Abstract
This review describes work from several research groups in which ultrasound is being used to target the peripheral nervous system and perform neuromodulation noninvasively. Although these techniques are in their infancy compared to implant-based and electrical nerve stimulation, if successful this new noninvasive method for neuromodulation could solve many of the challenges facing the field of bioelectronic medicine. The work outlined herein shows results in which two different (potentially therapeutic) targets are stimulated, a neuroimmune pathway within the spleen and a nutrient/sensory pathway within the liver. Both data and discussion are provided that compare this new noninvasive technique to implant-based nerve stimulation.
PERIPHERAL NERVE STIMULATION AND NEUROMODULATION
Peripheral nerve stimulation has been used to treat neuropathic pain since the 1960s, and over the last 20 years has developed into a major clinical and industrial market. Traditionally, implantable electronic devices have been used, and the primary clinical applications have been treatment of severe and chronic pain (in place of invasive surgical procedures or opioid treatment) (Slavin 2008; Wolter 2014; Chakravarthy et al. 2016; Meier 2017). Until recently, secondary use (or testing) in applications beyond pain management has been limited to other neural or neuromuscular conditions and pathologies, including migraine headaches, depression, epilepsy, and urinary incontinence (Schuster and Rapoport 2016; Guzman-Negron and Goldman 2017; Johnson and Wilson 2018). In the past 10 years, peripheral nerve stimulation has undergone a “renaissance” with standard tools (such as cervical vagus nerve stimulation [VNS]) being tested in clinical trials, in which the target of stimulation are end-organ nerve reflexes that modulate molecular (and not neuromuscular) targets (Marchall et al. 2015; Pavlov and Tracey 2015; Willemze et al. 2015; Yuan and Silberstein 2016; Chavan et al. 2017). This novel use of medical devices to target specific physiological or molecular pathways (similar to drugs) is known as bioelectronic medicine (Tracey 2016; Olofsson and Tracey 2017).
In one recent clinical trial, VNS has been applied to stimulate a neural reflex that inhibits the production of cytokines (i.e., an inflammatory reflex) (Koopman et al. 2016). Preclinical studies have shown that this reflex is dependent on a vagus nerve signal that reaches the spleen, alters concentration of norepinephrine and acetylcholine, and modulates the release and production of cytokines by resident (and migrating) macrophages (Borovikova et al. 2000; Tracey 2009, 2016). One of the main cytokines under regulation by this reflex is tumor necrosis factor (TNF). Interestingly, TNF is the target of biologic therapies that inhibit TNF signaling in rheumatoid arthritis ([RA] and other chronic inflammatory disease) patients (Koopman et al. 2016). In RA, symptomatic relief can be achieved in up to 50% of patients using anti-TNF biologics, and recently a first-of-its-kind study has shown that VNS significantly inhibited TNF production for up to 84 days in a pilot study in RA patients (with a concomitant decrease in disease severity as measured by standardized clinical composite scores) (Koopman et al. 2016). These first studies have been rapidly followed by a series of observations and discoveries of local nerve reflexes modulating the output of an end-organ or gland. Many of these new discoveries provides the opportunity for therapeutic replacement or augmentation of a drug therapy. These latest studies include discovery of a sciatic/vagus pathway that modulates production of immune-modulating catecholamines from the adrenal gland (Torres-Rosas et al. 2014), a group of peripheral sensory pathways that convey metabolic information (i.e., nutrient concentrations, gut-derived satiety signals and adiposity-related hormones) to the brain for maintenance of energy and glucose homeostasis (Roh et al. 2016), and a local intestinal nerve circuit required to activate tissue protective gene expression programs in resident macrophages upon bacterial infection (Gabanyi et al. 2016). Each of these discoveries carries implications on the neural components involved with maintenance of tissue/organ homeostasis, and potentially the pathogenesis of tissue/organ related chronic diseases.
However, development of therapeutic techniques from these important discoveries requires significant investment in future research and development. Several research investment entities (i.e., NIH SPARC; DARPA ElectRx) (commonfund.nih.gov/Sparc; www.darpa.mil/program/electrical-prescriptions) have recently made it a goal to enable precise end-organ neuromodulation (in which specific signals are sent to a single organ to affect its physiological function). Additional, corporate funding entities (i.e., GSK-Google, GE-Feinstein) (fortune.com/2016/08/01/google-alphabet-glaxosmithkline-bioelectronics; www.innovateli.com/ge-brings-good-things-bioelectronics-research) have also taken up this challenge, with an intent to eventually decode the language of communication between nerves and organs, and to learn to modulate the signals to create novel therapies. However, precision stimulation and neuromodulation with implant technologies remains an unsolved challenge, as smaller devices (i.e., those that can be implanted closer to a target organ) remain in development (Mei and Irazoqui 2014). Alternative methods for axon-level specific modulation remain confined to experimental molecular and genetic techniques (such as optogenetics [Deisseroth 2015] or nanoparticle-based approaches [Temel and Jahanshahi 2015]). In addition, the optimal stimulation tools and parameters for clinical use remain unknown. It is improbable that the same level or type of neuromodulation is required to effect end-tissue function at different organ targets. What are the basal neural signals required for homeostasis within these different natural reflexes, and which are activated only in response to a specific external stimulus or associated with disease pathogenesis? What are the optimal parameters to specifically stimulate the local nerve reflex, and how many times should stimulation take place to generate this therapeutic effect without any nonbeneficial side effects? Answers to these (and other) questions are necessary to build new tools that provides both precision stimulation (i.e., modulation of specific end organs and physiological functions) and paths toward clinical translation.
In this review, we first summarize work from a group of researchers who have begun to uniquely apply ultrasound energy within organs to provide a form of precision neuromodulation (Gigliotti et al. 2013; Cotero et al. 2019; Zachs et al. 2019). We discuss what is known about the use of ultrasound in these neuromodulation applications, and how it compares to current forms of electrical stimulation techniques. We show some direct comparisons between the ultrasound and implant-based electrical stimulation techniques (used within the same pre-clinical models of disease) (Cotero et al. 2019). Finally, we offer some commentary on general lessons learned from this use of ultrasound and how it might be applied to continue development of more precise and clinically impactful neuromodulation technologies.
TECHNICAL CHALLENGES IN ACHIEVING PRECISION NEUROMODULATION WITH CURRENT NONINVASIVE TECHNOLOGIES
For VNS, the first approaches used to develop noninvasive stimulation protocols (i.e., tools capable of stimulating vagus nerve pathways externally, without an implantable device) adapted technology from transcutaneous electrical nerve stimulation (TENS) devices (Ben-Menachem et al. 2015; Frangos et al. 2015; Anfinogenova 2016). These new transcutaneous VNS (tVNS) devices had a modified form or location/placement of the electrodes compared to TENS (i.e., designs that allowed targeting the electrical stimulus around superficial projections of the vagus nerve at the ear or neck; Ben-Menachem et al. 2015; Frangos et al. 2015; Anfinogenova 2016) and modified electrical stimulation parameters/waveforms. The proposed mechanism of action for these transcutaneous stimulation systems differ from TENS. For example, the auricular stimulation devices are thought to stimulate sensory afferent fibers of an auricular branch of the vagus nerve, rather than the branches that directly innervate the target organs (e.g., spleen, intestine, or liver) (Frangos et al. 2015). It is proposed that stimulation of these sensory fibers ascend to the nucleus tractus solitaris (NTS), where they synapse with other nerve fibers (Frangos et al. 2015). However, the direct mapping of these connections is unknown, and the preclinical and/or clinical effectiveness of these alternative indirect forms of VNS remain controversial and may be target and/or application specific.
NEW INSIGHTS INTO THE USE OF NONINVASIVE ULTRASOUND NEUROMODULATION TECHNIQUES
Attempts at using ultrasound stimuli to elicit and alter action potential propagation in ex vivo nerves date back to the 1980s (Schelling et al. 1994). In these initial studies, the ultrasound energy was focused on the same large nerves (outside of the organ or target tissue) that traditionally host implanted electrodes (e.g., the cervical vagus nerve), and this strategy has produced many conflicting results. While some studies report that ultrasound was capable of eliciting action potentials in ex vivo nerves (Wright et al. 2017), others have shown that ultrasound energy is only capable of modifying electrically induced action potentials (Colucci et al. 2009). Many of the reports demonstrating ultrasound-induced action potential generation have been shown in a very limited set of conditions or include powers that may cause damage to nerves and/or surrounding tissue if performed in vivo. These studies include recent investigations that have failed to trigger neural pathway activation to organ targets using ultrasound (Juan et al. 2014), or used indirect methods of measuring nerve activation via neuromuscular pathways (i.e., measures of ultrasound-induced EMG signals or muscle movements) (Downs et al. 2018). In contrast, several reports have demonstrated successful modulation of end axons or nerve terminal activity in brain tissue (Tyler et al. 2008) and retina (Menz et al. 2013) or have shown that scanned ultrasound imaging may achieve an energy threshold required to stimulate sensory receptors within peripheral tissue (Gigliotti et al. 2013).
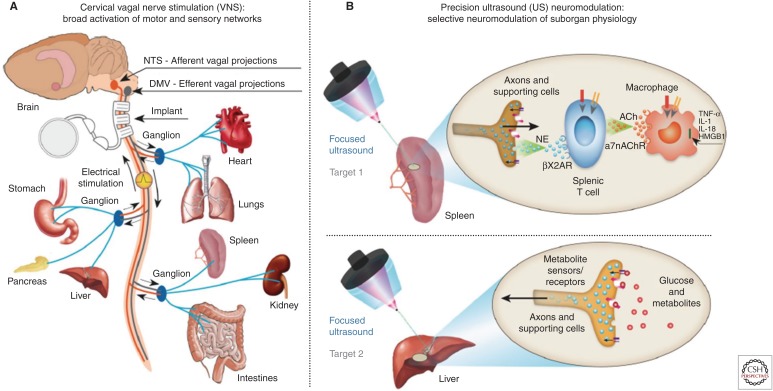
Considering these observations, several groups (including our own [Gigliotti et al. 2013; Cotero et al. 2019; Zachs et al. 2019]) have examined direct organ tissue stimulation to achieve noninvasive neuromodulation. Our group further explored suborgan targeting of the ultrasound stimulus using focused/pulsed ultrasound, to test for neuromodulation of areas of specific neural innervation within the organs themselves (Fig. 1; Cotero et al. 2019). The team chose to first stimulate areas of the spleen thought to contain the nerve innervation required for cholinergic anti-inflammatory pathway (CAP) signaling (as described above and in Fig. 1; Tracey 2002, 2009, 2016; Cotero et al. 2019). The team next chose to test for broader utility of the precision ultrasound neuromodulation concept by stimulating a second organ target (and proposed neural pathway) in the liver (Fig. 1; Cotero et al. 2019). This hepatic target is thought to contain neurons responsible for nutrient sensing and communication with the glucose regulation centers of the brain (Yi et al. 2010).
Figure 1.
Implant-based vagus nerve stimulation (VNS) versus precision ultrasound (US) neuromodulation (Cotero et al. 2019). (A) Schematic of the neurons within the vagus nerve, exemplary innervated organs, and the position used for VNS devices. (B) Schematic of precision organ-based neuromodulation in which the innervation points of known axonal populations are targeted for stimulation using focused pulsed US. (From Cotero et al. 2019; reprinted, with permission, from Nature Communications © 2019.)
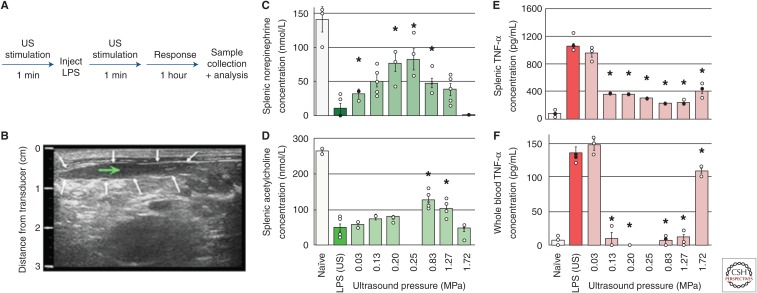
Figure 2 outlines data on ultrasound-based CAP modulation experiments in the lipopolysaccharide (LPS)-induced acute inflammation rodent model (Cotero et al. 2019). As discussed in the published manuscript, splenic concentrations of norepinephrine, acetylcholine, and TNF-α were measured, to monitor the output of each component in the CAP (Fig. 1B). As described above, electrical stimulation of this pathway is currently being studied as a nonpharmaceutical treatment for RA (Koopman et al. 2016). In this ultrasound study, an ultrasound transducer was focused directly on the spleen before and after LPS injection (for 1 min; Fig. 2A), using a second imaging transducer to align the ultrasound delivery to the center of the spleen (Fig. 2B; Cotero et al. 2019). The data in Figure 2C–F shows the measured CAP response of animals treated with ultrasound at varying stimulus powers compared to controls (i.e., animal receiving LPS but no ultrasound stimulation), demonstrating successful CAP activation using the ultrasound-based stimulation approach.
Figure 2.
Splenic ultrasound (US) Neuromodulation of the cholinergic anti-inflammatory pathway (CAP) (Cotero et al. 2019). (A) A timeline of the US CAP stimulation performed (with the US stimulus applied directly to the spleen). (B) US images were used to locate the US stimulus (white arrows—outline of the spleen; green arrow—target point for US stimulation). (C–E) Splenic concentrations of CAP signaling molecules, including norepinephrine (C), acetylcholine (D), and tumor necrosis factor α (TNF-α) (E) are shown for naïve animals, sham controls (lipopolysaccharide [LPS], US), and with US stimulation (0.03–1.72 MPa). (F) Whole-blood concentrations of TNF-α for the same conditions as E. The asterisks mark statistical significance using two-sided t-test versus LPS-only controls (threshold 0.05). (From Cotero et al. 2019; reprinted, with permission, from Nature Communications © 2019.)
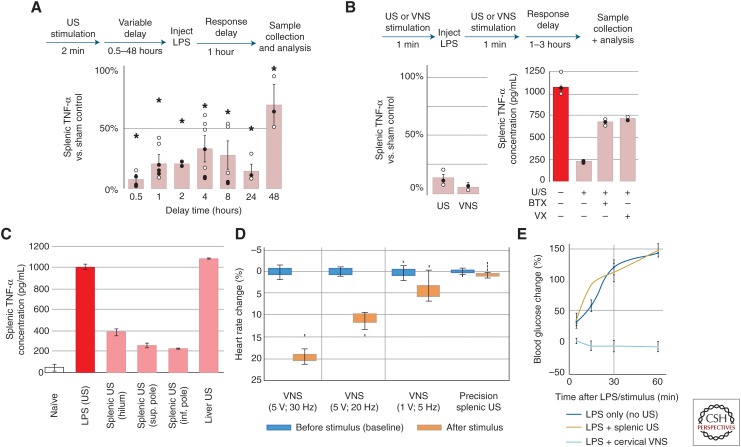
The previous work also found that splenic ultrasound was also protective when applied before LPS injection (Fig. 3A; Cotero et al. 2019) consistent with previous invasive VNS studies). And, when applied at the time of LPS administration, the splenic ultrasound treatment was found as effective in TNF reduction compared to traditional, invasive cervical VNS (Fig. 3B). Furthermore, studies showed (Fig. 3B) that both injection of α-bungarotoxin (BTX, a known antagonist for the α7nACh receptor central to CAP signaling) and vagotomy suppressed the effect of ultrasound stimulation on TNF-α concentration. This demonstrates that, like VNS-based CAP activation, optimal CAP modulation by ultrasound requires nerve and splenic α7nAChR signaling. Ultrasound stimulation of off-target sites (i.e., liver) did not modulate the LPS-induced TNF response; however, ultrasound stimulation at several distinct locations within the spleen provided similar modulation of the TNF response (Fig. 3C; Cotero et al. 2019).
Figure 3.
Comparison of splenic ultrasound (US) stimulus versus traditional cervical vagus nerve stimulation (VNS) stimulation of cholinergic anti-inflammatory pathway (CAP) (Cotero et al. 2019). (A) Splenic tumor necrosis factor (TNF) concentrations shown after protective US treatments with delay times from 0.5 to 48 h prior to lipopolysaccharide (LPS) injection. The asterisks mark statistical significance using two-sided t-test versus LPS only controls (threshold 0.05). (B) (left) Relative concentrations of splenic TNF are shown for US-stimulated (left) versus implant-based VNS treated animals. (right) Data showing the effect of α-bungarotoxin (BTX) or surgical (cervical) vagotomy on splenic concentrations of TNF after US stimulation. (C) Data showing specificity in the modulated TNF response for splenic versus liver US stimulation, but similar TNF response at different splenic stimulation locations. (D) Data comparing the effect of VNS and splenic US stimulation on heart rate. (E) Data confirming the VNS effect on LPS-induced hyperglycemia and absence of this effect when using splenic US stimulation. (From Cotero et al. 2019; reprinted, with permission, from Nature Communications © 2019.)
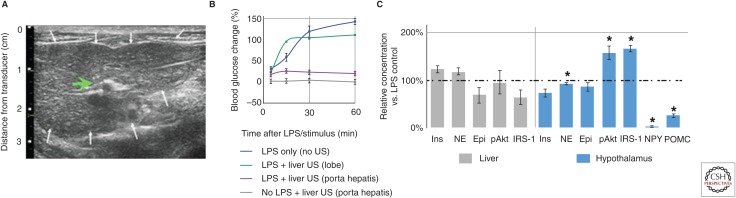
As described above (Fig. 1), it is known that cervical VNS stimulates both CAP pathways, and other vagus axonal projections terminating in other anatomical locations. Interestingly, it was found that while cervical VNS demonstrated an effect on heart rate at both 2- and 5-volt stimulation intensities, splenic ultrasound neuromodulation did not (Fig. 3D). In addition, the previously observed (Pavlov and Tracey 2012) secondary effect of cervical VNS on LPS-induced hyperglycemia persisted in the implant studies (Cotero et al. 2019), but ultrasound stimulation had no secondary effect on blood glucose concentration during the experiments. This supports the hypothesis that the observed metabolic effects of VNS are the result of other (non-CAP) vagus pathways (Pavlov and Tracey 2012; Roh et al. 2016; Cotero et al. 2019). Figure 4A shows another ultrasound-guided image, this time utilized to locate the ultrasound stimulus to the porta hepatis region of the liver (chosen because of the glucose-sensitive neurons known to innervate the region) (Roh et al. 2016). It was found that hepatic ultrasound stimulation did provide protection against the LPS-induced hyperglycemia (Fig. 4B), and interestingly this effect was anatomically specific (i.e., ultrasound stimulation at neighboring left or right lobes of the liver did not produce protection against hyperglycemia). In addition, hepatic concentrations of local signaling molecules associated with glucose metabolism did not show changes within the liver indicative of hepatic glycolysis/glyconeogenolysis (Fig. 4C); instead, ultrasound stimulation of the porta hepatis resulted in significantly reduced hypothalamic concentrations of NPY and POMC, resulting in increased hypothalamic insulin receptor substrate 1 (IRS-1) and protein kinase B (pAkt) activation (Fig. 4C). These results are consistent with activation of a pathway that modulates LPS-induced effect on energy metabolism through IRS1-PI3 signaling, and ultrasound mediated modulation of insulin signaling within the hypothalamus (Reyes et al. 2003; Kahn et al. 2006; Cani et al. 2007; Yi et al. 2010; Pavlov and Tracey 2012). In addition to the chemical data, the team has verified changes in activation of important hypothalamic nuclei using both DfMRI and cFos gene expression studies because of ultrasound stimulation (Cotero et al. 2019).
Figure 4.
Hepatic ultrasound (US) stimulation of pathways that affect glucose regulation (Cotero et al. 2019). (A) Example image of the liver used to focus the US stimulus (green arrow; white arrows—outline of the liver). (B) Data showing the effect of hepatic US stimulation on lipopolysaccharide (LPS)-induced hyperglycemia. Blood glucose concentrations compared to pre-injection concentration are shown, and LPS-induced hyperglycemia is prevented after US stimulation of the porta hepatis (purple), but not distal lobes (green). (C) Concentrations for molecules associated with metabolism in the liver and hypothalamus are shown compared to no ultrasound controls. The asterisks mark statistical significance using two-sided t-test versus LPS only controls (threshold 0.05). (From Cotero et al. 2019; reprinted, with permission, from Nature Communications © 2019.)
EXTENSION TO PRECLINICAL MODELS OF DISEASE
Splenic Stimulation and Inflammation
Several groups, including our own (Cotero et al. 2019), have begun to apply these observations of ultrasound peripheral neuromodulation to pre-clinical models of disease. Ultrasound application to the spleen has been shown to provide protection in both a renal ischemia-reperfusion (Gigliotti et al. 2013) (a model of acute kidney injury) and inflammatory arthritis model (Zachs et al. 2019). The Okusa group found that while attempting to precondition the renal vasculature before ischemia-reperfusion injury ([IRI] using a contrast enhanced ultrasound imaging procedure), the ultrasound energy reaching the neighboring spleen was enough to activate the CAP, and that CAP activation provided protection after the subsequent IRI (Gigliotti et al. 2013). Similar, to the results shown above (i.e., splenic ultrasound provided protection to LPS-induced inflammation up to 48 h prior to injection), the ultrasound treatment provided preservation of kidney function (as measured by post-IRI plasma creatinine) up to 2 days in advance of IRI. The Lim group recently utilized a daily whole organ splenic ultrasound treatment to activate CAP and reduced disease severity in a mouse model of inflammatory arthritis. Interestingly, the optimal ultrasound stimulation pressure found by Zachs et al. (2019) agrees well with optimal stimulation parameters shown above in the LPS model data, and longer stimulation durations per day were shown to drive greater therapeutic effects (Zachs et al. 2019).
Hepatic Stimulation in Metabolism and Type II Diabetes (T2D)
To further address the capabilities of hepatic US in the long-term management of metabolic disease, our group has also performed hepatic stimulation experiments in the inbred Zucker Diabetic Fatty rat model (ZDF rat). This model develops significant obesity at 4 wk of age and progressive development of hyperinsulemia, hyperlipidemia, and impaired glucose tolerance as a result of obesity (Shiota and Printz 2012). In this model, hyperinsulemia provides an early marker of disease progression (occurring well before 8 wk of age). This is followed by a decrease in insulin levels (as a result of β cell apoptosis) limiting the compensatory response by the pancreas to maintain euglycemia (a physiologic response suspected to occur in human T2D as well) (Shiota and Printz 2012). By 9 to 10 wk, the ZDF rat develops overt diabetes making the animal suitable for studying the pathophysiology, therapeutic intervention, and complications associated with T2D (Srinivasan and Ramarao 2007; Shiota and Printz 2012).
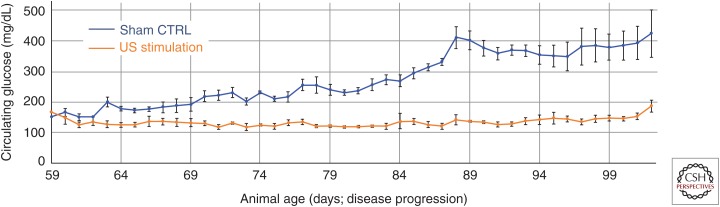
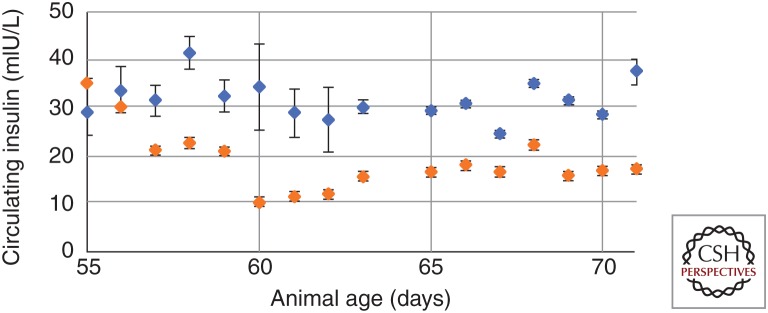
To study the effect of hepatic ultrasound stimulation (and its proposed effect on modulating insulin resistance/signaling within the hypothalamus), ZDF rats were treated with daily ultrasound targeted to the porta hepatis for multiple weeks (Fig. 5). In this study, ultrasound was applied for a total of 3 min daily (as three rounds of 1-min long sequences). When treatment was initiated at day 56 (8 wk of age) animals were still normoglycemic; however, within days the sham control animal began to show hyperglycemia (Fig. 5, blue) as expected in the ZDF model. Application of ultrasound prevented the increase in circulating glucose (Fig. 5, orange) and, interestingly, this prevention of hyperglycemia occurred concurrently with decreases in circulating insulin concentrations (Fig. 6). The maximum glucose levels remained significantly attenuated as compared to sham stimulated control animals (Fig. 5) for up to 45 days posttreatment initiation; this prolonged effect suggests that ultrasound hepatic neuromodulation is capable of long-term modulation of glucose homeostasis. Additional control experiments with nondiabetic lean Zucker animals (data not shown) also showed no effect of hepatic ultrasound stimulation on circulating glucose, suggesting that neuromodulation and signaling through the stimulated liver pathway does not directly lead to changes in blood glucose in the absence of a pathological or nonbaseline metabolic state (e.g., LPS-induced hyperglycemia or leptin signaling deficiencies/high fat diet). This is corroborated by the measured ultrasound-induced changes in insulin sensing and signaling to the hypothalamus in Figure 4 and Cotero et al. (2019).
Figure 5.
Effects of ultrasound (US) on glucose regulation in an animal model of obesity/diabetes. Data showing the effects of chronic US stimulation of the liver on glucose concentrations compared to nontreated littermates. US stimulation began day 56 and continued once daily for 20 days. The data shows a treatment-induced attenuation (orange) of the developing hyperglycemia starting at day 59 compared to a sham control group (blue).
Figure 6.
Hepatic ultrasound (US) stimulation does not modulate circulating glucose levels through insulin response. A significant decrease in circulating insulin was observed to occur in animals receiving US stimulation of the liver (orange) as compared to sham controls (blue), which suggests modulation of glucose levels via the insulin signaling pathways described above.
Glucose homeostasis refers to the equilibrium between blood glucose production, external glucose intake and glucose utilization. Multiple homeostatic mechanisms exist that keep blood glucose concentration within a narrow range, by triggering hormonal response (the most common being the glucose-insulin feedback loop) (Röder et al. 2016). Generally, increased blood glucose mediates a regulation in insulin secretion from the pancreatic β cells, and increased insulin acts to promote translocation of insulin-sensitive glucose transporters (GLUT) to the cell membrane within peripheral tissues (including liver, heart, intestine, muscle, and adipose) promoting glucose uptake. In this system, the liver serves as the principle organ for glucose storage, while muscle and adipose tissue serve relatively minor roles. In addition, elevations in circulating insulin also drives down-regulation of glucose-6-phosphatase, which prevents release of glucose from hepatic stores. However, Figure 4 showed that hepatic ultrasound stimulation (in the LPS model) failed to produce any meaningful change in phosphorylation of key biomarkers of local insulin signaling pathway (pIRS, pAkt, or pGLUT); our future reports will provide data for these key signaling molecules within the ZDF and additional diet-induced diabetes models. Furthermore, the hepatic ultrasound stimulation resulted in an observable decrease in circulating insulin (compared to sham controls [Fig. 6]) suggesting that circulating glucose reduction was not the result of an increase in pancreatic insulin secretion.
In prior literature, the brain–liver connection is well established (Yi et al. 2010), and the central nervous system (CNS) is known to control glucoregulatory response to both hyper- and hypoglycemia (Reyes et al. 2003; Kahn et al. 2006; Cani et al. 2007; Yi et al. 2010; Pavlov and Tracey 2012). In future reports, our team will outline specific alterations in the insulin signaling pathway within the diabetic animal models (before and after stimulation), and measures of the receptors and protein subunits that are known to be dysfunctional or reduced in both animal models and human T2D (i.e., IRS-1, Akt, and GLUT4). In addition, the team will provide data on ultrasound neuromodulation of the hallmarks of altered hypothalamic nerve signaling in diabetes, including activity of glucokinase and hypothalamic glucose uptake, as glucose uptake and conversion to glucose-6-phosphate has been proposed to be a crucial first step in the mechanistic response of glucose-sensing neurons (particularly glucose-excited neurons) to hyperglycemia (Cotero and Routh 2009; Cotero et al. 2010), as well as overall hypothalamic control of peripheral insulin sensitivity (Chan and Sherwin 2012). The team is also investigating other markers of the involvement of hypothalamic glucose-sensing neurons in our observed response to hepatic ultrasound neuromodulation and will report data on additional markers including hypothalamic GABA, BDNF, and glutamate. These neurotransmitters have been shown by single-cell gene expression analysis to be highly expressed in hypothalamic neurons (Miki et al. 2001; Kang et al. 2004) and their expression, in particular the differences in their expression, have been proposed as markers for glucose-sensing neuron activity (Miki et al. 2001; Karnani and Burdakov 2011).
SOME ADDITIONAL COMPARISONS OF HEPATIC ULTRASOUND TO IMPLANT-BASED ELECTRICAL NERVE STIMULATION
It is interesting to note that while the effects of splenic ultrasound stimulation have thus far mirrored results demonstrated with VNS implants, the liver studies described herein have shown several crucial differences. Past, VNS experiments measuring effects on metabolic processes have shown complex and contrary results (Stauss et al. 2015; Tanida et al. 2015; Meyers et al. 2016; Malbert et al. 2017). That is stimulation of the combined afferent and efferent cervical vagus has been shown to cause severe hyperglycemia in rodent models yet had no effect on stimulating overall insulin or glucagon secretion. In one set of studies, the observed hyperglycemia was associated with a marked change in hepatic glycogenolysis, which caused an increase in postprandial glucose output by the liver (Tanida et al. 2015; Meyers et al. 2016). Yet, other studies demonstrated that chronic VNS resulted in significant weight loss and reduced hepatic glucose release because of an alteration in the parasympathetic nervous system (Stauss et al. 2015). Another set of studies demonstrated that in a large animal model of insulin resistance, abdominal (but not cervical) VNS significantly increased whole-body glucose uptake and improved insulin sensitivity (Malbert et al. 2017). Thus, lack of precision (i.e., the ability to separately stimulate specific glucose-sensing versus efferent effector neurons) in implant-based VNS has resulted in a series of mixed results, in which the potential to alter changes in insulin/glucagon release by the pancreas, versus modulate glucose management by peripheral tissues is lost.
There is some existing evidence to enable separation of the function and effect of afferent vs. efferent input to the liver. In 1960, Shimazu et al. demonstrated the crucial role of autonomic neurons (originating in the hypothalamus) in the hepatic control of glucose and glycogen metabolism (Shimazu and Fukuda 1965; Levin et al. 1980) and since then, efforts in trans-neural tracing and immunohistochemistry (IHC) have provided considerable support to the hypothesis of a brain–liver connection in the regulation of liver metabolic function. Efferent nerve pathways to the liver originate primarily from hypothalamic regions in the brain; the ventromedial hypothalamus (VMH), lateral hypothalamic areas (LHA), and paraventricular nucleus (PVN), each eliciting a different effect on the liver. For example, stimulation of the VMH results in the activation of the sympathetic nervous system and signal propagation to the brain stem, which activates neural projections to the intermediolateral (IML) portion of the spinal column. From the IML, preganglionic neurons extend to the collateral ganglia, in the abdominal cavity. From this ganglion, postsynaptic nerves receive and carry the signal to the liver, causing an increase in glucose output (via increases in gluconeogenic enzymes) and a decrease in glycogen storage. In contrast, stimulation of the LHA activates the parasympathetic regions in the dorsal nucleus and brain stem where the signal travels along preganglionic parasympathetic nerves (which make up the hepatic vagus nerve) to the liver, causing an increase in glycogen synthesis and a reduction in total glucose output (via decreases in gluconeogenic enzymes). Finally, the PVN has been demonstrated to have direct connections to both the parasympathetic brain stem and the sympathetic neurons of the spinal cord, giving the PVN the capability to relay information along either autonomic pathway (Rogers and Nelson 1984; Yamashita et al. 1984; Lawrence and Pittman 1985). Afferent nerves are thought to be involved in the monitoring of metabolic signals (glucose, insulin, fatty acids) (Sakaguchi and Iwanaga 1982), amino acids (Torii and Niijima 2001), lipids (Randich et al. 2001), and circulating cytokines (Sakaguchi and Iwanaga 1982). These metabolic signals are encoded by hepatic nerves and transmitted along the afferent vagus nerve to the NTS of the hindbrain where they serve to provide relevant information of peripheral energy status. However, how these metabolic signals effect the various brain regions, resulting in efferent signals regulating systemic metabolism, are not well understood and often limited to examination of direct hormonal pathways, and currently only consist of studies examining regulatory response to hypoglycemia (Marston et al. 2011; Routh et al. 2014).
Direct electrical stimulation of the liver has yielded interesting results that primarily demonstrate a distinct dependency on pulse length of the applied stimulus. That is, application of a 4-mA, 300-μsec, and 70-msec stimulus, which corresponds to electrical stimulation parameters used in gastric stimulation for the reduction of nausea, results in a significant increase in circulating glucose (explained by a likely attenuation in whole body glucose uptake because of the observed decrease in insulin associated with the stimulus). However, application of a 4-mA, 300-μsec, and 25-msec stimulus, results in a decrease in glucose with an associated increase in circulating insulin (Chen et al. 2010). While in both instances there is a clear direct effect on hepatic tissues by the electrical stimulus resulting in local changes in glycogenesis and glycogenolysis, it is also clear that the stimulation of the present hepatic vagus nerve alters the secretion of insulin by the pancreas (Rozman et al. 2002; Rozman and Bunc 2004). This suggests an impact on not just local tissue but also the present neural pathways linking the liver to other metabolic organs, such as the pancreas. Although promising, these findings have not been clinically translated into a useful therapeutic approach. This is likely a result of the overall practicality involved in surgical placement of stimulation electrodes on the hepatic vagus nerve (versus the cervical vagus in VNS therapy) and difficulty in balancing sympathetic and parasympathetic nerves (Shimazu and Fukuda 1965).
SUMMARY AND PERSPECTIVE
The results and citations summarized herein represent the first few preclinical studies involving replacement of invasive electrical stimulation protocols with a noninvasive ultrasound-based neuromodulation stimulus. However, results have now been replicated by three separate groups in the splenic stimulation experiments. And, the first application of hepatic stimulation has provided a potentially “more precise” method of neuromodulation compared to previous electrical implant-based techniques. This suggests that precision ultrasound neuromodulation represents a new method for modulating peripheral nerves experimentally (that may enable a new wave of research on the contribution of peripheral sensing and signaling in health and disease). In addition, these results open a new set of engineering design considerations, in which noninvasive stimulation (versus pharmacological or electrical implant-based technologies) must interact with patients in ways that allow for controlled application of energy to specific anatomical targets of interest. The results described herein show that soon such devices may be part of the everyday therapies across a range of chronic human diseases. Finally, the potential for a new use of ultrasound (in a therapy modality that requires continued stimulation of the same anatomical region) will require trials to be conducted in a way that enables adequate investigation of the overall effects (both desired and off-target) of energy-based versus electrical based neuromodulation.
ACKNOWLEDGMENTS
The authors thank the research group of Hubert Lim for helpful discussions on the historical perspective of current and past ultrasound-based nerve stimulation studies. The authors would also like to thank our collaborators at the Feinstein Medical Research Institute (especially, Kevin Tracey and Sangeeta Chavan) for many helpful discussions on the cholinergic anti-inflammatory pathway. Portions of this research were developed with funding from the Defense Advanced Projects Agency (DARPA) HR0011-18-0040. The views, opinions, and/or findings expressed are those of the author and should not be interpreted as representing the official views or policies of the Department of Defense or the U.S. Government.
Footnotes
Editors: Valentin A. Pavlov and Kevin J. Tracey
Additional Perspectives on Bioelectronic Medicine available at www.perspectivesinmedicine.org
REFERENCES
- Anfinogenova Y. 2016. Vagus nerve stimulation: Invasive or noninvasive? Anatol J Cardiol 16: 811–812. 10.14744/AnatolJCardiol.2016.23113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Menachem E, Revesz D, Simon BJ, Silberstein S. 2015. Surgically implanted and non-invasive vagus nerve stimulation: A review of efficacy, safety, and tolerability. Eur J Neurol 22: 1260–1268. 10.1111/ene.12629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. 2000. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405: 458–462. 10.1038/35013070 [DOI] [PubMed] [Google Scholar]
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, et al. 2007. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56: 1761–1772. 10.2337/db06-1491 [DOI] [PubMed] [Google Scholar]
- Chakravarthy K, Nava A, Christo PJ, Williams K. 2016. Review of recent advances in peripheral nerve stimulation. Curr Pain Headache Rep 20: 60 10.1007/s11916-016-0590-8 [DOI] [PubMed] [Google Scholar]
- Chan O, Sherwin RS. 2012. Hypothalamic regulation of glucose stimulated insulin secretion. Diabetes 61: 564–565. 10.2337/db11-1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavan SS, Pavlov VA, Tracey KJ. 2017. Mechanisms and therapeutic relevance of neuro-immune communication. Immunity 46: 927 10.1016/j.immuni.2017.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Pasricha PJ, Yin J, Lin L, Chen JDZ. 2010. Hepatic electrical stimulation reduces blood glucose in diabetic rats. Neurogastroenterol Motil 22: 1109–e286. 10.1111/j.1365-2982.2010.01556.x [DOI] [PubMed] [Google Scholar]
- Colucci V, Strichartz G, Jolesz F, Vykhodtseva N, Hynynen K. 2009. Focused ultrasound effects on nerve action potential in vitro. Ultrasound Med Biol 35: 1737–1747. 10.1016/j.ultrasmedbio.2009.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotero V, Routh VH. 2009. Insulin blunts the response of glucose excited neurons in the ventrolateral ventromedial hypothalamic nucleus (V-VMN) to decreased glucose. Am J Physiol Endocrinol Metab 296: e1101–e1109. 10.1152/ajpendo.90932.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotero VE, Zhang BB, Routh VH. 2010. The response of glucose excited neurons in the ventromedial hypothalamus to decreased glucose is enhanced in a murine model of type 2 diabetes mellitus. Neuroendocrinol 22: 65–74. 10.1111/j.1365-2826.2009.01938.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotero V, Fan Y, Tsaava T, Kressel AM, Hancu I, Fitzgerald P, Wallace K, Kaanumalle S, Graf J, Rigby W, et al. 2019. Noninvasive sub-organ ultrasound stimulation for targeted neuromodulation. Nat Commun 10: 952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K. 2015. Optogenetics: 10 years of microbia opsins in neuroscience. Nat Neurosci 18: 1213–1225. 10.1038/nn.4091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs ME, Lee SA, Yang G, Kim S, Wang Q, Konofagou EE. 2018. Non-invasive peripheral nerve stimulation via focused ultrasound in vivo. Phys Med Biol 63: 35011 10.1088/1361-6560/aa9fc2 [DOI] [PubMed] [Google Scholar]
- Frangos E, Ellrich J, Komisaruk BR. 2015. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stim 8: 624–636. 10.1016/j.brs.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabanyi I, Muller PA, Feighery L, Oliveira TY, Costa-Pinto FA, Mucida D. 2016. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell 164: 378–391. 10.1016/j.cell.2015.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigliotti JC, Huang L, Ye H, Bajwa A, Chattrabhuti K, Lee S, Klibanov AL, Kalantari K, Rosin DL, Okusa MD. 2013. Ultrasound prevents renal ischemia-reperfusion injury by stimulating the splenic cholinergic anti-inflammatory pathway. J Am Soc Nephrol 24: 1451–1460. 10.1681/ASN.2013010084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman-Negron JM, Goldman HB. 2017. New devices and technologies for the management of overactive bladder. Curr Urol Rep 18: 94 10.1007/s11934-017-0739-y [DOI] [PubMed] [Google Scholar]
- Johnson RL, Wilson CG. 2018. A review of vagus nerve stimulation as a therapeutic intervention. J Inflamm Res 11: 203–213. 10.2147/JIR.S163248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan EJ, González R, Albors G, Ward MP, Irazoqui P. 2014. Vagus nerve modulation using focused pulsed ultrasound: Potential applications and preliminary observations in a rat. Int J Imaging Syst Technol 24: 67–71. 10.1002/ima.22080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn SE, Hull RL, Utzschneider KM. 2006. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444: 840–846. 10.1038/nature05482 [DOI] [PubMed] [Google Scholar]
- Kang L, Routh VH, Kuzhikandathil EV, Gaspers LD, Levin BE. 2004. Physiological and molecular characteristics of rat hypothalamic ventromedial nucleus glucosensing neurons. Diabetes 53: 549–559. 10.2337/diabetes.53.3.549 [DOI] [PubMed] [Google Scholar]
- Karnani M, Burdakov D. 2011. Multiple hypothalamic circuits sense and regulate glucose levels. Am J Physiol Regul Integr Comp Physiol 300: R47–R55. 10.1152/ajpregu.00527.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, Mehta AD, Levine YA, Faltys M, Zitnik R, et al. 2016. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci 113: 8284–8289. 10.1073/pnas.1605635113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence D, Pittman QJ. 1985. Interaction between descending paraventricular neurons and vagal motor neurons. Brain Res 332: 158–160. 10.1016/0006-8993(85)90399-3 [DOI] [PubMed] [Google Scholar]
- Levin BE, Triscari J, Sullivan AC. 1980. Abnormal sympatho-adrenal function and plasma catecholamines in obese Zucker rats. Pharmacol Biochem Behav 13: 107–113. 10.1016/0091-3057(80)90128-8 [DOI] [PubMed] [Google Scholar]
- Malbert CH, Picq C, Divoux JL, Henry C, Horowitz M. 2017. Obesity-associated alterations in glucose metabolism are reversed by chronic bilateral stimulation of the abdominal vagus nerve. Diabetes 66: 848–857. 10.2337/db16-0847 [DOI] [PubMed] [Google Scholar]
- Marchall R, Taylor I, Lahr C, Abell TL, Espinoza I, Gupta NK, Gomez CR. 2015. Bioelectrical stimulation for the reduction of inflammation in inflammatory bowel disease. Clin Med Insights Gastroenterol 8: 55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston OJ, Hurst P, Evans ML, Burdakov DI, Heisler LK. 2011. Neuropeptide Y cells represent a distinct glucose-sensing population in the lateral hypothalamus. Endocrinology 152: 4046–4052. 10.1210/en.2011-1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei H, Irazoqui PP. 2014. Miniaturizing wireless implants. Nat Biotechnol 32: 1008 10.1038/nbt.3038 [DOI] [PubMed] [Google Scholar]
- Meier K. 2017. Spinal cord stimulation: Background and clinical application. Scan J Pain 5: 115–181. 10.1007/978-3-319-08072-7_17 [DOI] [PubMed] [Google Scholar]
- Menz MD, Oralkan Ö, Khuri-Yakub PT, Baccus SA. 2013. Precise neural stimulation in the retina using focused ultrasound. J Neurosci 33: 4550–4560. 10.1523/jneurosci.3521-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers EE, Kronemberger A, Lira V, Rahmouni K, Stauss HM. 2016. Contrasting effects of afferent and efferent vagal nerve stimulation on insulin secretion and blood glucose regulation. Physiol Rep 4: e12718 10.14814/phy2.12718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T, Liss B, Minami K, Shiuchi T, Saraya A, Kashima Y, Horiuchi M, Ashcroft F, Minokoshi Y, Roeper J, et al. 2001. ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat Neurosci 4: 507–512. 10.1038/87455 [DOI] [PubMed] [Google Scholar]
- Olofsson PS, Tracey KJ. 2017. Bioelectronic medicine: Technology targeting molecular mechanisms for therapy. J Intern Med 282: 3–4. 10.1111/joim.12624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VA, Tracey KJ. 2012. The vagus nerve and the inflammatory reflex—Linking immunity and metabolism. Nat Rev Endocrinol 8: 743–754. 10.1038/nrendo.2012.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VA, Tracey KJ. 2015. Neural circuitry and immunity. Immunol Res 63: 38–57. 10.1007/s12026-015-8718-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randich A, Spraggins DS, Cox JE, Meller ST, Kelm GR. 2001. Jejunal or portal vein infusion of lipids increase hepatic vagal activity. Neuro Rep 12: 3101–3105. 10.1097/00001756-200110080-00024 [DOI] [PubMed] [Google Scholar]
- Reyes TM, Walker JR, DeCino C, Hogenesch JB, Sawchenko PE. 2003. Categorically distinct acute stressors elicit dissimilar transcriptional profiles in the paraventricular nucleus of the hypothalamus. J Neurosci 23: 5607–5616. 10.1523/jneurosci.23-13-05607.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röder PV, Wu B, Liu Y, Han W. 2016. Pancreatic regulation of glucose homeostasis. Exp Mol Med 48: e219 10.1038/emm.2016.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RC, Nelson DO. 1984. Neurons of the vagal division of the solitary nucleus activated by the paraventricular nucleus of the hypothalamus. J Auton Nerv Syst 10: 193–197. 10.1016/0165-1838(84)90057-2 [DOI] [PubMed] [Google Scholar]
- Roh E, Dong DK, Kim MS. 2016. Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism. Exp Mol Med 48: e216 10.1038/emm.2016.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routh VH, Hao L, Santiago AM, Sheng Z, Zhou C. 2014. Hypothalamic glucose sensing: Making ends meet. Front Syst Neurosci 8: 236 10.3389/fnsys.2014.00236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozman J, Bunc M. 2004. Modulation of visceral function by selective stimulation of the left vagus nerve in dogs. Exp Physiol 89: 717–725. 10.1113/expphysiol.2004.027953 [DOI] [PubMed] [Google Scholar]
- Rozman J, Zorko B, Bunc M, Žitko M. 2002. Stimulation of nerves innervating the dog's pancreas. Artif Organs 26: 241–243. 10.1046/j.1525-1594.2002.06942.x [DOI] [PubMed] [Google Scholar]
- Sakaguchi T, Iwanaga M. 1982. Effects of d-glucose anomers on afferent discharge in the hepatic vagus nerve. Experientia 38: 475–476. 10.1007/BF01952646 [DOI] [PubMed] [Google Scholar]
- Schelling G, Delius M, Gschwender M, Grafe P, Gambihler S. 1994. Extracorporeal shock waves stimulate frog sciatic nerves indirectly via a cavitation-mediated mechanism. Biophys J 66: 133–140. 10.1016/S0006-3495(94)80758-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster NM, Rapoport AM. 2016. New strategies for the treatment and prevention of primary headache disorders. Nat Rev Neurol 12: 635–650. 10.1038/nrneurol.2016.143 [DOI] [PubMed] [Google Scholar]
- Shimazu T, Fukuda A. 1965. Increased activities of glycogenolytic enzymes in liver after splanchnic-nerve stimulation. Science 150: 1607–1608. 10.1126/science.150.3703.1607 [DOI] [PubMed] [Google Scholar]
- Shiota M, Printz RL. 2012. Diabetes in Zucker diabetic fatty rat. Methods Mol Biol 933: 103–123. 10.1007/978-1-62703-068-7_8 [DOI] [PubMed] [Google Scholar]
- Slavin KV. 2008. Peripheral nerve stimulation for neuropathic pain. Neurotherapuetics 5: 100–110. 10.1016/j.nurt.2007.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan K, Ramarao P. 2007. Animal models in type 2 diabetes research: An overview. Ind J Med Res 125: 451–472. [PubMed] [Google Scholar]
- Stauss H, Meyers E, Glab T, Rahmouni K. 2015. Modulation of blood glucose concentration by vagal nerve stimulation. FASEB J 29(Suppl): 828.6.25411436 [Google Scholar]
- Tanida M, Yamamoto N, Morgan DA, Kurata Y, Shibamoto T, Rahmouni K. 2015. Leptin receptor signaling in the hypothalamus regulates hepatic autonomic nerve activity via phosphatidylinositol 3-kinase and AMP-activated protein kinase. J Neurosci 35: 474–484. 10.1523/jneurosci.1828-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temel Y, Jahanshahi A. 2015. Treating brain disorders with neuromodulation. Science 347: 1418–1419. 10.1126/science.aaa9610 [DOI] [PubMed] [Google Scholar]
- Torii K, Niijima A. 2001. Effect of lysine on afferent activity of the hepatic branch of the vagus nerve in normal and L-lysine-deficient rats. Physiol Behav 72: 685–690. 10.1016/S0031-9384(01)00426-7 [DOI] [PubMed] [Google Scholar]
- Torres-Rosas R, Yehia G, Peña G, Mishra P, del Rocio Thompson-Bonilla M, Moreno-Eutimio MA, Arriaga-Pizano LA, Isibasi A, Ulloa L. 2014. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat Med 20: 291–295. 10.1038/nm.3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ. 2002. The inflammatory reflex. Nature 420: 853–859. 10.1038/nature01321 [DOI] [PubMed] [Google Scholar]
- Tracey KJ. 2009. Reflex control of immunity. Nat Rev Immunol 9: 418–428. 10.1038/nri2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ. 2016. Reflexes in immunity. Cell 164: 343–344. 10.1016/j.cell.2016.01.018 [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Tufail Y, Finsterwald M, Tauchmann ML, Olson EJ, Majestic C. 2008. Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound. PLoS ONE 3: e3511 10.1371/journal.pone.0003511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemze RA, Luyer MD, Buurman WA, de Jonge WJ. 2015. Neural reflexes in intestinal inflammation: Hypothesis to viable therapy. Nat Gev Gastroenterol Hepatol 12: 353 10.1038/nrgastro.2015.56 [DOI] [PubMed] [Google Scholar]
- Wolter T. 2014. Spinal cord stimulation for neuropathic pain: Current perspectives. J Pain Res 7: 651–663. 10.2147/JPR.S37589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CJ, Haqshenas SR, Rothwell J, Saffari N. 2017. Unmyelinated peripheral nerves can be stimulated in vitro using pulsed ultrasound. Ultrasound Med Biol 43: 2269–2283. 10.1016/j.ultrasmedbio.2017.05.008 [DOI] [PubMed] [Google Scholar]
- Yamashita H, Inenaga K, Koizumi K. 1984. Possible projections from regions of paraventricular and supraoptic nuclei to the spinal cord: Electrophysiological studies. Brain Res 296: 373–378. 10.1016/0006-8993(84)90077-5 [DOI] [PubMed] [Google Scholar]
- Yi CX, la Fleur SE, Fliers E, Kalsbeek A. 2010. The role of the autonomic nervous liver innervation in the control of energy metabolism. Biochim Biophys Acta 1802: 416–431. 10.1016/j.bbadis.2010.01.006 [DOI] [PubMed] [Google Scholar]
- Yuan H, Silberstein SD. 2016. Vagus nerve and vagus nerve stimulation, a comprehensive review. Part I. Headache 56: 71–78. 10.1111/head.12647 [DOI] [PubMed] [Google Scholar]
- Zachs DP, Offutt SJ, Graham RS, Kim Y, Mueller J, Auger JL, Schuldt NJ, Kaiser CRW, Heiller AP, Dutta R, et al. 2019. Noninvasive ultrasound stimulation of the spleen to treat inflammatory arthritis. Nat Commun 10: 951. [DOI] [PMC free article] [PubMed] [Google Scholar]