Abstract
Stimulation of cell-surface receptors induces cytosolic Ca2+ ([Ca2+]i) increases that are detected and transduced by effector proteins for regulation of cell function. Intracellular Ca2+ release, via endoplasmic reticulum (ER) proteins inositol 1,4,5-trisphosphate receptors (IP3R) and ryanodine receptors (RyR), and Ca2+ influx, via store-operated Ca2+ entry (SOCE), contribute to the increase in [Ca2+]i. The amplitude, frequency, and spatial characteristics of the [Ca2+]i increases are controlled by the compartmentalization of proteins into signaling complexes such as receptor-signaling complexes and SOCE complexes. Both complexes include protein and lipid components, located in the plasma membrane (PM) and ER. Receptor signaling initiates in the PM via phospholipase C (PLC)-mediated hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2), and culminates with the activation of IP3R in the ER. Conversely, SOCE is initiated in the ER by Ca2+-sensing stromal interaction molecule (STIM) proteins, which then interact with PM channels Orai1 and TRPC1 to activate Ca2+ entry. This review will address how ER–PM junctions serve a central role in agonist regulation of SOCE.
Calcium (Ca2+) signaling mechanisms within a cell are tightly controlled to ensure generation of [Ca2+]i signals with appropriate magnitude as well as spatial and temporal characteristics that are required for regulating specific physiological functions. [Ca2+]i signals usually consist of an increase in cytosolic Ca2+ concentration, which is detected and transduced by effector proteins (Berridge 2006). In nonexcitable cells, such increases in [Ca2+]i result from the stimulation of cell-surface receptors, primarily G-protein-coupled (GPCR) or tyrosine-kinase-coupled receptors, which lead to activation of phospholipase C (PLC) and hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2). This is a critical initial step in the Ca2+-signaling cascade and results in the generation of two intracellular messengers: inositol 1,4,5-trisphosphate (IP3) and diacyl glycerol (DAG). DAG is retained in the plasma membrane (PM) where it modulates a number of cellular functions such as regulation of ion channels and enzyme activation. IP3, a soluble metabolite, diffuses in the cytosol and binds to its receptor, the IP3R, which is a Ca2+ channel localized in the endoplasmic reticulum (ER) membrane. The resulting release of Ca2+ from the ER lumen leads to an increase in [Ca2+]i, which is used to control many cellular functions, including ion channel regulation and gene expression (Mikoshiba 2015). Release of Ca2+ from the ER also causes a decrease in Ca2+ concentration within the ER lumen ([Ca2+]ER) and, depending on the magnitude, a substantial and sustained decrease in [Ca2+]ER can be deleterious to cells, affecting processes such as protein synthesis and cell proliferation (Mekahli et al. 2011). The ER-Ca2+ pool is finite, and maintaining long-term signaling requires refilling of the ER-Ca2+ stores via Ca2+ influx from the extracellular medium. In the absence of Ca2+ influx, the release of Ca2+ from the ER would be transient in nature because of progressive rundown of [Ca2+]ER and the lack of ER-Ca2+ store refilling by incoming extracellular Ca2+. This review will discuss current concepts pertaining to IP3-induced Ca2+ signaling and the store-operated Ca2+ entry (SOCE) pathway (Putney 1990, 2017).
SOCE is mediated by PM Ca2+ channels activated in response to decreases in [Ca2+]ER and inactivated by refilling of the ER-Ca2+ stores (Ambudkar 2006; Ambudkar et al. 2006; Putney 2017). The close coupling between SOCE and Ca2+ uptake into the ER suggests the formation of cellular microdomains, in which SOCE components in the PM are in close proximity to ER-Ca2+-signaling components, such as sarco/ER Ca2+ ATPase (SERCA) and IP3Rs. Many studies have now established that SOCE is regulated within ER–PM junctions that are formed by interactions of ER-resident proteins with the PM lipid or protein components. The PM protein Orai1 was identified as a critical component of SOCE as it forms the pore of the highly Ca2+-selective channel that is activated by ER-Ca2+ store depletion. This channel carries the Ca2+ release-activated Ca2+ current (ICRAC; Feske et al. 2006; Prakriya et al. 2006; Vig et al. 2006; Zhang et al. 2006; Hou et al. 2012; Yen et al. 2016). Orai1 has four transmembrane-spanning domains and assembles to form a hexameric channel that is activated by direct interaction with the ER proteins called STIMs, STIM1 and STIM2. Both STIM isoforms sense the decrease in [Ca2+]ER via amino-terminal Ca2+-binding domains, known as EF-hand domains, that are located in the ER lumen. Release of Ca2+ from the EF-hand domain triggers conformational changes to enable STIM1 or STIM2 protein clustering at the cell periphery where they recruit Orai1. The STIM carboxy-terminal tail mediates its binding to PM PIP2, whereas the cytosolic STIM–Orai1 activation region (SOAR; also known as CRAC activation domain [CAD]) interacts with and gates Orai1 (Liou et al. 2005; Roos et al. 2005; Brandman et al. 2007; Park et al. 2009; Yuan et al. 2009b; Bhardwaj et al. 2013). The STIM–Orai1 interaction, together with STIM1 scaffolding at the PM, culminates in the formation of ER–PM junctions that mark the sites of Ca2+ entry into the cell. Activation of clustered Orai1 channels generates higher [Ca2+]i near the cytosolic mouth of the channels (referred to as local increase in [Ca2+]i) than when the channels are diffusely spread within the PM (Samanta et al. 2015). Both local and global [Ca2+]i changes are used by cells to regulate distinct functions (Kar et al. 2011; Ong et al. 2012; Kar and Parekh 2015).
Transient receptor potential canonical (TRPC) channels, suggested as candidates for SOCE channels before the discovery of STIM and Orai, are also activated in response to receptor-stimulated PM PIP2 hydrolysis, and contribute to agonist-induced [Ca2+]i increases (Ambudkar et al. 2006; Abramowitz et al. 2007; Yuan et al. 2009a). TRPC channels regulate important physiological functions in several tissues such as exocrine glands, endothelium, cardiac muscle, neurons, and kidney cells (Ong et al. 2014). TRPC1, TRPC3, and TRPC4 have been shown to mediate Ca2+ entry following PM receptor stimulation and/or ER-Ca2+ store depletion. TRPC channel association with the Orai1/STIM1 complex is shown by the interaction of TRPC1 and TRPC3 with STIM1 (Yuan et al. 2007; Zeng et al. 2008). The localization of TRPC1 with Orai1/STIM1 in ER–PM junctions is a critical determinant of its activation (Pani et al. 2009; Cheng et al. 2011; de Souza et al. 2015). The polybasic domain of STIM1, specifically 684KK685, which is distinct from the SOAR/CAD domain, activates TRPC1 by binding to 639DD640 (Huang et al. 2006; Zeng et al. 2008). In cells expressing both TRPC1 and Orai1, both channels are activated by ER-Ca2+ depletion. Further, TRPC1-mediated Ca2+ entry enhances the [Ca2+]i increase induced by Orai1 and contributes to sustained elevation of [Ca2+]i (Ong et al. 2007). Importantly, Ca2+ signals generated by TRPC1 are used by cells for regulating functions distinct from those activated by Orai1; for example, NFAT is regulated by Ca2+ entry mediated by Orai1, whereas NF-κB regulation involves TRPC1. Local [Ca2+]i increase mediated by Orai1 is exclusively used for NFAT1 activation. The increase in [Ca2+]i detected by calmodulin leads to activation of calcineurin, dephosphorylation of NFAT1, and translocation of the transcription factor to the nucleus. Global Ca2+ signals resulting from TRPC1 function are detected by [Ca2+]i sensors, such as calmodulin kinase II, to cause NF-κB activation. Additionally, in exocrine gland cells, TRPC1-mediated Ca2+ entry is required for regulation of Ca2+-activated Cl– and K+ channel activities (Cheng et al. 2011; Hong et al. 2011; Ong et al. 2012; Sun et al. 2015).
The nature of SOCE-associated ER–PM junctions, their molecular components, and architecture, as well as their physiological relevance, are currently the subject of many studies in the field of Ca2+ signaling. Emerging data suggest that these junctions are not only involved in sensing agonist stimulation and regulating SOCE but also in compartmentalization of specific downstream targets and effector proteins that drive SOCE-dependent control of cell function. In the later sections of this article, we will discuss the role of ER–PM junctions as signaling hubs that coordinate agonist regulation of PIP2 hydrolysis with depletion of ER-Ca2+ store and activation of SOCE. Precise spatial and temporal coordination of these processes generates specific Ca2+ signals that regulate different cell functions.
AGONIST-DEPENDENT GENERATION OF [Ca2+]i SIGNALS
Signaling Complexes and ER–PM Junctions
Two major types of stimuli that trigger [Ca2+]i increases in nonexcitable cells are those acting on GPCRs (e.g., muscarinic and α1-adrenergic receptors) and those acting on tyrosine kinase-coupled receptors (e.g., growth factor receptors and T-cell receptor [TCR]; Berridge 2017). Activation of either class of PM receptors leads to the assembly of a receptor-associated signaling complex that includes G proteins, PLCs, and downstream targets such as adenylyl cyclases, RyR, and IP3R. A family of proteins that function as regulators of G protein signaling (RGS) has been suggested to serve as scaffolds in the assembly of such signaling complexes. Additionally, other scaffolding proteins (e.g., HOMER and A-kinase anchoring proteins [AKAPs]) and PM lipid domains have also been implicated in this process. Such association between proteins of the ER and PM results in the formation of ER–PM junctions. An important function of these junctions, which contain PM receptor-signaling proteins and the IP3R, ensures that IP3 is effectively delivered from the site of its synthesis in the PM to its receptor in the ER membrane with minimal diffusion or hydrolysis. Targeted delivery of IP3 increases the response speed and organizes the Ca2+ signals in spatially segregated areas within the cell (Delmas et al. 2002). Interaction of IP3Rs with other proteins, such as PLCs, receptor of activation protein C kinase 1 (RACK1), AKAPs, or HOMER, may bring them close to the PM receptor-signaling complex to facilitate IP3 delivery in a selective and rapid manner. Notably, some of these proteins also interact with TRPC channels and Orai1 (Ong et al. 2014; Prole and Taylor 2016). Thus, Ca2+ signaling is organized to be rapid and specific through compartmentalization of signaling proteins within privileged ER–PM junctions that are assembled to initiate the PLC cascade and IP3-induced Ca2+ release from the ER (Thillaiappan et al. 2019).
TRPC channels reside in PM lipid raft domains (LRDs), which are also populated by a number of receptor-coupled signaling proteins (Ong et al. 2014). These channels assemble and form signaling complexes with PLC, IP3R, G proteins, and scaffolding proteins such as RACK1 (TRPC3) and HOMER (TRPC1; Yuan et al. 2003; Bandyopadhyay et al. 2008; Ong et al. 2014). Taken together, these findings showed that PM Ca2+ influx channels, activated in response to PIP2 hydrolysis, are recruited into the same junctions as the signaling complex that causes hydrolysis of PIP2. Importantly, biochemical and total internal reflection microscopy (TIRFM) data show interactions between TRPC1 and TRPC3 with STIM1 and Orai1 (Ong et al. 2007; Yuan et al. 2007; Cheng et al. 2008, 2011; Zeng et al. 2008). These findings place Orai1/STIM1 together with TRPC channel within close proximity to the receptor-signaling complexes and IP3Rs. Further confirmation is provided by a recent study showing that native IP3Rs are preclustered in cellular areas in which STIM1 accumulates after store depletion (Thillaiappan et al. 2017). It has been suggested that this allows STIM1 to sense decreases in [Ca2+]ER near the IP3Rs, and rapidly respond to activate Orai1 within the same microdomain. This would also lead to regulation of TRPC1 function (further discussed below), which is directed to regions where Orai1/STIM1 cluster via a fast endocytic recycling pathway (de Souza et al. 2015) or interaction with PM caveolin-1 ([Cav-1]; Brazer et al. 2003; Pani et al. 2009). It is not clear whether the receptor and SOCE-signaling complex involve the formation of smaller “sub” or “nano” domains within a larger ER–PM junction or whether they are both segregated within a single large domain (summarized in the model shown in Fig. 1). Research in the field of lipid rafts has suggested that smaller rafts containing different components can undergo fusion to allow proteins seggregated in two different domains to be incorporated into one domain, enabling protein–protein interactions to occur in a regulated manner (Ong and Ambudkar 2015). Such a mechanism would bring agonist-signaling components within close proximity to SOCE components to facilitate interactions between them. There are no data to directly show this. However, it has been reported that the association of Orai1/STIM1 complexes with PM lipid domains may be altered following stimulation of Ca2+ entry (Maleth et al. 2014). Additionally, remodeling of ER–PM junctions has also been reported (further discussed below), which could reorganize the signaling complex and modulate protein function.
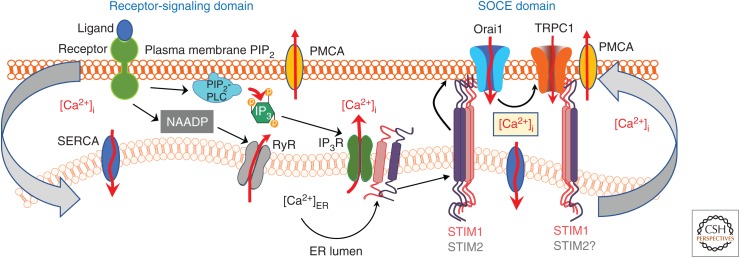
Figure 1.
Organization of endoplasmic reticulum–plasma membrane (ER–PM) junctions involved in agonist-regulated store-operated Ca2+ entry (SOCE). Binding of an agonist/ligand to its G-protein-coupled receptor in the PM triggers a signaling cascade that results in phospholipase C (PLC)-mediated hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) in the PM and generation of the second messenger inositol 1,4,5-trisphosphate (IP3). When IP3 binds to its receptor (IP3R) in the ER membrane, it causes release of Ca2+ from the ER-Ca2+ stores. Alternatively, the second messenger NAADP can activate the ryanodine receptors (RyRs) to cause Ca2+ release from the ER. The resulting decrease in Ca2+ concentration within the ER lumen ([Ca2+]ER) is sensed by stromal interaction molecules STIM1 and STIM2, which aggregate and translocate to the cell periphery to activate Orai1. Ca2+ entry via Orai1 raises the local [Ca2+] (denoted by boxed [Ca2+]i) near the channel pore, which is required for the trafficking of TRPC1 to and its insertion into the PM, as well as activation of NFAT signaling (not shown). Following channel insertion into the PM, TRPC1 is activated by STIM1. At the moment, it has not been shown that STIM2 also activates transient receptor potential canonical 1 (TRPC1). The Ca2+ entry via TRPC1 contributes to a global increase in [Ca2+]i, which is required for other downstream Ca2+-dependent processes such as NF-κB activation and fluid secretion in salivary glands. Additionally, the sarco/ER Ca2+ ATPase (SERCA) and PM Ca2+-ATPase (PMCA) pumps also modulate the [Ca2+]i by pumping Ca2+ into the ER or outside of the cell, respectively. Assembly of the proteins within these junctions coordinate PM–ER signaling, which then induces ER–PM signaling. Precise attunement of the functional and physical interactions between ER and PM proteins is critical for the cell.
Stimulus Intensities and Activation of SOCE
Agonist-induced, IP3-dependent release of Ca2+ from the ER causes [Ca2+]i elevation, the pattern and amplitude of which varies depending on the cell type and stimulus intensity. Low agonist concentrations typically trigger an oscillatory pattern of [Ca2+]i increase. Intermediary agonist concentrations induce either more frequent Ca2+ oscillations or Ca2+ oscillations arising from an elevated baseline. High agonist concentrations can cause a sustained elevation of [Ca2+]i (Bird and Putney 2005; Putney and Bird 2008; Ong et al. 2015). Both intracellular Ca2+ release via IP3Rs and extracellular Ca2+ influx via SOCE or other Ca2+ channels contribute to the elevations in [Ca2+]i. Several Ca2+ flux mechanisms in the cell can contribute to Ca2+ oscillations. In some cases, positive feedback regulation of PLC by Ca2+ causes IP3 levels to oscillate, which can drive periodic release of Ca2+ from the ER (Kawabata et al. 1996; Nash et al. 2001). However, Ca2+ oscillations have also been detected in cells with stable IP3 levels (Dupont et al. 2011). Importantly, [Ca2+]i oscillations in many cell types can be supported for a short time period in the absence of external Ca2+ because of repetitive Ca2+ release and reuptake into ER. Such [Ca2+]i oscillations are primarily controlled by regulation of IP3R function by IP3 and [Ca2+]i. The Ca2+-dependent activation of IP3Rs, followed by their Ca2+-dependent inhibition, can fully explain Ca2+ oscillations in most cells (Iino 1990; Bezprozvanny et al. 1991). In the absence of Ca2+ influx, there is an incremental loss of Ca2+ content in the ER during the oscillations as a result of the efflux of Ca2+ from the cell by the PM Ca2+ pump PMCA. Sustained Ca2+ oscillations or elevation in [Ca2+]i, which are critical for regulating most cellular functions, are dependent on Ca2+ entry, primarily via the SOCE mechanism (Parekh 2011; Thillaiappan et al. 2019).
SOCE modifies the pattern of Ca2+ oscillations by increasing the frequency and/or amplitude of the Ca2+ signal with a more sustained elevation of [Ca2+]i being generated at higher stimulus intensities. It is important to consider the status of ER-Ca2+ stores at different stimulus intensities. At low agonist concentrations, as a result of less IP3 generation and oscillatory [Ca2+]i, there is less depletion of ER-Ca2+ stores. Conversely, a higher agonist concentration produces a greater amount of IP3 and substantial depletion of [Ca2+]ER. Thus, SOCE modulation requires differential Ca2+ sensing of ER-Ca2+ stores across the range of [Ca2+]ER that result from agonist stimulation of cells. Although STIM1 and STIM2 share many properties, they have key differences that are critical in determining agonist regulation of SOCE. The different Ca2+ affinities of their EF-hand domains allow the proteins to differentially respond at high and low [Ca2+]ER, and regulate the response of SOCE to different stimulus intensities (Liou et al. 2005; Brandman et al. 2007; Carrasco and Meyer 2011; Ong et al. 2015; Subedi et al. 2018). STIM1 has a relatively high affinity for Ca2+ (about 200 µm) and thus can respond only when [Ca2+]ER is substantially decreased. Such STIM1-activating [Ca2+]ER decreases are typically achieved only with high agonist concentrations, or when SERCA function is compromised in cells. On the other hand, STIM2 has lower affinity for Ca2+ (around 400 µm) and can sense small changes in [Ca2+]ER (note that ambient [Ca2+]ER is around 600–800 µm). Thus, STIM2 is well placed to respond to low agonist concentrations that lead to small decreases in [Ca2+]ER, such as those observed in cells with [Ca2+]i oscillations. Together, STIM2 and STIM1 can regulate activation of SOCE across a wide range of agonist concentrations and consequent [Ca2+]ER depletion levels. Indeed, previous findings have shown that STIM2 tunes the sensitivity of SOCE activation at low agonist concentration (Ong et al. 2015). STIM2 has been shown to be important in maintaining both basal Ca2+ entry and Ca2+ influx in response to weak levels of stimulation (Brandman et al. 2007). Moreover, STIM1 is much more effective than STIM2 at gating Orai1, resulting in more Ca2+ entry via the channel, which is critical for cell function. STIM2 causes relatively weak Orai1 channel activation, which leads to less [Ca2+]i increase. [Ca2+]i signals generated by Orai1/STIM1 or Orai1/STIM2 complexes have been reported to cause activation of distinct cellular functions in mast cells (Kar et al. 2012; Thiel et al. 2013). Despite the contribution of STIM2 in Orai1 function, STIM1 has been established as the critical regulatory component for Orai1 activation. SOCE and SOCE-dependent cell function, at both low and high agonist concentration, are almost completely eliminated in cells lacking STIM1 (Luik et al. 2008; Oh-Hora et al. 2013; Ong et al. 2015; Subedi et al. 2018). A major question arising from these findings is how does STIM1 activate Orai1 at [Ca2+]ER that is not low enough to trigger its activation. Indeed, TIRFM shows that STIM1 does not respond to low [agonist] (Ong et al. 2015).
Recent findings have revealed a novel function for STIM2 in regulating SOCE. STIM2 mediates recruitment of STIM1 to ER–PM junctions, facilitating assembly of STIM1/Orai1 at low agonist concentrations (Ong et al. 2015; Subedi et al. 2018). Further, interaction of STIM1 with STIM2 causes a conformational change in the former that leads to STIM1-activating Orai1 even when [Ca2+]ER is not low enough to elicit a STIM1 response (Subedi et al. 2018). This scaffolding role for STIM2 appears to be critical for SOCE-dependent regulation of cell functions, such as cytokine synthesis in stimulated T lymphocytes, which is dependent on the assembly of Orai1/STIM1 complexes and strong Orai1 activation (Oh-Hora et al. 2008; Diercks et al. 2018). The relatively stronger PIP2-binding domain in STIM2 is a key determinant of its ability to cluster and generate ER–PM junctions in response to low level stimuli (Bhardwaj et al. 2013). Importantly, the carboxyl terminus of STIM2 has been shown to be in a more open conformation that allows the protein to be in an activated state to recruit and activate Orai1 in cells with minimal depletion of ER-Ca2+ stores (Subedi et al. 2018; Zheng et al. 2018). This ability of STIM2 to respond to small depletions of ER-Ca2+ stores and recruit Orai1/STIM1 under conditions in which STIM1 cannot respond on its own plays a critical role in the regulation of cell function.
REGULATION OF TRPC CHANNELS IN ER–PM JUNCTIONS
All TRPC channels are activated in response to stimulation of PIP2 hydrolysis by agonists. Some of them are activated by agonist-induced depletion of ER-Ca2+ stores (Ambudkar et al. 2006; Yuan et al. 2009a; Ong et al. 2014), whereas others are dependent on metabolites arising from receptor-stimulated PIP2 hydrolysis (Hurst et al. 1998; Liu et al. 2000; Strübing et al. 2001). The available data are the most consistent and strongest in support of a role for TRPC1 in SOCE. Nonetheless, a few studies have shown TRPC3 and TRPC4 to be activated by store depletion. Importantly, TRPC1 and TRPC3 are dependent on Orai1 and STIM1 for their function, as loss of either of these latter components eliminates TRPC-mediated Ca2+ entry. In contrast, knockdown of TRPC channels only causes partial reduction of Ca2+ entry. However, loss of TRPC function is reflected by a change in the phenotype of the current induced by store depletion. In cells expressing both TRPC and Orai channels, the current is typically nonselective, referred to as store-operated calcium current (ISOC). Loss of TRPC channels results in attenuation of the ISOC current. Instead, ICRAC carried by the residual Orai1 channel can be detected (Cheng et al. 2011). In cells containing both Orai1 and TRPC1, both channels contribute to [Ca2+]i increase. Nevertheless, Ca2+ entry mediated by each channel is used by cells to regulate distinct functions.
Several studies have shown that TRPC1 and Orai1 cocluster and interact with STIM1 within ER–PM junctions. STIM1 is required for clustering, as well as activation, of the channels. LRDs are enriched in cholesterol and sphingolipids, and have been proposed to provide a stable platform for the assembly of Ca2+-signaling complexes (Pani and Singh 2009; Lingwood and Simons 2010; Ong and Ambudkar 2012). The structural integrity of LRD appears to be vital for SOCE as disruption, using methyl-β-cyclodextrin to sequester cholesterol, has been shown to attenuate SOCE in many cell types. Moreover, LRDs in the PM are involved in localizing TRPC1 channels to the ER–PM junctions (Lockwich et al. 2000; Brownlow et al. 2004; Kannan et al. 2007; Jardin et al. 2008; Pani et al. 2008; Bomben et al. 2011). Disruption of LRD causes impairment of TRPC1 assembly with Orai1, STIM1, and the type 2 isoform of the inositol 1,4,5-triphosphate receptor ([IP3R2]; Brownlow and Sage 2003; Brownlow et al. 2004; Pani et al. 2008; Galan et al. 2010). TRPC1 and STIM1 partition into, and interact within, LRDs following store depletion (Lockwich et al. 2000; Berthier et al. 2004; Kannan et al. 2007; Pani et al. 2008; Formigli et al. 2009). Recruitment of TRPC1 into LRDs is mediated by Cav-1, whereas STIM1 is likely to interact directly with the PM phospholipids via its carboxy-terminal polybasic domain. Subsequent studies have shown that the Orai1/STIM1 complexes are also recruited into a microdomain that is PIP2-rich and contains Cav-1 (Jha et al. 2013; Maleth et al. 2014). Cav-1 has been also shown to regulate Orai1 function and trafficking (Yu et al. 2010; Yeh and Parekh 2015). These studies further highlight the importance of specialized PM domains in the assembly and regulation of SOCE channels.
Several studies have addressed the critical functional interaction between Orai1 and TRPC1. Strong evidence supports the suggestion that Ca2+ entry via Orai1 triggers recruitment of TRPC1 to the PM (Cheng et al. 2011). Thus, coordinated regulation of TRPC1 surface expression by Orai1 and gating by STIM1 provides a mechanism for rapidly modulating and amplifying Orai1-generated Ca2+ signals (Ong et al. 2012). By recruiting ion channels (e.g., TRPC channels) and other signaling components (e.g., adenylyl cyclase and NFAT-signaling complexes), Orai1 and STIM1 concertedly impact a variety of critical cell functions. The specific effector proteins that are involved in sensing the respective Ca2+ signals for regulating cell function are not yet known for TRPC1. Although AKAP79 provides a scaffold to coordinate Orai1-mediated NFAT activation (Kar et al. 2014), there are no data to show a role for AKAP79 in TRPC1-mediated regulation of cell function. Whereas other scaffolds, such as RACK1, Homer, and NHERF (Na+/H+ exchange regulatory factor), have been associated with TRPC proteins, it remains to be seen whether these represent signaling complexes in different ER–PM junctions (Ong et al. 2014).
ER-Ca2+ STORE RELEASE—A CRITICAL LINK BETWEEN RECEPTOR SIGNALING AND SOCE
IP3Rs
IP3 links receptor-stimulated, PLC-mediated, hydrolysis of PIP2 in the PM with ER-Ca2+ release via the IP3R. This critical step, leading to a decrease in [Ca2+]ER, is also the trigger for STIM/Orai1 clustering, TRPC recruitment, and activation of SOCE. Thus, signals are transmitted from the PM to the ER and back to the PM (denoted in the model in Fig. 1). The generation and concentration of IP3, as well as the location and activity of IP3R, are critical factors in the coordinated regulation of SOCE. IP3R function is exquisitely regulated by a number of different cellular factors. Although gating is primarily induced by IP3, Ca2+ exerts an important biphasic effect on IP3R, which enables the channel to be activated when [Ca2+]i is low but inhibited when [Ca2+]i is elevated above a certain threshold (Iino 1990; Bezprozvanny et al. 1991; Thillaiappan et al. 2019). IP3Rs are also regulated by cAMP-dependent protein kinase A (PKA). IP3R phosphorylation enhances IP3 binding affinity (Taylor 2017). Because Ca2+ entering via SOCE regulates Ca2+-dependent adenylyl cyclases within ER–PM junctions, IP3R in the vicinity of ER–PM junctions could be sensitized by cAMP to respond to low IP3 concentrations. Thus, there is a feedforward regulation of IP3R by SOCE via generation of cAMP and activation of PKA (Thillaiappan et al. 2019). Conversely, adenylyl cyclases that are inhibited by Ca2+ will be suppressed by SOCE, causing a decrease in ambient cAMP concentration and dampening of IP3R function (Willoughby et al. 2014).
It is important to note that although IP3Rs are localized throughout the ER, the distribution is not uniform. The location of IP3R in the cell determines the spatial characteristics of IP3-generated Ca2+ signals, and also likely defines the location of other signaling proteins that are regulated by the Ca2+ released from the ER. In polarized cells like exocrine gland acinar cells, IP3Rs are enriched in the apical region where the Ca2+ signal initiates following stimulation of cells (Yule et al. 1997; Lee et al. 2006; Petersen and Tepikin 2008). Importantly, STIM1, Orai1, and TRPC channels also overlap, at least partially, with IP3R in this region, suggesting close association between IP3Rs and the SOCE-signaling components in the PM (Hong et al. 2011). It has been reported that there are two populations of IP3R: mobile (∼75%) and immobile receptors. The latter group are found in discrete clusters at the ER membrane localized immediately beneath the PM. Interestingly, the small population of immobile IP3R clusters represent the sites of Ca2+ release induced by agonists, despite the population of mobile receptors. Further, following ER-Ca2+ release, STIM1 accumulates at ER–PM junctions that are located adjacent to immobile IP3Rs. These findings proposed that the immobile IP3R clusters are “licensed” receptors positioned to sense IP3 in the vicinity of the receptor-signaling complex in which it is generated. STIM1 localized near these IP3R can sense this decrease in local [Ca2+]ER and rapidly move to the ER–PM junctions to recruit and activate Orai1 (Thillaiappan et al. 2017). Together, these interesting findings place IP3R as a central Ca2+-signaling protein that links the receptor-mediated signaling events with those that result in SOCE activation. A key point to be considered is that both GPCR- and SOCE-associated signaling components are compartmentalized within ER–PM junctions. Localization of STIM1 in the close vicinity of IP3R suggests cross talk between these two functionally linked signaling complexes, in which the function of one protein may influence that of another. Alternatively, remodeling of SOCE-associated ER–PM junctions could bring the SOCE-associated signaling components close to the IP3R/GPCR-signaling complex. This would allow IP3R to rapidly respond to IP3 generation and STIM proteins to efficiently detect a decrease in the ER locally near the site of Ca2+ release, and activate Orai1 and TRPC channels. Currently, available data do not resolve between these two possible scenarios, which may not be mutually exclusive.
RyRs and T-Cell Receptor
TCR engagement leads to depletion of Ca2+ in the ER-Ca2+ stores followed by activation of SOCE, primarily via Orai1 (Lewis and Cahalan 1989; Zweifach and Lewis 1996). The cellular location where T cells make contact with antigen-presenting cell, the immunological synapse (IS), represents the signaling center in the cell. It has been shown that Orai1 and STIM1 are rapidly recruited to the IS, resulting in enhanced localized calcium influx at this site. Further, Orai1 and STIM1 colocalized with IS proteins, CD3, CD28, and LFA-1. Two types of T-lymphocyte potassium channel, Kv1.3 and KCa3.1, that are indirectly involved in functional Ca2+ signaling, are also recruited to the IS. Although SOCE is not required for IS formation, blocking Ca2+ entry compromises its long-term stability (Panyi et al. 2004; Beeton et al. 2006; Nicolaou et al. 2007). For example, SOCE is involved in the reorganization of actin, which helps regulate the intensity and duration of TCR signaling (Hartzell et al. 2016).
The earliest intracellular signals that occur after T-cell activation are local Ca2+ microdomains, which occur in the sub-PM region, close to where the TCR is activated. Ryanodine receptor type 1 (RyR1), NAADP, and Ca2+ entry contribute to these Ca2+ microdomains (Wolf et al. 2015; Wolf and Guse 2017). Recently, Ca2+ microdomain formation has also been described in unstimulated T cells, which require Orai1, STIM1, and STIM2, and which are preclustered within ER–PM junctions (Diercks et al. 2018). Following cell stimulation, the number of Ca2+ microdomains increased locally near the site of stimulation. This increase requires Orai1, STIM2, RyR1, and NAADP. The latter complex, involving ER-Ca2+ release, rapidly increases the number of Ca2+ microdomains, causing global spread of Ca2+ signals to promote full T-cell activation. Dependent on TCR stimulation, NAADP, IP3, and cADPR are formed sequentially to bind and activate their respective target Ca2+ channels in the ER such as RyRs and IP3Rs (Streb et al. 1983; Guse et al. 1999; Gasser et al. 2006). The preformed Orai1/STIM1/STIM2 complexes may enable the SOCE machinery in T cells to respond very quickly to ER-Ca2+ store depletion.
ACCESSORY PROTEINS WITHIN ER–PM JUNCTIONS—TETHERING AND LIPID TRANSFER PROTEINS
SOCE-associated ER–PM junctions are suggested to contribute to maintenance and/or replenishment of PM lipids, including sterols and phosphoinositides. Once in the junctions, STIM1 and Orai1 trigger the recruitment of proteins that induce remodeling of PM lipids as well as the underlying cytoskeleton (Jha et al. 2013; Sharma et al. 2013; Jing et al. 2015). Extended synaptotagmins (E-Syts1, -2, and -3) are ER proteins that tether to the PM and promote the formation and stabilization of ER–PM junctions. These proteins have a lipid-binding domain (SMP domain), which has been implicated in lipid transfer between membranes independently of vesicular transport (Herdman and Moss 2016). In E-Syts, the SMP domain binds phosphatidylcholine, phosphatidylethanolamine, phosphatidylinositol (PI), and phosphoserine (Schauder et al. 2014). The conserved domain 2 (C2 domain) has been shown to mediate the Ca2+-dependent binding of E-Syts to PM PIP2, which is required for tethering the proteins to the PM. The ER–PM gap and characteristics of the junctions are determined by the carboxy-terminal lengths of the proteins. E-Syts2 and -3 contribute to formation of constitutive junctions, which display further narrowing following stimulation. In contrast, E-Syt1 is primarily intracellular in unstimulated cells and recruited to ER–PM junctions after activation of SOCE, as it is anchored to PM PIP2 in a Ca2+-dependent manner (Min et al. 2007; Herdman and Moss 2016). Agonist stimulation of the cell induces formation of new junctions at which STIM1 colocalizes with Orai1 and E-Syt1. Although knockout of all three E-Syts is required to significantly reduce the number of ER–PM junctions, silencing E-Syt1 attenuates Orai1/STIM1 clustering (Giordano et al. 2013; Idevall-Hagren et al. 2015). However, the effect of E-Syt knockdown on SOCE has not been clearly established. Whereas one study shows that there is no effect on Orai1 function (Giordano et al. 2013), another shows diminished histamine-induced Ca2+ pulses (Chang et al. 2013). It can be suggested that ER–PM junctions containing E-Syts2 and -3 represent preformed sites where SOCE components can assemble immediately following ER-Ca2+ store depletion, whereas E-Syt1-dependent junctions represent newly formed junctions. Whether the function of E-Syts is determined by the stimulus intensity and how they contribute to STIM2 function are not yet known.
RASSF4, a member of the RASSF family of proteins that are proposed to function as adaptor or scaffolding proteins for large signaling complexes, modulates STIM1 translocation into the ER–PM junctions by regulating the ER–PM tethering functions of E-Syts2 and -3. Further, RASSF4 regulates the PM targeting of Arf6, an upstream regulator of phosphatidylinositol phosphate kinase ([PIP5K]; Chen et al. 2017). Loss of endogenous RASSF4 significantly reduces the steady-state levels of PIP2, leading to adverse effects on junction formation and stability and SOCE activation. Conversely, overexpression of RASFF4 enhanced the formation of ER–PM junctions and clustering of STIM1 and SOCE (Chen et al. 2017). Arf6 can activate phospholipase D (PLD) and, thus, act synergistically to increase PM-PIP2 levels. PLD also hydrolyzes phosphatidylcholine to generate phosphatidic acid (PA), which may act as a cofactor for activating PIP5K (Dickson 2017). PA also promotes recruitment of Nir2 to ER–PM junctions, which boosts the replenishment of PM PIP2 levels following PLC-mediated hydrolysis of PIP2. Both STIM1 and Nir2 colocalized with MAPPER, a marker for SOCE-associated ER–PM junctions (Chang et al. 2013). The synergistic actions of E-Syt1, Nir2, RASSF4, and Arf6 provide a positive feedback mechanism to replenish PM PI, PI-4-phosphate, and PIP2 at contact sites where SOCE occurs, enabling cells to respond to further agonist stimulation and prolonging the duration of Ca2+ signaling (Chang et al. 2013; Chen et al. 2017; Dickson 2017). It is interesting that Ca2+-induced feedforward regulation of E-Syts and other regulatory proteins involved in lipid transfer are localized in/or in close proximity to the SOCE domain. This allows PM PIP2 to be maintained at levels optimal for assembly of the STIM/Orai1 complex and PLC-stimulated hydrolysis following cell stimulation. Whether lipid microdomain(s) involved in agonist-stimulated PIP2 hydrolysis and SOCE vary and are differentially regulated needs further study.
CONCLUDING REMARKS
The nature of SOCE-associated ER–PM junctions, their molecular components and architecture, as well as their physiological relevance, are currently the subject of many studies in the field of Ca2+ signaling. Emerging data suggest that these junctions are not only hubs for sensing agonist stimulation and regulating SOCE but also for compartmentalizing specific downstream targets and effector proteins that drive SOCE-dependent regulation of cell function. Future studies should focus on the identification of the components involved in assembly and remodeling of these junctions. Furthermore, most of the currently available data have been obtained from studies with cell lines, using overexpressed proteins. Thus, information regarding the native status of these proteins and junctions is lacking. Further studies will be required to fill in this knowledge gap and increase our understanding of how receptor signaling, culminating in intracellular Ca2+ release, functionally and physically interact with, and regulate, SOCE. It is possible that tissue function will determine localization of the junctions as well as diversity in the components. Nevertheless, signaling from PM to the ER and, conversely, from the ER to the PM is central to agonist regulation of SOCE.
Footnotes
Editors: Geert Bultynck, Martin D. Bootman, Michael J. Berridge, and Grace E. Stutzmann
Additional Perspectives on Calcium Signaling available at www.cshperspectives.org
REFERENCES
- Abramowitz J, Yildirim E, Birnbaumer L. 2007. The TRPC family of ion channels: Relation to the TRP superfamily and role in receptor- and store-operated calcium entry. In TRP ion channel function in sensory transduction and cellular signaling cascades (ed. Liedtke WB, et al. ), pp. 1–16. Academic, Boca Raton, FL. [PubMed] [Google Scholar]
- Ambudkar IS. 2006. Ca2+ signaling microdomains: Platforms for the assembly and regulation of TRPC channels. Trends Pharmacol Sci 27: 25–32. 10.1016/j.tips.2005.11.008 [DOI] [PubMed] [Google Scholar]
- Ambudkar IS, Bandyopadhyay BC, Liu X, Lockwich TP, Paria B, Ong HL. 2006. Functional organization of TRPC-Ca2+ channels and regulation of calcium microdomains. Cell Calcium 40: 495–504. 10.1016/j.ceca.2006.08.011 [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay BC, Ong HL, Lockwich TP, Liu X, Paria BC, Singh BB, Ambudkar IS. 2008. TRPC3 controls agonist-stimulated intracellular Ca2+ release by mediating the interaction between inositol 1,4,5-trisphosphate receptor and RACK1. J Biol Chem 283: 32821–32830. 10.1074/jbc.M805382200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeton C, Wulff H, Standifer NE, Azam P, Mullen KM, Pennington MW, Kolski-Andreaco A, Wei E, Grino A, Counts DR, et al. 2006. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc Natl Acad Sci 103: 17414–17419. 10.1073/pnas.0605136103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. 2006. Calcium microdomains: Organization and function. Cell Calcium 40: 405–412. 10.1016/j.ceca.2006.09.002 [DOI] [PubMed] [Google Scholar]
- Berridge MJ. 2017. Lymphocyte activation in health and disease. Crit Rev Immunol 37: 439–462. 10.1615/CritRevImmunol.v37.i2-6.120 [DOI] [PubMed] [Google Scholar]
- Berthier A, Lemaire-Ewing S, Prunet C, Monier S, Athias A, Bessede G, Pais de Barros JP, Laubriet A, Gambert P, Lizard G, et al. 2004. Involvement of a calcium-dependent dephosphorylation of BAD associated with the localization of Trpc-1 within lipid rafts in 7-ketocholesterol-induced THP-1 cell apoptosis. Cell Death Differ 11: 897–905. 10.1038/sj.cdd.4401434 [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Watras J, Ehrlich BE. 1991. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature 351: 751–754. 10.1038/351751a0 [DOI] [PubMed] [Google Scholar]
- Bhardwaj R, Müller HM, Nickel W, Seedorf M. 2013. Oligomerization and Ca2+/calmodulin control binding of the ER Ca2+-sensors STIM1 and STIM2 to plasma membrane lipids. Biosci Rep 33: 833–845. 10.1042/BSR20130089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird GS, Putney JW Jr. 2005. Capacitative calcium entry supports calcium oscillations in human embryonic kidney cells. J Physiol 562: 697–706. 10.1113/jphysiol.2004.077289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomben VC, Turner KL, Barclay TT, Sontheimer H. 2011. Transient receptor potential canonical channels are essential for chemotactic migration of human malignant gliomas. J Cell Physiol 226: 1879–1888. 10.1002/jcp.22518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandman O, Liou J, Park WS, Meyer T. 2007. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell 131: 1327–1339. 10.1016/j.cell.2007.11.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazer SC, Singh BB, Liu X, Swaim W, Ambudkar IS. 2003. Caveolin-1 contributes to assembly of store-operated Ca2+ influx channels by regulating plasma membrane localization of TRPC1. J Biol Chem 278: 27208–27215. 10.1074/jbc.M301118200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlow SL, Sage SO. 2003. Rapid agonist-evoked coupling of type II Ins(1,4,5)P3 receptor with human transient receptor potential (hTRPC1) channels in human platelets. Biochem J 375: 697–704. 10.1042/bj20030929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlow SL, Harper AG, Harper MT, Sage SO. 2004. A role for hTRPC1 and lipid raft domains in store-mediated calcium entry in human platelets. Cell Calcium 35: 107–113. 10.1016/j.ceca.2003.08.002 [DOI] [PubMed] [Google Scholar]
- Carrasco S, Meyer T. 2011. STIM proteins and the endoplasmic reticulum–plasma membrane junctions. Annu Rev Biochem 80: 973–1000. 10.1146/annurev-biochem-061609-165311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CL, Hsieh TS, Yang TT, Rothberg KG, Azizoglu DB, Volk E, Liao JC, Liou J. 2013. Feedback regulation of receptor-induced Ca2+ signaling mediated by E-Syt1 and Nir2 at endoplasmic reticulum-plasma membrane junctions. Cell Rep 5: 813–825. 10.1016/j.celrep.2013.09.038 [DOI] [PubMed] [Google Scholar]
- Chen YJ, Chang CL, Lee WR, Liou J. 2017. RASSF4 controls SOCE and ER–PM junctions through regulation of PI(4,5)P2. J Cell Biol 216: 2011–2025. 10.1083/jcb.201606047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng KT, Liu X, Ong HL, Ambudkar IS. 2008. Functional requirement for Orai1 in store-operated TRPC1-STIM1 channels. J Biol Chem 283: 12935–12940. 10.1074/jbc.C800008200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng KT, Liu X, Ong HL, Swaim W, Ambudkar IS. 2011. Local Ca2+ entry via Orai1 regulates plasma membrane recruitment of TRPC1 and controls cytosolic Ca2+ signals required for specific cell functions. PLoS Biol 9: e1001025 10.1371/journal.pbio.1001025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas P, Wanaverbecq N, Abogadie FC, Mistry M, Brown DA. 2002. Signaling microdomains define the specificity of receptor-mediated InsP3 pathways in neurons. Neuron 34: 209–220. 10.1016/S0896-6273(02)00641-4 [DOI] [PubMed] [Google Scholar]
- de Souza LB, Ong HL, Liu X, Ambudkar IS. 2015. Fast endocytic recycling determines TRPC1-STIM1 clustering in ER–PM junctions and plasma membrane function of the channel. Biochim Biophys Acta 1853: 2709–2721. 10.1016/j.bbamcr.2015.07.019 [DOI] [PubMed] [Google Scholar]
- Dickson EJ. 2017. RASSF4: Regulator of plasma membrane PI(4,5)P2. J Cell Biol 216: 1879–1881. 10.1083/jcb.201706042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diercks BP, Werner R, Weidemüller P, Czarniak F, Hernandez L, Lehmann C, Rosche A, Krüger A, Kaufmann U, Vaeth M, et al. 2018. ORAI1, STIM1/2, and RYR1 shape subsecond Ca2+ microdomains upon T cell activation. Sci Signal 11: eaat0358 10.1126/scisignal.aat0358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont G, Combettes L, Bird GS, Putney JW. 2011. Calcium oscillations. Cold Spring Harb Perspect Biol 3: a004226 10.1101/cshperspect.a004226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. 2006. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441: 179–185. 10.1038/nature04702 [DOI] [PubMed] [Google Scholar]
- Formigli L, Sassoli C, Squecco R, Bini F, Martinesi M, Chellini F, Luciani G, Sbrana F, Zecchi-Orlandini S, Francini F, et al. 2009. Regulation of transient receptor potential canonical channel 1 (TRPC1) by sphingosine 1-phosphate in C2C12 myoblasts and its relevance for a role of mechanotransduction in skeletal muscle differentiation. J Cell Sci 122: 1322–1333. 10.1242/jcs.035402 [DOI] [PubMed] [Google Scholar]
- Galan C, Woodard GE, Dionisio N, Salido GM, Rosado JA. 2010. Lipid rafts modulate the activation but not the maintenance of store-operated Ca2+ entry. Biochim Biophys Acta 1803: 1083–1093. 10.1016/j.bbamcr.2010.06.006 [DOI] [PubMed] [Google Scholar]
- Gasser A, Bruhn S, Guse AH. 2006. Second messenger function of nicotinic acid adenine dinucleotide phosphate revealed by an improved enzymatic cycling assay. J Biol Chem 281: 16906–16913. 10.1074/jbc.M601347200 [DOI] [PubMed] [Google Scholar]
- Giordano F, Saheki Y, Idevall-Hagren O, Colombo SF, Pirruccello M, Milosevic I, Gracheva EO, Bagriantsev SN, Borgese N, De Camilli P. 2013. PI(4,5)P2-dependent and Ca2+-regulated ER–PM interactions mediated by the extended synaptotagmins. Cell 153: 1494–1509. 10.1016/j.cell.2013.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guse AH, da Silva CP, Berg I, Skapenko AL, Weber K, Heyer P, Hohenegger M, Ashamu GA, Schulze-Koops H, Potter BV, et al. 1999. Regulation of calcium signalling in T lymphocytes by the second messenger cyclic ADP-ribose. Nature 398: 70–73. 10.1038/18024 [DOI] [PubMed] [Google Scholar]
- Hartzell CA, Jankowska KI, Burkhardt JK, Lewis RS. 2016. Calcium influx through CRAC channels controls actin organization and dynamics at the immune synapse. eLife 5: e1485 10.7554/eLife.14850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdman C, Moss T. 2016. Extended-synaptotagmins (E-Syts); the extended story. Pharmacol Res 107: 48–56. 10.1016/j.phrs.2016.01.034 [DOI] [PubMed] [Google Scholar]
- Hong JH, Li Q, Kim MS, Shin DM, Feske S, Birnbaumer L, Cheng KT, Ambudkar IS, Muallem S. 2011. Polarized but differential localization and recruitment of STIM1, Orai1 and TRPC channels in secretory cells. Traffic 12: 232–245. 10.1111/j.1600-0854.2010.01138.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X, Pedi L, Diver MM, Long SB. 2012. Crystal structure of the calcium release-activated calcium channel Orai. Science 338: 1308–1313. 10.1126/science.1228757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. 2006. STIM1 carboxyl-terminus activates native SOC, Icrac and TRPC1 channels. Nat Cell Biol 8: 1003–1010. 10.1038/ncb1454 [DOI] [PubMed] [Google Scholar]
- Hurst RS, Zhu X, Boulay G, Birnbaumer L, Stefani E. 1998. Ionic currents underlying HTRP3 mediated agonist-dependent Ca2+ influx in stably transfected HEK293 cells. FEBS Lett 422: 333–338. 10.1016/S0014-5793(98)00035-0 [DOI] [PubMed] [Google Scholar]
- Idevall-Hagren O, Lu A, Xie B, De Camilli P. 2015. Triggered Ca2+ influx is required for extended synaptotagmin 1-induced ER-plasma membrane tethering. EMBO J 34: 2291–2305. 10.15252/embj.201591565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M. 1990. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca2+ release in smooth muscle cells of the guinea pig taenia caeci. J Gen Physiol 95: 1103–1122. 10.1085/jgp.95.6.1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardin I, Salido GM, Rosado JA. 2008. Role of lipid rafts in the interaction between hTRPC1, Orai1 and STIM1. Channels 2: 401–403. 10.4161/chan.2.6.7055 [DOI] [PubMed] [Google Scholar]
- Jha A, Ahuja M, Maléth J, Moreno CM, Yuan JP, Kim MS, Muallem S. 2013. The STIM1 CTID domain determines access of SARAF to SOAR to regulate Orai1 channel function. J Cell Biol 202: 71–79. 10.1083/jcb.201301148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, He L, Sun A, Quintana A, Ding Y, Ma G, Tan P, Liang X, Zheng X, Chen L, et al. 2015. Proteomic mapping of ER–PM junctions identifies STIMATE as a regulator of Ca2+ influx. Nat Cell Biol 17: 1339–1347. 10.1038/ncb3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan KB, Barlos D, Hauser CJ. 2007. Free cholesterol alters lipid raft structure and function regulating neutrophil Ca2+ entry and respiratory burst: Correlations with calcium channel raft trafficking. J Immunol 178: 5253–5261. 10.4049/jimmunol.178.8.5253 [DOI] [PubMed] [Google Scholar]
- Kar P, Parekh AB. 2015. Distinct spatial Ca2+ signatures selectively activate different NFAT transcription factor isoforms. Mol Cell 58: 232–243. 10.1016/j.molcel.2015.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar P, Nelson C, Parekh AB. 2011. Selective activation of the transcription factor NFAT1 by calcium microdomains near Ca2+ release-activated Ca2+ (CRAC) channels. J Biol Chem 286: 14795–14803. 10.1074/jbc.M111.220582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar P, Bakowski D, Di Capite J, Nelson C, Parekh AB. 2012. Different agonists recruit different stromal interaction molecule proteins to support cytoplasmic Ca2+ oscillations and gene expression. Proc Natl Acad Sci 109: 6969–6974. 10.1073/pnas.1201204109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar P, Samanta K, Kramer H, Morris O, Bakowski D, Parekh AB. 2014. Dynamic assembly of a membrane signaling complex enables selective activation of NFAT by Orai1. Curr Biol 24: 1361–1368. 10.1016/j.cub.2014.04.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata S, Tsutsumi R, Kohara A, Yamaguchi T, Nakanishi S, Okada M. 1996. Control of calcium oscillations by phosphorylation of metabotropic glutamate receptors. Nature 383: 89–92. 10.1038/383089a0 [DOI] [PubMed] [Google Scholar]
- Lee MY, Song H, Nakai J, Ohkura M, Kotlikoff MI, Kinsey SP, Golovina VA, Blaustein MP. 2006. Local subplasma membrane Ca2+ signals detected by a tethered Ca2+ sensor. Proc Natl Acad Sci 103: 13232–13237. 10.1073/pnas.0605757103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RS, Cahalan MD. 1989. Mitogen-induced oscillations of cytosolic Ca2+ and transmembrane Ca2+ current in human leukemic T cells. Cell Regul 1: 99–112. 10.1091/mbc.1.1.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingwood D, Simons K. 2010. Lipid rafts as a membrane-organizing principle. Science 327: 46–50. 10.1126/science.1174621 [DOI] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE Jr, Meyer T. 2005. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol 15: 1235–1241. 10.1016/j.cub.2005.05.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wang W, Singh BB, Lockwich T, Jadlowiec J, O'Connell B, Wellner R, Zhu MX, Ambudkar IS. 2000. Trp1, a candidate protein for the store-operated Ca2+ influx mechanism in salivary gland cells. J Biol Chem 275: 3403–3411. 10.1074/jbc.275.5.3403 [DOI] [PubMed] [Google Scholar]
- Lockwich TP, Liu X, Singh BB, Jadlowiec J, Weiland S, Ambudkar IS. 2000. Assembly of Trp1 in a signaling complex associated with caveolin-scaffolding lipid raft domains. J Biol Chem 275: 11934–11942. 10.1074/jbc.275.16.11934 [DOI] [PubMed] [Google Scholar]
- Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. 2008. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature 454: 538–542. 10.1038/nature07065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maléth J, Choi S, Muallem S, Ahuja M. 2014. Translocation between PI(4,5)P2-poor and PI(4,5)P2-rich microdomains during store depletion determines STIM1 conformation and Orai1 gating. Nat Commun 5: 5843–5853. 10.1038/ncomms6843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekahli D, Bultynck G, Parys JB, De Smedt H, Missiaen L. 2011. Endoplasmic-reticulum calcium depletion and disease. Cold Spring Harb Perspect Biol 3: a004317 10.1101/cshperspect.a004317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikoshiba K. 2015. Role of IP3 receptor signaling in cell functions and diseases. Adv Biol Regul 57: 217–227. 10.1016/j.jbior.2014.10.001 [DOI] [PubMed] [Google Scholar]
- Min SW, Chang WP, Sudhof TC. 2007. E-Syts, a family of membranous Ca2+-sensor proteins with multiple C2 domains. Proc Natl Acad Sci 104: 3823–3828. 10.1073/pnas.0611725104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash MS, Young KW, Challiss RA, Nahorski SR. 2001. Intracellular signalling. Receptor-specific messenger oscillations. Nature 413: 381–382. 10.1038/35096643 [DOI] [PubMed] [Google Scholar]
- Nicolaou SA, Neumeier L, Peng Y, Devor DC, Conforti L. 2007. The Ca2+-activated K+ channel KCa3.1 compartmentalizes in the immunological synapse of human T lymphocytes. Am J Physiol Cell Physiol 292: C1431–C1439. 10.1152/ajpcell.00376.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, Prakriya M, Feske S, Rao A. 2008. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol 9: 432–443. 10.1038/ni1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh-Hora M, Komatsu N, Pishyareh M, Feske S, Hori S, Taniguchi M, Rao A, Takayanagi H. 2013. Agonist-selected T cell development requires strong T cell receptor signaling and store-operated calcium entry. Immunity 38: 881–895. 10.1016/j.immuni.2013.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong HL, Ambudkar IS. 2012. Role of lipid rafts in the regulation of store-operated Ca2+ channels. In Cholesterol regulation of ion channels and receptors (ed. Levitan I, et al. ), pp. 69–90. Wiley, Hoboken, NJ. [Google Scholar]
- Ong HL, Ambudkar IS. 2015. Molecular determinants of TRPC1 regulation within ER–PM junctions. Cell Calcium 58: 376–386. 10.1016/j.ceca.2015.03.008 [DOI] [PubMed] [Google Scholar]
- Ong HL, Cheng KT, Liu X, Bandyopadhyay BC, Paria BC, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh BB, et al. 2007. Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. Evidence for similarities in store-operated and calcium release-activated calcium channel components. J Biol Chem 282: 9105–9116. 10.1074/jbc.M608942200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong HL, Jang SI, Ambudkar IS. 2012. Distinct contributions of Orai1 and TRPC1 to agonist-induced [Ca2+]i signals determine specificity of Ca2+-dependent gene expression. PLoS ONE 7: e47146 10.1371/journal.pone.0047146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong HL, de Souza LB, Cheng KT, Ambudkar IS. 2014. Physiological functions and regulation of TRPC channels. Handb Exp Pharmacol 223: 1005–1034. 10.1007/978-3-319-05161-1_12 [DOI] [PubMed] [Google Scholar]
- Ong HL, de Souza LB, Zheng C, Cheng KT, Liu X, Goldsmith CM, Feske S, Ambudkar IS. 2015. STIM2 enhances receptor-stimulated Ca2+ signaling by promoting recruitment of STIM1 to the endoplasmic reticulum–plasma membrane junctions. Sci Signal 8: ra3 10.1126/scisignal.2005748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani B, Singh BB. 2009. Lipid rafts/caveolae as microdomains of calcium signaling. Cell Calcium 45: 625–633. 10.1016/j.ceca.2009.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani B, Ong HL, Liu X, Rauser K, Ambudkar IS, Singh BB. 2008. Lipid rafts determine clustering of STIM1 in endoplasmic reticulum-plasma membrane junctions and regulation of store-operated Ca2+ entry (SOCE). J Biol Chem 283: 17333–17340. 10.1074/jbc.M800107200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani B, Ong HL, Brazer SC, Liu X, Rauser K, Singh BB, Ambudkar IS. 2009. Activation of TRPC1 by STIM1 in ER–PM microdomains involves release of the channel from its scaffold caveolin-1. Proc Natl Acad Sci 106: 20087–20092. 10.1073/pnas.0905002106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyi G, Vamosi G, Bacso Z, Bagdany M, Bodnar A, Varga Z, Gaspar R, Matyus L, Damjanovich S. 2004. Kv1.3 potassium channels are localized in the immunological synapse formed between cytotoxic and target cells. Proc Natl Acad Sci 101: 1285–1290. 10.1073/pnas.0307421100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh AB. 2011. Decoding cytosolic Ca2+ oscillations. Trends Biochem Sci 36: 78–87. 10.1016/j.tibs.2010.07.013 [DOI] [PubMed] [Google Scholar]
- Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. 2009. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell 136: 876–890. 10.1016/j.cell.2009.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen OH, Tepikin AV. 2008. Polarized calcium signaling in exocrine gland cells. Annu Rev Physiol 70: 273–299. 10.1146/annurev.physiol.70.113006.100618 [DOI] [PubMed] [Google Scholar]
- Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. 2006. Orai1 is an essential pore subunit of the CRAC channel. Nature 443: 230–233. 10.1038/nature05122 [DOI] [PubMed] [Google Scholar]
- Prole DL, Taylor CW. 2016. Inositol 1,4,5-trisphosphate receptors and their protein partners as signalling hubs. J Physiol 594: 2849–2866. 10.1113/JP271139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW Jr. 1990. Capacitative calcium entry revisited. Cell Calcium 11: 611–624. 10.1016/0143-4160(90)90016-N [DOI] [PubMed] [Google Scholar]
- Putney JW. 2017. Store-operated calcium entry: An historical overview. Adv Exp Med Biol 981: 205–214. 10.1007/978-3-319-55858-5_9 [DOI] [PubMed] [Google Scholar]
- Putney JW, Bird GS. 2008. Cytoplasmic calcium oscillations and store-operated calcium influx. J Physiol 586: 3055–3059. 10.1113/jphysiol.2008.153221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, et al. 2005. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol 169: 435–445. 10.1083/jcb.200502019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta K, Kar P, Mirams GR, Parekh AB. 2015. Ca2+ channel re-localization to plasma-membrane microdomains strengthens activation of Ca2+-dependent nuclear gene expression. Cell Rep 12: 203–216. 10.1016/j.celrep.2015.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauder CM, Wu X, Saheki Y, Narayanaswamy P, Torta F, Wenk MR, De Camilli P, Reinisch KM. 2014. Structure of a lipid-bound extended synaptotagmin indicates a role in lipid transfer. Nature 510: 552–555. 10.1038/nature13269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Quintana A, Findlay GM, Mettlen M, Baust B, Jain M, Nilsson R, Rao A, Hogan PG. 2013. An siRNA screen for NFAT activation identifies septins as coordinators of store-operated Ca2+ entry. Nature 499: 238–242. 10.1038/nature12229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streb H, Irvine RF, Berridge MJ, Schulz I. 1983. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature 306: 67–69. 10.1038/306067a0 [DOI] [PubMed] [Google Scholar]
- Strübing C, Krapivinsky G, Krapivinsky L, Clapham DE. 2001. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron 29: 645–655. 10.1016/S0896-6273(01)00240-9 [DOI] [PubMed] [Google Scholar]
- Subedi KP, Ong HL, Son GY, Liu X, Ambudkar IS. 2018. STIM2 induces activated conformation of STIM1 to control Orai1 function in ER–PM junctions. Cell Rep 23: 522–534. 10.1016/j.celrep.2018.03.065 [DOI] [PubMed] [Google Scholar]
- Sun Y, Birnbaumer L, Singh BB. 2015. TRPC1 regulates calcium-activated chloride channels in salivary gland cells. J Cell Physiol 230: 2848–2856. 10.1002/jcp.25017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CW. 2017. Regulation of IP3 receptors by cyclic AMP. Cell Calcium 63: 48–52. 10.1016/j.ceca.2016.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel M, Lis A, Penner R. 2013. STIM2 drives Ca2+ oscillations through store-operated Ca2+ entry caused by mild store depletion. J Physiol 591: 1433–1445. 10.1113/jphysiol.2012.245399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thillaiappan NB, Chavda AP, Tovey SC, Prole DL, Taylor CW. 2017. Ca2+ signals initiate at immobile IP3 receptors adjacent to ER–plasma membrane junctions. Nat Commun 8: 1505–1520. 10.1038/s41467-017-01644-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thillaiappan NB, Chakraborty P, Hasan G, Taylor CW. 2019. IP3 receptors and Ca2+ entry. Biochim Biophys Acta Mol Cell Res 1866: 1092–1100. 10.1016/j.bbamcr.2018.11.007 [DOI] [PubMed] [Google Scholar]
- Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, et al. 2006. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 312: 1220–1223. 10.1126/science.1127883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby D, Ong HL, De Souza LB, Wachten S, Ambudkar IS, Cooper DM. 2014. TRPC1 contributes to the Ca2+-dependent regulation of adenylate cyclases. Biochem J 464: 73–84. 10.1042/BJ20140766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf IMA, Guse AH. 2017. Ca2+ microdomains in T-lymphocytes. Front Oncol 7: 73–83. 10.3389/fonc.2017.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf IM, Diercks BP, Gattkowski E, Czarniak F, Kempski J, Werner R, Schetelig D, Mittrücker HW, Schumacher V, von Osten M, et al. 2015. Frontrunners of T cell activation: Initial, localized Ca2+ signals mediated by NAADP and the type 1 ryanodine receptor. Sci Signal 8: ra102 10.1126/scisignal.aab0863 [DOI] [PubMed] [Google Scholar]
- Yeh YC, Parekh AB. 2015. Distinct structural domains of caveolin-1 independently regulate CRAC channels and Ca2+ microdomain-dependent gene expression. Mol Cell Biol 35: 1341–1349. 10.1128/MCB.01068-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen M, Lokteva LA, Lewis RS. 2016. Functional analysis of Orai1 concatemers supports a hexameric stoichiometry for the CRAC channel. Biophys J 111: 1897–1907. 10.1016/j.bpj.2016.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Sun L, Machaca K. 2010. Constitutive recycling of the store-operated Ca2+ channel Orai1 and its internalization during meiosis. J Cell Biol 191: 523–535. 10.1083/jcb.201006022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, Kang SH, Dehoff MH, Schwarz MK, Seeburg PH, Muallem S, et al. 2003. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell 114: 777–789. 10.1016/S0092-8674(03)00716-5 [DOI] [PubMed] [Google Scholar]
- Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. 2007. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol 9: 636–645. 10.1038/ncb1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JP, Kim MS, Zeng W, Shin DM, Huang G, Worley PF, Muallem S. 2009a. TRPC channels as STIM1-regulated SOCs. Channels (Austin) 3: 221–225. 10.4161/chan.3.4.9198 [DOI] [PubMed] [Google Scholar]
- Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. 2009b. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol 11: 337–343. 10.1038/ncb1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yule DI, Ernst SA, Ohnishi H, Wojcikiewicz RJ. 1997. Evidence that zymogen granules are not a physiologically relevant calcium pool. Defining the distribution of inositol 1,4,5-trisphosphate receptors in pancreatic acinar cells. J Biol Chem 272: 9093–9098. 10.1074/jbc.272.14.9093 [DOI] [PubMed] [Google Scholar]
- Zeng W, Yuan JP, Kim MS, Choi YJ, Huang GN, Worley PF, Muallem S. 2008. STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Mol Cell 32: 439–448. 10.1016/j.molcel.2008.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. 2006. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc Natl Acad Sci 103: 9357–9362. 10.1073/pnas.0603161103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S, Ma G, He L, Zhang T, Li J, Yuan X, Nguyen NT, Huang Y, Zhang X, Gao P, et al. 2018. Identification of molecular determinants that govern distinct STIM2 activation dynamics. PLoS Biol 16: e2006898 10.1371/journal.pbio.2006898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifach A, Lewis RS. 1996. Calcium-dependent potentiation of store-operated calcium channels in T lymphocytes. J Gen Physiol 107: 597–610. 10.1085/jgp.107.5.597 [DOI] [PMC free article] [PubMed] [Google Scholar]