Abstract
Background
Resection of the primary tumour is a prerequisite for cure in patients with colorectal cancer, but hepatic metastasectomy has been used increasingly with curative intent. This national registry study examined prognostic factors for radically treated primary tumours, including the subgroup of patients undergoing liver metastasectomy.
Methods
Patients who had radical resection of primary colorectal cancer in 2009–2013 were identified in a population‐based Swedish colorectal registry and cross‐checked in a registry of liver tumours. Data on primary tumour and patient characteristics were extracted and prognostic impact was analysed.
Results
Radical resection was registered in 20 853 patients; in 38·7 per cent of those registered with liver metastases, surgery or ablation was performed. The age‐standardized relative 5‐year survival rate after radical resection of colorectal cancer was 80·9 (95 per cent c.i. 80·2 to 81·6) per cent, and the rate after surgery for colorectal liver metastases was 49·6 (46·0 to 53·2) per cent. Multivariable analysis identified lymph node status, multiple sites of metastasis, high ASA grade and postoperative complications after resection of the primary tumour as strong risk factors after primary resection and following subsequent liver resection or ablation. Age, sex and primary tumour location had no prognostic impact on mortality after liver resection.
Conclusion
Lymph node status and complications have a negative impact on outcome after both primary resection and liver surgery. Older age and female sex were underrepresented in the liver surgical cohort, but these factors did not influence prognosis significantly.
Prognostic factors for the primary colorectal tumour also affect the outcome after surgery for liver metastases. Although performance status and complications after primary resection also influence outcome after liver resection, other factors such as older age and female sex do not, but these patients are still underrepresented in the liver surgical cohort. Awareness of the prognostic impact in both cohorts will improve decision‐making.

Information from primary surgery influences liver resection outcomes.
Antecedentes
Para curar el cáncer colorrectal es necesaria la resección del tumor primario, pero cada día es más frecuente la realización de una metastasectomía hepática con intención curativa. Este estudio basado en un registro nacional analizó los factores pronósticos para los tumores primarios tratados con intención curativa, incluido un subgrupo de pacientes a los que se realizó una metastasectomía hepática.
Métodos
En el registro poblacional sueco de cáncer colorrectal se identificaron los pacientes a los que se realizó una resección primaria radical entre 2009‐2013 y se cotejaron con un registro de tumores hepáticos. Se obtuvieron los datos sobre el tumor primario y las características del paciente, y se analizó su influencia en el pronóstico.
Resultados
Se identificaron 20.853 pacientes con resección radical, de los que en un 38,9% se realizó la resección o ablación de metástasis hepáticas. La supervivencia relativa a 5 años, estandarizada por edad, después de la resección radical del cáncer colorrectal y después de la cirugía de las metástasis hepáticas colorrectales fue del 80,6% (i.c. del 95% 79,8‐81,3) y del 49,6% (i.c. del 95%: 46,0‐53,2), respectivamente. El análisis multivariable identificó la invasión ganglionar, las metástasis en varias localizaciones, una puntuación ASA alta y las complicaciones postoperatorias después de la resección del tumor primario como factores de riesgo tanto de la resección primaria como de la resección o ablación hepática. No tuvieron influencia sobre la mortalidad tras de la resección hepática ni la edad, el sexo o la ubicación del tumor primario.
Conclusión
El grado de invasión linfática y las complicaciones después de la resección primaria tuvieron un impacto negativo en los resultados tanto de la cirugía primaria, como de la cirugía hepática. Si bien la edad avanzada y el sexo femenino estaban infrarrepresentados en la cohorte de cirugía hepática, estos factores no influyeron significativamente en el pronóstico.
Introduction
Colorectal cancer is the third most common cancer globally and is estimated to cause around 9 per cent of all cancer deaths in the world (860 000 worldwide in 2018)1. A 5‐year survival rate of 50–69 per cent is expected in many high‐income countries2, and improvements in prognosis have been attributed to earlier detection, surgical technique and resection rate of primary, increasing use of systemic treatments and improvements in the treatment of stage IV disease, especially in older individuals3, 4, 5. A decline in the mortality rate of patients with stage IV disease, with an absolute 5‐year survival rate above 10 per cent during the past decade, has been associated with increased use of attempted curative treatment in some western European countries5, 6, 7, 8.
Liver metastases develop in 20–30 per cent of all patients with colorectal cancer9, 10. The indication for liver surgery has widened regarding number and size of metastases11, but is limited by the need for an adequate remnant liver volume and extrahepatic disease. The importance of liver surgery specialist participation in multidisciplinary boards has been emphasized, to increase the use of curative strategies12, 13, 14. Five‐year overall survival (OS) rates of 40–60 per cent after surgical resection or ablation of liver metastases have been reported15, 16, 17, whereas national cohort studies18, 19, 20, 21 indicate 5‐year survival rates of 25–45 per cent.
Age, high BMI and co‐morbidity are known risk factors for poor survival in patients with colorectal cancer22, 23, 24. Lymph node status of the primary tumour is a well recognized prognostic factor25, although preoperative chemotherapy might modify its importance26, 27. Lymphovascular and perineural invasion have also been attributed as independent factors for survival28, 29. It is proposed that the location of the primary colorectal tumour is associated with OS after surgery of colorectal liver metastases. Patients with right‐sided tumours have longer recurrence‐free survival than those with left‐sided tumours, but at recurrence the course of the disease seems to be more complicated with shorter OS30. Colorectal liver metastases (CRLM) from mucinous tumours, which are more common in the right colon, seem to be associated with shorter OS31. The right colon, evolving from the mid gut, and the left colon, evolving from the hind gut, are physiologically distinct and may also differ in genetic and immunopathological characteristics32, 33.
To determine whether liver metastases should be surgically resected, clinical risk scores and selection criteria based on chemotherapy response and technical aspects of operability have all been advocated34, 35, 36, 37. To reach an optimal decision, it is important to identify prognostic patient and primary tumour factors that are also prognostic for the decision regarding liver metastasectomy.
The aim of this study was to identify patient and tumour factors at the time of resection of the primary colorectal cancer that should be considered when treatment of liver metastases is being discussed.
Methods
Patients diagnosed with colorectal cancer in Sweden between 2009 and 2013 were identified in the Swedish Colorectal Cancer Register (SCRCR). Patient and tumour characteristics related to the primary surgical treatment included age, sex and location of tumour, TNM stage, and all known metastatic disease. If more than one colorectal cancer diagnosis was registered, the first date of diagnosis was used for data collection. Retrieved data were linked to the Swedish Registry for Liver Surgery (SweLiv), in which population‐based data on CRLM resections in Sweden have been collected since 2008. Information on patient and metastatic tumour characteristics and treatment was collected from the SweLiv register. When there was more than one event involving liver surgery, only the first event was considered. Data were collected from the clinical cancer registers in February 2018, and registry data were linked to the Swedish population register for survival analysis. For the liver‐treated cohort, follow‐up data were updated in March 2019. The cohort of patients with surgically treated (resection or ablation) liver metastases constituted a subgroup of the patients who had undergone an R0 resection. Survival was calculated from the date of diagnosis when comparing the cohorts, and from date of liver surgery when analysing the liver‐treated cohort. Synchronous metastases were defined as metastases reported within 30 days of surgery for the primary tumour.
The coverage of SCRCR, compared with that of the Swedish cancer registry, where registration is compulsory, has been shown previously38, 39 to be accurate for 97–99·9 per cent for the different years of the study. Good conformity with the source data has been reported for both registries.
Lymph node positivity of the primary tumour in histopathology reports was initially analysed by means of the N category, the ratio of the number of positive lymph nodes to the total number of lymph nodes removed (lymph node ratio (LNR)) and the log odds of positive lymph nodes (LODDS). Univariable analysis indicated that LNR showed the strongest correlation with survival and was used in the multivariable analyses.
A composite variable for severe postoperative 30‐day complications was generated by including postoperative care in the ICU, sepsis and reoperation (equivalent to Clavien–Dindo grade IIIB or above).
The study was approved by the ethical regional board in Gothenburg (189‐15). Patients were informed of the use of data for study purposes by the registries.
Statistical analysis
Differences in distributions of variables between R0 and liver‐treated cohorts were tested by Pearson's χ2 test. OS was determined by means of the Kaplan–Meier method, and relative survival using the Ederer II method40. Age standardization of relative survival was performed using the standard weight distributions for cancers (International Cancer Survival Standards)41. Mortality rates by sex, 1‐year age group and 1‐year calendar period for the general population in Sweden were used to estimate expected survival rates for the study populations. Relative risk (RR) for up to 5 years of follow‐up between different groups for OS was analysed using the Cox regression method, and presented as hazard ratio (HR) with 95 per cent confidence intervals. The proportional hazards assumption was assessed with Schoenfeld's test. It was violated (P < 0·050) for some of the co‐variables, and thus their HR must be interpreted as an average over the time interval studied. The RR between different groups for relative survival was analysed by Poisson regression and presented as the excess mortality rate ratio (EMRR) with 95 per cent confidence intervals42; after the Poisson regression, the Wald test was used to determine statistical significance. Both univariable and multivariable regressions were used. Variables in the univariable analyses included age, sex, BMI, location of tumour, TNM stage, metastatic pattern, tumour grade, mucinous tumour, vascular and neural invasion, acute or elective surgery, and perioperative/postoperative complications. Variables significant at P < 0·100 in the univariable analysis were tested through a backward stepwise selection process for their independent effect on OS. When there were overlapping prognostic factors, only one parameter was analysed further in the multivariable analyses. P < 0·050 was considered statistically significant. All statistical analyses were carried out with Stata® version 15.1 (StataCorp, College Station, Texas, USA).
Results
Between 2009 and 2013, 28 765 patients with colorectal cancer were registered in the SCRCR, of whom 6406 (22·3 per cent) had synchronous metastatic disease. The median duration of follow‐up was 6·4 years in the colorectal cancer cohort for those alive at the end of the follow‐up period. For those undergoing treatment for liver metastases, median follow‐up was 6·1 years for those alive at the end of follow‐up. Demographic data are presented in Table 1.
Table 1.
Primary tumour and patient characteristics
| R0 (n = 20 853) | R1 (n = 3139) | No surgery (n = 4773) | Liver‐treated cohort (n = 1325) | P † | |
|---|---|---|---|---|---|
| Age (years) * | 72 (14–101) | 73 (19–106) | 74 (26–100) | 66 (25–87) | < 0·001 |
| Sex | < 0·001 | ||||
| M | 10 939 (52·5) | 1601 (51·0) | 2632 (55·1) | 812 (61·3) | |
| F | 9914 (47·5) | 1538 (49·0) | 2141 (44·9) | 513 (38·7) | |
| BMI (kg/m 2 ) | 0·015 | ||||
| < 18·5 | 461 (2·2) | 124 (4·0) | 0 (0) | 16 (1·2) | |
| 18·5–25 | 8482 (40·7) | 1215 (38·7) | 13 (0·3) | 541 (40·8) | |
| > 25 | 9908 (47·5) | 1060 (33·8) | 16 (0·3) | 682 (51·5) | |
| Missing | 2002 (9·6) | 740 (23·6) | 4744 (99·4) | 86 (6·5) | |
| ASA grade | < 0·001 | ||||
| I–II | 14 403 (69·1) | 1716 (54·7) | 23 (0·5) | 1021 (77·1) | |
| III–IV | 5680 (27·2) | 993 (31·6) | 10 (0·2) | 272 (20·5) | |
| Missing | 770 (3·7) | 430 (13·7) | 4740 (99·3) | 32 (2·4) | |
| Location of primary tumour | < 0·001 | ||||
| Right colon | 6544 (31·4) | 930 (29·6) | 924 (19·4) | 268 (20·2) | |
| Transverse colon | 1163 (5·6) | 194 (6·2) | 198 (4·1) | 44 (3·3) | |
| Left colon | 6158 (29·5) | 915 (29·1) | 1581 (33·1) | 507 (38·3) | |
| Rectum | 6419 (30·8) | 1021 (32·5) | 1964 (41·1) | 467 (35·2) | |
| Multiple | 566 (2·7) | 71 (2·3) | 82 (1·7) | 39 (2·9) | |
| Missing | 3 (0·01) | 8 (0·3) | 24 (0·5) | 0 (0) | |
| pT status | < 0·001 | ||||
| pT0 | 238 (1·1) | 30 (1·0) | n.a. | 15 (1·1) | |
| pT1–2 | 5031 (24·1) | 201 (6·4) | 146 (11·0) | ||
| pT3 | 12 273 (58·9) | 800 (25·5) | 915 (69·1) | ||
| pT4 | 3241 (15·5) | 1211 (38·6) | 247 (18·6) | ||
| pTX | 70 (0·3) | 897 (28·6) | 2 (0·2) | ||
| pN status | < 0·001 | ||||
| pN0 | 11 934 (57·2) | 683 (21·8) | n.a. | 465 (35·1) | |
| pN1 | 5188 (24·9) | 548 (17·5) | 469 (35·4) | ||
| pN2–3 | 3454 (16·6) | 791 (25·2) | 387 (29·2) | ||
| pNX | 277 (1·3) | 1117 (35·6) | 4 (0·3) | ||
| M status | < 0·001 | ||||
| M0 | 18 513 (88·8) | 1768 (56·3) | 2078 (43·5) | 581 (43·8) | |
| M1 (synchronous) | 2340 (11·2) | 1371 (43·7) | 2695 (56·5) | 744 (56·2) | |
| Liver metastases only (synchronous) | 1229 (5·9) | 453 (14·4) | 1256 (26·3) | 611 (46·1) | |
| Lung metastases only (synchronous) | 286 (1·4) | 87 (2·8) | 186 (3·9) | 8 (0·6) | |
| Other metastases only (synchronous) | 380 (1·8) | 341 (10·9) | 142 (3·0) | 5 (0·4) | |
| Multiple metastases (synchronous) | 445 (2·1) | 490 (15·6) | 1111 (23·3) | 120 (9·1) | |
| Liver metastases only (metachronous) | 880 (4·2) | – | – | 436 (32·9) | |
| Lung metastases only (metachronous) | 725 (3·5) | – | – | 92 (6·9) | |
| Other metastases only (metachronous) | 546 (2·6) | – | – | 28 (2·1) | |
| Multiple metastases (metachronous) | 1012 (4·8) | – | – | 188 (14·2) |
Values in parentheses are percentages unless indicated otherwise;
values are median (range). R0, all patients with colorectal adenocarcinoma registered in the Swedish Colorectal Cancer Register (SCRCR) (2009–2013) as having a radical resection; R1, all patients registered in the SCRCR (2009–2013) as not having a radical resection; no surgery, all patients registered in the SCRCR (2009–2013) with colorectal cancer not treated surgically; liver‐treated cohort, all patients registered in the SCRCR (2009–2013) as radically resected and registered in the Swedish Registry for Liver Surgery (SweLiv) (2009–2016) as treated for colorectal liver metastases at some time point. n.a., Not applicable.
R0 versus liver‐treated cohort (Pearson's χ2 test).
Liver metastases were detected in 3427 (16·4 per cent) of the 20 853 patients in the R0 cohort (in either register), of whom 1325 (38·7 per cent) were registered in SweLiv as treated for liver metastases by resection or ablation therapy; these patients constitute the liver‐treated cohort. Significantly more men (1998 of 10 939, 18·3 per cent) than women (1429 of 9914, 14·4 per cent) with liver metastases were registered. There was a higher proportion of men in the liver‐treated cohort (61·3 per cent) than in the R0 cohort (52·5 per cent) (P < 0·001). In the liver‐treated cohort, 1224 patients underwent resection and 101 patients were treated with ablation only. Altogether, 731 patients were treated for synchronous metastases and 594 for metachronous metastases. There were significant differences in selection from the R0 cohort regarding age, sex, BMI, ASA grade, location of primary tumour, pT status, lymph node metastases and metastases (Table 1).
Survival
The 5‐year OS rate was 53·8 (95 per cent c.i. 53·2 to 54·3) per cent for all patients with colorectal cancer, 25·2 (23·7 to 26·8) per cent for the non‐radically resected group, and 15·2 (14·2 to 16·2) per cent for the non‐operated group. The 5‐year OS rate was 66·8 (66·2 to 67·5) per cent in the R0 cohort and 48·6 (45·8 to 51·3) per cent in the liver‐treated cohort. The age‐standardized 5‐year relative survival rate was 65·9 (65·2 to 66·6) per cent for all patients with colorectal cancer, 80·9 (80·2 to 81·6) per cent in the R0 cohort and 49·6 (46·0 to 53·2) per cent in the liver‐treated cohort.
Survival, stratified in quartiles based on time from diagnosis of the colorectal primary to surgery for liver metastases, was significantly worse only for patients in the third quartile (operated on after 0·76–1·54 years): age‐standardized 5‐year relative survival rate 44·0 (95 per cent c.i. 37·9 to 50·0) per cent versus 59·9 (50·8 to 67·9) per cent for patients in the fourth quartile (operated on after 1·54 years or more) (Fig. 1).
Figure 1.
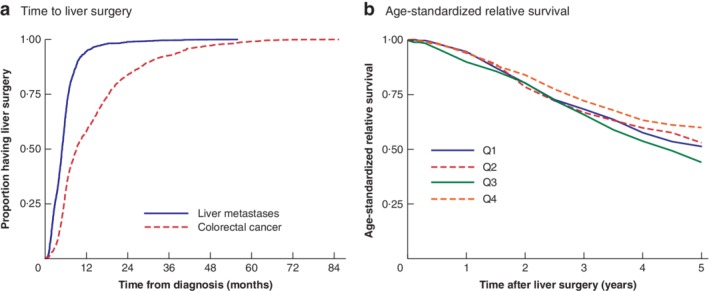
Time to liver surgery and age‐standardized relative survival a Time to liver surgery from diagnosis of liver metastases (median 5·0 months) and colorectal cancer (median 9·1 months). b Age‐standardized relative survival grouped by quartiles of time between diagnosis of colorectal cancer and liver surgery: quartile (Q) 1, 0–0·46 years (330 patients); Q2, 0·46–0·76 years (328 patients); Q3, 0·76–1·54 years (335 patients); Q4, 1·54–7·12 years (332 patients). The excess mortality rate ratio for Q3 versus Q4 was 1·44 (95 per cent c.i. 1·12 to 1·86). b P = 0·044 (Wald test).
Age‐standardized relative survival curves for basic patient characteristics (sex, age, ASA grade) and tumour characteristics (site, TNM categories) are shown in Figs 2 and 3. Synchronous metastases in the R0 cohort were associated with a 5‐year age‐standardized relative survival rate of 39·6 (95 per cent c.i. 36·7 to 42·4) per cent for isolated liver metastases and 54·5 (45·9 to 62·2) per cent for isolated lung metastases (Fig. 3 g).
Figure 2.

Relative survival in R0 and liver‐treated cohorts according to sex, age and ASA grade Age‐standardized relative survival in a R0 cohort and b liver‐treated cohort according to sex; relative survival in c R0 cohort and d liver‐treated cohort according to age; and age‐standardized relative survival in e R0 cohort and f liver‐treated cohort according to ASA grade.
Figure 3.
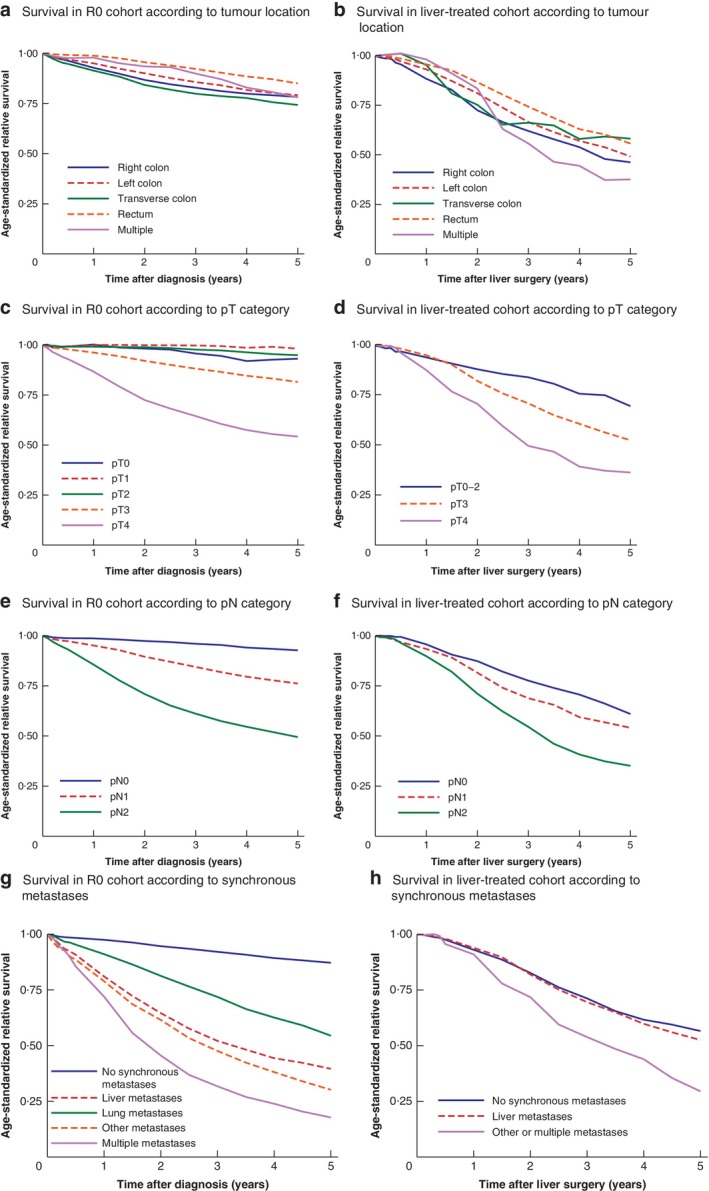
Age‐standardized relative survival in R0 and liver‐treated cohorts according to tumour location, pT and pN categories, and metastases at diagnosis of the primary tumour Survival in a R0 cohort and b liver‐treated cohort according to tumour location; survival in c R0 cohort and d liver‐treated cohort according to pT category; survival in e R0 cohort and f liver‐treated cohort according to pN category; survival in g R0 cohort and h liver‐treated cohort according to synchronous metastases at diagnosis of primary colorectal tumour.
Univariable and multivariable analyses
In the primary R0 cohort, pT category and lymph node positivity were the strongest risk factors for excess mortality in both univariable and multivariable analysis besides distant metastases. In multivariable analysis, EMRR values were 3·07 for pT4 tumours, 4·18 for LNR above 0·25, and 4·55 for metastases at multiple sites. For the liver‐treated cohort, LNR above 0·25 remained the strongest tumour‐associated risk factor (EMRR 2·03 in multivariable analysis). Tumour location in the right colon, tumour grade, vascular invasion and perineural invasion were also significant risk factors in the primary R0 cohort, but for the liver‐treated cohort primary tumour location was not a significant risk factor (Tables 2 and 3).
Table 2.
Relative survival and excess mortality rate ratio in the R0 cohort
| Univariable Poisson regression | Multivariable Poisson regression | |||||
|---|---|---|---|---|---|---|
| No. of patients (n = 20 853) | 5‐year age‐standardized relative survival (%)* | 5‐year EMRR* | P | 5‐year EMRR* | P | |
| Age (years) | ||||||
| < 50 | 1042 (5·0) | 79·5 (76·8, 81·9) | 1·11 (0·96, 1·28) | 0·168 | 1·07 (0·89, 1·29) | 0·456 |
| 50–74 | 11 113 (53·3) | 81·3 (80·4, 82·2) | 1·00 (reference) | 1·00 (reference) | ||
| ≥ 75 | 8698 (41·7) | 79·1 (77·5, 80·6) | 1·26 (1·15, 1·38) | < 0·001 | 1·37 (1·23, 1·53) | < 0·001 |
| Sex | ||||||
| M | 10 939 (52·5) | 80·1 (79·1, 81·2) | 1·00 (reference) | |||
| F | 9914 (47·5) | 81·6 (80·5, 82·5) | 0·98 (0·90, 1·07) | 0·134 | ||
| BMI (kg/m 2 ) | ||||||
| < 18·5 | 462 (2·2) | 65·6 (59·9, 70·8) | 2·18 (1·78, 2·66) | < 0·001 | 1·47 (1·14, 1·90) | 0·003 |
| 18·5–25 | 8484 (40·7) | 81·5 (80·4, 82·6) | 1·00 (reference) | 1·00 (reference) | 0·128 | |
| > 25 | 9907 (47·5) | 83·9 (82·8, 84·9) | 0·80 (0·72, 0·88) | < 0·001 | 0·93 (0·84, 1·02) | |
| Missing | 2000 (9·6) | |||||
| ASA grade | ||||||
| I–II | 14 403 (69·1) | 86·7 (85·9, 87·5) | 1·00 (reference) | 1·00 (reference) | ||
| III–IV | 5679 (27·2) | 67·8 (65·6, 70·0) | 2·96 (2·71, 3·24) | < 0·001 | 2·05 (1·86, 2·27) | < 0·001 |
| Missing | 771 (3·7) | |||||
| Location of primary tumour | ||||||
| Right colon | 6544 (31·4) | 78·3 (76·8, 79·8) | 1·13 (1·02, 1·25) | 0·016 | 1·32 (1·18, 1·49) | < 0·001 |
| Left colon | 6158 (29·5) | 79·2 (77·9, 80·5) | 1·00 (reference) | 0·001 | 1·00 (reference) | |
| Transverse colon | 1163 (5·6) | 74·3 (70·7, 77·6) | 1·32 (1·12, 1·56) | < 0·001 | 1·18 (0·96, 1·45) | 0·109 |
| Rectum | 6419 (30·8) | 85·1 (83·8, 86·3) | 0·62 (0·55, 0·70) | 0·430 | 1·22 (1·06, 1·40) | 0·006 |
| Multiple | 566 (2·7) | 78·2 (72·7, 82·6) | 0·89 (0·68, 1·18) | 1·26 (0·94, 1·67) | 0·120 | |
| Missing | 3 (0·01) | |||||
| pT status | ||||||
| pT0 | 238 (1·1) | 93·2 (77·4, 98·1) | 2·02 (0·41, 9·9) | 0·389 | 0·02 (0·00, ∝) | 0·861 |
| pT1 | 1426 (6·8) | 98·2 (94·6, 99·4) | 1·00 (reference) | 1·00 (reference) | ||
| pT2 | 3605 (17·3) | 94·9 (93·3, 96·2) | 2·32 (0·72, 7·46) | 0·157 | 1·05 (0·61, 1·79) | 0·869 |
| pT3 | 12 273 (58·9) | 81·6 (80·6, 82·5) | 10·0 (3·27, 30·6) | < 0·001 | 1·81 (1·10, 2·97) | 0·019 |
| pT4 | 3241 (15·5) | 54·2 (52·2, 56·2) | 33·2 (10·8, 101·5) | < 0·001 | 3·07 (1·86, 5·07) | < 0·001 |
| Missing | 70 (0·3) | |||||
| pN status | ||||||
| pN0 | 11 934 (57·2) | 92·7 (91·9, 93·4) | 1·00 (reference) | |||
| pN1 | 5188 (24·9) | 76·2 (74·7, 77·6) | 3·97 (3·43, 4·61) | < 0·001 | ||
| pN2 | 3454 (16·6) | 49·4 (47·6, 51·3) | 10·1 (8·82, 11·7) | < 0·001 | ||
| pNX | 277 (1·3) | |||||
| M status | ||||||
| M0 | 18 513 (88·8) | 86·1 (85·3, 86·9) | 1·00 (reference) | |||
| M1 (synchronous) | 2340 (11·2) | 34·4 (32·2, 36·5) | 7·39 (6·01, 8·01) | < 0·001 | ||
| Synchronous metastases | ||||||
| None | 18 513 (88·8) | 87·2 (86·4, 87·8) | 1·00 (reference) | 1·00 (reference) | ||
| Liver metastases only | 1229 (5·9) | 39·6 (36·7, 42·4) | 6·70 (6·08, 7·39) | < 0·001 | 3·55 (3·13, 4·03) | < 0·001 |
| Lung metastases only | 286 (1·4) | 54·5 (45·9, 62·2) | 4·24 (3·44, 5·23) | < 0·001 | 2·88 (2·23, 3·72) | < 0·001 |
| Other metastases only | 380 (1·8) | 30·2 (25·1, 35·4) | 8·46 (7·34, 9·76) | < 0·001 | 2·91 (2·41, 3·51) | < 0·001 |
| Multiple metastases | 445 (2·1) | 17·8 (14·3, 21·6) | 12·0 (10·6, 13·5) | < 0·001 | 4·55 (3·88, 5·34) | < 0·001 |
| LNR | ||||||
| 0 | 12278 (58·9) | 92·5 (91·6, 93·2) | 1·00 (reference) | 1·00 (reference) | ||
| > 0 to < 0·1 | 2906 (13·9) | 80·2 (78·3, 82·0) | 2·89 (2·45, 3·40) | < 0·001 | 1·55 (1·32, 1·82) | < 0·001 |
| 0·1 to < 0·25 | 2524 (12·1) | 68·4 (66·2, 70·5) | 5·10 (4·40, 5·92) | < 0·001 | 2·45 (2·11, 2·84) | < 0·001 |
| ≥ 0·25 | 2847 (13·7) | 45·3 (43·2, 47·3) | 11·1 (9·69, 12·7) | < 0·001 | 4·18 (3·65, 4·80) | < 0·001 |
| Missing | 298 (1·4) | |||||
| LODDS | ||||||
| ≤ −1·36 | 16 712 (80·1) | 88·0 (87·3, 88·9) | 1·00 (reference) | |||
| > −1·36 to −0·53 | 1932 (9·3) | 59·9 (57·2, 62·4) | 4·03 (3·62, 4·49) | < 0·001 | ||
| > −0·53 | 1898 (9·1) | 42·0 (39·5, 44·6) | 7·54 (6·86, 8·28) | < 0·001 | ||
| Missing | 311 (1·5) | |||||
| Tumour grade | ||||||
| High/mean | 16 009 (76·8) | 83·9 (83·0, 84·6) | 1·00 (reference) | 1·00 (reference) | ||
| Low | 3914 (18·8) | 68·5 (66·6, 70·3) | 2·35 (2·15, 2·56) | < 0·001 | 1·36 (1·23, 1·50) | < 0·001 |
| Missing | 930 (4·5) | |||||
| Vascular invasion | ||||||
| No | 14 357 (68·8) | 88·1 (87·3, 88·9) | 1·00 (reference) | 1·00 (reference) | ||
| Yes | 4933 (23·7) | 61·4 (59·8, 63·0) | 4·07 (3·71, 4·46) | < 0·001 | 1·24 (1·11, 1·38) | < 0·001 |
| Missing | 1563 (7·5) | |||||
| Perineural invasion | ||||||
| No | 14 958 (71·7) | 86·2 (85·4, 87·0) | 1·00 (reference) | 1·00 (reference) | ||
| Yes | 2714 (13·0) | 55·3 (53·2, 57·4) | 4·06 (3·71, 4·45) | < 0·001 | 1·36 (1·22, 1·52) | < 0·001 |
| Missing | 3181 (15·3) | |||||
| Mucinous tumour | ||||||
| No | 15 755 (75·6) | 81·8 (81·0, 82·6) | 1·00 (reference) | |||
| Yes | 3399 (16·3) | 77·9 (76·0, 79·7) | 1·34 (1·21, 1·49) | < 0·001 | ||
| Missing | 1699 (8·1) | |||||
| Acute or elective surgery | ||||||
| Elective | 18 205 (87·3) | 84·6 (83·8, 85·3) | 1·00 (reference) | 1·00 (reference) | ||
| Acute | 2641 (12·7) | 54·7 (52·4, 57·0) | 4·49 (4·13, 4·89) | < 0·001 | 1·90 (1·69, 2·13) | < 0·001 |
| Missing | 7 (0·03) | |||||
| Resection of other organ | ||||||
| No | 1059 of 1325 (79·9) | 83·0 (82·2, 83·8) | 1·00 (reference) | |||
| Yes | 266 of 1325 (20·1) | 69·3 (67·3, 71·3) | 1·90 (1·73, 2·08) | < 0·001 | ||
| Complication | ||||||
| No | 14 643 (70·2) | 84·0 (83·1, 84·8) | 1·00 (reference) | |||
| Yes | 6200 (29·7) | 73·8 (72·4, 75·2) | 2·18 (2·00, 2·38) | < 0·001 | ||
| Missing | 10 (0·05) | |||||
| Infection | ||||||
| No | 19 575 (93·9) | 81·8 (81·0, 82·5) | 1·00 (reference) | 1·00 (reference) | ||
| Yes | 1268 (6·1) | 67·6 (64·1, 70·8) | 2·59 (2·30, 2·91) | < 0·001 | 1·28 (1·09, 1·51) | 0·003 |
| Missing | 10 (0·05) | |||||
| Cardiovascular event | ||||||
| No | 19 956 (95·7) | 82·0 (81·2, 82·7) | 1·00 (reference) | 1·00 (reference) | ||
| Yes | 887 (4·3) | 56·2 (49·4, 62·3) | 4·62 (4·12, 5·17) | < 0·001 | 2·54 (2·14, 3·01) | < 0·001 |
| Missing | 10 (0·05) | |||||
| Primary intestinal perforation | ||||||
| No | 20 274 (97·2) | 81·3 (80·6, 82·0) | 1·00 (reference) | |||
| Yes | 577 (2·8) | 68·1 (63·1, 72·5) | 1·99 (1·66, 2·37) | < 0·001 | ||
| Missing | 2 (0·01) | |||||
| Perioperative bleeding (ml) | ||||||
| 0–400 | 15 025 (72·1) | 82·8 (81·9, 83·6) | 1·00 (reference) | 1·00 (reference) | ||
| > 400 | 4808 (23·1) | 75·9 (74·3, 77·4) | 1·45 (1·32, 1·59) | < 0·001 | 1·19 (1·07, 1·33) | 0·002 |
| Missing | 1020 (4·9) | |||||
| Sepsis, ICU admission, reoperation | ||||||
| No | 18 612 (89·2) | 82·3 (81·5, 83·0) | 1·00 (reference) | 1·00 (reference) | ||
| Yes | 2231 (10·7) | 69·3 (66·8, 71·6) | 2·36 (2·14, 2·61) | < 0·001 | 1·44 (1·26, 1·65) | < 0·001 |
| Missing | 10 (0·05) | |||||
Values in parentheses are percentages unless indicated otherwise;
values in parentheses are 95 per cent confidence intervals. EMRR, excess mortality rate ratio; LNR, lymph node ratio; LODDS, log odds of positive lymph nodes.
Table 3.
Relative survival and excess mortality rate ratio in the liver‐treated cohort
| Univariable Poisson regression | Multivariable Poisson regression | |||||
|---|---|---|---|---|---|---|
| No. of patients (n = 1325) | 5‐year age‐standardized relative survival (%)* | EMRR* | P | EMRR* | P | |
| Age (years) | ||||||
| < 50 | 121 (9·1) | 56·1 (46·6, 64·6) | 1·00 (0·75, 1·34) | 0·821 | ||
| 50–74 | 970 (73·2) | 53·3 (49·8, 56·7) | 1·00 (reference) | |||
| ≥ 75 | 234 (17·7) | 50·2 (41·9, 58·6) | 1·15 (0·90, 1·48) | 0·252 | ||
| Sex | ||||||
| M | 812 (61·3) | 52·1 (47·5, 56·5) | 1·00 (reference) | |||
| F | 513 (38·7) | 49·6 (43·8, 55·0) | 1·08 (0·90, 1·29) | 0·403 | ||
| BMI (kg/m 2 ) | ||||||
| < 18·5 | 16 (1·2) | 50·2 (22·6, 74·0) | 1·06 (0·48, 2·34) | 0·876 | ||
| 18·5–25 | 541 (40·8) | 50·5 (45·5, 55·6) | 1·00 (reference) | |||
| > 25 | 682 (51·5) | 53·1 (47·8, 58·2) | 0·94 (0·78, 1·13) | 0·506 | ||
| Missing | 86 (6·5) | |||||
| ASA grade | ||||||
| I–II | 1021 (77·1) | 54·3 (49·8, 58·6) | 1·00 (reference) | 1·00 (reference) | ||
| III–IV | 288 (21·7) | 43·5 (36·4, 50·3) | 1·54 (1·26, 1·88) | < 0·001 | 1·59 (1·28, 1·99) | < 0·001 |
| Missing | 16 (1·2) | |||||
| Location of primary tumour | ||||||
| Right colon | 268 (20·2) | 46·2 (39·0, 53·1) | 1·18 (0·93, 1·48) | 0·175 | ||
| Left colon | 507 (38·3) | 49·2 (43·8, 54·3) | 1·00 (reference) | |||
| Transverse colon | 44 (3·3) | 58·2 (39·6, 72·8) | 0·91 (0·53, 1·56) | 0·734 | ||
| Rectum | 467 (35·2) | 55·7 (48·2, 62·6) | 0·90 (0·74, 1·11) | 0·339 | ||
| Multiple | 39 (2·9) | 37·6 (22·2, 52·9) | 1·43 (0·89, 2·30) | 0·139 | ||
| pT status | ||||||
| pT0–2 | 161 (12·2) | 69·3 (56·2, 79·2) | 1·00 (reference) | |||
| pT3 | 915 (69·1) | 52·3 (48·0, 56·4) | 1·40 (1·02, 1·93) | 0·040 | ||
| pT4 | 247 (18·6) | 36·2 (29·3, 43·1) | 2·37 (1·67, 3·36) | < 0·001 | ||
| Missing | 2 (0·2) | |||||
| pN status | ||||||
| pN0 | 465 (35·1) | 61·0 (54·8, 66·5) | 1·00 (reference) | |||
| pN1 | 469 (35·4) | 54·1 (48·1, 59·7) | 1·47 (1·17, 1·86) | 0·001 | ||
| pN2 | 387 (29·2) | 35·1 (29·5, 40·8) | 2·38 (1·91, 2·98) | < 0·001 | ||
| pNX | 4 (0·3) | |||||
| M status | ||||||
| M0 | 581 (43·8) | 56·6 (51·3, 61·5) | 1·00 (reference) | |||
| M1 (synchronous) | 744 (56·2) | 48·0 (43·3, 52·5) | 1·24 (1·04, 1·49) | 0·018 | ||
| Synchronous metastases | ||||||
| None | 581 (43·8) | 56·6 (51·2, 61·6) | 1·00 (reference) | 1·00 (reference) | ||
| Liver metastases only | 611 (46·1) | 52·5 (47·2, 57·6) | 1·10 (0·90, 1·33) | 0·352 | 1·09 (0·88, 1·35) | 0·422 |
| Lung metastases only | 8 (0·6) | 10·8 (0·1, 50·8) | 1·96 (0·81, 4·76) | 0·138 | 2·31 (0·95, 5·62) | 0·065 |
| Other metastases only | 5 (0·4) | 65·3 (13·7, 95·9) | 0·69 (0·13, 3·74) | 0·668 | 0·86 (0·12, 6·30) | 0·882 |
| Multiple metastases | 120 (9·1) | 28·2 (18·6, 38·4) | 2·19 (1·68, 2·85) | < 0·001 | 1·80 (1·34, 2·42) | < 0·001 |
| LNR | ||||||
| 0 | 497 (37·5) | 60·7 (54·9, 66·0) | 1·00 (reference) | 1·00 (reference) | ||
| > 0 to < 0·1 | 259 (19·5) | 55·4 (47·3, 62·8) | 1·27 (0·97, 1·66) | 0·083 | 1·14 (0·84, 1·55) | 0·401 |
| 0·1 to < 0·25 | 271 (20·5) | 49·9 (40·8, 58·3) | 1·80 (1·40, 2·30) | < 0·001 | 1·48 (1·11, 1·98) | 0·007 |
| ≥ 0·25 | 289 (21·8) | 33·8 (27·6, 40·1) | 2·40 (1·91, 3·02) | < 0·001 | 2·03 (1·55, 2·65) | < 0·001 |
| Missing | 9 (0·7) | |||||
| LODDS | ||||||
| ≤ −1·36 | 927 (70·0) | 57·4 (53·0, 61·5) | 1·00 (reference) | |||
| > −1·36 to −0·53 | 213 (16·1) | 41·7 (32·1, 51·1) | 1·70 (1·36, 2·12) | < 0·001 | ||
| > −0·53 | 176 (13·3) | 31·1 (23·6, 38·9) | 2·19 (1·75, 2·73) | < 0·001 | ||
| Missing | 9 (0·7) | |||||
| Tumour grade | ||||||
| High/mean | 1085 (81·9) | 52·6 (48·6, 56·5) | 1·00 (reference) | 1·00 (reference) | ||
| Low | 185 (14·0) | 39·6 (31·4, 47·6) | 1·66 (1·32, 2·07) | < 0·001 | 1·39 (1·08, 1·77) | 0·009 |
| Missing | 55 (4·1) | |||||
| Vascular invasion | ||||||
| No | 710 (53·6) | 60·0 (54·6, 65·0) | 1·00 (reference) | 1·00 (reference) | ||
| Yes | 541 (40·8) | 40·4 (35·3, 45·5) | 1·85 (1·54, 2·21) | < 0·001 | 1·27 (1·03, 1·58) | 0·029 |
| Missing | 74 (5·6) | |||||
| Perineural invasion | ||||||
| No | 834 (62·9) | 57·1 (52·2, 61·7) | 1·00 (reference) | 1·00 (reference) | ||
| Yes | 311 (23·5) | 34·8 (29·0, 40·7) | 1·86 (1·54, 2·26) | < 0·001 | 1·25 (1·03, 1·58) | 0·046 |
| Missing | 180 (13·6) | |||||
| Mucinous tumour | ||||||
| No | 1093 (82·5) | 49·7 (45·9, 53·5) | 1·00 (reference) | |||
| Yes | 140 (10·6) | 53·6 (42·4, 63·6) | 0·99 (0·74, 1·32) | 0·943 | ||
| Missing | 92 (6·9) | |||||
| Acute or elective surgery | ||||||
| Elective | 1181 (89·1) | 53·2 (49·4, 56·9) | 1·00 (reference) | 1·00 (reference) | ||
| Acute | 144 (10·9) | 32·0 (24·7, 39·5) | 1·88 (1·48, 2·38) | < 0·001 | 1·97 (1·50, 2·58) | < 0·001 |
| Resection of other organ | ||||||
| No | 1059 (79·9) | 53·3 (49·4, 57·0) | 1·00 (reference) | |||
| Yes | 266 (20·1) | 42·9 (34·2, 51·3) | 1·34 (1·09, 1·64) | 0·004 | ||
| Complication | ||||||
| No | 945 (71·3) | 52·6 (48·4, 56·7) | 1·00 (reference) | |||
| Yes | 378 (28·5) | 46·9 (40·3, 53·2) | 1·24 (1·03, 1·49) | 0·025 | ||
| Missing | 2 (0·2) | |||||
| Infection | ||||||
| No | 1265 (95·5) | 51·7 (48·0, 55·2) | 1·00 (reference) | |||
| Yes | 58 (4·4) | 35·7 (23·8, 47·7) | 1·58 (1·10, 2·28) | 0·014 | ||
| Missing | 2 (0·2) | |||||
| Cardiovascular event | ||||||
| No | 1292 (97·5) | 51·7 (48·0, 55·3) | 1·00 (reference) | 1·00 (reference) | ||
| Yes | 31 (2·3) | 29·4 (15·2, 45·1) | 2·04 (1·25, 3·30) | 0·004 | 2·07 (1·15, 3·70) | 0·015 |
| Missing | 2 (0·2) | |||||
| Primary intestinal perforation | ||||||
| No | 1272 (96·0) | 51·8 (48·1, 55·3) | 1·00 (reference) | |||
| Yes | 42 (3·2) | 41·8 (28·8, 54·3) | 1·59 (1·02, 2·46) | 0·039 | ||
| Missing | 11 (0·8) | |||||
| Perioperative bleeding (ml) | ||||||
| 0–400 | 834 (62·9) | 52·4 (47·8, 56·7) | 1·00 (reference) | |||
| > 400 | 441 (33·3) | 49·2 (43·2, 54·9) | 1·03 (0·85, 1·24) | 0·757 | ||
| Missing | 50 (3·8) | |||||
| Sepsis, ICU admission, reoperation | ||||||
| No | 1201 (90·6) | 52·7 (48·9, 56·3) | 1·00 (reference) | 1·00 (reference) | ||
| Yes | 122 (9·2) | 33·9 (23·7, 44·4) | 1·74 (1·35, 2·24) | < 0·001 | 1·58 (1·18, 2·10) | 0·002 |
| Missing | 2 (0·2) | |||||
Values in parentheses are percentages unless indicated otherwise;
values in parentheses are 95 per cent confidence intervals. EMRR, excess mortality rate ratio; LNR, lymph node ratio; LODDS, log odds of positive lymph nodes.
Among patient factors at the time of resection of the primary tumour, several factors influenced survival in the R0 cohort. Older age, low BMI, ASA grade III–IV, acute (versus elective) surgery, cardiovascular events, serious postoperative complications and bleeding were significant risk factors in multivariable analysis (Table 2). For the liver‐treated cohort, age was not a significant risk factor, but ASA grade III–IV, acute surgery, cardiovascular events and postoperative complications remained significant in the multivariable analysis (Table 3). OS rates and HR values are presented in Tables S1 and S2 (supporting information).
When prognostic variables were evaluated over time comparing years 0–2 with years 2–5, HR values were higher in the R0 cohort for tumour site, tumour grade, acute versus elective surgery and severe complications in the first 2 years after surgery. For female patients, the HR value was more favourable 2–5 years after surgery. For the liver‐treated cohort, there were non‐proportional HR values for tumour location and tumour grade, with higher HRs in the first 2 years and for severe complications 2–5 years after liver surgery. All non‐proportional HRs are presented in Tables S3 and S4 (supporting information).
Discussion
This population‐based study highlights the importance of patient characteristics and primary tumour factors on survival after radical surgery for colorectal cancer and after surgery for liver metastases.
Liver surgery was performed in 4·6 per cent of all patients with colorectal cancer and in 6·4 per cent of those in the R0 cohort. In the R0 cohort, 38·7 per cent of patients with liver metastases underwent liver surgery. Despite high resection rates, survival was high with a 5‐year relative survival rate of 80·9 per cent in the colorectal R0 cohort and 49·6 per cent in the liver‐treated cohort. In Sweden, liver surgery is centralized to six university hospitals, and most patients with liver metastases are discussed in multidisciplinary tumour boards.
In the R0 cohort a greater than 40 per cent increase in excess mortality was observed in patients with ASA grades III–IV compared with grades I–II for pT3 and pT4 tumours, and for LNR above 0·1, acute resections and serious complications. In the liver‐treated cohort, EMRR values were generally lower than in the R0 cohort, but a greater than 40 per cent increase in EMRR remained for ASA grades III–IV, LNR above 0·1, acute primary tumour resections and serious complications at the time of primary tumour resection. By analysing the difference in EMRR over time, acute resection and serious postoperative complications continued to impact adversely on survival. A postoperative cardiovascular event after colorectal surgery more than doubled the risk of death after liver surgery, probably reflecting primary co‐morbidity indicating that a history of a risk factor is important in considering future liver surgery.
The impact of tumour factors did diminish over time, so these features should not influence treatment recommendations regarding late metachronous metastases.
Primary tumour location as a prognostic factor has attracted attention since reports43, 44 were made of increased KRAS and BRAF mutations in tumours of the right colon. Right‐sided tumours are also known to be more advanced when detected30, 32, 33. In the present study, excess mortality was observed for right‐sided colonic tumours, but this was not transmitted to the liver‐treated cohort.
LNR was the single most important local tumour factor for survival in the present study. In both cohorts, the increased risk observed for patients with cancer spread to vascular or neural spaces was minor in comparison with that for LNR, but registration might be underreported if pathologists were not aware of, or focused on, the importance of these factors28, 29.
Women underwent proportionally less liver surgery, although their survival was not inferior. Socioeconomic factors may be relevant here, but a prospective study in a primary R0 cohort would be needed to see whether there is a sex difference related to more aggressive spread19.
Age was inevitably a risk factor for death, but relative age did not significantly influence survival after liver surgery, even though only 2·7 per cent (234 of 8698) of those aged 75 years or above in the R0 cohort underwent resection compared with 9·0 per cent of younger patients. For the R0 cohort, patients aged 75 years and above did show an increased EMRR, indicating that those offered liver resection underwent further selection. A cohort study45 involving 186 patients aged 75 years or more treated by liver resection found a 5‐year OS rate of 28 per cent and a 5‐year cancer‐specific survival rate of 35 per cent. For the 234 patients aged 75 years or more who had liver surgery in the present study, the 5‐year OS rate was 38·6 per cent and the 5‐year relative survival rate was 50·2 per cent, confirming the value of this approach. Undertreatment of elderly patients has been cited as a significant contributor to poor results in population‐based studies of colorectal cancer survival46.
There was a difference in outcome in patients treated for synchronous or metachronous liver metastases. Patients with metastases that were treated surgically 0·76–1·54 years after diagnosis of the primary tumour had the worst outcome. This might reflect selection of synchronous metastases that needed to be downsized by systemic therapy to become resectable. Patients with metachronous tumours treated 1·54–7·12 years after diagnosis had a better prognosis, in accordance with other reports47. This difference was relatively small in the present study, and the study design could not distinguish between a selection effect or potentially better biology of metachronous tumours.
This study has limitations. The main weakness is that it was retrospective, although the data should have been entered contemporaneously. Registration of the primary tumours and of surgically treated liver metastases was high, but there is a risk that some metachronous metastases were missed. As the study focused on risk factors in a liver‐treated cohort, it is believed that the number of missing patients is likely to be small and unlikely to influence the results. The strengths of this study include a large study population, with a resected R0 cohort and liver‐resected group from a nationwide perspective. The Swedish colorectal cancer register had a coverage of 96–100 per cent during the study period48 and, although coverage for SweLiv was somewhat lower than that for the Swedish hospital register49, the real rate of liver interventions could be only a few percentage points higher at most.
Co‐morbidity, acute surgery for the primary tumour and complications after primary tumour surgery are risk factors for excess mortality after radical colorectal surgery that are transmitted to the liver intervention. LNR and concomitant or earlier metastases are also risk factors that should be considered when treatment for liver metastases is under consideration. Age and timing of metastases are of less importance.
Supporting information
Appendix S1. Supporting information
Acknowledgements
This study was not preregistered in an independent, institutional registry. All methods and data are available to other researchers on request.
The study was funded by Cancerfonden (CAN 2018/664) and by the Swedish government and county councils, the ALF agreement (ALFGBG‐722971).
P.S. received scholarships from Stig och Ragna Gorthons Stiftelse and Thelma Zoégas fond för medicinsk forskning.
Disclosure: The authors declare no conflict of interest.
Funding information
Cancerfonden, CAN 2018/664
Swedish government and county councils, the ALF agreement, ALFGBG‐722971
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M et al; CONCORD Working Group . Global surveillance of trends in cancer survival 2000–14 (CONCORD‐3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population‐based registries in 71 countries. Lancet 2018; 391: 1023–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allemani C, Rachet B, Weir HK, Richardson LC, Lepage C, Faivre J et al Colorectal cancer survival in the USA and Europe: a CONCORD high‐resolution study. BMJ Open 2013; 3: e003055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Snaebjornsson P, Jonasson L, Olafsdottir EJ, van Grieken NCT, Moller PH, Theodors A et al Why is colon cancer survival improving by time? A nationwide survival analysis spanning 35 years. Int J Cancer 2017; 141: 531–539. [DOI] [PubMed] [Google Scholar]
- 5. Benitez Majano S, Di Girolamo C, Rachet B, Maringe C, Guren MG, Glimelius B et al Surgical treatment and survival from colorectal cancer in Denmark, England, Norway, and Sweden: a population‐based study. Lancet Oncol 2019; 20: 74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brouwer NPM, Bos A, Lemmens V, Tanis PJ, Hugen N, Nagtegaal ID et al An overview of 25 years of incidence, treatment and outcome of colorectal cancer patients. Int J Cancer 2018; 143: 2758–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cavalli‐Bjorkman N, Lambe M, Eaker S, Sandin F, Glimelius B. Differences according to educational level in the management and survival of colorectal cancer in Sweden. Eur J Cancer 2011; 47: 1398–1406. [DOI] [PubMed] [Google Scholar]
- 8. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017; 66: 683–691. [DOI] [PubMed] [Google Scholar]
- 9. Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 2006; 244: 254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Engstrand J, Nilsson H, Stromberg C, Jonas E, Freedman J. Colorectal cancer liver metastases – a population‐based study on incidence. Management and survival. BMC Cancer 2018; 18: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Dam RM, Lodewick TM, van den Broek MA, de Jong MC, Greve JW, Jansen RL et al Outcomes of extended versus limited indications for patients undergoing a liver resection for colorectal cancer liver metastases. HPB (Oxford) 2014; 16: 550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wanis KN, Pineda‐Solis K, Tun‐Abraham ME, Yeoman J, Welch S, Vogt K et al Management of colorectal cancer with synchronous liver metastases: impact of multidisciplinary case conference review. Hepatobiliary Surg Nutr 2017; 6: 162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ioannidis A, Konstantinidis M, Apostolakis S, Koutserimpas C, Machairas N, Konstantinidis KM. Impact of multidisciplinary tumor boards on patients with rectal cancer. Mol Clin Oncol 2018; 9: 135–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van de Velde CJ, Boelens PG, Borras JM, Coebergh JW, Cervantes A, Blomqvist L et al EURECCA colorectal: multidisciplinary management: European consensus conference colon & rectum. Eur J Cancer 2014; 50: 1.e1–1.e34. [DOI] [PubMed] [Google Scholar]
- 15. Elferink MA, de Jong KP, Klaase JM, Siemerink EJ, de Wilt JH. Metachronous metastases from colorectal cancer: a population‐based study in North‐East Netherlands. Int J Colorectal Dis 2015; 30: 205–212. [DOI] [PubMed] [Google Scholar]
- 16. Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR et al Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 2004; 239: 818–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P et al; EORTC Gastro‐Intestinal Tract Cancer Group, Cancer Research UK, Arbeitsgruppe Lebermetastasen und‐tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM‐CAO), Australasian Gastro‐Intestinal Trials Group (AGITG), Federation Francophone de Cancerologie Digestive (FFCD). Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long‐term results of a randomised, controlled, phase 3 trial. Lancet Oncol 2013; 14: 1208–1215. [DOI] [PubMed] [Google Scholar]
- 18. Robertson DJ, Stukel TA, Gottlieb DJ, Sutherland JM, Fisher ES. Survival after hepatic resection of colorectal cancer metastases: a national experience. Cancer 2009; 115: 752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Norén A, Eriksson HG, Olsson LI. Selection for surgery and survival of synchronous colorectal liver metastases; a nationwide study. Eur J Cancer 2016; 53: 105–114. [DOI] [PubMed] [Google Scholar]
- 20. Morris EJ, Forman D, Thomas JD, Quirke P, Taylor EF, Fairley L et al Surgical management and outcomes of colorectal cancer liver metastases. Br J Surg 2010; 97: 1110–1118. [DOI] [PubMed] [Google Scholar]
- 21. Booth CM, Nanji S, Wei X, Biagi JJ, Krzyzanowska MK, Mackillop WJ. Surgical resection and peri‐operative chemotherapy for colorectal cancer liver metastases: a population‐based study. Eur J Surg Oncol 2016; 42: 281–287. [DOI] [PubMed] [Google Scholar]
- 22. Janssen‐Heijnen ML, Maas HA, Houterman S, Lemmens VE, Rutten HJ, Coebergh JW. Comorbidity in older surgical cancer patients: influence on patient care and outcome. Eur J Cancer 2007; 43: 2179–2193. [DOI] [PubMed] [Google Scholar]
- 23. Kaneko M, Sasaki S, Ozaki K, Ishimaru K, Terai E, Nakayama H et al Underweight status predicts a poor prognosis in elderly patients with colorectal cancer. Mol Clin Oncol 2016; 5: 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patel GS, Ullah S, Beeke C, Hakendorf P, Padbury R, Price TJ et al Association of BMI with overall survival in patients with mCRC who received chemotherapy versus EGFR and VEGF‐targeted therapies. Cancer Med 2015; 4: 1461–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Derwinger K, Carlsson G, Gustavsson B. A study of lymph node ratio as a prognostic marker in colon cancer. Eur J Surg Oncol 2008; 34: 771–775. [DOI] [PubMed] [Google Scholar]
- 26. van der Stok EP, Grunhagen DJ, Alberda WJ, Reitsma M, Rothbarth J, Verhoef C. The prognostic value of the primary tumor's nodal status after surgery for colorectal liver metastases in the era of effective systemic therapy. Dig Surg 2015; 32: 208–216. [DOI] [PubMed] [Google Scholar]
- 27. Duchalais E, Glyn Mullaney T, Spears GM, Kelley SR, Mathis K, Harmsen WS et al Prognostic value of pathological node status after neoadjuvant radiotherapy for rectal cancer. Br J Surg 2018; 105: 1501–1509. [DOI] [PubMed] [Google Scholar]
- 28. Cardona K, Mastrodomenico P, D'Amico F, Shia J, Gonen M, Weiser MR et al Detailed pathologic characteristics of the primary colorectal tumor independently predict outcome after hepatectomy for metastases. Ann Surg Oncol 2013; 20: 148–154. [DOI] [PubMed] [Google Scholar]
- 29. Spelt L, Sasor A, Ansari D, Andersson R. Pattern of tumour growth of the primary colon cancer predicts long‐term outcome after resection of liver metastases. Scand J Gastroenterol 2016; 51: 1233–1238. [DOI] [PubMed] [Google Scholar]
- 30. Sasaki K, Andreatos N, Margonis GA, He J, Weiss M, Johnston F et al The prognostic implications of primary colorectal tumor location on recurrence and overall survival in patients undergoing resection for colorectal liver metastasis. J Surg Oncol 2016; 114: 803–809. [DOI] [PubMed] [Google Scholar]
- 31. Vigano L, Russolillo N, Ferrero A, De Rosa G, Ferreri E, Forchino F et al Resection of liver metastases from colorectal mucinous adenocarcinoma: is this a different disease? Results of a case–control study. Ann Surg 2014; 260: 878–884. [DOI] [PubMed] [Google Scholar]
- 32. Missiaglia E, Jacobs B, D'Ario G, Di Narzo AF, Soneson C, Budinska E et al Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol 2014; 25: 1995–2001. [DOI] [PubMed] [Google Scholar]
- 33. Gao XH, Yu GY, Gong HF, Liu LJ, Xu Y, Hao LQ et al Differences of protein expression profiles, KRAS and BRAF mutation, and prognosis in right‐sided colon, left‐sided colon and rectal cancer. Sci Rep 2017; 7: 7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999; 230: 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Abdalla EK, Bauer TW, Chun YS, D'Angelica M, Kooby DA, Jarnagin WR. Locoregional surgical and interventional therapies for advanced colorectal cancer liver metastases: expert consensus statements. HPB (Oxford) 2013; 15: 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schreckenbach T, Malkomes P, Bechstein WO, Woeste G, Schnitzbauer AA, Ulrich F. The clinical relevance of the Fong and the Nordlinger scores in the era of effective neoadjuvant chemotherapy for colorectal liver metastasis. Surg Today 2015; 45: 1527–1534. [DOI] [PubMed] [Google Scholar]
- 37. Ayez N, Lalmahomed ZS, van der Pool AE, Vergouwe Y, van Montfort K, de Jonge J et al Is the clinical risk score for patients with colorectal liver metastases still useable in the era of effective neoadjuvant chemotherapy? Ann Surg Oncol 2011; 18: 2757–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pahlman L, Bohe M, Cedermark B, Dahlberg M, Lindmark G, Sjodahl R et al The Swedish rectal cancer registry. Br J Surg 2007; 94: 1285–1292. [DOI] [PubMed] [Google Scholar]
- 39. Moberger P, Sköldberg F, Birgisson H. Evaluation of the Swedish Colorectal Cancer Registry: an overview of completeness, timeliness, comparability and validity. Acta Oncol 2018; 57: 1611–1621. [DOI] [PubMed] [Google Scholar]
- 40. Ederer F, Axtell LM, Cutler SJ. The relative survival rate: a statistical methodology. Natl Cancer Inst Monogr 1961; 6: 101–121. [PubMed] [Google Scholar]
- 41. Corazziari I, Quinn M, Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer 2004; 40: 2307–2316. [DOI] [PubMed] [Google Scholar]
- 42. Dickman PW, Sloggett A, Hills M, Hakulinen T. Regression models for relative survival. Stat Med 2004; 23: 51–64. [DOI] [PubMed] [Google Scholar]
- 43. Xie MZ, Li JL, Cai ZM, Li KZ, Hu BL. Impact of primary colorectal cancer location on the KRAS status and its prognostic value. BMC Gastroenterol 2019; 19: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cushman‐Vokoun AM, Stover DG, Zhao Z, Koehler EA, Berlin JD, Vnencak‐Jones CL. Clinical utility of KRAS and BRAF mutations in a cohort of patients with colorectal neoplasms submitted for microsatellite instability testing. Clin Colorectal Cancer 2013; 12: 168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Booth CM, Nanji S, Wei X, Mackillop WJ. Management and outcome of colorectal cancer liver metastases in elderly patients: a population‐based study. JAMA Oncol 2015; 1: 1111–1119. [DOI] [PubMed] [Google Scholar]
- 46. Sorbye H, Cvancarova M, Qvortrup C, Pfeiffer P, Glimelius B. Age‐dependent improvement in median and long‐term survival in unselected population‐based Nordic registries of patients with synchronous metastatic colorectal cancer. Ann Oncol 2013; 24: 2354–2360. [DOI] [PubMed] [Google Scholar]
- 47. Landreau P, Drouillard A, Launoy G, Ortega‐Deballon P, Jooste V, Lepage C et al Incidence and survival in late liver metastases of colorectal cancer. J Gastroenterol Hepatol 2015; 30: 82–85. [DOI] [PubMed] [Google Scholar]
- 48. Regionala Cancercentrum Norr . Kvalitetsregisterrapporter 2009‐2013 https://www.cancercentrum.se/samverkan/cancerdiagnoser/tjocktarm-andtarm-och-anal/tjock--och-andtarm/kvalitetsregister/rapporter/ [accessed 2 November 2019].
- 49. Norén A, Sandström P, Gunnarsdottir K, Ardnor B, Isaksson B, Lindell G et al Identification of inequalities in the selection of liver surgery for colorectal liver metastases in Sweden. Scand J Surg 2018; 107: 294–301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information


