these data81, 82; however it was noted by others that, despite negative or low anti‐HBs levels, HBV‐specific T‐cell responses were found in children 10 years after their primary infant vaccination.83 In 2017, the FDA approved a new two‐dose HBV vaccine, HEPLISAV B (Dynavax Technologies), which consists of CpG‐adjuvanted recombinant HBsAg, for use in adults. Although this vaccine was tested in pregnant rats and found to be safe, no studies have been conducted in pregnant women or infants and may be an area for future clinical trials.84
In summary, poor vaccine response, vaccine failure, and breakthrough HBV infection have been reported up to 15 years after adequate infantile immunoprophylaxis. Although the evidence is limited, all successfully vaccinated infants born to mothers with HBsAg positivity (especially HBeAg+) should be followed long term (once every 5 years) until adolescence or early adulthood. It may also be prudent to provide an HBV booster, especially if at risk of ongoing exposure to HBsAg+ individuals (close household contacts).
HBV Immune Escape Mutants
The anti‐HBV surface antibodies induced by the current recombinant vaccine predominantly target the hydrophilic region of the major HBsAg protein, known as the antigenic determinant (“a” determinant) (amino acid residues 124‐147). A glycine to arginine substitution (G145R) is the archetypal immune escape mutant that markedly reduces the affinity of anti‐HBs binding and is able to survive despite high anti‐HBs titers.65, 85 Mutations that affect antigenicity outside of the major “a” determinant region could also affect the binding of circulating antibodies. These “downstream mutations” could affect the conformation of the major HBV S antigenic region and binding of neutralizing antibodies induced by the current vaccine.86, 87 Mutations, such as threonine to lysine at 118 (T118K), lysine to glutamine at 141 (K141E), aspartic acid to glycine at 144 (D144G), cysteine to arginine at 147 (C147R), and cysteine to arginine at 149 (C149R), were reported in association with vaccine escape.88 Overall, these mutants appear to replicate very slowly and have not significantly impacted the global immunization programs.89 Development of anti‐HBs antibodies requires T cell help. Therefore, mutations in CD4+ T‐cell epitopes can result in immune escape mutations affecting humoral immune response.90 Some of these include threonine to alanine at 23 (T23A), phenylalanine to serine at 20 (F20S), and phenylalanine to cysteine at 85 (F85C). Mutations in CD8+ T‐cell epitopes, such as proline to serine at 29 (P29S), serine to leucine at 34 (S34L), and glutamine to arginine at 181 (Q181R), have also been discovered.91, 92
Ayres et al.9 found significantly increased viral quasi‐species diversity in pregnancy despite short duration of LMV but not TDF therapy. Our study in 21 CHB carriers (5 on TDF) identified minor variants at residues associated with vaccine escape, drug resistance, and liver disease in pregnancy and/or postpartum,93 but no overt immunoprophylaxis failures were reported. It is unclear if vaccine failure mutants arise de novo in infants born to mothers who are HBsAg+ as a result of host immune response or if the mutants are transmitted by mothers and replicate in infants. One preliminary study of 4 mother–infant pairs with infant immunoprophylaxis failure showed low (<10%) amino acid substitutions in the “a” determinant of surface antigen in infants versus mothers (P > 0.05), suggesting passage of minor vaccine escape mutants from mother to infant.94 A recent report documented that, despite appropriate immunoprophylaxis in 44 children of mothers who were HBsAg+, 3/44 infants at 1 year of age showed OBI, including 1 child who subsequently developed overt (HBsAg+) CHB at 5 to 7 years of follow‐up.95 It should be noted that the mothers of children with OBI had high (>8 log10 copies/mL) antenatal viral loads. A previous randomized controlled trial in 259 vaccinated infants (n = 128 on HBIG; n = 131 on placebo) born to mothers with HBeAg+ CHB found that 42% of infants in the HBIG group developed OBI at 2 years of age,60 suggesting that follow‐up (every 5‐10 years) HBsAg testing should be considered in infants born to mothers who were highly viremic, as discussed above.
Immune Response and Trained Immunity in Neonates With CHB
An important mechanism of HBV persistence is from exhaustion of HBV‐specific CD8+ T‐cell responses, whereas robust polyclonal CD4+ and CD8+ T‐cell responses are associated with HBV clearance.96 Diminished expression of CD3+ T‐cell receptor zeta chain was associated with functionally defective CD8+ T cells producing less interferon‐γ (IFN‐γ) and reduced expression of the cytotoxicity marker CD107a in newborns who were HBsAg+ versus HBsAg− and healthy newborns.97 Shrivastava et al.98 demonstrated higher prevaccination levels of immature transitional B cells in 12 newborns who were HBsAg+ compared with infants who were HBsAg− born to mothers who were HBsAg+. The frequency of immature transitional B cells declined at 12 months after vaccination in newborns who were HBsAg+, whereas no changes were observed in HBsAg− and uninfected healthy newborns. These studies suggest weak T‐cell and B‐cell responses in infants with CHB. More convincing evidence now points toward a fully functional T‐cell response in infants. Serum cytokine profiling from neonates born to mothers with CHB revealed a cytokine signature compatible with a Th1‐like response (high levels of IL‐12 p40 and low levels of Th2 cytokines IL‐4, IL‐5, IL‐13, and IL‐10) and a decrease in proinflammatory cytokine profile (IL‐1β and IL‐6). Further, cord blood mononuclear cells from neonates who were HBV+ showed a stronger response compared to healthy controls to an unrelated bacterial challenge. Overall, these data suggest that HBV is able to induce “trained immunity” rather than a tolerogenic state in these infants.99 This also supports the findings of Tian et al.54 that maternal HBeAg “trains” Kupffer cells in utero, leading to HBV persistence, highlighting the immunomodulatory role of HBeAg. The concept of trained immunity, or priming of innate response by a prior pathogen or its components, is well established in many other infection and vaccination scenarios (Fig. 2).100, 101, 102
Figure 2.
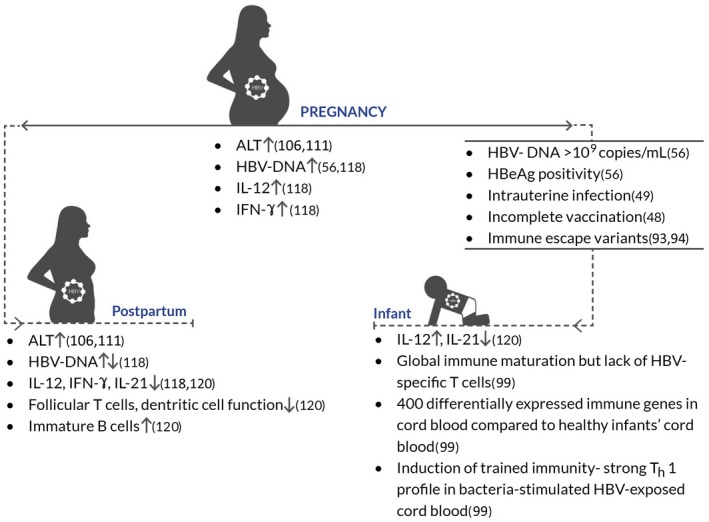
Schematic representation of immunologic changes in the peripartum period in mothers with CHB and their infants.
Virologic and Biochemical Flares in Pregnancy and Risk of Maternal Liver Fibrosis
Globally, especially in developed countries, more women are choosing to have children at an older age (>35 years).103 CHB carriers in the third and fourth decades are more likely to transition to HBeAg−/anti‐HBe+ (with undetectable or low HBV‐DNA) or harbor PC/BCP mutants (with fluctuating levels of moderate to high HBV‐DNA). In the latter scenario, monitoring for liver disease progression is important given the recognized risk of HBeAg− CHB in fibrosis progression and HCC development.
The clearance of HBV from infected cells is due to a complex interplay between different immune effector cell types. CD4+ T cells secrete cytokines that are responsible for development of efficient CD8+ T cells, which kill HBV‐infected hepatocytes through cytolytic and noncytolytic mechanisms. HBV‐specific CD4+ T cells also prime B cells to produce antibodies that neutralize free virus. However, this antiviral response is unsuccessful in patients with CHB.104 Immune‐mediated liver injury in HBV infection is initiated by antigen nonspecific cells, such as natural killer (NK) cells, whereas HBV‐specific CD4+ and CD8+ T cells are functionally exhausted.105
It is unknown if immune changes in pregnancy and postpartum impact the natural history of CHB. Higher rates of HBeAg loss (and HBsAg clearance) and biochemical‐hepatic flares with increased ALT levels have been reported, especially during early postpartum when immune reconstitution occurs.106, 107, 108, 109, 110 Most flares are self‐limited and do not require therapy, but some can be severe, resulting in liver failure.111, 112 The reported rates of ALT flares postpartum are variable owing to different definitions of ALT flare, patient characteristics, and antiviral therapy (Table 2). Studies from our group (N = 138; 60% Asian, 30% African) and other retrospective studies conducted in the United States and Australia (N = ~100; ~80% Asian) have reported hepatic flares during late pregnancy and early postpartum, even in the absence of antiviral treatment.106, 107, 113, 114 ALT flares are reported to be more common in HBeAg+ carriers versus women with HBeAg− CHB. Interestingly, a Taiwanese study determined that titers of HBeAg <1:650 in women who were HBeAg+ were associated with postpartum HBeAg clearance.115 Taken together, biochemical and virologic flares can occur in late pregnancy and postpartum in women with CHB, potentially increasing fibrosis risk, especially at an older maternal age and HBeAg− CHB. Thus, maternal postnatal monitoring for exacerbations of liver disease is necessary.
Table 2.
Recent Studies Evaluating Biochemical Flares in CHB During the Peripartum Period
| Study | Country | No. of Patients | Time Points for Reporting ALT Flares | Definition of Flares | Proportion of Flares (Untreated) | Proportion of Flares (Treated) | Time to Resolution of Flares After Delivery (Definition) | Rate of HBeAg Loss |
|---|---|---|---|---|---|---|---|---|
| Bzowej et al., 2019111 | USA | 158 | Twice in pregnancy <4, 4‐8, and 8‐12 months after delivery | >5 × ULN (<20 U/L) | 5/149 (3.4%) in pregnancy 4/92 (4.3%) postpartum | 1/49 (2%) in pregnancy 2/36 (5.6%) postpartum 5/29 (17.2%) (7‐14 weeks after NA cessation) | Not reported | 1/149 untreated 1/29 after treatment withdrawal |
| Jourdain et al., 201815 | Thailand | 154 (on TDF) 157 (placebo) | Postpartum ~6 months | >10 × ULN | N/A (study cohort includes TDF‐ or placebo‐treated patients) | 9/154 (6%) after TDF cessation 5/157 (3%) after placebo cessation | Not reported | Not reported |
| Kushner et al., 2018114 | USA | 310 | During pregnancy or within 6 months after delivery | >2 × ULN | 42/311 (14%) in pregnancy 22/134 (16%) postpartum* | 4/19 (21%) on NA therapy before pregnancy 3/19 (15%) after delivery | Not reported | Not reported |
| Chang et al., 2017110 | USA | 56 | Twice in pregnancy ~3‐6 months postpartum | >5 × ULN (19 U/L) or >3× baseline, whichever was higher | 7/43 (16%) in pregnancy 0/15 postpartum | 4/13 (31%) in pregnancy 3/9 (33%) postpartum (NA cessation at delivery) 4/18 (22%) who continued therapy postpartum 2/7 (29%) postpartum (NA cessation in the first trimester) | Within 12 months postpartum (<2 × ULN or similar to baseline ALT) | 1/9 (after therapy withdrawal postpartum) |
| Chang et al., 2016106 | USA | 113 | Twice in pregnancy and up to ~6 months postpartum | >5 × ULN (19 U/L) or >3× baseline, whichever was higher | 7/112 (6%) in pregnancy 5/51 (10%) postpartum | N/A (untreated women recruited to the study) | Within 12 months postpartum (<2 × ULN or similar to baseline ALT) | Not reported |
| Samadi Kochaksaraei et al., 2016113 | Canada | 161 | second trimester and ~3 months postpartum | >2 × ULN (19 U/L) | 7/138 (5%) postpartum | 4/23 (17.3%) postpartum | Not reported | Not reported |
| Giles et al., 2015107 | Australia | 126 | Twice (early and late) in pregnancy, 1.5‐3 months and 12 months postpartum | >2 × ULN or 2 × baseline ALT if higher than normal | 2/126 (1.6%) in pregnancy 27/108 (25%) postpartum | 4/7 (57%) postpartum | 9‐12 months | 2/30 |
| Nguyen et al., 2014108 | USA | 101 | Pregnancy, at birth, <3 months postpartum, and >3 months postpartum | 5 × ULN (19 U/L) | 4/14 (29%) postpartum | 22/44 (50%) postpartum, NA withdrawal at delivery 17/43 (40%) postpartum, NA withdrawal 3 months after delivery | 11‐12 months (normal or baseline) | 1/14 (untreated) 5/44 (early NA withdrawal) 1/43 (late NA withdrawal) |
Data for untreated flares not reported but includes 348/388 (90%) untreated cases.
Abbreviations: N/A, not applicable; ULN, upper limit of normal (defined as <19 or 20 U/L).
Immunologic Changes in the Peripartum Period in Mothers With CHB
In pregnancy, immune alterations, such as regulatory T‐cell (Treg) expansion and a distinct regulation of Th1, Th2, and Th17 cytokines, occur to prevent fetus rejection. This tolerance is reversed around parturition.116, 117 Our data show an increase in Th1 cytokines, IFN‐γ and IL‐12, and mild ALT flares in pregnant untreated HBeAg− carriers (n = 26) compared to healthy pregnant controls. A significant decline in these levels was noted postpartum in women with CHB (n = 12). Despite changes in the cytokine milieu in pregnancy versus postpartum, we found no impact on liver fibrosis risk, as determined by LSM using TE (FibroScan) in the peripartum period.117 Additionally, there was no significant change in serum viral load and qHBsAg levels. These results suggest inefficient antiviral responses by the maternal host immune system that prioritizes elimination of virus over fetal rejection. Interestingly, levels of proinflammatory chemokines (monocyte chemoattractant protein 1 and macrophage‐derived chemokine) increased postpartum, which might partially explain the mild ALT flares noted in our cohort.118 Both larger studies as well as ex vivo characterization of immune response using nonpregnant and pregnant PBMC samples are necessary to confirm the proposed mechanism.
A recent study by Li et al.119 evaluated cell proportions from PBMCs of women with HBeAg− (n = 23) versus HBeAg+ (n = 10) CHB alongside 190 healthy controls and studied their association with pregnancy outcomes. Compared to patients who were HBeAg−, a higher proportion of CD19+ B cells but lower frequency of CD3+CD4+ T cells was identified in patients who were HBeAg+. Peripheral NK cell inhibition was noted in HBeAg+ cases, as evidenced by reduced cytotoxicity against target cells, lower expression of activation receptor NK group 2, member D (NKG2D), and decreased production of cytotoxic molecules (granzyme B and perforin). These findings clearly point toward a differential immune response in women of childbearing age who were HBeAg− versus HBeAg+. No differences were seen in pregnancy outcomes (clinical pregnancy or early miscarriage) in women infected with HBV versus controls. Impaired dendritic cell function and decreased levels of follicular T cells (CD4+C‐X‐C chemokine receptor type 5+), plasma B cells (CD19+CD38+) within PBMCs, and low serum IL‐21 were found in mothers in association with MTCT (n = 22), whereas mothers who did not transmit HBV to their infants (n = 28) had a more functional immune cell profile (Fig. 2). Interestingly, newborns showed transcriptomic imprints of their mothers, suggesting that mothers’ immune signatures could be a potential marker for MTCT.120
Acute liver failure has been reported during pregnancy in CHB.112, 121 It is known that cortisol levels peak at term and delivery. A sudden decrease in cortisol levels postpartum is thought to have similar effects as withdrawal of steroid therapy causing HBV reactivation. There is little understanding regarding mechanisms of acute liver failure in pregnancy in the context of hepatitis B. There is some evidence that HBV core‐specific T cells may be involved114, 122 and hence indicate a defective HBV‐specific T‐cell response in the peripartum period. Although there is limited literature on immune response during the peripartum period in CHB, the data show differences in immune cell functions during pregnancy versus postpartum and also between mothers who were HBeAg+ versus HBeAg−. Further studies are necessary in a larger cohort and, if possible, serially collected samples from mothers and infants to clearly understand the dynamics of the host immune response in association with HBV flares and MTCT in these groups.
Discussion
Despite the availability of an effective HBV vaccine, approximately 10% of children born to mothers who are HBsAg+ with high serum HBV‐DNA levels (without antiviral therapy) during pregnancy are reported to develop CHB. Prior reports of vaccine failure may be related to incomplete or nonadherence to the recommended immunoprophylaxis schedule. The risk of OBI in successfully vaccinated infants is unclear.
Pregnancy impacts the host immune system, and it is unknown whether patients with CHB in the postpartum period are at greater (or lower) risk of immune‐mediated flares and liver disease (vs. before and during pregnancy). The risk of liver fibrosis progression in pregnant women may also be affected by advanced maternal age and the obesity epidemic in developed countries.
TDF is the treatment of choice for prevention of MTCT. The impact of short‐term TDF use in multiple gestations is unclear because most studies (especially in China) were conducted in women with single pregnancies. Recently, TDF‐resistant mutants were identified in a treatment‐naive Chinese patient with CHB.123 The potential risk of reselection of resistant variants by repeat TDF exposure is unknown but predicted to be extremely rare.
There are evolving data on enhanced prophylaxis strategies with the current HBV vaccine, recently approved adjuvanted HBV vaccines, novel viral biomarkers, and new anti‐HBV therapies that may have future applications in management of HBV in pregnancy and prevention of HBV MTCT.
Acknowledgment
We thank Rutali Joshi for her help with the graphic illustration.
Potential conflict of interest: Dr. Coffin is on the speakers’ bureau, has served on the advisory board, and has received grants from Gilead and GlaxoSmithKline. Dr. Joshi has nothing to report.
References
Author names in bold designate shared co‐first authorship.
- 1. World Health Organization . Global health sector strategy on viral hepatitis 2016‐2021. https://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en/. Published June 2016. Accessed September 2019. [Google Scholar]
- 2. World Health Organization . Hepatitis B. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b. Published July 18, 2019. Accessed August 2019. [Google Scholar]
- 3. Chang MS, Nguyen MH. Epidemiology of hepatitis B and the role of vaccination. Best Pract Res Clin Gastroenterol 2017;31:239‐247. [DOI] [PubMed] [Google Scholar]
- 4. Tran TT. Hepatitis B in pregnancy. Clin Infect Dis 2016;62(Suppl. 4):S314‐S317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coffin CS, Fung SK, Alvarez F, Cooper CL, Doucette KE, Fournier C, et al. Management of hepatitis B virus infection: 2018 guidelines from the Canadian Association for the Study of Liver Disease and Association of Medical Microbiology and Infectious Disease Canada. Can Liver J 2018;1:156‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560‐1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. European Association for the Study of the Liver . EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370‐398. [DOI] [PubMed] [Google Scholar]
- 8. Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian‐Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016;10:1‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ayres A, Yuen L, Jackson KM, Manoharan S, Glass A, Maley M, et al. Short duration of lamivudine for the prevention of hepatitis B virus transmission in pregnancy: lack of potency and selection of resistance mutations. J Viral Hepat 2014;21:809‐817. [DOI] [PubMed] [Google Scholar]
- 10. Song J, Yang F, Wang S, Tikande S, Deng Y, Tang W, et al. Efficacy and safety of antiviral treatment on blocking the mother‐to‐child transmission of hepatitis B virus: a meta‐analysis. J Viral Hepat 2019;26:397‐406. [DOI] [PubMed] [Google Scholar]
- 11. Agarwal K, Fung SK, Nguyen TT, Cheng W, Sicard E, Ryder SD, et al. Twenty‐eight day safety, antiviral activity, and pharmacokinetics of tenofovir alafenamide for treatment of chronic hepatitis B infection. J Hepatol 2015;62:533‐540. [DOI] [PubMed] [Google Scholar]
- 12. Gupta SK, Post FA, Arribas JR, Eron JJ, Wohl DA, Clarke AE, et al. Renal safety of tenofovir alafenamide vs. tenofovir disoproxil fumarate: a pooled analysis of 26 clinical trials. AIDS 2019;33:1455‐1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pan C, Chang TT, Bae SH, Brunetto M, Coffin C, Lau A, et al. Viral kinetics in women of child bearing potential with chronic hepatitis B virus following treatment with tenofovir alafenamide or tenofovir disoproxil fumarate. J Hepatol 2017;66(Suppl.):S258‐S259. [Google Scholar]
- 14. U .S. Food and Drug Administration . Pregnancy and lactation labeling (drugs) final rule. https://www.fda.gov/drugs/labeling/pregnancy-and-lactation-labeling-drugs-final-rule. Published September 11, 2019. Accessed October 2019. [Google Scholar]
- 15. Jourdain G, Ngo‐Giang‐Huong N, Harrison L, Decker L, Khamduang W, Tierney C, et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N Engl J Med 2018;378:911‐923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pan CQ, Duan Z, Dai E, Zhang S, Han G, Wang Y, et al.; China Study Group for the Mother‐to‐Child Transmission of Hepatitis B . Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med 2016;374:2324‐2334. [DOI] [PubMed] [Google Scholar]
- 17. Yuan J, Lin J, Xu A, Li H, Hu B, Chen J, et al. Antepartum immunoprophylaxis of three doses of hepatitis B immunoglobulin is not effective: a single‐centre randomized study. J Viral Hepat 2006;13:597‐604. [DOI] [PubMed] [Google Scholar]
- 18. Shi Z, Li X, Ma L, Yang Y. Hepatitis B immunoglobulin injection in pregnancy to interrupt hepatitis B virus mother‐to‐child transmission‐a meta‐analysis. Int J Infect Dis 2010;14:e622‐e634. [DOI] [PubMed] [Google Scholar]
- 19. Coffin CS, Zhou K, Terrault NA. New and old biomarkers for diagnosis and management of chronic hepatitis B virus infection. Gastroenterology 2019;156:355‐368.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hu B, Wang R, Fu J, Su M, Du M, Liu Y, et al. Integration of hepatitis B virus S gene impacts on hepatitis B surface antigen levels in patients with antiviral therapy. J Gastroenterol Hepatol 2018;33:1389‐1396. [DOI] [PubMed] [Google Scholar]
- 21. Samadi Kochaksaraei G, Congly SE, Matwiy T, Castillo E, Martin SR, Charlton CL, et al. Cost‐effectiveness of quantitative hepatitis B virus surface antigen testing in pregnancy in predicting vertical transmission risk. Liver Int 2016;36:1604‐1610. [DOI] [PubMed] [Google Scholar]
- 22. Fujiko M, Chalid MT, Turyadi ISI, Maghfira S, et al. Chronic hepatitis B in pregnant women: is hepatitis B surface antigen quantification useful for viral load prediction? Int J Infect Dis 2015;41:83‐89. [DOI] [PubMed] [Google Scholar]
- 23. Ganji A, Esmaeilzadeh A, Ghafarzadegan K, Helalat H, Rafatpanah H, Mokhtarifar A. Correlation between HBsAg quantitative assay results and HBV DNA levels in chronic HBV. Hepat Mon 2011;11:342‐345. [PMC free article] [PubMed] [Google Scholar]
- 24. Wiegand J, Wedemeyer H, Finger A, Heidrich B, Rosenau J, Michel G, et al. A decline in hepatitis B virus surface antigen (hbsag) predicts clearance, but does not correlate with quantitative hbeag or HBV DNA levels. Antivir Ther 2008;13:547‐554. [PubMed] [Google Scholar]
- 25. Cornberg M, Wong VW, Locarnini S, Brunetto M, Janssen HL, Chan HL. The role of quantitative hepatitis B surface antigen revisited. J Hepatol 2017;66:398‐411. [DOI] [PubMed] [Google Scholar]
- 26. Wen WH, Huang CW, Chie WC, Yeung CY, Zhao LL, Lin WT, et al. Quantitative maternal hepatitis B surface antigen predicts maternally transmitted hepatitis B virus infection. Hepatology 2016;64:1451‐1461. [DOI] [PubMed] [Google Scholar]
- 27. Rokuhara A, Matsumoto A, Tanaka E, Umemura T, Yoshizawa K, Kimura T, et al. Hepatitis B virus RNA is measurable in serum and can be a new marker for monitoring lamivudine therapy. J Gastroenterol 2006;41:785‐790. [DOI] [PubMed] [Google Scholar]
- 28. Patel NH, Joshi SS, Lau KCK, Castillo E, Coffin CS. Analysis of serum hepatitis B virus RNA levels in a multiethnic cohort of pregnant chronic hepatitis B carriers. J Clin Virol 2019;111:42‐47. [DOI] [PubMed] [Google Scholar]
- 29. Kollmann TR, Levy O, Montgomery RR, Goriely S. Innate immune function by toll‐like receptors: distinct responses in newborns and the elderly. Immunity 2012;37:771‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bertoletti A, Hong M. Age‐dependent immune events during HBV infection from birth to adulthood: an alternative interpretation. Front Immunol 2014;5:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koumbi L, Bertoletti A, Anastasiadou V, Machaira M, Goh W, Papadopoulos NG, et al. Hepatitis B‐specific T helper cell responses in uninfected infants born to HBsAg+/HBeAg− mothers. Cell Mol Immunol 2010;7:454‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Publicover J, Goodsell A, Nishimura S, Vilarinho S, Wang Z, Avanesyan L, et al. IL‐21 is pivotal in determining age‐dependent effectiveness of immune responses in a mouse model of human hepatitis B. J Clin Invest 2011;121:1154‐1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Publicover J, Gaggar A, Nishimura S, Van Horn CM, Goodsell A, Muench MO, et al. Age‐dependent hepatic lymphoid organization directs successful immunity to hepatitis B. J Clin Invest 2013;123:3728‐3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chou HH, Chien WH, Wu LL, Cheng CH, Chung CH, Horng JH, et al. Age‐related immune clearance of hepatitis B virus infection requires the establishment of gut microbiota. Proc Natl Acad Sci U S A 2015;112:2175‐2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xu DZ, Yan YP, Choi BC, Xu JQ, Men K, Zhang JX, et al. Risk factors and mechanism of transplacental transmission of hepatitis B virus: a case‐control study. J Med Virol 2002;67:20‐26. [DOI] [PubMed] [Google Scholar]
- 36. Liu J, Feng Y, Wang J, Li X, Lei C, Jin D, et al. An “immune barrier” is formed in the placenta by hepatitis B immunoglobulin to protect the fetus from hepatitis B virus infection from the mother. Hum Vaccin Immunother 2015;11:2068‐2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bhat P, Anderson DA. Hepatitis B virus translocates across a trophoblastic barrier. J Virol 2007;81:7200‐7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vyas AK, Ramakrishna U, Sen B, Islam M, Ramakrishna G, Patra S, et al. Placental expression of asialoglycoprotein receptor associated with hepatitis B virus transmission from mother to child. Liver Int 2018;38:2149‐2158. [DOI] [PubMed] [Google Scholar]
- 39. Lin HH, Lee TY, Chen DS, Sung JL, Ohto H, Etoh T, et al. Transplacental leakage of HBeAg‐positive maternal blood as the most likely route in causing intrauterine infection with hepatitis B virus. J Pediatr 1987;111:877‐881. [DOI] [PubMed] [Google Scholar]
- 40. Bai GQ, Li SH, Yue YF, Shi L. The study on role of peripheral blood mononuclear cell in HBV intrauterine infection. Arch Gynecol Obstet 2011;283:317‐321. [DOI] [PubMed] [Google Scholar]
- 41. Shao Q, Zhao X, Yao Li MD. Role of peripheral blood mononuclear cell transportation from mother to baby in HBV intrauterine infection. Arch Gynecol Obstet 2013;288:1257‐1261. [DOI] [PubMed] [Google Scholar]
- 42. Hu XL, Zhou XP, Qian YL, Wu GY, Ye YH, Zhu YM. The presence and expression of the hepatitis B virus in human oocytes and embryos. Hum Reprod 2011;26:1860‐1867. [DOI] [PubMed] [Google Scholar]
- 43. Nie R, Jin L, Zhang H, Xu B, Chen W, Zhu G. Presence of hepatitis B virus in oocytes and embryos: a risk of hepatitis B virus transmission during in vitro fertilization. Fertil Steril 2011;95:1667‐1671. [DOI] [PubMed] [Google Scholar]
- 44. Wong VC, Lee AK, Ip HM. Transmission of hepatitis B antigens from symptom free carrier mothers to the fetus and the infant. Br J Obstet Gynaecol 1980;87:958‐965. [DOI] [PubMed] [Google Scholar]
- 45. Cheung KW, Seto MT, Wong SF. Towards complete eradication of hepatitis B infection from perinatal transmission: review of the mechanisms of in utero infection and the use of antiviral treatment during pregnancy. Eur J Obstet Gynecol Reprod Biol 2013;169:17‐23. [DOI] [PubMed] [Google Scholar]
- 46. Yao GB. Importance of perinatal versus horizontal transmission of hepatitis B virus infection in China. Gut 1996;38(Suppl. 2):S39‐S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Indolfi G, Easterbrook P, Dusheiko G, Siberry G, Chang MH, Thorne C, et al. Hepatitis B virus infection in children and adolescents. Lancet Gastroenterol Hepatol 2019;4:466‐476. [DOI] [PubMed] [Google Scholar]
- 48. Kanji JN, Penner RE, Giles E, Goodison K, Martin SR, Marinier E, et al. Horizontal transmission of hepatitis B virus from mother to child due to immune escape despite immunoprophylaxis. J Pediatr Gastroenterol Nutr 2019;68:e81‐e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yi P, Chen R, Huang Y, Zhou RR, Fan XG. Management of mother‐to‐child transmission of hepatitis B virus: propositions and challenges. J Clin Virol 2016;77:32‐39. [DOI] [PubMed] [Google Scholar]
- 50. Beasley RP, Stevens CE, Shiao IS, Meng HC. Evidence against breast‐feeding as a mechanism for vertical transmission of hepatitis B. Lancet 1975;2:740‐741. [DOI] [PubMed] [Google Scholar]
- 51. de Martino M, Appendino C, Resti M, Rossi ME, Muccioli AT, Vierucci A. Should hepatitis B surface antigen positive mothers breast feed? Arch Dis Child 1985;60:972‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wong F, Pai R, Van Schalkwyk J, Yoshida EM. Hepatitis B in pregnancy: a concise review of neonatal vertical transmission and antiviral prophylaxis. Ann Hepatol 2014;13:187‐195. [PubMed] [Google Scholar]
- 53. Milich DR, Jones JE, Hughes JL, Price J, Raney AK, McLachlan A. Is a function of the secreted hepatitis B e antigen to induce immunologic tolerance in utero? Proc Natl Acad Sci U S A 1990;87:6599‐6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tian Y, Kuo CF, Akbari O, Ou JH. Maternal‐derived hepatitis B virus e antigen alters macrophage function in offspring to drive viral persistence after vertical transmission. Immunity 2016;44:1204‐1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bai G, Wang Y, Zhang L, Tang Y, Fu F. The study on the role of hepatitis B virus X protein and apoptosis in HBV intrauterine infection. Arch Gynecol Obstet 2012;285:943‐949. [DOI] [PubMed] [Google Scholar]
- 56. Wiseman E, Fraser MA, Holden S, Glass A, Kidson BL, Heron LG, et al. Perinatal transmission of hepatitis B virus: an Australian experience. Med J Aust 2009;190:489‐492. [DOI] [PubMed] [Google Scholar]
- 57. Zou H, Chen Y, Duan Z, Zhang H, Pan C. Virologic factors associated with failure to passive‐active immunoprophylaxis in infants born to HBsAg‐positive mothers. J Viral Hepat 2012;19:e18‐e25. [DOI] [PubMed] [Google Scholar]
- 58. Raimondo G, Pollicino T, Romanò L, Zanetti AR. A 2010 update on occult hepatitis B infection. Pathol Biol (Paris) 2010;58:254‐257. [DOI] [PubMed] [Google Scholar]
- 59. Coppola N, Onorato L, Iodice V, Starace M, Minichini C, Farella N, et al. Occult HBV infection in HCC and cirrhotic tissue of HBsAg‐negative patients: a virological and clinical study. Oncotarget 2016;7:62706‐62714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pande C, Sarin SK, Patra S, Kumar A, Mishra S, Srivastava S, et al. Hepatitis B vaccination with or without hepatitis B immunoglobulin at birth to babies born of HBsAg‐positive mothers prevents overt HBV transmission but may not prevent occult HBV infection in babies: a randomized controlled trial. J Viral Hepat 2013;20:801‐810. [DOI] [PubMed] [Google Scholar]
- 61. Yi W, Pan CQ, Hao J, Hu Y, Liu M, Li L, et al. Risk of vertical transmission of hepatitis B after amniocentesis in HBs antigen‐positive mothers. J Hepatol 2014;60:523‐529. [DOI] [PubMed] [Google Scholar]
- 62. Pan CQ, Zou H‐B, Chen Y, Zhang X, Zhang H, Li J, et al. Cesarean section reduces perinatal transmission of hepatitis B virus infection from hepatitis B surface antigen‐positive women to their infants. Clin Gastroenterol Hepatol 2013;11:1349‐1355. [DOI] [PubMed] [Google Scholar]
- 63. Seeger C, Mason WS. Molecular biology of hepatitis B virus infection. Virology 2015;479‐480:672‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tong S, Revill P. Overview of hepatitis B viral replication and genetic variability. J Hepatol 2016;64(Suppl.):S4‐S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gao S, Duan ZP, Coffin CS. Clinical relevance of hepatitis B virus variants. World J Hepatol 2015;7:1086‐1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Inui A, Komatsu H, Sogo T, Nagai T, Abe K, Fujisawa T. Hepatitis B virus genotypes in children and adolescents in Japan: before and after immunization for the prevention of mother to infant transmission of hepatitis B virus. J Med Virol 2007;79:670‐675. [DOI] [PubMed] [Google Scholar]
- 67. Liu SL, Dong Y, Zhang L, Li MW, Wo J, Lu LW, et al. Influence of HBV gene heterogeneity on the failure of immunization with HBV vaccines in eastern China. Arch Virol 2009;154:437‐443. [DOI] [PubMed] [Google Scholar]
- 68. Carman WF, Jacyna MR, Hadziyannis S, Karayiannis P, McGarvey MJ, Makris A, et al. Mutation preventing formation of hepatitis B e antigen in patients with chronic hepatitis B infection. Lancet 1989;2:588‐591. [DOI] [PubMed] [Google Scholar]
- 69. Hayashi S, Khan A, Simons BC, Homan C, Matsui T, Ogawa K, et al. An association between core mutations in hepatitis B virus genotype F1b and hepatocellular carcinoma in Alaskan native people. Hepatology 2019;69:19‐33. [DOI] [PubMed] [Google Scholar]
- 70. Kao JH, Chen PJ, Lai MY, Chen DS. Basal core promoter mutations of hepatitis B virus increase the risk of hepatocellular carcinoma in hepatitis B carriers. Gastroenterology 2003;124:327‐334. [DOI] [PubMed] [Google Scholar]
- 71. Liu S, Xie J, Yin J, Zhang H, Zhang Q, Pu R, et al. A matched case‐control study of hepatitis B virus mutations in the preS and core promoter regions associated independently with hepatocellular carcinoma. J Med Virol 2011;83:45‐53. [DOI] [PubMed] [Google Scholar]
- 72. Papadakis MA, Elefsiniotis IS, Vlahos G, Daskalakis G, Barbatis C, Antsaklis A. Intrauterine‐transplacental transmission of hepatitis B virus (HBV) from hepatitis B e antigen negative (precore mutant, G1896A) chronic HBV infected mothers to their infants. Preliminary results of a prospective study. J Clin Virol 2007;38:181‐183. [DOI] [PubMed] [Google Scholar]
- 73. Cheng H, Su H, Wang S, Shao Z, Men K, Li M, et al. Association between genomic heterogeneity of hepatitis B virus and intrauterine infection. Virology 2009;387:168‐175. [DOI] [PubMed] [Google Scholar]
- 74. Joshi SS, Gao S, Castillo E, Coffin CS. Presence of precore (C)/C promoter mutants in peripheral blood mononuclear cells of chronic hepatitis B (CHB) carriers during pregnancy does not correlate with increased risk of liver disease in 4 years of follow‐up. Dig Dis Sci 2019; 10.1007/s10620-019-05745-w. [DOI] [PubMed] [Google Scholar]
- 75. Wu TW, Lin HH, Wang LY. Chronic hepatitis B infection in adolescents who received primary infantile vaccination. Hepatology 2013;57:37‐45. [DOI] [PubMed] [Google Scholar]
- 76. Wang J, He Y, Jin D, Liu J, Zheng J, Yuan N, et al. No response to hepatitis B vaccine in infants born to HBsAg(+) mothers is associated to the transplacental transfer of HBsAg. Infect Dis (Lond) 2017;49:576‐583. [DOI] [PubMed] [Google Scholar]
- 77. Cao M, Wu Y, Wen S, Pan Y, Wang C, Zhang X, et al. 20 μg hepatitis B vaccination reduced the risk of low responsiveness in infants with HLA‐II risk genotype of HBsAg positive mothers. Infect Genet Evol 2018;63:243‐248. [DOI] [PubMed] [Google Scholar]
- 78. Machaira M, Papaevangelou V, Vouloumanou EK, Tansarli GS, Falagas ME. Hepatitis B vaccine alone or with hepatitis B immunoglobulin in neonates of HBsAg+/HBeAg− mothers: a systematic review and meta‐analysis. J Antimicrob Chemother 2015;70:396‐404. [DOI] [PubMed] [Google Scholar]
- 79. Yue Y, Meng J, Zhang S. Mechanism of peripheral blood mononuclear cell invasion by HBV on artificial immunization in newborns. Chin Med J (Engl) 2002;115:1380‐1382. [PubMed] [Google Scholar]
- 80. Chen X, Gui X, Zhang L, Huang F, Zhong H, Pang Z, et al. Maternal anti‐HBVs suppress the immune response of infants to hepatitis B vaccine. J Viral Hepat 2016;23:955‐960. [DOI] [PubMed] [Google Scholar]
- 81. Gupta I, Ratho RK. Immunogenicity and safety of two schedules of hepatitis B vaccination during pregnancy. J Obstet Gynaecol Res 2003;29:84‐86. [DOI] [PubMed] [Google Scholar]
- 82. Hu Y, Wu Q, Xu B, Zhou Z, Wang Z, Zhou YH. Influence of maternal antibody against hepatitis B surface antigen on active immune response to hepatitis B vaccine in infants. Vaccine 2008;26:6064‐6067. [DOI] [PubMed] [Google Scholar]
- 83. Carollo M, Palazzo R, Bianco M, Pandolfi E, Chionne P, Fedele G, et al. Hepatitis B specific T cell immunity induced by primary vaccination persists independently of the protective serum antibody level. Vaccine 2013;31:506‐513. [DOI] [PubMed] [Google Scholar]
- 84. No Authors Listed . A two‐dose hepatitis B vaccine for adults (Heplisav‐B). Med Lett Drugs Ther 2018;60:17‐18. [PubMed] [Google Scholar]
- 85. Cooreman MP, van Roosmalen MH, te Morsche R, Sünnen CM, de Ven EM, Jansen JB, et al. Characterization of the reactivity pattern of murine monoclonal antibodies against wild‐type hepatitis B surface antigen to G145R and other naturally occurring “a” loop escape mutations. Hepatology 1999;30:1287‐1292. [DOI] [PubMed] [Google Scholar]
- 86. Wu C, Deng W, Deng L, Cao L, Qin B, Li S, et al. Amino acid substitutions at positions 122 and 145 of hepatitis B virus surface antigen (HBsAg) determine the antigenicity and immunogenicity of HBsAg and influence in vivo HBsAg clearance. J Virol 2012;86:4658‐4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Chaouch H, Taffon S, Villano U, Equestre M, Bruni R, Belhadj M, et al. Naturally occurring surface antigen variants of hepatitis B virus in Tunisian patients. Intervirology 2016;59:36‐47. [DOI] [PubMed] [Google Scholar]
- 88. Kwei K, Tang X, Lok AS, Sureau C, Garcia T, Li J, et al. Impaired vrion secretion by hepatitis B virus immune escape mutants and its rescue by wild‐type envelope proteins or a second‐site mutation. J Virol 2013;87:2352‐2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Basuni AA, Butterworth L, Cooksley G, Locarnini S, Carman WF. Prevalence of HBsAg mutants and impact of hepatitis B infant immunisation in four Pacific Island countries. Vaccine 2004;22:2791‐2799. [DOI] [PubMed] [Google Scholar]
- 90. Qin Y, Liao P. Hepatitis B virus vaccine breakthrough infection: surveillance of S gene mutants of HBV. Acta Virol 2018;62:115‐121. [DOI] [PubMed] [Google Scholar]
- 91. Larralde O, Dow B, Jarvis L, Davidson F, Petrik J. Hepatitis B escape mutants in Scottish blood donors. Med Microbiol Immunol 2013;202:207‐214. [DOI] [PubMed] [Google Scholar]
- 92. Ye Q, Shang S, Li W. A new vaccine escape mutant of hepatitis B virus causes occult infection. Hum Vaccin Immunother 2015;11:407‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Virine B, Osiowy C, Gao S, Wang T, Castillo E, Martin SR, et al. Hepatitis B virus (HBV) variants in untreated and tenofovir treated chronic hepatitis B (CHB) patients during pregnancy and post‐partum follow‐up. PLoS ONE 2015;10:e0140070. Erratum in: PLoS One 2015;10:e0145898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wang X, Deng W, Qian K, Deng H, Huang Y, Tu Z, et al. Quasispecies characters of hepatitis B virus in immunoprophylaxis failure infants. Eur J Clin Microbiol Infect Dis 2018;37:1153‐1162. [DOI] [PubMed] [Google Scholar]
- 95. Eilard A, Andersson M, Ringlander J, Wejstål R, Norkrans G, Lindh M. Vertically acquired occult hepatitis B virus infection may become overt after several years. J Infect 2019;78:226‐231. [DOI] [PubMed] [Google Scholar]
- 96. Bertoletti A, Ferrari C. Adaptive immunity in HBV infection. J Hepatol 2016;64(Suppl.):S71‐S83. [DOI] [PubMed] [Google Scholar]
- 97. Shrivastava S, TrehanPati N, Patra S, Kottilil S, Pande C, Trivedi SS, et al. Increased regulatory T cells and impaired functions of circulating CD8 T lymphocytes is associated with viral persistence in hepatitis B virus‐positive newborns. J Viral Hepat 2013;20:582‐591. [DOI] [PubMed] [Google Scholar]
- 98. Shrivastava S, TrehanPati N, Kottilil S, Sarin SK. Decline in immature transitional B cells after hepatitis B vaccination in hepatitis B positive newborns. Pediatr Infect Dis J 2013;32:792‐794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hong M, Sandalova E, Low D, Gehring AJ, Fieni S, Amadei B, et al. Trained immunity in newborn infants of HBV‐infected mothers. Nat Commun 2015;6:6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Domínguez‐Andrés J, Joosten LA, Netea MG. Induction of innate immune memory: the role of cellular metabolism. Curr Opin Immunol 2019;56:10‐16. [DOI] [PubMed] [Google Scholar]
- 101. Walk J, de Bree LCJ, Graumans W, Stoter R, van Gemert GJ, van de Vegte‐Bolmer M, et al. Outcomes of controlled human malaria infection after BCG vaccination. Nat Commun 2019;10:874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Netea MG, van der Meer JW. Trained immunity: an ancient way of remembering. Cell Host Microbe 2017;21:297‐300. [DOI] [PubMed] [Google Scholar]
- 103. Molina‐García L, Hidalgo‐Ruiz M, Arredondo‐López B, Colomino‐Ceprián S, Delgado‐Rodríguez M, Martínez‐Galiano JM. Maternal age and pregnancy, childbirth and the puerperium: obstetric results. J Clin Med 2019;8:pii:E672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Tan A, Koh S, Bertoletti A. Immune response in hepatitis B virus infection. Cold Spring Harb Perspect Med 2015;5:a021428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Li H, Zhai N, Wang Z, Song H, Yang Y, Cui A, et al. Regulatory NK cells mediated between immunosuppressive monocytes and dysfunctional T cells in chronic HBV infection. Gut 2018;67:2035‐2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Chang CY, Aziz N, Poongkunran M, Javaid A, Trinh HN, Lau D, et al. Serum alanine aminotransferase and hepatitis B DNA flares in pregnant and postpartum women with chronic hepatitis B. Am J Gastroenterol 2016;111:1410‐1415. [DOI] [PubMed] [Google Scholar]
- 107. Giles M, Visvanathan K, Lewin S, Bowden S, Locarnini S, Spelman T, et al. Clinical and virological predictors of hepatic flares in pregnant women with chronic hepatitis B. Gut 2015;64:1810‐1815. [DOI] [PubMed] [Google Scholar]
- 108. Nguyen V, Tan PK, Greenup A‐J, Glass A, Davison S, Samarasinghe D, et al. Anti‐viral therapy for prevention of perinatal HBV transmission: extending therapy beyond birth does not protect against post‐partum flare. Aliment Pharmacol Ther 2014;39:1225‐1234. [DOI] [PubMed] [Google Scholar]
- 109. ter Borg MJ, Leemans WF, de Man RA, Janssen HL. Exacerbation of chronic hepatitis B infection after delivery. J Viral Hepat 2008;15:37‐41. [DOI] [PubMed] [Google Scholar]
- 110. Chang CY, Aziz N, Poongkunran M, Javaid A, Trinh HN, Lau DT, et al. Serum aminotransferase flares in pregnant and postpartum women with current or prior treatment for chronic hepatitis B. J Clin Gastroenterol 2018;52:255‐261. [DOI] [PubMed] [Google Scholar]
- 111. Bzowej NH, Tran TT, Li R, Belle SH, Smith CI, Khalili M, et al.; Hepatitis B Research Network (HBRN) . Total alanine aminotransferase (ALT) flares in pregnant North American women with chronic hepatitis B infection: results from a prospective observational study. Am J Gastroenterol 2019;114:1283‐1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Yang YB, Li XM, Shi ZJ, Ma L. Pregnant woman with fulminant hepatic failure caused by hepatitis B virus infection: a case report. World J Gastroenterol 2004;10:2305‐2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Samadi Kochaksaraei G, Castillo E, Osman M, Simmonds K, Scott AN, Oshiomogho JI, et al. Clinical course of 161 untreated and tenofovir‐treated chronic hepatitis B pregnant patients in a low hepatitis B virus endemic region. J Viral Hepat 2016;23:15‐22. [DOI] [PubMed] [Google Scholar]
- 114. Kushner T, Shaw PA, Kalra A, Magaldi L, Monpara P, Bedi G, et al. Incidence, determinants and outcomes of pregnancy‐associated hepatitis B flares: a regional hospital‐based cohort study. Liver Int 2018;38:813‐820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Lin HH, Wu WY, Kao JH, Chen DS. Hepatitis B post‐partum e antigen clearance in hepatitis B carrier mothers: correlation with viral characteristics. J Gastroenterol Hepatol 2006;21:605‐609. [DOI] [PubMed] [Google Scholar]
- 116. La Rocca C, Carbone F, Longobardi S, Matarese G. The immunology of pregnancy: regulatory T cells control maternal immune tolerance toward the fetus. Immunol Lett 2014;162:41‐48. [DOI] [PubMed] [Google Scholar]
- 117. Guleria I, Sayegh MH. Maternal acceptance of the fetus: true human tolerance. J Immunol 2007;178:3345‐3351. [DOI] [PubMed] [Google Scholar]
- 118. Joshi SS, Wong D, Castillo E, Swain MG, Coffin CS. Peripartum cytokine flares in a multiethnic cohort of chronic hepatitis B carriers does not correlate with hepatitis B virus suppression or increased risk of liver disease. Am J Reprod Immunol 2017; 10.1111/aji.12707. [DOI] [PubMed] [Google Scholar]
- 119. Li L, Wang L, Huang C, Diao L, Zhang Y, Zhang X, et al. Chronic hepatitis B infection alters peripheral immune response in women with reproductive failure. Am J Reprod Immunol 2019;81:e13083. [DOI] [PubMed] [Google Scholar]
- 120. Vyas AK, Negi P, Patra S, Maras JS, Ramakrishna G, Sarin SK, et al. Maternal immunity influences vertical transmission of hepatitis B to newborns. Hepatol Commun 2019;3:795‐811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Singhal A, Kanagala R, Jalil S, Wright HI, Kohli V. Chronic HBV with pregnancy: reactivation flare causing fulminant hepatic failure. Ann Hepatol 2011;10:233‐236. [PubMed] [Google Scholar]
- 122. Liaw YF. Hepatitis flares and hepatitis B e antigen seroconversion: implication in anti‐hepatitis B virus therapy. J Gastroenterol Hepatol 2003;18:246‐252. [DOI] [PubMed] [Google Scholar]
- 123. Cho WH, Lee HJ, Bang KB, Kim SB, Song IH. Development of tenofovir disoproxil fumarate resistance after complete viral suppression in a patient with treatment‐naïve chronic hepatitis B: a case report and review of the literature. World J Gastroenterol 2018;24:1919‐1924. [DOI] [PMC free article] [PubMed] [Google Scholar]


