Abstract
Background:
Spinal cord stimulation (SCS) is widely used to treat chronic pain by inhibiting sympathetic activity; however, it is unknown whether it exerts a prokinetic effect on gastric motility. Our aim was to explore effects and possible mechanisms of SCS on glucagon-induced gastric dysmotility and dysrhythmia.
Methods:
Seven female dogs with electrodes chronically placed on the dorsal column of the spinal cord between T10 and T12 segments were studied in 2 randomized sessions (glucagon + sham-SCS, glucagon + SCS). SCS at T10 using a set of optimized stimulation parameters was performed for 30 minute immediately after glucagon injection. The antral manometry, electrogastrogram, and electrocardiogram were recorded to assess gastric contractions, gastric slow waves (GSW), and autonomic functions, respectively.
Key Results:
(a) Compared to baseline, glucagon decreased antral motility index (MI) (6315 ± 565 vs 3243 ± 775, P < 0.001), reduced the percentage of normal GSW (89 ± 3% vs 58 ± 3%, P < 0.01), and increased sympathetic activity (0.25 ± 0 0.06 vs 0.60 ± 0.07, P < 0.01). (b) The sympathetic activity was negatively correlated with antral MI (r = −0.558; P < 0.01) and the percentage of gastric normal slow wave (r = −0.616; P < 0.01). (c) SCS prevented the glucagon-induced impairment in antral hypomotility (MI: 5770 ± 927 vs 5521 ± 1238, P > 0.05) and GSW abnormalities (% of normal waves: 84 ± 4% vs 79 ± 6%, P > 0.05) and sympathetic activity (0.27 ± 0.03 vs 0.33 ± 0.07, P > 0.05).
Conclusion:
Spinal cord stimulation dramatically improves glucagon-induced impairment in gastric contractions and slow waves by inhibiting sympathetic activity.
Keywords: autonomic function, gastric motility, gastric slow waves, neuromodulation, spinal cord stimulation
1 |. INTRODUCTION
Gastric dysmotility is a major pathophysiology factor in gastroparesis, functional dyspepsia (FD), and postoperative ileus (POI); it includes gastric myoelectric activity (slow wave) dysrhythmia and gastric contractile hypomotility. The prevalence of functional dyspepsia is 10%‐40% in Western countries and 5%‐30% in Asia.1 The prevalence of postoperative ileus is between 10% and 30% for abdominal surgery.2 Evidence suggests that 30%−60% of patients with diabetes develop clinical signs of visceral autonomic neuropathy of which gastroparesis is one form after 10–20 years of clinically apparent diabetes.3 Slow waves dysrhythmia in gastroparesis and FD due to interstitial cells of Cajal (ICC) depletion, damage, or network disruption was noted to have strong associations with gastric functional disorders.4 ICC are considered as the pacemaker cells that generate slow waves5 and are innervated by the intrinsic enteric nervous system6 that is regulated by the extrinsic vagal and sympathetic nerves.7 Abnormalities in the autonomic function assessed by heart rate variability (HRV) were reported in symptomatic patients with diabetic gastroparesis,8 FD,9 or POI.10
Medications approved to treat gastrointestinal disturbance or restore gastrointestinal motility are very limited. Cisapride, a 5-HT4 agonist, was noted to improve fundic accommodation and meal-related symptoms in FD but it has been removed from the market11 due to its possible cardiovascular adverse events.12 Metoclopramide is the only drug currently used in the treatment of gastroparesis; however, this is associated with the adverse reactions in a considerable number of patients.13 Alvimopan and prucalopride were shown to ameliorate POI disorders but RCTs are needed to reduce methodological biases14 and to investigate which kind of patient would gain the most from this medicine.15
Alternative and complementary medicine has been used more and more often to treat gastrointestinal diseases.16 Enterra gastric electric stimulation has been reported to ameliorate nausea and vomiting in patients with refractory gastroparesis but it does not improve gastric motility.17 Gastric pacing with an implantable pulse generator improved gastric slow wave dysrhythmia and gastric emptying impaired by glucagon in dogs.18 However, there is no commercialized device. Electroacupuncture (EA) has been reported to improve gastrointestinal motility in both preclinical and clinical studies.19–21 However, EA is not practiced in major medical centers or hospitals in USA.22 And another problem with EA is the use of needles that limits widespread applications since the procedure has to be administrated by healthcare professionals. Transcutaneous electrical acustimulation is a newly developed method of EA by using surface electrodes instead of needles. It has been shown to improve gastroparesis and stress-induced gastric slow waves dysrhythmia via activating vagal nerve and inhibiting sympathovagal ratio.23,24 However, so far there is no commercial device available.
Spinal cord stimulation (SCS) using an implantable pulse generator (IPG) has been widely used in treating patients with neuropathic pain and is known to inhibit sympathetic activity.25,26 Recently, SCS using an external stimulator was reported to improve emptying in diabetic rats via suppressing sympathetic activity and enhancing vagal activity in a rodent model of type 1 diabetes.27
The aim of this study was to explore the therapeutic potential of SCS using a commercially available IPG for gastroparesis by studying the effects of SCS on ameliorating glucagon-induced gastric dysrhythmia and antral hypomotility and the possible mechanisms involving autonomic functions in dogs.
2|. MATERIALS AND METHODS
2.1 |. Animal preparation
Seven healthy female hound-mix dogs (18–25 kg) were used in the study. After overnight fasting, the dog was anesthetized with thiopental (11 mg/kg, ip) and anesthesia was maintained with IsoFlo (1.5% isoflurane, inhalation). A laminectomy was performed to expose the T9 thoracic segment of the spinal cord. T9 was located at the 4 levels higher than T13 which was found by finding the highest caudal-most rib. A paddle stimulation electrode lead (CoverEdge 32, Boston Scientific Company) was inserted from T9 and placed on the dorsal column level of T10-T12. The electrode lead was connected to an IPG (Precision Spectra, Boston Scientific) which was placed under the skin located at the back close to the scapula.
After the laminectomy, an abdominal surgical procedure was performed to place recording electrodes and gastric cannulas. For recording gastric pace-making activity, a pair of 28-gauge cardiac pacing wires (A&E Medical, Farmingdale, NJ) was implanted on the gastric serosa along the great curvature 4 cm above the pylorus with an interval of 1cm. The electrode connecting wires (insulated part of the wire) were fixed to the serosa by non-absorbable sutures and tunneled through the anterior abdominal wall subcutaneously along the right side of the trunk and brought out at the back close to the neck. For measuring gastric contractions via an intraluminal manometric catheter, a cannula with an inner diameter of 1cm was placed in the middle of the anterior side of the stomach 15 cm above the pylorus, which provided access to the luminal side of the stomach when it was opened.
Antibiotics (Cefpodoxime, 5–10 mg/kg) and pain medications (Buprenorphine SR, 0.03–0.06 mg/kg) were given for a period of 7 and 3 days, respectively, immediately after the surgery. The animals were closely monitored (twice a day) for a period of at least 2 weeks. No experiments were performed before the dogs were completely recovered from these surgical procedures (usually about 2 weeks). The study was approved by the Animal Care and Use Committees of the University of Johns Hopkins and performed at the University of Johns Hopkins (protocol number: DO16M318).
2.2 |. Spinal cord stimulation
The SCS stimuli were delivered by an implanted pulse generator (Precision Spectra™, Boston Scientific Corp.) and programed via a wireless wand (Bionic Navigator 3D software with Illumina 3D Programming, Boston Scientific Corp.). T10 was selected to be the stimulation location, and the following parameters were used for stimulation: pulse train on time of 2 second, off time of 3 second, pulse width of 0.5 ms, frequency of 20 Hz, and an intensity of 90% of the motor threshold (MT: tonic contraction of the abdominal muscles; the actual amplitude was 0.4–1.0 mA). These choices were based on a previous test that systematically optimized stimulation location and parameters.28 The SCS was performed for 30 minute immediately after the injection of glucagon via a pair of bipolar electrodes, two inner electrodes of the spinal paddle lead located at T10 (CoverEdge™, Boston Scientific Corp), surgically inserted in the dorsal column.
2.3 |. Experimental protocol
Before the formal experiment, all dogs were brought to the laboratory for getting used to the experimental environment. The experiment was composed of two randomized sessions (glucagon, and glucagon + SCS). The glucagon session (or called sham-SCS session) was designed to investigate the effects of glucagon on the gastric slow wave, antral contraction, and autonomic functions. After an overnight fast, the dogs were brought to the laboratory and fed with one can of dog food (Pedigree chopped chicken, 421 Kcal/can). Immediately after the meal, antral contractions, gastric pace-making activity, and the electrocardiogram (ECG) were simultaneously recorded (see detail below) for 30 minute, and then, glucagon (0.1 mg/kg, ip) was injected, and the recordings were made for one more hour.
The glucagon plus SCS session was designed to investigate the effects of SCS on the glucagon-induced gastric slow wave dysrhythmia, antral hypomotility, and autonomic functions disorders. This protocol of this session was the same as the glucagon session except that SCS was performed during the first 30 minute after the glucagon injection.
2.4 |. Recording and analysis of gastric pace-making activity
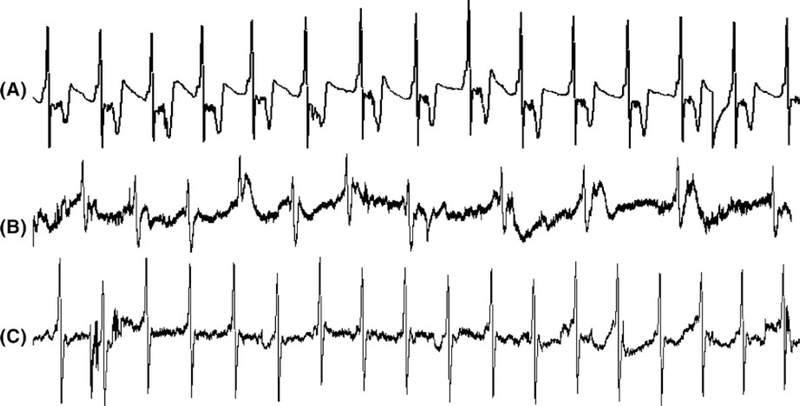
Gastric pace-making activity was recorded by a special Biopac amplifier (Acqknowledge, EOG 100A; Biopac Systems, Inc Santa Barbara, CA) with a frequency range of 0.016–30 Hz via the implanted serosal electrodes. The signal was offline analyzed using a special spectral analysis software package (Ningbo MedKinetic Inc, Ningbo, Zhejiang, China) and following parameters were derived: percentage of bradygastria (percentage of time during which the dominant frequency of the gastric slow wave was in the range of 0.5–4.0 cycles/min (cpm), percentage of normal slow waves (4.0–6.0 cpm) and the percentage of tachygastria (6.0–12.0 cpm).29,30 As seen in Figure 1, the gastric slow wave recording was of a high signal to noise ratio without major noises. Therefore, no special signal processing methods were needed before the spectral analysis of the signal. Occasionally, respiration and cardiac interferences might present in the recording but they did not affect the spectral analysis of the gastric slow waves as their frequencies were higher than the interested frequency range of the gastric slow waves.
FIGURE 1.
Typical tracings of gastric slow waves after the meal. A, Recording of 3 min gastric slow waves at baseline. B, Recording of dysrhythmia 0–3 min after glucagon injection. C, Recording of 0–3 min slow waves after glucagon injection with SCS
2.5 |. Recording and analysis of gastric contractions
Antral manometry was performed after a solid-state manometric instrument with 10 circumferential sensors spaced at 1-cm intervals and an outer diameter of 4.2 mm (Ningbo Maida Medical Device Inc, Ningbo, China) was inserted into the antrum through the implanted cannula. Antral contractile signals were recorded by a multi-channel recorder (MedView360 V2.0; Ningbo Maida Medical Device Inc). All recordings were displayed on a computer monitor and were analyzed by a customized software package to derive the following two parameters31: (a) MI (motility index): defined as the total number of contractions times the summation of all contraction peaks and then divided by the analyzed time period and (b) AUC (area under contractions): defined as the summation of the area under the curve of all contractions divided by the analyzed time period; these two parameters were used to represent the contractile strength of the distal stomach. Channel 5 was selected for the analysis due to its highest quality of the recording. The manometric signal was clean without noises or interference, and therefore, no signal processing methods were needed before the above-mentioned quantitative analysis.
2.6 |. Recording and analysis of autonomic functions
A special amplifier with a recording frequency of 100 Hz (model 2283 Fti Universal Fetrode Amplifier; UFI, Morro Bay, CA) was used to record the ECG signal from cutaneous electrodes: (a) The dog’s skin where electrodes were to be placed was cleaned with special skin-prep gel (Nuprep; Weaver and Company, Aurora); (b) conductive gel (Ten20; Weaver and Company, Aurora) was applied to reduce electrode-skin impedance; and (c) three regular ECG electrodes were placed at the apical, the inside of the right hind leg, and the offside of the apical.32 After the deletion of the recording with severe motion artifacts due to occasional movement of the animal, the heart rate variability (HRV) signal was derived using a custom-made software by identifying R peaks and calculating consecutive RR intervals from the ECG; then, the power spectrum analysis of the HRV signal was performed to calculate the power in different frequency bands.33,34 The power in the low-frequency (LF) band (0.04–0.15 Hz) reflects mainly sympathetic activity, whereas the power in the high-frequency (HF) band (0.15–0.50 Hz) stands purely parasympathetic or vagal activity.33,34 In this study, the standardized values were used, that is, LF was defined as LF/(HF + LF) and HF as HF/(HF + LF).33,34 The SCS stimulation (output current of <1 mA) did not generate any artifacts in the ECG recording nor the gastric slow waves/manometric recording.
2.7 |. Statistical analysis
All data are expressed as mean ± standard error (SE). One way analysis of variance was used to compare the data among the different periods. Least significant difference was used to assess the difference between two periods. P < 0.05 was considered statistical significance. Pearson’s correlation analysis was performed to assess the correlation between gastric motility and autonomic functions. Data were analyzed by statistical software SPSS 19.0.
3|. RESULTS
3.1 |. Effects of SCS on glucagon-induced gastric slow wave dysrhythmia
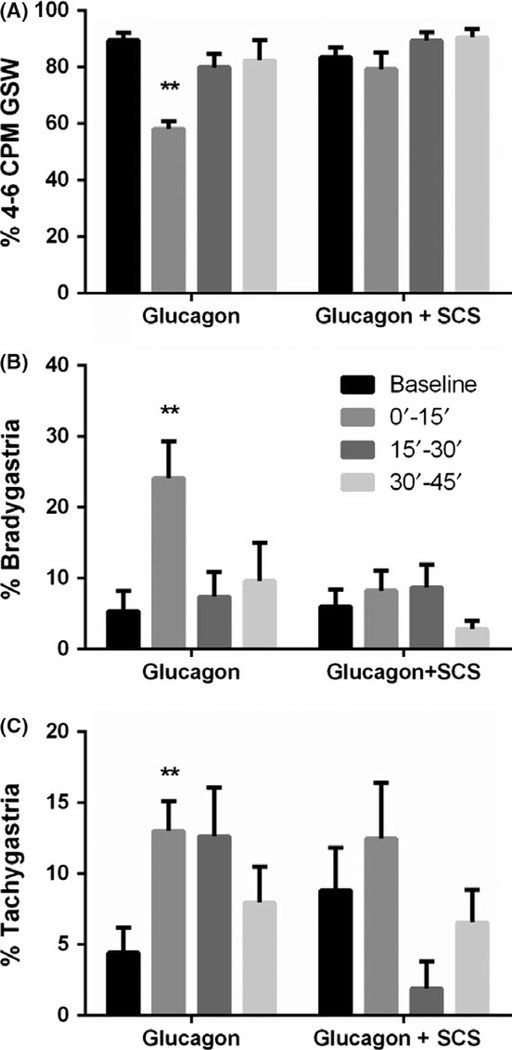
Glucagon provoked gastric dysrhythmia in all seven dogs (Figure 2). Compared to the baseline, glucagon decreased the percentage of 4–6 cpm normal slow waves by 35% (P < 0.01, ANOVA; Figure 2A) and increased the percentage of bradygastria by 3.5 times (P < 0.01, ANOVA; Figure 2B) and tachygastria by almost 2 times (P < 0.05, ANOVA; Figure 2C) in the first 15-minute period after the glucagon injection. The percentages of normal slow wave and bradygastria recovered during the second 15 minute but the percentage of tachygastria did not. Figure 1A presents a typical tracing of regular gastric slow waves at the baseline and irregular slow waves (Figure 1B) after the glucagon injection. It shows that the dysrhythmia appeared as early as 1 minute after the glucagon injection.
FIGURE 2.
Gastric slow waves during different recording periods in the glucagon and glucagon + SCS sessions. Glucagon decreased the % N GSW (A) and increased % bradygastria (B) and % tachygastria (C) and SCS improved it via suppressing Bradygastria (vs baseline, **P < 0.01)
Spinal cord stimulation prevented the gastric dysrhythmia induced by glucagon. As shown in Figure 2, when SCS was performed (0–15’ and 15’−30’), the injection of glucagon was not able to reduce the percentage of 4–6 cpm normal slow waves or increase the percentage of bradygastria or tachygastria. The tracing showing the normal slow waves after the glucagon injection in the SCS session is presented in Figure 1C.
3.2 |. Effects of SCS on glucagon-induced hypomotility
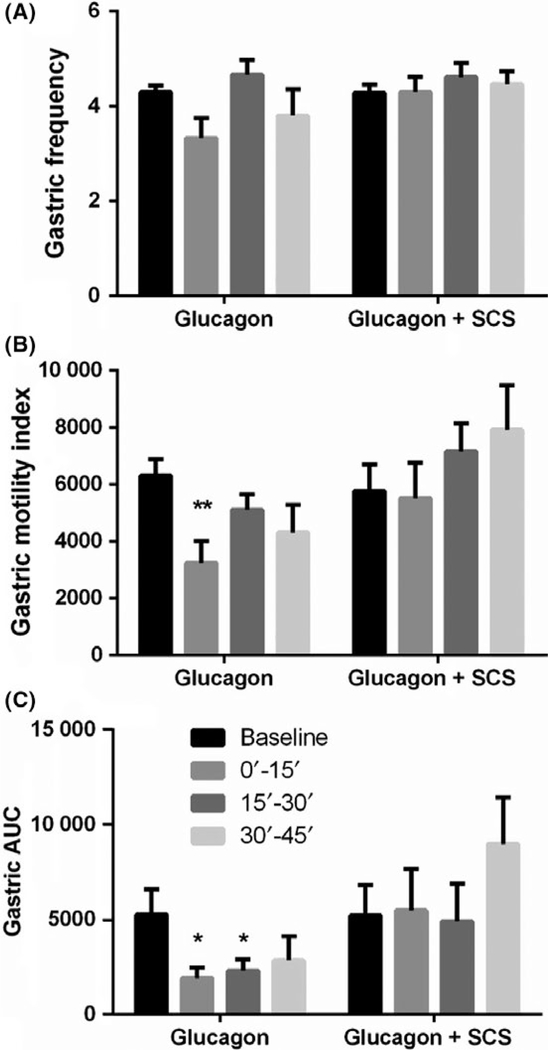
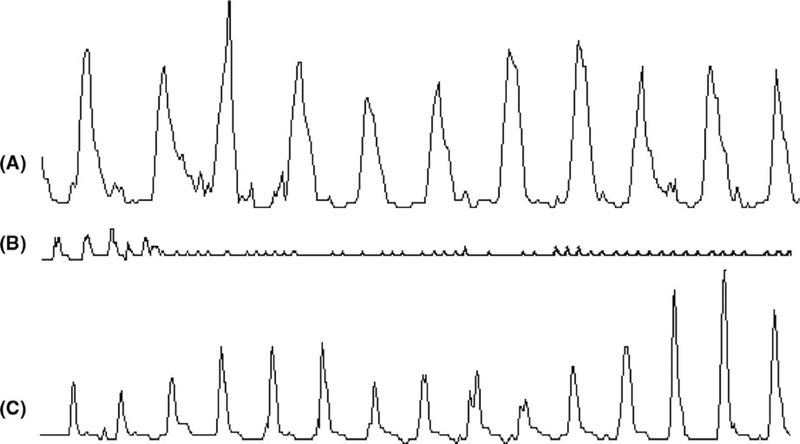
Glucagon induced gastric hypomotility in all 7 dogs (Figure 4). Compared to the baseline, glucagon decreased the MI by 49% (P < 0.01, ANOVA; Figure 4B) and AUC by 64% (P < 0.05, ANOVA; Figure 4C) during the first 15-minute period after the glucagon injection. The MI and AUC gradually recovered in the next period but did not return to the baseline; as shown in the figure, the AUC during the second 15 minute after the glucagon injection was still significantly lower than the baseline. Figure 3A presents typical tracings of regular gastric contractions at the baseline, and Figure 3B presents reduced gastric contractions after the glucagon injection. It shows that gastric contractions were inhibited as early as 1 minute after the glucagon.
FIGURE 4.
Effects of SCS on postprandial antral motility. Glucagon decreased the gastric contractile MI (B) and AUC (C) and SCS improved it. No significant change of gastric contractile frequency was noted in glucagon or glucagon + SCS session (A). (vs baseline, *P < 0.05; **P < 0.01)
FIGURE 3.
Manometric tracing showing the effects of SCS on glucagon-induced hypomotility. A, Recording of 3-min antral contractions at baseline; B, Recording of first 3-min impaired antral contractions right after glucagon injection; C, Recording of 3-min antral contractions after glucagon injection with SCS
Spinal cord stimulation prevented gastric hypomotility induced by glucagon. As shown in Figure 4, SCS prevented the glucagon-induced reduction in the MI (Figure 4B) and AUC (Figure 4C) of gastric contractions during the first 15 minute after the glucagon injection. Typical tracings showing the improvement in gastric contractions with SCS are presented in Figure 3C. The MI and AUC gradually recovered in the last period after the glucagon injection but not up to the baseline values in the glucagon session. In the SCS + glucagon session, however, the MI and AUC during the last period were even higher than the baseline.
3.3 |. Mechanisms of SCS involving autonomic functions
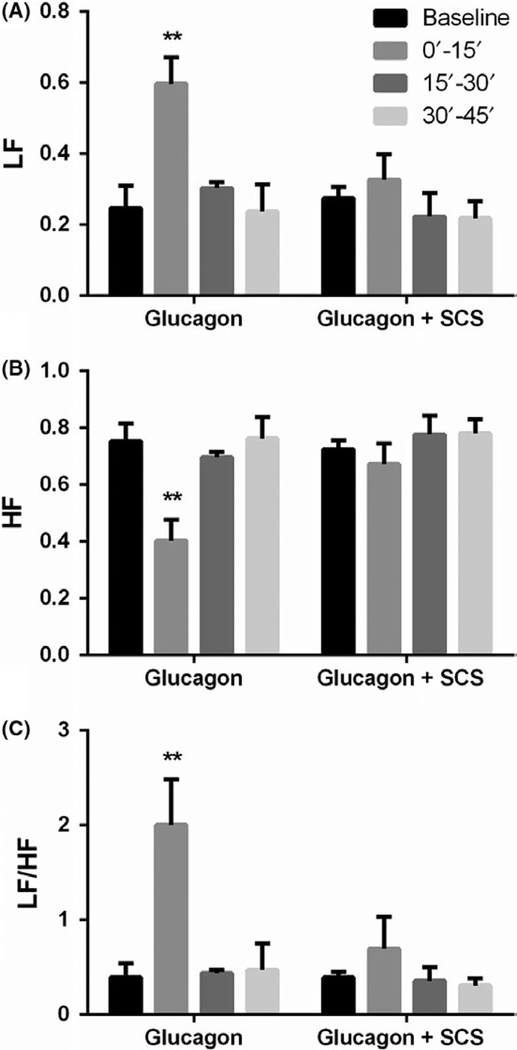
Glucagon injection increased sympathetic activity (LF) and decreased vagal activity (HF) in all seven dogs. Compared to baseline, glucagon increased the LF by 1.4 times (P < 0.01, ANOVA; Figure 5A) and LF/HF by 4 times (P < 0.01, ANOVA; Figure 5C) but decreased the HF by 46% (P < 0.01, ANOVA; Figure 5B) during the first 15-minute period after the glucagon injection.
FIGURE 5.
Effects of SCS on glucagon-induced autonomic function disorders. Glucagon increased LF and LF/HF but decreased HF; SCS increased HF (B) but decreased LF (A) and LF/HF (C). (vs baseline, **P < 0.01)
Spinal cord stimulation prevented the alterations induced by glucagon. As shown in Figure 5, in the SCS plus glucagon session, the glucagon injection did not induce an increase in sympathetic activity (Figure 5A), a decrease in vagal activity (Figure 5B), or an increase in the sympathy-vagal ratio (Figure 5C).
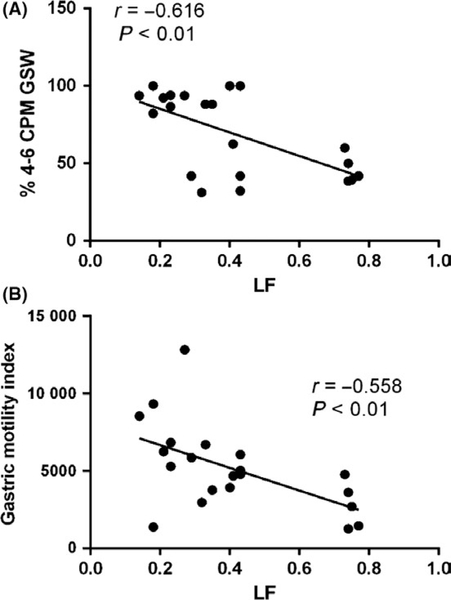
Interesting, the autonomic functions assessed from the spectral analysis of HRV were found to be correlated with the percentage of normal gastric slow waves and the MI of the gastric contractions. In the glucagon session, during the three 15-minute periods of the baseline recording, the LF was negatively correlated with the percentage of normal slow waves (r = −0.861, P < 0.05) and positively correlated with the percentage of bradygastria (r = 0.857, P < 0.05); during the three 15-minute periods after the glucagon injection without SCS, the LF was negatively correlated with the percentage of normal gastric slow wave (r = −0.616, P < 0.01; Figure 6A) and positively correlated with the percentage of bradygastria (r = 0.359, P < 0.01) as well as the percentage of tachygastria (r = 0.408, P < 0.01). During the same periods, the LF was negatively correlated with antral contraction MI (r = −0.558, P < 0.01) (Figure 6B), suggesting the role of autonomic function in the regulation effects on gastric slow waves and motility.
FIGURE 6.
Correlation between sympathetic activity and gastric motility during three consecutive 15‐min periods after glucagon without SCS. LF was negatively correlated with % N GSW (A) and gastric motility (B)
During the three 15-minute periods after glucagon with SCS, the LF was negatively correlated with the percentage of normal gastric slow waves (r = −0.528, P < 0.05) as well as the gastric MI (r = −0.471, P < 0.05).
4 |. DISCUSSION
In this study, SCS with optimized stimulation location and parameters improved glucagon-induced gastric dysrhythmia and hypomotility, decreased sympathetic activity, and sympathovagal balance in dogs. Further, sympathetic activity was noted to be negatively correlated with the percentage of gastric normal slow waves and contractile MI, suggesting an autonomic mechanism of SCS.
Gastric motility disorders are commonly seen in patients with gastroparesis, FD, and POI. However, treatment options for gastric dysmotility or gastroparesis are very limited in the clinical setting due to lack of prokinetics, side effects, or therapeutic devices. It is well known that gastric motility was innervated with the intrinsic enteric nerve which was modulated by the extrinsic autonomic nerve. SCS was reported to inhibit sympathetic activity35,36 and noted to improve postoperative ileus in rats.37 Recently, we reported the acceleration of gastric emptying in normal and diabetic rats.27 To the best of our knowledge, this is the first time to investigate the effects of SCS using a commercially available IPG on gastric slow waves and contractions impaired by glucagon in dogs. If proven effective, the clinically established SCS method using the IPG might provide a novel therapy for gastroparesis which is of great clinical significance.
Glucagon was wildly used to induce GI motility disorders in animals.18,38,39 Glucagon is a peptide hormone, released from α cells of the pancreatic islets. It increases blood glucose by raising the concentration of glucose and fatty acids in the bloodstream via its action on the liver (causing the liver to convert stored glycogen into glucose). The inhibitory effect of exogenous glucagon on gastric contractions and gastric slow waves is believed to be attributed to hyperglycemia acting via the vagal reflex pathway.40,41 The effects of glucagon on gastric motility in this canine study were consistent with previous studies. The injection of glucagon immediately and substantially inhibited antral contractions; both MI and AUC were dramatically reduced. It also severely impaired gastric slow waves: a substantial reduction in the percentage of normal 4–6 cpm slow waves and a substantial increase in bradygastria. Similar autonomic mechanisms were implicated in this study: The glucagon injection dramatically reduced vagal efferent activity and increased sympathetic activity assessed by the spectral analysis of HRV.
The major finding of this study was the prevention or normalization of glucagon-induced suppression in antral contractions. To the best of our knowledge, this was the first direct evidence showing the prokinetic effect of SCS using a commercially available IPG on gastric motility in large animals. Previously, SCS was reported to accelerate gastric emptying in rodent models of diabetes27 and postoperative ileus.37 However, in these rodent studies, SCS was performed using a unipolar ball electrode placed in the spinal cord and an external stimulator; no direct gastric contractions were measured. In an attempt to improve gastric motility, electrical stimulation was directly applied to the stomach in a few previous studies.42,43 In a similar canine study, gastric electrical stimulation synchronized with gastric slow waves was reported to improve the glucagon-impaired antral contractile MI via the cholinergic pathway in fed dogs.42 This previous study and the current study have demonstrated that gastric motility may be improved either by enhancing the vagal activity or by suppressing sympathetic activity. In addition, electrical stimulation at acupuncture points (or electroacupuncture, EA) has also been reported to improve gastric motility in animals44–46 and gastric motility and dyspeptic symptoms in patients with functional dyspepsia.23,47 In diabetic rats, EA at ST36 was reported to improve in vitro antral muscle contractions via the stem cell factor/c-kit pathway.44 In dogs, EA was noted to ameliorate impaired antral contraction induced by rectal distention via the autonomic and opioid pathways.45 In patients with functional dyspepsia, EA at PC6 and ST36 improved gastric emptying of solids measured by scintigraphy.46 However, patient compliance is one of the major problems with the widespread clinical application of EA: Ideally, EA should be performed daily but this would not be practical and too expensive as well. In addition, EA is not practiced in most of hospitals or gastroenterology clinics.
In addition to its prokinetic effect on gastric contractions, SCS was also found to improve glucagon-induced gastric dysrhythmia. The percentage of normal gastric slow waves dropped substantially from higher than 89% at the baseline to 58% after the glucagon injection. Gastric dysrhythmia has been observed in several clinical settings, including gastroparesis,8 patients with FD,9 and patients with POI10 and plays an important pathophysiological role in gastric dysmotility. Some prokinetic agents, such as domperidone, erythromycin, and cisapride, were reported to be able to improve gastric dysrhythmia.48–50 However, cisapride and domperidone have not been available due to their adverse effects, and erythromycin has limited effects on the improvement of symptoms. Neuromodulation and alternative medicine have received more and more attention in basic and clinical research in recent years, and its application in improving gastric dysrhythmia was investigated in a number of studies. Improvement of gastric dysrhythmia with gastric electric stimulation (GES) has been investigated in both animals and humans. In dogs, GES with a long pulse (pulse width in the unit of milliseconds) was noted to normalize the atropine-induced gastric dysrhythmia,51 as well as glucagon-induced gastric dysrhythmia.52 In humans, two-channel GES with a long pulse delivered at a frequency of 10% higher the intrinsic frequency is able to normalize gastric dysrhythmia and accelerate gastric emptying in patients with diabetic gastroparesis.53 Electroacupuncture (EA) is a widely used complimentary treatment for functional gastrointestinal diseases. EA at ST36 was reported to ameliorate gastric dysrhythmia in diabetic rats54 and rats with thermal injuries.21 Clinically, EA at ST36 was reported to increase the percentage of normal slow waves in diabetic patients55 and in healthy controls.56 Transcutaneous electroacupuncture is a newly developed method of EA which has been shown to improve gastric myoelectrical activity in patients with systemic sclerosis57 and stress-induced gastric dysrhythmia in healthy control.24 While these methods are completely different from the method of SCS, we proposed in this study, their mechanisms of action involved in the ameliorating effect on gastric dysrhythmia were similar to the SCS method in this study: Most of these previous studies suggested an autonomic mechanism involving both vagal and sympathetic activity.
Our findings seemed to suggest that both glucagon-induced gastric dysmotility (antral hypomotility and gastric dysrhythmia) and SCS-induced improvement in gastric motility were mediated via the autonomic mechanisms. It is well known that the stomach is innervated with the intrinsic enteric nervous system and extrinsic vagal and sympathetic nerves.58 The extrinsic sympathetic activation releases noradrenaline from its axons to the intrinsic enteric nervous system, mainly the myenteric and submucosal plexuses, inhibiting gastric motility by suppressing transmitter release from enteric nerve terminals via presynaptic alpha 2 adrenoceptors.59 Whereas the parasympathetic activation releases acetylcholine to enhance gastric motility via its widespread connection in the enteric nervous system.60 Enteric motor neurons innervated by extrinsic sympathetic and parasympathetic nerves determined the patterns of gastrointestinal contractions via inhibiting or activating smooth muscle cells and interstitial cells.61. In the current study, parasympathetic activity and sympathovagal balance were analyzed by the spectral analysis of the HRV derived from the ECG, a non-invasive, accurate, and reliable method.62,63 We found that the treatment of glucagon increased sympathetic activity and sympathovagal balance (LF/HF) and decreased vagal activity (HF) and that the SCS was able to prevent or normalize these glucagon-induced alterations in the autonomic functions. Similar autonomic mechanisms were also reported in previous studies in different clinical settings. Assessed by the same method of spectral analysis of HRV, SCS was found to decrease the sympathovagal ratio in patients with refractory angina64 and in diabetic rats.27 Assessed by total body norepinephrine, SCS was also noted to decrease overall sympathetic activity in patients with anginal pectoris.36 Linderoth et al65 reported that SCS dilated peripheral blood vessels via inhibiting efferent sympathetic activity involving nicotinic transmission in the ganglia and the postganglionic alpha 1-adrenoreceptors. The reduction of sympathovagal ratio and/or increase of vagal activity have been reported to be associated with the improvement in gastrointestinal motility in animals and in patients.21,54,66,67 In addition, we found that the vagal activity was positively correlated with the percentage of normal gastric slow waves and contractile MI, and negatively correlated with gastric dysrhythmia. These interesting correlations further suggested the autonomic mechanisms involved in the improvement of gastric dysmotility with the SCS.
4.1 |. Clinical perspectives
Our results suggested that SCS might be used to improve gastric dysrhythmia and antral hypomotility. IPGs for SCS are commercially available and have been widely used for treating neuropathic pain. The implementation of SCS is relatively easy, safe, and minimally invasive. SCS may be considered as a potentially novel therapy for gastroparesis or other gastric dysmotility disorders. Clinical studies are warranted to investigate the therapeutic potential of SCS for gastroparesis.
In conclusion, SCS is able to improve glucagon-induced gastric motility disorders. This effect is attributed to its enhancement in vagal activity and inhibition in sympathetic activity. Further studies are warranted to investigate its potential for treating gastric motility disorders, such as gastroparesis.
Key Points.
Key question: Treatment options for gastric dysmotility or gastroparesis are very limited in the clinical setting due to lack of medications, side effects, or therapeutic devices.
Results: SCS with optimized stimulation location and parameters improved glucagon-induced gastric dysrhythmia and hypomotility, decreased sympathetic activity and sympathovagal balance in dogs.
Importance of these results: SCS may be considered as a potentially novel therapy for gastroparesis or other gastric dysmotility disorders.
ACKNOWLEDGMENTS
This work was supported by a grant from the NIH SPARC program (U18TR001920). The spinal cord stimulator was provided by Boston Scientific Company. Prof. Jieyun Yin provided technical assistances, such as EGG, HRV, and manometry recording.
Funding information
Foundation for the National Institutes of Health, Grant/Award Number: U18TR001920
Footnotes
DISCLOSURE
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Enck P, Azpiroz F, Boeckxstaens G, et al. Functional dyspepsia. Nat Rev Dis Primers. 2017;3:17081. [DOI] [PubMed] [Google Scholar]
- 2.Venara A, Neunlist M, Slim K, et al. Postoperative ileus: Pathophysiology, incidence, and prevention. J Vise Surg. 2016;153:439–446. [DOI] [PubMed] [Google Scholar]
- 3.Sato T, Kitahara F, Nakamura T, Kojima Y, Fujino MA. [Peptic ulcer in patients with diabetes mellitus]. Nihon Rinsho. 2002;60:1580–1584. [PubMed] [Google Scholar]
- 4.O’Grady G, Wang TH, Du P, Angeli T, Lammers WJ, Cheng LK. Recent progress in gastric arrhythmia: pathophysiology, clinical significance and future horizons. Clin Exp Pharmacol Physiol. 2014;41:854–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Helden DF, Laver DR, Holdsworth J, Imtiaz MS. Generation and propagation of gastric slow waves. Clin Exp Pharmacol Physiol. 2010;37:516–524. [DOI] [PubMed] [Google Scholar]
- 6.Hirst GD, Edwards FR. Role of interstitial cells of Cajal in the control of gastric motility. J Pharmacol Sei. 2004;96:1–10. [DOI] [PubMed] [Google Scholar]
- 7.Schemann M, Grundy D. Electrophysiological identification of vagally innervated enteric neurons in guinea pig stomach. Am J Physiol. 1992;263:G709–G718. [DOI] [PubMed] [Google Scholar]
- 8.Stocker A, Abell TL, Rashed H, Kedar A, Boatright B, Chen J. Autonomic evaluation of patients with gastroparesis and neuro-stimulation: comparisons of direct/systemic and indirect/cardiac measures. Gastroenterology Res. 2016;9:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lorena SL, Figueiredo MJ, Almeida JR, Mesquita MA. Autonomic function in patients with functional dyspepsia assessed by 24-hour heart rate variability. Dig Dis Sei. 2002;47:27–31. [DOI] [PubMed] [Google Scholar]
- 10.Amar D, Fleisher M, Pantuck CB, et al. Persistent alterations of the autonomic nervous system after noncardiac surgery. Anesthesiology. 1998;89:30–42. [DOI] [PubMed] [Google Scholar]
- 11.Tack J, Broeckaert D, Coulie B, Janssens J. The influence of cisapride on gastric tone and the perception of gastric distension. Aliment Pharmacol Ther. 1998;12:761–766. [DOI] [PubMed] [Google Scholar]
- 12.Tack J, Camilleri M, Chang L, et al. Systematic review: cardiovascular safety profile of 5-HT(4) agonists developed for gastrointestinal disorders. Aliment Pharmacol Ther. 2012;35:745–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Navas CM, Patel NK, Lacy BE. Gastroparesis: medical and therapeutic advances. Dig Dis Sci. 2017;62:2231–2240. [DOI] [PubMed] [Google Scholar]
- 14.Traut U, Brügger L, Kunz R, et al. Systemic prokinetic pharmacologic treatment for postoperative adynamic ileus following abdominal surgery in adults. Cochrane Database Syst Rev. 2008;(1):CD004930. [DOI] [PubMed] [Google Scholar]
- 15.Liu S, Peng S, Hou X, Ke M, Chen JD. Transcutaneous electroacupuncture improves dyspeptic symptoms and increases high frequency heart rate variability in patients with functional dyspepsia. Neurogastroenterol Motil. 2008;20:1204–1211. [DOI] [PubMed] [Google Scholar]
- 16.Jdz C, Yin J, Hou X, Takahashi T. Complementary and alternative therapies for functional gastrointestinal diseases 2016. Evid Based Complement Alternat Med. 2017;2017:2089165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maranki JL, Lytes V, Meilahn JE, et al. Predictive factors for clinical improvement with Enterra gastric electric stimulation treatment for refractory gastroparesis. Dig Dis Sci. 2008;53:2072–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu J, Ross RA, McCallum RW, Chen JD. Two-channel gastric pacing with a novel implantable gastric pacemaker accelerates glucagon-induced delayed gastric emptying in dogs. Am J Surg. 2008;195:122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang J-F, Fang J-Q, Shao X-M, et al. Electroacupuncture treatment partly promotes the recovery time of postoperative ileus by activating the vagus nerve but not regulating local inflammation. Sci Rep. 2017;7:39801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Huang H, Xu X, Chen JD. Effects and possible mechanisms of acupuncture at ST36 on upper and lower abdominal symptoms induced by rectal distension in healthy volunteers. Am J Physiol Regul Integr Comp Physiol. 2012;303:R209–R217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song J, Yin J, Sallam HS, Bai T, Chen Y, Chen JD. Electroacupuncture improves burn-induced impairment in gastric motility mediated via the vagal mechanism in rats. Neurogastroenterol Motil. 2013;25:807–807e635. [DOI] [PubMed] [Google Scholar]
- 22.Meng ZQ, Garcia MK, Chiang JS, et al. Electroacupuncture to prevent prolonged postoperative ileus: a randomized clinical trial. World J Gastroenterol. 2010;16:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji T, Li X, Lin L, et al. An alternative to current therapies of functional dyspepsia: self-administrated transcutaneous electroacupuncture improves dyspeptic symptoms. Evid Based Complement Alternat Med. 2014;2014:832523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang N, Song G, Chen J, et al. Ameliorating effects and autonomic mechanisms of needle-less transcutaneous electrical stimulation at ST36 on stress-induced impairment in gastric slow waves. J Gastroenterol Hepatol. 2015;30:1574–1581. [DOI] [PubMed] [Google Scholar]
- 25.Kapural L, Narouze SN, Janicki TI, Mekhail N. Spinal cord stimulation is an effective treatment for the chronic intractable visceral pelvic pain. Pain Med. 2006;7:440–443. [DOI] [PubMed] [Google Scholar]
- 26.Greenwood-Van MB, Johnson AC, Foreman RD, Linderoth B. Attenuation by spinal cord stimulation of a nociceptive reflex generated by colorectal distention in a rat model. Auton Neurosci. 2003;104:17–24. [DOI] [PubMed] [Google Scholar]
- 27.Song GQ, Sun Y, Foreman RD, Chen JD. Therapeutic potential of spinal cord stimulation for gastrointestinal motility disorders: a preliminary rodent study. Neurogastroenterol Motil. 2014;26:377–384. [DOI] [PubMed] [Google Scholar]
- 28.Tu Lei, Zhang B, Ji F, et al. A novel method of spinal cord stimulation for treating gastroparesis a priliminary study in dogs. DDW Abstract. 2018. Sa1560. [Google Scholar]
- 29.Dai F, Lei Y, Chen JD. Inhibitory effects of desvenlafaxine on gastric slow waves, antral contractions, and gastric accommodation mediated via the sympathetic mechanism in dogs. Am J Physiol Gastrointest Liver Physiol. 2011;301:G707–G712. [DOI] [PubMed] [Google Scholar]
- 30.Sun Y, Song GQ, Yin J, Lei Y, Chen JD. Effects and mechanisms of gastrointestinal electrical stimulation on slow waves: a systematic canine study. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1392–R1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu H, Chen JD. Implantable gastric stimulation inhibits gastric motility via sympathetic pathway in dogs. Obes Surg. 2005;15:95–100. [DOI] [PubMed] [Google Scholar]
- 32.Song J, Yin J, Chen J. Needleless transcutaneous electroacupuncture improves rectal distension-induced impairment in intestinal motility and slow waves via vagal mechanisms in dogs. Int J Clin Exp Med. 2015;8:4635–4646. [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang B, Xu F, Hu P, et al. Needleless transcutaneous electrical acustimulation: a pilot study evaluating improvement in postoperative recovery. Am J Gastroenterol. 2018;113:1026–1035. [DOI] [PubMed] [Google Scholar]
- 34.Lei Y, Chen JD. Effects of dual pulse gastric electrical stimulation on gastric tone and compliance in dogs. Dig Liver Dis. 2009;41: 277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eliasson T, Mannheimer C, Waagstein F, et al. Myocardial turnover of endogenous opioids and calcitonin-gene-related peptide in the human heart and the effects of spinal cord stimulation on pacing-induced angina pectoris. Cardiology. 1998;89:170–177. [DOI] [PubMed] [Google Scholar]
- 36.Norrsell H, Eliasson T, Mannheimer C, et al. Effects of pacing-induced myocardial stress and spinal cord stimulation on whole body and cardiac norepinephrine spillover. Eur Heart J. 1997;18:1890–1896. [DOI] [PubMed] [Google Scholar]
- 37.Maher J, Johnson AC, Newman R, et al. Effect of spinal cord stimulation in a rodent model of post-operative ileus. Neurogastroenterol Motil. 2009;21(672–7):e33–e34. [DOI] [PubMed] [Google Scholar]
- 38.Song GQ, Chen JD. Gastric electrical stimulation on gastric motility in dogs. Neuromodulation. 2011;14:271–277; discussion 277. [DOI] [PubMed] [Google Scholar]
- 39.Chen JD, Qian L, Ouyang H, Yin J. Gastric electrical stimulation with short pulses reduces vomiting but not dysrhythmias in dogs. Gastroenterology. 2003;124:401–409. [DOI] [PubMed] [Google Scholar]
- 40.Ouyang X, Li S, Foreman R, et al. Hyperglycemia-induced small intestinal dysrhythmias attributed to sympathovagal imbalance in normal and diabetic rats. Neurogastroenterol Motil. 2015;27:406–415. [DOI] [PubMed] [Google Scholar]
- 41.Browning KN. Modulation of gastrointestinal vagal neurocircuits by hyperglycemia. Front Neurosci. 2013;7:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu H, Sallam H, Chen DD, Chen JD. Therapeutic potential of synchronized gastric electrical stimulation for gastroparesis: enhanced gastric motility in dogs. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1875–R1881. [DOI] [PubMed] [Google Scholar]
- 43.Li H, Chen Y, Liu S, Hou XH. Long-pulse gastric electrical stimulation protects interstitial cells of Cajal in diabetic rats via IGF-1 signaling pathway. World J Gastroenterol. 2016;22:5353–5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Y, Xu J, Liu S, Hou X. Electroacupuncture at ST36 increases contraction of the gastric antrum and improves the SCF/c-kit pathway in diabetic rats. Am J Chin Med. 2013;41:1233–1249. [DOI] [PubMed] [Google Scholar]
- 45.Chen J, Song GQ, Yin J, Koothan T, Chen JD. Electroacupuncture improves impaired gastric motility and slow waves induced by rectal distension in dogs. Am J Physiol Gastrointest Liver Physiol. 2008;295:G614–G620. [DOI] [PubMed] [Google Scholar]
- 46.Xu S, Hou X, Zha H, Gao Z, Zhang Y, Chen JD. Electroacupuncture accelerates solid gastric emptying and improves dyspeptic symptoms in patients with functional dyspepsia. Dig Dis Sci. 2006;51:2154–2159. [DOI] [PubMed] [Google Scholar]
- 47.Xu F, Tan Y, Huang Z, Zhang N, Xu Y, Yin J. Ameliorating effect of transcutaneous electroacupuncture on impaired gastric accommodation in patients with postprandial distress syndrome-predominant functional dyspepsia: a pilot study. Evid Based Complement Alternat Med. 2015;2015:168252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koch KL, Stern RM, Stewart WR, Vasey MW. Gastric emptying and gastric myoelectrical activity in patients with diabetic gastroparesis: effect of long-term domperidone treatment. Am J Gastroenterol. 1989;84:1069–1075. [PubMed] [Google Scholar]
- 49.Chen JD, Ke MY, Lin XM, Wang Z, Zhang M. Cisapride provides symptomatic relief in functional dyspepsia associated with gastric myoelectrical abnormality. Aliment Pharmacol Ther. 2000;14:1041–1047. [DOI] [PubMed] [Google Scholar]
- 50.Rothstein RD, Alavi A, Reynolds JC. Electrogastrography in patients with gastroparesis and effect of long-term cisapride. Dig Dis Sci. 1993;38:1518–1524. [DOI] [PubMed] [Google Scholar]
- 51.Qian L, Lin X, Chen JD. Normalization of atropine-induced postprandial dysrhythmias with gastric pacing. Am J Physiol. 1999;276:G387–G392. [DOI] [PubMed] [Google Scholar]
- 52.Xu X, Qian L, Chen JD. Anti-dysrhythmic effects of long-pulse gastric electrical stimulation in dogs. Digestion. 2004;69:63–70. [DOI] [PubMed] [Google Scholar]
- 53.Lin Z, Sarosiek I, Forster J, Ross RA, Chen JD, McCallum RW. Two-channel gastric pacing in patients with diabetic gastroparesis. Neurogastroenterol Motil. 2011;23:912–912e396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yin J, Chen J, Chen JD. Ameliorating effects and mechanisms of electroacupuncture on gastric dysrhythmia, delayed emptying, and impaired accommodation in diabetic rats. Am J Physiol Gastrointest Liver Physiol. 2010;298:G563–G570. [DOI] [PubMed] [Google Scholar]
- 55.Chang CS, Ko CW, Wu CY, Chen GH. Effect of electrical stimulation on acupuncture points in diabetic patients with gastric dysrhythmia: a pilot study. Digestion. 2001;64:184–190. [DOI] [PubMed] [Google Scholar]
- 56.Chou JW, Chang YH, Chang CS, Chen GH. The effect of different frequency electrical acustimulation on gastric myoelectrical activity in healthy subjects. Hepatogastroenterology. 2003;50:582–586. [PubMed] [Google Scholar]
- 57.McNearney TA, Sallam HS, Hunnicutt SE, Doshi D, Chen JD. Prolonged treatment with transcutaneous electrical nerve stimulation (TENS) modulates neuro-gastric motility and plasma levels of vasoactive intestinal peptide (VIP), motilin and interleukin-6 (IL-6) in systemic sclerosis. Clin Exp Rheumatol. 2013;31:140–150. [PubMed] [Google Scholar]
- 58.Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286–294. [DOI] [PubMed] [Google Scholar]
- 59.Tan LL, Bornstein JC, Anderson CR. The neurochemistry and innervation patterns of extrinsic sensory and sympathetic nerves in the myenteric plexus of the C57Bl6 mouse jejunum. Neuroscience. 2010;166:564–579. [DOI] [PubMed] [Google Scholar]
- 60.Berthoud HR, Jedrzejewska A, Powley TL. Simultaneous labeling of vagal innervation of the gut and afferent projections from the visceral forebrain with dil injected into the dorsal vagal complex in the rat. J Comp Neurol. 1990;301:65–79. [DOI] [PubMed] [Google Scholar]
- 61.Sanders KM, Koh SD, Ro S, Ward SM. Regulation of gastrointestinal motility-insights from smooth muscle biology. Nat Rev Gastroenterol Hepatol. 2012;9:633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berntson GG, Thomas bigger J, Eckberg DL, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. [DOI] [PubMed] [Google Scholar]
- 63.Kamath MV, Fallen EL. Power spectral analysis of heart rate variability: a noninvasive signature of cardiac autonomic function. Crit Rev Biomed Eng. 1993;21:245–311. [PubMed] [Google Scholar]
- 64.Anselmino M, Ravera L, De luca A, et al. Spinal cord stimulation and 30-minute heart rate variability in refractory angina patients. Pacing Clin Electrophysiol. 2009;32:37–42. [DOI] [PubMed] [Google Scholar]
- 65.Linderoth B, Herregodts P, Meyerson BA. Sympathetic mediation of peripheral vasodilation induced by spinal cord stimulation: animal studies of the role of cholinergic and adrenergic receptor subtypes. Neurosurgery. 1994;35:711–719. [DOI] [PubMed] [Google Scholar]
- 66.Jin H, Liu J, Foreman RD, Chen JD, Yin J. Electrical neuromodulation at acupoint ST36 normalizes impaired colonic motility induced by rectal distension in dogs. Am J Physiol Gastrointest Liver Physiol. 2015;309:G368–G376. [DOI] [PubMed] [Google Scholar]
- 67.Sallam H, McNearney TA, Doshi D, Chen JD. Transcutaneous electrical nerve stimulation (TENS) improves upper GI symptoms and balances the sympathovagal activity in scleroderma patients. Dig DisSci. 2007;52:1329–1337. [DOI] [PubMed] [Google Scholar]