Abstract
Thermal burn injuries in patients alcohol intoxicated result in greater morbidity and mortality. Murine models combining ethanol and localized thermal burn injury reproduce the systemic toxicity seen in human subjects, which consists of both acute systemic cytokine production with multiple organ dysfunction, as well as a delayed systemic immunosuppression. However, the exact mechanisms for these acute and delayed effects are unclear. These studies sought to define the role of the lipid mediator Platelet-activating factor (PAF) in the acute and delayed effects of intoxicated burn injury. Combining ethanol and thermal burn injury resulted in increased enzymatic PAF generation in a keratinocyte cell line in vitro, human skin explants ex vivo, as well as in murine skin in vivo. Further, the acute increase in inflammatory cytokines such as IL-6, and the systemic immunosuppressive effects of intoxicated thermal burn injury, were suppressed in mice lacking PAF receptors. Together, these studies provide a potential mechanism and novel treatment strategies for the augmented toxicity and immunosuppressive effects of thermal burn injury in the setting of acute ethanol exposure, which involves the pleotropic lipid mediator PAF.
Introduction
Thermal burn injury causes significant morbidity and mortality. More than 100,000 patients are hospitalized in the U.S. annually for severe burn injuries (Choudry et al., 2006). Ethanol use at the time of injury is found in almost half of burn patients, and this population is nearly twice as likely to suffer from morbid complications (Silver et al., 2008; Grobmyer et al., 1996; Haum et al., 1995; McGill et al., 1995; Davis et al., 2013; Friedmann, 2013; Howland et al., 1987). The complications found in patients who had moderate to high blood ethanol levels at the time of the thermal burn injury include over 50% more surgical procedures, a doubling of the risk of severe infectious complications, and length of inpatient hospitalization. It should be noted that the majority of these intoxicated burn patients are not chronic alcoholics, but binge drinkers (McGill et al., 1995; Davis et al., 2013; Howland et al., 1987).
Murine models have been used to characterize the acute inflammatory and immunosuppressive effects observed in humans (Faunce et al., 1997; Faunce et al., 1998; Faunce et al., 2003; Bird et al., 2008; Li et al., 2011; Chen et al., 2013). In particular, intoxicated burn injury results in an increased level of systemic cytokines with IL-6 being a key cytokine in these murine models. IL-6 is elevated acutely in blood serum and peripheral organs including the lungs and small intestine. This IL-6 activation is believed to trigger both acute inflammation and longer term immunosuppressive effects as administration of anti-IL-6 antibodies can attenuate markers of acute pulmonary and gut associated inflammation as well as delayed-type hypersensitivity immunosuppression associated with the intoxicated burn injury in mice (Li et al., 2011; Chen et al., 2013; Zahs et al., 2013).
We believe there are several lines of evidence that support a causal role for the lipid mediator Platelet-activating factor (1-alkyl-2-acetyl-glycerophosphocholine; PAF [reviewed in (Palgan et al., 2015; Shimizu, 2009; Stafforini et al., 2003)] in this injury. First, PAF, which acts through its specific ligand binding of a single G-protein coupled receptor (PAF-R), contributes to systemic inflammatory syndromes from acute allergy response to septic shock, which are symptomatically similar acute findings found in intoxicated burn injury. Second, PAF mediates systemic immunosuppression in response to many environmental stressors, including cigarette smoke exposure and ultraviolet B radiation (Waltersheid et al., 2002; Zhang et al., 2005; Wolf et al., 2006; Zhang et al., 2008; Sahu et al., 2013; Ferracini et al., 2015). Third, thermal burn injury has been shown to generate PAF in keratinocytes (Alappatt et al., 2000). Finally, we believe ethanol can promote PAF production as ethanol exposure has been demonstrated to increase the activity of phospholipase A2 (PLA2) enzymes (in particular cytosolic type IV [cPLA2]), which serve as a major enzyme for the remodeling pathway of PAF biosynthesis (Lee et al., 2004; Tajuddin et al., 2014). Furthermore, a previous study has demonstrated that ethanol augments PAF biosynthesis in endothelial cells in response to ATP (Magai et al., 2001). Subsequently, this study was designed to determine if there is a role for the PAF system in the acute pro-inflammatory and delayed immunosuppressive effects of the intoxicated burn injury. We have found that intoxicated burn promotes PAF production in keratinocytes and that both the acute inflammatory cytokine production and the systemic immunosuppressive effects associated with this injury are suppressed in PAF-receptor deficient mice. These results suggest a PAF-mediated mechanism for the morbidities associated with intoxicated injury, and point toward the PAF system as a novel therapeutic target for this all too frequent injury.
Results
Intoxicated burn injury results in increased PAF in a keratinocyte cell line.
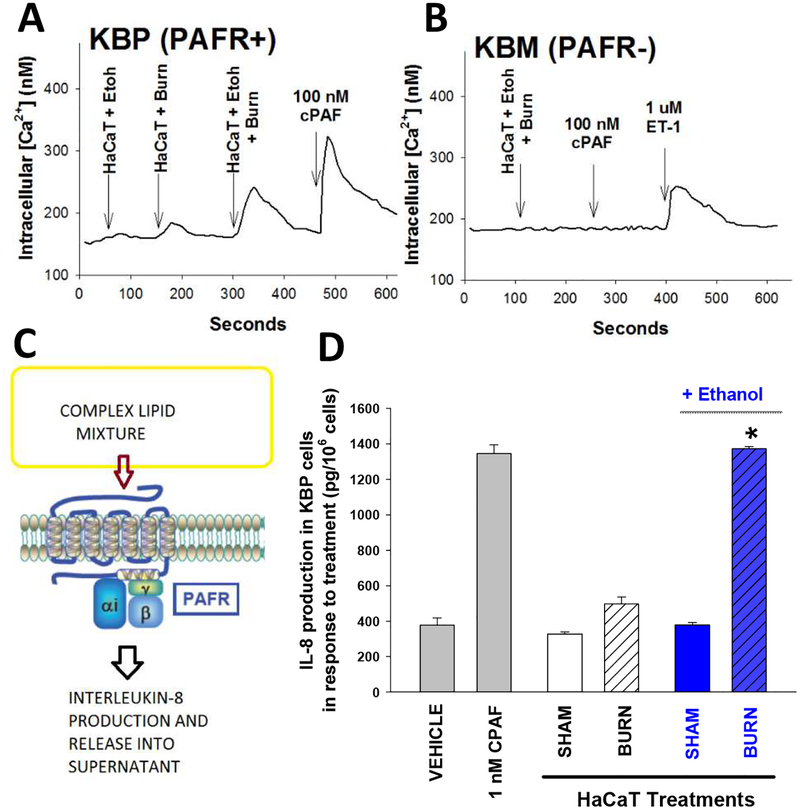
Our previous in vitro studies have demonstrated that thermal burn injury stimulates enzymatic PAF biosynthesis (Alappatt et al., 2000). Thus, our first experiments were designed to test the hypothesis that combining ethanol with thermal burn injury results in enhanced PAF biosynthesis. For these studies, we used the human keratinocyte-derived cell line HaCaT (Boukamp et al., 1988). As multiple glycerophosphocholines exert PAF-R agonistic effects (Marathe et al., 2001; Marathe et al., 2005), our first studies measured total PAF-R biochemical activity using the PAF-R-negative epidermoid cell line KB transduced with PAF-R (KBP) or control MSCV2.1 retrovirus (KBM) (Pet et al., 1998). As shown in Figure 1, addition of complex lipid mixtures containing PAF-R agonists to KBP cells results in an immediate intracellular calcium (Ca2+) mobilization response, as well as delayed IL-8 production, both of which are useful methodologies to quantify PAF-R agonistic activity (Zhang et al., 2005; Sahu et al., 2013; Ferracini et al., 2015; Sahu et al., 2014). Using this KBP-KBM model system, incubation of HaCaT keratinocytes with ethanol before thermal burn injury resulted in augmented PAF-R agonist formation as documented by increased intracellular Ca2+ mobilization selectively in PAF-R-expressing KBP cells (Fig 1A,B) as well as IL-8 accumulation (Fig 1D) upon exposure to lipid extracts of the treated HaCaT keratinocytes. Similar to the Ca2+ mobilization studies, lipid extracts derived from HaCaT cells did not result in IL-8 release in PAF-R-negative KBM cells (Supplemental Fig S1). Testing various ethanol doses ranging from 0.25%−2% revealed that doses above 1% combined with thermal burn injury resulted in the most PAF agonistic activity generated (Supplemental Fig S2). Treatment of primary cultures of undifferentiated human keratinocytes with ethanol + thermal burn injury resulted in qualitatively similar findings of increased PAF agonistic activity generated as seen in HaCaT cells (see Supplemental Fig S3).
Figure 1. EtOH exposure augments PAF-R agonistic activity in response to thermal burn injury in HaCaT cells.
HaCaT cells were subjected to 90°C water bath × 2 min, or sham injury either under normal conditions or following a 30 min pre-incubation with 1% EtOH (BLUE). Five min following injury treatment, the lipids were extracted and PAF-R agonistic activity assessed. A,B). Examples of PAF-R calcium mobilization assays. PAF-R-expressing (A) KBP or (B) PAF-R-negative KBM cells were loaded with Fura-2 AM, and treated with lipid extracts derived from 5 × 106 HaCaT cells, and CPAF or endothelin-1 (ET-1) used at the end of the assay as positive controls. C,D). Measurement of IL-8 release by KBP cells. C) Model of KBP-IL-8 assay where a complex mixture (lipid extracts) containing PAF agonists are incubated with PAF-R-positive KBP cells and IL-8 released used as surrogate of PAF-R activation. D) Lipid extracts from 5 × 106 HaCaT cells previously treated with thermal injury ± ethanol or 1 nM CPAF as positive control were incubated with KBP cells and 6h later IL-8 measured in the supernatants as a surrogate for PAF-R activation. The data are the Mean ± SE IL-8 production in KBP cells (pg/106 KBP cells) from three separate experiments. *Denotes statistically significant (P<0.05) changes in levels of PAF-R agonistic activity from burn values w/o EtOH exposure.
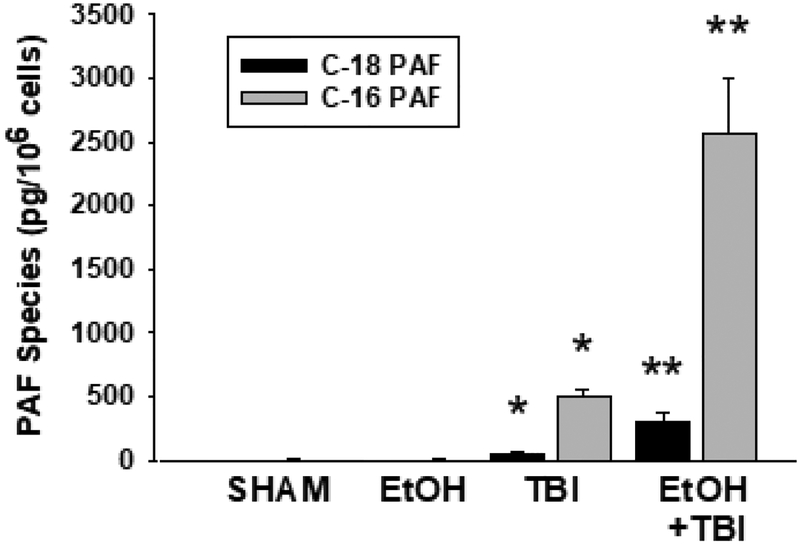
PAF-R agonists consist of both enzymatically produced sn-2 acetyl species as well as non-enzymatically generated sn-2 oxidatively modified species (Shimizu, 2009; Marathe et al., 2001; Marathe et al., 2005; Sahu et al., 2014). To define the exact species formed in response to combining ethanol + thermal burn injury, we employed mass spectrometry using deuterated internal standards as previously described (Sahu et al., 2014; Yao et al., 2012). As shown in Figure 2 (for the major PAF species 1-hexadecyl-2-acetyl GPC and 1-octadecyl-2-acetyl GPC) and Table S1 in supplemental (for a total of eight different sn-2 GPC species), combining ethanol and thermal burn injury resulted in approximately 3–4 fold increase of these PAF species. However, we did not detect increased levels of oxidized GPCs or PAF precursor lyso-PAF. The PAF-R biochemical activity combined with the quantitative analysis of PAF-R agonists indicates that the PAF-R biochemical activity resides in enzymatic sn-2 acetyl GPC (PAF) rather than non-enzymatic oxidized GPC.
Figure 2. EtOH exposure + thermal burn injury results in augmented PAF species accumulation in HaCaT cells.
HaCaT cells were subjected to thermal burn injury (TBI) ± EtOH as outlined in Fig. 1. Five min following injury treatment, the lipids were extracted and PAF species (1-octadecyl-2-acetyl-GPC [C-18 PAF] and hexadecyl-2-acetyl-GPC [C-16 PAF]) was quantified by mass spectrometry using deuterated internal standards. The data are Mean ± SE PAF levels (pg/106 HaCaT cells) from five separate experiments. *Denotes statistically significant (P<0.05) changes in levels of PAF from control (sham-treated) values; **Denotes statistically significant (P<0.05) changes in levels of PAF in comparison to burn w/o EtOH exposure.
Ethanol activates phospholipase A2 and is incorporated into PAF.
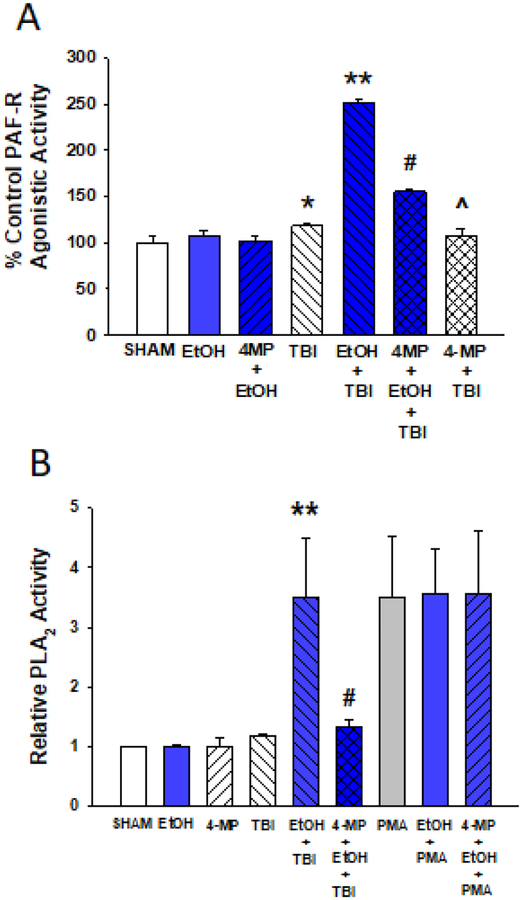
To define the mechanism by which ethanol augments PAF biosynthesis, we tested whether or not the metabolism of ethanol impacts PAF production. Using an alcohol dehydrogenase inhibitor (4-methylpyrazole; 4-MP) we found that 4-MP blocks PAF agonist formation in response to combining ethanol + thermal burn injury, but it had no discernable effects on PAF produced in response to thermal burn injury alone (Figure 3A). This result suggests that ethanol metabolites mediate augmented PAF biosynthesis. Ethanol can be metabolized to acetylCoA, which is the source of the sn-2 acetate in PAF (Shimizu, 2009; Snyder et al., 1996). Therefore, we tested the hypothesis that the excess ethanol provides acetyl-CoA which is incorporated into newly synthesized PAF by incubating HaCaT cells with deuterium-labelled ethanol ([2,2,2-D3]-ethanol (99 atom% D). Mass spectrometry was used to selectively assay the incorporation of the deuterium label into the PAF. Carbamoyl-PAF (CPAF) was used as the internal standard for the quantitation of the deuterium-labeled PAF. As shown in Supplementary Table S2, we were able to identify deuterium-labelled PAF (with three additional mass units). However, the amount of deuterium-labelled PAF was only about 8% of total PAF produced following combined ethanol + thermal burn injury in HaCaT cells. These results indicate that while the metabolism of ethanol enhances PAF production, only a small component of the PAF generated in response to combined ethanol + thermal burn injury is due to incorporation of ethanol-derived acetyl-CoA into PAF.
Figure 3. 4-MP pretreatment blocks augmentation of EtOH on PAF-R agonist formation and PLA2 enzyme activity in response to thermal burn injury in HaCaT cells.
A. PAF-R agonistic activity. HaCaT cells were pretreated with 2 mM 4-MP, or vehicle (SHAM) for 30 min before treatment with 1% EtOH (BLUE) for an additional 30 min. Cells were then subjected to thermal burn injury (TBI), and 5 min following injury the lipids were extracted and normalized to HaCaT cell number, and PAF-R agonistic activity determined as measurement of IL-8 released in KBP cells. The data are the Mean ± SD % control PAF-R agonists measured from IL-8 production in KBP cells (pg/106 KBP cells) from a single experiment representative of four separate experiments. B. PLA2 enzymatic activity. HaCaT cells were treated exactly as in A., except that 10nM PMA was also used. The data are the Mean ± SE PLA2 enzymatic activity normalized to Sham Control values from 4–5 separate experiments. *Denotes statistically significant (P<0.05) changes in levels of % IL-8 production or normalized PLA2 activity in comparison to sham values; ** Denotes statistically significant (P<0.01) changes in comparison to burn injury alone; # Denotes statistically significant (P<0.01) changes in comparison to EtOH + TBI. ^ Denotes not significantly different from TBI.
PLA2 is a key enzyme for PAF biosynthesis in the remodeling pathway (Shimizu, 2009). As ethanol has been reported to activate PLA2 in several model systems (Lee et al., 2004; Tajuddin et al., 2014), we next tested the effects of thermal burn injury alone or in the presence of the ethanol on PLA2 activity in HaCaT cells. As shown in Figure 3B, neither ethanol nor thermal burn injury treatment alone modulated PLA2 activity. However, the combination of thermal burn injury with ethanol resulted in an increased PLA2 enzymatic activity. Of interest, the level of PLA2 enzyme activity in response to thermal burn + ethanol resembled the potent stimulus (Balsinde et al., 1997) phorbol myristate acetate (PMA). Consistent with our studies indicating that 4-MP blocked ethanol augmentation of PAF production, pretreatment with 4-MP blocked the ability of ethanol to augment PLA2 activity induced by thermal burn injury (Fig 3B). Neither ethanol nor 4-MP pretreatment modulated PMA-induced PLA2 activity. Moreover, none of these stimuli affected the activity of the PAF and Ox-GPC metabolizing enzyme PAF-acetylhydrolase in HaCaT cells (Supplementary Fig S4). These results suggest that ethanol metabolism augments thermal burn injury-induced PAF production via PLA2 activation using the PAF remodeling pathway.
Combination of ethanol + thermal burn injury augments PAF generation in both murine and human skin.
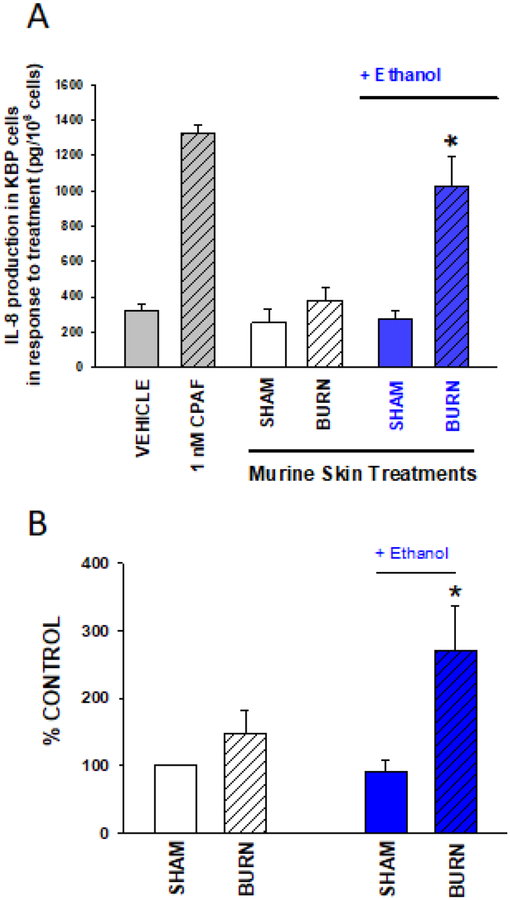
The in vitro results in keratinocytes lead us to determine if an intoxicated burn injury could induce PAF production in intact skin tissue. Initial studies used a previously published murine model of intraperitoneal injection of 400 ul of 20% ethanol (2.4 g/kg) followed 30 minutes later by thermal burn injury to skin (Faunce et al., 1997; Faunce et al., 1998). Anesthetized C57BL/6 mice were treated with saline or ethanol i.p., 30 minutes prior to a thermal burn injury of approximately 12–15% body surface area. Immediately following the injury, lipids were extracted from the injured epidermis and tested for PAF-R agonistic activity using the KBP IL-8 assay (as in Fig 1). Enhanced PAF-R agonist formation was observed in the combined ethanol + burn injury group relative to controls (Figure 4A). To similarly assess if ethanol + burn injury elevates PAF-R agonistic activity in human skin, we treated discarded human skin tissue from body contouring surgeries (e.g., abdominoplasties) with topical 20% ethanolic solution (or control) 1 hour prior to a thermal burn injury using a 90 deg C heated metal block applied for 8 seconds. Similarly to the in vivo skin, the epidermal lipid extracts from the combined ethanol + thermal burn skin augmented PAF-R agonist formation relative to the thermal burn control. Similar to our findings with HaCaT cells (Supplementary Fig S1), lipid extracts from skin specimens did not induce IL-8 in PAF-R-negative KBM cells (data not shown). These studies demonstrate that ethanol augments murine and human epidermal PAF production in response to thermal burn injury.
Figure 4. EtOH exposure augments PAF-R agonistic activity in response to thermal burn injury in murine skin in vivo and human skin ex vivo.
A. Murine skin. The dorsal back skin of groups of 5–6 anesthetized C57BL6 mice were subjected to thermal burn injury (8 second treatment with 90°C heated iron blocks) or sham following a 30 min exposure to i.p. PBS vehicle or 2.4g/kg EtOH (BLUE). Five min following injury treatment to mice, the skin was harvested and lipids were extracted and normalized to 10mg weight of skin. B. Human skin. Human skin abdominoplasty specimens were pre-treated with topical 20% EtOH in PBS (BLUE) or PBS alone and 1h later treated with an 8 sec thermal burn injury or sham control as outlined in A. Five min following thermal burn injury, skin was harvested and lipids extracted and normalized to 25mg weight of skin. PAF-R agonistic activity in both murine and human skin samples was assessed by measurement of IL-8 release in KBP cells as in Fig 1. The data are A. Mean ± SD IL-8 production in KBP cells (pg/106 KBP cells) from 5–6 mice in each group, and B. Mean ± SE % control human skin PAF-R agonistic activity from four separate experiments. *Denotes statistically significant (P <0.05) changes in levels of PAF-R agonist activity from control values w/o EtOH exposure.
PAF-R activation mediates acute systemic inflammation in response to intoxicated burn injury.
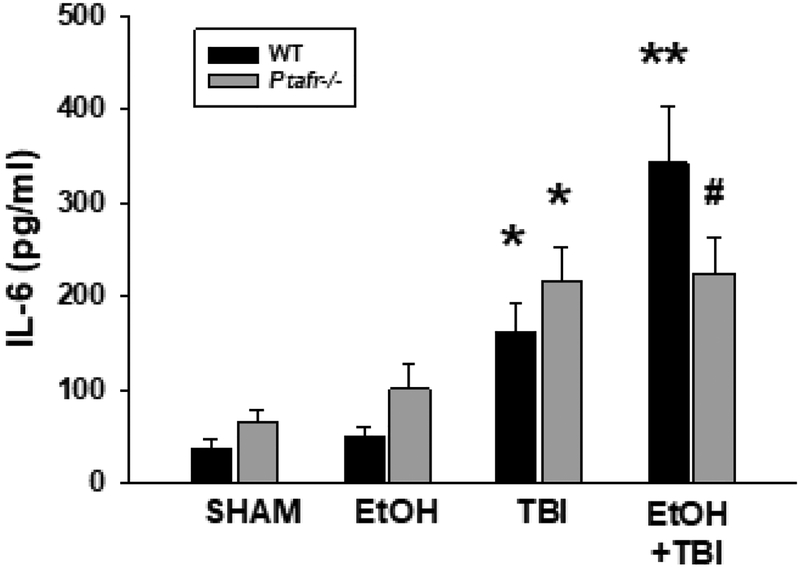
Previous studies have demonstrated that intoxicated thermal burn results in an enhanced systemic inflammatory response (e.g. IL-6 activation), relative to controls (Faunce et al., 1997; Faunce et al., 1998; Faunce et al., 2003; Bird et al., 2008; Li et al., 2011; Chen et al., 2013; Zahs et al., 2013). Given our in vitro and in vivo findings that ethanol + thermal burn injury augments PAF production, coupled with the well characterized role of PAF in mediating inflammatory responses (Shimizu 2009; Stafforini et al., 2003; Zhang et al., 2005), we sought to define the role of the PAF-R in the acute inflammatory response to intoxicated thermal burn injury. Utilizing the wild-type or PAF-R-deficient (Ptafr−/−) mice (Ishii et al., 1998) we found (Figure 5) that ethanol exposure alone did not result in increased serum IL-6 levels in either genotype. Moreover, thermal burn injury increased serum IL-6 levels in both PAF-R-positive and –negative mice, but the combination of ethanol + thermal burn injury triggered increased IL-6 levels above thermal burn injury alone only in PAF-R-expressing mice. Serum was also assayed for 22 other inflammation-related cytokines using cytokine array, which not only confirmed our IL-6 findings, it revealed similar profiles of augmented levels of MIP-β, MCP-1, G-CSF, and TNF-α in combined ethanol + thermal burn injury over thermal burn injury alone selectively in wild-type mice (see Supplemental Table S2). These studies suggest that the acute systemic toxicity associated with murine thermal injury in the setting of ethanol intoxication involves PAF-R activation.
Figure 5. EtOH exposure augments serum IL-6 levels in response to thermal burn injury in a PAF-R-dependent manner.
Wild-type or PAF-R-deficient (Ptafr−/−) mice underwent treatment with either vehicle or EtOH, then 30 min later subjected to TBI or Sham treatment. 14 h later mice were harvested and serum levels of IL-6 measured by ELISA. The data represent Mean ± SD IL-6 from a total of 6–8 mice in each group. *Denotes statistically significant (P<0.05) changes in levels of IL-6 from sham values; ** Denotes statistically significant (P<0.05) changes in comparison to burn injury alone; # Denotes no statistically significant changes in comparison to TBI alone.
PAF-R activation mediates the delayed immunosuppression associated with intoxicated thermal burn injury.
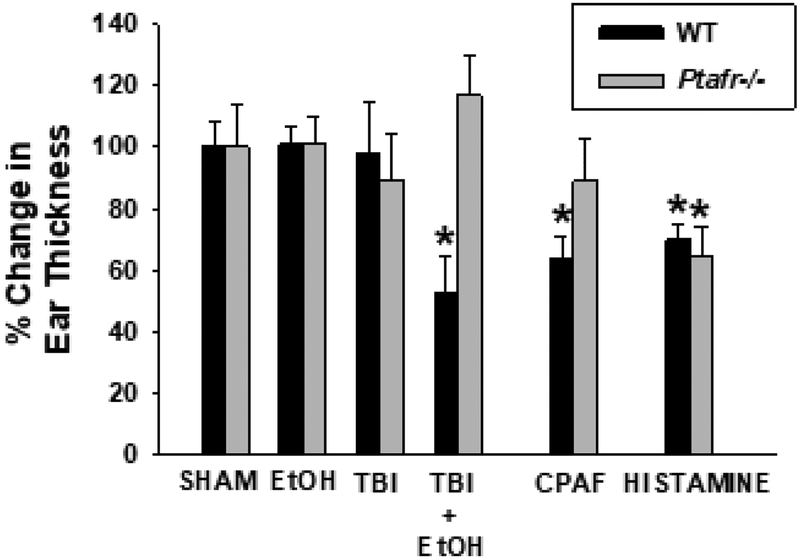
Combining ethanol and localized thermal burn injury in mice results in systemic immunosuppression as shown by decreasing contact hypersensitivity responses to the chemical dinitrofluorobenzene (DNFB) (Faunce et al., 1997; Faunce et al., 1998). Of note, multiple environmental stressors that trigger PAF agonist formation including ultraviolet B radiation (Walterscheid et al., 2002; Marathe et al., 2005), photodynamic therapy (Ferracini et al., 2015), chemotherapy (Sahu et al., 2014) and cigarette smoke (Sahu et al., 2013) all induce systemic immunosuppression in a PAF-R-dependent manner. The next studies were designed to test if the combined ethanol + thermal burn injury immunosuppression is due to the enhanced skin PAF generation from this clinically-relevant combination. To assess the effects of the injury on immune competence, Ptafr−/− and wild-type mice received an intoxicated thermal burn injury or control treatments and were then subjected to the well-established delayed-type hypersensitivity protocol (Zhang et al., 2005; Zhang et al., 2008; Ferracini et al., 2015). Briefly, five days after treatment, the mice were sensitized with the chemical DNFB topically applied to the shaved non-thermal burn-injured part of the upper back (to test for systemic immunosuppression) and challenged 9 days later with DNFB applied to the ears. The intensity of the immune response to DNFB was measured by change in the ear thickness prior to and 24 h after the delayed challenge. Animals were also injected with PAF-R agonist CPAF or histamine as controls for PAF-dependent and independent immunosuppression, respectively. As shown in Figure 6, only the intoxicated burn injury of wild-type mice suppressed the delayed hypersensitivity response, suggesting that intoxicated burn-mediated immuno-suppression is PAF-R dependent.
Figure 6. Thermal burn injury results in enhanced immunosuppression when combined with EtOH in a PAF-R-dependent manner.
Wild-type or PAF-R-deficient (Ptafr−/−) mice underwent treatment with either vehicle or EtOH, then 30 min later subjected to TBI or Sham treatment. Some mice were treated with 250ng CPAF i.p. or 200 μg Histamine s.c. as positive controls for inhibition of contact hypersensitivity reactions to allergen DNFB (systemic immunosuppression). Five days later the mice were sensitized on non-injured back skin with DNFB, and 9 days later ear elicitation reactions to DNFB were obtained. The data are the Mean ± SD % change in ear thickness of the elicitation reactions obtained from sham-treated animals using 7–10 mice in each group. *Denotes significant (p <0.05) differences between value compared to sham control. Please note only Histamine treatment resulted in an inhibition of CHS reactions to DNFB in Ptafr−/− mice.
Discussion
Thermal burn injuries are commonly associated with ethanol intoxication, and the presence of ethanol results in a much poorer prognosis (Choudry et al., 2006; Silver et al., 2008; Grobmyer et al., 1996; Haum et al., 1995; McGill et al., 1995). In particular, the combination of acute ethanol intoxication followed by thermal burn injury in humans exhibit augmented systemic cytokine production, especially IL-6. Of interest, increased serum IL-6 levels are correlated with worse prognosis in patients (Bird et al., 2008; Albright et al., 2012). Murine model systems combining systemic ethanol and thermal burn injury reproduce many of the pathologies seen in humans (Faunce et al., 1997; Faunce et al., 1998; Faunce et al., 2003; Bird et al., 2008; Li et al., 2011). These include the systemic toxicity with inflammation associated with multiple organs (Bird et al., 2008; Li et al., 2011; Chen et al., 2013; Zahs et al., 2013). In addition, murine studies have demonstrated that combining ethanol and localized thermal burn injury results in systemic immunosuppression (Faunce et al., 1997; Faunce et al., 1998). Intoxication by intraperitoneal injection or oral gavage equally potentiates the organ damage and inflammation following thermal burn injury (Chen et al., 2013). The exact mechanism of the acute effects is unclear, though neutralizing antibodies against IL-6 have been demonstrated to be protective (Faunce et al., 1998; Zahs et al., 2013). One potential mechanism for the acute systemic injury of intoxicated burn injury involves facilitating intestinal permeability, causing the gut/liver axis to generate IL-6 (Zahs et al., 2012; Early et al., 2015). It should be noted that PAF has been reported to augment gut permeability (Tan et al., 2000; Akyürek et al., 2005; Keely et al., 2010), potentially providing a direct link between excess skin-generated PAF and systemic toxicity.
The present studies provide evidence that the lipid mediator PAF is involved in both the acute systemic cytokine production as well as the delayed immunosuppressive effects of ethanol + thermal burn injury. PAF production in response to thermal burn injury in a keratinocyte cell line in vitro as well as human skin ex vivo and murine skin in vivo are all increased dramatically in response to short-term incubation with ethanol. Use of PAF-R deficient mice confirm that the increased PAF being generated from skin in response to ethanol + thermal burn injury is involved in the systemic effects of this combination.
Unlike pro-oxidative stressors such as UVB which as our group has reported generates both enzymatic PAF and non-enzymatic oxidized glycerophosphocholines in keratinocytes (Marathe et al., 2005), our mass spectrometry-based structural studies indicate that the vast majority of the combination of ethanol and thermal burn injury-generated PAF is produced enzymatically. PAF is synthesized enzymatically via two separate pathways (Shimizu, 2009). The remodeling pathway which is associated with cellular stimulation from a variety of sources consists of PLA2 followed by an acetyl-CoA-dependent acetyltransferase (LPCAT) that acetylates a lyso-PAF intermediate to form PAF. A second pathway for PAF synthesis involves a de novo pathway, which consists of three separate enzymes: 1-alkyl-2-lyso-sn-glycero-3-phosphate (alkyllyso-GP): acetyl-CoA acetyltransferase, 1-alkyl-2-acetyl-sn-glycero-3-phosphate phosphohydrolase, and 1-alkyl-2-acetyl-sn-glycerol (alkylacetyl-G):CDP-choline cholinephosphotransferase. This de novo pathway appears to be more constitutively active, generating low levels of PAF, though it can be activated by phorbol esters (Shimizu, 2009; Snyder et al., 1996; Snyder, 1997). It should be noted that keratinocytes use the remodeling pathway to synthesize PAF (Travers et al., 1996). Consistent with the notion that ethanol augmentation of PAF biosynthesis involves the remodeling pathway, pretreatment of HaCaT cells with the LPCAT inhibitor TSI-01 at a dose that inhibits PAF production in A23187-stimulated macrophages (Tarui et al., 2014), blocks PAF production in response to ethanol + thermal burn injury (Supplementary Figure S-5). Of interest, TSI-01 exerted only partial inhibitory effects on PAF-R agonist generation in response to treatment with tert-butyl hydroperoxide (Figure S-5), which confirms our previous studies that the majority of PAF-R agonists from this pro-oxidative stressor are due to non-enzymatic processes (Travers, 1999).
The present studies provide evidence that short-term ethanol exposure augments thermal burn injury-induced PAF biosynthesis through several mechanisms. First, ethanol pre-incubation followed by thermal burn injury results in augmented PLA2 enzymatic activity, yet ethanol alone has no effect. These findings suggest involvement of PLA2 and thus the remodeling pathway. The attenuating effects of LPCAT inhibitor TSI-01 also suggest involvement of the remodeling pathway. The second, albeit minor mechanism that appears to be in play is suggested by our deuterated ethanol studies, which demonstrate a small amount of the PAF (~8%) is due to ethanol metabolized to acetyl-CoA. Of interest, keratinocytes do express both alcohol dehydrogenase and aldehyde dehydrogenase enzymes, though at lower amounts (~20%) than hepatocytes (Cheung et al., 1999). Our finding that the alcohol dehydrogenase inhibitor 4-MP blocks the ethanol augmentation of both PAF production and PLA2 activation by thermal burn injury suggests that the effect of ethanol in this system is due to a down-stream metabolite.
Recent studies have implicated PAF agonists in chronic ethanol toxicity. In particular, chronic ethanol administration in rats and mice results in increased levels of oxidized glycerophosphocholines and PAF in plasma (Yang et al., 2010; Liu et al., 2013). Moreover, PAF-R-deficient mice are protected from chronic ethanol-mediated kidney damage (Latchoumycandane et al., 2015). In these chronic ethanol models, non-enzymatic oxidized glycerophosphocholine PAF agonists appear to play important roles, whereas the present acute ethanol + thermal injury model involves enzymatic PAF agonists.
The enhanced toxicity of combining ethanol with thermal burn injury might not be a unique process. Indeed, preclinical studies combining acute radiation + thermal burn injury were found to result in similar findings to ethanol + thermal burn injury (Palmer et al., 2013). Given that radiation is a potent stimulus for PAF production (Sahu et al., 2016), it is possible that the PAF system could also be involved in this process.
Thermal burn injuries are a significant source of morbidity and mortality. Ethanol intoxication at the time of the thermal burn injury is a relatively common occurrence, and is associated with poorer patient outcomes (Choudry et al., 2006; Silver et al., 2008; Grobmyer et al., 1996; Haum et al., 1995; McGill et al., 1995; Davis et al., 2013). The present studies provide a potential mechanism involving the PAF system for many of both the acute pro-inflammatory and delayed immunosuppressive effects associated with combining ethanol and thermal burn injury. These findings could provide the impetus for novel therapies to address the enhanced morbidity and mortality associated with intoxicated burn patients.
Materials and Methods
All chemicals were obtained from Sigma-Aldrich (St. Louis, MO) unless indicated otherwise. HaCaT keratinocyte-derived cell line was grown in DMEM high glucose media with 10% FCS as described (Marathe et al., 2005). PAF-R-negative KB cells were rendered PAF-R-positive (KBP) by transducing the MSCV2.1 retrovirus encoding the human leukocyte PAF-R and PAF-R-deficient (KBM) by transducing with the vector alone and grown in DMEM high glucose media with supplements as described previously (Pei et al., 1998). Cell lines were regularly tested for mycoplasma. HaCaT cells were grown to approximately 80–90% confluence in 10 cm dishes, and washed three times with Hanks Balanced Salt Solution (HBSS) and then incubated with 2 ml of pre-warmed (37 °C) HBSS with 10mg/ml fatty acid-free BSA with/without 1% ethanol. After 30 min, the cells were treated by placement onto a 90 °C water bath for two minutes. In some experiments, 100 μM 4-MP was preincubated for 30 min before addition of ethanol +/− thermal burn injury. The incubations were quenched by addition of 2 ml of ice-cold methanol followed by methylene chloride, and lipids extracted as described (Marathe et al., 2005; Yao et al., 2012).
Mice
Female C57BL/6-wild type mice (PAF-R expressing; age 6–8 week) were purchased from The Charles River Laboratories. Age-matched female PAFR-deficient (Ptafr−/−) mice on a C57BL/6 background, generated as described (Isshi et al., 1998), were a kind gift of Professor Takao Shimizu (Department of Biochemistry, University of Tokyo). All mice were housed under specific pathogen-free conditions and all procedures were approved by the Institutional Animal Care and Use Committees of Indiana University School of Medicine and Wright State University.
Calcium mobilization studies
The presence of systemic PAF-R agonists in lipid extracts derived from HaCaT cells was assessed by the ability of lipid extracts to induce an intracellular Ca2+ mobilization response in FURA-2 AM-loaded PAF-R-expressing KBP cells, but not in PAFR-deficient KBM cells as described (Ferracini et al., 2015; Pei et al., 1998; Sahu et al., 2014). PAF-R agonistic activity in lipid extracts was quantified by measuring IL-8 released into the supernatants of KBP vs KBM cells as reported (Ferracini et al., 2015; Sahu et al., 2014).
Mass spectrometry studies
Mass spectrometry was performed on HaCaT cell samples using the AB Sciex (Foster City, CA) triple quadrupole QTRAP® 5500 mass spectrometer, equipped with a CTC-PAL autosampler and a Shimadzu HPLC as described (Yao et al, 2012; Sahu et al., 2014). In some experiments D3 Ethanol (>99% purity) from Sigma Aldrich was used instead of ethanol.
PLA2/PAF-AH enzyme assays.
PLA2 and PAF-AH enzyme activity was measured in HaCaT cells using specific assays (Cayman Chemical, Inc) exactly as per manufacturer’s recommendations.
Thermal burn injury in murine and human skin
All procedures involving mice were approved by the Animal Care and Use Committees of Indiana University School of Medicine and Wright State University. Wild-type or Ptafr−/− C57BL/6J mice were anesthetized with ketamine/xylazine (100 and 10mg/kg, respectively) and fur removed from dorsal back skin by shaving with clippers. The mice were given 0.4ml of 20% ethanol (2.4g/kg) in distilled water or water alone. Thirty minutes later, the dorsal skin of the mice were treated with 8 second exposure of stainless steel metal heated to 90 °C. Mice were then given 1 ml of normal saline i.p. and buprimorphine i.m. To measure PAF-R agonists, 5 min post thermal burn injury burned skin from wild-type mice was treated with liquid nitrogen using a cryo-spray (Brymill, Inc., Ellington, CT). The epidermal skin was curetted and the contents weighed before lipid extraction. In experiments measuring acute serum cytokines or contact hypersensitivity studies, the mice were allowed to awaken. In some experiments the mice were euthanized at 14h post injury and blood serum isolated and assayed for IL-6 by ELISA (R &D, Minneapolis, MN). In some experiments multiple cytokines were assayed using a multiplex system (BioRad). In other experiments, contact hypersensitivity studies were performed using dinitrofluorobenzene (DNFB) as previously described (Zhang et al., 2008; Sahu et al., 2013). Briefly, mice were sensitized to 50 μl of DNFB on dorsal back skin at least 2 cm away from the burn site, five days post thermal burn injury. Nine days later the mice were anesthetized and ear thickness measured using Mitutuyo calipers. 20 μl DNFB was applied to one ear and 4:1 acetone: olive oil applied to one ear. 24 h later the mice were anesthetized and ear thickness re-assessed. In some experiments mice were not sensitized to DNFB yet treated with DNFB on ears as control.
Human skin tissue
De-identified skin was obtained from contouring surgeries (abdominoplasties and brachiplasties) (Travers et al., 2010; Fahy et al., 2017). Skin was washed and fat trimmed, and placed at 37 °C in PBS. Skin was treated with 20% ethanol in PBS, or PBS alone (100 μl per 2 × 2 cm area). After one hour incubation, skin was treated with thermal burn injury with heated metal for 10 seconds as described for the murine studies. After 5 min, skin was treated with liquid nitrogen with a Cryac, and epidermis curetted and weighed. The lipids were extracted (and normalized to 25 mg wet tissue) and PAF-R agonists quantified using IL-8 release in KBP vs KBM cells (Ferracini et al., 2015; Pei et al., 1998; Sahu et al., 2014).
Statistics
All statistical calculations were performed using Prism 6. Statistical significance was determined between individual groups using student’s T-test, with significance listed as p <0.05.
Supplementary Material
Acknowledgements
This research was supported in part by grants from the National Institutes of Health grant R01 HL062996 (JBT), K22 ES023850 (RPS), Veteran’s Administration Merit Award 5I01BX000853 (JBT), and JW was supported by T32 AR062495.
References cited
- Akyürek N, Salman B, Irkörücü O, Tezcaner T, Azili C, Erdem O, et al. The effect of platelet activating factor antagonist BN 52021 on bacterial translocation and ICAM-I expression in experimental obstructive jaundice. J Invest Surg 2005;8:247–56. [DOI] [PubMed] [Google Scholar]
- Alappatt C, Johnson CA, Clay KL, Travers JB. Acute keratinocyte damage stimulates platelet-activating factor production. Arch Dermatol Res 2000;292:256–9. [DOI] [PubMed] [Google Scholar]
- Albright JM, Davis CS, Bird MD, Ramirez L, Kim H, Burnham EL, et al. The acute pulmonary inflammatory response to the graded severity of smoke inhalation injury. Crit Care Med 2012;40:1113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsinde J, Balboa MA, Insel PA, Dennis EA. Differential regulation of phospholipase D and phospholipase A2 by protein kinase C in P388D1 macrophages. Biochem J 1997;321:805–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird MD, Kovacs EJ. Organ-specific inflammation following acute ethanol and burn injury. J Leuk Biol 2008;84:607–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol 1988;106:761–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MM, Bird MD, Zahs A, Deburghgraeve C, Posnik B, Davis C, et al. Pulmonary inflammation after ethanol exposure and burn injury is attenuated in the absence of IL-6. Alcohol 2013;47:223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MM, Palmer JL, Ippolito JA, Choudhry MA, Kovacs EJ. Intoxication by intraperitoneal injection or oral gavage equally potentiates postburn organ damage and inflammation. Mediat Inflamm 2013;2013:971481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung C, Smith CK, Hoog J-O, Hotchkiss SAM. Expression and localization of human alcohol and aldehyde dehydrogenase enzymes in skin. Biochem Biophys Res Comm 1999;261:100–7. [DOI] [PubMed] [Google Scholar]
- Choudhry MA, Chaudry IH. Alcohol intoxication and post-burn complications. Front in Bioscience 2006;11:998–1005. [DOI] [PubMed] [Google Scholar]
- Davis CS, Esposito TJ, Palladino-Davis AG, Rychlik K, Schermer CR, Gamelli RL, et al. Implications of alcohol intoxication at the time of burn and smoke inhalation injury: an epidemiologic and clinical analysis. J Burn Care & Res 2013; 34:120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley ZM, Akhtar S, Green SJ, Naqib A, Khan O, Cannon AR, et al. Burn injury alters the intestinal microbiome and increases gut permeability and bacterial translocation. PLoS ONE 2015;10:e0129996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahy K, Liu L, Rapp CM, Borchers C, Bihl JC, Chen Y, et al. UVB-generated microvesicle particles: A novel pathway by which a skin-specific stimulus could exert systemic effects. Photochem Photobiol 2017;93:937–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faunce DE, Gregory MS, Kovacs EJ. Effects of acute ethanol exposure on cellular immune responses in a murine model of thermal injury. J Leuk Biol 1997;62:733–40. [DOI] [PubMed] [Google Scholar]
- Faunce DE, Gregory MS, Kovacs EJ. Acute ethanol exposure prior to thermal injury results in decreased T-cell responses mediated in part by increased production of IL-6. Shock 1998;10:135–40. [DOI] [PubMed] [Google Scholar]
- Faunce DE, Garner JL, Llanas JN, Patel PJ, Gregory MS, Duffner LA, et al. Effect of acute ethanol exposure on the dermal inflammatory response after burn injury. Alcoholism: Clin Exp Res. 2003;27:1199–1206. [DOI] [PubMed] [Google Scholar]
- Ferracini M, Sahu RP, Harrison KA, Waeiss RA, Murphy RC, Jancar S, et al. Topical photodynamic therapy induces systemic immunosuppression via generation of platelet-activating factor agonists. J Invest Dermatol 2015;135:321–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann PD. Clinical practice. Alcohol use in adults. New Engl J Med 2013;368: 365–73. [DOI] [PubMed] [Google Scholar]
- Grobmyer SR, Maniscalco SP, Purdue GF, Hunt JL. Alcohol, drug intoxication, or both at the time of burn injury as a predictor of complications and mortality in hospitalized patients with burns. J Burn Care Rehabil 1996;17:532–9. [DOI] [PubMed] [Google Scholar]
- Haum A, Perbix W, Hack HJ, Stark GB, Spilker G, Doehn M. Alcohol and drug abuse in burn injuries. Burns 1995; 21:194–9. [DOI] [PubMed] [Google Scholar]
- Howland J, Hingson R. Alcohol as a risk factor for injuries or death due to fires and burns: review of the literature. Pub Health Rep 1987;102:475–83. [PMC free article] [PubMed] [Google Scholar]
- Ishii S, Kuwaki T, Nagase T, Maki K, Tashiro F, Sunaga S, et al. Impaired anaphylactic responses with intact sensitivity to endotoxin in mice lacking a platelet-activating factor receptor. J Exp Med 1998;187:1779–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keely S, Glover LE, Weissmueller T, MacManus CF, Fillon S, Fennimore B, et al. Hypoxia-inducible factor-dependent regulation of platelet-activating factor receptor as a route for gram-positive bacterial translocation across epithelia. Mol Biol Cell 2010;21:538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchoumycandane C, Nagy LE, McIntyre TM. Myeloperoxidase formation of PAF receptor ligands Induces PAF receptor-dependent kidney injury during ethanol consumption. Free Radic Biol Med 2015;86:179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Lee JH, Han HJ. Ethanol-inhibited [3H]thymidine incorporation via protein kinase C-p44/42 mitogen-activated protein kinase/phospholipase A2 signal pathway in renal proximal tubule cells. Alcoholism: Clin Exp Res 2004;28:1172–9. [DOI] [PubMed] [Google Scholar]
- Li X, Akhtar S, Kovacs EJ, Gamelli RL, Choudhry MA. Inflammatory response in multiple organs in a mouse model of acute alcohol intoxication and burn injury. J Burn Care & Res 2011;32:489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Li W, Chen R, McIntyre TM. Circulating biologically active oxidized phospholipids show on-going and increased oxidative stress in older male mice. Redox Biol 2013;1:110–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magai RM, Shukla SD. Metabolic Fate of [14C]-Ethanol into Endothelial Cell Phospholipids Including Platelet-Activating Factor, Sphingomyelin and Phosphatidylethanol. J Biomed Sci 2001;8:143–50. [DOI] [PubMed] [Google Scholar]
- Marathe GK, Prescott SM, Zimmerman GA, McIntyre TM. Oxidized LDL contains inflammatory PAF-like phospholipids. Trends Cardiovasc Med 2001;11:139–42. [DOI] [PubMed] [Google Scholar]
- Marathe GK, Johnson C, Billings SD, Southall MD, Pei Y, Spandau D, et al. Ultraviolet B radiation generates platelet-activating factor-like phospholipids underlying cutaneous damage. J Biol Chem 2005;280:35448–57. [DOI] [PubMed] [Google Scholar]
- McGill V, Kowal-Vern A, Fisher SG, Kahn S, Gamelli RL. The impact of substance use on mortality and morbidity from thermal injury. J Trauma 1995;38:931–4. [DOI] [PubMed] [Google Scholar]
- Pałgan K, Bartuzi Z. Platelet activating factor in allergies. Int J Immunopathol Pharmacol 2015;28:584–9. [DOI] [PubMed] [Google Scholar]
- Palmer JL, Deburghgraeve CR, Bird MD, Hauer-Jensen M, Chen MM, Yong S, et al. Combined radiation and burn injury results in exaggerated early pulmonary inflammation. Radiat Res 2013;180:276–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y, Barber LA, Murphy RC, Johnson CA, Kelley S, Williams DA et al. Activation of the epidermal platelet-activating factor receptor results in cytokine and cyclooxygenase-2 biosynthesis. J Immunol 1998;161:1954–61. [PubMed] [Google Scholar]
- Sahu RP, Harrison KA, Weyerbacher J, Murphy RC, Konger RL, Garrett JE, et al. Radiation therapy generates immunosuppressive Platelet-activating Factor agonists. Oncotarget 2016;7:20788–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu RP, Petrache I, Van Demark MJ, Rashid BM, Ocana JA, Tang Y, et al. Cigarette smoke exposure inhibits contact hypersensitivity via the generation of platelet-activating factor agonists. J Immunol 2013;190:2447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu RP, Ocana JA, Harrison KA, Ferracini M, Touloukian CE, Al-Hassani M, et al. Chemotherapeutic agents subvert tumor immunity by generating Platelet-activating Factor agonists. Cancer Res 2014;74:7069–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T Lipid mediators in health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu Rev Pharmacol Toxicol 2009;49:123–50. [DOI] [PubMed] [Google Scholar]
- Silver GM, Albright JM, Schermer CR, Halerz M, Conrad P, Ackerman PD, et al. Adverse clinical outcomes associated with elevated blood alcohol levels at the time of burn injury. J Burn Care & Res 2008;29:784–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder F, Fitzgerald V, Blank ML. Biosynthesis of platelet-activating factor and enzyme inhibitors. Adv Exp Med & Biol 1996;416:5–10. [DOI] [PubMed] [Google Scholar]
- Snyder F CDP-choline:alkylacetylglycerol cholinephosphotransferase catalyzes the final step in the de novo synthesis of platelet-activating factor. Biochim. Biophys. Acta 1997;1348:111–6. [DOI] [PubMed] [Google Scholar]
- Stafforini DM, McIntyre TM, Zimmerman GA, Prescott SM. Platelet-activating factor, a pleiotrophic mediator of physiological and pathological processes. Crit Rev Clin Lab Sci 2003;40:643–72. [DOI] [PubMed] [Google Scholar]
- Tajuddin N, Moon KH, Marshall SA, Nixon K, Neafsey EJ, Kim HY, et al. Neuroinflammation and neurodegeneration in adult rat brain from binge ethanol exposure: abrogation by docosahexaenoic acid. PLoS ONE 2014;9:e101223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X-D, Chang H, Qu X-W, Caplan M, Gonzalez-Crussi F, Hsueh W. Platelet-activating factor increases mucosal permeability in rat intestine via tyrosine phosphorylation of E-cadherin. Br J Pharmacol 2000;129:1522–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarui M, Shindou H, Kumagai K, Morimoto R, Harayama T, Hashidate T, et al. Selective inhibitors of a PAF biosynthetic enzyme lysophosphatidylcholine acyltransferase 2. J Lipid Res 2014;55:1386–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers JB, Harrison KA, Johnson CA, Clay KL, Morelli JG, Murphy RC. Platelet-activating Factor biosynthesis induced by various stimuli in human HaCaT keratinocytes. J Invest Dermatol 1996;107:88–94. [DOI] [PubMed] [Google Scholar]
- Travers JB. Evidence that reactive oxygen species can signal through the epidermal platelet-activating factor receptor. J Invest Dermatol 1999;112:279–83. [DOI] [PubMed] [Google Scholar]
- Travers JB, Kozman A, Mousdicas N, Saha C, Landis M, Al-Hassani M, et al. Infected atopic dermatitis lesions contain pharmacologic amounts of lipoteichoic acid. J Allergy Clin Immunol 2010;125:146–52.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walterscheid JP, Ullrich SE, Nghiem DX. Platelet-activating factor, a molecular sensor for cellular damage, activates systemic immune suppression. J Exp Med. 2002;195:171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf P, Nghiem DX, Walterscheid JP, Byrne S, Matsumura Y, Matsumura Y, et al. Platelet-activating factor is crucial in psoralen and ultraviolet A-induced immune suppression, inflammation, and apoptosis. Am J Pathol 2006;169:795–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Latchoumycandane C, McMullen MR, Pratt BT, Zhang R, Papouchado BG, et al. Chronic alcohol exposure increases circulating bioactive oxidized phospholipids. J Biol Chem 2010;285:22211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Harrison KA, Al-Hassani M, Murphy RC, Rezania S, Konger RL, et al. Platelet-activating factor agonists mediate Xeroderma Pigmentosum A photosensitivity. J Biol Chem 2012;287:9311–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahs A, Bird MD, Ramirez L, Turner JR, Choudhry MA, Kovacs EJ. Inhibition of long myosin light-chain kinase activation alleviates intestinal damage after binge ethanol exposure and burn injury. Am J Physiol Gastrointest Liver Physiol 2012; 303:G705–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahs A, Bird MD, Ramirez L, Choudry MA, Kovacs EJ. Anti-IL-6 antibody treatment but not IL-6 knockout improves intestinal barrier function and reduces inflammation after binge ethanol exposure and burn injury. Shock 2013;39: 373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Mousdicas N, Yi Q, Al-Hassani M, Billings SD, Perkins SM, et al. Staphylococcal lipoteichoic acid inhibits delayed-type hypersensitivity reactions via the platelet-activating factor receptor. J Clin Invest 2005;115:2855–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Yao Y, Konger RL, Sinn AL, Cai S, Pollok KE, Travers JB. UVB radiation-mediated inhibition of contact hypersensitivity reactions is dependent on the platelet-activating factor system. J Invest Dermatol 2008;128:1780–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.