Abbreviations
- 2D/3D
two/three‐dimensional
- AUROC
area under the receiver operating characteristic curve
- F2
fibrosis stage 2
- MRE
magnetic resonance elastography
- MRI‐PDFF
magnetic resonance imaging–proton density fat fraction
- NAS
nonalcoholic fatty liver disease activity score
- NASH
nonalcoholic steatohepatitis
Nonalcoholic steatohepatitis (NASH) has become the most common cause of chronic liver disease in the United States and the European Union, the leading cause of liver transplant in women, and the second leading cause of transplant in men.1, 2 NASH is the advanced form of nonalcoholic fatty liver disease (NAFLD), the dominant feature of which is steatosis.3 In NASH, steatosis is accompanied by inflammation and hepatocyte injury in the form of ballooning, which can lead to fibrosis. Patients with NASH with stage 2 fibrosis (F2) or higher have worse clinical outcomes than those with no or mild fibrosis. The association of poorer prognosis with fibrosis has led to a shift from emphasis on treating the entire NASH spectrum to focusing on NASH with at least F2 fibrosis. F2 fibrosis has also become the main criterion for entry into phase 3 clinical trials of NASH.3 Emphasis on fibrosis in NASH also is a feature of a recent white paper from the U.S. Food and Drug Administration in which the approval of NASH drugs has required meeting two main histologic outcomes from phase 3 trials: either resolution of NASH without worsening fibrosis or improvement of fibrosis by one stage without worsening of NASH (Fig. 1).3 On the other hand, early phase trials, especially phase 2a trials, have adopted changes in noninvasive tests, especially in magnetic resonance imaging–proton density fat fraction (MRI‐PDFF) as primary outcome, and liver enzymes, inflammatory and fibrosis biomarkers, and magnetic resonance elastography (MRE) changes as secondary outcomes4 MRI techniques have rapidly become the preferred methods for evaluating NASH because of the invasive nature and poor patient acceptance of liver biopsy and increasingly evident advantages of MRI. MRI can accurately assess the degree of steatosis and fibrosis (cross‐sectional); show dynamic changes within a relatively short time (which support the proof‐of‐concept of action of many agents, especially those that target steatosis); and potentially detect changes in steatosis at a minimum and possibly also of steatohepatitis and fibrosis (the last by using MRE). They are also accurate in patients who are overly obese.5
Figure 1.
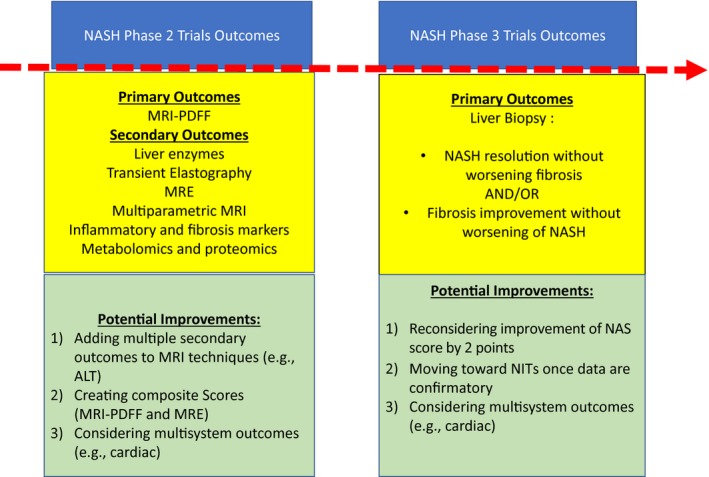
Primary outcomes of NASH phase 2 and phase 3 clinical trials. Abbreviations: ALT, alanine aminotransferase; NIT, non invasive testing.
Biomarkers are usually categorized based on: (a) diagnostic ability, (b) monitoring ability, (c) prognostic ability, and (d) ability to assess response to pharmacologic treatment. As mentioned, MRI‐PDFF and MRE are accurate in diagnosing and assessing the severity of NASH, and their ability to detect longitudinal changes (monitoring) is being investigated. Data from paired liver biopsy and MRI techniques support the use of MRI. A proof‐of‐concept single‐center study found that a 29% reduction in liver fat, as seen on MRI‐PDFF, was associated with a 2‐point decrease in the NAFLD activity score (NAS).6 However, in a recent secondary analysis of 54 patients collected from multiple centers where selonsertib was tested for 24 weeks, MRI‐PDFF changes showed an area under the receiver operating characteristic curve (AUROC) of 0.70 for predicting a 2‐point decrease in the NAS (P = 0.08), while that of two‐dimensional (2D) MRE was 0.66.7 Also, a recent analysis of pooled data found that MR spectroscopy changes correlated with changes in steatosis but not with changes in resolution of NASH or a decrease in the NAS by 2 points.8 MR spectroscopy changes also did not correlate with changes in inflammation, ballooning, or fibrosis. It was also found that only those patients who had resolution of steatosis had improvement (modest) in lobular inflammation. In contrast, a recent, randomized, controlled trial of MGL‐3196 (a thyroid hormone receptor B) versus placebo for 36 weeks found that in patients who received MGL‐3196, MRI‐PDFF (≥30% fat reduction) at week 12 predicted resolution of NASH at week 36 (P = 0.001). MGL‐3196 PDFF responders were also more likely to have a reduction in other components of NASH (ballooning, inflammation).9
In a recent work from a cohort of patients who had bariatric surgery, Allen et al.5 used combined 3D‐MRE measurement of shear stiffness at 60 Hz, damping ratio at 40 Hz, and MRI‐PDFF techniques (called multiparametric MRE) to try to improve detection of the NAS. The authors detected NASH (AUROC, 0.73) and NAS (AUROC, 0.82) by 3D‐MRE compared with detecting NASH (AUROC, 0.61) and NAS (AUROC, 0.64) by 2D‐MRE in the same cohort.5 These differences in results with the two MRI techniques are plausible, as combining techniques may detect more components of the histologic features than would a single technique alone (MRI‐PDFF mainly detects steatosis, whereas MRE detects fibrosis and possibly ballooning and inflammation).
In this issue of Hepatology Communications, Allen et al.10 followed up on their use of multiparametric MRE in a longitudinal study of a small cohort of patients who had bariatric surgery who had lost weight (median body mass index decrease from 45 to 32 kg/m2) and underwent baseline and 1‐year liver biopsy as well as multiparametric MRE. The authors found a stronger correlation between changes in the multiparametric MRE and NAS (r = 0.73, P < 0.001) than in MRI‐PDFF (r = 0.69, P < 0.001) or MRE correlations (r = 0.43, P = 0.009) alone. They proposed that multiparametric MRE is more accurate than MRI‐PDFF as a biomarker to monitor longitudinal changes in response to pharmacologic therapy or life‐style intervention of NASH. However, the study had several limitations: (1) the bariatric surgery cohort may not represent other patients with NASH; (2) the cohort included few patients with NASH (only 13 had NASH and 7 had a NAS of 4 or more), so the cohort did not represent the current targeted populations in clinical trials (NAS ≥4 and ≥F2); (3) the relationships between the proposed technique and each histologic component of NASH (steatosis, lobular inflammation, and ballooning) were not reported; and (4) the study lacked a placebo group for comparison. Because of these problems, the study does not support wide application of multiparametric MRE in NASH clinical trials, especially with the limited availability of 3D MREs compared with that of 2D MREs. Nevertheless, use of this multiparametric MRE in NASH has promise, and this innovative approach deserves critical analysis in large‐scale studies. The study by Allen and colleagues is noteworthy because it is one of few longitudinal studies with paired biopsies, MRI‐PDFF, and MRE techniques in a cohort that showed improvement in histology. Moreover, it was performed by an experienced center in advanced imaging techniques in NASH.
The ideal noninvasive tests in NASH, mainly MRI techniques, will have high precision that accurately reflects the primary outcomes required for drug approval (NASH resolution without worsening fibrosis or with improvement in fibrosis without worsening NASH). This precision will give greater confidence in using it in phase 3 trials. On the other hand, use of the current histologic outcomes may change, especially because resolution of NASH is difficult to achieve. For instance, a decrease of the NAS by 2 points has been recently shown to correlate with improvement in fibrosis and thus might be reconsidered an outcome,11 and multisystem outcomes (such as cardiac outcomes) have been proposed. Nevertheless, the endpoints and outcomes for phase 3 trials are beyond the scope of discussion of this editorial.
The available data on MRI‐PDFF and MRE call for awareness of the pros and cons of using MRI findings as primary outcomes in early phase trials. While more data are emerging and optimization of these techniques is ongoing, it is crucial to understand their current benefits and which research should be conducted. Approaches that might be used to enhance these outcomes are the following:
Enrich early phase trials with secondary outcomes, such as alanine aminotransferase changes or changes in other inflammatory and fibrosis markers. Data from clinical trials and weight‐loss cohorts have indicated that changes in these clinical markers correlate with histologic response.12
Conduct larger studies to assess the performance of MRI‐PDFF and MRE and their correlation with histologic changes, preferably in randomized controlled trials. An example of these trials is the analysis of MRI data from the trials of farnesoid X nuclear receptor ligand obeticholic acid and MGL‐3196 for treatment of noncirrhotic NASH.
Consider combining other techniques with MRI‐PDFF and MRE to create “composite scores,” which may improve prediction of histologic changes. An example could be the combining of serum alanine aminotransferase values with MRI‐PDFF and MRE findings because normalization of the enzyme values has correlated with histologic improvement.12
In conclusion, MRI techniques can accurately diagnose and stage NASH and are useful in monitoring disease response. Large studies are ongoing to enrich their longitudinal performance in monitoring response of the disease to treatments. Given the advances in drug development for NASH, the MRI techniques and their utility should be interpreted based on the available literature while results from ongoing studies are awaited.
Potential conflict of interest: Dr. Alkhouri is on the speakers’ bureau for Intercept and Gilead; he advises Intercept, Gilead, Allergan, and Pfizer; he has received grants from Intercept, Gilead, Genfit, Allergan, Galmed, and Madrigal. Dr. Mazen Noureddin owns stock in Anaetos and Viking; he is on the speakers’ bureau for Abbott and Echosens; he advises Abbott, Gilead, Novartis, Allergan, Intercept, Pfizer, Blade, Echosens North America, OWL, Terns, Roche, and Siemens; he has received grants from Gilead, Novartis, Allergan, BMS, Galmed, Galectin, Genfit, Emanta, Shire, and Zydus. Dr. Schattenberg has received grants from Gilead; he consults for Gilead, BMS, Boehringer Ingelheim, Echosens, Galmed, Genfit, Intercept, IQVIA, Madrigal, and Novartis. Dr. Nabil Noureddin has nothing to report.
See Article on Page https://doi.org/10.1002/hep4.1446.
References
Author names in bold designate shared co‐first authorship.
- 1. Setiawan VW, Stram DO, Porcel J, Lu SC, Le Marchand L, Noureddin M. Prevalence of chronic liver disease and cirrhosis by underlying cause in understudied ethnic groups: the multiethnic cohort. Hepatology 2016;64:1969‐1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Noureddin M, Vipani A, Bresee C, Todo T, Kim IK, Alkhouri N, et al. NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic and gender variances. Am J Gastroenterol 2018;113:1649‐1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sanyal AJ. Past, present and future perspectives in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol 2019;16:377‐386. [DOI] [PubMed] [Google Scholar]
- 4. Younossi ZM, Loomba R, Anstee QM, Rinella ME, Bugianesi E, Marchesini G, et al. Diagnostic modalities for nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and associated fibrosis. Hepatology 2018;68:349‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Allen AM, Shah VH, Therneau TM, Venkatesh SK, Mounajjed T, Larson JJ, et al. The role of three‐dimensional magnetic resonance elastography in the diagnosis of nonalcoholic steatohepatitis in obese patients undergoing bariatric surgery. Hepatology 2018. 10.1002/hep.30483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patel J, Bettencourt R, Cui J, et al. Association of noninvasive quantitative decline in liver fat content on MRI with histologic response in nonalcoholic steatohepatitis. Therap Adv Gastroenterol 2016;9:692‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jayakumar S, Middleton MS, Lawitz EJ, Mantry PS, Caldwell SH, Arnold H, et al. Longitudinal correlations between MRE, MRI‐PDFF, and liver histology in patients with non‐alcoholic steatohepatitis: analysis of data from a phase II trial of selonsertib. J Hepatol 2019;70:133‐141. [DOI] [PubMed] [Google Scholar]
- 8. Bril F, Barb D, Lomonaco R, Lai J, Cusi K. Change in hepatic fat content measured by MRI does not predict treatment‐induced histological improvement of steatohepatitis. J Hepatol 2019; pii:S0168‐8278(19)30584‐7. [DOI] [PubMed] [Google Scholar]
- 9. Harrison SA, Bashir MR, Guy CD, Zhou R, Moylan CA, Frias JP, et al. Resmetirom (MGL‐3196) for the treatment of non‐alcoholic steatohepatitis: a multicentre, randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet 2019;394:2012–2024. [DOI] [PubMed] [Google Scholar]
- 10. Alina M, Allen AM, Shah VH, Therneau TM, Venkatesh SK, Mounajjed T, et al. Multiparametric magnetic resonance elastography improves the detection of NASH regression following bariatric surgery. Hepatol Commun 2020;4:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brunt EM, Kleiner DE, Wilson LA, Sanyal AJ, Neuschwander‐Tetri BA; Nonalcoholic Steatohepatitis Clinical Research Network. Improvements in histologic features and diagnosis associated with improvement in fibrosis in nonalcoholic steatohepatitis: results from the Nonalcoholic Steatohepatitis Clinical Research Network treatment trials. Hepatology 2019;70:522‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vilar‐Gomez E, Calzadilla‐Bertot L, Friedman SL, Gra‐Oramas B, Gonzalez‐Fabian L, Lazo‐Del Vallin S, et al. Serum biomarkers can predict a change in liver fibrosis 1 year after lifestyle intervention for biopsy‐proven NASH. Liver Int 2017;37:1887‐1896. [DOI] [PubMed] [Google Scholar]


