Abstract
Background:
Though over one-third of veterans suffer from obesity and its associated comorbidities, bariatric surgery (deleted: is seldom offered) is less commonly offered than in other populations.
Methods:
We reviewed surgical outcomes using CPRS/Vista data of (“deleted 308) 315 Roux-en-Y gastric bypass (RYGB) cases performed at a major VA Medical Center (1995–2017).
Results:
Patients were 69% male, with an average age 52 (65% over 50), and were followed for an average of 8 years; 158 (51%) underwent laparoscopic surgery, and the remaining open. Outcomes were: 30-day mortality- Open: 1.3%, Lap: 0%; anatomic leak-open: 0.3%, Lap: 0%. A total of 32 (10%) Clavien-Dindo ≥3 complications occurred. At 5 and 15 years, average BMI decreased from 47 preoperatively to 33.3 and 31 respectively, while excess body weight loss was 68%, and 80%, respectively. Co-morbidity resolution rates were between 70 and 80% diabetes, sleep apnea, hyperlipidemia, GERD, (delete – hypertension), and NASH.
Conclusions:
RYGB offers sustained, long-term weight loss with significant resolution of major comorbidities in older veterans, with acceptably low morbidity and mortality.
Keywords: Roux-en-Y gastric bypass, Elderly, Veteran, Obesity, Co-morbidity
Introduction
Morbid obesity and its related comorbidities are widely prevalent in the veteran population, with estimates of 36–37% of veteran primary care patients meeting criteria for obesity, though only 54% carry a recorded diagnosis. 1,2 It is striking that a recent study of VA care practices for obese patients showed that despite the prevalence of obesity, only one third receive education or counseling for obesity, 0.4% were prescribed weight-loss medications, and (delete - a dismal) 0.2% of patients received bariatric surgery.1
Some studies in the elderly veteran population have shown that Roux-en-Y gastric bypass (RYGB) yields durable weight loss, with decreases of 24% and 29% loss from baseline weight at 5 and 10 years. However, studies of long-term outcomes such as this one often do not examine resolution of co-morbidities.3 The effects on age on bariatric surgery outcomes has also not been widely studied. Only small series have showed modestly low complication rates in elderly populations with minimal morality.4
Perception of veteran patients as too high risk or elderly may be preventing referrals for surgery. Thus, the objective of this study was to assess long term outcomes on weight loss, comorbidity resolution, and complications after RYGB on our series of (deleted “elderly, male”) older, male-predominant, veteran population.
Material and methods
As part of our quality review process, we prospectively followed and recorded outcomes of all patients undergoing Roux-en-Y gastric bypass at the San Francisco Veterans’ Affairs (VA) Medical Center (1995–2018). Data was recorded in and collected from the Computerized Patient Record System (CPRS), generally during follow-up clinic appointments. We prospectively collected and reviewed quality assurance outcomes following bariatric surgery. Data reviewed included: surgery date, age, gender, height, weight, surgical follow-up information, vitals, progress notes, pharmacy, and laboratory data from 315 cases in 310 patients. Patients’ weights (kg) were measured by health care professionals during clinic visits. BMI was calculated as weight (kg) divided by height (m2).
Resolution or improvement of co-morbidities were based on clinical follow up in our Bariatric clinic, as well primary care physician notes, active medications, and laboratory values. Since the most rapid period of weight loss occurs in the first 18 months, we examined outcomes at a minimum of 18-month follow-up; but, if comorbidities showed resolution or improvement sooner, this outcome was recorded. For example, NASH was diagnosed by elevated liver funciton tests (usually AST or ALT), cross-sectional imaging, or liver biopsy. Resolution was defined as normalization of liver enzymes. For diabetes, dosage and number of pre-operative oral hypoglycemic agents, insulin dosage, and HbA1c levels was recorded. Post-operatively, “resolution” of diabetes was defined as cessation of insulin and oral hypoglycemic agents with normalization of HbA1c; diabetes “improvement” was defined as decreased need for anti-glycemic agents (discontinuation of insulin and/or requiring lower dosages or fewer oral hypoglycemic agents). We analyzed immediate and long-term outcomes (mean 8 years, range 0.5–15 years) for open (1995–2015) and laparoscopic (2005–2018) RYGB procedures.
Statistical analysis
Statistical analysis was completed using analysis of variance (ANOVA) for interval data, chi-square analysis for ordinal and nominal data. Statistical analysis was performed using the SPSS statistical program (IBM, V21).
Results
Of our cohort of 315 cases in 310 patients, 219 (69.2%) were male, 95 (30.1%) female, and 1 (0.3%) transgender. The average age was 52 years old, with 65% of the cohort being in their 5th decade or older. The mean follow-up was 8 years (range 0.5–15 years). Complete or ≥18 month outcomes were available in 289 (92%) cases for meaningful interpretation of long-term data. The mean initial BMI and weight were 47 kg/m2 and 317 pounds respectively (Table 1). 146 (46.3%) cases were open and were conducted from 1995 to 2015, while 164 (52%) were laparoscopic, conducted from 2005 to 2017. There were 5 cases (1.6%) that were converted from laparoscopic to open, 4 of whom had undergone prior laparotomies; these were included in the open group for analysis. Of all the procedures, 288 were RYGB alone, 16 were RYGB with a concomitant procedure (4 cholecystectomy, 4 gastrectomy, 1 hiatal hernia repair, 1 Heller myotomy), and 11 were revisional or redo RYGB.
Table 1.
Patient demographics and surgical procedures.
| Number (%) | |
|---|---|
| Number of cases | 315 |
| Male | 219 (69.2%) |
| Female | 95 (30.1) |
| Transgender | 1 (0.3%) |
| Age (years, mean) | 52.2 |
| Age by Decade | |
| 20 | 4 |
| 30 | 30 |
| 40 | 76 |
| 50 | 127 |
| 60 | 76 |
| 70 | 2 |
| Follow-up (years, mean) | 8 years |
| Initial BMI (kg/m,2 mean) | 47 |
| Initial weight (lbs, mean) | 312 |
| Surgical Technique | |
| Open | 146 (47.1%) |
| Laparoscopic | 164(51.3) |
| Conversion to Open | 5 (1.6%) |
| Procedures | |
| RYGB | 288 |
| + Cholecystectomy | 10 |
| + Partial/wedge Gastrectomy | 4 |
| + Hiatal hernia repair | 1 |
| + Heller myotomy | 1 |
| Redo/Revision RYGB | 11 |
While minor complications were more common, major morbidity and mortality was low. Two patients (0.6%) died within thirty days of their operation, both underwent open RYGB early in the cohort (2001 and 2004). 97 patients (31%) developed complications: 66 cases (21%) with Clavien-Dindo grade 1–2 and 32 cases (10%) grade ≥3. Of the grade ≥3 complications, 22 (69%) were in open cases while 10 (31%) were in laparoscopic cases. Anastomotic leak occurred in one patient (0.3%), following open RYGB. This patient had two prior Nissen fundoplication procedures. No anastomotic leaks occurred in the laparoscopic RYGB group (Table 2). Two patients had gastric remnant staple-line leaks (0.6%), one each open and laparoscopic group.
Table 2.
Complications in cohort by operative technique.
| All (n = 315) | Open (n =151) | Laparoscopic (n = 164) | |
|---|---|---|---|
| 30-day Mortality | 2 (0.6%) | 2 (1.3%) | 0 |
| Complications | 97 (31%) | 50 (33%) | 47 (29%) |
| Clavien-Dindo 1 or 2 | 65 (21%) | 28 (19%)a | 37 (23%) |
| Clavien-Dindo ≥ 3 | 32 (10%) | 22 (15%) | 10 (6.1%) |
| Anastomotic Leak at gastrojejunostomy | 1 (0.3%) | 1 (0.7%)b | 0 |
Includes 3 laparoscopic converted to open cases.
Redo case (history of 2 prior Nissen fundoplications).
Patients had sustained long-term weight loss. From an average pre-operative BMI of 47 kg/m2, at 5, 10 and 15 years, the average BMI decreased from 33.3, 33.7 and 31 respectively. Excess body weight loss compared to baseline followed similar trends at 5, 10 and 15 years, with rates being 68%, 68% and 80% respectively.
Preoperative comorbid conditions were highly prevalent with the most common being: diabetes 52%, gastroesophageal reflux disease (GERD) 39%, obstructive sleep apnea (OSA) 65%, hyperlipidemia (HLD) 48%, hypertension (HTN) 75%, and degenerative disc disease (DJD) 76% (Fig. 1A). Most patients had multiple comorbidities with the median of 7.5 and mean of 8.3 conditions (Fig. 1B).
Fig. 1.
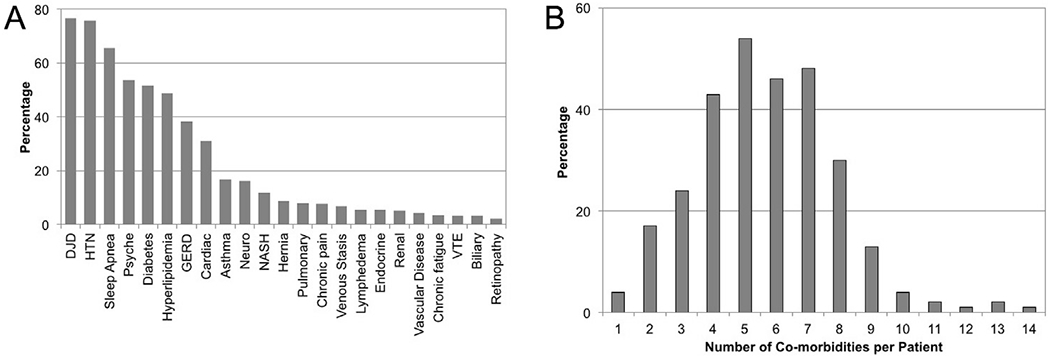
Preoperative Comorbid Conditions. Comorbidity abbreviations: DJD - degenerative joint disease, HTN - hypertension, Psyche - psychiatric disease (e.g. depression, anxiety, PTSD, schizophrenia, bipolar), HLD - hyperlipidemia, GERD - gastroesophageal reflux disease, Cardiac (CAD, CHF, arrythmia), Neuro - neurologic disease (e.g. stroke, peripheral neuropathy, spinal stenosis, sciatica), Endocrine (hypothyroidism, hyperparathyroidism, hyperaldosteronism, hypogonadism), and VTE - venous thromboembolic disease.
Among patients with meaningful follow-up data (18 months or more), most had dramatic resolution or improvement in their co-morbidities (Fig. 2A). NASH resolved in 83% of cases, 80% of cases with diabetes had complete diabetes resolution, and more than 70% of patients had resolution of sleep apnea, GERD, asthma, (delete – hypertension) and hyperlipidemia. In patients with diabetes pre-op, HgbA1c levels also fell with resolution of diabetes (see Fig. 2B).
Fig. 2.
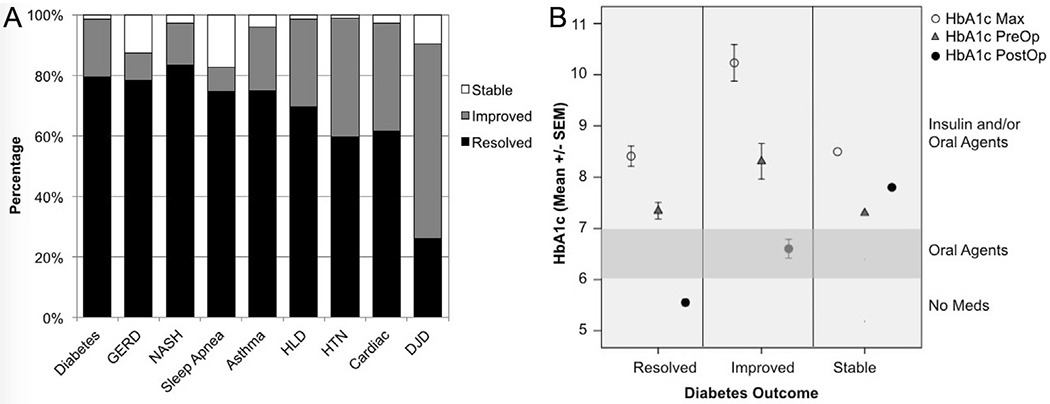
Medical Co-Morbidity Outcomes and HbA1c Trends 2A. Outcomes of patients’ comorbidities as classified by whether their medical issues remained: Stable (i.e. Diabetes, GERD, HLD, HTN: still requiring medications or OSA still requiring CPAP, NASH: AST and ALT unchanged), Improved (i.e. fewer medications, improved liver function tests), or Resolved (i.e. off medications or CPAP usage). 2B: Outcomes for patients with diabetes: Stable (no change in medications), Improved (off insulin and/or on fewer oral hypoglycemic agents), or Resolved (discontinuation of all diabetes medications). HbA1c Max - maximum preoperative HbA1c level, HbA1c PreOp - immediately preoperative HbA1c level, HbA1c PostOp – postoperative HbA1c level (most recent).
Resolution and/or improvement in diabetes was associated with decreased HgbA1c levels (Fig. 2B). We compared maximum levels, preoperative, and post-operative follow-up HgbA1c levels. Cases with diabetes resolution (and discontinuation of diabetic medications) had the lowest HgbA1c levels. Similarly, among cases with improvement in diabetes, HgbA1c levels also decreased (Fig. 2B). Among 123 cases with diabetes resolution, average HbA1c decreased significantly from 8.4% (max) and 7.3% preoperative, to 5.5% post-operatively (p = 0.0001). Among 29 cases with diabetes improvement (off insulin and/or fewer oral hypoglycemic agents), average HbA1c decreased from 10.2% (max) and 8.3% preoperatively to 6.6% post-operatively (p = 0.0001). Only one case with diabetes failed to show improvement in their diabetes, and demonstrated stable HbA1c levels (max, preoperative, and post-operative follow-up).
Long term weight loss was robust. At 5 and 15 years, average BMI decreased from 47 preoperatively to 33.3 and 31 respectively (Fig. 3A), while excess body weight loss was 68%, and 80%, respectively (Fig. 3B). Thus weight loss following the Roux-Y gastric bypass procedure, was excellent and durable.
Fig. 3.
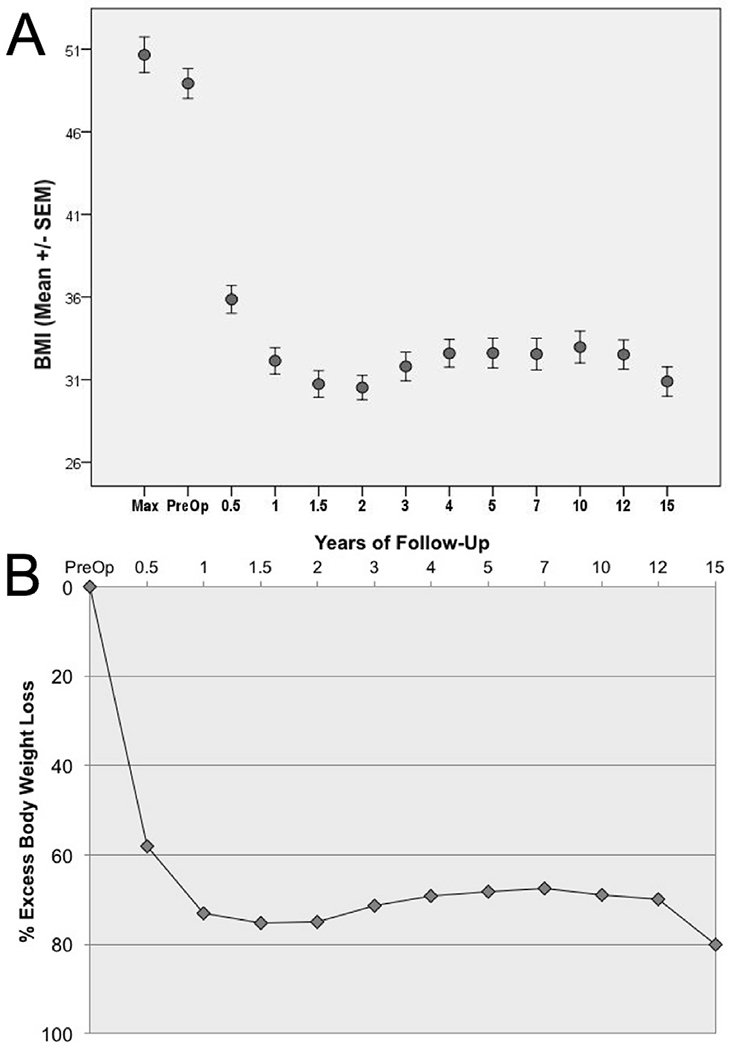
Long-term weight loss following RYGB. 3A: Body mass index trends pre- and post-operatively: 3B: Percentage excess body weight loss trends pre- and post-operatively.
Discussion
Compared to the general population of patients undergoing bariatric surgery, who are predominantly female, younger, with fewer medical morbidities, our study examines the long-term outcomes (weight loss and comorbidity resolution/improvement) in an older, male-predominant Veteran population. We utilize a robust follow-up approach to (deleted: trend) record outcomes and monitor patients, and most of our data comes from this follow-up documentation. In addition, the structure of the VA healthcare system facilitates excellent patient follow-up. In the United States, the veteran healthcare system is unique in the ability to capture long-term data on patients based on the Veterans Integrated System Technology Architecture nationwide electronic health record6. This allows accurate assessment of comorbidities by having access to up-to-date medication lists, lab values, and primary care progress notes. Difficulty obtaining complete follow-up is a common issue for bariatric programs. This study uniquely provides outcomes with more complete and long-term nature of follow-up.
In this study, we (deleted: showed) demonstrated that RYGB is safe and highly effective achieving sustained weight loss, even in an older veteran population. Our overall complication rate was 31%, Clavein-Dindo grade ≥3 was 10%, and 30-day mortality was 0.6%, with no deaths in the laparoscopic group. This is within range of or slightly better that reported outcomes in a larger series comparison of NSQIP data analyzing bariatric surgery outcomes in 12 Veterans’ Affairs medical centers, reporting a complication rate of 19.7% and 30-day mortality of 1.4%. (replaced “Though some have found that male gender is a risk factor for increased mortality7-8, we found very low mortality rates, especially with the laparoscopic approach.” with the following) In addition, other studies have reported that male gender is a risk factor for increased mortality,7,8 but we found very low mortality rates, especially utilizing the laparoscopic approach. Our population was older; the average age in our series was 52, and two-thirds of patients were in their fifth decade or older. Studies looking specifically at patients >65 years undergoing bariatric surgery (both sleeve gastrectomy and RYGB) report much higher complication rates of 9–14%.9,10 Grey et al.4 reported a complication rate of 5.8% in their RYGB group with an average age of 64 and 3.25-year follow-up. Additionally, a comparison of RYGB outcomes compared to the private sector showed an odds ratio of 2.29 for increased postoperative morbidity for VA patients; however, since this study the VA instituted regionalization of bariatric centers, thus lowering associated morbidity and mortality.11
Our patients obtained sustained weight loss as far as 15 years post-operatively, with a 16 kg/m2 decrease in BMI and 80% decrease in excess body weight loss. In comparison, other series of gastric bypass with a follow-up of greater than 5 years report a range of 50–72% EBWL5 and a large meta-analysis of bariatric surgery showed BMI decreased from 12 to 17 kg/m2 at 5 years.12
Perhaps even more importantly (deleted “that”) than weight loss, for these medically complex veterans, was that 70–80% of patients had profound resolution or improvement of their co-morbidities. Particularly for diabetes and HbA1c data: 80% had resolution of their diabetes, 19% had improved disease, and only one patient did not improve. This is comparable to published rates of diabetes remission rates of 80%.13,14 It was striking how quickly patients’ diabetes resolved after surgery, with (deleted “many”) most patients at discharge already on fewer hypoglycemic agents. We also noticed that of the patients who did have weight regain, they often did not have recurrence of their diabetes. Since cardiovascular disease is so prevalent in the VA population, RYGB offers the ability to cure diabetes, and thus improve their overall health and long term survival with survival rates increased by 30–40%, primary by decreasing cardiovascular and cancer risk.15,16 More-over, the effect of gastric bypass to improve insulin sensitivity is unique compared to patients who undergo sleeve gastrectomy and medical management per the STAMPEDE trial.17 Additionally, among cases with equivalent weight loss, more sleeve patients (deleted duplicate word “patients) require supplemental medications.13,14
In the past decade, bariatric surgery trends show increased rates of sleeve gastrectomy comprising 58% of bariatric operations performed in 2016.18 Some authors have raised concerns about the increased adoption of sleeve gastrectomy because of reports of new GERD and Barrett’s esophagus development.19–21 These concerns may be particularly relevant in the older, male veteran population, since risk factors for esophageal carcinoma (alcohol, tobacco use, etc.) are high in veteran populations. In addition, the RYGB effectively eliminates diabetes, which facilitates the reduction of other serious medical conditions. However, studies directly correlating sleeve gastrectomy with esophageal cancer have not yet been done.22 Close follow-up of patients is essential, especially among high risk groups.
(Deleted: In the current study, we have shown) In conclusion, the current study demonstrates that RYGB is safe and effective for weight loss and comorbidity resolution in an older, veteran population. Our data supports increased referrals of veterans for consideration of bariatric surgery and RYGB in particular.
DISCLOSURES & Acknowledgements
This was an unfunded study. V.L. and L.S. have no disclosures. A.L.S.'s effort was supported by the National Institutes of Health (R01 DK107629 and R21 DK112126). A.L.S. also has non-grant research support for studies related to bariatric surgery, but not for the study reported in this manuscript: calcium citrate for a research study donated by Bariatric Advantage and soluble corn fiber and maltodextrin for a research study donated by Tate & Lyle.
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.amjsurg.2019.07.027.
References
- 1.Noël PH, Copeland LA, Pugh MJ, et al. Obesity diagnosis and care practices in the Veterans health administration. J Gen Intern Med. 2010. February;25(6):510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Das SR, Kinsinger LS, Yancy WS Jr, et al. Obesity prevalence among veterans at Veterans Affairs medical facilities. Am J Prev Med. 2005. April;28(3):291–294. [DOI] [PubMed] [Google Scholar]
- 3.Maciejewski ML, Arterburn DE, Scoyoc LV, et al. Bariatric surgery and long-term durability of weight loss. JAMA Surg. 2016. November;151(11):1046–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray KD, Moore MD, Bellorin O, et al. Increased metabolic benefit for obese, elderly patients undergoing roux-en-y gastric bypass vs sleeve gastrectomy. Obes Surg. 2018. March;28(3):636–642. [DOI] [PubMed] [Google Scholar]
- 5.Brethauer S, Herron D, Demaria EJ, et al. Bariatric metabolic surgery: advances continue. Bull Am Coll Surg. 2019. February;103(2). [Google Scholar]
- 6.Livingston EH, Arterburn D, Schifftner TL, et al. National surgical quality improvement program analysis of bariatric operations: modifiable risk factors contribute to bariatric surgical adverse outcomes. J Am Coll Surg. 2006. November;203(5):625–633. [DOI] [PubMed] [Google Scholar]
- 7.Livingston EH. Procedure, incidence and complication rates of bariatric surgery in the United States. Am J Surg. 2004;199:541–551. [DOI] [PubMed] [Google Scholar]
- 8.Mason EE, Renquist JEGS. Perioperative risk and safety of surgery for severe obesity. Am J Clin Nutr. 1992;55, 573S–76S. [DOI] [PubMed] [Google Scholar]
- 9.Elbahrawy A, Bougie A, Loiselle SE, et al. Medium to long-term outcomes of bariatric surgery in older adults with super obesity. m Surg Obes Relat Dis. 2018. April;14(4):470–476. [DOI] [PubMed] [Google Scholar]
- 10.Susmallian S, Raziel A, Barnea R, et al. Bariatric Surgery in older adults: should there be an age limit? Medicine. 2019;98, 3(e13824). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lautz DB, Jackson TD, Clancy KA, et al. Bariatric operations in veterans Affairs and selected university medical centers: results of the patient safety in surgery study. J Am Coll Surg. 2007. April;204:1261–1272. [DOI] [PubMed] [Google Scholar]
- 12.Chang SH, Stoll CRT, Song J, et al. The effective and risks of bariatic surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014;149(3):275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376(7): 641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brethauer SA, Aminian A, Romero-Talamas H, et al. Can diabetes be surgically cured? Long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann Surg. 2013;258(4):628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753–761. [DOI] [PubMed] [Google Scholar]
- 16.Sjostrom L Review of the key results from the Swedish Obese Subjects (SOS) trial–a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273(3):219–234. [DOI] [PubMed] [Google Scholar]
- 17.Kashyap SR, Bhatt DL, Wolski K, et al. Metabolic effects of bariatric surgery in patients with moderate obesity and type 2 diabetes: analysis of a randomized control trial comparing surgery with intensive medical treatment. Diabetes Care. 2013;36(8):2175–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khorgami Z, Shoar S, Andalib A, et al. Trends in utilization of bariatric surgery, 2010–2014: sleeve gastrectomy dominates. Surg Obes Relat Dis. 2017;13(5): 774–778. [DOI] [PubMed] [Google Scholar]
- 19.Felsenreich DM, Kefurt R, Schermann M, et al. Reflux, sleeve dilation, and Barrett’s esophagus after laparoscopic sleeve gastrectomy: long-term follow-up. Obes Surg. 2017. December;27(12):3092–3101. [DOI] [PubMed] [Google Scholar]
- 20.Genco A, Soricelli E, Casella G, et al. Gastroesophageal reflux disease and Barrett’s esophagus after laparoscopic sleeve gastrectomy: a possible, under-estimated long-term complication. Surg Obes Relat Dis. 2017. April;13(4): 568–574. [DOI] [PubMed] [Google Scholar]
- 21.Hammad TA, Thrift AP, El-Serag HB, et al. Missed opportunities for screening and surveillance of barrett’s esophagus in veterans with esophageal adenocarcinoma. Dig Dis Sci. 2019. February;64(2):367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El Khoury L, Benvenga R, Romero R, et al. Esophageal adenocarcinoma in Barrett’s esophagus after sleeve gastrectomy: case report and literature review. Int J Surg Case Rep. 2018;52:132–136. [DOI] [PMC free article] [PubMed] [Google Scholar]


