Abstract
Proteinopathies are degenerative diseases in which specific proteins adopt deleterious conformations, leading to the dysfunction and demise of distinct cell types. They comprise some of the most significant diseases of aging—from Alzheimer's disease to Parkinson's disease to type 2 diabetes—for which not a single disease-modifying or preventative strategy exists. Here, we survey approaches in tractable cellular and organismal models that bring us toward a more complete understanding of the molecular consequences of protein misfolding. These include proteome-scale profiling of genetic modifiers, as well as transcriptional and proteome changes. We describe assays that can capture protein interactomes in situ and distinct protein conformational states. A picture of cellular drivers and responders to proteotoxicity emerges from this work, distinguishing general alterations of proteostasis from cellular events that are deeply tied to the intrinsic function of the misfolding protein. These distinctions have consequences for the understanding and treatment of proteinopathies.
Proteinopathies are degenerative diseases in which specific proteins aggregate in distinct cell types. The most common and devastating diseases of the group include the neurodegenerative disorders, Alzheimer's disease (AD), and Parkinson's disease (PD). But beyond these, type 2 diabetes mellitus, inclusion body myopathy, and systemic amyloidoses are also proteinopathies. Outside of the rare condition, transthyretin amyloidosis (Maurer et al. 2018), no disease-modifying treatments exist for these diseases, and the public health significance cannot be overstated. They collectively cost the United States around $500 billion annually, and AD alone affects 4.5 million Americans.
Increasingly, it is becoming appreciated that a “one-size-fits-all” treatment strategy may not be feasible for proteinopathies. Substantial variability among individual genomes, cells, and protein conformations may lead to unique disease processes in individual patients (Jarosz and Khurana 2017). Broadly speaking, these diseases are thought to arise from an interplay between genetic factors and environmental “hits” that result in accumulation of misfolded proteins (Fig. 1A). In some informative cases, aggressive early-onset disease is driven deterministically by a dominant mutation or locus multiplication in the gene encoding the aggregating protein, tying that protein aggregation process causally to the disease. Once the protein-folding pathology is induced, there are toxic responses within the cell to the misfolding and mislocalization of proteins (Fig. 1B). A distinction can be made between responses that relate to the intrinsic function of the misfolded protein and general responses to misfolded proteins, the latter commonly referred to as disruption of the proteostasis machinery (Balch et al. 2008; Labbadia and Morimoto 2015). Finally, there may be toxic interactions among cells. For neurodegenerative diseases, these interactions may result in disruption of neuronal circuitry, abnormal interactions between neurons and glial or immune cells (Fig. 1D; Liddelow et al. 2017; Salter and Stevens 2017). These may be driven by the pathologic templating and propagation of misfolded protein conformers in prion-like fashion between cells (Fig. 1C; Guo and Lee 2014; Jarosz and Khurana 2017).
Figure 1.
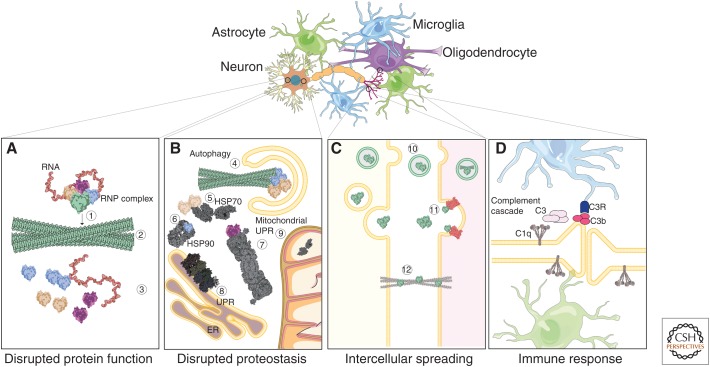
A summary of consequences of protein aggregation in proteinopathies. The brain is comprised of neurons and glial cells (astrocytes, oligodendrocytes, and microglia). Glio–neuronal interactions are critical for appropriate brain development and increasingly recognized as pivotal for neurodegeneration also. Here, we illustrate four consequences of protein misfolding: (A) Direct consequences of aggregation of a protein. In this example, one (green) protein component of a ribonucleoprotein (RNP) complex aggregates as a result of an environmental challenge, deterministic point mutations, or various genetic factors (1). This leads to formation of amyloid fibrils of the protein (2), thus relieving other protein/RNA members from the complex (3). Toxicity results from either loss-of-function of the RNP complex, or from toxic gain-of-function of misfolded or mislocalized RNP complex proteins. (B) Effects of protein aggregation on the cellular homeostasis machinery. Members of protein complexes require each other for correct folding and chaperoning. The first member of the complex forms an amyloid aggregate and is degraded in this illustration through engulfment by autophagic membranes (4). A second with exposed hydrophobic cores attracts Hsp70 for refolding (5). Another protein (blue) attracts Hsp90 to exposed hydrophilic surfaces (6). Another member (purple) is ubiquitinated and degraded by the proteasome (7). Sequestration of these chaperones or overwhelmed protein degradation pathways can lead to a vicious cycle that results in misfolding and abnormal accumulation of other proteins, eventually triggering an unfolded protein response (UPR) in the endomembrane system (8) or mitochondria (9). (C) Intercellular spread or self-templating of amyloid fibrils. Fibrils can be secreted (10) or taken up from the extracellular matrix by receptor-mediated endocytosis (11). Nanotubes between cells can also mediate transfer of fibrils from one cell to another (12). (D) Neuroinflammation and complement activation by neurons (via C1q expression) and glia results in C3b deposition at the surface of neurons. Microglia expressing C3 receptors can engulf such neurons, thus contributing to neurodegeneration. ER, endoplasmic reticulum.
Even within this elemental view of neurodegenerative disease pathogenesis, there are many unanswered questions. We discuss some of these in this review, including:
What biological approaches can help us complete the genetic “map” of proteinopathies? Genetic factors have been pivotal in directing rational research in the proteinopathy field, but most of the heritability of these diseases remain unexplained.
Are genetic factors that inform us about disease risk and initiation equally informative about mechanisms of disease progression? Or are important cellular and intercellular responses not captured by genetic analysis? Vulnerability factors involved in disease risk or initiation might be good preventative targets, but may not be good targets in late stages of the disease. On the other hand, if these diseases progress in an orderly fashion, distinct cell types may be in different disease states at any given time, and thus there may be a scope for preventative and reversal strategies simultaneously.
How can we develop methods to systematically understand and target different toxic protein conformations? Much attention has been given to distinct biophysical forms, or conformers, of toxic proteins, and methods are sorely needed to tractably model these in the laboratory.
The answers to some of these questions may lie in methods that can capture systematically the molecular consequences of protein misfolding. A reductionist approach could plausibly use a variety of cellular assays to fully deconstruct cellular consequences in cellular models. In time, this could be achievable in different cell types and in more complex models to examine intercellular interactions. In this review, we describe distinct methods we and others have employed for such analysis. We focus on neurodegenerative proteinopathy. Some emphasis is placed on α-synuclein, the protein that misfolds in synucleinopathies including PD, because it has been extensively analyzed with these methods.
MAPPING PROTEOTOXICITY: GENETIC DETERMINANTS AND CELLULAR RESPONSES
To study proteinopathies, we can, on the one hand, systematically catalog the genetic requirements for proteotoxicity. This approach aims to identify the cellular setup—through forcing the absence or elevated expression of a specific gene—that exacerbates (“enhances”) or ameliorates (“suppresses”) toxicity associated with a misfolding protein. Most often in model systems, the proteotoxicity is induced by overexpression of a protein that is prone to misfolding, in accordance with the customary autosomal dominant and toxic gain-of-function mechanisms in proteinopathies. In a genome-wide screen, gene knockouts/deletions or gene overexpression for approximately every coding sequence are queried for their effect on a proteotoxic phenotype. However, genetic screens are typically not designed to report on cellular responses to protein misfolding. This is because the gene deletion or overexpression generally occurs before, or concomitant with, expression of the toxic protein. Rather, cellular response can be investigated by systematically measuring the changes that occur at the transcript and protein level (transcriptomics, proteomics) as a consequence of proteotoxicity.
To gain a global view of the cellular effects of protein misfolding, data sets from multiple “omics” approaches (e.g., genetic screens, transcriptomics, metabolomics, proteome-scale protein–protein interactions, ChIP-seq) can be integrated through bioinformatic methods (Fig. 2A; Tuncbag et al. 2016). Such in silico approaches can point to cellular pathway(s) most affected by a particular proteotoxic protein, and unveil relevant genes that might not have been recovered in individual screens, thereby increasing our understanding of the cytotoxic mechanism. As noted above, some of the genetic requirements or molecular consequences may reflect a general proteostatic response within a cell, whereas others relate to the intrinsic function of the protein that misfolds.
Figure 2.
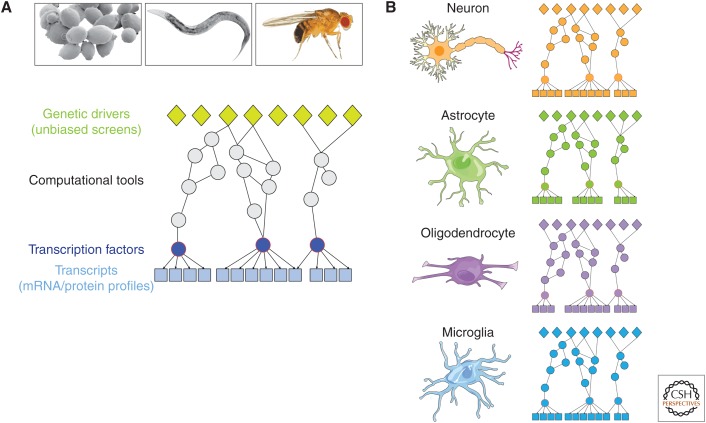
Building a global view of cellular response to proteotoxicity. (A) Bioinformatic methods can be applied to bridge data sets from genome-wide genetic screens and transcription profiles, thereby integrating genetic drivers and cellular response to proteotoxicity, and augmenting the list of molecular candidates implicated in the cytotoxic mechanism. Network-building algorithms can identify “hidden nodes” that are not recovered in individual screens. Such approaches are currently feasible in model organisms where multiple “omics” data sets are already available, but can one day be applied to human disease-relevant cell types (e.g., human neurons) as genome-wide genetic and molecular data sets become available. (B) Different cell types (e.g., neurons, astrocytes, oligodendrocytes, microglia) likely exhibit different molecular network profiles due to differential gene expression, so within proteotoxicity models, it will be important to obtain cell-specific data and build cell-type-specific networks. mRNA, messenger RNA.
Genetic Screens
In proteinopathies, the principal aggregating protein (or proteins) are distinct in each disease, for example, α-synuclein in PD, β-amyloid and tau in AD, huntingtin in Huntington's disease (HD), TDP-43 in amyotrophic lateral sclerosis (ALS), prion protein (PrP) in prion diseases (e.g., Creutzfeldt–Jakob disease), and islet amyloid polypeptide (IAPP) in type 2 diabetes. Many of these proteinopathies have been modeled by transgenic overexpression in genetically tractable model systems, including baker's yeast cells (Table 1; for review, see Khurana and Lindquist 2010). The tractability of yeast cells is offset by lack of specialized neuronal features, and tractable metazoan organisms, including Caenorhabditis elegans and Drosophila melanogaster, have helped fill in that significant gap. Genome-wide and candidate genetic screens in these model organisms have been facilitated by high-throughput technologies such as knockout or overexpression libraries for every protein-coding gene (Saccharomyces cerevisiae), RNA interference (C. elegans, D. melanogaster), and transgenic stocks with P-element insertion mutations (D. melanogaster). Published data sets of genetic modifiers from high- and low-throughput screens against nine neurodegenerative proteotoxicities in yeast, flies, and worms have been cataloged and annotated in NeuroGeM (Na et al. 2013). Here, we distill four key insights from screens in proteinopathy models.
Table 1.
Modeling human proteinopathies in genetically tractable model organisms
| Misfolding protein | Proteinopathy | Model organism | References |
|---|---|---|---|
| α-Synuclein | Parkinson's disease | Saccharomyces cerevisiae | Outeiro and Lindquist 2003; Willingham et al. 2003; Dixon et al. 2005; Zabrocki et al. 2005; Sharma et al. 2006 |
| Drosophila melanogaster | Feany and Bender 2000; Auluck et al. 2002 | ||
| Caenorhabditis elegans | Lakso et al. 2003; Cao et al. 2005; Kuwahara et al. 2006; Hamamichi et al. 2008; van Ham et al. 2008 | ||
| β-Amyloid | Alzheimer's disease | S. cerevisiae | Bagriantsev and Liebman 2006; Caine et al. 2007; von der Haar et al. 2007; Treusch et al. 2011; D'Angelo et al. 2013 |
| D. melanogaster | Finelli et al. 2004; Iijima et al. 2004; Crowther et al. 2005; Iijima et al. 2008 | ||
| C. elegans | Link 1995; Link et al. 2003 | ||
| tau | Alzheimer's disease, FTDP-17 | S. cerevisiae | Vandebroek et al. 2005 |
| D. melanogaster | Williams et al. 2000; Wittmann et al. 2001; Jackson et al. 2002; Blard et al. 2007 | ||
| C. elegans | Kraemer et al. 2003; Miyasaka et al. 2005; Brandt et al. 2009 | ||
| huntingtin | Huntington's disease | S. cerevisiae | Krobitsch and Lindquist 2000; Muchowski et al. 2000; Duennwald et al. 2006a,b; Solans et al. 2006; Kayatekin et al. 2014 |
| D. melanogaster | Jackson et al. 1998 | ||
| C. elegans | Faber et al. 1999; Parker et al. 2001 | ||
| Polyglutamine (polyQ) | Polyglutamine-expansion disorders (e.g., spinocerebellar ataxia type 1, type 3) | S. cerevisiae | Fernandez-Funez et al. 2000; Meriin et al. 2002, 2003 |
| D. melanogaster | Warrick et al. 1998; Kazemi-Esfarjani and Benzer 2000; Marsh et al. 2000 | ||
| C. elegans | Satyal et al. 2000; Brignull et al. 2006 | ||
| TDP-43 | Amyotrophic lateral sclerosis | S. cerevisiae | Johnson et al. 2008; Elden et al. 2010; Kim et al. 2014 |
| D. melanogaster | Kim et al. 2014 | ||
| FUS | Amyotrophic lateral sclerosis | S. cerevisiae | Fushimi et al. 2011; Ju et al. 2011; Kryndushkin et al. 2011; Sun et al. 2011 |
| SOD1 | Amyotrophic lateral sclerosis | S. cerevisiae | Rabizadeh et al. 1995; Ticozzi et al. 2011 |
| D. melanogaster | Watson et al. 2008; Bahadorani et al. 2013; Gallart-Palau et al. 2016; Şahin et al. 2017 | ||
| C. elegans | Oeda et al. 2001; Gidalevitz et al. 2009; Wang et al. 2009 | ||
| Islet amyloid polypeptide (IAPP) | Type 2 diabetes | S. cerevisiae | Kayatekin et al. 2018 |
First, genetic screens in simple model organisms have uncovered disease-relevant biology. Homologs of known human disease risk factors have emerged as genetic modifiers (for reviews, see Khurana and Lindquist 2010; Kryndushkin and Shewmaker 2011; Tenreiro et al. 2017). For example, genetic screens in the yeast synuclein models recovered orthologs of multiple confirmed or putative risk factors for human parkinsonism, including ATP13A2/PARK9, RAB7L1/PARK16, VPS35/PARK17, EIF4G1/PARK18, and SYNJ1/PARK20, with validation of the findings in models from worm to rodent to induced pluripotent stem cell (iPSc)-derived neurons (Gitler et al. 2009; Dhungel et al. 2015; Khurana et al. 2017). Similarly, a genome-wide screen against β-amyloid (Aβ) toxicity in yeast recovered homologs of multiple risk factors of sporadic AD (including YAP1802, the yeast homolog of PICALM) as suppressors of toxicity, and these were also found to modify Aβ toxicity in worms and primary rodent neuronal models (Treusch et al. 2011). Remarkably, the identification of the yeast homolog of ATXN2 as a modifier of TDP-43 toxicity in yeast led to the identification of this gene as a robust risk factor for ALS in humans (Elden et al. 2010) and a modulator of neurodegeneration in a mouse model of that disease (Becker et al. 2017).
Second, the same gene modifiers are not necessarily recovered in genetic screens of the same proteotoxicity in different model organisms, but often the same cellular pathways emerge. For example, genes in the vesicle trafficking pathway appear as modifiers of α-synuclein toxicity in both yeast and C. elegans, even if precisely the same genes do not emerge as modifiers in the different models. A case in point is the gene encoding the vacuolar assembly/sorting protein VPS41, which emerged as a modifier of α-synuclein pathology in an RNAi screen in C. elegans and was subsequently validated to be neuroprotective (Hamamichi et al. 2008; Ruan et al. 2010; Harrington et al. 2012). The homolog of VPS41 did not emerge as a genetic modifier of α-synuclein in yeast genetic screens to date, although YCK3, which encodes the kinase that phosphorylates Vps41, as well as a host of other genes involved in endolysosomal trafficking were identified as modifiers (Khurana et al. 2017). The same genes may fail to be recovered in different screens of the same proteotoxicity due to technical limitations of the screens (i.e., if the screens have low genome coverage), or for biological reasons (e.g., redundant gene function, or differences in genetic requirement across strain backgrounds in the same species).
Third, there is little overlap in genetic modifiers against different proteotoxicities (e.g., α-synuclein, Aβ, TDP-43, tau, polyglutamine-expansion models), suggesting that each protein exerts different intrinsic genetic requirements and/or cellular responses despite a common protein misfolding pathology in human disease (Jarosz and Khurana 2017; Khurana et al. 2017). For example, genetic modifiers against α-synuclein toxicity in yeast are enriched in processes related to lipid metabolism and vesicle trafficking (Outeiro and Lindquist 2003), whereas modifiers against polyglutamine-expanded huntingtin are enriched for genes related to stress response, protein folding, and ubiquitin-dependent protein catabolism (Willingham et al. 2003). Along the same lines, kinases and phosphatases are the major class of genetic modifiers identified in a D. melanogaster model of tauopathy, but not of the polyglutamine disease model SCA3 (Shulman and Feany 2003). These observations are consistent with hyperphosphorylated and aggregated tau as hallmark pathology in AD and frontotemporal dementia (FTD). Meta-analysis of published genetic screen data sets indicate that this trend holds true when comparing polyglutamine disease models (e.g., HD, SCA1, SCA3, SCA7) versus AD models (Aβ): polyglutamine disease models share many genetic modifiers that are not seen in AD models, consistent with a common underlying biology linking polyglutamine expansion (Na et al. 2013).
Fourth, despite the common molecular dysfunction (protein misfolding), genes in the proteostasis network do not appear as a major class of modifiers overlapping proteotoxicities (e.g., α-synuclein, Aβ, and TDP-43) (Jarosz and Khurana 2017). Although proteostasis network components that are broadly protective against misfolded and aggregation-prone proteins do emerge as modifiers (Nollen et al. 2004; Silva et al. 2011), it is estimated that such genes account for only 3% of genetic modifiers across yeast, worm, and flies (Na et al. 2013). There are many possible explanations for their underrepresentation, including the setup strategy for genetic screens, the essential functions of certain genes in this pathway, and the redundancy of others. We return to this point again below.
Finally, the advent of CRISPR-Cas9 genome editing has extended the reach for high-throughput genetic screens against proteotoxicities (e.g., Kramer et al. 2018). For example, most genome-wide screens to date have been limited to looking at single genetic modifiers. In practice, combinatorial effects could be equally critical and valuable for functional genomics. This would be of particular interest for genes with redundant function, whereby knockdown of one gene is compensated by a second gene, masking the genetic modifier effect of the first. Recently, screening technology that combined randomized guide RNAs (gRNAs) with CRISPR-Cas9-based transactivation (so-called “CRISPRa” technology) was used for unbiased screening of transcriptional networks that modify α-synuclein toxicity in yeast (Chen et al. 2017). In this approach, the gRNA recruits a transactivating protein complex to the 5′ untranslated region (UTR) of a number of gene targets, enabling combinatorial effects of gene expression to be probed. Genes with altered expression in the presence of top gRNA hits from the screen were then shown to suppress α-synuclein toxicity in yeast and neuronal models. Most of the genes recovered in this way were not previously identified in single-gene overexpression or deletion screens (but tellingly had overlap in gene ontology [GO] categories). This study underscores the importance of orthogonal approaches to obtain a complete understanding of the cellular requirements and consequences of protein misfolding.
With CRISPR/Cas9-based approaches, whole-genome genetic screens previously conceivable only in tractable genetic organisms are now also within reach in human cells (Khurana et al. 2015). For example, Kramer and colleagues used the CRISPR-Cas9 system to conduct genome-wide knockout screens in human cell models of C9ORF72 dipeptide-repeat (DPR) protein toxicity associated with ALS and FTD (Kramer et al. 2018). Their cellular model of C9ORF72 DPR toxicity consisted of exposing K562 cells to proline-arginine and glycine-arginine synthetic polymers. Suppressors and enhancers of C9ORF72 DPR toxicity were validated in a secondary, pooled CRISPR-Cas9 screen in mouse primary neurons. Screen hits were enriched in endoplasmic reticulum (ER) stress, proteasome, nuclear transport, RNA processing, and chromatin modification pathways. One of the suppressors, TMX2, which encodes the ER-resident transmembrane thioredoxin protein, was further validated in human neurons. Knockdown of TMX2 improved survival of motor neurons derived from a C9orf72 ALS patient's iPScs (Kramer et al. 2018).
Transcriptomics
A systematic assessment of 179 environmental and genetic perturbations in yeast (an organism where such comprehensive data are uniquely available) surprisingly revealed that transcriptional responses are largely distinct from genetic modulators when yeast cells were subjected to distinct stressors or environmental perturbations (Yeger-Lotem et al. 2009). Genetic hits were biased toward genes that encode regulatory proteins, whereas transcriptional profiling hits were biased toward genes involved in metabolic processes. The same study confirmed this exclusive nature of genetic and transcriptional data sets in a yeast model of α-synuclein toxicity. Genetic modifiers were dominated by genes encoding vesicle trafficking proteins. In contrast, messenger RNA (mRNA) profiling revealed up-regulated genes with oxidoreductase activities, and down-regulated genes relating to mitochondrial activity and ribosomal response to stress (Yeger-Lotem et al. 2009; Su et al. 2010). Notably, down-regulation of genes related to mitochondrial activity (along with dysregulation of lipid, and membrane transport) was also detected by genome-wide expression profiling in a D. melanogaster transgenic model of α-synuclein toxicity (Scherzer et al. 2003), and in expression analysis of postmortem substantia nigra tissue of PD patients (Hauser et al. 2005; Mandel et al. 2005; Duke et al. 2006; Zheng et al. 2010). Gene-expression analyses in individuals affected by PD also revealed perturbation of other cellular functions including chaperones, ubiquitin-proteasome protein degradation, vesicle trafficking, iron transport, synaptic transmission, oxidative stress, and dopamine metabolism (Hauser et al. 2005; Mandel et al. 2005; Duke et al. 2006; Zheng et al. 2010; Borrageiro et al. 2018). At least in the case of PD and α-synucleinopathy, these findings reveal a surprisingly conserved difference between transcriptional profiles (dominated by metabolic and mitochondrial genes) and genetic modifiers (dominated by vesicle trafficking genes) from model organisms to PD patients.
Proteomics
Proteomics is another important component to consider when capturing the macromolecular environment in the presence of a perturbation. Many studies have conducted quantitative proteome profiling in the presence of proteotoxicity in cellular and animal models, and patient brain samples (for review, see Kasap et al. 2017). Notably, proteomic analyses of PD research models concur with transcriptional profiling data in detecting dysregulation of proteins associated with mitochondrial function compared to controls. This holds true in Drosophila or mouse models expressing transgenic α-synuclein A53T or A30P mutations, or neurotoxin-induced neurodegeneration in rodents (e.g., MPTP, 6-OHDA) (Poon et al. 2005; Xun et al. 2007a,b; Diedrich et al. 2008; Lessner et al. 2010). Proteins in other molecular pathways are also significantly altered in models of PD, including calcium metabolism, actin cytoskeleton, membrane trafficking, and ribosomal components (Kasap et al. 2017).
Differences in protein levels between pathologic and normal conditions can provide useful insights for discovery of disease biomarkers or therapeutic targets. Proteomic and genetic analyses can inform druggable targets when used in a coordinated fashion. A recent study utilized this approach to identify the molecular mechanism through which calcineurin and FK506 contribute to α-synuclein toxicity in yeast and rat α-synuclein models (Van der Perren et al. 2015; Caraveo et al. 2017). Calcineurin is a calcium-calmodulin dependent phosphatase. A thorough genetic analysis revealed that increased activity of calcineurin is associated with α-synuclein toxicity in cellular models, and the calcineurin inhibitor tacrolimus/FK506 accordingly rescued this toxicity (Caraveo et al. 2014). Calcineurin is inhibited by tacrolimus/FK506 through the formation of a ternary complex with the FK506-binding protein FKBP12 (Liu et al. 1991). Mass spectrometry-based phosphoproteomics analysis in the yeast model demonstrated that FKBP12 contributes to α-synuclein toxicity by regulating calcineurin activity and promoting dephosphorylation of proteins involved in endocytosis, vesicle trafficking, actin reorganization, and ion channel regulation (Caraveo et al. 2017). Conversely, inhibiting the functional interaction between FKBP12 and calcineurin with low doses of tacrolimus restored phosphorylation of calcineurin- and FKBP12-dependent substrates involved in vesicular trafficking in an in vivo rat model of PD, resulting in restored dopamine transporter trafficking and dopamine secretion at presynaptic terminals. At the organismal level, tacrolimus improved behavioral deficits related to α-synuclein toxicity (Van der Perren et al. 2015; Caraveo et al. 2017). In this case, proteomic analysis provided critical insights into mechanisms through which genetic and pharmacologic targets relate to proteotoxicities.
ResponseNet: Integrating Genetic and Transcriptional Data
Because genetic modifiers and transcriptional responses are frequently distinct (see above), computational methods have been used to combine these two data sets to develop a coherent view of the cellular response to a proteotoxicity. Yeger-Lotem et al. (2009) developed an algorithm (ResponseNet) to bridge the data obtained from genome-wide screens and transcriptional profiling, and thereby capture a unified cellular map of the proteins and genes responding to cellular perturbation. ResponseNet identifies molecular-interaction pathways connecting genetic screen hits and differentially expressed genes through known protein–protein interactions (Lan et al. 2011; Basha et al. 2013). This method can theoretically be extended to other types of “omics” data sets across different organisms and proteotoxicities (Fig. 2A). Furthermore, different cell types will likely exhibit different molecular network profiles, so within proteotoxicity models it will be important to generate cell-type-specific networks (Fig. 2B) and to use molecular interaction data that are cell-type and tissue-specific. Basha and colleagues have recently assembled such tissue-specific molecular interaction data in a database called DifferentialNet (Basha et al. 2018). Cell-type-specific networks may reveal differences in network connectivities that provide mechanistic insight into differential vulnerability to proteotoxicity across cell types, a unifying but poorly understood feature of proteinopathies.
MAPPING TO FIND MISSING HERITABILITY IN PROTEINOPATHIES
Despite decades of human genetic studies, the set of genes responsible for disease risk of many proteinopathies remains incomplete. Genes responsible for disease risk have traditionally been identified through family-based genetic linkage analysis and population-based genome-wide association studies (GWAS) (Cui et al. 2010; Flint 2013). Linkage analysis is generally better suited to detect rare variants with large effects, as in Mendelian disorders (e.g., HD). More recently, whole-exome sequencing has been favored to search for such variants. In contrast, GWAS are geared toward detecting common variants with smaller effects. GWAS have successfully uncovered causal genetic loci for a variety of complex genetic disorders (e.g., type 2 diabetes, schizophrenia) (Visscher et al. 2017). However, when the effect size and frequencies of the known causative loci are combined for a disease, they often explain only a small fraction of the heritability of the disease. Even in monogenic disorders like HD, our knowledge of genetic variants that modify disease onset or progression is far from complete (Arning 2016; Holmans et al. 2017). Put another way, a large proportion of genetic variation predisposing to proteinopathies or modulating their progression is unaccounted for.
What are the other genetic loci that are affecting disease susceptibility? This conundrum is referred to as the problem of “missing heritability” (Manolio et al. 2009). Many ideas have been put forth to explain it (Manolio et al. 2009; Eichler et al. 2010; Brookfield 2013). GWAS are limited in identifying single-nucleotide polymorphisms (SNPs) in linkage disequilibrium with the “true” gene locus conferring risk and may never pinpoint the causal gene (MacArthur et al. 2014; Schaid et al. 2018). Furthermore, traditional methods for evaluating common variants, such as replication of the association across populations, are not readily applicable for rare variants. Rare variants may be private to a family or small population and are thereby difficult to replicate in another population, leaving the status of the variant in doubt (Lupski et al. 2011; Casals and Bertranpetit 2012). In addition, for both classes of variant, combinatorial effects can be elusive.
As noted above, genetic screens against proteotoxicities in model organisms are enriched in homologs of known human genetic risk factors for neurodegenerative diseases. It is thus plausible that complete cellular dissection of proteotoxicity (through the different methods outlined in this review) could provide a candidate list of genes enriched in true human genetic modifiers. This kind of Bayesian approach—Bayesian because it begins with a list of genes a priori more likely to be involved with the disease process—may be synergistic with traditional human genetic analysis. Because tests to identify association between rare or common variants and complex genetic disease are statistically underpowered, one method to sidestep this limitation is by reducing the number of comparisons in genetic association studies through integration of information from available biological data sets in model organisms or cellular models. This idea is gaining traction and has recently been tested in the PD field. For example, in a recent GWAS meta-analysis of PD cases and controls, candidate causal genes were assigned to novel PD-associated risk loci (P < 5 × 10−8) by using a “neurocentric” scoring-based strategy that takes into account multiple levels of experimental evidence, including expression quantitative trait locus (eQTL) and tissue-specific gene-expression data from central nervous system (CNS) cell types in mice and neurologically relevant phenotypic annotations in Drosophila (Chang et al. 2017).
TransposeNet: Transposing Molecular Networks across Species
If model organisms are utilized for functional genomics, a clear challenge arises in assigning cross-species homology. Assigning homology of genes and networks across species is a nontrivial exercise, particularly when sequences of homologous genes diverge over evolution. Ideally, a molecular network identified in one species could be “transposed” to another. A recent study achieved this for three genome-wide screens against α-synuclein toxicity in yeast. A computational approach (TransposeNet) was developed to transpose rich yeast molecular networks from yeast to human (Khurana et al. 2017).
Prior to network generation, TransposeNet comprised three steps (Fig. 3, steps 1 through 3). First, human homologs to yeast genes were assembled through consideration not just of sequence, but also of protein structure and known molecular interactions. This model of homology accounted better for sequence divergence across evolution, and demonstrably increased assignment of homologs across species. Only homologs expressed in brain were included. Second, molecular interaction data linking these human gene “nodes” were curated. However, human molecular interaction data (particularly genetic interactions) are sparse and result in fragmented, incomplete networks. To address this, an augmentation method was developed as a third component of TransposeNet: known molecular interaction data between yeast genes and proteins were transposed across species to augment the sparse human data sets. This enriched data set emerged as a critical component to generate network “coherence,” namely, the appropriate interconnection of the majority of the original genetic hits into a single network on which genes/proteins of like function are linked together in close proximity.
Figure 3.
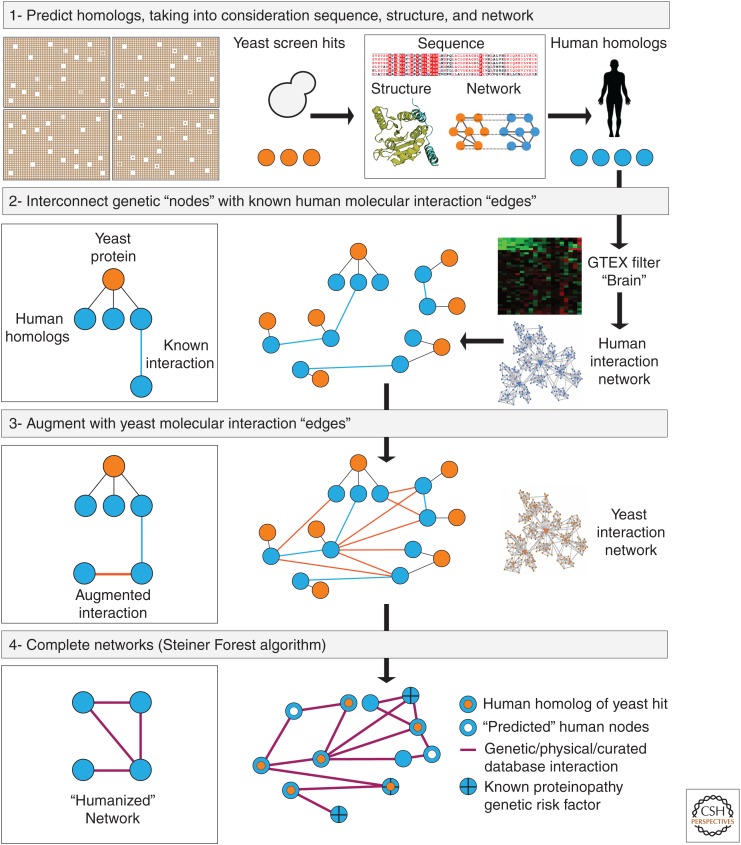
TransposeNet is a computational approach that may enable integration of cross-species molecular data to build a more coherent view of proteotoxicity. In this depiction of a recently published study (Khurana et al. 2017), modifiers recovered from genetic screens of proteotoxicity in yeast were “transposed” into the context of the human proteome. (1) Human homologs of yeast modifiers were generated through cross-species consideration of sequence, structure, and protein–protein interactions. (2,3) Genetic and physical interactions (“edges”) between these human genes/protein “nodes” were curated not just from the relatively sparse existing human molecular interaction data sets (2) but also through augmentation of the much richer data set of homologous interactions in the yeast proteome (3). (4) A network was generated to connect the nodes through edges, employing a method known as the Steiner Forest prize-collecting algorithm. The advantage of this method is that it solves a “hairball” of interactions in the most efficient, robust way and introduces “predicted” nodes to do so. The “predicted” nodes can capture biology that was missed in the original screen. In the published example for α-synuclein toxicity, the method captured interactions between many genetic risk factors for Parkinson's disease through specific molecular pathways. It is presumed that the tool can be used to focus attention on specific human genes as potential genetic risk factors, aiding functional genomics efforts.
Finally, having assembled the original genetic hits and known yeast/human molecular interaction data, the TransposeNet networks themselves were generated through an optimization framework based on the prize-collecting Steiner Forest algorithm (Fig. 3, step 4). The algorithm identifies the simplest way to connect the gene/protein “nodes” of the network through the most robust known protein–protein or genetic interactions. The algorithm also inserts genes (“predicted nodes”) to solve the network problem in the most efficient way, thereby connecting genes not included in the original data set in the network. The resultant “humanized” networks were more complete and appropriately linked genes of related function together. Importantly, underscoring the strength of the approach, the resulting “humanized” network included human disease genes with no clear homologs in yeast as predicted nodes. The humanized network of α-synuclein toxicity modifiers thus revealed molecular connections between multiple different genetic forms of parkinsonism, and predicted pathologies (e.g., mRNA translation defect) that were validated in patient-derived neurons. Importantly the network approach could also infer connections to druggable targets for synucleinopathy (including calcineurin, described above, and another target Nedd4), even when those druggable targets were not recovered by the genetic screens from which the networks were generated. This type of computational approach may provide a blueprint for translating findings in model organisms for functional genomics and pharmacogenomics in human proteinopathies.
A “DRIVER-RESPONDER” ARCHITECTURE IN PROTEOTOXICITY
Distinguishing Causation and Progression in Neurodegeneration
Where abundant molecular interactome data are available, genetic and transcriptional data are largely nonoverlapping, as noted above. Genetic screens to identify modifiers of proteotoxicities may be more geared toward identifying factors that either drive disease processes or vulnerability. In contrast, transcriptional profiling may be more germane for identifying responders to the toxic insult. It is worth remembering that genetic modifiers in simple yeast screens are often introduced into the yeast cell prior to the proteotoxic insult (or simultaneous with it), just as they would be in a human born with a gene variant. In contrast, transcriptional profiling assesses the response at various intervals after the toxic protein is overexpressed.
There is mounting evidence that drivers and responders to proteotoxicities may be different. For example, in HD, the archetypal polyglutamine-expansion disorder, it is well known that the longer the polyglutamine tract (or the CAG repeat that encodes it), the earlier the onset of the disease. However, a recent study found that disease duration is independent of this repeat length (Keum et al. 2016). Thus, factors other than the polyglutamine tract, whether genetic or otherwise, are more important in determining disease course and progression. Alternatively, the polyglutamine tract may also be important in driving progression, but in cells or circuits that are distinct from those involved in disease initiation.
Likewise, as noted above, in the case of α-synuclein toxicity in cellular models or in patients, genetic hits are enriched in genes encoding proteins involved in vesicle trafficking, but transcriptional responses are enriched in genes encoding proteins localized to the mitochondria and genes involved in oxidative metabolism. Interestingly, an emerging literature is revealing mechanistic links between trafficking and mitochondria. For example, α-synuclein itself has been localized to mitochondria-associated ER membranes (Guardia-Laguarta et al. 2014), and binds to the mitochondria-associated tethering protein Vapb to disrupt mitochondrial function (Paillusson et al. 2017). Mutation of the Parkinson's gene VPS35 (that genetically interacts with α-synuclein) leads to mitochondrial fragmentation. This has been attributed to abnormal interactions between mutant Vps35 and mitochondrial dynamin-like protein (DLP1) (Wang et al. 2016). The endocytic and actin cytoskeletal machinery are intimately related, and perturbations induced by α-synuclein in the actin cytoskeleton lead to mitochondrial dysfunction (Ordonez et al. 2018). Beyond mitochondrial dysfunction, it is conceivable that “responders” to different proteotoxic stressors include other components of the proteostasis network, for instance the unfolded protein response, chaperones, proteasomal and lysosomal degradation machineries, and so forth.
Another major thrust of current neurodegenerative disease research involves factors outside neurons, and even outside the nervous system, that may be pivotal in disease progression. These types of disease mechanisms may not be accessible to modeling within model organisms that lack specialized biology. For example, there is deepening interest, in the prion-like self-templating behavior of amyloids and their ability to spread trans-synaptically or between neurons and glial cells (Brettschneider et al. 2015; Jarosz and Khurana 2017). Increasing evidence suggests that, just as for bona fide prions like PrP, specific conformers of proteins prone to misfold may have tropism for certain cells and circuits (Falcon et al. 2018). This could explain how distinct disease processes may result from the misfolding of the same protein (Jarosz and Khurana 2017; Peng et al. 2018).
There has also been burgeoning interest in neuroimmune interactions, in particular the potential role of microglial dysfunction. This has been partly driven by the recovery of many genes enriched in microglia in human genetic studies of neurodegenerative disease (Salter and Stevens 2017) in conjunction with elegant cellular studies that distinguish normal from pathogenic interactions between microglia and neurons (Krasemann et al. 2017). Even more recently, there has been growing appreciation that viral infection may have a role to play in the initiation and progression of neurodegeneration (Eimer et al. 2018). It is plausible that neuroimmune mechanisms may be involved in neurodegenerative disease progression after an initiating proteotoxic stress. Alternatively, in late-onset “sporadic” forms of the disease it is conceivable that protein misfolding is initiative through either defective neuroimmune mechanisms or perturbed clearance of toxic proteins from extracellular spaces.
This distinction between drivers and responders to proteotoxic stress may have significant implications for therapy. Genetic modifiers may alter vulnerability to protein misfolding and cytotoxicity, but they may miss critical proteostasis pathways that respond to proteotoxic stress. It is intriguing, for example, that for the most part the central proteostasis machineries (chaperones and so forth) are not commonly implicated in human genetic studies of neurodegenerative disease, and also in genetic modifier screens of proteotoxicity in model organisms. This is surprising because these pathways are known to be critical to the handling of misfolded proteins and deeply tied to pathways of aging. But this does not necessarily detract from the potential importance of these pathways. It could be, for example, that the core components of the chaperone machinery are essential for all of protein biogenesis and required to support embryonic development, and thus detrimental mutations would not be recovered. Or it could be that alterations in these pathways do not affect predisposition to disease but modulate severity or rate of progression. Conventional case-control human genetic studies would miss these factors, and yet they might be very important for therapeutics. Likewise, genetic screens in cellular models such as yeast cells could miss key genetic drivers of pathologies in aged organisms, and screening at different time points in organisms that can be aged (like the fly or worm) may be useful for identifying these factors. Finally, many of these factors may act in combination. As noted above, human genetic analysis of proteinopathies has far from exhausted a search for combinatorial effects of genes, and the majority of heritability factors remain as yet unrecovered.
Spatial Mapping to Identify Drivers
One intriguing feature that has emerged from the genetic architecture of neurodegenerative diseases is how little overlap there is between distinct diseases. With the possible exception of apolipoprotein E genotype, there is not a single genetic risk factor known to definitively predispose to different degenerative proteinopathies. It is possible that major defects in the proteostasis machinery that could predispose to misfolding and aggregation of multiple toxic proteins would be lethal, or that there is enough redundancy in the machinery to protect against the effect of such mutations.
Another possibility is that the genetic modifiers of a proteotoxicity relate in part to the intrinsic function of the protein that misfolds. Put another way, modulation of pathways intrinsically related to this function are major drivers of disease. Considerable evidence supports this possibility, particularly for proteins like huntingin and ataxin proteins (reviewed recently in Jarosz and Khurana 2017). Recently, this hypothesis was indirectly tested via mapping of interacting proteins. For example, Hughes and colleagues exploited protein interaction data of huntingtin protein (via yeast two-hybrid and affinity pull down-mass spectrometry) to identify candidate genetic modifiers of huntingtin in a Drosophila model of HD. They thereby connected genetic drivers to functional interactors of the misfolding protein (Kaltenbach et al. 2007). Proximity biotinylation labeling with an ascorbate peroxidase (APEX) tag (Han et al. 2018) enables this kind of analysis in living neurons, and was recently applied to α-synuclein (Chung et al. 2017). Genetic modifiers of α-synuclein toxicity in yeast cells (“genetic map” as described above) were cross-compared to proteins <10 nm radius from α-synuclein in neurons (Chung et al. 2017; Khurana et al. 2017). The spatial and genetic maps significantly overlapped, most clearly for vesicle trafficking and mRNA-binding proteins. Thus, the intrinsic location and protein interactions of α-synuclein are directly related to its mechanism of toxicity when it misfolds. This may turn out to be a general theme, explaining in part the exquisite specificity of protein-misfolding pathologies.
CAPTURING SPECIFIC PROTEIN CONFORMERS IN LIVING CELLS
Underlying the conceptual framework of drivers and responders to neurodegeneration, there is a physical dimension at the level of biomolecules that needs to be understood. The methods we describe above delineate proteins and pathways mediating the cellular effects of toxic proteins. But at the molecular level, these cellular networks need to be resolved as specific protein–protein interactions, and as interactions between proteins in specific conformational states. To achieve this level of understanding, there are a growing number of approaches to assess alterations of protein structure and protein–protein interactions in living cells.
It is straightforward to grasp the disruption of a network if a mutation arises in an “executive domain’’ of a protein, such as a catalytic site, a well-folded protein-interaction domain or a posttranslational modification site. However, there are numerous Mendelian mutations that are mapped to intrinsically disordered regions (IDRs), or low-complexity domains (LCDs) (Castello et al. 2013). Nowhere is this clearer than the case of ALS, where mutations of TDP-43 and FUS proteins are concentrated in LCDs (Harrison and Shorter 2017). Although IDRs seem to have low information content, they had been well-recognized in yeast prions and polyglutamine-containing proteins as the primary source of prionogenic potential. For instance, conversion to a prion conformation in yeast depends on long stretches of asparagines and glutamines (N/Q) (Halfmann and Lindquist 2010), and computational algorithms trained on these LCD sequences enabled the discovery of novel prions (Alberti et al. 2009). But beyond sequence, the last decade has brought remarkable biophysical insights into the nature of intrinsically disordered proteins. These included seminal observations of the gel-like properties of nuclear pore complexes (Frey et al. 2006) and the liquid behaviors of P-granules of C. elegans oocytes (Brangwynne et al. 2009). A new understanding of the material properties of proteins has emerged in which phase separation contributes to cellular organization through the formation of membraneless organelles (MLOs). Moreover, this process is highly sensitive to mutation of such proteins (Shin et al. 2017; Boeynaems et al. 2018). Although the nomenclature for this phenomenon is as abundant as the newly discovered MLOs (prion-like aggregation, liquid–liquid phase separation, droplets, ribonucleoprotein bodies, phases, granules, etc.), the term “condensates” has been widely accepted in the field (Banani et al. 2017) as it is inclusive of all the physical states (liquid, gel, amyloid fibrils) of biological polymers.
Condensates are widely thought to organize the cellular milieu in a spatiotemporal manner. Classical cellular compartments are now recognized as MLOs; P-bodies/stress granules (Decker and Parker 2012), Cajal bodies (Nizami et al. 2010), nucleolus (Feric et al. 2016), and Balbiani bodies (Boke et al. 2016). Some of these granules exist constitutively, such as P-bodies, although their numbers increase upon mRNA decay-related stress. In contrast, stress granules quickly form de novo upon translation-related stress (Protter and Parker 2016). Although these physiologic protein accumulations are carefully controlled by the cell, their inherent metastability gives them a dangerous edge. Point mutations in the IDRs (Harrison and Shorter 2017), malfunctioning of the chaperone machinery (Mateju et al. 2017), aging (Alberti and Carra 2018), ATP depletion (Patel et al. 2017), pH changes (Munder et al. 2016), or cellular crowding (Delarue et al. 2018) can drive these metastable proteins into pathological aggregates. Cells can cope with such aggregates to a certain extent through degradation (autophagy/proteasomal degradation) (Balchin et al. 2016), or sequestration (JUNQ/INQ or IPODs) (Miller et al. 2015). However, beyond a point, the cell succumbs and, as noted above, proteotoxicity can result and may be amplified when some conformations are templated and spread within or between cells (Jarosz and Khurana 2017). Abnormal protein conformation may thus be critical to both the genesis and progression of proteinopathies. It is, therefore, imperative to integrate this conformational information into our understanding of cellular pathologies in proteinopathies.
Technologies to Measure and Control Aggregation Complexes and States in Living Cells
Traditional biochemical methods for assaying protein aggregation may entail lysis of the cell but many tools have been developed for in vivo detection of condensation. Within intact cells, our knowledge has mostly arisen from skillful usage of fluorescent microscopy of tagged candidate proteins. In vivo dynamics of these condensates are measured by FRAP (fluorescence recovery after photobleaching) complemented by in vitro droplet formation assays with purified components (Brangwynne et al. 2009; Li et al. 2012; Caudron and Barral 2013; Zeng et al. 2016). Complementation of split fluorescent proteins have also been extensively utilized to track α-synuclein aggregations in vivo (Lázaro et al. 2014). Frequently, these condensates are a complex mixture of proteins and RNA. APEX methods (noted above) can be used to identify the heterogeneous composition of granules before or after condensation events. Stress-dependent composition of stress granules, for example, have been identified through proximity labeling of G3BP1-APEX (Markmiller et al. 2018). In some instances, solid cores of condensates are strong enough for extraction and proteomic analysis (Jain et al. 2016). Hubstenberger et al. (2017) have developed a fluorescence-activated particle-sorting method to check for the composition of P-bodies before and after stress conditions by labeling LSM14.
Amyloids can be investigated by methods developed in the prion field. Semidenaturing agarose gel electrophoresis (SDD-AGE) or filter trap assays have been extensively used to screen hundreds of wild yeast strains for [PSI+] prion (Halfmann et al. 2012), or to search for amyloidogenic propensities of Q/N rich proteins in the yeast proteome (Alberti et al. 2010). However, a major challenge is to examine these entities in vivo with minimal intrusion. Recently, Newby and colleagues developed a highly sensitive, dynamic in vivo protein aggregation tracking method called yeast transcriptional reporting of aggregating protein (yTRAP). yTRAP makes use of a synthetic zinc finger (ZnF) fold and an activator moiety attached to a protein under investigation (Newby et al. 2017). ZnF recognizes a highly specific cognate DNA sequence upstream of a fluorescent reporter. This simple logic allows channeling the aggregation state of a protein to a fluorescent output (Fig. 4A). The modular nature of yTRAP permits tracking of multiple prion aggregations simultaneously and its high sensitivity, combined with flow cytometry, allows differentiating different strains of the same prion, a crucial distinction especially for the neurodegeneration field.
Figure 4.
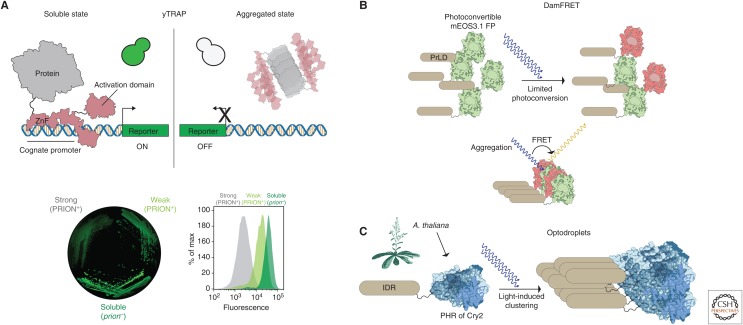
New technologies to measure and control protein condensation in vivo. (A) Yeast transcriptional reporting of aggregating protein (yTRAP) relies on tagging a protein of interest with a synthetic zinc finger (ZnF), which binds specifically to its cognate promoter. This yTRAP module contains an activation domain of VP16. In the soluble state, proteins diffuse freely and activate the reporter (in this case, mNeonGreen but could be easily interchanged or another reporter added for dual sensing of proteins). Upon aggregation, the signal is lost. The fluorescence can be measured sensitively with a CCD camera or flow cytometry. Three different strains of a yeast prion can be separated with this method. (B) DamFRET (distributed amphifluoric FRET). A protein contacting a prion-like domain (PrLD) is attached to mEOS3.1. Upon conversion of a subpopulation of mEOS1, two fluorophores can act as FRET pairs, if they are in close proximity. Therefore, the FRET signal acts as a proxy for concentration-dependent nucleation of prion-like proteins. Optodroplets rely on the photolyase homology region (PHR) domain of Cry2 protein of Arabidopsis thaliana. This domain self-associates upon exposure to blue light. The intrinsically disordered regions (IDRs) can be induced in vivo for supersaturation.
Importantly, many condensates contain RNA molecules, and low-complexity regions are highly represented in RNA-binding proteins (RBPs) (Castello et al. 2013). The unexpected finding that numerous RBPs contained IDRs and could be precipitated in biotinylated isoxazole from cell lysates opened up intense research into the biophysical properties of RNP granules (Han et al. 2012; Kato et al. 2012). Certain RBPs with IDR regions, especially components of stress granules, can solidify in vivo, a process known as maturation or hardening, causing loss of their protein/RNA clients, thus altering cellular “ribostasis” (Ramaswami et al. 2013). Hardened stress granules have been implicated in ALS pathology (Li et al. 2013). To find aggregation-prone RBPs, Newby et al. created a library of yTRAP strains for RBPs in the yeast proteome. This allowed simultaneous measurement of RBP aggregation states upon a chemical or environmental stress, such as chaperone inhibition or overexpression. Moreover, the library permitted tracking of coaggregation events in a time-resolved manner (Newby et al. 2017). It will be crucial to extend these libraries to other protein families, such as transcription factors, and transfer the methodology to mammalian systems.
Although amyloids are extremely stable, their nucleation is rare due to kinetic barriers. An important question in amyloidogenesis is how initial protofilaments form and self-template. To track these rare nucleation events in vivo, the Halfmann laboratory developed DamFRET (Khan et al. 2018). This method relies on a photoconvertible mEos3.1 fluorescent tag. Semiconversion of mEOS1 allows a single tag to represent a protein of interest as FRET pairs. Therefore, an increasing FRET signal occurs when the tagged protein becomes more tightly aggregated (Fig. 4B). With this single tag and flow cytometry, the aggregation of any protein can be measured as a function of its concentration. Kinetic barriers for aggregation can certainly be overcome by overexpression of the protein under investigation; however, its supersaturation may occur in the cell because of overcrowding, change of cellular milieu, failure of protein control mechanisms, or aging as discussed above. Therefore, it is highly desirable to have precise spatiotemporal control of nucleation events in vivo by means other than overexpression. Optodroplets, developed for this purpose, are chimeric fusions of proteins under investigation and a blue light–inducible oligomerization domain of Cry2 from Arabidopsis thaliana (Fig. 4C). Using this technique, Shin and colleagues determined the in vivo dynamics of triggered condensations and their solidifications in time (Shin et al. 2017).
Collectively, these powerful and complementary approaches set the stage for detailed molecular dissection of protein aggregation states and multiprotein complexes.
CONCLUDING REMARKS
Here, we surveyed some methods that are enabling proteome-scale dissection of proteotoxic mechanisms at the cellular and organismal level. As noted above, the emerging biological data sets promise to, in turn, help us focus on specific gene variants emerging from larger-scale genetic studies for which pure human genetic analysis will most likely become an exercise in diminishing returns.
Driven by the classical tools of molecular biology, the preponderance of knowledge to date has (not surprisingly) been at the gene, transcript, and protein level. But chemical genetic profiling (Piotrowski et al. 2017), metabolomic profiling (Evers et al. 2017), and survey of chromatin and epigenetic factors (De Jager et al. 2018) are revealing novel mechanisms. For instance, recent elegant work has connected genomic instability, chromatin relaxation, and the aberrant activation of transposable genetic elements in tauopathy, providing entirely new perspectives on how age-related cellular changes might amplify the toxic effects of misfolded proteins (Guo et al. 2018; Sun et al. 2018). Computational approaches are also being employed to make better biological sense of the “hairballs” of large-scale interaction networks and to interrelate different large-scale data sets (Khurana et al. 2017; Kedaigle and Fraenkel 2018).
For all of their utility, the data sets described in this review have largely emerged from model systems that have not captured human cellular biology or the specific protein conformations that give rise to disease. There is a growing appreciation that specificity of proteinopathies is driven by distinct conformational states of proteins. These may be unique among diseases (Woerman et al. 2018), differ between individual patients, and be critically dependent upon cellular milieu (Peng et al. 2018). These unique conformers have not been modeled in cellular or organism models, nor have they been profiled in a human cellular context. But this will now change as technologies that capture both human host cell and toxic protein strain are available.
At the level of capturing human cell biology, somatic-cell reprogramming and the advent of iPSc is enabling the generation of patient-specific cells and distinct cell types from these patients. Cells can be arrayed in cocultures in specific orientations, aided by microfluidics or 3D laser printing. Stunning organoid technologies enable 3D patient-specific tissues to be generated too. These technologies are becoming available for the cells and tissues most affected in proteinopathies, from CNS (Lancaster and Knoblich 2014) to pancreatic islets (Zhou and Melton 2018) to muscle (Pourquié et al. 2018). At the level of capturing disease- and patient-specific conformer, biochemical approaches that capture and amplify these from brain (Peng et al. 2018) and spinal fluid (Shahnawaz et al. 2017) are being developed. Elusive cell types, such as microglia, can now be generated from iPSc and incorporated into 3D organoids (Muffat et al. 2016).
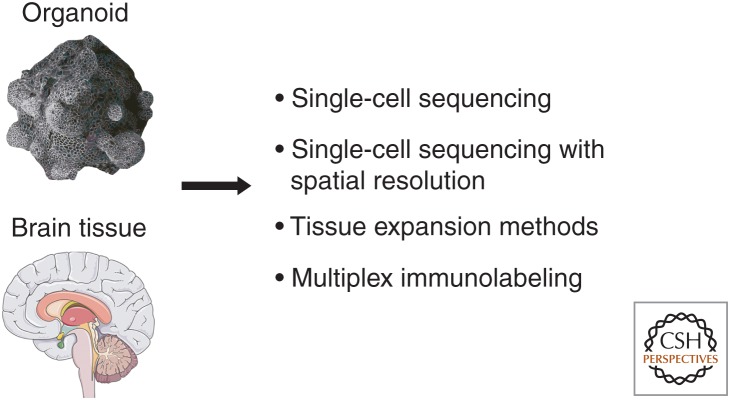
The amalgamation of these distinct technologies will provide an unprecedented view of proteotoxicity, amenable to the many methods outlined in this review. But a new wave of technologies will render it possible to profile 2D and 3D models, or patient-derived postmortem human tissue, in a spatially specific way (Fig. 5). Single-cell sequencing technologies are poised to reveal somatic mosaicism at the level of genome (Evrony et al. 2012) and transcriptome (Ecker et al. 2017). Next-generation barcoding and sequencing have now also been applied to achieve transcriptional profiling of spatially resolved intact tissue, with methods such as fluorescence in situ sequencing (FISSEQ) (Lee 2017) and highly multiplexed error-robust FISH (MERFISH) (Moffitt et al. 2016) that can now be applied to 2D and 3D models of proteinopathy or human tissue samples. Stunning developments in microscopy will enhance our capacity to profile and understand proteinopathies in 3D, for example, the ability to expand tissue with expansion microscopy (Chen et al. 2015; Ku et al. 2016) or to combine clearing of lipids with multiplex antibody analysis (Murray et al. 2015).
Figure 5.
Emerging sequencing and imaging technologies. An unprecedented view of 3D postmortem tissue and stem-cell-derived organoid models is emerging through breakthrough techniques. These include single-cell DNA sequencing (Lodato et al. 2015) and RNA sequencing (Ecker et al. 2017). Our visualization of gene expression will be increasingly spatially resolved with more widespread use of techniques that can visualize hundreds or thousands of messenger RNA (mRNA) transcripts in tissue, including fluorescent in situ sequencing (FISSEQ) (Lee 2017) and highly multiplexed error-robust FISH (MERFISH) (Moffitt et al. 2016). Visualization will be enhanced by tissue-expansion methods (Chen et al. 2015; Ku et al. 2016; Wang et al. 2018) and methods like SWITCH that combine delipidation of tissue with multiplex antibody analysis (Murray et al. 2015).
In a time of aging populations, the personal and societal consequences of proteinopathies, from AD to PD to diabetes, will become even more devastating. It is heartening that our understanding of these diseases, from factors that drive them to those that mediate progression, is commensurately increasing. Unbiased methodology at the level of cell, tissue, and organism promises to enrich our understanding of underlying biological mechanisms. We predict that sorely needed preventative and therapeutic options for patients will follow closely behind.
ACKNOWLEDGMENTS
We apologize to colleagues whose work could not be cited owing to space limitations. We thank Dr. Mel Feany for critical reading of the manuscript. Work in our laboratory is supported by the New York Stem Cell Foundation, the National Ataxia Foundation, the Dr B.R. and Dr C.R. Shetty Initiative for Parkinsonian Disorders, the Barbara Bloom Ranson Fund for Multiple System Atrophy (MSA) Research and the Brigham Research Institute of Brigham and Women's Hospital. In addition, V.K. is supported by a National Ataxia Foundation Young Investigator Award and the 2018 Bishop Dr. Karl Golser Award. I.L. is supported by awards from the American Parkinson Disease Association (APDA) and PhRMA Foundation, and E.H. by the Human Frontier Science Program (HFSP, LT000717/2015-L). V.K. is a Robertson Investigator of the New York Stem Cell Foundation. Some of the graphics in this chapter were adapted from Servier Medical Art templates available at https://smart.servier.com. Servier Medical Art is licensed under a Creative Commons Attribution 3.0 Unported License.
Footnotes
Editors: Richard I. Morimoto, F. Ulrich Hartl, and Jeffery W. Kelly
Additional Perspectives on Protein Homeostasis available at www.cshperspectives.org
REFERENCES
- Alberti S, Carra S. 2018. Quality control of membraneless organelles. J Mol Biol 430: 4711–4729. 10.1016/j.jmb.2018.05.013 [DOI] [PubMed] [Google Scholar]
- Alberti S, Halfmann R, King O, Kapila A, Lindquist S. 2009. A systematic survey identifies prions and illuminates sequence features of prionogenic proteins. Cell 137: 146–158. 10.1016/j.cell.2009.02.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti S, Halfmann R, Lindquist S. 2010. Biochemical, cell biological, and genetic assays to analyze amyloid and prion aggregation in yeast. Methods Enzymol 470: 709–734. 10.1016/S0076-6879(10)70030-6 [DOI] [PubMed] [Google Scholar]
- Arning L. 2016. The search for modifier genes in Huntington disease—Multifactorial aspects of a monogenic disorder. Mol Cell Probes 30: 404–409. 10.1016/j.mcp.2016.06.006 [DOI] [PubMed] [Google Scholar]
- Auluck PK, Chan HY, Trojanowski JQ, Lee VM, Bonini NM. 2002. Chaperone suppression of α-synuclein toxicity in a Drosophila model for Parkinson's disease. Science 295: 865–868. 10.1126/science.1067389 [DOI] [PubMed] [Google Scholar]
- Bagriantsev S, Liebman S. 2006. Modulation of Aβ42 low-n oligomerization using a novel yeast reporter system. BMC Biol 4: 32 10.1186/1741-7007-4-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahadorani S, Mukai ST, Rabie J, Beckman JS, Phillips JP, Hilliker AJ. 2013. Expression of zinc-deficient human superoxide dismutase in Drosophila neurons produces a locomotor defect linked to mitochondrial dysfunction. Neurobiol Aging 34: 2322–2330. 10.1016/j.neurobiolaging.2013.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch WE, Morimoto RI, Dillin A, Kelly JW. 2008. Adapting proteostasis for disease intervention. Science 319: 916–919. 10.1126/science.1141448 [DOI] [PubMed] [Google Scholar]
- Balchin D, Hayer-Hartl M, Hartl FU. 2016. In vivo aspects of protein folding and quality control. Science 353: aac4354 10.1126/science.aac4354 [DOI] [PubMed] [Google Scholar]
- Banani SF, Lee HO, Hyman AA, Rosen MK. 2017. Biomolecular condensates: organizers of cellular biochemistry. Nat Rev Mol Cell Biol 18: 285–298. 10.1038/nrm.2017.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basha O, Tirman S, Eluk A, Yeger-Lotem E. 2013. ResponseNet2.0: Revealing signaling and regulatory pathways connecting your proteins and genes—Now with human data. Nucleic Acids Res 41: W198–W203. 10.1093/nar/gkt532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basha O, Shpringer R, Argov CM, Yeger-Lotem E. 2018. The DifferentialNet database of differential protein–protein interactions in human tissues. Nucleic Acids Res 46: D522–D526. 10.1093/nar/gkx981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker LA, Huang B, Bieri G, Ma R, Knowles DA, Jafar-Nejad P, Messing J, Kim HJ, Soriano A, Auburger G, et al. 2017. Therapeutic reduction of ataxin-2 extends lifespan and reduces pathology in TDP-43 mice. Nature 544: 367–371. 10.1038/nature22038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blard O, Feuillette S, Bou J, Chaumette B, Frébourg T, Campion D, Lecourtois M. 2007. Cytoskeleton proteins are modulators of mutant tau-induced neurodegeneration in Drosophila. Hum Mol Genet 16: 555–566. 10.1093/hmg/ddm011 [DOI] [PubMed] [Google Scholar]
- Boeynaems S, Alberti S, Fawzi NL, Mittag T, Polymenidou M, Rousseau F, Schymkowitz J, Shorter J, Wolozin B, Van Den Bosch L, et al. 2018. Protein phase separation: A new phase in cell biology. Trends Cell Biol 28: 420–435. 10.1016/j.tcb.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boke E, Ruer M, Wühr M, Coughlin M, Lemaitre R, Gygi SP, Alberti S, Drechsel D, Hyman AA, Mitchison TJ. 2016. Amyloid-like self-assembly of a cellular compartment. Cell 166: 637–650. 10.1016/j.cell.2016.06.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrageiro G, Haylett W, Seedat S, Kuivaniemi H, Bardien S. 2018. A review of genome-wide transcriptomics studies in Parkinson's disease. Eur J Neurosci 47: 1–16. 10.1111/ejn.13760 [DOI] [PubMed] [Google Scholar]
- Brandt R, Gergou A, Wacker I, Fath T, Hutter H. 2009. A Caenorhabditis elegans model of tau hyperphosphorylation: Induction of developmental defects by transgenic overexpression of Alzheimer's disease-like modified tau. Neurobiol Aging 30: 22–33. 10.1016/j.neurobiolaging.2007.05.011 [DOI] [PubMed] [Google Scholar]
- Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, Gharakhani J, Julicher F, Hyman AA. 2009. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324: 1729–1732. 10.1126/science.1172046 [DOI] [PubMed] [Google Scholar]
- Brettschneider J, Del Tredici K, Lee VM, Trojanowski JQ. 2015. Spreading of pathology in neurodegenerative diseases: A focus on human studies. Nat Rev Neurosci 16: 109–120. 10.1038/nrn3887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brignull HR, Moore FE, Tang SJ, Morimoto RI. 2006. Polyglutamine proteins at the pathogenic threshold display neuron-specific aggregation in a pan-neuronal Caenorhabditis elegans model. J Neurosci 26: 7597–7606. 10.1523/jneurosci.0990-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookfield JF. 2013. Quantitative genetics: heritability is not always missing. Curr Biol 23: R276–R278. 10.1016/j.cub.2013.02.040 [DOI] [PubMed] [Google Scholar]
- Caine J, Sankovich S, Antony H, Waddington L, Macreadie P, Varghese J, Macreadie I. 2007. Alzheimer's Aβ fused to green fluorescent protein induces growth stress and a heat shock response. FEMS Yeast Res 7: 1230–1236. 10.1111/j.1567-1364.2007.00285.x [DOI] [PubMed] [Google Scholar]
- Cao S, Gelwix CC, Caldwell KA, Caldwell GA. 2005. Torsin-mediated protection from cellular stress in the dopaminergic neurons of Caenorhabditis elegans. J Neurosci 25: 3801–3812. 10.1523/jneurosci.5157-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraveo G, Auluck PK, Whitesell L, Chung CY, Baru V, Mosharov EV, Yan X, Ben-Johny M, Soste M, Picotti P, et al. 2014. Calcineurin determines toxic versus beneficial responses to α-synuclein. Proc Natl Acad Sci 111: E3544–E3552. 10.1073/pnas.1413201111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraveo G, Soste M, Cappelleti V, Fanning S, van Rossum DB, Whitesell L, Huang Y, Chung CY, Baru V, Zaichick S, et al. 2017. FKBP12 contributes to α-synuclein toxicity by regulating the calcineurin-dependent phosphoproteome. Proc Natl Acad Sci 114: E11313–E11322. 10.1073/pnas.1711926115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casals F, Bertranpetit J. 2012. Human genetic variation, shared and private. Science 337: 39–40. 10.1126/science.1224528 [DOI] [PubMed] [Google Scholar]
- Castello A, Fischer B, Hentze MW, Preiss T. 2013. RNA-binding proteins in Mendelian disease. Trends Genet 29: 318–327. 10.1016/j.tig.2013.01.004 [DOI] [PubMed] [Google Scholar]
- Caudron F, Barral Y. 2013. A super-assembly of Whi3 encodes memory of deceptive encounters by single cells during yeast courtship. Cell 155: 1244–1257. 10.1016/j.cell.2013.10.046 [DOI] [PubMed] [Google Scholar]
- Chang D, Nalls MA, Hallgrímsdóttir IB, Hunkapiller J, van der Brug M, Cai F; International Parkinson's Disease Genomics Consortium; 23andMe Research Team, Kerchner GA, Ayalon G, et al. 2017. A meta-analysis of genome-wide association studies identifies 17 new Parkinson's disease risk loci. Nat Genet 49: 1511-1516. 10.1038/ng.3955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Tillberg PW, Boyden ES. 2015. Optical imaging. Expansion microscopy. Science 347: 543–548. 10.1126/science.1260088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Farzadfard F, Gharaei N, Chen WCW, Cao J, Lu TK. 2017. Randomized CRISPR-Cas transcriptional perturbation screening reveals protective genes against α-synuclein toxicity. Mol Cell 68: 247–257 e245. 10.1016/j.molcel.2017.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CY, Khurana V, Yi S, Sahni N, Loh KH, Auluck PK, Baru V, Udeshi ND, Freyzon Y, Carr SA, et al. 2017. In situ peroxidase labeling and mass-spectrometry connects α-synuclein directly to endocytic trafficking and mRNA metabolism inneurons. Cell Syst 4: 242–250 e244. 10.1016/j.cels.2017.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther DC, Kinghorn KJ, Miranda E, Page R, Curry JA, Duthie FA, Gubb DC, Lomas DA. 2005. Intraneuronal Aβ, non-amyloid aggregates and neurodegeneration in a Drosophila model of Alzheimer's disease. Neuroscience 132: 123–135. 10.1016/j.neuroscience.2004.12.025 [DOI] [PubMed] [Google Scholar]
- Cui Y, Li G, Li S, Wu R. 2010. Designs for linkage analysis and association studies of complex diseases. Methods Mol Biol 620: 219–242. 10.1007/978-1-60761-580-4_6 [DOI] [PubMed] [Google Scholar]
- D'Angelo F, Vignaud H, Di Martino J, Salin B, Devin A, Cullin C, Marchal C. 2013. A yeast model for amyloid-β aggregation exemplifies the role of membrane trafficking and PICALM in cytotoxicity. Dis Model Mech 6: 206–216. 10.1242/dmm.010108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker CJ, Parker R. 2012. P-bodies and stress granules: possible roles in the control of translation and mRNA degradation. Cold Spring Harb Perspect Biol 4: a012286 10.1101/cshperspect.a012286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jager PL, Ma Y, McCabe C, Xu J, Vardarajan BN, Felsky D, Klein HU, White CC, Peters MA, Lodgson B, et al. 2018. A multi-omic atlas of the human frontal cortex for aging and Alzheimer's disease research. Sci Data 5: 180142 10.1038/sdata.2018.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delarue M, Brittingham GP, Pfeffer S, Surovtsev IV, Pinglay S, Kennedy KJ, Schaffer M, Gutierrez JI, Sang D, Poterewicz G, et al. 2018. mTORC1 controls phase separation and the biophysical properties of the cytoplasm by tuning crowding. Cell 174: 338–349.e320. 10.1016/j.cell.2018.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhungel N, Eleuteri S, Li LB, Kramer NJ, Chartron JW, Spencer B, Kosberg K, Fields JA, Stafa K, Adame A, et al. 2015. Parkinson's disease genes VPS35 and EIF4G1 interact genetically and converge on α-synuclein. Neuron 85: 76–87. 10.1016/j.neuron.2014.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrich M, Mao L, Bernreuther C, Zabel C, Nebrich G, Kleene R, Klose J. 2008. Proteome analysis of ventral midbrain in MPTP-treated normal and L1cam transgenic mice. Proteomics 8: 1266–1275. 10.1002/pmic.200700754 [DOI] [PubMed] [Google Scholar]
- Dixon C, Mathias N, Zweig RM, Davis DA, Gross DS. 2005. α-Synuclein targets the plasma membrane via the secretory pathway and induces toxicity in yeast. Genetics 170: 47–59. 10.1534/genetics.104.035493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duennwald ML, Jagadish S, Giorgini F, Muchowski PJ, Lindquist S. 2006a. A network of protein interactions determines polyglutamine toxicity. Proc Natl Acad Sci 103: 11051–11056. 10.1073/pnas.0604548103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duennwald ML, Jagadish S, Muchowski PJ, Lindquist S. 2006b. Flanking sequences profoundly alter polyglutamine toxicity in yeast. Proc Natl Acad Sci 103: 11045–11050. 10.1073/pnas.0604547103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke DC, Moran LB, Kalaitzakis ME, Deprez M, Dexter DT, Pearce RK, Graeber MB. 2006. Transcriptome analysis reveals link between proteasomal and mitochondrial pathways in Parkinson's disease. Neurogenetics 7: 139–148. 10.1007/s10048-006-0033-5 [DOI] [PubMed] [Google Scholar]
- Ecker JR, Geschwind DH, Kriegstein AR, Ngai J, Osten P, Polioudakis D, Regev A, Sestan N, Wickersham IR, Zeng H. 2017. The BRAIN Initiative Cell Census Consortium: Lessons learned toward generating a comprehensive brain cell atlas. Neuron 96: 542–557. 10.1016/j.neuron.2017.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler EE, Flint J, Gibson G, Kong A, Leal SM, Moore JH, Nadeau JH. 2010. Missing heritability and strategies for finding the underlying causes of complex disease. Nat Rev Genet 11: 446–450. 10.1038/nrg2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer WA, Vijaya Kumar DK, Navalpur Shanmugam NK, Rodriguez AS, Mitchell T, Washicosky KJ, György B, Breakefield XO, Tanzi RE, Moir RD. 2018. Alzheimer's disease-associated β-amyloid is rapidly seeded by Herpesviridae to protect against brain infection. Neuron 99: 56–63.e53. 10.1016/j.neuron.2018.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elden AC, Kim HJ, Hart MP, Chen-Plotkin AS, Johnson BS, Fang X, Armakola M, Geser F, Greene R, Lu MM, et al. 2010. Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature 466: 1069–1075. 10.1038/nature09320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers BM, Rodriguez-Navas C, Tesla RJ, Prange-Kiel J, Wasser CR, Yoo KS, McDonald J, Cenik B, Ravenscroft TA, Plattner F, et al. 2017. Lipidomic and transcriptomic basis of lysosomal dysfunction in progranulin deficiency. Cell Rep 20: 2565–2574. 10.1016/j.celrep.2017.08.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrony GD, Cai X, Lee E, Hills LB, Elhosary PC, Lehmann HS, Parker JJ, Atabay KD, Gilmore EC, Poduri A, et al. 2012. Single-neuron sequencing analysis of L1 retrotransposition and somatic mutation in the human brain. Cell 151: 483–496. 10.1016/j.cell.2012.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber PW, Alter JR, MacDonald ME, Hart AC. 1999. Polyglutamine-mediated dysfunction and apoptotic death of a Caenorhabditis elegans sensory neuron. Proc Natl Acad Sci 96: 179–184. 10.1073/pnas.96.1.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon B, Zhang W, Murzin AG, Murshudov G, Garringer HJ, Vidal R, Crowther RA, Ghetti B, Scheres SHW, Goedert M. 2018. Structures of filaments from Pick's disease reveal a novel tau protein fold. Nature 561: 137–140. 10.1038/s41586-018-0454-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feany MB, Bender WW. 2000. A Drosophila model of Parkinson's disease. Nature 404: 394–398. 10.1038/35006074 [DOI] [PubMed] [Google Scholar]
- Feric M, Vaidya N, Harmon TS, Mitrea DM, Zhu L, Richardson TM, Kriwacki RW, Pappu RV, Brangwynne CP. 2016. Coexisting liquid phases underlie nucleolar subcompartments. Cell 165: 1686–1697. 10.1016/j.cell.2016.04.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Funez P, Nino-Rosales ML, de Gouyon B, She WC, Luchak JM, Martinez P, Turiegano E, Benito J, Capovilla M, Skinner PJ, et al. 2000. Identification of genes that modify ataxin-1-induced neurodegeneration. Nature 408: 101–106. 10.1038/35040584 [DOI] [PubMed] [Google Scholar]
- Finelli A, Kelkar A, Song HJ, Yang H, Konsolaki M. 2004. A model for studying Alzheimer's Aβ42-induced toxicity in Drosophila melanogaster. Mol Cell Neurosci 26: 365–375. 10.1016/j.mcn.2004.03.001 [DOI] [PubMed] [Google Scholar]
- Flint J. 2013. GWAS. Curr Biol 23: R265–R266. 10.1016/j.cub.2013.01.040 [DOI] [PubMed] [Google Scholar]
- Frey S, Richter RP, Gorlich D. 2006. FG-rich repeats of nuclear pore proteins form a three-dimensional meshwork with hydrogel-like properties. Science 314: 815–817. 10.1126/science.1132516 [DOI] [PubMed] [Google Scholar]
- Fushimi K, Long C, Jayaram N, Chen X, Li L, Wu JY. 2011. Expression of human FUS/TLS in yeast leads to protein aggregation and cytotoxicity, recapitulating key features of FUS proteinopathy. Protein Cell 2: 141–149. 10.1007/s13238-011-1014-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallart-Palau X, Ng CH, Ribera J, Sze SK, Lim KL. 2016. Drosophila expressing human SOD1 successfully recapitulates mitochondrial phenotypic features of familial amyotrophic lateral sclerosis. Neurosci Lett 624: 47–52. 10.1016/j.neulet.2016.05.006 [DOI] [PubMed] [Google Scholar]
- Gidalevitz T, Krupinski T, Garcia S, Morimoto RI. 2009. Destabilizing protein polymorphisms in the genetic background direct phenotypic expression of mutant SOD1 toxicity. PLoS Genet 5: e1000399 10.1371/journal.pgen.1000399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler AD, Chesi A, Geddie ML, Strathearn KE, Hamamichi S, Hill KJ, Caldwell KA, Caldwell GA, Cooper AA, Rochet JC, et al. 2009. α-Synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nat Genet 41: 308–315. 10.1038/ng.300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardia-Laguarta C, Area-Gomez E, Rub C, Liu Y, Magrane J, Becker D, Voos W, Schon EA, Przedborski S. 2014. α-Synuclein is localized to mitochondria-associated ER membranes. J Neurosci 34: 249–259. 10.1523/jneurosci.2507-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JL, Lee VM. 2014. Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nat Med 20: 130–138. 10.1038/nm.3457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Jeong HH, Hsieh YC, Klein HU, Bennett DA, De Jager PL, Liu Z, Shulman JM. 2018. Tau activates transposable elements in Alzheimer's disease. Cell Rep 23: 2874–2880. 10.1016/j.celrep.2018.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfmann R, Lindquist S. 2010. Epigenetics in the extreme: Prions and the inheritance of environmentally acquired traits. Science 330: 629–632. 10.1126/science.1191081 [DOI] [PubMed] [Google Scholar]
- Halfmann R, Jarosz DF, Jones SK, Chang A, Lancaster AK, Lindquist S. 2012. Prions are a common mechanism for phenotypic inheritance in wild yeasts. Nature 482: 363–368. 10.1038/nature10875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamamichi S, Rivas RN, Knight AL, Cao S, Caldwell KA, Caldwell GA. 2008. Hypothesis-based RNAi screening identifies neuroprotective genes in a Parkinson's disease model. Proc Natl Acad Sci 105: 728–733. 10.1073/pnas.0711018105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Li J, Ting AY. 2018. Proximity labeling: Spatially resolved proteomic mapping for neurobiology. Curr Opin Neurobiol 50: 17–23. 10.1016/j.conb.2017.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han TW, Kato M, Xie S, Wu LC, Mirzaei H, Pei J, Chen M, Xie Y, Allen J, Xiao G, et al. 2012. Cell-free formation of RNA granules: Bound RNAs identify features and components of cellular assemblies. Cell 149: 768–779. 10.1016/j.cell.2012.04.016 [DOI] [PubMed] [Google Scholar]
- Harrington AJ, Yacoubian TA, Slone SR, Caldwell KA, Caldwell GA. 2012. Functional analysis of VPS41-mediated neuroprotection in Caenorhabditis elegans and mammalian models of Parkinson's disease. J Neurosci 32: 2142–2153. 10.1523/jneurosci.2606-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison AF, Shorter J. 2017. RNA-binding proteins with prion-like domains in health and disease. Biochem J 474: 1417–1438. 10.1042/BCJ20160499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser MA, Li YJ, Xu H, Noureddine MA, Shao YS, Gullans SR, Scherzer CR, Jensen RV, McLaurin AC, Gibson JR, et al. 2005. Expression profiling of substantia nigra in Parkinson disease, progressive supranuclear palsy, and frontotemporal dementia with parkinsonism. Arch Neurol 62: 917–921. 10.1001/archneur.62.6.917 [DOI] [PubMed] [Google Scholar]
- Holmans PA, Massey TH, Jones L. 2017. Genetic modifiers of Mendelian disease: Huntington's disease and the trinucleotide repeat disorders. Hum Mol Genet 26: R83–R90. 10.1093/hmg/ddx261 [DOI] [PubMed] [Google Scholar]
- Hubstenberger A, Courel M, Bénard M, Souquere S, Ernoult-Lange M, Chouaib R, Yi Z, Morlot JB, Munier A, Fradet M, et al. 2017. P-body purification reveals the condensation of repressed mRNA regulons. Mol Cell 68: 144–157.e145. 10.1016/j.molcel.2017.09.003 [DOI] [PubMed] [Google Scholar]
- Iijima K, Liu HP, Chiang AS, Hearn SA, Konsolaki M, Zhong Y. 2004. Dissecting the pathological effects of human Aβ40 and Aβ42 in Drosophila: A potential model for Alzheimer's disease. Proc Natl Acad Sci 101: 6623–6628. 10.1073/pnas.0400895101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima K, Chiang HC, Hearn SA, Hakker I, Gatt A, Shenton C, Granger L, Leung A, Iijima-Ando K, Zhong Y. 2008. Aβ42 mutants with different aggregation profiles induce distinct pathologies in Drosophila. PLoS ONE 3: e1703 10.1371/journal.pone.0001703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson GR, Salecker I, Dong X, Yao X, Arnheim N, Faber PW, MacDonald ME, Zipursky SL. 1998. Polyglutamine-expanded human huntingtin transgenes induce degeneration of Drosophila photoreceptor neurons. Neuron 21: 633–642. 10.1016/S0896-6273(00)80573-5 [DOI] [PubMed] [Google Scholar]
- Jackson GR, Wiedau-Pazos M, Sang TK, Wagle N, Brown CA, Massachi S, Geschwind DH. 2002. Human wild-type tau interacts with wingless pathway components and produces neurofibrillary pathology in Drosophila. Neuron 34: 509–519. 10.1016/S0896-6273(02)00706-7 [DOI] [PubMed] [Google Scholar]
- Jain S, Wheeler JR, Walters RW, Agrawal A, Barsic A, Parker R. 2016. ATPase-modulated stress granules contain a diverse proteome and substructure. Cell 164: 487–498. 10.1016/j.cell.2015.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosz DF, Khurana V. 2017. Specification of physiologic and disease states by distinct proteins and protein conformations. Cell 171: 1001–1014. 10.1016/j.cell.2017.10.047 [DOI] [PubMed] [Google Scholar]
- Johnson BS, McCaffery JM, Lindquist S, Gitler AD. 2008. A yeast TDP-43 proteinopathy model: Exploring the molecular determinants of TDP-43 aggregation and cellular toxicity. Proc Natl Acad Sci 105: 6439–6444. 10.1073/pnas.0802082105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju S, Tardiff DF, Han H, Divya K, Zhong Q, Maquat LE, Bosco DA, Hayward LJ, Brown RH Jr, Lindquist S, et al. 2011. A yeast model of FUS/TLS-dependent cytotoxicity. PLoS Biol 9: e1001052 10.1371/journal.pbio.1001052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach LS, Romero E, Becklin RR, Chettier R, Bell R, Phansalkar A, Strand A, Torcassi C, Savage J, Hurlburt A, et al. 2007. Huntingtin interacting proteins are genetic modifiers of neurodegeneration. PLoS Genet 3: e82 10.1371/journal.pgen.0030082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasap M, Akpinar G, Kanli A. 2017. Proteomic studies associated with Parkinson's disease. Expert Rev Proteomics 14: 193–209. 10.1080/14789450.2017.1291344 [DOI] [PubMed] [Google Scholar]
- Kato M, Han TW, Xie S, Shi K, Du X, Wu LC, Mirzaei H, Goldsmith EJ, Longgood J, Pei J, et al. 2012. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell 149: 753–767. 10.1016/j.cell.2012.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayatekin C, Matlack KE, Hesse WR, Guan Y, Chakrabortee S, Russ J, Wanker EE, Shah JV, Lindquist S. 2014. Prion-like proteins sequester and suppress the toxicity of huntingtin exon 1. Proc Natl Acad Sci 111: 12085–12090. 10.1073/pnas.1412504111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayatekin C, Amasino A, Gaglia G, Flannick J, Bonner JM, Fanning S, Narayan P, Barrasa MI, Pincus D, Landgraf D, et al. 2018. Translocon declogger Ste24 protects against IAPP oligomer-induced proteotoxicity. Cell 173: 62–73.e69. 10.1016/j.cell.2018.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazemi-Esfarjani P, Benzer S. 2000. Genetic suppression of polyglutamine toxicity in Drosophila. Science 287: 1837–1840. 10.1126/science.287.5459.1837 [DOI] [PubMed] [Google Scholar]
- Kedaigle A, Fraenkel E. 2018. Turning omics data into therapeutic insights. Curr Opin Pharmacol 42: 95–101. 10.1016/j.coph.2018.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keum JW, Shin A, Gillis T, Mysore JS, Abu Elneel K, Lucente D, Hadzi T, Holmans P, Jones L, Orth M, et al. 2016. The HTT CAG-expansion mutation determines age at death but not disease duration in Huntington disease. Am J Hum Genet 98: 287–298. 10.1016/j.ajhg.2015.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan T, Kandola TS, Wu J, Venkatesan S, Ketter E, Lange JJ, Rodríguez Gama A, Box A, Unruh JR, Cook M, et al. 2018. Quantifying nucleation in vivo reveals the physical basis of prion-like phase behavior. Mol Cell 71: 155–168.e157. 10.1016/j.molcel.2018.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana V, Lindquist S. 2010. Modelling neurodegeneration in Saccharomyces cerevisiae: Why cook with baker's yeast? Nat Rev Neurosci 11: 436–449. 10.1038/nrn2809 [DOI] [PubMed] [Google Scholar]
- Khurana V, Tardiff DF, Chung CY, Lindquist S. 2015. Toward stem cell-based phenotypic screens for neurodegenerative diseases. Nat Rev Neurol 11: 339–350. 10.1038/nrneurol.2015.79 [DOI] [PubMed] [Google Scholar]
- Khurana V, Peng J, Chung CY, Auluck PK, Fanning S, Tardiff DF, Bartels T, Koeva M, Eichhorn SW, Benyamini H, et al. 2017. Genome-scale networks link neurodegenerative disease genes to α-synuclein through specific molecular pathways. Cell Syst 4: 157–170 e114. 10.1016/j.cels.2016.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Raphael AR, LaDow ES, McGurk L, Weber RA, Trojanowski JQ, Lee VM, Finkbeiner S, Gitler AD, Bonini NM. 2014. Therapeutic modulation of eIF2α phosphorylation rescues TDP-43 toxicity in amyotrophic lateral sclerosis disease models. Nat Genet 46: 152–160. 10.1038/ng.2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer BC, Zhang B, Leverenz JB, Thomas JH, Trojanowski JQ, Schellenberg GD. 2003. Neurodegeneration and defective neurotransmission in a Caenorhabditis elegans model of tauopathy. Proc Natl Acad Sci 100: 9980–9985. 10.1073/pnas.1533448100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer NJ, Haney MS, Morgens DW, Jovičić A, Couthouis J, Li A, Ousey J, Ma R, Bieri G, Tsui CK, et al. 2018. CRISPR-Cas9 screens in human cells and primary neurons identify modifiers of C9ORF72 dipeptide-repeat-protein toxicity. Nat Genet 50: 603–612. 10.1038/s41588-018-0070-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasemann S, Madore C, Cialic R, Baufeld C, Calcagno N, El Fatimy R, Beckers L, O'Loughlin E, Xu Y, Fanek Z, et al. 2017. The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 47: 566–581 e569. 10.1016/j.immuni.2017.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krobitsch S, Lindquist S. 2000. Aggregation of huntingtin in yeast varies with the length of the polyglutamine expansion and the expression of chaperone proteins. Proc Natl Acad Sci 97: 1589–1594. 10.1073/pnas.97.4.1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryndushkin D, Shewmaker F. 2011. Modeling ALS and FTLD proteinopathies in yeast: An efficient approach for studying protein aggregation and toxicity. Prion 5: 250–257. 10.4161/pri.17229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryndushkin D, Wickner RB, Shewmaker F. 2011. FUS/TLS forms cytoplasmic aggregates, inhibits cell growth and interacts with TDP-43 in a yeast model of amyotrophic lateral sclerosis. Protein Cell 2: 223–236. 10.1007/s13238-011-1525-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku T, Swaney J, Park JY, Albanese A, Murray E, Cho JH, Park YG, Mangena V, Chen J, Chung K. 2016. Multiplexed and scalable super-resolution imaging of three-dimensional protein localization in size-adjustable tissues. Nat Biotechnol 34: 973–981. 10.1038/nbt.3641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara T, Koyama A, Gengyo-Ando K, Masuda M, Kowa H, Tsunoda M, Mitani S, Iwatsubo T. 2006. Familial Parkinson mutant α-synuclein causes dopamine neuron dysfunction in transgenic Caenorhabditis elegans. J Biol Chem 281: 334–340. 10.1074/jbc.M504860200 [DOI] [PubMed] [Google Scholar]
- Labbadia J, Morimoto RI. 2015. The biology of proteostasis in aging and disease. Annu Rev Biochem 84: 435–464. 10.1146/annurev-biochem-060614-033955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakso M, Vartiainen S, Moilanen AM, Sirviö J, Thomas JH, Nass R, Blakely RD, Wong G. 2003. Dopaminergic neuronal loss and motor deficits in Caenorhabditis elegans overexpressing human α-synuclein. J Neurochem 86: 165–172. 10.1046/j.1471-4159.2003.01809.x [DOI] [PubMed] [Google Scholar]
- Lan A, Smoly IY, Rapaport G, Lindquist S, Fraenkel E, Yeger-Lotem E. 2011. ResponseNet: Revealing signaling and regulatory networks linking genetic and transcriptomic screening data. Nucleic Acids Res 39: W424–W429. 10.1093/nar/gkr359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Knoblich JA. 2014. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 345: 1247125 10.1126/science.1247125 [DOI] [PubMed] [Google Scholar]
- Lázaro DF, Rodrigues EF, Langohr R, Shahpasandzadeh H, Ribeiro T, Guerreiro P, Gerhardt E, Kröhnert K, Klucken J, Pereira MD, et al. 2014. Systematic comparison of the effects of α-synuclein mutations on its oligomerization and aggregation. PLoS Genet 10: e1004741 10.1371/journal.pgen.1004741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH. 2017. De novo gene expression reconstruction in space. Trends Mol Med 23: 583–593. 10.1016/j.molmed.2017.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessner G, Schmitt O, Haas SJ, Mikkat S, Kreutzer M, Wree A, Glocker MO. 2010. Differential proteome of the striatum from hemiparkinsonian rats displays vivid structural remodeling processes. J Proteome Res 9: 4671–4687. 10.1021/pr100389u [DOI] [PubMed] [Google Scholar]
- Li P, Banjade S, Cheng HC, Kim S, Chen B, Guo L, Llaguno M, Hollingsworth JV, King DS, Banani SF, et al. 2012. Phase transitions in the assembly of multivalent signalling proteins. Nature 483: 336–340. 10.1038/nature10879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YR, King OD, Shorter J, Gitler AD. 2013. Stress granules as crucibles of ALS pathogenesis. J Cell Biol 201: 361–372. 10.1083/jcb.201302044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, et al. 2017. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541: 481–487. 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link CD. 1995. Expression of human β-amyloid peptide in transgenic Caenorhabditis elegans. Proc Natl Acad Sci 92: 9368–9372. 10.1073/pnas.92.20.9368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link CD, Taft A, Kapulkin V, Duke K, Kim S, Fei Q, Wood DE, Sahagan BG. 2003. Gene expression analysis in a transgenic Caenorhabditis elegans Alzheimer's disease model. Neurobiol Aging 24: 397–413. 10.1016/S0197-4580(02)00224-5 [DOI] [PubMed] [Google Scholar]
- Liu J, Farmer JD Jr, Lane WS, Friedman J, Weissman I, Schreiber SL. 1991. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 66: 807–815. 10.1016/0092-8674(91)90124-H [DOI] [PubMed] [Google Scholar]
- Lodato MA, Woodworth MB, Lee S, Evrony GD, Mehta BK, Karger A, Lee S, Chittenden TW, D'Gama AM, Cai X, et al. 2015. Somatic mutation in single human neurons tracks developmental and transcriptional history. Science 350: 94–98. 10.1126/science.aab1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupski JR, Belmont JW, Boerwinkle E, Gibbs RA. 2011. Clan genomics and the complex architecture of human disease. Cell 147: 32–43. 10.1016/j.cell.2011.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur DG, Manolio TA, Dimmock DP, Rehm HL, Shendure J, Abecasis GR, Adams DR, Altman RB, Antonarakis SE, Ashley EA, et al. 2014. Guidelines for investigating causality of sequence variants in human disease. Nature 508: 469–476. 10.1038/nature13127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel S, Grunblatt E, Riederer P, Amariglio N, Jacob-Hirsch J, Rechavi G, Youdim MB. 2005. Gene expression profiling of sporadic Parkinson's disease substantia nigra pars compacta reveals impairment of ubiquitin-proteasome subunits, SKP1A, aldehyde dehydrogenase, and chaperone HSC-70. Ann NY Acad Sci 1053: 356–375. 10.1196/annals.1344.031 [DOI] [PubMed] [Google Scholar]
- Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, McCarthy MI, Ramos EM, Cardon LR, Chakravarti A, et al. 2009. Finding the missing heritability of complex diseases. Nature 461: 747–753. 10.1038/nature08494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markmiller S, Soltanieh S, Server KL, Mak R, Jin W, Fang MY, Luo EC, Krach F, Yang D, Sen A, et al. 2018. Context-dependent and disease-specific diversity in protein interactions within stress granules. Cell 172: 590–604.e513. 10.1016/j.cell.2017.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh JL, Walker H, Theisen H, Zhu YZ, Fielder T, Purcell J, Thompson LM. 2000. Expanded polyglutamine peptides alone are intrinsically cytotoxic and cause neurodegeneration in Drosophila. Hum Mol Genet 9: 13–25. 10.1093/hmg/9.1.13 [DOI] [PubMed] [Google Scholar]
- Mateju D, Franzmann TM, Patel A, Kopach A, Boczek EE, Maharana S, Lee HO, Carra S, Hyman AA, Alberti S. 2017. An aberrant phase transition of stress granules triggered by misfolded protein and prevented by chaperone function. EMBO J 36: 1669–1687. 10.15252/embj.201695957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, et al. 2018. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 379: 1007–1016. 10.1056/NEJMoa1805689 [DOI] [PubMed] [Google Scholar]
- Meriin AB, Zhang X, He X, Newnam GP, Chernoff YO, Sherman MY. 2002. Huntington toxicity in yeast model depends on polyglutamine aggregation mediated by a prion-like protein Rnq1. J Cell Biol 157: 997–1004. 10.1083/jcb.200112104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meriin AB, Zhang X, Miliaras NB, Kazantsev A, Chernoff YO, McCaffery JM, Wendland B, Sherman MY. 2003. Aggregation of expanded polyglutamine domain in yeast leads to defects in endocytosis. Mol Cell Biol 23: 7554–7565. 10.1128/MCB.23.21.7554-7565.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SB, Mogk A, Bukau B. 2015. Spatially organized aggregation of misfolded proteins as cellular stress defense strategy. J Mol Biol 427: 1564–1574. 10.1016/j.jmb.2015.02.006 [DOI] [PubMed] [Google Scholar]
- Miyasaka T, Ding Z, Gengyo-Ando K, Oue M, Yamaguchi H, Mitani S, Ihara Y. 2005. Progressive neurodegeneration in C. elegans model of tauopathy. Neurobiol Dis 20: 372–383. 10.1016/j.nbd.2005.03.017 [DOI] [PubMed] [Google Scholar]
- Moffitt JR, Hao J, Bambah-Mukku D, Lu T, Dulac C, Zhuang X. 2016. High-performance multiplexed fluorescence in situ hybridization in culture and tissue with matrix imprinting and clearing. Proc Natl Acad Sci 113: 14456–14461. 10.1073/pnas.1617699113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchowski PJ, Schaffar G, Sittler A, Wanker EE, Hayer-Hartl MK, Hartl FU. 2000. Hsp70 and hsp40 chaperones can inhibit self-assembly of polyglutamine proteins into amyloid-like fibrils. Proc Natl Acad Sci 97: 7841–7846. 10.1073/pnas.140202897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muffat J, Li Y, Yuan B, Mitalipova M, Omer A, Corcoran S, Bakiasi G, Tsai LH, Aubourg P, Ransohoff RM, et al. 2016. Efficient derivation of microglia-like cells from human pluripotent stem cells. Nat Med 22: 1358–1367. 10.1038/nm.4189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munder MC, Midtvedt D, Franzmann T, Nüske E, Otto O, Herbig M, Ulbricht E, Muller P, Taubenberger A, Maharana S, et al. 2016. A pH-driven transition of the cytoplasm from a fluid- to a solid-like state promotes entry into dormancy. eLife 5: e09347 10.7554/eLife.09347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray E, Cho JH, Goodwin D, Ku T, Swaney J, Kim SY, Choi H, Park YG, Park JY, Hubbert A, et al. 2015. Simple, scalable proteomic imaging for high-dimensional profiling of intact systems. Cell 163: 1500–1514. 10.1016/j.cell.2015.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na D, Rouf M, O'Kane CJ, Rubinsztein DC, Gsponer J. 2013. NeuroGeM, a knowledgebase of genetic modifiers in neurodegenerative diseases. BMC Med Genomics 6: 52 10.1186/1755-8794-6-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby GA, Kiriakov S, Hallacli E, Kayatekin C, Tsvetkov P, Mancuso CP, Bonner JM, Hesse WR, Chakrabortee S, Manogaran AL, et al. 2017. A genetic tool to track protein aggregates and control prion inheritance. Cell 171: 966–979.e918. 10.1016/j.cell.2017.09.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizami Z, Deryusheva S, Gall JG. 2010. The Cajal body and histone locus body. Cold Spring Harb Perspect Biol 2: a000653 10.1101/cshperspect.a000653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollen EA, Garcia SM, van Haaften G, Kim S, Chavez A, Morimoto RI, Plasterk RH. 2004. Genome-wide RNA interference screen identifies previously undescribed regulators of polyglutamine aggregation. Proc Natl Acad Sci 101: 6403–6408. 10.1073/pnas.0307697101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeda T, Shimohama S, Kitagawa N, Kohno R, Imura T, Shibasaki H, Ishii N. 2001. Oxidative stress causes abnormal accumulation of familial amyotrophic lateral sclerosis-related mutant SOD1 in transgenic Caenorhabditis elegans. Hum Mol Genet 10: 2013–2023. 10.1093/hmg/10.19.2013 [DOI] [PubMed] [Google Scholar]
- Ordonez DG, Lee MK, Feany MB. 2018. α-Synuclein induces mitochondrial dysfunction through spectrin and the actin cytoskeleton. Neuron 97: 108–124 e106. 10.1016/j.neuron.2017.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outeiro TF, Lindquist S. 2003. Yeast cells provide insight into α-synuclein biology and pathobiology. Science 302: 1772–1775. 10.1126/science.1090439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillusson S, Gomez-Suaga P, Stoica R, Little D, Gissen P, Devine MJ, Noble W, Hanger DP, Miller CCJ. 2017. α-Synuclein binds to the ER-mitochondria tethering protein VAPB to disrupt Ca2+ homeostasis and mitochondrial ATP production. Acta Neuropathol 134: 129–149. 10.1007/s00401-017-1704-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JA, Connolly JB, Wellington C, Hayden M, Dausset J, Neri C. 2001. Expanded polyglutamines in Caenorhabditis elegans cause axonal abnormalities and severe dysfunction of PLM mechanosensory neurons without cell death. Proc Natl Acad Sci 98: 13318–13323. 10.1073/pnas.231476398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Malinovska L, Saha S, Wang J, Alberti S, Krishnan Y, Hyman AA. 2017. ATP as a biological hydrotrope. Science 356: 753–756. 10.1126/science.aaf6846 [DOI] [PubMed] [Google Scholar]
- Peng C, Gathagan RJ, Covell DJ, Medellin C, Stieber A, Robinson JL, Zhang B, Pitkin RM, Olufemi MF, Luk KC, et al. 2018. Cellular milieu imparts distinct pathological α-synuclein strains in α-synucleinopathies. Nature 557: 558–563. 10.1038/s41586-018-0104-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowski JS, Li SC, Deshpande R, Simpkins SW, Nelson J, Yashiroda Y, Barber JM, Safizadeh H, Wilson E, Okada H, et al. 2017. Functional annotation of chemical libraries across diverse biological processes. Nat Chem Biol 13: 982–993. 10.1038/nchembio.2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon HF, Frasier M, Shreve N, Calabrese V, Wolozin B, Butterfield DA. 2005. Mitochondrial associated metabolic proteins are selectively oxidized in A30P α-synuclein transgenic mice—A model of familial Parkinson's disease. Neurobiol Dis 18: 492–498. 10.1016/j.nbd.2004.12.009 [DOI] [PubMed] [Google Scholar]
- Pourquié O, Al Tanoury Z, Chal J. 2018. The long road to making muscle in vitro. Curr Top Dev Biol 129: 123–142. 10.1016/bs.ctdb.2018.03.003 [DOI] [PubMed] [Google Scholar]
- Protter DS, Parker R. 2016. Principles and properties of stress granules. Trends Cell Biol 26: 668–679. 10.1016/j.tcb.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabizadeh S, Gralla EB, Borchelt DR, Gwinn R, Valentine JS, Sisodia S, Wong P, Lee M, Hahn H, Bredesen DE. 1995. Mutations associated with amyotrophic lateral sclerosis convert superoxide dismutase from an antiapoptotic gene to a proapoptotic gene: Studies in yeast and neural cells. Proc Natl Acad Sci 92: 3024–3028. 10.1073/pnas.92.7.3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswami M, Taylor JP, Parker R. 2013. Altered ribostasis: RNA-protein granules in degenerative disorders. Cell 154: 727–736. 10.1016/j.cell.2013.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Q, Harrington AJ, Caldwell KA, Caldwell GA, Standaert DG. 2010. VPS41, a protein involved in lysosomal trafficking, is protective in Caenorhabditis elegans and mammalian cellular models of Parkinson's disease. Neurobiol Dis 37: 330–338. 10.1016/j.nbd.2009.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Şahin A, Held A, Bredvik K, Major P, Achilli TM, Kerson AG, Wharton K, Stilwell G, Reenan R. 2017. Human SOD1 ALS mutations in a Drosophila knock-in model cause severe phenotypes and reveal dosage-sensitive gain- and loss-of-function components. Genetics 205: 707–723. 10.1534/genetics.116.190850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MW, Stevens B. 2017. Microglia emerge as central players in brain disease. Nat Med 23: 1018–1027. 10.1038/nm.4397 [DOI] [PubMed] [Google Scholar]
- Satyal SH, Schmidt E, Kitagawa K, Sondheimer N, Lindquist S, Kramer JM, Morimoto RI. 2000. Polyglutamine aggregates alter protein folding homeostasis in Caenorhabditis elegans. Proc Natl Acad Sci 97: 5750–5755. 10.1073/pnas.100107297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaid DJ, Chen W, Larson NB. 2018. From genome-wide associations to candidate causal variants by statistical fine-mapping. Nat Rev Genet 19: 491–504. 10.1038/s41576-018-0016-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzer CR, Jensen RV, Gullans SR, Feany MB. 2003. Gene expression changes presage neurodegeneration in a Drosophila model of Parkinson's disease. Hum Mol Genet 12: 2457–2466. 10.1093/hmg/ddg265 [DOI] [PubMed] [Google Scholar]
- Shahnawaz M, Tokuda T, Waragai M, Mendez N, Ishii R, Trenkwalder C, Mollenhauer B, Soto C. 2017. Development of a biochemical diagnosis of Parkinson disease by detection of α-synuclein misfolded aggregates in cerebrospinal fluid. JAMA Neurol 74: 163–172. 10.1001/jamaneurol.2016.4547 [DOI] [PubMed] [Google Scholar]
- Sharma N, Brandis KA, Herrera SK, Johnson BE, Vaidya T, Shrestha R, Debburman SK. 2006. α-Synuclein budding yeast model: Toxicity enhanced by impaired proteasome and oxidative stress. J Mol Neurosci 28: 161–178. 10.1385/JMN:28:2:161 [DOI] [PubMed] [Google Scholar]
- Shin Y, Berry J, Pannucci N, Haataja MP, Toettcher JE, Brangwynne CP. 2017. Spatiotemporal control of intracellular phase transitions using light-activated optoDroplets. Cell 168: 159–171 e114. 10.1016/j.cell.2016.11.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman JM, Feany MB. 2003. Genetic modifiers of tauopathy in Drosophila. Genetics 165: 1233–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MC, Fox S, Beam M, Thakkar H, Amaral MD, Morimoto RI. 2011. A genetic screening strategy identifies novel regulators of the proteostasis network. PLoS Genet 7: e1002438 10.1371/journal.pgen.1002438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solans A, Zambrano A, Rodríguez M, Barrientos A. 2006. Cytotoxicity of a mutant huntingtin fragment in yeast involves early alterations in mitochondrial OXPHOS complexes II and III. Hum Mol Genet 15: 3063–3081. 10.1093/hmg/ddl248 [DOI] [PubMed] [Google Scholar]
- Su LJ, Auluck PK, Outeiro TF, Yeger-Lotem E, Kritzer JA, Tardiff DF, Strathearn KE, Liu F, Cao S, Hamamichi S, et al. 2010. Compounds from an unbiased chemical screen reverse both ER-to-Golgi trafficking defects and mitochondrial dysfunction in Parkinson's disease models. Dis Model Mech 3: 194–208. 10.1242/dmm.004267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Diaz Z, Fang X, Hart MP, Chesi A, Shorter J, Gitler AD. 2011. Molecular determinants and genetic modifiers of aggregation and toxicity for the ALS disease protein FUS/TLS. PLoS Biol 9: e1000614 10.1371/journal.pbio.1000614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Samimi H, Gamez M, Zare H, Frost B. 2018. Pathogenic tau-induced piRNA depletion promotes neuronal death through transposable element dysregulation in neurodegenerative tauopathies. Nat Neurosci 21: 1038–1048. 10.1038/s41593-018-0194-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenreiro S, Franssens V, Winderickx J, Outeiro TF. 2017. Yeast models of Parkinson's disease-associated molecular pathologies. Curr Opin Genet Dev 44: 74–83. 10.1016/j.gde.2017.01.013 [DOI] [PubMed] [Google Scholar]
- Ticozzi N, Tiloca C, Morelli C, Colombrita C, Poletti B, Doretti A, Maderna L, Messina S, Ratti A, Silani V. 2011. Genetics of familial amyotrophic lateral sclerosis. Arch Ital Biol 149: 65–82. 10.4449/aib.v149i1.1262 [DOI] [PubMed] [Google Scholar]
- Treusch S, Hamamichi S, Goodman JL, Matlack KE, Chung CY, Baru V, Shulman JM, Parrado A, Bevis BJ, Valastyan JS, et al. 2011. Functional links between Aβ toxicity, endocytic trafficking, and Alzheimer's disease risk factors in yeast. Science 334: 1241–1245. 10.1126/science.1213210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuncbag N, Gosline SJ, Kedaigle A, Soltis AR, Gitter A, Fraenkel E. 2016. Network-based interpretation of diverse high-throughput datasets through the omics integrator software package. PLoS Comput Biol 12: e1004879 10.1371/journal.pcbi.1004879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandebroek T, Vanhelmont T, Terwel D, Borghgraef P, Lemaire K, Snauwaert J, Wera S, Van Leuven F, Winderickx J. 2005. Identification and isolation of a hyperphosphorylated, conformationally changed intermediate of human protein tau expressed in yeast. Biochemistry 44: 11466–11475. 10.1021/bi0506775 [DOI] [PubMed] [Google Scholar]
- Van der Perren A, Macchi F, Toelen J, Carlon MS, Maris M, de Loor H, Kuypers DR, Gijsbers R, Van den Haute C, Debyser Z, et al. 2015. FK506 reduces neuroinflammation and dopaminergic neurodegeneration in an α-synuclein-based rat model for Parkinson's disease. Neurobiol Aging 36: 1559–1568. 10.1016/j.neurobiolaging.2015.01.014 [DOI] [PubMed] [Google Scholar]
- van Ham TJ, Thijssen KL, Breitling R, Hofstra RM, Plasterk RH, Nollen EA. 2008. C. elegans model identifies genetic modifiers of α-synuclein inclusion formation during aging. PLoS Genet 4: e1000027 10.1371/journal.pgen.1000027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher PM, Wray NR, Zhang Q, Sklar P, McCarthy MI, Brown MA, Yang J. 2017. 10 years of GWAS discovery: biology, function, and translation. Am J Hum Genet 101: 5–22. 10.1016/j.ajhg.2017.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Haar T, Jossé L, Wright P, Zenthon J, Tuite MF. 2007. Development of a novel yeast cell-based system for studying the aggregation of Alzheimer's disease-associated Aβ peptides in vivo. Neurodegener Dis 4: 136–147. 10.1159/000101838 [DOI] [PubMed] [Google Scholar]
- Wang J, Farr GW, Hall DH, Li F, Furtak K, Dreier L, Horwich AL. 2009. An ALS-linked mutant SOD1 produces a locomotor defect associated with aggregation and synaptic dysfunction when expressed in neurons of Caenorhabditis elegans. PLoS Genet 5: e1000350 10.1371/journal.pgen.1000350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Wang X, Fujioka H, Hoppel C, Whone AL, Caldwell MA, Cullen PJ, Liu J, Zhu X. 2016. Parkinson's disease-associated mutant VPS35 causes mitochondrial dysfunction by recycling DLP1 complexes. Nat Med 22: 54–63. 10.1038/nm.3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Moffitt JR, Zhuang X. 2018. Multiplexed imaging of high-density libraries of RNAs with MERFISH and expansion microscopy. Sci Rep 8: 4847 10.1038/s41598-018-22297-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrick JM, Paulson HL, Gray-Board GL, Bui QT, Fischbeck KH, Pittman RN, Bonini NM. 1998. Expanded polyglutamine protein forms nuclear inclusions and causes neural degeneration in Drosophila. Cell 93: 939–949. 10.1016/S0092-8674(00)81200-3 [DOI] [PubMed] [Google Scholar]
- Watson MR, Lagow RD, Xu K, Zhang B, Bonini NM. 2008. A Drosophila model for amyotrophic lateral sclerosis reveals motor neuron damage by human SOD1. J Biol Chem 283: 24972–24981. 10.1074/jbc.M804817200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DW, Tyrer M, Shepherd D. 2000. Tau and tau reporters disrupt central projections of sensory neurons in Drosophila. J Comp Neurol 428: 630–640. [DOI] [PubMed] [Google Scholar]
- Willingham S, Outeiro TF, DeVit MJ, Lindquist SL, Muchowski PJ. 2003. Yeast genes that enhance the toxicity of a mutant huntingtin fragment or α-synuclein. Science 302: 1769–1772. 10.1126/science.1090389 [DOI] [PubMed] [Google Scholar]
- Wittmann CW, Wszolek MF, Shulman JM, Salvaterra PM, Lewis J, Hutton M, Feany MB. 2001. Tauopathy in Drosophila: Neurodegeneration without neurofibrillary tangles. Science 293: 711–714. 10.1126/science.1062382 [DOI] [PubMed] [Google Scholar]
- Woerman AL, Watts JC, Aoyagi A, Giles K, Middleton LT, Prusiner SB. 2018. α-Synuclein: Multiple system atrophy prions. Cold Spring Harb Perspect Med 8: a024588 10.1101/cshperspect.a024588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xun Z, Sowell RA, Kaufman TC, Clemmer DE. 2007a. Lifetime proteomic profiling of an A30P α-synuclein Drosophila model of Parkinson's disease. J Proteome Res 6: 3729–3738. 10.1021/pr0700504 [DOI] [PubMed] [Google Scholar]
- Xun Z, Sowell RA, Kaufman TC, Clemmer DE. 2007b. Protein expression in a Drosophila model of Parkinson's disease. J Proteome Res 6: 348–357. 10.1021/pr060488o [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeger-Lotem E, Riva L, Su LJ, Gitler AD, Cashikar AG, King OD, Auluck PK, Geddie ML, Valastyan JS, Karger DR, et al. 2009. Bridging high-throughput genetic and transcriptional data reveals cellular responses to α-synuclein toxicity. Nat Genet 41: 316–323. 10.1038/ng.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabrocki P, Pellens K, Vanhelmont T, Vandebroek T, Griffioen G, Wera S, Van Leuven F, Winderickx J. 2005. Characterization of α-synuclein aggregation and synergistic toxicity with protein tau in yeast. FEBS J 272: 1386–1400. 10.1111/j.1742-4658.2005.04571.x [DOI] [PubMed] [Google Scholar]
- Zeng M, Shang Y, Araki Y, Guo T, Huganir RL, Zhang M. 2016. Phase transition in postsynaptic densities underlies formation of synaptic complexes and synaptic plasticity. Cell 166: 1163–1175 e1112. 10.1016/j.cell.2016.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Liao Z, Locascio JJ, Lesniak KA, Roderick SS, Watt ML, Eklund AC, Zhang-James Y, Kim PD, Hauser MA, et al. 2010. PGC-1α, a potential therapeutic target for early intervention in Parkinson's disease. Sci Transl Med 2: 52ra73 10.1126/scitranslmed.3001059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Melton DA. 2018. Pancreas regeneration. Nature 557: 351–358. 10.1038/s41586-018-0088-0 [DOI] [PMC free article] [PubMed] [Google Scholar]