Abstract
Animals can smell odors from the external environment or from their mouth via two routes: orthonasal and retronasal, respectively. Little is known about how the brain processes orthonasal and retronasal odors associated with taste, but a new study has revealed an important role for the gustatory cortex in such odor processing.
Imagine the best meal you have ever consumed; or if you prefer, imagine the worst food you’ve ever had. Your memories and descriptions are likely filled with rich expressions of far more than simple mixtures of sweet, salty, bitter, sour and savory. The experience of eating, or drinking, involves a combination of tastes, smells, sights, mouth feels and even sounds. Smells, in particular, occupy a central role in the tasting experience. Olfaction is such an important partner to gustation, and plays such a central role in the experience of flavor, that many languages erroneously confuse the two experiences [1], illustrated by comments such as “I love the taste of cinnamon in grandma’s pie”.
Odors have a dual relationship with taste: they can either precede it or accompany it. The former happens when we smell the aroma of the food in front of us through what is known as orthonasal smelling. The latter happens when food in the mouth liberates volatile molecules that are carried by air through the pharynx into the nose, a process called retronasal smelling. In this issue of Current Biology, Blankenship et al. [2] present novel results on how orthonasal and retronasal odors are associated with taste and processed by the gustatory cortex.
The relationship of orthonasal and retronasal smelling to taste has been extensively investigated in behavioral studies. Take, for example, the phenomenon of taste-potentiated odor aversion. In conditioned taste aversion, an animal rapidly learns to avoid a taste that precedes gastric malaise. When an odor precedes gastric malaise, the aversion is relatively weak [3]; however, when the taste and odor simultaneously precede gut illness, aversive learning extends to both stimuli [4]. These studies also provided crucial evidence on the importance of the route of odor presentation. Indeed, the effectiveness of the same odor is different if it is presented with the ingested solution (retronasal) or in a cup in the external environment (orthonasal) [5].
Despite the enormous biological relevance of the interaction between taste and olfaction, the neural circuits responsible for processing orthonasal odors, retronasal odors and taste are not yet fully understood. Indeed, only relatively recently have researchers started to study how the brain integrates these two modalities. For decades, studies on the neural basis of chemosensation focused on either olfaction or taste. Odor processing has been studied in the olfactory system, regardless of whether odors traveled via the orthonasal or retronasal route, and taste processing has been investigated in the gustatory system. Early work on the neural basis of odor-taste integration postulated that signals traveled two distinct sensory streams to converge exclusively onto neurons in the orbitofrontal cortex [6]. This view became very influential and established the often-cited notion that the integration between taste and olfaction was performed exclusively by a ‘high order’ area, such as the orbitofrontal cortex. Recent work, however, has contradicted this established view and indicates that the two streams of chemosensory information are not entirely separated, but that interplay between taste and olfaction is already occurring well before signals reach the orbitofrontal cortex. Neurons in the olfactory cortex can respond to taste [7] and neurons in the gustatory cortex can respond to odors [8–10]. Indeed, odors delivered both orthonasally as well as directly into the mouth through a cannula can change firing rates of gustatory cortex neurons.
While this body of work led to a radical revision of the models of chemosensory integration (see [11] for a review on sensory integration), it still largely relied on the assumption that ortho- and retronasally smelled odors travel through the same neural pathways, albeit inducing different patterns of activation [12]. Pioneering functional magnetic resonance imaging (fMRI) experiments in humans shifted this view. By directly comparing responses to orthonasally and retronasally delivered odors, the researchers showed that the route of odor presentation has significant effects on the patterns of brain activation [13]. The results presented in this issue of Current Biology by Blankenship et al. [2] significantly extend this work, showing that the rodent brain treats ortho- and retronasally smelled odors very differently when it comes to associating them with taste. Furthermore, the authors provide the first evidence of a behavioral task in which the gustatory cortex is necessary for processing retronasal, but not orthonasal, odors (Figure 1A,B).
Figure 1. Gustatory cortex engagement in different odor–taste paradigms.
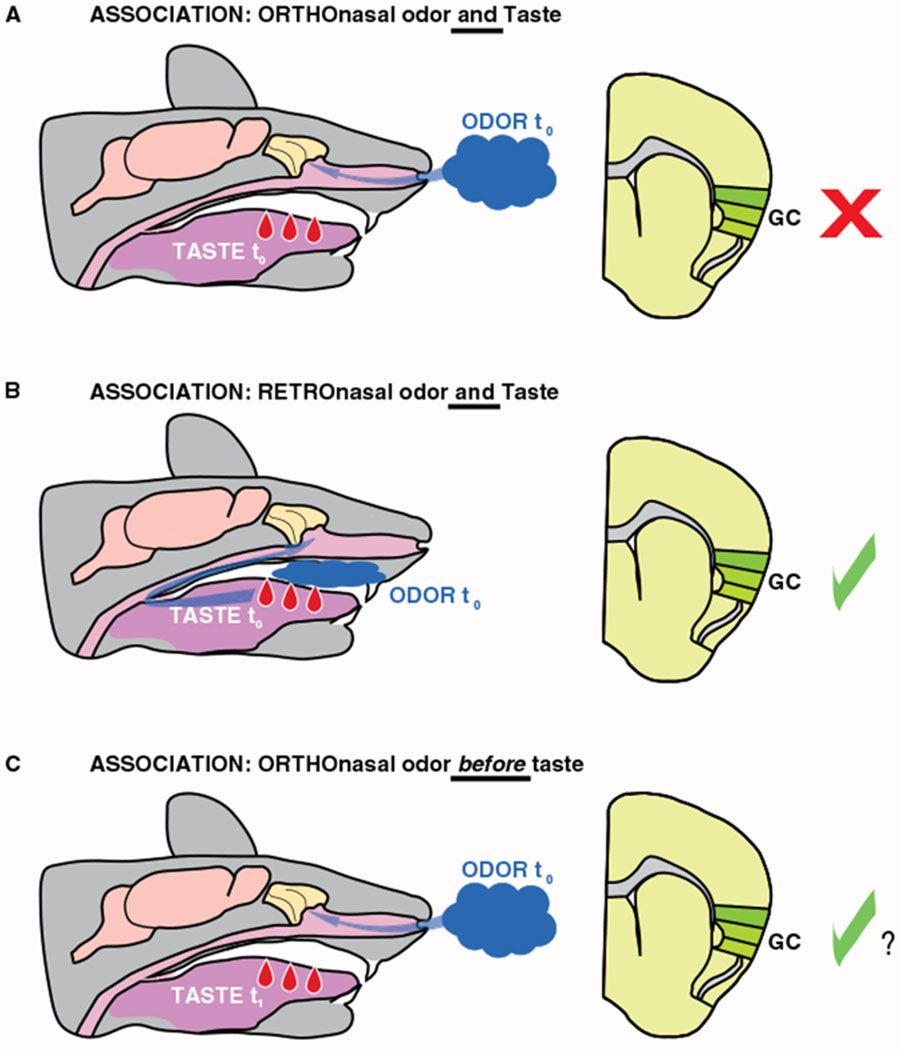
(A) Schematic sagittal section of the mouse head (left) depicting simultaneous presentation of orthonasal odor and taste (t0). In this issue of Current Biology, Blankenship et al. [2] report that associations between orthonasal odors and taste are slower to establish than associations between retronasal odors and taste. In addition, they demonstrate that the gustatory cortex is not necessary for the expression of an odor preference following association between an orthonasal odor and saccharin (right: coronal section featuring gustatory cortex, GC). (B) The gustatory cortex is necessary for the expression of a preference developed with the simultaneous presentation of retronasal odor and taste. (C) Organisms can learn pairings when orthonasal odors precede (t0) taste (t1) and activity in gustatory cortex is recruited. The extent to which timing and route of odor exposure interact with taste and the circumstances in which gustatory cortex is necessary have yet to be exhaustively studied. We hypothesize that the gustatory cortex will play a causal role in the expression of these associations, based on prior studies on the involvement of gustatory cortex in mediating behaviors triggered by predictive cues.
Blankenship et al. [2] devised a very elegant behavioral paradigm, which relies on odor preference learning to investigate differences in the processing of retronasally and orthonasally delivered odors. The paradigm consists of three phases: baseline, training and testing. At baseline, rats are exposed to two odors upon entering each of two nose ports. In one condition, the two odors are orthonasally delivered via an olfactometer, which puffs odorized air in front of the nose of the rat. In the second condition, both odors are retronasally delivered via an intraoral cannula, which delivers an odorized solution directly into the mouth. In both cases, odor presentation is paired with water delivery through the intraoral cannula.
This design ensures the same type of intraoral stimulation in both conditions and maintains similar levels of motivation for rats to enter the nose port. Within each condition, the preference for one of the two odors is assessed by comparing the number of entries in each port. After assessing baseline preference, rats are trained for either two or three sessions to associate one odor, either orthonasally or retronasally delivered, with a sweet tastant (saccharin) and the other odor with water. Finally, the preference is again tested by comparing nose poking for the two ports. The authors used four conditions in which baseline/training/testing relied exclusively on orthonasal odor, only on retronasal odor, on retronasal training/orthonasal testing, and on a combined ortho-/retronasal training with orthonasal testing.
The results of these experiments show that: rats develop a preference for retronasal-paired odors with fewer testing sessions than for orthonasal odors; training a retronasal odor–sucrose pairing does not generalize to an orthonasal test of the same odor; and training with a combined retro-/orthonasal delivery leads to the rapid development of a preference when tested orthonasally. This set of results is consistent with orthonasal and retronasal odors evoking different percepts — see [14] for recent evidence of the “Duality of Smell” for humans — and establishes a privileged interaction between retronasal odors and taste in preference learning.
In a second, and extremely important, set of experiments, Blankenship et al. [2] used optogenetic silencing to test the role of gustatory cortex. They found that inactivation of the gustatory cortex selectively affects the expression of retronasally trained and tested preference (Figure 1B), while sparing orthonasally (Figure 1A) and combined orthonasally/retronasally developed preference. These results demonstrate an entirely new function of the gustatory cortex in the expression of odor preference learning for retronasally delivered odors.
The Blankenship et al. [2] study has broad implications for theories of taste processing, olfactory processing and flavor formation. It provides evidence for the necessity of the gustatory cortex in processing intraoral stimuli related to food independently of the specific modality (olfaction or taste). In light of these and other results, it may be more appropriate to view what we now call gustatory cortex as a cortical area devoted to the experience of eating (and drinking). The results of Blankenship et al. [2] suggest a potential role of the gustatory cortex in creating associations between intraoral stimuli. However, temporal inactivation experiments during training will be required to prove the role of gustatory cortex in learning.
At first glance, the lack of involvement of gustatory cortex in mediating the expression of preferences for orthonasal odors seems at odds with the literature. Orthonasally smelled odors can acquire gustatory qualities, presumably through learning [15]. Both human imaging as well as single unit electrophysiology in rodents indicate that orthonasally delivered odors can recruit the gustatory cortex [9,13]. Furthermore, the neural representation of an orthonasally delivered odor expands upon learning that the odor cue predicts the availability of sucrose [9]. It seems highly unlikely that odor cue responses in gustatory cortex would not play any behavioral role, considering that inactivating gustatory cortex during a taste predictive, audiovisual cue reduces conditioned responses toward a food port [16].
The solution to this apparent contradiction may reside in the importance of relative timing between olfactory and gustatory stimulation. When food is consumed, retronasal activation of olfactory receptor neurons and intraoral activation of taste receptor cells happen simultaneously. In this context, as both odor and taste refer to food, the two stimuli congruently point to the same object. Orthonasal smelling, on the other hand, typically precedes gustatory stimulation, rather than accompanying it (Figure 1C). Orthonasally smelled odors are cues that anticipate taste, allowing animals to predict the available food and decide the course of action. The simultaneous presentation of orthonasal odor and intraoral taste delivery is unlikely to mimic natural forms of odor–taste integration.
This ecological perspective may explain the slower rate of learning seen for orthonasal odors — described by Blankenship et al. [2] and in classical work on taste potentiated odor aversion [5] — as well as the lack of gustatory cortex involvement in the expression of preferences for orthonasal odors. The importance of timing in flavor perception has been well established by psychophysical and behavioral experiments (reviewed in [17]), and we suggest that this parameter is critical in determining odor–taste associations as well as the recruitment of gustatory cortex. Of course, only further experiments will be able to tell us if this is the case.
In conclusion, the Blankenship et al. [2] article is a milestone in the groundbreaking series of studies from these groups on odor/taste integration [7,18–20]. The strength of this work resides not only in its originality and elegance, but also in its combined ecological and neurobiological perspective. Indeed, embracing the inherently complex sensory nature of foraging, consumption and ingestion has allowed the authors to provide a decisive contribution to our understanding of the cortex in flavor perception.
REFERENCES
- 1.Rozin P (1982). “Taste-smell confusions” and the duality of the olfactory sense. Percept. Psychophys 31, 397–401. [DOI] [PubMed] [Google Scholar]
- 2.Blankenship ML, Grigorova M, Katz DB, and Maier JX (2019). Retronasal, but not orthonasal, odor perception requires taste cortex. Curr. Biol 29, 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hankins WG, Garcia J, and Rusiniak KW (1973). Dissociation of odor and taste in baitshyness. Behav. Biol 8, 407–419. [DOI] [PubMed] [Google Scholar]
- 4.Palmerino CC, Rusiniak KW, and Garcia J (1980). Flavor-illness aversions: The peculiar roles of odor and taste in memory for poison. Science 208, 753–755. [DOI] [PubMed] [Google Scholar]
- 5.Bouton ME, Jones DL, McPhillips SA, and Swartzentruber D (1986). Potentiation and overshadowing in odor-aversion learning: Role of method of odor presentation, the distal-proximal cue distinction, and the conditionability of odor. Learn. Motiv 17, 115–138. [Google Scholar]
- 6.Rolls ET (2016). Flavor: Brain processing In Flavor: From Food to Behaviors, Wellbeing and Health, Etiévant P, et al. , eds. (New York: Woodhead Publishing; ), pp. 143–160. [Google Scholar]
- 7.Maier JX, Wachowiak M, and Katz DB (2012). Chemosensory convergence on primary olfactory cortex. J. Neurosci 32, 17037–17047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott TR, and Plata-Salaman CR (1999). Taste in the monkey cortex. Physiol. Behav 67, 489–511. [DOI] [PubMed] [Google Scholar]
- 9.Vincis R, and Fontanini A (2016). Associative learning changes cross-modal representations in the gustatory cortex. eLife 5, e16420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samuelsen CL, and Fontanini A (2017). Processing of intraoral olfactory and gustatory signals in the gustatory cortex of awake rats. J. Neurosci 37, 244–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verhagen JV, and Engelen L (2006). The neurocognitive bases of human multimodal food perception: Sensory integration. Neurosci. Biobehav. Rev 30, 613–650. [DOI] [PubMed] [Google Scholar]
- 12.Gautam SH, and Verhagen JV (2012). Retronasal odor representations in the dorsal olfactory bulb of rats. J. Neurosci 32, 7949–7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Small DM, Gerber JC, Mak YE, and Hummel T (2005). Differential neural responses evoked by orthonasal versus retronasal odorant perception in humans. Neuron 18, 593–605. [DOI] [PubMed] [Google Scholar]
- 14.Hannum M, Stegman MA, Fryer JA, and Simons CT (2018). Differential olfactory percepts evoked by orthonasal and retronasal odorant delivery. Chem. Senses 43, 515–521. [DOI] [PubMed] [Google Scholar]
- 15.Stevenson RJ, Prescott J, and Boakes RA (1995). The acquisition of taste properties by odors. Learn. Motiv 26, 1–23. [Google Scholar]
- 16.Kusumoto-Yoshida I, Liu H, Chen BT, Fontanini A, and Bonci A (2015). Insular cortex mediates responses to cues. Proc. Natl. Acad. Sci. USA 112, 1190–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Small DM, and Prescott J (2005). Odor/taste integration and the perception of flavor. Exp. Brain Res 166, 345–357. [DOI] [PubMed] [Google Scholar]
- 18.Maier JX (2017). Single-neuron responses to intraoral delivery of odor solutions in primary and gustatory cortex. J. Neurophysiol 117, 1293–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fortis-Santiago Y, Rodwin BA, Neseliler S, Piette CE, and Katz DB (2009). State dependence of olfactory perception as a function of taste cortical inactivation. Nat. Neurosci 13, 158–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maier JX, Blankenship ML, Li JX, and Katz DB (2015). A multisensory network for olfactory processing. Curr. Biol 25, 2642–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]


