Abstract
Biologics are among the most commonly prescribed medications for several chronic inflammatory diseases. Tumor necrosis factor alpha inhibitors, more so than other agents, have been observed to cause drug‐induced liver injury. Additionally, because the approval and popularity of checkpoint inhibitors have grown, similar patterns of liver injury have been documented, with a majority of cases describing immune‐mediated hepatitis. Although the exact mechanism of injury is unknown, various host and medication characteristics play a role in the outcome of the molecular cascade invoked by biologics. Prognosis is usually favorable with cessation of the offending agent, but cases of acute liver failure requiring liver transplantation have also been observed. Therefore, algorithms have been created to assist clinicians in treating drug‐induced autoimmune hepatitis, mostly with corticosteroids. Additionally, case reports have documented successfully rechallenging patients with a different biologic without recurrence of liver injury, but data are limited. Further investigation is warranted regarding the potential for cross‐reactivity and mechanism of injury to develop guidelines to aid clinicians in further management of these patients.
Abbreviations
- AIH
autoimmune hepatitis
- ALF
acute liver failure
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- ANA
antinuclear antibody
- AST
aspartate aminotransferase
- CD
cluster of differentiation
- CTLA‐4
cytotoxic T lymphocyte antigen 4
- DILI
drug‐induced liver injury
- FDA
U.S. Food and Drug Administration
- HBc
hepatitis B core antigen
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- IBD
inflammatory bowel disease
- IgG
immunoglobulin G
- IL
interleukin
- PD‐1
programmed cell death receptor 1
- PD‐L1
programmed cell death ligand 1
- RA
rheumatoid arthritis
- TNF‐α
tumor necrosis factor alpha
- ULN
upper limit of normal
A 63‐year‐old African American man with ulcerative colitis (UC) presented to the clinic with persistently elevated liver enzymes after receiving three doses of infliximab 5 mg/kg (470 mg/dose) due to multiple UC flare‐ups. His presenting bilirubin was 16 mg/dL (normal <1.2 mg/dL), alkaline phosphatase (ALP) 464 U/L (<115 U/L), alanine aminotransferase [ALT] 1,164 U/L (<55 U/L), and aspartate aminotransferase (AST) 896 U/L (<34 U/L). At follow‐up visits, his aminotransferases stabilized but his bilirubin continued to uptrend. Initial work up for etiology of the liver injury was negative, including a negative antinuclear antibody (ANA) and smooth muscle actin and normal immunoglobulin G (IgG). A liver biopsy showed cholestatic hepatitis with patchy lobular necrosis, ductopenia with marked duct injury, and mild steatosis without fibrosis.
He was admitted to the hospital 2 weeks later with a bilirubin of 55.3 mg/dL and a Model for End‐Stage Liver Disease score of 38, consistent with subfulminant liver failure. He underwent a liver transplantation 14 weeks after his first dosage of infliximab. The explanted liver pathology showed severe lobular cholestasis with patchy hepatocyte necrosis with severe bile duct injury as well as patchy bile duct loss (Fig. 1). No fibrosis was identified. The extrahepatic and large bile ducts were sampled and did not show evidence of primary sclerosing cholangitis. No florid duct lesions or granulomas that would suggest primary biliary cholangitis were identified. A diagnosis of autoimmune hepatitis (AIH) was unlikely given the lack of positive autoantibodies, prominence of plasma cells, interface activity, and fibrosis along with the presence of marked cholestasis and duct injury/loss with minimal inflammation on the explanted liver. Instead, these findings are consistent with the first published case of infliximab‐induced vanishing bile duct syndrome and subsequent liver failure that required a liver transplantation.1
Figure 1.
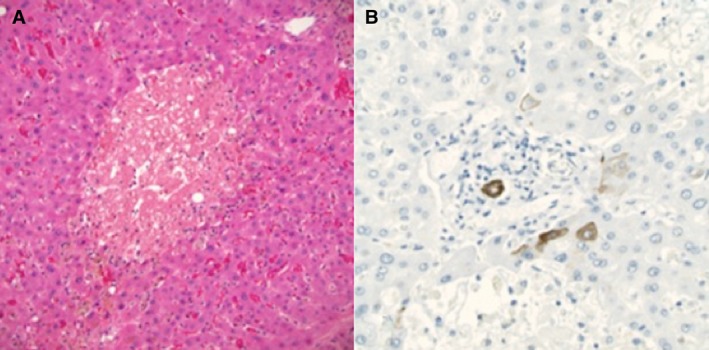
Pathology from the explanted liver, November 2018. (A) Extensive hepatocyte dropout and marked cholestasis with minimal lobular lymphocytic inflammation and no fibrosis. Hematoxylin and eosin stain, magnification ×200. (B) Cytokeratin 7 immunostain (magnification ×400) highlights the ductal epithelium of a severely degenerative interlobular bile duct in a portal area. There is minimal staining of periportal immature hepatocytes. No significant ductular reaction is present, which would also stain with the immunostain, indicating minimal reconstitution of the ducts.
Tumor Necrosis Factor Alpha Inhibitors
Tumor necrosis factor alpha (TNF‐α) is a protein produced by lymphocytes and macrophages that has both beneficial and harmful effects due to its inflammatory, proliferative, apoptotic, and antitumor effects.2 In the 1990s, TNF‐α inhibitors were developed to combat the underlying biologic disease processes seen in rheumatoid arthritis (RA) and Crohn's disease. Infliximab was the first agent to be approved by the U.S. Food and Drug Administration (FDA) in 1998 for the treatment of Crohn’s disease.3, 4 Initially, FDA labels for these TNF‐α inhibitors only included cautions on injection site reactions, headache, nausea, rash, and arthralgias.5 It was not until an FDA postmarketing surveillance program that documented over 130 reports of liver injury from either infliximab or etanercept treatment within 5 years did the labels start to include hepatobiliary adverse effects.6
Although the underlying mechanism of biologic‐induced liver injury remains unknown, TNF‐α inhibitors lead to an abundance of lymphocytes by preventing the normal suppression of B‐cell production and apoptosis of cluster of differentiation (CD)8+ T cells.7 Additionally, TNF‐α acts on two receptors (TNF receptor 1 and 2) to manage opposing effects (Fig. 2). When TNF‐α is inhibited, the balance between effector and regulatory T cells is altered and can result in either liver injury or regeneration. The tipping of the scale depends probably on genetics and the immunologic status of the host.7 Additionally, it is still unclear why infliximab seems to more commonly elicit AIH compared to other TNF‐α inhibitors. Some believe that this might be due to infliximab's mouse–human chimeric antibody make‐up compared to the full human make‐up of the remaining biologics, although this remains speculative.8, 9, 10, 11, 12
Figure 2.
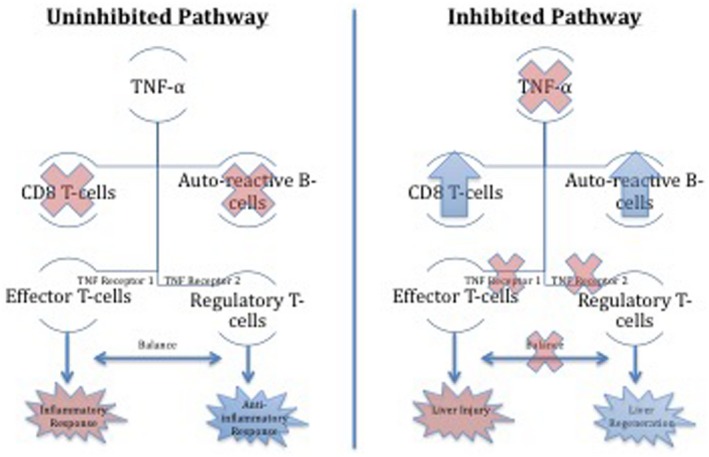
Mechanism of anti‐TNF‐α‐induced liver injury. Inhibition of the TNF‐α pathway disrupts the balance between an inflammatory and anti‐inflammatory response through receptors on T cells. Depending on host characteristics, this imbalance may either lead to liver injury or liver regeneration.
Pharmacokinetics and pharmacodynamics also play a role in the clearance and half‐life of biologics, which impact the possibility of adverse effects. Patients who are predisposed to faster clearance/short half‐life, such as those with severe underlying inflammatory disease, low albumin, or high body weight, may need shorter dose intervals, which might put them at higher risk of drug‐induced liver injury (DILI).13
In this review, we describe the hepatotoxicity associated with biologics, with an emphasis on TNF‐α inhibitors and checkpoint inhibitors. Our purpose is to help clinicians identify potential adverse effects of these biologics on the liver, identify strengths and weakness in current literature, and aid in diagnosis and management if such hepatotoxic events occur.
A bibliographical search was performed in PubMed using the following key words: biologic, TNF inhibitors, drug‐related liver injury, drug‐induced liver injury, liver injury, infliximab, etanercept, adalimumab, golimumab, certolizumab, checkpoint inhibitors, case report, and case series. Reference lists from studies found were also searched for further relevant studies.
All study designs from 2001 to 2018 that suggested a pattern of DILI injury regardless of method of evaluation were included, such as FDA reports, case reports, case series, and literature reviews. These findings are summarized in Table 1.
Table 1.
Types of Biologics and Potential Adverse Effects on the Liver
| Name | Mechanism of Action | Potential Hepatotoxic Effects |
|---|---|---|
| Infliximab | TNF‐α inhibitor (monoclonal mouse–human chimeric antibody) | Hepatocellular and cholestatic patterns of injury, reactivation of hepatitis B, AIH, ALF, vanishing bile duct syndrome |
| Etanercept | TNF‐α inhibitor (human form of TNF‐α receptor fused with IgG) | Hepatocellular and cholestatic patterns of injury, reactivation of hepatitis B, AIH |
| Adalimumab | TNF‐α inhibitor (monoclonal antibody) | Hepatocellular and cholestatic patterns of injury, reactivation of hepatitis B, AIH |
| Certolizumab | TNF‐α inhibitor (Fab fragment of a monoclonal antibody) | Reactivation of hepatitis B |
| Golimumab | TNF‐α inhibitor (human monoclonal immunoglobulin antibody) | Hepatocellular pattern of injury, reactivation of hepatitis B |
| Rituximab | CD20 surface antigen antagonist (chimeric mouse/human monoclonal antibody) | Hepatocellular and cholestatic patterns of injury, reactivation of hepatitis B, AIH, ALF |
| Tocilizumab | IL‐6 receptor antagonist (human monoclonal antibody) | Hepatocellular pattern of injury |
| Anakinra | IL‐1 receptor antagonist (recombinant) | Hepatocellular and biliary pattern of injury, ALF |
| Abatacept | CTLA‐4 antagonist (human fusion protein of the cell‐surface marker CTLA‐4 and immunoglobulin) | Hepatocellular pattern of injury, reactivation of hepatitis B, AIH |
| Ipilimumab | CTLA‐4 blockade checkpoint inhibitor (human monoclonal antibody) | Hepatocellular pattern of injury, immune‐mediated hepatic injury, fibrin ring granulomas |
| Nivolumab | PD‐1 blockade checkpoint inhibitor (human monoclonal antibody) | Hepatocellular and biliary pattern of injury, immune‐mediated hepatic injury |
| Pembrolizumab | PD‐1 blockade checkpoint inhibitor (humanized monoclonal antibody) | Hepatocellular and biliary pattern of injury, immune‐mediated hepatic injury, vanishing bile duct syndrome |
| Cemiplimab | PD‐1 blockade checkpoint inhibitor (monoclonal antibody) | Hepatocellular pattern of injury, immune‐mediated hepatic injury |
| Atezolizumab | PD‐L1 blockade checkpoint inhibitor (humanized monoclonal antibody) | Hepatocellular pattern of injury, immune‐mediated hepatic injury |
| Avelumab | PD‐L1 blockade checkpoint inhibitor (humanized monoclonal antibody) | Hepatocellular pattern of injury, immune‐mediated hepatic injury |
| Durvalumab | PD‐L1 blockade checkpoint inhibitor (humanized monoclonal antibody) | Hepatocellular pattern of injury, immune‐mediated hepatic injury |
Patterns of Liver Injury Related to TNF‐α Inhibitor Use
The FDA released a report in 2003 regarding elevated aminotransferases between 2 and 3 times the upper limit of normal (ULN) during TNF‐α inhibitor treatment, with a prevalence of 37%‐42% compared to 29%‐36% in control groups.14 Reviews since then have reported complications related to TNF‐α inhibitors,15 including instances of hepatitis or cholestasis,16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 acute liver failure (ALF),27, 28, 29, 30, 31 fulminant hepatitis B reactivation,30, 31, 32 induction of autoimmune/immune‐mediated hepatitis,33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44 and even the need for emergent liver transplantation.45, 46 Among 176 patients with inflammatory bowel disease (IBD) treated with infliximab, transaminitis occurred in 36% of patients, of whom 73% of cases had spontaneous resolution even while continuing the medication.26
Of the nine cases of TNF‐α inhibitor‐induced ALF, 8 of the described patients were taking infliximab while the other patient was on adalimumab.27, 45, 47, 48, 49, 50 The latency between initial drug exposure and ALF ranged from 3 days to 1 year. The pattern of liver injury was determined in seven cases, of which four were found to have hepatocellular injury, two with cholestatic injury, and one with mixed injury.47 In three of these nine cases, ANA went from negative before TNF‐α inhibitor initiation to positive during ALF.27, 45, 47, 48, 49, 50 Liver biopsy was performed in seven cases, which showed clear histologic autoimmune features. Six of the nine cases went on to emergent transplantation, with the other three recovering liver function with steroid therapy. Given the wide range of latency, this may suggest an idiosyncratic DILI rather than a dose‐dependent toxicity, which has been described in other studies as well.5, 51, 52
Ghabril et al.23 analyzed 34 cases of DILI related to TNF‐α inhibitors and found the most common presentation to be an autoimmune phenotype with improvement after drug withdrawal.23 In large retrospective studies, mild DILI (defined as ALT >2× ULN and/or ALP>2× ULN) was reported to have a 0.2%‐11% incidence, with the majority implicating infliximab.51, 53, 54 The absolute risk of DILI for patients that were started on infliximab was found in 1 of 120 users, 1 of 270 for adalimumab, and 1 of 430 for etancercept.53 The median duration of TNF‐α inhibitors therapy was 3.3 months for those who developed DILI, mostly after four infusions of infliximab, and this time line was corroborated in a review of 26 cases of infliximab‐induced hepatocellular injury by Ghabril et al.23, 54
The most common form of liver injury related to TNF‐α inhibitors has been shown to be hepatocellular injury with autoantibodies, mostly with positive ANA and some with anti‐smooth muscle antibody, and histologic features consistent with AIH. Development of this pattern of injury has been reported in up to 92% of TNF‐α inhibitor‐induced DILI.5, 23, 51, 54, 55, 56 Additionally, there seems to be a lack of cross‐reactivity between TNF‐α inhibitors, possibly due to the difference in molecular structure of these agents. For instance, a patient with infliximab‐induced hepatocellular injury that resolved after cessation of infliximab was then successfully transitioned to adalimumab without DILI,52 which has also been reported in other studies.23, 52, 54, 57, 58, 59 Additionally, even though ANA can be used to diagnose AIH, positive ANA status before the initiation of therapy did not seem to place the patient at greater risk of developing DILI.54 Out of the patients that developed an AIH‐like picture from TNF‐α inhibitors, at least half of the patients required steroids while the other half recovered after stopping treatment.23, 53, 54
There have also been several case series that demonstrated more severe DILI, defined as a more prolonged cholestasis or acute liver injury.60 One study from Taiwan investigated serious hepatic events, defined as an event requiring hospitalization, and reported 0.75 events per 100 person‐years on adalimumab and 0.39 events per 100 person‐years on etanercept.61 Another study described eight cases of TNF‐α inhibitor‐induced hepatitis that was defined as ALT >10× ULN out of a cohort of 600 patients, with 7 of them related to infliximab.55
Steatosis and steatohepatitis have also been reported in patients taking TNF‐α blockers, which can improve once treatment has been stopped.62, 63 Another study of 48 patients with psoriatic arthritis with hepatic steatosis before initiation of TNF‐α inhibitor therapy found worsening hepatic steatosis in those with persistent symptoms.64 Although the mechanisms of TNF‐α inhibitor‐induced nonalcoholic fatty liver disease is unclear, more patatin‐like phospholipase domain containing 3 (PNPLA3) polymorphism I148M, a DNA sequence associated with increased hepatic fat level and inflammation, was found in patients who developed liver test abnormalities compared to those who did not.62
Specific TNF‐α Inhibitors
Infliximab
Infliximab, a monoclonal mouse–human chimeric antibody to TNF‐α is currently used for IBD and RA. Although the overall risk of DILI with TNF‐α inhibitors is low, there have been a disproportionate amount of liver injury reports related to infliximab compared to other drugs of the same class.47 When AST and ALT elevations develop, this usually occurs after two to five infusions and can be transient and asymptomatic but do not always improve after cessation of infliximab.61 A hepatocellular pattern of injury is often seen along with induction of antibodies, including ANA, smooth muscle antibody, and anti‐double‐stranded DNA. Lupus‐like symptoms or AIH usually accompany these antibodies. Liver biopsy often shows interface hepatitis, focal liver cell necrosis, and intense mononuclear cell infiltrates that can include plasma cells.
Multiple studies have shown that 64%‐80% of patients with infliximab‐induced AIH did not improve with cessation of the drug alone and required steroids.61, 65 Patients have usually recovered after treatment, and relapse was not observed after completing a course of steroids.65 There have also been documented cases of cholestatic forms of liver injury ranging from within a few days to 24 weeks after initiating therapy with infliximab.16
Etanercept
Liver injury related to etanercept is less well documented compared to infliximab. It is a human form of TNF‐α receptor fused with IgG used to treat rheumatologic conditions. Transient low elevations of ALT have been documented along with cholestatic and hepatocellular patterns of injury.12 Similarly to infliximab, induction of AIH and ANA autoantibodies has been noted. Latency has been reported to be as short as 2 weeks to as long as several years from initial dose of etanercept.12
Two cases have described etanercept‐induced AIH.66, 67 One patient had systemic‐onset juvenile idiopathic arthritis, and the other had RA with Sjogren’s syndrome. They were found to have elevated AST/ALT, total bilirubin, and gamma‐glutamyl transpeptidase (GGT) along with positive antibodies. Liver biopsies showed extensive portal and periportal interface lymphoplasmacytic infiltration and panlobular hepatitis along with single‐cell necrosis, rosette formation, and collections of syncytial multinucleated hepatocytes. Both patients were diagnosed with drug‐induced AIH and responded well to prednisone after discontinuing etanercept.66, 67
Adalimumab
Adalimumab is a monoclonal antibody used to treat RA and IBD. Although it seems that adalimumab is less frequently associated with liver injury than infliximab, it has also been associated with transient and mild serum aminotransferase elevations, reactivation of hepatitis B virus (HBV), AIH, and even cholestatic liver injury.12 Two cases have been reported of patients developing drug‐induced AIH within 2‐3 months of initiation of therapy.68, 69 One patient was treated for psoriatic arthritis and Crohn’s disease, while the other was treated for RA. Both experienced elevated liver enzymes, positive ANA, and elevated IgG, with liver biopsies showing portal fibrosis, periportal lymphocytic infiltrates, and interface hepatitis. Both patients had an AIH score of 7, consistent with a definite diagnosis of AIH.68, 69
Adalimumab was also demonstrated to cause cholestatic liver injury in a patient with chronic hepatitis C virus and RA who was treated with adalimumab due to RA reactivation.70 At the third month of treatment, the patient began having pruritus with liver function tests showing elevations in GGT, ALP, and AST. A liver biopsy showed portal dystrophy, portal inflammation with eosinophils, lobular hepatitis, and microabscesses.70
Certolizumab and Golimumab
Certolizumab is a humanized recombinant Fab fragment of a monoclonal antibody to TNF‐α; it is used to treat RA and IBD and has been associated with a low rate of transaminitis, similar to the rate found with placebo therapy.12 Although it is speculated that, similar to other TNF‐α inhibitors, it may cause reactivation of HBV, no cases have been documented thus far. Golimumab is a human monoclonal immunoglobulin antibody to TNF‐α and is similarly used to treat RA and IBD. In controlled trials, serum ALT elevations occurred in up to 8% of patients versus 1%‐3% of subjects on placebo. These elevations were mostly asymptomatic and self‐limited. Only rarely did patients have to stop therapy because of elevations above 5 times the ULN.12 There have been no published cases thus far of acute liver injury due to golimumab.
Other Biologics
CD20 Surface Antigen Antagonists
Rituximab, a chimeric mouse/human monoclonal antibody to the CD20 surface antigen on pre‐B and mature B lymphocytes, has been approved for treating RA along with lymphoma and leukemia. A majority of documented adverse reactions have described a self‐limited hepatocellular pattern of injury in 10%‐15% of patients but often resolve even with continuing therapy.71 One case also describes an episode of cholestatic liver injury after being treated with rituximab.72 Rituximab is known to cause reactivation of HBV and can rarely cause ALF.73
Notably, rituximab has also been used for treatment of refractory AIH. In a pilot study from Canada, 6 patients with refractory AIH (due to persistent disease or intolerable side effects from prednisone or azathioprine) were treated with rituximab with success.74 Transaminases and IgG levels decreased with liver biopsies after 1 year, showing improvement in inflammation as well. These findings were corroborated with case reports as well as single‐center studies.74
Interleukin‐6 Receptor Antagonists
Tocilizumab is a humanized monoclonal antibody to the interleukin (IL)‐6 receptor that has been used for RA. A hepatocellular pattern of liver injury has been documented in several case reports, with liver biopsies showing focal necrosis with steatosis and early fibrosis with other etiologies of injury excluded.75, 76 In one case, tocilizumab was continued given normalization of liver enzymes and remained stable at 6 months of follow‐up. In another case, treatment was discontinued and N‐acetylcysteine was given until normalization of enzymes. There has also been a case describing ALF requiring liver transplant in a patient who was treated with tocilizumab for RA.77 Along with an otherwise negative work up, the explanted liver showed liver necrosis with ongoing cell death and no signs of proliferating hepatocytes, which suggests a possible medication‐induced etiology of injury.
IL‐1 Receptor Antagonists
Anakinra is a recombinant IL‐1 receptor antagonist that can be used for treating RA and juvenile idiopathic arthritis but also has been used for treating adult‐onset Still’s disease, given the benefit in reducing liver inflammation. Anakinra has been linked to acute liver injury that mimics acute viral hepatitis, with a hepatocellular pattern of serum enzyme elevations and jaundice, as described in multiple case reports.75, 78, 79 One of these case reports used the Roussel Uclaf Causality Assessment Method evaluation, a scale used to predict whether liver injury can be attributed to a certain medication, which suggested anakinra‐induced liver injury80. Discontinuation of anakinra led to normalization of liver enzymes; other possible etiologies of injury were excluded. Two patients with adult‐onset Still’s disease developed ALF during treatment with anakinra but resolved following withdrawal of the medication.80, 81 Although Still’s disease itself can be associated with the development of hepatitis, the improvement in clinical symptoms and liver injury once the culprit medication was stopped suggested the etiology was due to anakinra. Lastly, three cases of hepatitis in patients treated for juvenile idiopathic arthritis were also thought to be due to anakinra.82 However, given the young age of these patients, there were multiple confounding factors, and this pattern of injury may have been due to a typical childhood infection that presented differently given the altering of the immune response from anakinra.
Biologic Disease‐Modifying Antirheumatic Drug
Abatacept is a selective, costimulation modulator, recombinant human fusion protein of the cell‐surface marker cytotoxic T lymphocyte antigen 4 (CTLA‐4) and modified Fc region of human IgG that interferes with T‐cell activation and is used to treat RA. Abatacept selectively inhibits T‐cell activation by binding to CD80 and CD86. In prelicensure controlled trials, a hepatocellular pattern of injury occurred in about 3% of patients but was asymptomatic and self‐limited. There are risks for reactivation of chronic HBV as well, but one case report documents an instance of drug‐induced AIH that responded well to corticosteroid therapy.83
Checkpoint Inhibitors
This class of medications targets specific points in the cell cycle to increase immune reactivity that aid in treating certain malignancies. Some of these medications block CTLA‐4, others block programmed cell death receptor 1 (PD‐1), and others also block programmed cell death ligand 1 (PD‐L1). Hepatotoxicity has been reported to occur from 2% to 10% of patients within 3‐6 months of starting treatment, with a mixed pattern of injury.84 The first subgroup of the checkpoint inhibitors, which includes ipilimumab, blocks the CTLA‐4 antigen on cytotoxic T lymphocytes. Asymptomatic hepatitis has been seen following treatment, and if detected early, skipping a dose and waiting 3 weeks or more until the next dose can be acceptable if liver tests improve.85 Liver histology has often revealed focal or confluent necrosis with prominent lymphocytic infiltrates of activated T cells, which is consistent with an immune‐mediated hepatic injury.86 In a study of 11 cases of suspected ipilimumab‐associated hepatitis, the distribution was found to be panlobular and resembled AIH.86 Another study assessed the liver injury associated with ipilimumab, and no positive autoantibodies or IgG elevations were observed; stopping the immunotherapy and starting corticosteroids normalized the liver enzymes.87 Although there were some shared features with AIH on liver histology, there were also differences that led the authors to believe that this was a separate idiosyncratic pattern of injury. Fibrin ring granulomas have also been described and are considered pathognomonic for ipilimumab‐induced hepatic injury.84
Other checkpoint inhibitors, including nivolumab, pembrolizumab, and cemiplimab, are PD‐1 antagonists that aim to modulate T‐cell immune reactivity. Five patients treated with nivolumab, similar to ipilimumab, had immune‐mediated liver injury that, after further investigation, was considered to be distinct from AIH.87 A sustained liver injury marked by grade 4 ALT elevation has been reported to be refractory to steroid therapy, but the hepatocellular pattern of injury resolved about 5 months after discontinuation of nivolumab.88 Another patient with worsening liver enzymes after nivolumab treatment for metastatic melanoma had a liver biopsy showing focal ductopenia and periductal fibrosis consistent with a pattern of biliary injury, so treatment was stopped.89 A similar pattern was seen with pembrolizumab, and despite prednisolone for presumed autoimmune‐like hepatitis, bilirubin and ALP continued to worsen, which led to a biopsy and the eventual diagnosis of acute‐onset vanishing bile duct syndrome secondary to pembrolizumab.89 Additionally, a patient with pulmonary metastatic liver cancer was treated with pembrolizumab and despite improvement of metastatic lesions, developed ALF due to immune‐related hepatitis.90 This article also describes a patient with progressive epithelioid mesothelioma who was treated with pembrolizumab but soon developed a cholestatic pattern of injury with ductal loss on biopsy that ceased after the medication was stopped.89
The PD‐L1 inhibitors, such as atezolizumab, avelumab, and durvalumab, increase immune reactivity to tumor neoantigens by blocking PD‐L1 to treat bladder, lung, and metastatic cancers. Similar to other checkpoint inhibitors, immune‐mediated hepatitis along with hepatocellular patterns of injury have been documented.
Combination therapy of the PD‐1 blocking antibody nivolumab and the anti‐CTLA‐4 drug ipilimumab has been reported to result in immune‐related hepatitis, which may be managed with steroids.91, 92 In one study, 25%‐30% all‐grade hepatitis and 15% incidence of grade 3 toxicity were found within the first 6 to 12 weeks after initiation of dual therapy.93 However, 40% of patients who discontinued combination therapy for toxicities experienced recurrent or distinct toxicities with anti‐PD‐1 monotherapy resumption.91 These data suggest that even with dual immune therapy, either ipilimumab or nivolumab may be the primary culprit in driving specific toxicities due to a high rate of 21% of clinically distinct adverse effects following anti‐PD‐1 rechallenge.91 Therefore, the concept of “immune priming” by combination therapy may predispose patients to other toxicities.91, 94
Despite these documented cases of checkpoint inhibitor‐induced DILI, evaluation of other causes of liver injury should be considered. One study involving 491 patients treated with pembrolizumab noted 14% of patients with liver injury, a majority of which was attributed to hepatic metastases rather than DILI; those with liver injury had lower tumor response and higher mortality, likely due to more advanced cancer before treatment. Therefore, thorough assessment for other etiologies of liver injury should be completed to not only assist with diagnosis but also with management regarding appropriate use of immunosuppressive therapy.95
Management of Biologic‐Induced Liver Injury
A previous consensus among experts for the safe use of infliximab in IBD gave recommendations that liver tests should be obtained before initiating treatment, after induction, and every 4 months while on maintenance treatment.46 Additionally, the statement suggests avoiding or discontinuing infliximab therapy in patients found to have a transaminitis >3 times the ULN. However, despite following such recommendations, severe liver injury with ALF has been reported.27 Therefore, a new algorithm has been proposed.
According to these recommendations, the patient should be evaluated for underlying previous liver disease with baseline liver tests along with hepatitis screening. Next is the initiation of the TNF‐α inhibitor therapy followed by rechecking liver tests every 2 weeks for 8 weeks. If they are normal, repeat every 8 weeks for the duration of treatment. However, if these tests are abnormal, monitoring must be done based on which value is above normal limits. If there is an isolated increase of ALT <3 times the ULN without jaundice, recheck the hepatic panel every 2 weeks until resolution. If ALT >3 times the ULN (regardless of any increase in bilirubin or presence of jaundice), it is important to rule out other causes of elevated values and proceed with ultrasound or biopsy based on the resulting differential.26
At this point, if liver tests continue to worsen, the drug should be discontinued. N‐acetyl‐cysteine has been shown to improve transplant‐free survival for patients not only with acetaminophen‐induced ALF but also with non‐acetaminophen ALF; however, patients with liver injury due to biologics have not been included in these studies.96, 97, 98 Corticosteroids have also been administered with better results in patients with DILI or AIH from TNF‐α inhibitor therapy.5, 54, 61 Data from a single‐center retrospective cohort study support these findings and also showed that corticosteroids were most effective for severe liver injury with peak total bilirubin >243 μmol/L.99 Generally, steroids were given after about 1 week in patients with severe DILI with two different schedules.
One option is a corticosteroid titration schedule with a reduction of the daily dose over several weeks (methylprednisolone 60‐120 mg/day or prednisone 40‐60 mg/day for 3‐5 days and then prednisone 20 mg/day tapered by 5‐10 mg weekly thereafter).99 The other option is a corticosteroid pulse therapy using methylprednisolone 60‐120 mg/day for 3‐5 days.99 Steroids not only decreased the mortality rate but also shortened the time duration to recovery.99 Because data show a correlation between higher total bilirubin and the severity of liver disease, early identification of signs of liver failure are important to prevent delay in therapy, especially in those who are prone to developing ALF.100 Usually, corticosteroid therapy is associated with rapid improvement and can be discontinued with resolution of clinical and laboratory signs of injury, which can occur within 3‐6 months of treatment, followed by histologic resolution 3‐8 months later.5, 54, 61, 101
Management of Checkpoint Inhibitor‐Induced Liver Injury
The American Society of Clinical Oncology created a clinical practice guideline to assist clinicians managing immune‐related adverse events.84 Regarding checkpoint inhibitor‐induced hepatitis, the society recommends monitoring AST, ALT, and bilirubin before each infusion and/or weekly if there are any mild liver function test elevations. For patients with grade 1 hepatotoxicity (AST or ALT >ULN to 3× ULN and/or total bilirubin >ULN to 1.5× ULN), clinicians should continue to offer checkpoint inhibitor therapy with close monitoring with the aforementioned tests 1‐2 times weekly while ruling out other etiology and offering supportive symptom control.
If there is grade 2 toxicity (AST or ALT >3 to <5× ULN and/or total bilirubin >1.5 to <3× ULN), therapy should be held temporarily and resumed only if toxicity improves to grade 1 or less.84 Additionally, 0.5 to 1 mg/kg/day prednisone or equivalent should be started if the liver function tests remain elevated with clinical symptoms over the next 3 to 5 days. Frequency of laboratory monitoring should be increased to every 3 days. Checkpoint inhibitor therapy may be resumed if symptoms and liver injury improve to grade 1 or less on ≤10 mg/day of corticosteroid, which should be tapered over at least 1 month.
Grade 3 hepatotoxicity (symptomatic dysfunction, fibrosis found on a liver biopsy, compensated cirrhosis, reactivation of chronic hepatitis, AST or ALT 5‐20× ULN, and/or total bilirubin 3‐10× ULN) should result in permanent discontinuation of checkpoint inhibitors along with immediately starting 1 to 2 mg/kg/day of methylprednisolone or equivalent with a plan to taper over 4 to 6 weeks.84 If there is no improvement in 3 days, mycophenolate mofetil or azathioprine can be offered. Monitoring through laboratory tests should be increased to daily or every other day with possible inpatient monitoring.
Grade 4 hepatotoxicity (decompensated liver function, AST OR ALT >20× ULN, and/or total bilirubin >10× ULN) should be managed by permanently discontinuing checkpoint inhibitor therapy and immediately administering 2 mg/kg/day of methylprednisolone equivalent with a plan to taper over 4 to 6 weeks once symptoms improve to grade 1 or less. However, it has to be acknowledged that these recommendations are not evidence based and hopefully await further studies.
If there is no improvement over 3 days, mycophenolate mofetil should be started as well. Daily laboratory results should be monitored along with the option of inpatient monitoring, and infliximab should be avoided given the risk of development of liver failure.84
Prevention of HBV Reactivation
Reactivation of HBV can be defined as a rise in HBV DNA compared to baseline and reverse seroconversion from hepatitis B surface antigen (HBsAg) negative to HBsAg positive for patients who are HBsAg negative and anti‐hepatitis B core antigen (HBc) positive.102 In moderate to high‐prevalent regions of HBV, HBV testing (HBsAg and anti‐HBc) is recommended before initiation of immunosuppressive therapy.102 Three randomized controlled trials support starting prophylaxis before initiation of immunosuppressive therapy for patients who are HBsAg positive and anti‐HBc positive, given the high risk of reactivation.102 On the other hand, patients who are HBsAg negative but anti‐HBc positive can be monitored closely for reactivation with ALT, HBV DNA, and HBsAg to screen for treatment. The only exceptions would be for patients receiving anti‐CD20 antibody therapy or stem cell transplantation for which prophylaxis is recommended. When indicated, regardless of baseline HBV‐DNA level, prophylactic therapy should be started as soon as possible or at latest at the time of immunosuppressive therapy. Preferred choice of agent is usually a first‐line nucleoside/nucleotide analogue due to multiple meta‐analyses showing reduced reactivation, mortality, and therapy interruption.102 The most commonly studied duration for prophylactic therapy is around 6‐12 months, but because reactivation has been reported past 12 months, further monitoring should be considered for up to 12 months after cessation of anti‐HBV therapy.
Re‐initiation of TNF‐α Therapy
Certain patients who have developed liver injury from one TNF‐α inhibitor have been able to tolerate re‐initiation of TNF‐α inhibitor treatment after resolution of the initial injury.28, 33, 54, 61, 103 For example, 2 patients who had been treated with infliximab for corticosteroid‐dependent IBD developed DILI, but after discontinuing infliximab they were successfully transitioned to other agents. Both patients were successfully started on adalimumab without any signs of cross‐reactivity, and liver tests remained normal for 6 months following initiation.28, 33 Similar cases have also been described with patents being treated for rheumatologic conditions. One patient being treated for seronegative RA with etanercept was switched to adalimumab after a year due to loss of efficacy but was soon diagnosed with adalimumab‐induced AIH.68 After steroid therapy and normalization of liver enzymes after 6 weeks, she was started on abatacept without further elevation of liver enzymes.68 Two other patients with spondyloarthropathy found to have infliximab‐induced liver injury were also successfully transitioned to etanercept without recurrence of original injury.21, 57
Due to limited data available, there is no consensus on when to rechallenge patients with documented episodes of biologic‐induced liver injury. Based on successful case reports of switching agents without recurrence, the common practice has been to stop the offending medication and wait to rechallenge until the patient was asymptomatic with resolution of laboratory signs of injury. This was achieved both by removing the offending agent in some cases and in others with treatment with corticosteroids. There are no current guidelines or recommendations on minimal time needed before initiation after normalization of symptoms and laboratory values.
Conclusions
Biologics remain one of the main treatment alternatives for several chronic inflammatory diseases. TNF‐α inhibitors have been more frequently documented in causing liver injury when assessing its clinical use and safety risk compared with other biologics. There may be various factors that play a role in developing such toxic effects, such as both host and medication characteristics, that may confound findings. Prognosis is favorable with cessation of the offending drug and corticosteroids, specifically in patients with autoimmune features. Recent case reports have also supported rechallenging patients with a different TNF‐α inhibitor after resolution of the initial liver injury that have not resulted in recurrence of the original liver injury.
Although there are cases documenting a successful transitioning and rechallenging from one TNF‐α inhibitor to another without recurrence of the initial observed liver injury, more information is needed to further investigate cross‐reactivity. This may also lead to further discovery regarding the mechanism of action of liver injury secondary to TNF‐α inhibitors given the skewed documentation revealing various forms of infliximab‐induced liver injury.
Potential conflict of interest: Nothing to report.
References
- 1. Shah P, Larson B, Wishingrad M, Nissen N, Bjornsson E, Sundaram V. Now you see it, now you don’t: a case report of infliximab‐induced vanishing bile duct syndrome. ACG Case Rep J 2019;6:e00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aggarwal BB, Gupta SC, Kim JH. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood 2012;119:651‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, et al. A short‐term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med 1997;337:1029‐1035. [DOI] [PubMed] [Google Scholar]
- 4. U.S. Food and Drug Administration . Drug safety information: drug safety resources for healthcare professionals https://www.fda.gov/drugs/information-healthcare-professionals-drugs/drug-safety-information. Published February 22, 2019. Accessed February 2019. [Google Scholar]
- 5. French JB, Bonacini M, Ghabril M, Foureau D, Bonkovsky HL. Hepatotoxicity associated with the use of anti‐TNF‐α agents. Drug Saf 2016;39:199‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. U.S. Food and Drug Administration. Drug Induced Liver Injury Rank (DILIRank) Dataset . https://www.fda.gov/science-research/liver-toxicity-knowledge-base-ltkb/drug-induced-liver-injury-rank-dilirank-dataset. Accessed February 2019.
- 7. Lopetuso LR, Mocci G, Marzo M, D’Aversa F, Rapaccini GL, Guidi L, et al. Harmful effects and potential benefits of anti‐tumor necrosis factor (TNF)‐alpha on the liver. Int J Mol Sci 2018;19:pii: E2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Antoniou C, Dessinioti C, Katsambas A, Stratigos AJ. Elevated triglyceride and cholesterol levels after intravenous antitumour necrosis factor‐alpha therapy in a patient with psoriatic arthritis and psoriasis vulgaris. Br J Dermatol 2007;156:1090‐1091. [DOI] [PubMed] [Google Scholar]
- 9. Spanakis E, Sidiropoulos P, Papadakis J, Ganotakis E, Katsikas G, Karvounaris S, et al. Modest but sustained increase of serum high density lipoprotein cholesterol levels in patients with inflammatory arthritides treated with infliximab. J Rheumatol 2006;33:2440‐2446. [PubMed] [Google Scholar]
- 10. Castro KR, Aikawa NE, Saad CG, Moraes JC, Medeiros AC, Mota LM, et al. Infliximab induces increase in triglyceride levels in psoriatic arthritis patients. Clin Dev Immunol 2011;2011:352686 10.1155/2011/352686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koutroubakis IE, Oustamanolakis P, Malliaraki N, Karmiris K, Chalkiadakis I, Ganotakis E, et al. Effects of tumor necrosis factor alpha inhibition with infliximab on lipid levels and insulin resistance in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol 2009;21:283‐288. [DOI] [PubMed] [Google Scholar]
- 12. Tumor Necrosis Factor Antagonists . LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. www.livertox.nih.gov. Published 2012. Accessed February 2019.
- 13. Mould DR. The pharmacokinetics of biologics: a primer. Dig Dis 2015;33(Suppl. 1):61‐69. [DOI] [PubMed] [Google Scholar]
- 14. U.S. Food and Drug Administration, Center for Drug Evaluation and Research, Arthritis Advisory Committee . https://www.fda.gov/media/99121/download. Accessed February 2019. [Google Scholar]
- 15. Ierardi E, Valle ND, Nacchiero MC, De Francesco V, Stoppino G, Panella C. Onset of liver damage after a single administration of infliximab in a patient with refractory ulcerative colitis. Clin Drug Investig 2006;26:673‐676. [DOI] [PubMed] [Google Scholar]
- 16. Menghini VV, Arora AS. Infliximab‐associated reversible cholestatic liver disease. Mayo Clin Proc 2001;76:84‐86. [DOI] [PubMed] [Google Scholar]
- 17. Soto‐Fernández S, González‐Carro P, De Pedro‐Esteban A, Legaz‐Huidobro ML, Pérez‐Roldán F, Roncero García‐Escribano O, et al. Infliximab‐induced hepatitis in a patient with Crohn’s disease [in Spanish]. Gastroenterol Hepatol 2006;29:321‐322. [DOI] [PubMed] [Google Scholar]
- 18. Wahie S, Alexandroff A, Reynolds NJ. Hepatitis: a rare, but important, complication of infliximab therapy for psoriasis. Clin Exp Dermatol 2006;31:460‐461. [DOI] [PubMed] [Google Scholar]
- 19. Becker H, Willeke P, Domschke W, Gaubitz M. Etanercept tolerance in a patient with previous infliximab‐induced hepatitis. Clin Rheumatol 2008;27:1597‐1598. [DOI] [PubMed] [Google Scholar]
- 20. Kluger N, Girard C, Guillot B, Bessis D. Efficiency and safety of etanercept after acute hepatitis induced by infliximab for psoriasis. Acta Derm Venereol 2009;89:332‐334. [DOI] [PubMed] [Google Scholar]
- 21. Thiéfin G, Morelet A, Heurgué A, Diebold MD, Eschard JP. Infliximab‐induced hepatitis: absence of cross‐toxicity with etanercept. Joint Bone Spine 2008;75:737‐739. [DOI] [PubMed] [Google Scholar]
- 22. Carlsen KM, Riis L, Madsen OR. Toxic hepatitis induced by infliximab in a patient with rheumatoid arthritis with no relapse after switching to etanercept. Clin Rheumatol 2009;28:1001‐1003. [DOI] [PubMed] [Google Scholar]
- 23. Ghabril M, Bonkovsky HL, Kum C, Davern T, Hayashi PH, Kleiner DE, et al.;US Drug‐Induced Liver Injury Network . Liver injury from tumor necrosis factor‐α antagonists: analysis of thirty‐four cases. Clin Gastroenterol Hepatol 2013;11:558‐564.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moum B, Konopski Z, Tufteland KF, Jahnsen J. Occurrence of hepatoxicicty and elevated liver enzymes in a Crohn’s disease patient treated with infliximab. Inflamm Bowel Dis 2007;13:1584‐1586. [DOI] [PubMed] [Google Scholar]
- 25. Poulin Y, Thérien G. Drug‐induced hepatitis and lupus during infliximab treatment for psoriasis: case report and literature review. J Cutan Med Surg 2010;14:100‐104. [DOI] [PubMed] [Google Scholar]
- 26. Rossi RE, Parisi I, Despott EJ, Burroughs AK, O’Beirne J, Conte D, et al. Anti‐tumour necrosis factor agent and liver injury: literature review, recommendations for management. World J Gastroenterol 2014;20:17352‐17359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kinnunen U, Färkkilä M, Mäkisalo H. A case report: ulcerative colitis, treatment with an antibody against tumor necrosis factor (infliximab), and subsequent liver necrosis. J Crohns Colitis 2012;6:724‐727. [DOI] [PubMed] [Google Scholar]
- 28. Haennig A, Bonnet D, Thebault S, Alric L. Infliximabinduced acute hepatitis during Crohn’s disease therapy: absence of cross‐toxicity with adalimumab. Gastroenterol Clin Biol 2010;34:e7‐e8. [DOI] [PubMed] [Google Scholar]
- 29. Caussé S, Bouquin R, Wylomanski S, Flamant M, Joubert M, Dréno B, et al. Infliximab‐induced hepatitis during treatment of vulvar Crohn’s disease [in French]. Ann Dermatol Venereol 2013;140:46‐51. [DOI] [PubMed] [Google Scholar]
- 30. Ostuni P, Botsios C, Punzi L, Sfriso P, Todesco S. Hepatitis B reactivation in a chronic hepatitis B surface antigen carrier with rheumatoid arthritis treated with infliximab and low dose methotrexate. Ann Rheum Dis 2003;62:686‐687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Michel M, Duvoux C, Hezode C, Cherqui D. Fulminant hepatitis after infliximab in a patient with hepatitis B virus treated for an adult onset still’s disease. J Rheumatol 2003;30:1624‐1625. [PubMed] [Google Scholar]
- 32. Esteve M, Saro C, González‐Huix F, Suarez F, Forné M, Viver JM. Chronic hepatitis B reactivation following infliximab therapy in Crohn’s disease patients: need for primary prophylaxis. Gut 2004;53:1363‐1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cravo M, Silva R, Serrano M. Autoimmune hepatitis induced by infliximab in a patient with Crohn’s disease with no relapse after switching to adalimumab. BioDrugs 2010;24(Suppl. 1):25‐27. [DOI] [PubMed] [Google Scholar]
- 34. Arai O, Omoto K, Notohara K, Shibata N, Kuboki M, Ikeda H. A case of infliximab‐related liver damage‐case report and literature review [in Japanese]. Nihon Shokakibyo Gakkai Zasshi 2013;110:104‐111. [PubMed] [Google Scholar]
- 35. Mancini S, Amorotti E, Vecchio S, Ponz de Leon M, Roncucci L. Infliximab‐related hepatitis: discussion of a case and review of the literature. Intern Emerg Med 2010;5:193‐200. [DOI] [PubMed] [Google Scholar]
- 36. Saleem G, Li SC, MacPherson BR, Cooper SM. Hepatitis with interface inflammation and IgG, IgM, and IgA anti‐double‐stranded DNA antibodies following infliximab therapy: comment on the article by Charles et al. Arthritis Rheum 2001;44:1966‐1968. [DOI] [PubMed] [Google Scholar]
- 37. Germano V, Picchianti Diamanti A, Baccano G, Natale E, Onetti Muda A, Priori R, et al. Autoimmune hepatitis associated with infliximab in a patient with psoriatic arthritis. Ann Rheum Dis 2005;64:1519‐1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ozorio G, McGarity B, Bak H, Jordan AS, Lau H, Marshall C. Autoimmune hepatitis following infliximab therapy for ankylosing spondylitis. Med J Aust 2007;187:524‐526. [DOI] [PubMed] [Google Scholar]
- 39. Marques M, Magro F, Cardoso H, Carneiro F, Portugal R, Lopes J, et al. Infliximab‐induced lupus‐like syndrome associated with autoimmune hepatitis. Inflamm Bowel Dis 2008;14:723‐725. [DOI] [PubMed] [Google Scholar]
- 40. Fairhurst DA, Sheehan‐Dare R. Autoimmune hepatitis associated with infliximab in a patient with palmoplantar pustular psoriaisis. Clin Exp Dermatol 2009;34:421‐422. [DOI] [PubMed] [Google Scholar]
- 41. Doyle A, Forbes G, Kontorinis N. Autoimmune hepatitis during infliximab therapy for Crohn’s disease: a case report. J Crohns Colitis 2011;5:253‐255. [DOI] [PubMed] [Google Scholar]
- 42. Goldfeld DA, Verna EC, Lefkowitch J, Swaminath A. Infliximab‐induced autoimmune hepatitis with successful switch to adalimumab in a patient with Crohn’s disease: the index case. Dig Dis Sci 2011;56:3386‐3388. [DOI] [PubMed] [Google Scholar]
- 43. Subramaniam K, Chitturi S, Brown M, Pavli P. Infliximab‐induced autoimmune hepatitis in Crohn’s disease treated with budesonide and mycophenolate. Inflamm Bowel Dis 2011;17:E149‐E150. [DOI] [PubMed] [Google Scholar]
- 44. Goujon C, Dahel K, Bérard F, Guillot I, Gunera‐Saad N, Nicolas JF. Autoimmune hepatitis in two psoriasis patients treated with inflixmab. J Am Acad Dermatol 2010;63:e43‐e44. [DOI] [PubMed] [Google Scholar]
- 45. Tobon GJ, Cañas C, Jaller JJ, Restrepo JC, Anaya JM. Serious liver disease induced by infliximab. Clin Rheumatol 2007;26:578‐581. [DOI] [PubMed] [Google Scholar]
- 46. Miehsler W, Novacek G, Wenzl H, Vogelsang H, Knoflach P, Kaser A, et al.; Austrian Society of Gastroenterology and Hepatology . A decade of infliximab: the Austrian evidence based consensus on the safe use of infliximab in inflammatory bowel disease. J Crohns Colitis 2010;4:221‐256. [DOI] [PubMed] [Google Scholar]
- 47. Kok B, Lester ELW, Lee WM, Hanje AJ, Stravitz RT, Girgis S, et al.; United States Acute Liver Failure Study Group . Acute liver failure from tumor necrosis factor‐a antagonists: report of four cases and literature review. Dig Dis Sci 2018;63:1654‐1666. [DOI] [PubMed] [Google Scholar]
- 48. Hagel S, Bruns T, Theis B, Herrmann A, Stallmach A. Subacute liver failure induced by adalimumab. Int J Clin Pharmacol Ther 2011;49:38‐40. [DOI] [PubMed] [Google Scholar]
- 49. Parra RS, Feitosa MR, Machado VF, Ramalho LN, da Rocha JJ, Feres O. Infliximab‐associated fulminant hepatic failure in ulcerative colitis: a case report. J Med Case Rep 2015;9:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Forker R, Escher M, Stange EF. A 20‐year‐old woman with ulcerative colitis and acute liver failure [in German]. Internist (Berl) 2017;58:982‐989. [DOI] [PubMed] [Google Scholar]
- 51. Shelton E, Chaudrey K, Sauk J, Khalili H, Masia R, Nguyen DD, et al. New onset idiosyncratic liver enzyme elevations with biological therapy in inflammatory bowel disease. Aliment Pharmacol Ther 2015;41:972‐979. [DOI] [PubMed] [Google Scholar]
- 52. Parekh R, Kaur N. Liver injury secondary to anti‐TNF‐alpha therapy in inflammatory bowel disease: a case series and review of the literature. Case Rep Gastrointest Med 2014;2014:956463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Koller T, Galambosova M, Filakovska S, Kubincova M, Hlavaty T, Toth J, et al. Drug‐induced liver injury in inflammatory bowel disease: 1‐year prospective observational study. World J Gastroenterol 2017;23:4102‐4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bjornsson ES, Gunnarsson BI, Grondal G, Jonasson JG, Einarsdottir R, Ludviksson BR, et al. Risk of drug‐induced liver injury from tumor necrosis factor antagonists. Clin Gastroenterol Hepatol 2015;13:602‐608. [DOI] [PubMed] [Google Scholar]
- 55. Rodrigues S, Lopes S, Magro F, Cardoso H, Horta e Vale AM, Marques M, et al. Autoimmune hepatitis and anti‐tumor necrosis factor alpha therapy: a single center report of 8 cases. World J Gastroenterol 2015;21:7584‐7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Parisi I, O’Beirne J, Rossi RE, Tsochatzis E, Manousou P, Theocharidou E, et al. Elevated liver enzymes in inflammatory bowel disease: the role and safety of infliximab. Eur J Gastroenterol Hepatol 2016;28:786‐791. [DOI] [PubMed] [Google Scholar]
- 57. García Aparicio AM, Rey JR, Sanz AH, Alvarez JS. Successful treatment with etanercept in a patient with hepatotoxicity closely related to infliximab. Clin Rheumatol 2007;26:811‐813. [DOI] [PubMed] [Google Scholar]
- 58. Massarotti M, Marasini B. Successful treatment with etanercept of a patient with psoriatic arthritis after adalimumab related hepatotoxicity. Int J Immunopathol Pharmacol 2009;22:547‐549. [DOI] [PubMed] [Google Scholar]
- 59. Björnsson ES, Bergmann O, Jonasson JG, Grondal G, Gudbjornsson B, Olafsson S. Drug‐induced autoimmune hepatitis: response to corticosteroids and lack of relapse after cessation of steroids. Clin Gastroenterol Hepatol 2017;15:1635‐1636. [DOI] [PubMed] [Google Scholar]
- 60. Hoffmann JH, Knoop C, Enk AH, Hadaschik EN. Routine laboratory parameter dynamics and laboratory adverse events in psoriasis patients on long‐term treatment with adalimumab, etanercept, and ustekinumab. Acta Derm Venereol 2017;97:705‐710. [DOI] [PubMed] [Google Scholar]
- 61. Chiu YM, Tang CH, Hung ST, Yang YW, Fang CH, Lin HY. A real‐world risk analysis of biological treatment (adalimumab and etanercept) in a country with a high prevalence of tuberculosis and chronic liver disease: a nationwide population‐based study. Scand J Rheumatol 2017;46:236‐240. [DOI] [PubMed] [Google Scholar]
- 62. Feagins LA, Flores A, Arriens C, Park C, Crook T, Reimold A, et al. Nonalcoholic fatty liver disease: a potential consequence of tumor necrosis factor‐inhibitor therapy. Eur J Gastroenterol Hepatol 2015;27:1154‐1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. McGowan CE, Jones P, Long MD, Barritt AS 4th. Changing shape of disease: nonalcoholic fatty liver disease in Crohn’s disease‐a case series and review of the literature. Inflamm Bowel Dis 2012;18:49‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Di Minno MN, Iervolino S, Peluso R, Russolillo A, Lupoli R, Scarpa R, et al.; CaRRDS Study Group . Hepatic steatosis and disease activity in subjects with psoriatic arthritis receiving tumor necrosis factor‐alpha blockers. J Rheumatol 2012;39:1042‐1046. [DOI] [PubMed] [Google Scholar]
- 65. Asmundsson J, Valgeirsson KB, Bjornsson HK, Bjornsson ES. Drug‐induced autoimmune hepatitis caused by infliximab: clinical features and comparison with patients with genuine autoimmune hepatitis [Abstract]. Gastroenterology 2019;156(Suppl. 1):S1203. [Google Scholar]
- 66. Fathalla B, Goldsmith DP, Pascasio JM, Baldridge A. Development of autoimmune hepatitis in a child with systemic‐onset juvenile idiopathic arthritis during therapy with etanercept. J Clin Rheumatol 2008;14:297‐298. [DOI] [PubMed] [Google Scholar]
- 67. Harada K, Akai Y, Koyama S, Ikenaka Y, Saito Y. A case of autoimmune hepatitis exacerbated by the administration of etanercept in the patient with rheumatoid arthritis. Clin Rheumatol 2008;27:1063‐1066. [DOI] [PubMed] [Google Scholar]
- 68. Grasland A, Sterpu R, Boussoukaya S, Mahe I. Autoimmune hepatitis induced by adalimumab with successful switch to abatacept. Eur J Clin Pharmacol 2012;68:895‐898. [DOI] [PubMed] [Google Scholar]
- 69. Adar T, Mizrahi M, Pappo O, Scheiman‐Elazary A, Shibolet O. Adalimumab‐induced autoimmune hepatitis. J Clin Gastroenterol 2010;44:e20‐e22. [DOI] [PubMed] [Google Scholar]
- 70. Frider B, Bruno A, Ponte M, Amante M. Drug‐induced liver injury caused by adalimumab: a case report and review of the bibliography. Case Reports Hepatol 2013;2013:406901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Toprak SK, Karakus S. Rituximab‐related reversible hepatocellular damage. Turk J Haematol 2012;29:422‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Latus J, Klein R, Koetter I, Schwab M, Fritz P, Kimmel M, et al. Cholestatic liver disease after rituximab and adalimumab and the possible role of cross‐reacting antibodies to Fab 2 fragments. PLoS One 2013;8:e78856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Qazilbash MH, Qu Z, Hosing C, Couriel D, Donato M, Giralt S, et al. Rituximab‐induced acute liver failure after an allogeneic transplantation for chronic myeloid leukemia. Am J Hematol 2005;80:43‐45. [DOI] [PubMed] [Google Scholar]
- 74. Terziroli Beretta‐Piccoli B, Mieli‐Vergani G, Vergani D. Autoimmune hepatitis: standard treatment and systematic review of alternative treatments. World J Gastroenterol 2017;23:6030‐6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Mahamid M, Mader R, Safadi R. Hepatotoxicity of tocilizumab and anakinra in rheumatoid arthritis: management decisions. Clin Pharmacol 2011;3:39‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Drepper M, Rubbia‐Brandt L, Spahr L. Tocilizumab‐induced acute liver injury in adult onset Still’s disease. Case Reports Hepatol 2013;2013:964828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Anger F, Wiegering A, Wagner J, Lock J, Baur J, Haug L, et al. Toxic drug‐induced liver failure during therapy of rheumatoid arthritis with tocilizumab subcutaneously: a case report. Rheumatology (Oxford) 2017;56:1628‐1629. [DOI] [PubMed] [Google Scholar]
- 78. Taylor SA, Vittorio JM, Martinez M, Fester KA, Lagana SM, Lobritto SJ, et al. Anakinra‐induced acute liver failure in an adolescent patient with Still’s disease. Pharmacotherapy 2016;36:e1‐e4. [DOI] [PubMed] [Google Scholar]
- 79. Diallo A, Mekinian A, Boukari L, Mouas H, Zamy M, Nahon P, et al. Severe hepatitis in a patient with adult‐onset Still’s disease treated with anakinra [in French]. Rev Med Interne 2013;34:168‐170. [DOI] [PubMed] [Google Scholar]
- 80. Ahmed O, Brahmania M, Alsahafi M, Alkhowaiter S, Erb S. Anakinra hepatotoxicity in a patient with adult‐onset Still’s disease. ACG Case Rep J 2015;2:173‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Aly L, Iking‐Konert C, Quaas A, Benten D. Subacute liver failure following anakinra treatment for adult‐onset Still disease. J Rheumatol 2013;40:1775‐1777. [DOI] [PubMed] [Google Scholar]
- 82. Canna S, Frankovich J, Higgins G, Narkewicz MR, Nash SR, Hollister JR, et al. Acute hepatitis in three patients with systemic juvenile idiopathic arthritis taking interleukin‐1 receptor antagonist. Pediatr Rheumatol Online J 2009;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Iwanaga N, Origuchi T, Terada K, Ueki Y, Kamo Y, Kinoshita N, et al. Rheumatoid arthritis complicated with severe liver injury during treatment with abatacept. Mod Rheumatol 2014;24:874‐876. [DOI] [PubMed] [Google Scholar]
- 84. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al.; National Comprehensive Cancer Network . Management of immune‐related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1714‐1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Weber J. Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother 2009;58:823‐830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Johncilla M, Misdraji J, Pratt DS, Agoston AT, Lauwers GY, Srivastava A, et al. Ipilimumab‐associated hepatitis: clinicopathologic characterization in a series of 11 cases. Am J Surg Pathol 2015;39:1075‐1084. [DOI] [PubMed] [Google Scholar]
- 87. Zen Y, Yeh MM. Hepatotoxicity of immune checkpoint inhibitors: a histology study of seven cases in comparison with autoimmune hepatitis and idiosyncratic drug‐induced liver injury. Mod Pathol 2018;31:965‐973. [DOI] [PubMed] [Google Scholar]
- 88. Matsubara T, Nishida T, Higaki Y, Tomita R, Shimakoshi H, Shimoda A, et al. Nivolumab induces sustained liver injury in a patient with malignant melanoma. Intern Med 2018;57:1789‐1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Doherty GJ, Duckworth AM, Davies SE, Mells GF, Brais R, Harden SV, et al. Severe steroid‐resistant anti‐PD1 T‐cell checkpoint inhibitor‐induced hepatotoxicity driven by biliary injury. ESMO Open 2017;2:e000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wu Z, Lai L, Li M, Zhang L, Zhang W. Acute liver failure caused by pembrolizumab in a patient with pulmonary metastatic liver cancer: a case report. Medicine (Baltimore) 2017;96:e9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Pollack MH, Betof A, Dearden H, Rapazzo K, Valentine I, Brohl AS, et al. Safety of resuming anti‐PD‐1 in patients with immune‐related adverse events (irAEs) during combined anti‐CTLA‐4 and anti‐PD1 in metastatic melanoma. Ann Oncol 2018;29:250‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tripathi A, Kaymakcalan MD, LeBoeuf NR, Harshman LC. Programmed cell death‐1 pathway inhibitors in genitourinary malignancies: specific side‐effects and their management. Curr Opin Urol 2016;26:548‐555. [DOI] [PubMed] [Google Scholar]
- 93. Ziemer M, Koukoulioti E, Beyer S, Simon JC, Berg T. Managing immune checkpoint‐inhibitor‐induced severe autoimmune‐like hepatitis by liver‐directed topical steroids. J Hepatol 2017;66:657‐659. [DOI] [PubMed] [Google Scholar]
- 94. Menzies AM, Johnson DB, Ramanujam S, Atkinson VG, Wong ANM, Park JJ, et al. Anti‐PD‐1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol 2017;28:368‐376. [DOI] [PubMed] [Google Scholar]
- 95. Tsung I, Dolan R, Lao CD, Fecher L, Riggenbach K, Yeboah‐Korang A, et al. Liver injury if most commonly due to hepatic metastases rather than drug hepatotoxicity during pembrolizumab immunotherapy. Aliment Pharmacol Ther 2019;50:800‐808. [DOI] [PubMed] [Google Scholar]
- 96. Lee WM, Hynan LS, Rossaro L, Fontana RJ, Stravitz RT, Larson AM, et al.; Acute Liver Failure Study Group . Intravenous N‐acetylcysteine improves transplant‐free survival in early stage non‐acetaminophen acute liver failure. Gastroenterology 2009;137:856‐864, 864.e851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lee WM. Drug‐induced acute liver failure. Clin Liver Dis 2013;17:575‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. European Association for the Study of the Liver . Clinical practice guidelines: drug‐induced liver injury. J Hepatol 2019;70:1222‐1261. [DOI] [PubMed] [Google Scholar]
- 99. Hu PF, Wang PQ, Chen H, Hu XF, Xie QP, Shi J, et al. Beneficial effect of corticosteroids for patients with severe drug‐induced liver injury. J Dig Dis 2016;17:618‐627. [DOI] [PubMed] [Google Scholar]
- 100. Hu PF, Xie WF. Corticosteroid therapy in drug‐induced liver injury: pros and cons. J Dig Dis 2019;20:122‐126. [DOI] [PubMed] [Google Scholar]
- 101. Czaja AJ. Drug‐induced autoimmune‐like hepatitis. Dig Dis Sci 2011;56:958‐976. [DOI] [PubMed] [Google Scholar]
- 102. Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560‐1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Averbukh LD, Wu GY. Role of biologics in the development of autoimmune hepatitis: a review. J Clin Transl Hepatol. 2018;6:402‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]


