Abstract
The cerebral cortex is the hallmark of the mammalian nervous system, and its large size and cellular diversity in humans supports our most sophisticated cognitive abilities. Although the basic cellular organization of the cortex is conserved across mammals, cells have diversified during evolution. An increasingly integrated taxonomy of cell types, especially with the advent of single cell transcriptomic data, has revealed an unprecedented variety of human cortical cell subtypes. Here, we broadly review the cellular composition and diversity of the mammalian brain, and how progenitor pools generate cell subtypes during development. We then discuss human cortical cells that are distinct from rodent cells, as well as the challenges and advantages of using model systems to study human cell types in health and disease.
Keywords: Cerebral cortex, cell types, single cell transcriptomics, development, evolution, organoids
Introduction
Cells are the building blocks of the nervous system, and diversity in their composition, electrophysiological properties, and connectivity is the basis for both information processing as well as evolutionary modification of neural circuits [1,2]. The classification of neural cells began more than 100 years ago with drawings based upon Nissl and Golgi stains that enabled early anatomists to describe the major types of brain cells: neurons, glia, endothelial cells, and ependymal cells [3]. Pioneering neuroanatomists classified neurons with short, local axons as interneurons and those with long, distant connections as projection neurons (PNs). During the second half of the 20th century, neuroscientists began organizing these major cell types into molecularly distinct subtypes, for example classifying neurons as excitatory or inhibitory based upon the expression of glutamate or gamma-aminobutyric acid (GABA), respectively [4]. Methods enabling the identification of specific neuronal connections expanded earlier descriptions of, for example, PNs into intracerebrally projecting (IcPN) or subcerebrally projecting (ScPN) subtypes, and each of these can be further subdivided (Figure 1). More recently, single cell molecular profiling has divided cell types by gene expression clusters, adding another dimension to our classification of cell subtypes. As research continues to integrate multiple sources of evidence in pursuit of a conclusive cellular taxonomy [5], comparative research and studies in model systems that recapitulate specific aspects of cellular growth and differentiation have improved our understanding of the cellular composition of the human brain in health and disease.
Figure 1. Basic cellular organization of the neocortex is conserved but varies across mammals.
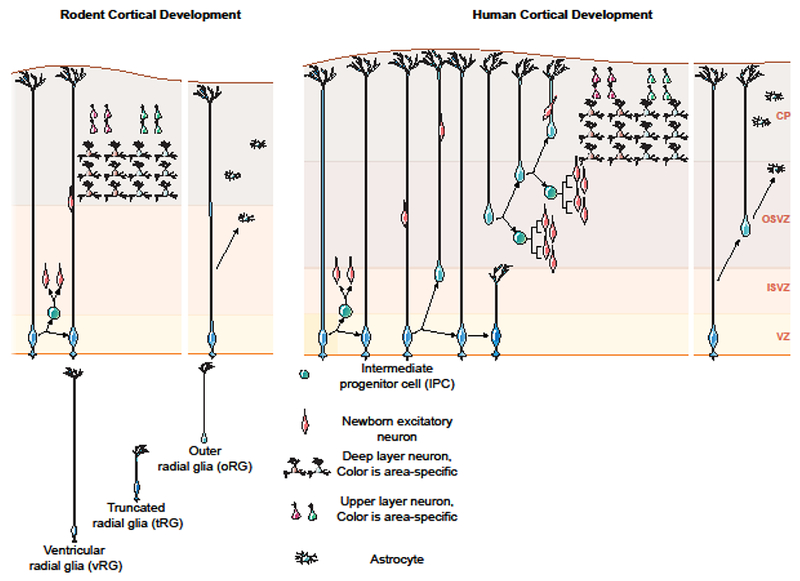
A schematic overview of the major morphological and functional classes of neurons and glia in the adult rodent and human neocortex. The cerebral cortex is traditionally subdivided into 6 layers, each of which contains a unique set of neurons and connections. The major types of neurons and macroglia are depicted in their laminar distributions by shape and color. Glutamatergic excitatory neurons can be subdivided into three major groups: pyramidal intracerebral projection neurons (IcPNs, green); pyramidal subcerebral projection neurons (ScPNs, blue); and layer 4 spiny stellate cells (gray). Layer 5B of certain areas of the human but not rodent neocortex contain large or giant modified pyramidal neurons such as Betz cells in motor cortices, Meynert cells in primary visual cortex, and von Economo spindle neurons and fork cells in fronto-insular cortices. GABAergic inhibitory neurons are diverse, depicted here as a single class (orange). Macroglia consist of oligodendrocytes, and a single class of protoplasmic astroctyes in rodents parallels interlaminar (layer 1), protoplasmic (layer 2-4), and varicose projection (layers 5, 6) astrocytes in humans. See main text for additional details.
The cerebral cortex or neocortex emerged in mammals to constitute a majority of the brain and is organized horizontally into ~6 layers of cells that are connected vertically into columns [6]. Several evolutionary trends contributed to the specific changes in cellular composition of the human neocortex that is now home to our most sophisticated mental activities, like language and theory of mind [1,7]. First, although the human brain is not the largest, most heavily populated or highly interconnected mammalian brain, the human neocortex is the largest and most cell dense among extant primates [8]. Second, the corpus callosum emerged as a major interhemispheric pathway in placental mammals, beyond the anterior and hippocampal commissures [9], to become the largest axonal projection system in humans [10]. Third, the neocortex expanded during primate evolution to create many novel functional areas, particularly association-type cortical fields [1], where neurons exhibit more synapses and complex dendritic morphologies [11]. Fourth, the expansion of association cortex and the corpus callosum is reflected in an increased thickness of the supragranular layers and the number of their resident neurons [12], although early researchers also noted a diversification of infragranular layer neurons [13]. These evolutionary processes generated a radiation of cortical neuron subtypes that has likely driven the emergence of human cognition and behavior.
Over a century of research has revealed that all cortical cell types arise from a limited pool of progenitor cells, although we do not currently have a census of cortical cell subtypes. Modeling work holds great promise to disentangle the extent and impact of human cortical cellular diversity, and the addition of single cell RNA sequencing (scRNA-seq) has helped to clarify the exact number of cell subtypes through an increasingly comprehensive taxonomy. There is increasing evidence for changes in the developmental features, morphology, molecular profiles, and number of cell subtypes over human neocortical evolution. In this review, we summarize how cellular diversity is generated during development, outline what is currently known about the cellular composition of the human neocortex based upon comparison to other species, particularly rodents, and then discuss the use of model systems to understand human cortical cells in health and disease.
Development of human cerebral cortical cell types
The enormous diversity of adult cortical cell types arises progressively through development, and can be traced back to just a few neural stem and progenitor cell types at the onset of neurogenesis. All cortical neurons and glia derive from neuroepithelial cells that form the rostral neural tube following neurulation [14]. Transcription factors acting in opposing spatial gradients, including EMX2 and PAX6, NR2F1 (aka Coup-TF1) and SP8, are expressed by neuroepithelial cells in the anterior neural tube and impart positional identity to cells [15,16]. Neuroepithelial cells at this stage have not been subclassified, although scRNA-seq may reveal distinguishing marker gene clusters [*17].
Neocortical neurogenesis starts around seven postconceptional weeks [18], when neuroepithelial cells lining the ventricles start to generate nascent neurons and gradually transform into radial glia (RG) that constitute a proliferative ventricular zone. The ventricular radial glia (vRG) generate both neurons and astrocytes during a protracted period of proliferation that extends over ~3 months. RG often divide asymmetrically to self-renew and produce a non-RG daughter cell. vRGs can generate neurons directly, but they primarily generate secondary progenitor cells that in turn produce neurons [19,20]. vRG cells produce intermediate progenitor cells (IPCs) in rodents, and, in humans and other large-brain mammals [21,22], they also produce outer RGs (oRGs), or basal progenitors. IPCs and oRGs move out of the ventricular zone and constitute an expanded, secondary proliferative region, the subventricular zone (SVZ). The SVZ can be subdivided into inner (iSVZ) and outer zones (oSVZ), where most IPCs and oRGs are located [23]. The oSVZ is the predominant source of neurons for the upper cortical layers [23,**24] (Figure 2). Approximately half-way through cortical neurogenesis, a wave of oRG production occurs via horizontal cleavage of vRG cells that leads to inheritance of the basal vRG fibers by the newly-generated oRG cells [25]. The vRG cells re-grow basal fibers, but these generally stop short of the pia within the oSVZ or intermediate zone, often ending on blood vessels [*26].
Figure 2. Development of human cortical cell types.
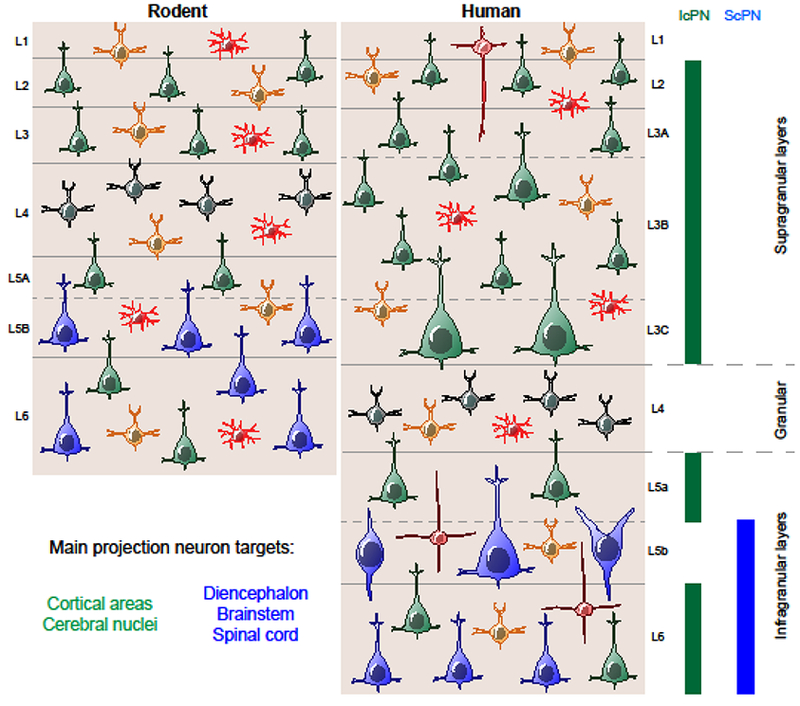
The cortex develops when radial glia give rise to neurons via intermediate progenitor cells. These cells give rise to newborn neurons that migrate to the cortical plate and generate the cortical excitatory neurons in an inside out fashion, with deep layer excitatory neurons generated first followed by the upper layer excitatory neurons. These excitatory neurons have recently been described to be area-specific and to emerge early in development. The human cortex consists of a ventricular zone, and an expanded outer subventricular zone compared to the rodent. Humans have additional subtypes of radial glia, the outer radial glia and truncated radial glia that emerge at later stages of development. After the peak of neurogenesis, radial glia engage in gliogenesis, producing astrocytes.
The oRG cells in non-human primates have been subdivided into four morphotypes based on the presence of basal and/or apical processes [27]. However, scRNA-seq datasets have not yet confirmed distinct cell identities related to these morphologies, and it is uncertain whether these represent true distinct, or intermediate oRG cell subtypes. Possible distinctions might also exist within the IPC population or between IPCs that divide only once, and transient amplifying progenitors that divide multiple times, but these remain to be identified, and could possibly be revealed with scRNA-seq data. scRNA-seq studies of human cortical progenitor cells have demonstrated relatively little transcriptional diversity within cortical RG populations, but have highlighted that significant regional gene differences do emerge in the postmitotic neuron populations as immature neurons migrate into the cortical plate and begin to mature [**24].
Recent studies have also identified many shared and certain derived features of developing human cortical neurons [28]. For instance, nitric oxide synthase 1 (NOS1) protein is transiently translated under the control of FMRP, a protein disrupted in fragile X syndrome, in layer 5 neurons forming minicolumns in the midfetal frontal operculum, a neocortical region comprising prospective orofacial motor and speech/language areas [29]. This distinct molecular pattern, not evident in mice or monkeys nor in fragile X syndrome patients who suffer from intellectual disability, suggests that evolutionarily divergent features of developing cortical cells may be impacted in disorders affecting human cognition. Accordingly, an increasingly fine-grain cellular taxonomy holds great promise to reveal exactly how a few progenitor cell subtypes generate an incredible diversity of cortical cell types, how developing cortical neurons progressively acquire shared and derived features, and whether these complex developmental processes are affected in human neurological and psychiatric disorders.
Evolution of human cerebral cortical cell types
Excitatory neurons are the largest group of cortical neurons, and recent work reaffirms the importance of neurodevelopmental regional patterning to cell type identity, confirming the major mammalian cell types while also demonstrating the potential for rare subtypes to emerge. For example, recent work using scRNA-seq identified 24 excitatory neuron subtypes in a single human neocortical area [**30], and interestingly, most of these corresponded to subtypes already identified in the mouse neocortex [32]. In addition, mammalian PNs are subdivided by connectivity into IcPNs or ScPNs, and then into callosal or intrahemispheric association and corticostriatal PNs, as well as cortico-spinal, -bulbar, -tectal, and -thalamic PNs [31,32]. Furthermore, ScPNs segregate to infragranular layers and IcPNs primarily reside in supragranular layers, although some IcPNs are infragranular (Figure 1). In parallel, recent work has reinforced the view that subtypes of excitatory neurons may be specific to areas or even layers within areas [**30,33,*34]. Modifications to both laminar position and targets, for example, supports classifying callosally projecting PNs as a distinct class of cells because they differ from other commissural or intrahemispheric PNs [35-39]. However, previous neuroanatomical studies have shown that the relative proportion of ScPNs,which contain some of the longest axonal projections in the nervous system, is decreased in humans compared to mice, and that in certain cortical areas these cells have undergone a dramatic increase in size and modification in morphology, for example the Betz, Meynert, von Economo, and fork cells [13]. Consideration of the agreement and disagreement between multiple sources of evidence in an increasingly complete cellular taxonomy will be crucial to understanding how to identify rare but potentially influential subtypes within a broadly conserved cellular population.
Early anatomists described a richer variety of inhibitory compared to excitatory neuron subtypes that modern molecular biologists have now shown reflects ancient neurotransmitter profiles. Specifically, genomic tools have shown that all amniotes have similar classes of inhibitory neurons, but that mammalian cortical excitatory neurons are new cell types generated by the diversification of ancestral gene-regulatory programs [**40]. Recent cortical scRNA-seq data suggests the presence of 24 excitatory and nearly 50 inhibitory neuron subtypes [**30], and even by morphology, early anatomists described only 2-3 excitatory but ~7 types of inhibitory neurons [3]. Indeed, all cortical inhibitory neurons have local connections and can be successfully classified by neurotransmitter and neuropeptide composition into 3 major groups: parvalbumin, serotonin, or somatostatin [5]. However, contemporary studies have identified interneuron subtypes in humans not found in rodents, such as layer 1 rosehip interneurons [**41], and a rare subpallial-derived inhibitory neuron subtype present in humans but not nonhuman African great apes (i.e., common chimpanzee, bonobo and gorilla) [**42]. Thus, while major inhibitory neuron subtypes are conserved across amniotes, changes in rare subtypes in humans may modulate human circuit function.
Research has increasingly shown that macroglia are more than passive support cells [43], and recent work has discovered human-specific mutations that may shed light on the functional transformation of these cell types in the human brain. Astrocytes play a varied neurobiological role and have diversified into multiple subtypes. For instance, all mammals have protoplasmic astrocytes in cortical gray matter, and fibrous astrocytes throughout the white matter. However, comparative work has shown that gray matter astrocytes diversified into infragranular layer varicose projection astrocytes and layer 1 interlaminar astrocytes in humans and chimpanzees, but neither cell type is present in rodents [44]. Furthermore, protoplasmic astrocytes in primates extend 10 times the number of processes across a 2.6 times larger volume of tissue than in rodents. Although the specific physiological consequences of these changes may be unclear, macroglia like astrocytes have diversified over the course of human evolution and likely support higher brain metabolism [7].
In vitro modeling of human cerebral cortical cell types
The diversity of human neocortical cells presents significant challenges for both in vivo and in vitro models. However, comparative studies can provide insight to alternative, complementary biological processes and hs the potential to reveal the scale and scope of human cortical cellular diversity. Roughly comparable cortical circuits are present in mice and humans because while the cellular composition and organization of cortical circuitry may be distinct, the major cell types as well as the basics of each neural circuit are shared (see Figure 1). For example, recent scRNA-seq work shows correspondence between most neuron types in human and mouse neocortex, and that excitatory PNs can be further subdivided into tens of subclasses based upon transcriptional identity, although there may be variation in homologous cell types [**30]. ScRNA-seq profiling of mouse oligodendrocyte has revealed a number of sub-classes in what was previously thought to be a uniform cell type [**45]. Furthermore, non-neuronal cell types in the mouse represent an approximation of what we may discover to be relevant to human brain function during development and in the presence of disease. For example, studies in the mouse have identified layer- [46] and area-specific subtypes of astrocytes [33], that have long been thought to be relatively uniform across the cortex. Interestingly, experimentally altering pyramidal neuron layering abrogates these differences, suggesting that excitatory neuron identity influences astrocyte layer identity. In addition, areal subtypes suggest that the process of circuit formation may be essential to the determination of pyramidal neuron identity, highlighting the importance of developmental studies to unravel the mechanisms of cellular diversity. Furthermore, the conservation of major cell types between humans and rodents, but the divergence in gene expression within these populations, hints that regulatory programs gave rise to distinct cellular transcriptomes between species [*47]. Additional studies of the epigenetic landscape in developing and adult cortex may shed light on this question as initial reports indicate that the chromatin landscape closely reflects transcriptional identity in the adult human [48]. Thus rodent models can play an important role in clarifying the identity and function of human cortical cells by serving as a platform in which to observe the interplay between gene expression, connectivity, and physiology across development.
The human brain has undergone substantial reorganization over the course of evolution, and thus human-based experiments are essential to understand the unique aspects of human cortical biology. One way of approaching these issues involves the generation of cortical organoids from pluripotent human stem cells. These 3D stem cell-derived structures contain rosettes of cortical progenitors that can generate a diversity of cortical cell types [49,50]. A number of recent advances have improved the ability to carry out long term experiments that model physiological activity [*51], astrocyte generation [*52], and oligodendrocyte formation [53]. For example, investigators are creating ganglionic eminence organoids that can be fused with cortical organoids to model interneuron migration and integration [54]. These models enable the addition of genetic manipulation and functional outcome measures to studies of cortical cell type development. However, the fidelity of these models to normal development is still under investigation, and additional innovations will likely improve the generation of cortical columns, regions, and early circuit formation.
Organoids are a model system derived from a developmental perspective, but a number of studies and applications require characterization of adult neurons. As such, key innovations in transdifferentiation have enabled the creation and subsequent exploration of adult human neurons derived from cultures of fibroblasts or induced pluripotent cells [55]. New approaches have found that depending on the set of transcription factors used for reprogramming, distinct subtypes of neurons that resemble in vivo populations emerge [56]. Understanding cortical cell types in both development and the adult will be essential to characterize disease-associated vulnerabilities. Recently, for example, the outbreak of Zika virus was modeled in slice culture [*17,57] and organoids [58], leading to the identification of cellular vulnerabilities in cortical progenitor cells.
Summary
The human neocortex has expanded dramatically and exhibits an unprecedented degree of cellular heterogeneity, with recent work estimating it may contain on the order of 100 cell types. The newly-emerging molecular toolkit provides important evidence to catalogue the cellular composition of the humans neocortex, and current studies seek to develop a comprehensive cell type taxonomy in order to identify how the brain has evolved as well as how it may be compromised in disease. In this review, we have discussed how the unprecedented variety of adult human cortical cell types is generated from only a few progenitor cell types, that different mechanisms may act upon distinct subpopulations of neurons to influence subtype identity, and how judicious use of in vivo and in vitro methods will dramatically improve our understanding of the full variety of human cortical cell types.
Highlights:
Human cerebral cortex contains 100s of cell types
Basic cellular organization of the cortex is conserved but varies across mammals
Human neural progenitors are highly diversified during cortical development
Homologous cell types exhibit divergent features in humans, including area-specific excitatory neurons
Distinct human interneurons may switch between somatostatin and dopamine
Acknowledgments:
We apologize to the colleagues whose work in the field could not be cited because of space restrictions. This work was supported, in part, by grants from the National Institutes of Health and the Simons Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited:
- 1.Krubitzer L, Kaas J: The evolution of the neocortex in mammals: how is phenotypic diversity generated? Curr Opin Neurobiol 2005, 15:444–453. [DOI] [PubMed] [Google Scholar]
- 2.Preuss TM: Human brain evolution: from gene discovery to phenotype discovery. Proc Natl Acad Sci U S A 2012, 109 Suppl 1:10709–10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nieuwenhuys R: The neocortex. An overview of its evolutionary development, structural organization and synaptology. Anat Embryol (Berl) 1994, 190:307–337. [DOI] [PubMed] [Google Scholar]
- 4.Jones EG: Neurotransmitters in the cerebral cortex. J Neurosurg 1986, 65:135–153. [DOI] [PubMed] [Google Scholar]
- 5.Lodato S, Arlotta P: Generating Neuronal Diversity in the Mammalian Cerebral Cortex. Annu Rev Cell Dev Biol 2015, 31:699–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaas JH: Evolution of columns, modules, and domains in the neocortex of primates. Proc Natl Acad Sci U S A 2012, 109 Suppl 1:10655–10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherwood CC, Bauernfeind AL, Bianchi S, Raghanti MA, Hof PR: Human brain evolution writ large and small. Prog Brain Res 2012, 195:237–254. [DOI] [PubMed] [Google Scholar]
- 8.Sousa AMM, Meyer KA, Santpere G, Gulden FO, Sestan N: Evolution of the Human Nervous System Function, Structure, and Development. Cell 2017, 170:226–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.JI Johnson, Kirsch JA, Switzer RC: Phylogeny through brain traits: fifteen characters which adumbrate mammalian genealogy. Brain Behav Evol 1982, 20:72–83. [DOI] [PubMed] [Google Scholar]
- 10.Aboitiz F: Brain connections: interhemispheric fiber systems and anatomical brain asymmetries in humans. Biol Res 1992, 25:51–61. [PubMed] [Google Scholar]
- 11.Elston GN: Cortex, cognition and the cell: new insights into the pyramidal neuron and prefrontal function. Cereb Cortex N Y N 1991 2003, 13:1124–1138. [DOI] [PubMed] [Google Scholar]
- 12.Suárez R, Paolino A, Fenlon LR, Morcom LR, Kozulin P, Kurniawan ND, Richards LJ: A pan-mammalian map of interhemispheric brain connections predates the evolution of the corpus callosum. Proc Natl Acad Sci U S A 2018, 115:9622–9627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seeley WW, Merkle FT, Gaus SE, Craig ADB, Allman JM, Hof PR, Economo CV: Distinctive neurons of the anterior cingulate and frontoinsular cortex: a historical perspective. Cereb Cortex N Y N 1991 2012, 22:245–250. [DOI] [PubMed] [Google Scholar]
- 14.Nikolopoulou E, Galea GL, Rolo A, Greene NDE, Copp AJ: Neural tube closure: cellular, molecular and biomechanical mechanisms. Dev Camb Engl 2017, 144:552–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bishop KM, Rubenstein JLR, O’Leary DDM: Distinct actions of Emx1, Emx2, and Pax6 in regulating the specification of areas in the developing neocortex. J Neurosci Off J Soc Neurosci 2002, 22:7627–7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Leary SJB, Poulis BAD, von Aderkas P: Identification of two thaumatin-like proteins (TLPs) in the pollination drop of hybrid yew that may play a role in pathogen defence during pollen collection. Tree Physiol 2007, 27:1649–1659. [DOI] [PubMed] [Google Scholar]
- 17.*.Onorati M, Li Z, Liu F, Sousa AMM, Nakagawa N, Li M, Dell’Anno MT, Gulden FO, Pochareddy S, Tebbenkamp ATN, et al. : Zika Virus Disrupts Phospho-TBK1 Localization and Mitosis in Human Neuroepithelial Stem Cells and Radial Glia. Cell Rep 2016, 16:2576–2592.The authors used neuroepithelial stem cells as a model to study the effect of the Zika virus, and together with analyses of brain slices, revealed that this virus impairs the mitosis and survival of neuroepithelial stem cells and radial glia.
- 18.Silbereis JC, Pochareddy S, Zhu Y, Li M, Sestan N: The Cellular and Molecular Landscapes of the Developing Human Central Nervous System. Neuron 2016, 89:248–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haubensak W, Attardo A, Denk W, Huttner WB: Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc Natl Acad Sci U S A 2004, 101:3196–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noctor SC, Martínez-Cerdeño V, Ivic L, Kriegstein AR: Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci 2004, 7:136–144. [DOI] [PubMed] [Google Scholar]
- 21.Fietz SA, Kelava I, Vogt J, Wilsch-Bräuninger M, Stenzel D, Fish JL, Corbeil D, Riehn A, Distler W, Nitsch R, et al. : OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat Neurosci 2010, 13:690–699. [DOI] [PubMed] [Google Scholar]
- 22.Hansen DV, Lui JH, Parker PRL, Kriegstein AR: Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 2010, 464:554–561. [DOI] [PubMed] [Google Scholar]
- 23.Smart IHM, Dehay C, Giroud P, Berland M, Kennedy H: Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb Cortex N Y N 1991 2002, 12:37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.**.Nowakowski TJ, Bhaduri A, Pollen AA, Alvarado B, Mostajo-Radji MA, Di Lullo E, Haeussler M, Sandoval-Espinosa C, Liu SJ, Velmeshev D, et al. : Spatiotemporal gene expression trajectories reveal developmental hierarchies of the human cortex. Science 2017, 358:1318–1323.This paper uses single-cell sequencing during the peak stages of neurogenesis to identify major cell populations during human cortical development. One major finding from this paper is the emergence of area-specific excitatory neurons at developmental stages, prior to the onset of sensory experience.
- 25.LaMonica BE, Lui JH, Hansen DV, Kriegstein AR: Mitotic spindle orientation predicts outer radial glial cell generation in human neocortex. Nat Commun 2013, 4:1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.*.Nowakowski TJ, Pollen AA, Sandoval-Espinosa C, Kriegstein AR: Transformation of the Radial Glia Scaffold Demarcates Two Stages of Human Cerebral Cortex Development. Neuron 2016, 91:1219–1227.This paper highlights the emergence of morphologically distinct truncated radial glia at later stages of neurogenesis. These cells correspond transcriptionally to cells identified in single-cell datasets and suggest a diversification of progenitor populations as the transition from neurogenesis to gliogenesis occurs.
- 27.Betizeau M, Cortay V, Patti D, Pfister S, Gautier E, Bellemin-Ménard A, Afanassieff M, Huissoud C, Douglas RJ, Kennedy H, et al. : Precursor diversity and complexity of lineage relationships in the outer subventricular zone of the primate. Neuron 2013, 80:442–457. [DOI] [PubMed] [Google Scholar]
- 28.Raju CS, Spatazza J, Stanco A, Larimer P, Sorrells SF, Kelley KW, Nicholas CR, Paredes MF, Lui JH, Hasenstaub AR, et al. : Secretagogin is Expressed by Developing Neocortical GABAergic Neurons in Humans but not Mice and Increases Neurite Arbor Size and Complexity. Cereb Cortex N Y N 1991 2018, 28:1946–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwan KY, Sestan N, Anton ES: Transcriptional co-regulation of neuronal migration and laminar identity in the neocortex. Dev Camb Engl 2012, 139:1535–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.**.Hodge RD, Bakken TE, Miller JA, Smith KA, Barkan ER, Graybuck LT, Close JL, Long B, Penn O, Yao Z, et al. : Conserved cell types with divergent features between human and mouse cortex. bioRxiv 2018, doi: 10.1101/384826.Using single-cell or single-nuclei sequencing, the authors compare mouse and human cell types in the visual cortex and medial temporal gyrus, respectively. They discover a large conservation of broad cell types but very distinctive markers, and significantly different signatures and subpopulations in non-neuronal cell types. Additionally, they find an expansion of suprgranular cell types in the human and cell types that cross layer boundaries.
- 31.Fame RM, MacDonald JL, Macklis JD: Development, specification, and diversity of callosal projection neurons. Trends Neurosci 2011, 34:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leone DP, Srinivasan K, Chen B, Alcamo E, McConnell SK: The determination of projection neuron identity in the developing cerebral cortex. Curr Opin Neurobiol 2008, 18:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeisel A, Hochgerner H, Lönnerberg P, Johnsson A, Memic F, van der Zwan J, Häring M, Braun E, Borm LE, La Manno G, et al. : Molecular Architecture of the Mouse Nervous System. Cell 2018, 174:999–1014.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.*.Saunders A, Macosko EZ, Wysoker A, Goldman M, Krienen FM, de Rivera H, Bien E, Baum M, Bortolin L, Wang S, et al. : Molecular Diversity and Specializations among the Cells of the Adult Mouse Brain. Cell 2018, 174:1015–1030.e16.The authors use broad scale single-cell sequencing to identify major populations of cell types across the mouse brain. They identify areally defined cortical excitatory neurons that corroborate other findings in mouse and developing human.
- 35.Chun JJ, Nakamura MJ, Shatz CJ: Transient cells of the developing mammalian telencephalon are peptide-immunoreactive neurons. Nature 1987, 325:617–620. [DOI] [PubMed] [Google Scholar]
- 36.deAzevedo LC, Hedin-Pereira C, Lent R: Callosal neurons in the cingulate cortical plate and subplate of human fetuses. J Comp Neurol 1997, 386:60–70. [DOI] [PubMed] [Google Scholar]
- 37.Jones EG, Burton H, Porter R: Commissural and cortico-cortical “columns” in the somatic sensory cortex of primates. Science 1975, 190:572–574. [DOI] [PubMed] [Google Scholar]
- 38.Wise SP: The laminar organization of certain afferent and efferent fiber systems in the rat somatosensory cortex. Brain Res 1975, 90:139–142. [DOI] [PubMed] [Google Scholar]
- 39.Fries W, Keizer K, Kuypers HG: Large layer VI cells in macaque striate cortex (Meynert cells) project to both superior colliculus and prestriate visual area V5. Exp Brain Res 1985, 58:613–616. [DOI] [PubMed] [Google Scholar]
- 40.**.Tosches MA, Yamawaki TM, Naumann RK, Jacobi AA, Tushev G, Laurent G: Evolution of pallium, hippocampus, and cortical cell types revealed by single-cell transcriptomics in reptiles. Science 2018, 360:881–888.This paper used single-cell RNA sequencing to provide evidence for an ancient homology between the mammalian cerebral cortex and the avian or reptilian dorsal ventricular ridge. The authors describe the commonality of cell types across all amniotes, from turtles to birds to humans, and in particular an ancient diversification of inhibitory neuron types.
- 41.**.Boldog E, Bakken TE, Hodge RD, Novotny M, Aevermann BD, Baka J, Bordé S, Close JL, Diez-Fuertes F, Ding S-L, et al. : Transcriptomic and morphophysiological evidence for a specialized human cortical GABAergic cell type. Nat Neurosci 2018, 21:1185–1195.Using single-nuclei sequencing of layer 1 cells, the authors discover a transcriptionally and morphologically distinct GABAergic cell type they define as a "rosehip" cell. This cell type is of yet unknown function but has not been found in the rodent.
- 42.**.Sousa AMM, Zhu Y, Raghanti MA, Kitchen RR, Onorati M, Tebbenkamp ATN, Stutz B, Meyer KA, Li M, Kawasawa YI, et al. : Molecular and cellular reorganization of neural circuits in the human lineage. Science 2017, 358:1027–1032.The authors generated sequencing data from across the human, chimpanzee, and macaque brain that revealed human-specific patterns of differential gene expression in genes coding transcription factors, ion channels, and neurotransmitter biosynthesis enzymes and receptors.
- 43.Fields RD, Woo DH, Basser PJ: Glial Regulation of the Neuronal Connectome through Local and Long-Distant Communication. Neuron 2015, 86:374–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oberheim NA, Takano T, Han X, He W, Lin JHC, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, et al. : Uniquely hominid features of adult human astrocytes. J Neurosci Off J Soc Neurosci 2009, 29:3276–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.**.Zeisel A, Muñoz-Manchado AB, Codeluppi S, Lönnerberg P, La Manno G, Juréus A, Marques S, Munguba H, He L, Betsholtz C, et al. : Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 2015, 347:1138–1142.Using single-cell sequencing in the adult mouse, this paper describes the mouse nervous system. Not only do they identify cortical area specific excitatory neurons, but they also define brain region specific astrocyte populations.
- 46.Lanjakornsiripan D, Pior B-J, Kawaguchi D, Furutachi S, Tahara T, Katsuyama Y, Suzuki Y, Fukazawa Y, Gotoh Y: Layer-specific morphological and molecular differences in neocortical astrocytes and their dependence on neuronal layers. Nat Commun 2018, 9:1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.*.Arendt D, Musser JM, Baker CVH, Bergman A, Cepko C, Erwin DH, Pavlicev M, Schlosser G, Widder S, Laubichler MD, et al. : The origin and evolution of cell types. Nat Rev Genet 2016, 17:744–757.This review paper nicely frames cell type definitions in terms of specialized access to the genome, and describes the evolution of cell types and cell functions in terms of changes of co-regulatory complexes to enable distinctive privileged access to areas of the genome.
- 48.Lake BB, Chen S, Sos BC, Fan J, Kaeser GE, Yung YC, Duong TE, Gao D, Chun J, Kharchenko PV, et al. : Integrative single-cell analysis of transcriptional and epigenetic states in the human adult brain. Nat Biotechnol 2018, 36:70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, Sekiguchi K, Adachi T, Sasai Y: Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 2011, 472:51–56. [DOI] [PubMed] [Google Scholar]
- 50.Lancaster MA, Renner M, Martin C-A, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA: Cerebral organoids model human brain development and microcephaly. Nature 2013, 501:373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.*.Quadrato G, Nguyen T, Macosko EZ, Sherwood JL, Min Yang S, Berger DR, Maria N, Scholvin J, Goldman M, Kinney JP, et al. : Cell diversity and network dynamics in photosensitive human brain organoids. Nature 2017, 545:48–53.This publication models photosensitive cells using long term organoid culture. Combined with single-cell RNA-sequencing, the authors demonstrate some functional applications for organoids to model aspects of organismal function, such as photosensation.
- 52.*.Sloan SA, Darmanis S, Huber N, Khan TA, Birey F, Caneda C, Reimer R, Quake SR, Barres BA, Paşca SP: Human Astrocyte Maturation Captured in 3D Cerebral Cortical Spheroids Derived from Pluripotent Stem Cells. Neuron 2017, 95:779–790.e6.This paper explores the maturation gradients of astrocytes in terms of their emergence in long term cortical spheroid generation. The authors demonstrate the utility of long term in vitro systems to study processes of astrocyte development that are difficult to access from primary tissue and may have evolved in primates compared to rodents.
- 53.Madhavan M, Nevin ZS, Shick HE, Garrison E, Clarkson-Paredes C, Karl M, Clayton BLL, Factor DC, Allan KC, Barbar L, et al. : Induction of myelinating oligodendrocytes in human cortical spheroids. Nat Methods 2018, 15:700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiang Y, Tanaka Y, Patterson B, Kang Y-J, Govindaiah G, Roselaar N, Cakir B, Kim K-Y, Lombroso AP, Hwang S-M, et al. : Fusion of Regionally Specified hPSC-Derived Organoids Models Human Brain Development and Interneuron Migration. Cell Stem Cell 2017, 21:383–398.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, Citri A, Sebastiano V, Marro S, Südhof TC, et al. : Induction of human neuronal cells by defined transcription factors. Nature 2011, 476:220–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsunemoto R, Lee S, Szűcs A, Chubukov P, Sokolova I, Blanchard JW, Eade KT, Bruggemann J, Wu C, Torkamani A, et al. : Diverse reprogramming codes for neuronal identity. Nature 2018, 557:375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Retallack H, Di Lullo E, Arias C, Knopp KA, Laurie MT, Sandoval-Espinosa C, Mancia Leon WR, Krencik R, Ullian EM, Spatazza J, et al. : Zika virus cell tropism in the developing human brain and inhibition by azithromycin. Proc Natl Acad Sci U S A 2016, 113:14408–14413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, Yao B, Shin J, Zhang F, Lee EM, et al. : Zika Virus Infects Human Cortical Neural Progenitors and Attenuates Their Growth. Cell Stem Cell 2016, 18:587–590. [DOI] [PMC free article] [PubMed] [Google Scholar]


