Abstract
Alpha‐Klotho (αKlotho), produced by the kidney and selected organs, is essential for tissue maintenance and protection. Homozygous αKlotho‐deficiency leads to premature multi‐organ degeneration and death; heterozygous insufficiency leads to apoptosis, oxidative stress, and increased injury susceptibility. There is inconsistent data in the literature regarding whether αKlotho is produced locally in the lung or derived from circulation. We probed murine and human lung by immunohistochemistry (IHC) and immunoblot (IB) using two monoclonal (anti‐αKlotho Kl1 and Kl2 domains) and three other common commercial antibodies. Monoclonal anti‐Kl1 and anti‐Kl2 yielded no labeling in lung on IHC or IB; specific labeling was observed in kidney (positive control) and also murine lungs following tracheal delivery of αKlotho cDNA, demonstrating specificity and ability to detect artificial pulmonary expression. Other commercial antibodies labeled numerous lung structures (IHC) and multiple bands (IB) incompatible with known αKlotho mobility; labeling was not abolished by blocking with purified αKlotho or using lungs from hypomorphic αKlotho‐deficient mice, indicating nonspecificity. Results highlight the need for rigorous validation of reagents. The lung lacks native αKlotho expression and derives full‐length αKlotho from circulation; findings could explain susceptibility to lung injury in extrapulmonary pathology associated with reduced circulating αKlotho levels, for example, renal failure. Conversely, αKlotho may be artificially expressed in the lung, suggesting therapeutic opportunities.
Keywords: human, immunoblot, immunohistochemistry, inhalational cDNA delivery, mice, monoclonal antibodies
Abbreviations
- IB
immunoblot
- IHC
immunohistochemistry
- PBS
phosphate-buffered saline
- PLGA
poly-lactic-co-glycolic acid
1. INTRODUCTION
The lung interfaces with the exterior via an enormous surface area with constant exposure to pollutants, chemicals, biological toxins, fluctuating temperatures, allergens, microbial pathogens, and the highest oxygen tensions of any internal organ.1 The lung also receives the entire cardiac output bearing waste products from the periphery. In addition, lung parenchyma experiences mechanical stress with each respiratory cycle and vascular distention with each cardiac cycle. Thus, the lung has high needs for cytoprotection and is generously bestowed with endogenous and blood‐derived antioxidants.2, 3, 4 One essential cytoprotective protein is αKlotho, a member of the multifunctional Klotho gene family (α, β, and γ).5 Only αKlotho is secreted into body fluids (blood,6 cerebrospinal fluid,7 and urine8), and is derived from the cleavage of transmembrane αKlotho by secretases.9, 10, 11 Transmembrane αKlotho is a co‐receptor for the circulating mineral‐regulating hormone fibroblast growth factor (FGF)2312, 13 while the released soluble αKlotho serves the antioxidative and cytoprotective functions in distal organs including the lung.14 Homozygous αKlotho hypomorphic (kl/kl) mice with negligible plasma αKlotho levels are small in body size and succumb to multi‐organ failure at 2‐3 months of age.5 Heterozygous kl/+ mice (one normal allele) with ~50% of normal plasma αKlotho levels have normal lifespan, histology, and function in most organs,5 except that their lungs show age‐exacerbated degenerative changes, air space enlargement, elevated compliance, increased apoptosis15, 16 and oxidative DNA damage,17 highlighting the lung's exquisite sensitivity to circulating αKlotho. Exogenous recombinant αKlotho protects the lung and cultured lung cells from oxidative stress.17, 18 The highly enriched αKlotho content in human induced pluripotent stem cell secretome significantly contributes to protection of lung cells and lungs from hyperoxic injury.19 Multiple laboratories have shown direct or indirect αKlotho actions in the lung using in vitro systems.20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 Cumulative literature unequivocally supports a pivotal cytoprotective role of αKlotho in the lung.
Circulating soluble αKlotho is derived mainly from the kidney.32, 33 αKlotho mRNA and protein are abundantly expressed in distal and to a lesser extent proximal renal tubules.32, 34 There is controversy concerning whether αKlotho present in the lung is produced by resident lung cells or derived from the circulation. Several lung cell lines show αKlotho mRNA expression by RT‐PCR but none express αKlotho protein.21, 26, 29, 31 On the other hand, Kuro‐o and colleagues discovered that αKlotho could not detect αKlotho transcript in the intact lung,5 a finding independently reproduced by our group.17 Despite the absence of mRNA, αKlotho protein expression was reported in lungs and large airways by several groups using commercial antibodies.23, 26, 30 The discrepant in vitro and in vivo results, complicated by uncertain sensitivity and specificity of the various anti‐αKlotho antibodies used in different studies, significantly impede progress in the understanding of αKlotho biology.
To resolve the above discrepancies and clarify the source of the documented αKlotho actions in the lung, we probed normal murine and human lungs, lungs from hypomorphic αKlotho‐deficient (kl/kl) mice, and lungs from wild‐type mice following inhalational delivery of αKlotho cDNA, using two validated monoclonal antibodies against the Kl1 and Kl2 domains of αKlotho protein, and three other frequently used commercial antibodies. Our aims were twofold: a) To determine the sensitivity and specificity of αKlotho protein detection by immunoprecipitation (IP), immunoblot (IB), and immunohistochemistry (IHC) using commonly available antibodies, and b) To definitively prove whether αKlotho protein is endogenously expressed in the lung.
2. METHODS
2.1. Animals
All experimental protocols were approved by the University of Texas Southwestern Medical Center Institutional Animal Care and Use Committee. Wild‐type and hypomorphic αKlotho‐deficient (kl/kl) mice (129/Sv background)5, 35 were bred in our laboratory. Human lung and kidney tissues were obtained from the Lung Tissue Research Consortium of the National Heart, Lung and Blood Institute.
The mice were anesthetized by intraperitoneal (i.p.) injection of ketamine (100 mg/kg), xylazine (10 mg/kg), and acepromazine (2 mg/kg,) killed by exsanguination, and the organs perfused with phosphate‐buffered saline (PBS). One kidney was frozen in liquid nitrogen; the other was fixed in 4% paraformaldehyde. The right lung was removed and snap‐frozen in liquid nitrogen for immunoblotting. The left lung was fixed by tracheal instillation of 4% paraformaldehyde at a constant airway pressure (25 cmH2O).
2.2. Antibodies and reagents
Rat monoclonal antibodies (Antibody 1: clone‐KM2076, anti‐αKlotho Kl1 domain; Antibody 2: clone‐KM2119, anti‐αKlotho Kl2 domain) were generously gifted by Dr Makoto Kuro‐o (Jichi Medical University, Tochigi, Japan)36; these are also available commercially (KO603 and KO604, respectively, TransGenic Inc, Fukuoka, Japan). The other commercial antibodies were: Antibody 3: Rat anti‐mouse αKlotho monoclonal MAB1819 (R&D Systems); Antibody 4: Rabbit polyclonal anti‐mouse αKlotho ab203576 (Abcam); Antibody 5: Rat monoclonal anti‐mouse αKlotho sc74205 (Santa Cruz, Dallas TX). For IP, a synthetic anti‐Klotho antibody sb48 (also termed sb106) was used.6
Recombinant αKlotho protein containing the ectodomain of mouse αKlotho (amino acid number 31‐982) with C‐terminal V5 and 6xHis tags was generated and purified in our laboratory in mammalian cells as described previously.37
2.3. IP and IB
Total lung or kidney lysate was prepared as previously described.37 Thirty micrograms of protein of lysate was solubilized in Laemmli's sample buffer and subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis. After transferring to polyvinylidene difluoride membranes, proteins were immunoblotted with different primary antibodies and β‐actin for loading control. Signal was visualized using the enhanced chemiluminescence (ECL) kit (Perkin‐Elmer LAS, Inc).
2.4. IHC
For epitope retrieval, paraformaldehyde‐fixed paraffin‐embedded tissue sections were pretreated with 0.01 mol/L citrate buffer (pH 6.0) in a microwave oven for 14 minutes, including a boiling period of 1.5 minutes to enhance antigen retrieval. Tissue sections were washed with PBS (15 minutes), followed by 0.1% TritonX‐100 (10 minutes), incubated with a blocking solution (PBS, 3% BSA, 10% donkey serum; 40 minutes), then reacted with the primary antibody or neutralized primary antibodies (4°C overnight). Neutralization of primary antibody was achieved by incubation with purified mouse αklotho (molar ratio of αKlotho protein: antibody 4:1, 22°C × 1 hour). Peptides encompassing the known epitopes for Antibody 1 (peptide FRDTEALR in Kl1 region) and Antibody 2 (peptide LEVQEMTD in Kl2 region) were also used for blocking. After washing with PBS (3 × 20 minutes), sections were incubated with Alexa fluor 555‐coupled donkey anti‐rat IgG antibody (Invitrogen, Carlsbad, CA, USA) × 1 hour, counterstained with Alexa Fluor 488‐phalloidin (Invitrogen) for filamentous actin and DAPI Fluoromount‐G (SouthernBiotech, Birmingham, AL, USA) for nuclei, and examined with a Zeiss LSM880 microscope.
2.5. Pulmonary αKlotho cDNA delivery
To demonstrate our ability to detect αKlotho expression in the lung, adult 129/Sv mice (5 mo old, Charles River, Wilmington, MA, USA) were anesthetized (ketamine 50 mg/kg, xylazine 5 mg/kg, i.p.) and intubated. Heart rate and oxygen saturation were monitored (Kent Scientific Torrington, CT, USA). Full‐length mouse αKlotho with a C‐terminal FLAG tag inserted before the stop codon were cloned into expression vector (pEF1/myc‐His[A], Life Technologies). αKlotho cDNA or the vector (control) was encapsulated within poly‐lactic‐co‐glycolic acid (PLGA) nanoparticles, kindly prepared by Kytai Nguyen's laboratory (University of Texas Arlington) following established methods.38, 39 Nanoparticles (0.2 mg) were suspended in sterile saline (50 µL), sonicated for 2 minutes (300VT ultrasonic homogenizer, BioLogics, Manassas, VA, USA), aerosolized and delivered into the trachea (MicroSprayer Model IA‐1C, High Pressure Syringe FMJ‐250, Penn‐Century, Wyndmoor, PA, USA). A total of nine mice were used. Four mice received αKlotho‐containing nanoparticles; two each were studied 4 and 7 days later. Five mice received vector‐containing nanoparticles; two were studied after 4 days later and three were studied 7 days later. Mice were deeply anesthetized (ketamine 100 mg/kg, xylazine 10 mg/kg, acepromazine 2 mg/kg, i.p.) and killed by exsanguination. The right lung was removed and snap‐frozen in liquid nitrogen. The left lung was fixed by tracheal instillation of 4% paraformaldehyde at a constant airway pressure (25 cmH2O). αKlotho expression in the lungs was probed by IHC and immunoblotting as described above.
3. RESULTS
3.1. IB and IP
Mouse lung lysate was immunoblotted with each of the five antibodies. Labeling specificity was tested by preincubation with purified recombinant αKlotho. Kidney lysate served as positive control. The monoclonal Antibodies 1 and 2 did not show labeling with lung lysate regardless of the exposure times while Antibodies 3, 4, and 5 labeled multiple bands that are not compatible with αKlotho's electrophoretic mobility (a broad band approximately 130 kD; Figure 1A). Moreover, labeling by Antibodies 3‐5 was not blocked by incubation with purified αKlotho protein (Figure 1A). To test if αKlotho is expressed in limited regions or the signal may be “diluted” in whole lung lysates, we isolated peripheral and central lung regions for IB by Antibody 1 and again observed no specific staining even though strong labeling was present in the kidney lysate on the same blot (Figure 1B). To test if αKlotho expression is age‐dependent, we performed IB on lungs from neonatal mice (day P5) with Antibody 1 and observed no staining in the lung either (data not shown). To ensure that low protein abundance is not the reason for the lack of labeling by Antibody 1, lysate from the entire mouse lung was immunoprecipitated with asynthetic anti‐Klotho antibody sb48 (previously termed sb106) known to perform well in IP,6 then immunoblotted with each of the five antibodies Even with the concentration and a long exposure, no specific bands were identifiable from the lung using Antibodies 1 and 2 while the expected positive labeling of the kidney (130 kD) as control clearly verifies the utility and validity of Antibodies 1 and 2 (Figure 1C). We also ran lung and kidney lysates on the same gel, transferred them to the same PDVF membrane, incubated with the same antibody solutions, and then cut the filters to separate the lung and kidney lanes for long (lung) and short (kidney) exposure, and we did not observe any signal from the lung (data not shown). Antibodies 3, 4, and 5 yielded inconsistent and uninterpretable results in kidney and lung.
Figure 1.
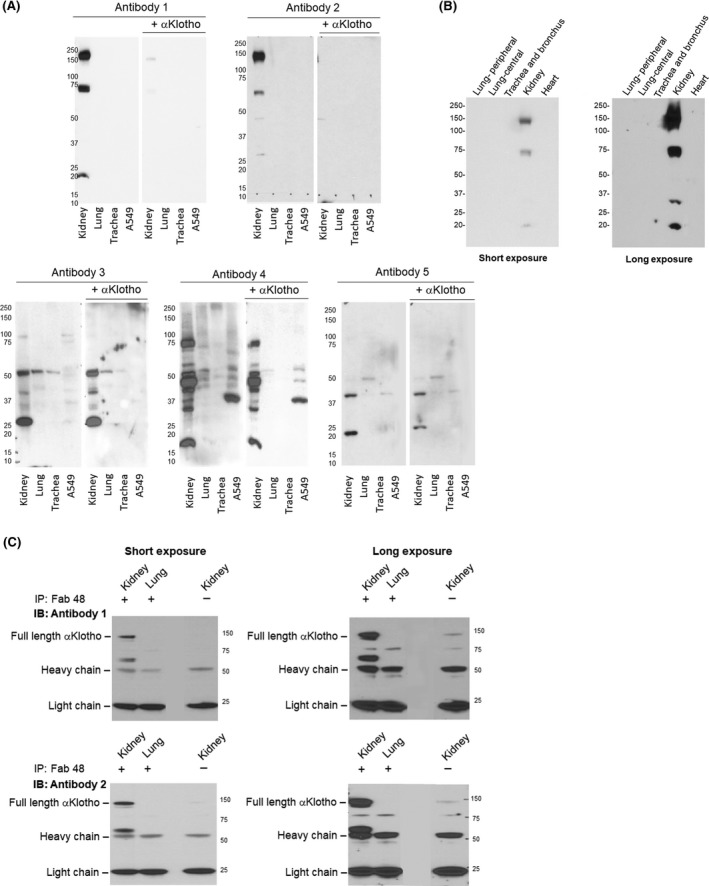
Immunoprecipitation‐immunoblot and for αKlotho in murine lung and A549 lung epithelial cells. Studies were performed with five antibodies. Antibody 1: Rat monoclonal Anti‐αKlotho Kl1 (KM2076). Antibody 2: Rat monoclonal Anti‐αKlotho Kl2 (KM2119). Antibody 3: R&D Cat# MAB1819; Antibody 4: Abcam Cat# ab203576; Antibody 5: Santa Cruz Cat# sc74205. Three wild‐type mice were used. (A), Representative immunoblot of lung lysate and A549 lung epithelial cell line probed with each antibody and blockade with purified recombinant αKlotho. Kidney lysate served as positive control. (B), Representative immunoblot of αKlotho in samples taken from different regions of the lung and probed using Antibody 1. Kidney served as positive control and the heart as negative control. (C), Representative immunoprecipitation (IP)‐immunoblot (IB) of αKlotho immunoprecipitated from lung lysate of two animals using a well validated synthetic anti‐αKlotho antibody sb48 (synonymous with sb106)6, and blotted with Antibodies 1 to 5. In (B) and (C), both a short and a long exposure are shown (left and right panels, respectively). Kidney lysate served as positive control
3.2. IHC
Several published reports described αKlotho expression in lung parenchyma by IHC and very intense labeling particularly in the airways using various commercial antibodies.23, 26, 30 As in the case of IB and IP (Figure 1), no αKlotho labeling was detected in wild‐type mouse lung by IHC using Antibody 1 or 2, contradicting the widespread labeling observed with Antibody 3, 4, or, 5 (Figure 2A, upper row). Use of standard antigen retrieval techniques could not bring out staining by Antibody 1 (not shown). Preincubation of the antibodies with purified recombinant αKlotho did not abolish labeling by commercial Antibody 3, 4, or 5 except for minor diminution of labeling by Antibody 3 (Figure 2A, lower row), indicating that these are likely nonspecific binding.
Figure 2.
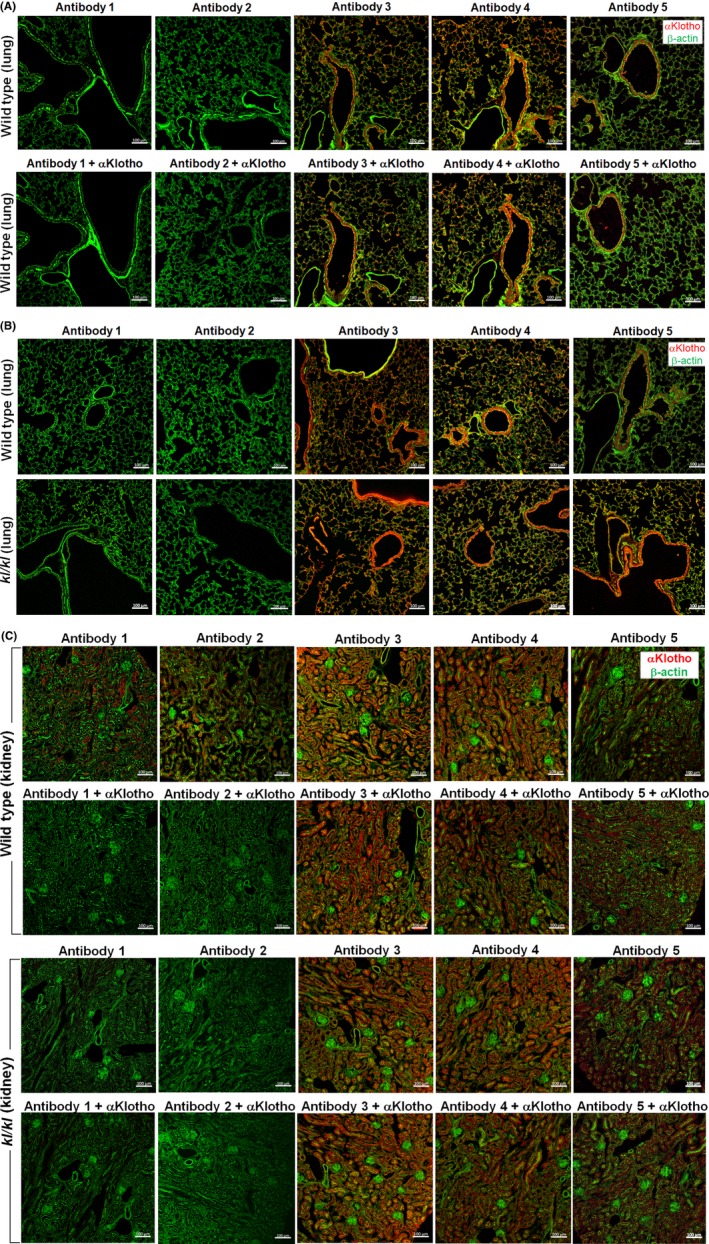
Immunohistochemistry for αKlotho expression in the lung and kidney of wild‐type and kl/kl hypomorphic αKlotho‐deficient mice. Studies were performed with five antibodies. Antibody 1: Rat monoclonal Anti‐αKlotho Kl1 (KM2076). Antibody 2: Rat monoclonal Anti‐αKlotho Kl2 (KM2119). Antibody 3: R&D Cat# MAB1819; Antibody 4: Abcam Cat# ab203576; Antibody 5: Santa Cruz Cat# sc74205. Three wild‐type and 2 kl/kl mice were used. (A), Representative images of lungs from wild‐type mice probed with the five antibodies (upper row) and blockade with purified recombinant αKlotho (lower row). (B), Representative images of lungs probed with the five antibodies in wild‐type (upper row) and kl/kl (lower row) mice. (C), Kidney sections served as control for αKlotho expression probed by IHC using five antibodies in wild‐type (upper two rows) and homozygous hypomorphic kl/kl (lower 2 rows) mice, with and without blockade by incubation with purified recombinant αKlotho. Bar = 100 µm
Further testimony on the specificity, or lack thereof, of these antibodies was provided by the IHC results in the kl/kl hypomorphs that express negligible αKlotho. As in wild‐type mice, there was no staining in the lung of kl/kl mice using Antibody 1 or 2, suggesting again that the positive signal observed in these lungs using Antibody 3, 4, or 5 is nonspecific (Figure 2B). Furthermore, the positive labeling of kidney in wild‐type mice using Antibody 1 or 2 was completely blocked by recombinant αKlotho, attesting to their specificity, whereas the positive labeling using Antibody 3, 4, or 5 was not blocked (Figure 2C, upper two rows). If αKlotho is genuinely expressed in the lung, αKlotho labeling should be decreased in kl/kl mice as its 5'‐flanking region is disrupted. Indeed, a lack of αKlotho labeling in kidney sections from kl/kl mice was demonstrated by IHC using Antibodies 1 or 2. In contrast, widespread labeling was still observed using Antibodies 3, 4, and 5 that were not blocked by recombinant αKlotho (Figure 2C, lower 2 rows).
To exclude the possibility that peculiarities of the lung precludes Antibody 1 or 2 from reacting with αKlotho and that our results may represent false negatives, we examined a situation when αKlotho protein is artificially expressed in murine lung by tracheal delivery of nanoparticles bearing αKlotho cDNA (Figure 3). We previously validated this inhalational approach showing successful pulmonary cDNA delivery and sustained expression and bioactivity of the erythropoietin receptor.38 At 4 and 7 days following αKlotho cDNA delivery, αKlotho protein expression was clearly detected by IB using Antibody 1 or 2, showing the characteristic 130 kD band while no 130 kD signal was observed in the lungs of vector‐treated animals (Figure 3A). Similarly, in animals treated with αKlotho cDNA, intense positive αKlotho labeling in lung tissue was detected by IHC using Antibody 1 or 2 (Figure 3B) localized within the cytoplasm of airway and alveolar septal cells (Figure 3C). Thus, αKlotho protein expression in lung cells may be artificially induced and specifically detected using Antibodies 1 and 2.
Figure 3.
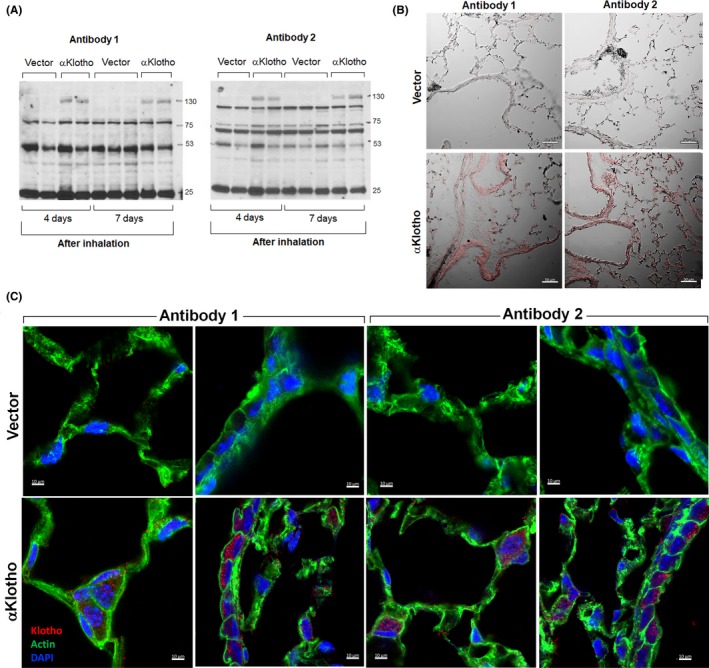
Detection of αKlotho protein expression in murine lung following tracheal delivery of nanoparticles containing αKlotho cDNA or vector. Four mice received αKlotho‐containing nanoparticles; two were studied 4 d later and two were studied 7 d later. Five mice received vector‐containing nanoparticles; two were studied 4 d later and three were studied 7 d later. (A), One lung was lyzed and immunoblotted with Antibody 1 (monoclonal anti‐Kl1, KM2076) or 2 (monoclonal anti‐Kl2, KM2119), showing expression of full‐length αKlotho (130 kD) in lungs of αKlotho‐treated mice and absent expression in vector‐treated mice. (B), The other lung was fixed for αKlotho expression by fluorescence immunohistochemistry. Representative images (differential interference contrast) at 4 d following delivery are shown. Bar = 50 µm. (C), High magnification confocal micrographs show cytoplasmic localization of αKlotho within airway and alveolar septal cells in lung tissue of vector‐ and αKlotho‐treated mice, probed using Antibodies 1 and 2. Bar = 10 µm
We extended these studies from murine to human lung and kidney tissue, by probing with each of the five antibodies and blockading by incubation with purified αKlotho protein (Figure 4). In adult human lung (upper 2 rows), no labeling was observed with Antibody 1 or 2, whereas nonspecific staining was observed using Antibody 3, 4, or 5 that was not blocked. In human kidney (lower 2 rows), specific labelling was observed using Antibody 1 or 2 that was blocked by incubation with purified αKlotho protein, whereas the positive labeling by Antibody 3, 4, or 5 was not blocked. These results show that native αKlotho expression was similarly present in both murine and human kidneys and similarly absent in murine and human lungs.
Figure 4.
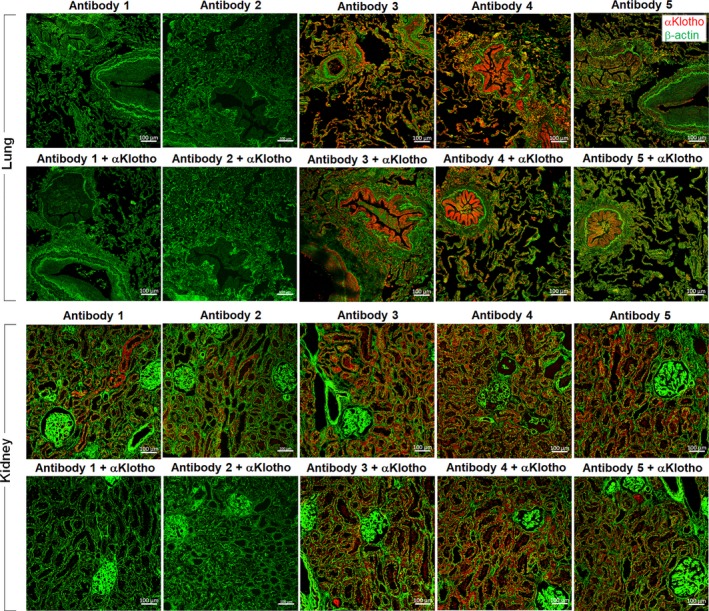
Immunohistochemistry of αKlotho expression in human lung and kidney. Paraformaldehyde‐fixed human lung and kidney sections from two subjects were probed with five antibodies; specificity was tested by preincubation with purified recombinant αKlotho. Antibody 1: Rat monoclonal Anti‐αKlotho Kl1 (KM2076). Antibody 2: Rat monoclonal Anti‐αKlotho Kl2 (KM2119). Antibody 3: R&D Cat# MAB1819; Antibody 4: Abcam Cat# ab203576; Antibody 5: Santa Cruz Cat# sc74205. Representative images are shown. Bar = 100 µm
4. DISCUSSION
4.1. Summary of the findings
We present novel data disproving the notion of native αKlotho protein expression in murine and human lung by meticulously ensuring that the monoclonal Antibodies 1 (anti‐Kl1, KM2076) and 2 (anti‐Kl2, KM2119) are sensitive and specific in detecting αKlotho natively expressed in the kidney and artificially expressed in the lung. We further demonstrate the nonspecificity of several commercial anti‐αKlotho antibodies commonly used in previously published studies that reported positive αKlotho expression in lung tissue. While Antibodies 1 and 2 yielded no staining on IHC, the other antibodies labeled numerous lung structures. While IB using Antibodies 1 and 2 was negative in murine lung, the other antibodies produced multiple bands incompatible with the electrophoretic mobility of full‐length αKlotho that were not blocked by preincubation with purified recombinant αKlotho protein or using lung tissue from kl/kl hypomorphic mice, arguing against the specificity of Antibodies 3, 4, and 5. In contrast, labeling with Antibody 1 or 2 in the kidney, which is known to express abundant αKlotho, was completely abrogated by purified αKlotho protein, indicating specificity. We conclude that the lung normally does not express full‐length αKlotho; the unequivocal findings of αKlotho‐mediated in vivo cytoprotection in the lung is derived from circulating αKlotho produced mainly by the kidney or exogenous sources including the delivery of conditioned media of human induced pluripotent stem cells that are enriched in αKlotho content.17, 18, 19 Nevertheless, pulmonary αKlotho protein may be experimentally expressed by targeted delivery of αKlotho cDNA to the lung and specifically detected using commercially available monoclonal anti‐Kl1 and anti‐Kl2 antibodies. We cannot rule out the possibility that αKlotho taken up by the lung from the circulation can be processed in lung cells to smaller fragments. These results highlight the need for rigorous validation of each reagent used in αKlotho research to avoid reaching erroneous conclusions.
4.2. Organ‐specific αKlotho expression
In addition to the kidney, αKlotho is endogenously expressed in the parathyroid gland,40 brain,41 breast,42 gonads,43 and sino‐atrial node.44 Some organs do not express αKlotho but clearly derive benefits from circulating αKlotho. For example, the myocardium does not express αKlotho yet undergoes pathologic remodeling in genetic or acquired states of circulating αKlotho deficiency45; repletion with recombinant αKlotho protein rescues the cardiomyopathy in vivo and in vitro.37, 45 Thus, the absence of native αKlotho expression in the heart does not diminish the biological significance of circulating αKlotho action on the myocardium. Our findings present a parallel scenario in the lung where the absence of native αKlotho does not lessen the biological significance of circulating αKlotho action on the lung. In another scenario, there is an existing controversy regarding whether αKlotho is actively produced by vascular smooth muscle cells or is derived from circulation46, 47, 48, 49, 50, 51 even though αKlotho has clearly been shown to protect vascular smooth muscle cells from vascular calcification52, 53; the uncertainty regarding the source of αKlotho production has impeded the progress in the field.53
4.3. αKlotho actions in the lung
Pleiotropic actions of αKlotho include antioxidation and antiapoptosis,17, 54 antifibrosis,55 enzymatic activity,34 growth factor regulation,16 ion transport,56 mineral metabolism,57 stem cell function,58 tumor suppression,59 autophagy activation,60 and cell maintenance.61 αKlotho inhibits Wnt signaling,62 and reciprocally interacts with erythropoietin receptor to enhance cytoprotection.63 In lung epithelial cells, αKlotho activates the Nrf2 network of antioxidant proteins to alleviate injury.17, 18 Circulating αKlotho protects alveolar endothelium, and rapidly crosses the septum to reach the epithelium.64, 65 αKlotho gene disruption leads to premature multi‐organ degeneration and death5 whereas overexpression extends life span.35 Circulating αKlotho levels inversely correlate with local oxidative DNA damage in the lung.17, 18 Homozygous αKlotho‐deficient hypomorphic (kl/kl) mice die at 8‐12 weeks of age; their lungs are friable with enlarged air spaces, decreased elastic recoil and heightened apoptosis.16 These mice exhibit reduced hematopoietic stem cells and cytoprotective molecules,58 suggesting diminished repair and regenerative ability. Hemizygous αKlotho haplo‐insufficient (kl/+) mice (~50% of normal circulating αKlotho level) show age‐exacerbated oxidative damage, enlarged air spaces,17 elevated lung compliance, and increased apoptosis.15, 16 Acquired circulating αKlotho deficiency, for example, in renal disease, predisposes to secondary lung injury18 and exacerbates coexisting hemodynamic, metabolic, and pro‐inflammatory factors contributing to lung dysfunction. In rats with ischemia‐reperfusion kidney injury8 and severely reduced plasma αKlotho level, acute lung injury develops quickly18; repletion of circulating αKlotho alleviates pulmonary complications independent of the severity of kidney injury.18 By inference, age‐related decline in renal αKlotho synthesis may also heighten susceptibility to lung injury in the elderly.
4.4. αKlotho detection
Endogenous αKlotho transcripts have been detected in cultured lung cells transfected with αKlotho cDNA; yet none of the lung cell lines express αKlotho at baseline.21, 26, 29, 31 Li et al25 performed IHC using an unspecified polyclonal anti‐αKlotho antibody, and reported co‐localization with alveolar macrophages and decreased staining in lungs of smokers and patients with chronic obstructive pulmonary disease (COPD); however, control specimens showed high background intensity, and specificity of staining was not established. Gao et al 23 performed IHC and IB with commercial antibodies reporting intense αKlotho staining in the lungs of healthy nonsmokers compared to markedly reduced staining in smokers and COPD patients, whereas αKlotho staining intensity was similar in ozone‐exposed mice compared to air‐exposed controls. Also using commercial anti‐αKlotho antibodies,66 αKlotho staining was detected by IHC and IB in bronchial epithelium of cystic fibrosis patients. However, in that study66 the two detected bands (~65 and ~80 kD) were below the expected size of full‐length/secreted αKlotho (~130 kD) and no control IHC was shown. Furthermore, the detected bands66 are inconsistent with the single band (130 kD) shown in another study by the same group using the same antibody that probed airway epithelia from COPD patients.30 Usuda et al67 using monoclonal anti‐Kl1 (KM2076) detected αKlotho expression by IHC in 33% of resected lung cancer specimens, and suggested that expression predicts good outcome. There was no description or data validating antibody specificity in the above studies.
Both transmembrane and secreted full–length αKlotho are glycoproteins migrating around ~130 kD.7 A 65‐70 kD band reported in the literature68 likely represents fragments containing the Kl1 domain. Compatible with our findings in Klotho protein is the fact that we were unable to detect Klotho mRNA in normal murine lungs.17 In the Protein Atlas database from the Human Protein Atlas,69 Klotho mRNA is reported to be present at very low levels. The small discrepancy between findings in these two studies is likely due to different amounts of tissue, PCR primers, conditions, and cycle numbers. As the earlier reported presence of αKlotho mRNA expression by RT‐PCR in alveolar macrophages25 could not be confirmed by protein expression,68 it may be that partially processed transcripts are primed and amplified by the highly sensitive RT‐PCR but not translated,70 whereas the existence of a short αKlotho protein translated from alternatively spliced transcript published in earlier studies may represent illegitimate splicing as has been reported for other genes.71 There is good evidence that αKlotho mRNA may be transcribed but not translated into protein. Mencke et al72 provided convincing data that the so‐called “spliced Klotho mRNA” is actually destined for degradation by nonsense‐mediated mRNA decay and not translated into protein. RT‐PCR is very sensitive and could amplify non‐specific targets especially when a high cycle number is used. Thus, it is risky to draw conclusions based solely on the presence of αKlotho mRNA without corroboration by the corresponding protein expression.
Our previous study demonstrated the sensitivity and specificity of anti‐Kl1 (KM2076) for detecting serum αKlotho by IP and IB.17 Multiple synthetic anti‐αKlotho antibodies, for example, sb106 (now called sb48) have been developed for use in immunoprecipitation and on unfixed cells6; however, synthetic antibodies only detect native nondenatured αKlotho and cannot detect denatured proteins by IHC and IB even in the kidney with its abundant αKlotho expression. The ability of sb48 (sb106) to label unfixed transfected cells may be due to the highly abundant overexpression but a major reason is explained by lack of fixation.6 In contrast, KM2076 and KM2019 are well proven to label denatured Klotho. Enzyme‐linked immunosorbent assays (ELISA) are known to exhibit suboptimal specificity and sensitivity in detecting αKlotho in serum6, 73 and cannot detect αKlotho in tissue lysates. To date, no laboratory including ours (data not shown) has been able to detect native αKlotho by IP followed by mass spectrometry using validated antibodies (including KM2076 and KM2019) from any tissue including the kidney.
4.5. Significance of absent endogenous αKlotho expression in the lung
Our finding of the absence of native αKlotho production in the normal lung carries significant physiological impact. Karl Popper logically emphasized that no amount of positive experimental outcomes can absolutely confirm a scientific theory, but a single reproducible counterproof is decisive in showing the theory to be incorrect.74 Not only is a negative finding just “as true” as a positive one, it actually possesses greater power in supporting conclusions. By resolving the controversy regarding the source of αKlotho in the lung, these results permit accurate data interpretation of studies designed to elucidate the organ‐specific mechanisms of action of this essential protein.
Like the myocardium, the lung depends heavily on circulating αKlotho for maintenance and protection, rather than being equipped with its own αKlotho protein expression. The lack of native local αKlotho production does not diminish the biological importance of αKlotho in lung homeostasis or contradict the cumulative literature overwhelmingly supporting αKlotho as essential for lung health. Given the continuous physico‐chemical insults imposed on the lung, it is not surprising that local endogenous cytoprotective mechanisms need to be supplemented by circulating factors such as kidney‐derived αKlotho to neutralize the toxicity of blood‐borne whole‐body waste products traversing the lung. As renal αKlotho production declines with age or disease, an imbalance between pulmonary toxin delivery and cytoprotective capacity is created that predisposes to lung degeneration and dysfunction. Local or systemic diseases, for example, diabetes mellitus and cardiovascular disorders, that cause renal impairment further reduce αKlotho synthesis and accelerate widespread age‐exacerbated organ degeneration in the lung. Primary acute or chronic lung disease may secondarily impair renal function and reduce circulating αKlotho level, thereby aggravating lung degeneration in a vicious cycle. Thus, circulating αKlotho delivery to the lung is a plausible mechanism of pulmonary‐renal crosstalk and explains an important aspect of reciprocal interdependence between these two organs.
4.6. Conclusions
We provide unequivocal proof that a) αKlotho protein is not normally expressed in murine or human lung tissue, although expression may be artificially induced; b) therefore, the biological actions of αKlotho on the lung is normally derived from circulating αKlotho and c) the reported αKlotho detection by several commonly used commercial antibodies are nonspecific artefacts. These results resolve a major controversy of pulmonary αKlotho expression, and promote valid methodology and accurate data interpretation for future studies in this emergent field. It is absolutely critical to advance the current understanding of the role of αKlotho in pulmonary physiology and pathobiology; however, validated reagents must be used and these are accessible to all investigators. In addition, the validity of any new reagent for elucidating αKlotho expression must be rigorously established to avoid reaching erroneous conclusions. This caveat broadly applies to the study of αKlotho biology in extrapulmonary organs as in the lung. In terms of physiological significance, the dependence of the lung on extrapulmonary source of αKlotho for health maintenance and cytoprotection heightens the susceptibility to lung injury from either direct insult or secondary development of acute respiratory distress syndrome in renal failure or other extrapulmonary diseases associated with reduced circulating αKlotho levels. Whether there is local pulmonary expression of αKlotho in pathological states remains to be investigated. Finally, the ability to artificially express αKlotho in the lung via inhalational cDNA delivery holds promise for noninvasive translational interventions designed to fortify αKlotho‐mediated cytoprotection in the lung.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
Jianning Zhang, Khoa Cao, Liping Li and Johanne V. Pastor performed experiments, and analyzed and interpreted the data. Orson W. Moe and Connie CW Hsia conceived and designed the project, supervised the performance of experiments and the analysis and interpretation of the results, and wrote the manuscript. All the authors read and approved the manuscript.
ACKNOWLEDGEMENTS
We thank the staff of the Animal Resources Centerat UT Southwestern Medical Center for excellent veterinary care, Dr Kytai Nguyen for provision of reagents, and Paddy P. Moe for helpful discussion and encouragement. Supported by the National Heart, Lung and Blood Institute Grants R01 HL134373 and U01 HL111146, and the National Institute of Diabetes and Digestive and Kidney Diseases Grants R01 DK092461, R01 DK091392 and P30 DK079328 (O'Brien Kidney Research Center), and the Lung Tissue Research Consortium, the National Center for Research Resources. The content of this manuscript is solely the authors' responsibility and does not necessarily represent official views of the funding agencies.
Zhang J, Cao K, Pastor JV, Li L, Moe OW, Hsia CCW. Alpha‐Klotho, a critical protein for lung health, is not expressed in normal lung. FASEB BioAdvances. 2019;1:675–687. 10.1096/fba.2019-00016
Jianning Zhang and Khoa Cao share equal contribution.
Funding information
Supported by the National Heart, Lung and Blood Institute Grants R01 HL134373 and U01 HL111146, the National Institute of Diabetes and Digestive and Kidney Diseases Grants R01 DK092461, R01 DK091392 and P30 DK079328 (O'Brien Kidney Research Center), and the Lung Tissue Research Consortium, the National Center for Research Resources.
REFERENCES
- 1. Hsia C, Ravikumar P, Ye J. Acute lung injury complicating acute kidney injury: A model of endogenous alphaKlotho deficiency and distant organ dysfunction. Bone. 2017;100(Special Issue: Kidney and Bone):100‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Poljsak B, Fink R. The protective role of antioxidants in the defence against ROS/RNS‐mediated environmental pollution. Oxid Med Cell Longev. 2014;2014:671539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Valavanidis A, Vlachogianni T, Fiotakis K, Loridas S. Pulmonary oxidative stress, inflammation and cancer: respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int J Environ Res Public Health. 2013;10:3886‐3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Araneda OF, Tuesta M. Lung oxidative damage by hypoxia. Oxid Med Cell Longev. 2012;2012:856918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kuro‐o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45‐51. [DOI] [PubMed] [Google Scholar]
- 6. Barker SL, Pastor J, Carranza D, et al. The demonstration of alphaKlotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrol Dial Transplant. 2015;30:223‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Imura A, Iwano A, Tohyama O, et al. Secreted Klotho protein in sera and CSF: implication for post‐translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565:143‐147. [DOI] [PubMed] [Google Scholar]
- 8. Hu MC, Shi M, Zhang J, Quinones H, Kuro‐o M, Moe OW. Klotho deficiency is an early biomarker of renal ischemia‐reperfusion injury and its replacement is protective. Kidney Int. 2010;78:1240‐1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen CD, Tung TY, Liang J, et al. Identification of cleavage sites leading to the shed form of the anti‐aging protein klotho. Biochemistry. 2014;53:5579‐5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci USA. 2007;104:19796‐19801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bloch L, Sineshchekova O, Reichenbach D, et al. Klotho is a substrate for alpha‐, beta‐ and gamma‐secretase. FEBS Lett. 2009;583:3221‐3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Urakawa I, Yamazaki Y, Shimada T, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770‐774. [DOI] [PubMed] [Google Scholar]
- 13. Kurosu H, Ogawa Y, Miyoshi M, et al. Regulation of fibroblast growth factor‐23 signaling by klotho. J Biol Chem. 2006;281:6120‐6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hu MC, Shiizaki K, Kuro‐o M, Moe OW. Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol. 2013;75:503‐533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Suga T, Kurabayashi M, Sando Y, et al. Disruption of the klotho gene causes pulmonary emphysema in mice. Defect in maintenance of pulmonary integrity during postnatal life. Am J Respir Cell Mol Biol. 2000;22:26‐33. [DOI] [PubMed] [Google Scholar]
- 16. Ishii M, Yamaguchi Y, Yamamoto H, Hanaoka Y, Ouchi Y. Airspace enlargement with airway cell apoptosis in klotho mice: a model of aging lung. J Gerontol A Biol Sci Med Sci. 2008;63:1289‐1298. [DOI] [PubMed] [Google Scholar]
- 17. Ravikumar P, Ye J, Zhang J, et al. alpha‐Klotho protects against oxidative damage in pulmonary epithelia. Am J Physiol Lung Cell Mol Physiol. 2014;307:L566‐L575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ravikumar P, Li L, Ye J, et al. alphaKlotho deficiency in acute kidney injury contributes to lung damage. J Appl Physiol (1985). 2016;120:723‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gazdhar A, Ravikumar P, Pastor J, et al. Alpha‐Klotho enrichment in induced pluripotent stem cell secretome contributes to antioxidative protection in acute lung injury. Stem Cells. 2018;36:616‐625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen B, Wang X, Zhao W, Wu J. Klotho inhibits growth and promotes apoptosis in human lung cancer cell line A549. J Exp Clin Cancer Res. 2010;29:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen B, Ma X, Liu S, Zhao W, Wu J. Inhibition of lung cancer cells growth, motility and induction of apoptosis by Klotho, a novel secreted Wnt antagonist, in a dose‐dependent manner. Cancer Biol Ther. 2012;13:1221‐1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang Y, Chen L, Huang G, et al. Klotho sensitizes human lung cancer cell line to cisplatin via PI3k/Akt pathway. PLoS ONE. 2013;8:e57391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gao W, Yuan C, Zhang J, et al. Klotho expression is reduced in COPD airway epithelial cells: effects on inflammation and oxidant injury. Clin Sci (Lond). 2015;129:1011‐1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shin IS, Shin HK, Kim JC, Lee MY. Role of Klotho, an antiaging protein, in pulmonary fibrosis. Arch Toxicol. 2015;89:785‐795. [DOI] [PubMed] [Google Scholar]
- 25. Li L, Wang Y, Gao W, et al. Klotho reduction in alveolar macrophages contributes to cigarette smoke extract‐induced inflammation in chronic obstructive pulmonary disease. J Biol Chem. 2015;290:27890‐27900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ibi T, Usuda J, Inoue T, Sato A, Takegahara K. Klotho expression is correlated to molecules associated with epithelial‐mesenchymal transition in lung squamous cell carcinoma. Oncol Lett. 2017;14:5526‐5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim SJ, Cheresh P, Eren M, et al. Klotho, an antiaging molecule, attenuates oxidant‐induced alveolar epithelial cell mtDNA damage and apoptosis. Am J Physiol Lung Cell Mol Physiol. 2017;313:L16‐L26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li L, Zhang M, Zhang L, Cheng Y, Tu X, Lu Z. Klotho regulates cigarette smoke‐induced autophagy: implication in pathogenesis of COPD. Lung. 2017;195:295‐301. [DOI] [PubMed] [Google Scholar]
- 29. Chen B, Liang Y, Chen L, et al. Overexpression of Klotho inhibits HELF fibroblasts SASP‐related protumoral effects on non‐small cell lung cancer cells. J Cancer. 2018;9:1248‐1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Krick S, Grabner A, Baumlin N, et al. Fibroblast growth factor 23 and Klotho contribute to airway inflammation. Eur Respir J. 2018;52:1800236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blake DJ, Reese CM, Garcia M, Dahlmann EA, Dean A. Soluble extracellular Klotho decreases sensitivity to cigarette smoke induced cell death in human lung epithelial cells. Toxicol In Vitro. 2015;29:1647‐1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hu MC, Shi M, Zhang J, et al. Renal production, uptake, and handling of circulating alphaKlotho. J Am Soc Nephrol. 2016;27:79‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lindberg K, Amin R, Moe OW, et al. The kidney is the principal organ mediating klotho effects. J Am Soc Nephrol. 2014;25:2169‐2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hu MC, Shi M, Zhang J, et al. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 2010;24:3438‐3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kurosu H, Yamamoto M, Clark JD, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829‐1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kato Y, Arakawa E, Kinoshita S, et al. Establishment of the anti‐Klotho monoclonal antibodies and detection of Klotho protein in kidneys. Biochem Biophys Res Commun. 2000;267:597‐602. [DOI] [PubMed] [Google Scholar]
- 37. Hu MC, Shi M, Gillings N, et al. Recombinant alpha‐Klotho may be prophylactic and therapeutic for acute to chronic kidney disease progression and uremic cardiomyopathy. Kidney Int. 2017;91:1104‐1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ravikumar P, Menon JU, Punnakitikashem P, et al. Nanoparticle facilitated inhalational delivery of erythropoietin receptor cDNA protects against hyperoxic lung injury. Nanomedicine. 2016;12:811‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Menon JU, Ravikumar P, Pise A, Gyawali D, Hsia CC, Nguyen KT. Polymeric nanoparticles for pulmonary protein and DNA delivery. Acta Biomater. 2014;10:2643‐2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fan Y, Liu W, Bi R, et al. Interrelated role of Klotho and calcium‐sensing receptor in parathyroid hormone synthesis and parathyroid hyperplasia. Proc Natl Acad Sci USA. 2018;115:E3749‐E3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Clinton SM, Glover ME, Maltare A, et al. Expression of klotho mRNA and protein in rat brain parenchyma from early postnatal development into adulthood. Brain Res. 2013;1527:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wolf I, Levanon‐Cohen S, Bose S, et al. Klotho: a tumor suppressor and a modulator of the IGF‐1 and FGF pathways in human breast cancer. Oncogene. 2008;27:7094‐7105. [DOI] [PubMed] [Google Scholar]
- 43. Li SA, Watanabe M, Yamada H, Nagai A, Kinuta M, Takei K. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct. 2004;29:91‐99. [DOI] [PubMed] [Google Scholar]
- 44. Takeshita K, Fujimori T, Kurotaki Y, et al. Sinoatrial node dysfunction and early unexpected death of mice with a defect of klotho gene expression. Circulation. 2004;109:1776‐1782. [DOI] [PubMed] [Google Scholar]
- 45. Hu MC, Shi M, Cho HJ, et al. Klotho and phosphate are modulators of pathologic uremic cardiac remodeling. J Am Soc Nephrol. 2015;26:1290‐1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cheng L, Zhang L, Yang J, Hao L. Activation of peroxisome proliferator‐activated receptor gamma inhibits vascular calcification by upregulating Klotho. Exp Ther Med. 2017;13:467‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu L, Liu Y, Zhang Y, et al. High phosphate‐induced downregulation of PPARgamma contributes to CKD‐associated vascular calcification. J Mol Cell Cardiol. 2018;114:264‐275. [DOI] [PubMed] [Google Scholar]
- 48. Nakahara T, Kawai‐Kowase K, Matsui H, et al. Fibroblast growth factor 23 inhibits osteoblastic gene expression and induces osteoprotegerin in vascular smooth muscle cells. Atherosclerosis. 2016;253:102‐110. [DOI] [PubMed] [Google Scholar]
- 49. Mencke R, Harms G, Mirković K, et al. Membrane‐bound Klotho is not expressed endogenously in healthy or uraemic human vascular tissue. Cardiovasc Res. 2015;108:220‐231. [DOI] [PubMed] [Google Scholar]
- 50. Yamada S, Giachelli CM. Vascular calcification in CKD‐MBD: roles for phosphate, FGF23, and Klotho. Bone. 2017;100:87‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chang JR, Guo J, Wang Y, et al. Intermedin1‐53 attenuates vascular calcification in rats with chronic kidney disease by upregulation of alpha‐Klotho. Kidney Int. 2016;89:586‐600. [DOI] [PubMed] [Google Scholar]
- 52. Hu MC, Shi M, Zhang J, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22:124‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hu MC, Kuro‐o M, Moe OW. alphaKlotho and vascular calcification: an evolving paradigm. Curr Opin Nephrol Hypertens. 2014;23:331‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yamamoto M, Clark JD, Pastor JV, et al. Regulation of oxidative stress by the anti‐aging hormone klotho. J Biol Chem. 2005;280:38029‐38034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hu MC, Kuro‐o M, Moe OW. Renal and extrarenal actions of Klotho. Semin Nephrol. 2013;33:118‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cha SK, Hu MC, Kurosu H, Kuro‐o M, Moe O, Huang CL. Regulation of renal outer medullary potassium channel and renal K(+) excretion by Klotho. Mol Pharmacol. 2009;76:38‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Semba RD, Moghekar AR, Hu J, et al. Klotho in the cerebrospinal fluid of adults with and without Alzheimer's disease. Neurosci Lett. 2014;558:37‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vadakke Madathil S, Coe LM, Casu C, Sitara D. Klotho deficiency disrupts hematopoietic stem cell development and erythropoiesis. Am J Pathol. 2014;184:827‐841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tang X, Wang Y, Fan Z, et al. Klotho: a tumor suppressor and modulator of the Wnt/beta‐catenin pathway in human hepatocellular carcinoma. Lab Invest. 2016;96:197‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shi M, Flores B, Gillings N, et al. alphaKlotho mitigates progression of AKI to CKD through activation of autophagy. J Am Soc Nephrol. 2016;27:2331‐2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kusaba T, Okigaki M, Matui A, et al. Klotho is associated with VEGF receptor‐2 and the transient receptor potential canonical‐1 Ca2+ channel to maintain endothelial integrity. Proc Natl Acad Sci USA. 2010;107:19308‐19313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kuro‐o M. Klotho as a regulator of oxidative stress and senescence. Biol Chem. 2008;389:233‐241. [DOI] [PubMed] [Google Scholar]
- 63. Hu MC, Shi M, Cho HJ, et al. The erythropoietin receptor is a downstream effector of Klotho‐induced cytoprotection. Kidney Int. 2013;84:468‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nagai R, Saito Y, Ohyama Y, et al. Endothelial dysfunction in the klotho mouse and downregulation of klotho gene expression in various animal models of vascular and metabolic diseases. Cell Mol Life Sci. 2000;57:738‐746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Saito Y, Nakamura T, Ohyama Y, et al. In vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochem Biophys Res Commun. 2000;276:767‐772. [DOI] [PubMed] [Google Scholar]
- 66. Krick S, Baumlin N, Aller SP, et al. Klotho inhibits interleukin‐8 secretion from cystic fibrosis airway epithelia. Sci Rep. 2017;7:14388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Usuda J, Ichinose S, Ishizumi T, et al. Klotho predicts good clinical outcome in patients with limited‐disease small cell lung cancer who received surgery. Lung Cancer. 2011;74:332‐337. [DOI] [PubMed] [Google Scholar]
- 68. Han X, Li L, Yang J, King G, Xiao Z, Quarles LD. Counter‐regulatory paracrine actions of FGF‐23 and 1,25(OH)2 D in macrophages. FEBS Lett. 2016;590:53‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. The Human Protein Atlas. https://www.proteinatlas.org/ENSG00000133116-KL/tissue/lung.
- 70. Cooper DN, Berg LP, Kakkar VV, Reiss J. Ectopic (illegitimate) transcription: new possibilities for the analysis and diagnosis of human genetic disease. Ann Med. 1994;26:9‐14. [DOI] [PubMed] [Google Scholar]
- 71. Wimmer K, Eckart M, Rehder H, Fonatsch C. Illegitimate splicing of the NF1 gene in healthy individuals mimics mutation‐induced splicing alterations in NF1 patients. Hum Genet. 2000;106:311‐313. [DOI] [PubMed] [Google Scholar]
- 72. Mencke R, Harms G, Moser J, et al. Human alternative Klotho mRNA is a nonsense‐mediated mRNA decay target inefficiently spliced in renal disease. JCI Insight. 2017;2(20):e94375 10.1172/jci.insight.94375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Neyra JA, Moe OW, Pastor J, et al. Performance of soluble Klotho assays in clinical samples of kidney disease. Clin Kidney J. 2019:1‐10. 10.1093/ckj/sfz085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Popper K. The logic of scientific discovery. Abingdon‐on‐Thames: Routledge; 1959. [Google Scholar]


