Abstract
Background
Increased uptake of robotic surgery has led to interest in learning curves for robot‐assisted procedures. Learning curves, however, are often poorly defined. This systematic review was conducted to identify the available evidence investigating surgeon learning curves in robot‐assisted surgery.
Methods
MEDLINE, Embase and the Cochrane Library were searched in February 2018, in accordance with PRISMA guidelines, alongside hand searches of key congresses and existing reviews. Eligible articles were those assessing learning curves associated with robot‐assisted surgery in patients.
Results
Searches identified 2316 records, of which 68 met the eligibility criteria, reporting on 68 unique studies. Of these, 49 assessed learning curves based on patient data across ten surgical specialties. All 49 were observational, largely single‐arm (35 of 49, 71 per cent) and included few surgeons. Learning curves exhibited substantial heterogeneity, varying between procedures, studies and metrics. Standards of reporting were generally poor, with only 17 of 49 (35 per cent) quantifying previous experience. Methods used to assess the learning curve were heterogeneous, often lacking statistical validation and using ambiguous terminology.
Conclusion
Learning curve estimates were subject to considerable uncertainty. Robust evidence was lacking, owing to limitations in study design, frequent reporting gaps and substantial heterogeneity in the methods used to assess learning curves. The opportunity remains for the establishment of optimal quantitative methods for the assessment of learning curves, to inform surgical training programmes and improve patient outcomes.
A broad systematic literature review was performed to characterize the current evidence base and appraise the methods used to measure and define learning curves for surgeons performing robot‐assisted surgery, taking a holistic, panspecialty view. The learning curve estimates identified are subject to considerable uncertainty, and robust evidence was often lacking due to limitations in study design and frequent reporting gaps. Thus, the opportunity remains for the establishment of optimal quantitative methods for the assessment of learning curves, which may inform surgical training programmes and improve patient outcomes.

Little consistency between studies
Antecedentes
La aceptación creciente de la cirugía robótica ha generado interés en las curvas de aprendizaje para los procedimientos asistidos por robot. Sin embargo, las curvas de aprendizaje a menudo están mal definidas. Esta revisión sistemática se realizó para identificar la evidencia disponible en relación a las curvas de aprendizaje del cirujano en la cirugía asistida por robot.
Métodos
En Febrero de 2018, se realizaron búsquedas en MEDLINE, Embase y Cochrane Library, de acuerdo con las recomendaciones PRISMA, junto con búsquedas manuales de congresos clave y de revisiones ya existentes. Los artículos elegibles fueron aquellos que evaluaron las curvas de aprendizaje asociadas con la cirugía asistida por robot efectuada en pacientes.
Resultados
Las búsquedas bibliográficas identificaron 2.316 registros de los cuales 68 cumplían los criterios de elegibilidad y correspondían a 68 estudios primarios. De estos 68 estudios, 49 evaluaron las curvas de aprendizaje basadas en datos de pacientes de 10 especialidades quirúrgicas. Los 49 estudios eran todos estudios observacionales, en su mayoría de un solo brazo (35/49 (71%)) e incluían pocos cirujanos. Las curvas de aprendizaje mostraban una notable heterogeneidad, variando entre procedimientos, estudios y parámetros analizados. Los estándares de presentación de informes fueron generalmente deficientes, con solo 17/49 (35%) cuantificando la experiencia previa. Los métodos utilizados para evaluar la curva de aprendizaje fueron heterogéneos, a menudo carecían de validación estadística y usaban terminología ambigua.
Conclusión
Las estimaciones de la curva de aprendizaje estaban sujetas a una considerable incertidumbre, careciendo de evidencia robusta por las limitaciones en el diseño del estudio, lagunas de información en los artículos y heterogeneidad sustancial en los métodos utilizados para evaluar las curvas de aprendizaje. Queda pendiente establecer métodos cuantitativos óptimos para evaluar las curvas de aprendizaje, informar de los programas de formación quirúrgica y mejorar los resultados del paciente.
Introduction
Learning curves describe the rate of progress in gaining experience or new skills and are widely reported in surgery. Surgeons typically exhibit improvements in performance over time, often followed by a plateau where minimal/limited additional improvement is observed1. Generally, surgical learning curves are measured as a change in an operative variable (which can be considered a surrogate for surgeon performance) over a series of procedures. Studies investigating learning curves for surgical procedures are becoming increasingly important, as learning curves can have substantial impact on surgical metrics, clinical outcomes and cost–benefit decisions.
There has been particular interest in learning curves in robot‐assisted surgery, especially in gynaecology and urology2, 3. Despite the reported operative benefits and improved hospital experience provided by robot‐assisted surgery compared with traditional minimally invasive approaches4, 5, uptake of robotic technology has been slow, largely due to high capital and maintenance costs, and uncertainty regarding the potential benefits of robot‐assisted approaches over conventional laparoscopic approaches. For example, robot‐assisted approaches have been associated with longer operating times for many procedure types6. A large proportion of these comparative studies, however, may have been generated from surgeons who were still learning the robotic technology in question4, potentially underestimating the full benefits of robotic assistance. Robot‐assisted approaches have the potential to expedite surgeon learning, but methods used to measure and define learning curves seem inconsistent1. Studies evaluating the learning curve for surgical procedures often aim to determine the number of sequential procedures that comprise the learning curve, or that are required to ‘overcome’ the learning curve (sometimes referred to as the learning curve length). To achieve this aim, studies often define a particular threshold in surgeon performance. A common threshold includes reaching a plateau in performance, yet the performance thresholds used are highly inconsistent1. The way learning curves are described can lead to misinterpretation. Terms such as ‘overcome’, for instance, could be considered misnomers, implying surgeons have mastered a procedure, for which certain performance thresholds may not provide sufficient evidence. For example, a plateau in performance does not necessarily equate to high‐quality performance; it only implies that a surgeon is no longer improving1.
There remains a need to understand better the learning curve of robot‐assisted surgery and broadly characterize how learning curves are defined and reported. This systematic review was performed to characterize the current evidence base and appraise the methods used to define and measure learning curves for surgeons performing robot‐assisted surgery, taking a holistic, panspecialty view.
Methods
This systematic review was conducted in accordance with a prespecified protocol and the PRISMA guidelines7.
MEDLINE, MEDLINE In‐Process, Embase, the Cochrane Database of Systematic Reviews, the Cochrane Central Register of Controlled Trials, and the NHS Economic Evaluation Database were searched. Surgical training has evolved alongside the rapid development of robotic technologies. As such, database searches were limited to the period from 1 January 2012 to 5 February 2018, in order to capture studies investigating learning curves in the context of training relevant to current practice. The search terms used are provided in Tables S1 and S2 (supporting information). The two most recent abstract books of relevant surgical congresses were also searched from 1 January 2016 to 14 February 2018. This review considered only primary research, and excluded review articles. Supplementary hand searches of the bibliographies of relevant systematic reviews were conducted to identify any primary studies not identified elsewhere.
The review process was performed by two independent reviewers, who assessed the titles and abstracts of all search results (stage 1), as well as the full texts of all potentially eligible studies identified in the first stage (stage 2). In the event of discrepancies, the two reviewers came to a consensus for each decision. In the absence of a consensus, a third independent reviewer resolved any disagreements.
Eligible publications included any randomized or non‐randomized, comparative or observational studies involving wet‐ or dry‐lab testing, simulations, patients or registry/economic analyses that performed a learning curve analysis (consisting of a graph and/or reported data for at least 4 time points) of surgeons performing robot‐assisted surgery in any specialty. Studies were required to report learning curve results from more than one surgeon alone or as part of a surgical team, of any specialization (robot‐assisted, laparoscopic or open). In the absence of reporting the number of surgeons involved, included studies were required to have multiple authors. Only studies that included at least 20 surgical procedures in the analysis of the learning curve were considered. At least one of the following metrics had to be reported: time to plateau/number of ‘phases’ in the learning curve; statistical differences in metrics assessed over time; or learning percentages. Detailed eligibility criteria are shown in Table S3 (supporting information). The studies reported in this review are restricted to learning curve analyses of procedures on patients.
Data extraction and quality assessment
For each eligible study, data were extracted into a prespecified grid by one reviewer, with verification by a second, independent reviewer. Where there was a discrepancy, the two reviewers attempted to come to a consensus; a third reviewer resolved any disagreements in the absence of a consensus.
Captured data included study design, methodology, surgeon experience, robotic technology used and the metric measured to evaluate the learning curve. Information relating to the learning curve itself was captured, including the number of phases of the curve, the number of operations per phase and the number of procedures to overcome the learning curve (denoted in this review as the point where the chosen performance threshold was considered to have been overcome). Where reported, the specific performance threshold used was captured. If the learning curve had not been overcome within the study period, the number of procedures to overcome the learning curve was reported to be greater than the total number included in the study period.
The quality of each eligible study was assessed using either the UK National Institute for Health and Care Excellence (NICE) RCT checklist8 or a modified version of the Downs and Black checklist for non‐randomized studies9.
Results
A total of 2316 records from electronic database searches, conference abstract searches and hand searches were identified. Of these, 281 full‐text articles were assessed for eligibility, of which 213 were excluded (Table S4 , supporting information). The remaining 68 records (reporting on 68 unique studies) were found to meet the eligibility criteria, 49 of which reported on patient data and are presented here (Fig. 1).
Figure 1.

PRISMA diagram for the systematic literature review
Characteristics of included articles
Characteristics of the 49 eligible studies presenting learning curves derived from patient procedures are presented in Table 1 10–58. All were observational in design. Data were analysed retrospectively in 40 of 49 studies (82 per cent), and the remaining nine studies (18 per cent) were prospective in design. The majority (35 of 49, 71 per cent) were single‐arm studies, and the remainder (14 of 49, 29 per cent) were comparative. Among the 41 studies that explicitly defined the number of robotic surgeons per study, the number of participating robotic surgeons was generally small. Most studies (33 of 41, 80 per cent) included fewer than five robotic surgeons, over half of which (18 of 33, 55 per cent) included fewer than three (Fig. 2). The captured studies spanned ten surgical specialties (Fig. 3). Learning curves were reported most frequently for urology, general surgery and gynaecology.
Table 1.
Details of studies included in the systematic literature review
| Reference | Design | Surgical specialty | Procedure(s)/task(s) performed | Study arms (surgeon experience)* | No. of robotic surgeons |
|---|---|---|---|---|---|
| Albergotti et al.10 | Retrospective single‐arm observational | ORL | TORS | Single arm | 3 |
| Arora et al.11 | Retrospective single‐arm observational | Urology | RKT | Single arm | n.r. |
| Benizri et al.12 | Retrospective controlled observational | General | RDP versus LDP | RDP | 2 |
| Bindal et al.13 | Retrospective single‐arm observational | Bariatric | LRRYGB and TRRYGB | Single arm | 2 |
| Binet et al.14 | Retrospective single‐arm observational | Paediatric | Robot‐assisted fundoplications | Single arm | 2 |
| Boone et al.15 | Retrospective single‐arm observational | General | RPD | Single arm | 4 |
| Chang et al.16 | Retrospective controlled observational | Urology | RARP | RARP (open experience) | 1 |
| RARP (open/laparoscopic experience) | 1 | ||||
| RARP (laparoscopic experience) | 1 | ||||
| Ciabatti et al.17 | Prospective single‐arm observational | ORL | Transaxillary robot‐assisted thyroid surgery | Single arm | n.r. |
| D'Annibale et al.18 | Retrospective single‐arm observational | Urology | Robot‐assisted adrenalectomy | Single arm | n.r. |
| Davis et al.19 | Retrospective controlled observational | Urology | RARP versus ORP | RARP | 744 |
| Dhir et al.20 | Retrospective single‐arm observational | General | Robot‐assisted HAI pump placement | Single arm | n.r. |
| Esposito et al.21 | Retrospective single‐arm observational | Paediatric | REVUR | Single arm | 4 |
| Fahim et al.22 | Retrospective single‐arm observational | Thoracic | RATS | Single arm | 3 |
| Fossati et al.23 | Retrospective single‐arm observational | Urology | RARP | Single arm | 4 |
| Geller et al.24 | Retrospective single‐arm observational | Gynaecology | RSC | Single arm | 2 |
| Good et al.25 | Retrospective controlled observational | Urology | RARP versus LRP | RARP | 1 |
| Goodman et al.26 | Retrospective single‐arm observational | Cardiovascular | Robot‐assisted mitral valve repair | Single arm | 2 |
| Guend et al.27 | Retrospective single‐arm observational | Colorectal | Robotic colorectal resection | Single arm | 4 |
| Kamel et al.28 | Retrospective single‐arm observational | Thoracic | RAT | Single arm | 4 |
| Kim et al.29 | Retrospective controlled observational | Colorectal | RRS | RRS (laparoscopically inexperienced) | 1 |
| RRS (laparoscopically experienced) | 1 | ||||
| Lebeau et al.30 | Prospective controlled observational | Urology | RARP | RARP (expert laparoscopic surgeons) | 1 |
| RARP (junior surgeons) | 1 | ||||
| Linder et al.31 | Retrospective single‐arm observational | Gynaecology | RSC | Single arm | 2 |
| Lopez et al.32 | Retrospective controlled observational | Gynaecology | Robot‐assisted single‐site laparoscopic hysterectomy versus LESS hysterectomy | Robot‐assisted single‐site laparoscopic hysterectomy | 3 |
| Lovegrove et al.33 | Prospective single‐arm observational | Urology | RARP | Single arm | 15 |
| Luciano et al.34 | Retrospective controlled observational | Gynaecology | Robot‐assisted versus laparoscopic versus vaginal versus abdominal hysterectomy | Robot‐assisted hysterectomy | 1315 |
| Meyer et al.35 | Retrospective single‐arm observational | Thoracic | Robotic lobectomy | Single arm | 2 |
| Myers et al.36 | Retrospective single‐arm observational | Gynaecology | RSC | Single arm | 2 |
| Nelson et al.37 | Retrospective single‐arm observational | General | RC | Single arm | 8 |
| Odermatt et al.38 | Retrospective controlled observational | Colorectal | Robot‐assisted TME versus laparoscopic TME | Robot‐assisted TME | 2 |
| Park et al.39 | Prospective single‐arm observational | General | Less than total robot‐assisted thyroidectomy | Single arm | 2 |
| Park et al.40 | Retrospective single‐arm observational | General | RAG | Single arm | 3 |
| Paulucci et al.41 | Retrospective single‐arm observational | Urology | RAPN | Single arm | 2 |
| Pietrabissa et al.42 | Prospective single‐arm observational | General | SSRC | Single arm | 5 |
| Pulliam et al.43 | Retrospective controlled observational | Gynaecology | RSC versus LSC | RSC | 3 |
| Riikonen et al.44 | Retrospective single‐arm observational | Urology | RALP | Single arm | 12 |
| Sarkaria et al.45 | Retrospective single‐arm observational | General | RA‐GPEHR | Single arm | n.r. |
| Schatlo et al.46 | Retrospective single‐arm observational | Orthopaedic | Robot‐assisted placement of pedicle screws | Single arm | 13 |
| Shakir et al.47 | Retrospective single‐arm observational | General | RDP | Single arm | 3 |
| Sivaraman et al.48 | Retrospective controlled observational | Urology | RARP versus LRP | RARP | 9 |
| Sood et al.49 | Prospective controlled observational | Urology | RKT | RKT (extensive RKT experience, limited OKT experience) | 1 |
| RKT (extensive RKT and OKT experience) | 1 | ||||
| RKT (limited RKT experience, extensive OKT experience) | 1 | ||||
| Tasian et al.50 | Prospective controlled observational | Urology | Robot‐assisted pyeloplasty | Robot‐assisted pyeloplasty (fellow surgeon) | 4 |
| Robot‐assisted pyeloplasty (attending surgeon) | 1 | ||||
| Tobis et al.51 | Retrospective single‐arm observational | Urology | RAPN | Single arm | 3 |
| van der Poel et al.52 | Retrospective single‐arm observational | Urology | LND during RARP | Single arm | 2 |
| Vidovszky et al.53 | Prospective single‐arm observational | General | SSRC | Single arm | n.r. |
| White et al.54 | Prospective single‐arm observational | ORL | TORS | Single arm | n.r. |
| Woelk et al.55 | Retrospective single‐arm observational | Gynaecology | Robot‐assisted hysterectomy | Single arm | 2 |
| Wolanski et al.56 | Retrospective controlled observational | Urology | RALP versus LRP | RALP | 2 |
| Zhou et al.57 | Retrospective single‐arm observational | General | RAG | Single arm | 2 |
| Zureikat et al.58 | Retrospective single‐arm observational | General | Robot‐assisted pancreatic resections | Single arm | n.r. |
Surgeon experience only stated in studies with multiple robotic study arms. ORL, otorhinolaryngology; TORS, transoral robot‐assisted surgery; RKT, robot‐assisted kidney transplantation; n.r., not reported; RDP, robot‐assisted distal pancreatectomy; LDP, laparoscopic distal pancreatectomy; LRRYGB, laparoscopic robot‐assisted Roux‐en‐Y gastric bypass; TRRYGB, totally robot‐assisted Roux‐en‐Y gastric bypass; RPD, robot‐assisted pancreatoduodenectomy; RARP, robot‐assisted radical prostatectomy; ORP, open radical prostatectomy; HAI, hepatic artery infusion; REVUR, robot‐assisted extravesical ureteral reimplantation; RATS, robot‐assisted thoracic surgery; RSC, robot‐assisted sacrocolpopexy; LRP, laparoscopic radical prostatectomy; RAT, robot‐assisted thymectomy; RRS, robot‐assisted rectal cancer surgery; LESS, laparoendoscopic single‐site; RC, robot‐assisted cholecystectomy; TME, total mesorectal excision; RAG, robot‐assisted gastrectomy; RAPN, robot‐assisted partial nephrectomy; SSRC, single‐site robot‐assisted cholecystectomy; LSC, laparoscopic sacrocolpopexy; RALP, robot‐assisted laparoscopic prostatectomy; RA‐GPEHR, robot‐assisted giant para‐oesophageal hernia repair; RKT, robot‐assisted kidney transplantation; OKT, open kidney transplantation; LND, lymph node dissection.
Figure 2.
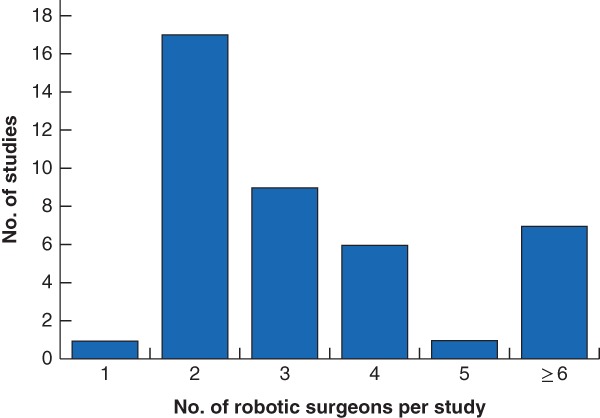
Bar chart showing the number of surgeons in robot‐assisted study arms per study One study enrolled only a single robot‐assisted surgeon, but was considered eligible because the total number of enrolled surgeons was greater than one (the study also evaluated a surgeon who performed procedures laparoscopically).
Figure 3.
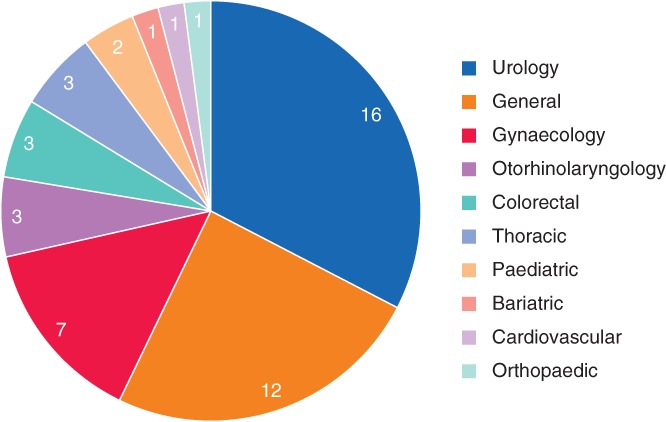
Pie chart of surgical specialties captured in the systematic literature review Values indicate the number of studies involving the specialty.
Learning curve metrics
Time‐based metrics were the most commonly reported variables used to assess the learning curve, reported by 42 of the 49 studies (86 per cent). Other measures, including length of hospital stay, morbidity and mortality rates, and procedure‐specific metrics, were reported less commonly (Fig. 4). Of the categories of metrics captured, duration of surgery, length of stay (LOS) and complication rate were reported most frequently within each category. The number of procedures required to overcome the learning curve for these metrics is shown in Table 2.
Figure 4.
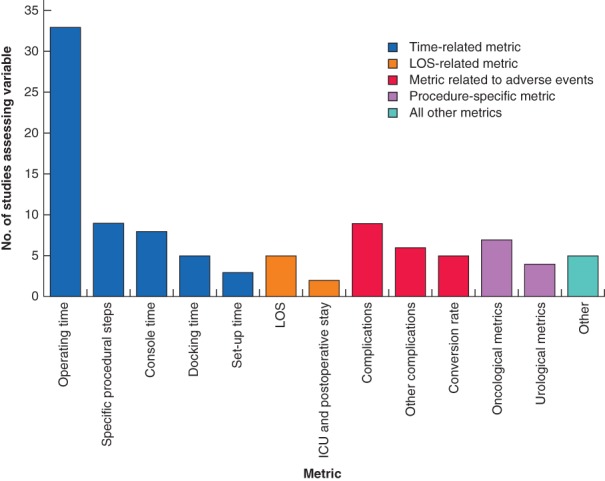
Bar chart of the learning curve metrics assessed across the captured studies LOS, length of stay.
Table 2.
Learning curve results for duration of surgery, length of stay and complication rate
| Metric | Procedure | No. of robotic surgeons | Procedures to overcome learning curve (subgroup)* | Specific threshold in surgeon performance | Reference |
|---|---|---|---|---|---|
| Operating time | Urology | ||||
| RARP | 2 | > 15 (expert laparoscopic surgeon) | No. of procedures to reach break point | Lebeau et al.30 | |
| 15 (junior surgeon) | |||||
| 3 | 140 (open experience) | No. of procedures to reach plateau | Chang et al.16 | ||
| 40 (open/laparoscopic experience) | |||||
| > 79 (laparoscopic experience) | |||||
| Robot‐assisted pyeloplasty | 4 | 37 (fellows) | No. of procedures to achieve median operating time of an attending surgeon | Tasian et al.50 | |
| Robot‐assisted adrenalectomy | n.r. | 12 | No. of procedures to reach plateau | D'Annibale et al.18 | |
| RAPN | 3 | > 100 | Identifying a plateau effect | Tobis et al.51 | |
| RALP | 2 | 20 | No. of procedures to approach median operative duration of LRP and presence of a plateau effect | Wolanski et al.56 | |
| General surgery | |||||
| Robot‐assisted HAI pump placement | n.r. | 8 | No. of procedures to reach a decline in CUSUM curve | Dhir et al.20 | |
| RPD | 4 | 80 | No. of procedures to reach a decline in CUSUM curve | Boone et al.15 | |
| n.r. | > 132 | n.r. | Zureikat et al.58 | ||
| Robot‐assisted thyroidectomy | 2 | 19; 20 | No. of procedures to reach plateau | Park et al.39 | |
| RDP | 2 | > 11 | n.r. | Benizri et al.12 | |
| 3 | 40 | No. of procedures to reach a decline in CUSUM curve | Shakir et al.47 | ||
| n.r. | > 83 | n.r. | Zureikat et al.58 | ||
| RAG | 3 | 8·2 | No. of procedures to reach stabilization | Park et al.40 | |
| 2 | 14; 21 | No. of procedures to reach a decline in CUSUM curve | Zhou et al.57 | ||
| RC | 8 | > 6 | n.r. | Nelson et al.37 | |
| SSRC | 5 | 0 | n.r. | Pietrabissa et al.42 | |
| Gynaecology | |||||
| RSC | 2 | > 24; 60 | No. of procedures to reach plateau | Linder et al.31 | |
| 3 | 0 | Moving block technique to detect a significant drop‐off | Pulliam et al.43 | ||
| Robot‐assisted hysterectomy | 1315 | > 150 | n.r. | Luciano et al.34 | |
| Colorectal | |||||
| Robot‐assisted colorectal resection | 4 | 74 (early adapter) | No. of procedures to reach a CUSUM curve phase change | Guend et al.27 | |
| 25–30 (later adapters) | |||||
| Robot‐assisted TME | 2 | 7; 15 | No. of procedures to achieve comparable performance to laparoscopy via CUSUM analysis | Odermatt et al.38 | |
| RRS | 2 | 17 (laparoscopically inexperienced) | No. of procedures to reach plateau | Kim et al.29 | |
| 0 (laparoscopically experienced) | |||||
| Thoracic | |||||
| RAT | 4 | 15–20 | No. of procedures to reach plateau | Kamel et al.28 | |
| Robot‐assisted lobectomy | 2 | 15 | No. of procedures to reach plateau | Meyer et al.35 | |
| Paediatric | |||||
| Laparoscopic robot‐assisted fundoplications | 2 | 25 | No. of procedures to reach plateau | Binet et al.14 | |
| REVUR | 4 | 7–8 | No. of procedures to reach plateau | Esposito et al.21 | |
| Cardiovascular | |||||
| Robot‐assisted mitral valve repair | 2 | > 404 | No. of procedures to reach plateau | Goodman et al.26 | |
| Length of stay | Colorectal | ||||
| Robot‐assisted TME | 2 | 0; 15 | No. of procedures to achieve comparable performance to laparoscopy via CUSUM analysis | Odermatt et al.38 | |
| Thoracic | |||||
| Robot‐assisted lobectomy | 2 | > 185 | No. of procedures to reach plateau | Meyer et al.35 | |
| Complications | Urology | ||||
| LND during RALP | 2 | > 400 | No. of procedures to reach plateau | van der Poel et al.52 | |
| General surgery | |||||
| Robot‐assisted pancreatic resections | n.r. | > 132 | n.r. | Zureikat et al.58 | |
| Gynaecology | |||||
| RSC | 2 | 0 | Proficiency set at less than 10% complication rate | Myers et al.36 | |
| 2 | > 24; 84 | Proficiency defined as point where CUSUM curve crossed and consistently stayed below reference line of 0 (based on expected complication rate) | Linder et al.31 | ||
| Robot‐assisted hysterectomy | 2 | 12; 14 | Proficiency defined as the point where CUSUM curve crossed lower control limit H0 | Woelk et al.55 | |
| 1315 | > 150 | n.r. | Luciano et al.34 | ||
| Colorectal | |||||
| Robot‐assisted TME | 2 | 0; 15 | No. of procedures to achieve comparable performance to laparoscopy via CUSUM analysis | Odermatt et al.38 | |
| Cardiovascular | |||||
| Robot‐assisted mitral valve repair | 2 | > 404 | No. of procedures to reach plateau | Goodman et al.26 |
Studies that did not report whether the learning curve had or had not been overcome within the study period were not included in this table.
For studies that reported a consistent improvement in metrics across the course of the study, it was assumed that the learning curve had not been overcome within the study period. If the learning curve had not been overcome, the number of procedures to overcome the learning curve was reported to be greater than (>) the total number of procedures in the study period. If the learning curve was reported to have been overcome (or surgeons were reported to be proficient/competent) before study initiation, the number of procedures required to overcome the learning curve was recorded as zero. Where results were reported separately for individual surgeons with no clear differences in previous experience, learning curve estimates are reported separately, separated by a semicolon; where the experience of surgeons was intentionally different, individual experience is reported as separate rows and experience level is stated in brackets. RARP, robot‐assisted radical prostatectomy; n.r., not reported; RAPN, robot‐assisted partial nephrectomy; RALP, robot‐assisted laparoscopic prostatectomy; LRP, laparoscopic radical prostatectomy; HAI, hepatic artery infusion; CUSUM, cumulative sum; RPD, robot‐assisted pancreatoduodenectomy; RDP, robot‐assisted distal pancreatectomy; RAG, robot‐assisted gastrectomy; RC, robot‐assisted cholecystectomy; SSRC, single‐site robot‐assisted cholecystectomy; RSC, robot‐assisted sacrocolpopexy; TME: total mesorectal excision; RRS, robot‐assisted rectal cancer surgery; RAT, robot‐assisted thymectomy; REVUR, robot‐assisted extravesical ureteral reimplantation; LND, lymph node dissection.
Duration of surgery
Across 33 studies that investigated the learning curve based on duration of surgery, 27 reported whether the learning curve had been overcome. In 21 of these 27 studies (78 per cent), at least one of the included surgeons was reported to have overcome the learning curve, with the remaining six studies (22 per cent) stating that the learning curve had not been overcome by any surgeon within the number of procedures in the study period (Table 2).
Among studies where the learning curve was not overcome by any surgeon, the number of procedures assessed over the study period varied significantly, with a range of 6–404 patients12, 26, 34, 37, 51, 58. Of the studies in which the learning curve had been overcome, 12–140 patients were needed for urological procedures16, 18, 30, 50, 56, 0–80 for general surgical procedures (where 0 suggests no apparent learning process required, if the surgeon was already experienced)15, 20, 39, 40, 42, 47, 57, 0–60 for gynaecological procedures31, 43, 0–74 for colorectal procedures27, 29, 38, 15–20 for thoracic procedures28, 35 and 7–25 for paediatric procedures14, 21.
Among the 27 studies that reported whether the learning curve had been overcome, learning curve analyses were conducted for 22 unique procedures, of which only five (23 per cent) were supported by multiple studies. In such instances, the number of patients required to overcome the learning curve for these varied substantially between studies. For example, three studies evaluated the learning curve for duration of surgery for robot‐assisted distal pancreatectomy. In one study47 the learning curve was overcome after 40 patients, whereas in the other two12, 58 it had not been overcome within the study period (of 11 and 83 patients).
Length of stay
Of five studies reporting LOS, two reported on whether the learning curve had been overcome by at least one robotic surgeon (Table 2). One study38 estimated that the number of patients required to overcome the learning curve was between 0 and 15. In the other study35, the learning curve was not overcome within a study period of 185 patients.
Complications
Of nine studies assessing complications, eight reported whether the learning curve was overcome for complication rate. Of these, five26, 31, 34, 52, 58 found that the learning curve for complications had not been overcome for at least one robotic surgeon within the study period. With the exception of one study31, which included a surgeon with a study period of only 24 patients, these studies generally involved large patient numbers (132–404), and spanned urology, general surgery, gynaecology and cardiovascular specialties (Table 2). Only four studies reported that the learning curve for complications had been overcome. The numbers of procedures were estimated as: 0–84 for robot‐assisted sacrocolpopexy31, 36, 12–14 for robot‐assisted hysterectomy55 and 0–15 for robot‐assisted total mesorectal excision38.
Clinical metrics
Of the 49 studies, eight (16 per cent) evaluated whether the learning curve for clinical metrics had been overcome (Table 3). Metrics included oncology‐specific metrics such as surgical margin status and recurrence rate, and urology‐specific metrics such as urinary continence. The number of procedures to overcome the learning curve varied substantially. Of the two studies assessing urinary continence after robot‐assisted radical prostatectomy, one25 reported that 100 procedures were required to overcome the learning curve, whereas in the other23 the learning curve was not overcome by any of the four robotic surgeons, with study periods ranging from 112 to 541 patients. For all other clinical metrics, the learning curve was overcome by at least one surgeon during the study period, with a wide range of 0–300 patients to achieve this target.
Table 3.
Learning curve results for clinical metrics
| Metric group | Specific metric | Procedure | No. of robotic surgeons | Procedures to overcome learning curve (subgroup)* | Specific threshold in surgeon performance | Reference |
|---|---|---|---|---|---|---|
| Urinary continence | Probability of UC recovery after 1 year | RARP | 4 | > 112; > 411; > 413; > 541 | No. of procedures to reach plateau | Fossati et al.23 |
| Early UC | RARP | 1 | 100 | No. of procedures to reach plateau | Good et al.25 | |
| Renal function | Serum creatinine level | RKT | 3 | 0 (extensive RKT, limited OKT) | No. of procedures to reach ‘transition point’ in CUSUM curve | Sood et al.49 |
| 0 (extensive RKT/OKT) | ||||||
| 3 (limited RKT, extensive OKT) | ||||||
| Glomerular filtration rate | RKT | 3 | 0 (extensive RKT, limited OKT) | No. of procedures to reach ‘transition point’ in CUSUM curve | Sood et al.49 | |
| 0 (extensive RKT/OKT) | ||||||
| 3 (limited RKT, extensive OKT) | ||||||
| Biochemical recurrence | Biochemical recurrence | RARP | 9 | 100 | No. of procedures to reach ‘transition point’ in CUSUM curve | Sivaraman et al.48 |
| Surgical margins | Positive surgical margins | RARP | 9 | 100 | No. of procedures to reach ‘transition point’ in CUSUM curve | Sivaraman et al.48 |
| Apical positive surgical margins | RARP | 1 | 0 | No. of procedures to reach plateau | Good et al.25 | |
| Node positivity rate | LND during RALP | 2 | 300 | No. of procedures to reach plateau | van der Poel et al.52 | |
| Initial margin status | TORS | 3 | 15; 22; > 68 | No. of procedures to reach inflection point in CUSUM curve | Albergotti et al.10 | |
| Final positive surgical margins | TORS | 3 | 25; 27; > 37 | No. of procedures to reach inflection point in CUSUM curve | Albergotti et al.10 | |
| Lymph node yield | No. of removed nodes | LND during RALP | 2 | 250 | No. of procedures to reach plateau | van der Poel et al.52 |
| Lymph node harvest | RPD | 4 | 80 | No. of procedures to reach significant improvement | Boone et al.15 | |
| Lymph node harvest | Robot‐assisted TME | 2 | 0 | No. of procedures to achieve comparable performance to laparoscopy via CUSUM analysis | Odermatt et al.38 |
Studies that did not report whether the learning curve had or had not been overcome within the study period are not included in this table.
For studies that reported a consistent improvement in metrics across the course of the study, it was assumed that the learning curve had not been overcome within the study period. If the learning curve had not been overcome, the number of procedures to overcome the learning curve was reported to be greater than (>) the total number of procedures in the study period. If the learning curve was reported to have been overcome (or surgeons were reported to be proficient/competent) before study initiation, the number of procedures to overcome the learning curve was recorded as zero. Where results were reported separately for individual surgeons with no clear differences in previous experience, learning curve estimates are reported separately, separated by a semicolon; where the experience of surgeons was intentionally different, individual experience is reported as separate rows and experience level is stated in brackets. UC, urinary continence; RARP, robot‐assisted radical prostatectomy; RKT, robot‐assisted kidney transplantation; OKT, open kidney transplantation; CUSUM, cumulative sum; LND, lymph node dissection; RALP, robot‐assisted laparoscopic prostatectomy; TORS, transoral robotic surgery; RPD, robot‐assisted pancreatoduodenectomy; TME, total mesorectal excision.
Within‐study comparison between metrics
Some studies in the review evaluated the learning curve using more than one metric. The number of patients to overcome the learning curve was sometimes inconsistent between metrics. For example, of the two studies35, 38 that reported whether or not the learning curve was overcome for both duration of surgery and LOS, one35 indicated that substantially greater procedural experience was required for LOS, with more than 170 additional patients required to overcome the learning curve based on this metric.
Standards of reporting
The overall standard of reporting and level of detail provided in the included studies was low, often lacking sufficient information to interpret the learning curve for robot‐assisted procedures. For example, although 34 of the 49 studies (69 per cent) made some acknowledgement relating to the previous experience of included surgeons (Table 4), the detail in the reporting was mixed, with only 17 of 49 studies (35 per cent) quantifying previous experience of robot‐assisted (8 of 49, 16 per cent), laparoscopic (13 of 49, 27 per cent) or open (6 of 49, 12 per cent) operations completed. Only four of 49 studies (8 per cent) indicated whether simulation, dry lab or cadaver training had been completed before patient enrolment.
Table 4.
Studies reporting previous surgeon experience
| Surgeon's previous procedure experience | |||||
|---|---|---|---|---|---|
| Reference | Robot‐assisted | Laparoscopic | Open | Approach not defined | Simulated robotic training before study |
| Albergotti et al.10 | ✘ | ✘ | ✘ | ? | ✘ |
| Arora et al.11 | ? | ✘ | ✘ | ? | ✘ |
| Benizri et al.12 | ✘ | ✘ | ✘ | ✘ | ? |
| Bindal et al.13 | ✘ | ? | ✘ | ✘ | ✘ |
| Binet et al.14 | ✘ | ? | ✘ | ✘ | ✘ |
| Chang et al.16 | ✘ | ? | ? | ✘ | ✘ |
| Dhir et al.20 | ✘ | ✘ | ? | ✘ | ✘ |
| Geller et al.24 | ✓ | ✘ | ✘ | ? | ? |
| Good et al.25 | ✓ | ✓ | ✓ | ✘ | ? |
| Goodman et al.26 | ✓ | ✘ | ? | ✘ | ✓ |
| Guend et al.27 | ? | ? | ✘ | ✘ | ✘ |
| Kamel et al.28 | ✘ | ? | ✘ | ✘ | ✘ |
| Kim et al.29 | ✘ | ✓ | ✓ | ✘ | ? |
| Lebeau et al.30 | ✘ | ✓ | ✓ | ✘ | ✘ |
| Lopez et al.32 | ✓ | ✓ | ✘ | ✘ | ✘ |
| Lovegrove et al.33 | ? | ✘ | ✘ | ✘ | ✘ |
| Meyer et al.35 | ✘ | ✓ | ✘ | ✘ | ✘ |
| Odermatt et al.38 | ✘ | ✓ | ✘ | ✘ | ✓ |
| Park et al.40 | ✘ | ✓ | ✘ | ✘ | ✘ |
| Park et al.39 | ✓ | ✓ | ✓ | ✘ | ✘ |
| Pietrabissa et al.42 | ✓ | ✘ | ✘ | ✘ | ✓ |
| Pulliam et al.43 | ? | ? | ✘ | ✘ | ✘ |
| Riikonen et al.44 | ✘ | ? | ? | ✘ | ✘ |
| Sarkaria et al.45 | ✘ | ? | ✘ | ✘ | ✘ |
| Shakir et al.47 | ? | ? | ✘ | ✘ | ✘ |
| Sivaraman et al.48 | ✘ | ✓ | ✘ | ✘ | ✘ |
| Sood et al.49 | ✓ | ✘ | ✓ | ✘ | ✘ |
| Tasian et al.50 | ? | ✘ | ✘ | ✘ | ✘ |
| Tobis et al.51 | ✓ | ✓ | ✘ | ✘ | ✘ |
| van der Poel et al.52 | ✘ | ✓ | ✓ | ✘ | ? |
| Woelk et al.55 | ? | ✘ | ✘ | ? | ✘ |
| Wolanski et al.56 | ✘ | ✓ | ? | ✘ | ✓ |
| Zhou et al.57 | ✘ | ✓ | ✘ | ✘ | ✘ |
| Zureikat et al.58 | ✘ | ✘ | ✘ | ? | ✘ |
✓, Study quantified the amount of previous experience (procedures, days of simulated training); ?, study acknowledged previous experience but did not quantify it; ✘, study did not acknowledge previous experience.
Variability was observed in the performance thresholds used to measure the learning curve. For example, among the 27 studies that defined whether the learning curve for duration of surgery had been overcome (Table 2), the most common performance threshold used was the number of procedures needed to reach a plateau in performance, but other thresholds included the number of procedures to reach a change in phase, or the number to achieve a predetermined skill threshold set by an expert surgeon, and some studies did not specify the performance thresholds used (Fig. 5 a). In nine of the 27 studies (33 per cent), no statistical or quantitative assessment of the learning curve was reported beyond visual fit or a qualitative description (data not reported). Similar variation in learning curve definitions was observed for analyses of LOS and complication rates (Fig. 5 b,c).
Figure 5.
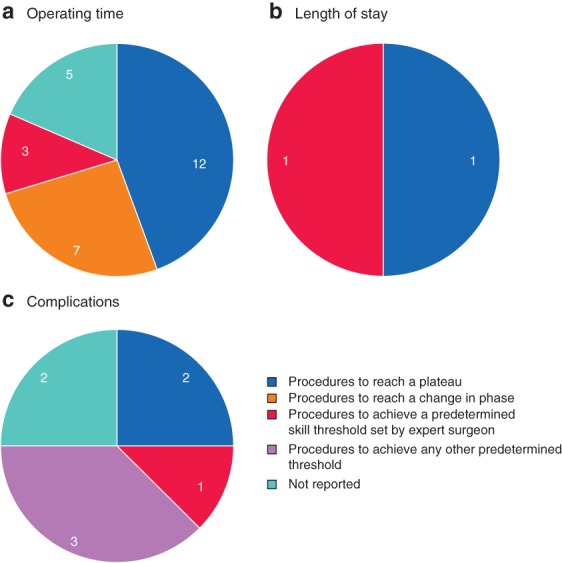
Pie chart of methods used to define the point at which learning curve was overcome a Operating time; b length of stay; c complications. Values indicate the number of studies using the method.
Several studies used these methods to define whether surgeons had achieved a high level of performance, characterized by terms such as proficiency or competency. However, these terms were used inconsistently. In 13 of 14 studies (93 per cent) that employed performance terms, at least one term was used to describe the point where the learning curve had been overcome; ‘proficiency’ was used for this purpose in ten studies22, 27, 28, 31, 36, 38, 39, 42, 50, 55, ‘competency’ in five studies10, 27, 33, 36, 49 and ‘expertise’ in one study50. In the four studies that reported more than one performance term, the terms were either used interchangeably36, 50 or assigned divergent definitions33, 49.
Although quality assessment of included studies (Table S5 , supporting information) revealed a relatively low risk of bias for several quality assessment items, risk of bias was unclear for a large proportion of the questions, suggesting poor reporting of methodology. In particular, the risk of bias with respect to the blinding of subjects, external validity of included populations and study centres, and statistical power was either high or could not be determined (at least 45 of the 49 studies, 92 per cent).
Discussion
This review identified substantial variation in the lengths of learning curves, included metrics and methods employed to assess the learning curve, as well as the reporting of the analyses and terminology used across ten surgical specialties. Reported learning curve estimates are therefore subject to substantial uncertainty, and the generalizability of these findings is limited.
The results of the 49 eligible studies suggested that surgeon learning curves were complex. They varied significantly between studies, procedures and specific metrics assessed. A variety of factors could account for much of the variation in reported learning curve length.
The surgeon's previous experience may have been a significant factor; in three of five studies comparing the operating time learning curves of robotic surgeons, those with greater experience required fewer procedures to overcome their learning curve16, 29, 38. Although the captured studies often compared surgeons with different experience levels, such as trainees versus those who had completed training or robotic versus laparoscopic surgeons, studies generally did not report the participants' specific grade or training experience.
Robotic training programmes are becoming increasingly common to enable surgeons to overcome the learning curve faster59, 60. In addition to previous experience, participation in specific training programmes may influence the learning process. Although based on a small sample size, Guend and colleagues27 reported that a lower procedure volume was required to overcome the learning curve for robot‐assisted colorectal resections for three surgeons who had participated in an institutional training programme (25–30 procedures each), compared with the volume required for an earlier surgeon who joined the institution before the programme was established (74 procedures). Few studies, however, provided details of their training programmes, where these existed. Recent training programmes have considered innovations such as feedback loops that aim to provide specific recommendations for improvement, shortening the time required to achieve adequate performance61.
Differences in procedural complexity may also have contributed to variation in the learning curves observed. In surgical practice, following initial improvement and subsequent stabilization of performance, a decline in performance is often observed62. This decline is thought to reflect the point at which, following mastery of simpler procedures, surgeons take on more challenging, technically complex procedures, that impact on a learning curve63.
Some procedures are inherently more complex and challenging than others. Studies64, 65, 66 of simulated robotic training tasks have observed learning curves of different duration for tasks of varying complexity. Learning curves are likely to be influenced by numerous observed and unobserved confounders. To account better for such differences, and to permit comparisons between studies, enhanced reporting of surgeon baseline characteristics, experience and procedure complexity is required.
This review has highlighted a number of limitations associated with reporting learning curves for robot‐assisted surgery. Many studies failed to describe the characteristics of the surgeons, patients or methods of assessment in sufficient detail to make valid comparisons between studies or to enable a study to be reproduced.
Although the majority of captured studies were determined to be of reasonable methodological quality, the studies were observational and usually included few surgeons. These study designs are associated with significant drawbacks, particularly with respect to confounding and selection bias67, 68, although this is expected given that they are more suited to measuring learning curves. Regarding sources of bias in the included studies, the risk of bias was unclear for a large proportion of the quality assessment items, particularly in relation to blinding, external validity and statistical power, suggesting poor reporting of methodology.
There was little consistency in the performance thresholds used to measure the learning curve, making between‐study comparisons challenging. A large proportion of studies measured the number of procedures required to reach a plateau in surgeon performance. Although a variety of methods can define quantitatively the point at which a plateau is reached69, 70, 71, 72, there is currently no widely accepted and validated method, and some studies used visual fit alone1. The number of procedures required to overcome learning curves reported are subject to considerable uncertainty. Several studies measured the number of procedures to achieve a threshold set by experts. These were sometimes based on the performance of expert robotic surgeons50, whereas others38, 56 included expert laparoscopic surgeons. Many studies did not report the specific performance thresholds used to measure the learning curve, precluding any ability to make comparisons.
These methods were frequently used to define the points at which surgeons reached ‘proficiency’, ‘competency’ or other related terms. These terms, however, were used inconsistently or interchangeably36, 50, or used with distinct definitions33, 49. In one study49 competency was used to describe performance that reached a steady state or plateau, whereas proficiency described further improvement after plateau and mastery as the achievement of outcomes better than the set target value. These definitions are not well aligned with guidelines for assessing surgical competence73, 74, recommended by the US Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties, nor the criteria developed for procedure‐based assessment in the Intercollegiate Surgical Curriculum Programme in the UK.
The mismatch between the performance thresholds used to measure the learning curve and the terminology used to describe the results of the analyses can lead to misinterpretation. The term ‘overcome’ was commonly used to describe a point when surgeons reached a given performance threshold, implying a high level of performance. These thresholds, particularly time to plateau, are often simplistic and may not capture sufficient evidence about the learning process to support this implication. A plateau in performance does not always equate with high‐quality performance, as surgeons will not necessarily plateau at the same level1. Likewise, using thresholds of performance to define terms such as competence and proficiency could be considered inappropriate, as these terms also imply a specific level of performance. A recent study61 investigated proficiency‐based progression training programmes in which residents who failed to show progressive improvement (reached a plateau in performance) were not considered proficient unless they had achieved predetermined proficiency benchmarks set by experienced surgeons.
The lack of consistency in methods used to describe surgical performance and the use of simplistic and inappropriate methods adds to the complexity of interpreting learning curves. Using thresholds that provide meaningful measures of surgeon performance alongside standardized terminology seems vital to realize the full potential of learning curve analyses for optimization of surgical training programmes.
Time‐based variables were the most common metrics used to assess learning curves, as is the case for other systematic reviews assessing surgical learning curves in other contexts63, 75. Although common across learning curve analyses, the present review suggests that variation can exist between the learning curve profiles of different metrics for a given procedure, with recovery and safety metrics (LOS, complications) exhibiting substantially longer learning curves than those for operating time, often with continued improvement for extended periods of time after the learning curve for operating time has been overcome. Given that improvements in clinical outcomes may be important drivers for the uptake of robot‐assisted approaches, the value of comparisons based on learning curves for operating time alone is unclear. In addition, the metrics captured may not directly measure surgical performance, with surrogate markers, such as operating time and LOS, reported frequently. Real‐time automated performance metrics, coupled with machine learning algorithms to process automatically collected data, may enable more direct measurement of surgeon performance76.
Several data gaps were identified in the reported data. Many of the identified studies enrolled fully trained surgeons, investigating the transferability of skills for conversion from laparoscopic or open surgery to robot‐assisted procedures. These studies may be of limited value for informing the design of surgical training programmes. The limited data investigating the learning curves of trainee surgeons may result in missed opportunities for the optimization of programmes to accelerate the training of surgeons who are novices with robot‐assisted devices. No study reported data related to the economic impact of the learning curve, such as training costs or financial impacts of suboptimal outcomes.
This systematic review was a broad, exploratory search of the literature reporting on the surgeon learning curve for robot‐assisted surgery, with broad search terms and eligibility criteria. Incomplete and variable reporting created challenges for data synthesis, especially given some of the exploratory and subjective outcomes this review intended to identify. The exploratory nature of the review may have introduced a number of limitations, which may have resulted in relevant data being overlooked. For example, to include evidence of suitable quality, studies that involved fewer than 20 surgical procedures in total (across surgical approaches or surgeons) were excluded, regardless of the number of robot‐assisted procedures completed by any one surgeon or included within the learning curve analysis. Studies were captured only if they reported actual learning curve data (as a graph or table presenting at least 4 time points), so that studies reporting potentially relevant data (for example the economic impact of the learning curve) could have been excluded if they did not meet these criteria. Studies that included just a single surgeon were also excluded, as the innate differences in technical ability that may exist between surgeons were anticipated to limit the reliability of the data reported in these studies. Only the learning curves of robotic procedures were considered in this review, and although the review did not set out to compare robotic‐assisted procedures with other surgical approaches, this prevented any conclusions to be drawn regarding lengths of the learning curve for robot‐assisted versus non‐robotic procedures. Only studies originally published in English were included, and database searches were limited to 2012–2018 in order to capture evidence most relevant to present‐day training practices. Although comparisons between robot‐assisted and other surgical approaches are warranted, studies with appropriate evaluation methods, standardized terminology and necessary context are essential for robust comparisons to be made. These kinds of study should provide better estimates of learning curves for robot‐assisted procedures, enhance surgical training programmes and improve patient outcomes.
Supporting information
Table S1. Search terms for MEDLINE, MEDLINE In‐Process, MEDLINE Epub Ahead of Print and Embase
Table S2. Search terms for Cochrane Library databases (searched via Wiley Online platform)
Table S3. Full eligibility criteria
Table S4. List of studies excluded at full‐text review and reasons for exclusion
Table S5. Quality assessment of non‐randomized articles using Downs and Black checklist
Acknowledgements
This study was funded by Ethicon, USA.
R.D. and S.R. are paid employees of Ethicon and shareholders of Johnson & Johnson. A.J.P., C.J.A.R. and W.J.M. are paid employees of Costello Medical, UK, which was contracted by Ethicon, USA, to undertake the review. Support for third‐party writing assistance for this article, provided by A.J.P., C.J.A.R. and W.J.M., Costello Medical UK, was also funded by Ethicon and shareholders of Johnson & Johnson. N.A.S. has participated in advisory boards of Verb Surgical, Intuitive Surgical, Medtronic and CMR Surgical. D.A.H. is an independent consultant for the Johnson & Johnson Institute, Verily Life Sciences and the Gerson Lehrman Group. He has received research funding from the Olympus Corporation. N.A.S. and D.A.H. received no compensation for their participation in this research.
Disclosure: The authors declare no other conflict of interest.
Funding information:
Ethicon, USA
Presented as a poster to the 24th Annual International Meeting of the International Society of Pharmacoeconomics and Outcomes Research, New Orleans, Louisiana, USA, May 2019
References
- 1. Khan N, Abboudi H, Khan MS, Dasgupta P, Ahmed K. Measuring the surgical ‘learning curve’: methods, variables and competency. BJU Int 2014; 113: 504–508. [DOI] [PubMed] [Google Scholar]
- 2. Randell R, Alvarado N, Honey S, Greenhalgh J, Gardner P, Gill A. Impact of robotic surgery on decision making: perspectives of surgical teams. AMIA Annu Symp Proc 2015; 2015: 1057–1066. [PMC free article] [PubMed] [Google Scholar]
- 3. Lanfranco AR, Castellanos AE, Desai JP, Meyers WC. Robotic surgery: a current perspective. Ann Surg 2004; 239: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cundy TP, Harling L, Hughes‐Hallett A, Mayer EK, Najmaldin AS, Athanasiou T et al Meta‐analysis of robot‐assisted vs conventional laparoscopic and open pyeloplasty in children. BJU Int 2014; 114: 582–594. [DOI] [PubMed] [Google Scholar]
- 5. Agrusa A, Romano G, Navarra G, Conzo G, Pantuso G, Buono GD et al Innovation in endocrine surgery: robotic versus laparoscopic adrenalectomy. Meta‐analysis and systematic literature review. Oncotarget 2017; 8: 102 392–102 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Turchetti G, Palla I, Pierotti F, Cuschieri A. Economic evaluation of da Vinci‐assisted robotic surgery: a systematic review. Surg Endosc 2012; 26: 598–606. [DOI] [PubMed] [Google Scholar]
- 7. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Institute for Health and Care Excellence (NICE) . Single Technology Appraisal: User Guide for Company Evidence Submission Template. NICE: London, 2015. [Google Scholar]
- 9. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J Epidemiol Community Health 1998; 52: 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Albergotti WG, Gooding WE, Kubik MW, Geltzeiler M, Kim S, Duvvuri U et al Assessment of surgical learning curves in transoral robotic surgery for squamous cell carcinoma of the oropharynx. JAMA Otolaryngol Head Neck Surg 2017; 143: 542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arora S, Tugcu V, Sood A, Bhandari M, Ahlawat R, Menon M. Learning curve of a new surgical procedure: experience from a new center adopting robotic kidney transplant. J Urol 2017; 197: e73–e74. [Google Scholar]
- 12. Benizri EI, Germain A, Ayav A, Bernard JL, Zarnegar R, Benchimol D et al Short‐term perioperative outcomes after robot‐assisted and laparoscopic distal pancreatectomy. J Robot Surg 2014; 8: 125–132. [DOI] [PubMed] [Google Scholar]
- 13. Bindal V, Gonzalez‐Heredia R, Masrur M, Elli EF. Technique evolution, learning curve, and outcomes of 200 robot‐assisted gastric bypass procedures: a 5‐year experience. Obes Surg 2015; 25: 997–1002. [DOI] [PubMed] [Google Scholar]
- 14. Binet A, Fourcade L, Amar S, Alzahrani K, Cook AR, Braïk K et al Robot‐assisted laparoscopic fundoplications in pediatric surgery: experience review. Eur J Pediatr Surg 2019; 29: 173–178. [DOI] [PubMed] [Google Scholar]
- 15. Boone BA, Zenati M, Hogg ME, Steve J, Moser AJ, Bartlett DL et al Assessment of quality outcomes for robotic pancreaticoduodenectomy: identification of the learning curve. JAMA 2015; 150: 416–422. [DOI] [PubMed] [Google Scholar]
- 16. Chang Y, Qu M, Wang L, Yang B, Chen R, Zhu F et al Robotic‐assisted laparoscopic radical prostatectomy from a single Chinese center: a learning curve analysis. Urology 2016; 93: 104–111. [DOI] [PubMed] [Google Scholar]
- 17. Ciabatti PG, Burali G, D'Ascanio L. Single‐incision robot‐assisted transaxillary surgery for early‐stage papillary thyroid cancer. Ann Otol Rhinol Laryngol 2012; 121: 811–815. [DOI] [PubMed] [Google Scholar]
- 18. D'Annibale A, Lucandri G, Monsellato I, De Angelis M, Pernazza G, Alfano G et al Robotic adrenalectomy: technical aspects, early results and learning curve. Int J Med Robot 2012; 8: 483–490. [DOI] [PubMed] [Google Scholar]
- 19. Davis JW, Kreaden US, Gabbert J, Thomas R. Learning curve assessment of robot‐assisted radical prostatectomy compared with open‐surgery controls from the premier perspective database. J Endourol 2014; 28: 560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dhir M, Zenati MS, Padussis JC, Jones HL, Perkins S, Clifford AK et al Robotic assisted placement of hepatic artery infusion pump is a safe and feasible approach. J Surg Oncol 2016; 114: 342–347. [DOI] [PubMed] [Google Scholar]
- 21. Esposito C, Masieri L, Steyaert H, Escolino M, Cerchione R, La Manna A et al Robot‐assisted extravesical ureteral reimplantation (REVUR) for unilateral vesico‐ureteral reflux in children: results of a multicentric international survey. World J Urol 2018; 36: 481–488. [DOI] [PubMed] [Google Scholar]
- 22. Fahim C, Hanna W, Waddell T, Shargall Y, Yasufuku K. Robotic‐assisted thoracoscopic surgery for lung resection: the first Canadian series. Can J Surg 2017; 60: 260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fossati N, Di Trapani E, Gandaglia G, Dell'Oglio P, Umari P, Buffi NM et al Assessing the impact of surgeon experience on urinary continence recovery after robot‐assisted radical prostatectomy: results of four high‐volume surgeons. J Endourol 2017; 31: 872–877. [DOI] [PubMed] [Google Scholar]
- 24. Geller EJ, Lin FC, Matthews CA. Analysis of robotic performance times to improve operative efficiency. J Minim Invasive Gynecol 2013; 20: 43–48. [DOI] [PubMed] [Google Scholar]
- 25. Good DW, Stewart GD, Laird A, Stolzenburg JU, Cahill D, McNeill SA. A critical analysis of the learning curve and postlearning curve outcomes of two experience‐ and volume‐matched surgeons for laparoscopic and robot‐assisted radical prostatectomy. J Endourol 2015; 29: 939–947. [DOI] [PubMed] [Google Scholar]
- 26. Goodman A, Koprivanac M, Kelava M, Mick SL, Gillinov AM, Rajeswaran J et al Robotic mitral valve repair: the learning curve. Innovations 2017; 12: 390–397. [DOI] [PubMed] [Google Scholar]
- 27. Guend H, Widmar M, Patel S, Nash GM, Paty PB, Guillem JG et al Developing a robotic colorectal cancer surgery program: understanding institutional and individual learning curves. Surg Endosc 2017; 31: 2820–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kamel MK, Rahouma M, Stiles BM, Nasar A, Altorki NK, Port JL. Robotic thymectomy: learning curve and associated perioperative outcomes. J Laparoendosc Adv Surg Tech A 2017; 27: 685–690. [DOI] [PubMed] [Google Scholar]
- 29. Kim IK, Kang J, Park YA, Kim NK, Sohn SK, Lee KY. Is prior laparoscopy experience required for adaptation to robotic rectal surgery?: feasibility of one‐step transition from open to robotic surgery. Int J Colorectal Dis 2014; 29: 693‐699. [DOI] [PubMed] [Google Scholar]
- 30. Lebeau T, Rouprêt M, Ferhi K, Chartier‐Kastler E, Bitker MO, Richard F et al The role of a well‐trained team on the early learning curve of robot‐assisted laparoscopic procedures: the example of radical prostatectomy. Int J Med Robot 2012; 8: 67–72. [DOI] [PubMed] [Google Scholar]
- 31. Linder BJ, Anand M, Weaver AL, Woelk JL, Klingele CJ, Trabuco EC et al Assessing the learning curve of robotic sacrocolpopexy. Int Urogynecol J 2016; 27: 239–246. [DOI] [PubMed] [Google Scholar]
- 32. Lopez S, Mulla ZD, Hernandez L, Garza DM, Payne TN, Farnam RW. A comparison of outcomes between robotic‐assisted, single‐site laparoscopy versus laparoendoscopic single site for benign hysterectomy. J Minim Invasive Gynecol 2016; 23: 84–88. [DOI] [PubMed] [Google Scholar]
- 33. Lovegrove C, Novara G, Mottrie A, Guru KA, Brown M, Challacombe B et al Structured and modular training pathway for robot‐assisted radical prostatectomy (RARP): validation of the RARP assessment score and learning curve assessment. Eur Urol 2016; 69: 526–535. [DOI] [PubMed] [Google Scholar]
- 34. Luciano AA, Luciano DE, Gabbert J, Seshadri‐Kreaden U. The impact of robotics on the mode of benign hysterectomy and clinical outcomes. Int J Med Robot 2016; 12: 114–124. [DOI] [PubMed] [Google Scholar]
- 35. Meyer M, Gharagozloo F, Tempesta B, Margolis M, Strother E, Christenson D. The learning curve of robotic lobectomy. Int J Med Robot 2012; 8: 448–452. [DOI] [PubMed] [Google Scholar]
- 36. Myers EM, Geller EJ, Connolly A, Bowling JM, Matthews CA. Robotic sacrocolpopexy performance and cumulative summation analysis. Female Pelvic Med Reconstr Surg 2014; 20: 83–86. [DOI] [PubMed] [Google Scholar]
- 37. Nelson EC, Gottlieb AH, Müller HG, Smith W, Ali MR, Vidovszky TJ. Robotic cholecystectomy and resident education: the UC Davis experience. Int J Med Robot 2014; 10: 218–222. [DOI] [PubMed] [Google Scholar]
- 38. Odermatt M, Ahmed J, Panteleimonitis S, Khan J, Parvaiz A. Prior experience in laparoscopic rectal surgery can minimise the learning curve for robotic rectal resections: a cumulative sum analysis. Surg Endosc 2017; 31: 4067–4076. [DOI] [PubMed] [Google Scholar]
- 39. Park JH, Lee J, Hakim NA, Kim HY, Kang SW, Jeong JJ et al Robotic thyroidectomy learning curve for beginning surgeons with little or no experience of endoscopic surgery. Head Neck 2015; 37: 1705–1711. [DOI] [PubMed] [Google Scholar]
- 40. Park SS, Kim MC, Park MS, Hyung WJ. Rapid adaptation of robotic gastrectomy for gastric cancer by experienced laparoscopic surgeons. Surg Endosc 2012; 26: 60–67. [DOI] [PubMed] [Google Scholar]
- 41. Paulucci DJ, Krane LS, Hemal AK, Badani KK. Beyond the learning curve: robotic partial nephrectomy outcomes continue to improve with surgeon experience. J Urol 2016; 195: e476. [Google Scholar]
- 42. Pietrabissa A, Sbrana F, Morelli L, Badessi F, Pugliese L, Vinci A et al Overcoming the challenges of single‐incision cholecystectomy with robotic single‐site technology. Arch Surg 2012; 147: 709–714. [DOI] [PubMed] [Google Scholar]
- 43. Pulliam SJ, Weinstein MM, Wakamatsu MM. Minimally invasive apical sacropexy: a retrospective review of laparoscopic and robotic operating room experiences. Female Pelvic Med Reconstr Surg 2012; 18: 122–126. [DOI] [PubMed] [Google Scholar]
- 44. Riikonen J, Kaipia A, Petas A, Horte A, Koskimäki J, Kähkönen E et al Initiation of robot‐assisted radical prostatectomies in Finland: impact on centralization and quality of care. Scand J Urol 2016; 50: 149–154. [DOI] [PubMed] [Google Scholar]
- 45. Sarkaria IS, Latif MJ, Bianco VJ, Bains MS, Rusch VW, Jones DR et al Early operative outcomes and learning curve of robotic assisted giant paraesophageal hernia repair. Int J Med Robot 2017; 13: e1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schatlo B, Martinez R, Alaid A, von Eckardstein K, Akhavan‐Sigari R, Hahn A et al Unskilled unawareness and the learning curve in robotic spine surgery. Acta Neurochir (Wien) 2015; 157: 1819–1823. [DOI] [PubMed] [Google Scholar]
- 47. Shakir M, Boone BA, Polanco PM, Zenati MS, Hogg ME, Tsung A et al The learning curve for robotic distal pancreatectomy: an analysis of outcomes of the first 100 consecutive cases at a high‐volume pancreatic centre. HPB 2015; 17: 580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sivaraman A, Sanchez‐Salas R, Prapotnich D, Yu K, Olivier F, Secin FP et al Learning curve of minimally invasive radical prostatectomy: comprehensive evaluation and cumulative summation analysis of oncological outcomes. Urol Oncol 2017; 35: 149. [DOI] [PubMed] [Google Scholar]
- 49. Sood A, Ghani KR, Ahlawat R, Modi P, Abaza R, Jeong W et al Application of the statistical process control method for prospective patient safety monitoring during the learning phase: robotic kidney transplantation with regional hypothermia (IDEAL phase 2a–b). Eur Urol 2014; 66: 371–378. [DOI] [PubMed] [Google Scholar]
- 50. Tasian GE, Wiebe DJ, Casale P. Learning curve of robotic assisted pyeloplasty for pediatric urology fellows. J Urol 2013; 190: 1622–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tobis S, Venigalla S, Knopf JK, Scosyrev E, Erturk EN, Golijanin DJ et al Robot‐assisted partial nephrectomy: analysis of the first 100 cases from a single institution. J Robot Surg 2012; 6: 139–147. [DOI] [PubMed] [Google Scholar]
- 52. van der Poel HG, de Blok W, Tillier C, van Muilekom E. Robot‐assisted laparoscopic prostatectomy: nodal dissection results during the first 440 cases by two surgeons. J Endourol 2012; 26: 1618–1624. [DOI] [PubMed] [Google Scholar]
- 53. Vidovszky TJ, Carr AD, Farinholt GN, Ho HS, Smith WH, Ali MR. Single‐site robotic cholecystectomy in a broadly inclusive patient population: a prospective study. Ann Surg 2014; 260: 134–141. [DOI] [PubMed] [Google Scholar]
- 54. White HN, Frederick J, Zimmerman T, Carroll WR, Magnuson JS. Learning curve for transoral robotic surgery: a 4‐year analysis. JAMA Otolaryngol Head Neck Surg 2013; 139: 564–567. [DOI] [PubMed] [Google Scholar]
- 55. Woelk JL, Casiano ER, Weaver AL, Gostout BS, Trabuco EC, Gebhart JB. The learning curve of robotic hysterectomy. Obstet Gynecol 2013; 121: 87–95. [DOI] [PubMed] [Google Scholar]
- 56. Wolanski P, Chabert C, Jones L, Mullavey T, Walsh S, Gianduzzo T. Preliminary results of robot‐assisted laparoscopic radical prostatectomy (RALP) after fellowship training and experience in laparoscopic radical prostatectomy (LRP). BJU Int 2012; 110: 64–70. [DOI] [PubMed] [Google Scholar]
- 57. Zhou J, Shi Y, Qian F, Tang B, Hao Y, Zhao Y et al Cumulative summation analysis of learning curve for robot‐assisted gastrectomy in gastric cancer. J Surg Oncol 2015; 111: 760–767. [DOI] [PubMed] [Google Scholar]
- 58. Zureikat AH, Moser AJ, Boone BA, Bartlett DL, Zenati M, Zeh HJ III . 250 robotic pancreatic resections: safety and feasibility. Ann Surg 2013; 258: 554–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bric J, Connolly M, Kastenmeier A, Goldblatt M, Gould JC. Proficiency training on a virtual reality robotic surgical skills curriculum. Surg Endosc 2014; 28: 3343–3348. [DOI] [PubMed] [Google Scholar]
- 60. Gomez PP, Willis RE, Van Sickle KR. Development of a virtual reality robotic surgical curriculum using the da Vinci Si surgical system. Surg Endosc 2015; 29: 2171–2179. [DOI] [PubMed] [Google Scholar]
- 61. Angelo RL, Ryu RK, Pedowitz RA, Beach W, Burns J, Dodds J et al A proficiency‐based progression training curriculum coupled with a model simulator results in the acquisition of a superior arthroscopic Bankart skill set. Arthroscopy 2015; 31: 1854–1871. [DOI] [PubMed] [Google Scholar]
- 62. Hopper AN, Jamison MH, Lewis WG. Learning curves in surgical practice. Postgrad Med J 2007; 83: 777–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pernar LIM, Robertson FC, Tavakkoli A, Sheu EG, Brooks DC, Smink DS. An appraisal of the learning curve in robotic general surgery. Surg Endosc 2017; 31: 4583–4596. [DOI] [PubMed] [Google Scholar]
- 64. Yang K, Perez M, Hossu G, Hubert N, Perrenot C, Hubert J. ‘Alarm‐corrected’ ergonomic armrest use could improve learning curves of novices on robotic simulator. Surg Endosc 2017; 31: 100–106. [DOI] [PubMed] [Google Scholar]
- 65. Walliczek U, Förtsch A, Dworschak P, Teymoortash A, Mandapathil M, Werner J et al Effect of training frequency on the learning curve on the da Vinci Skills Simulator. Head Neck 2016; 38(Suppl 1): E1762–E1769. [DOI] [PubMed] [Google Scholar]
- 66. Harrison P, Raison N, Abe T, Watkinson W, Dar F, Challacombe B et al The validation of a novel robot‐assisted radical prostatectomy virtual reality module. J Surg Educ 2018; 75: 758–766. [DOI] [PubMed] [Google Scholar]
- 67. Jepsen P, Johnsen SP, Gillman MW, Sørensen HT. Interpretation of observational studies. Heart 2004; 90: 956–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. StatsDirect . Prospective vs. Retrospective Studies https://www.statsdirect.com/help/basics/prospective.htm [accessed 22 November 2018].
- 69. Pusic MV, Boutis K, Pecaric MR, Savenkov O, Beckstead JW, Jaber MY. A primer on the statistical modelling of learning curves in health professions education. Adv Health Sci Educ Theory Pract 2017; 22: 741–759. [DOI] [PubMed] [Google Scholar]
- 70. Yelle LE. The learning curve: historical review and comprehensive survey. Decis Sci 1979; 10: 302–328. [Google Scholar]
- 71. Feldman LS, Cao J, Andalib A, Fraser S, Fried GM. A method to characterize the learning curve for performance of a fundamental laparoscopic simulator task: defining ‘learning plateau’ and ‘learning rate’. Surgery 2009; 146: 381–386. [DOI] [PubMed] [Google Scholar]
- 72. Adam MA, Thomas S, Youngwirth L, Hyslop T, Reed SD, Scheri RP et al Is there a minimum number of thyroidectomies a surgeon should perform to optimize patient outcomes? Ann Surg 2017; 265: 402–407. [DOI] [PubMed] [Google Scholar]
- 73. Satava RM, Gallagher AG, Pellegrini CA. Surgical competence and surgical proficiency: definitions, taxonomy, and metrics. J Am Coll Surg 2003; 196: 933–937. [DOI] [PubMed] [Google Scholar]
- 74. Mendes da Costa T. Procedure‐based assessments: an appropriate assessment tool? Bull R Coll Surg England 2014; 96: 236–238. [Google Scholar]
- 75. Khan N, Abboudi H, Khan MS, Dasgupta P, Ahmed K. Measuring the surgical ‘learning curve’: methods, variables and competency. BJU Int 2014; 113: 504–508. [DOI] [PubMed] [Google Scholar]
- 76. Hung AJ, Chen J, Gill IS. Automated performance metrics and machine learning algorithms to measure surgeon performance and anticipate clinical outcomes in robotic surgery. JAMA Surg 2018; 153: 770–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Search terms for MEDLINE, MEDLINE In‐Process, MEDLINE Epub Ahead of Print and Embase
Table S2. Search terms for Cochrane Library databases (searched via Wiley Online platform)
Table S3. Full eligibility criteria
Table S4. List of studies excluded at full‐text review and reasons for exclusion
Table S5. Quality assessment of non‐randomized articles using Downs and Black checklist


