Abstract
The inappropriate deposition of extracellular matrix within the heart (termed cardiac fibrosis) is associated with nearly all types of heart disease, including ischemic, hypertensive, diabetic, and valvular. This alteration in the composition of the myocardium can physically limit cardiomyocyte contractility and relaxation, impede electrical conductivity, and hamper regional nutrient diffusion. Fibrosis can be grossly divided into 2 types, namely reparative (where collagen deposition replaces damaged myocardium) and reactive (where typically diffuse collagen deposition occurs without myocardial damage). Despite the widespread association of fibrosis with heart disease and general understanding of its negative impact on heart physiology, it is still not clear when collagen deposition becomes pathologic and translates into disease symptoms. In this review, we have summarized the current knowledge of cardiac fibrosis in human patients and experimental animal models, discussing the mechanisms that have been deduced from the latter in relation to the former. Because assessment of the extent of fibrosis is paramount both as a research tool to further understanding and as a clinical tool to assess patients, we have also summarized the current state of noninvasive/minimally invasive detection systems for cardiac fibrosis. Albeit not exhaustive, our aim is to provide an overview of the current understanding of cardiac fibrosis, both clinically and experimentally.
INTRODUCTION
The heart is a perpetually working muscle whose function is to pump oxygenated blood throughout the body in order to maintain the viability of all organs, including the heart itself. It is a complex organ that is made up of a variety of cells including cardiomyocytes, vascular smooth muscle cells, endothelial cells, macrophages, and others. Fibroblasts are among the most abundant cell types in the heart. While the exact percentage remains controversial, they have been reported to comprise as many as half of the cells in the heart in rodent species.1 Although cardiac fibroblasts serve a variety of purposes, their main role is to generate and maintain a scaffold infrastructure that holds the heart together, transduces the shortening of individual cardiomyocytes into efficient muscle pump activity, and helps anchor in place other cardiac cells that regulate cardiomyocyte function.2,3 Given the close association between the heart’s fibrous tissue infrastructure and cardiomyocytes, which are the contractile units of the heart, changes in the properties of the fibrous mesh can adversely affect both the heart’s pumping function and its filling properties. Not surprisingly, alterations in either the quality or quantity of the fibrous tissue infrastructure contribute substantially to the development and severity of heart failure.
REPARATIVE VS REACTIVE FIBROSIS
To provide the scaffold necessary to hold the heart together and coordinate its function as a pump, cardiac fibroblasts must ultimately produce a variety of provisional and structural proteins, the most important of which are the collagens, particularly collagen I (Col I) and collagen III (Col III). Collectively, these extracellular matrix (ECM) proteins define the fibrous meshwork of the heart. Abnormalities in the ECM can occur due to abnormal quantities of ECM proteins (both excesses and deficiencies), alterations in ECM quality (eg, changes in crosslinking), and changes in the proportion of the various individual components of the ECM (including both alterations in the proportion of noncollagen to collagen matrix components4 and in the relative amounts of Col I to Col III). Regulation of the amount and composition of the ECM is a dynamic process involving both the production and degradation of collagen molecules. Cardiac fibrosis, which is a cause or companion of many cardiovascular diseases, occurs when there is an imbalance between these processes so that the production of ECM proteins exceeds their degradation.
There are 2 types of fibrosis in the heart.5 Cardiac fibrosis that develops in response to a loss of cardiomyocytes is considered to be reparative fibrosis. This type of fibrosis is stimulated by myocyte necrosis and is an essential reparative response to injury and cell death. In the heart, perhaps the most prominent and relevant example of this is the generation of a replacement scar for a segment of myocardium that has undergone extensive cardiomyocyte death as a consequence of a myocardial infarction (MI). The timely formation of an adequate replacement scar in the infarct zone is a critical response and the failure for this to occur enhances the likelihood of post-MI myocardial rupture, a complication that is usually fatal. Replacement of devitalized bulging myocardium in the infarct zone by stiffer, less distensible fibrous tissue also limits post-MI dilatation. In controlling the increase in ventricular radius, deposition of a replacement scar helps to limit increases in wall stress in the chamber. As wall stress is an important stimulus for further maladaptive remodeling of the ventricle, expeditious formation of the replacement scar would also be expected to impact a patient’s subsequent clinical course.
In contrast to replacement fibrosis, reactive fibrosis is the term used for the diffuse deposition of collagen throughout the myocardium. It occurs in the absence of cell death and can be stimulated by prolonged periods of stress or by exposure to profibrotic mediators. Imposition of a pressure load on the heart, as occurs with aortic stenosis or systemic hypertension,6,7 increases wall stress in the left ventricle and has been shown to promote reactive fibrosis in the chamber. Activation of neurohormonal systems, both intracardiac and systemic, which give rise to increased levels of mediators that stimulate cardiac fibroblasts to produce ECM proteins (eg, angiotensin II (Ang II), aldosterone, catecholamines), also produces reactive fibrosis in the heart.8,9 Furthermore, diseases or conditions that trigger an inflammatory response, either systemically or locally, can cause reactive fibrosis to develop. As will be discussed subsequently, these include obesity, diabetes, metabolic syndrome, infections of the heart, drugs, and radiation. The pattern of ECM deposition in reactive fibrosis, while more diffuse than with reparative fibrosis, can vary. Depending on the stimulus, reactive fibrosis can develop in a relatively homogeneous pattern throughout the myocardium (interstitial fibrosis), while in other situations it may be more prominent in the tissue surrounding intracardiac blood vessels (ie, perivascular fibrosis). In systemic hypertension, there is a gradient of fibrosis in the left ventricle from the endocardial to epicardial surface, reflecting the gradient in wall stress seen in the chamber as a result of the increased pressure load.
The distinction between reparative and reactive fibrosis is important as these processes have different triggers, mechanisms, and consequences. However, distinct separation between them in the complex environment of human heart disease is not straightforward. For instance, while it is widely recognized that reparative fibrosis is responsible for the generation of replacement scar in the setting of an MI, it probably also plays a role in the development of the diffuse intracardiac fibrosis caused by microischemia in patients with small vessel disease.10,11 The 2 forms of fibrosis also likely coexist in many patients. An example of this is in the post-MI heart, where in addition to the reparative fibrosis that develops to generate replacement scar, there is diffuse reactive fibrosis in segments of myocardium that are distant from the infarct zone.12
EFFECTS OF FIBROSIS ON THE HEART
Whereas reparative fibrosis serves an important role in maintaining the integrity of the heart after an MI, reactive fibrosis is clearly a less beneficial process. In the normal heart, cardiomyocytes are integrated into contractile units by a well-defined fibrous network. This fibrous mesh helps transduce the function of individual cells into an effective organ pump. Excess amounts of ECM can adversely affect contractile performance of the heart by disrupting normal electrical conduction pathways. The resulting conduction abnormalities seen on electrocardiograms (eg, bundle branch block patterns) alter the well-ordered coordination of mechanical activity that is needed for optimal cardiac function.13 Excessive fibrous tissue in the heart also affects the transduction of force from individual cardiomyocytes into forceful and well-coordinated pump function.14 The deposition of fibrous tissue around small nutrient blood vessels in the heart (ie, perivascular fibrosis) can further impair cardiac function by causing local areas of microischemia to develop. Cardiac fibrosis has been shown to play a major role in determining the level of myocardial stiffness in patients affected by heart failure with preserved ejection fraction (HFpEF).15,16 Zile et al found that in biopsy specimens of human left ventricular myocardial tissue taken from patients with HFpEF, there were increases in collagen volume fraction of ~5-fold compared to that in patients with hypertension alone, due mainly to increases in insoluble collagen. They further showed a significant positive correlation between the level of collagen-dependent tissue stress and echocardiography-derived measures of diastolic dysfunction (late atrial diameter and estimated pulmonary artery wedge pressure).17 Excess amounts of collagen in the ECM can also impact diastolic function by impairing elastic recoil of the myocardium as the myocytes relax. Deposition of ECM throughout the heart can predispose to arrhythmias, both through reentry and other mechanisms. While there is a recognized association between interstitial fibrosis, ventricular arrhythmias, and sudden cardiac death,18-21 fibrosis in the wall of the atria has been described as playing a role in the development of atrial fibrillation.22-24
CAUSES OF FIBROSIS IN THE HUMAN HEART
The loss of cardiomyocytes stimulates the development of reparative fibrosis in the heart. Coronary artery disease that leads to the development of an MI is the prototypic initiator of reparative fibrosis but other conditions that result in cell death can cause this to occur. Disease in small intramyocardial coronary arteries that causes areas of microischemia can result in a more diffuse distribution of reparative fibrosis. Cardiac contusion resulting in myocardial necrosis can stimulate the development of a replacement scar in areas where cardiomyocytes have been lost. Infection of the heart by viruses and other pathogens and toxic effects of absorbed or ingested agents (eg, alcohol) can also lead to cardiomyocyte loss and development of replacement fibrosis.
Reactive fibrosis is seen in the heart as individuals age25,26 and it plays an important role in causing stiffening of the heart and development of HFpEF. This process is accentuated by conditions that increase pressure load on the heart. Thus, patients with systemic hypertension or aortic stenosis are prone to develop stiff left ventricles due to the deposition of interstitial fibrosis.6,7 Reactive fibrosis in noninfarcted zones of the heart is also a key component in post-MI cardiac remodeling, and it is stimulated by a host of factors including global increases in left ventricular wall stress as well as neurohormonal and other inflammatory influences.27 Diffuse cardiac fibrosis has been found in patients with diabetes, obesity, and metabolic syndrome.28-30
A variety of drugs that act as serotonergic receptor agonists including anorectics, antimigraine drugs, anti-parkinson drugs, and recreational drugs have been associated with myocardial and cardiac valvular fibrosis.31,32 Carcinoid tumors of the gut that secrete large amounts of serotonin into the systemic venous circulation33 and ingestion of foods with high serotonin content have been associated with fibrosis in the right side of the heart. Cardiac fibrosis has been shown to be stimulated by smoking and can develop as a result of passive second hand inhalation of cigarette smoke.34 It has also been reported in patients with heart disease due to genetic mutations as well as in athletes.20,35
Cardiac fibrosis can be seen in cancer patients treated with a variety of chemotherapeutic agents such as anthracyclines/anthraquinones, cyclophosphamide, trastuzumab, and other monoclonal antibody-based tyrosine kinase inhibitors and antimetabolites.36 It can also develop as a consequence of radiation therapy when there is exposure of the heart.
ANIMAL MODELS OF CARDIAC FIBROSIS AND MECHANISMS INVOLVED
Most, if not all, of the mechanisms of cardiac fibrosis have been elucidated through animal models and in vitro cell culture systems. A summary of some of the experimental animal models that have been used to represent the human condition are shown in Table I. Albeit not exhaustive, some critical mechanistic details that have been garnered from these models are outlined below.
Table I.
Animal models of cardiac fibrosis
| Human condition | Animal model | Cardiac fibrosis characteristics | Reference |
|---|---|---|---|
| Ischemia | Permanent occlusion of coronary artery* |
|
37,38 |
| Temporary occlusion of coronary artery (ischemia/reperfusion)* |
|
39,40 | |
| Hypertension or pressure overload | Macaca fascicularis model of progressive hypertension up to 88 wk |
|
41 |
| Transverse aortic constriction (TAC) in mice and rats* |
|
42-45 | |
| Spontaneous hypertensive rats (SHR) given 8% salt for 5 wk |
|
46 | |
| L-NAME† administration in drinking water |
|
47,48 | |
| Diabetes | Western diet in Sprague Dawley rats for 18 wk |
|
49 |
| Type I diabetes induced by streptozotocin injection* |
|
50,51 | |
| Drug/chemical | Chronic ethanol consumption (4%; up to 14 wk) in mice |
|
52 |
| Doxorubicin-induced cardiac fibrosis in rats |
|
53 | |
| Butyrylcholinesterase knockout mice receiving intraperitoneal cocaine (20 mg/kg) daily for 7 d |
|
54 | |
| Other | 5-oxoprolinase (OPLAH) null mice |
|
55 |
| Rats with 2-mo aldosterone treatment and 1% NaCl/0.3% KCl drinking water |
|
56 | |
| Mouse obesity/diabetes model (homozygous leptin receptor inactivation; db/db mice) at 6–12 mo of age‡ |
|
57 | |
| Cryoinjury of myocardium in adult and neonatal mice |
|
58 | |
| Angiotensin II infusion in rats and mice |
|
59 |
This model has been used extensively in the literature.
L-NAME, L-NG-nitroarginine methyl ester, a broad inhibitor of nitric oxide synthases; LV, left ventricle; RV, right ventricle.
This also could be considered a diabetes model.
Transforming growth factor-β.
The pleiotropic transforming growth factor-β (TGF-β) family, consisting of TGF-β1, −β2, and −β3, possesses diverse functions within the body, and its stimulating effects on fibrosis (especially TGF-β1) are well known.60,61 In fact, TGF-β1 has been called the master regulator of fibrosis. TGF-βs are expressed by many cell types, but they are secreted as inactive, latent forms. These latent forms consist of the homodimeric TGF-β subunits, each noncovalently bound to its N-terminal prodomain (termed TGF-β latency-associated protein [LAP]), and with the latter linked to latent TGF-β binding protein (LTBP, existing as 4 isoforms) by disulfide bonds (reviewed in62). The N-terminus of LTBP contains an ECM binding domain that anchors the inactive complex to the ECM. The homodimeric TGF-β cannot bind its receptor until it is released from this inactive complex. There are multiple mechanisms capable of activating latent TGF-β, including protease release (eg, matrix metalloproteinase (MMP)-2 and MMP-963), thrombospondin-1 binding,64 reactive oxygen species,65 pH extremes that denature LAP,66 integrin binding,67 and mechanical separation of LTBP and LAP on stiff matrices.68
Binding of TGF-β1 to its cell-surface, type II receptor (TβRII) causes recruitment and transphosphorylation of the type I receptor (ALK5 and/or ALK1 in some cell types69). In the canonical pathway, this leads to Smad phosphorylation and activation (eg., Smad2 and Smad3 are activated by ALK5, while Smad1, Smad5, and Smad8 are activated by ALK1). These activated Smads then bind to Smad4, and the complex translocates into the nucleus to modify transcription. Inhibitory Smads (Smad6 and Smad7) can be upregulated subsequently to feedback negatively on the signaling pathway. In the noncanonical pathways, TβRII can activate other signaling pathways, such as those involving mitogen-activated protein kinases (MAPKs), phosphatidylinositol-3-kinase activation of AKT, and activation of RhoA leading to stabilization of F-actin. (See Derynck and Zhang70 for a more thorough review of TGF-β signaling.)
TGF-β has pleiotropic effects throughout the body. Within the heart, it is known to stimulate cardiac fibroblasts to exhibit a profibrotic phenotype. These changes include myofibroblast conversion (see below) with resultant increases in collagen secretion,71 decreases in collagen degradation,72,73 and increases in synthesis of other profibrotic mediators (eg, see next section below). Regarding cardiomyocytes, TGF-β is known to induce a hypertrophic response.74,75 The effects of TGF-β on endothelial cells are complex, inducing angiogenesis, angiostasis, or endothelial-to-mesenchymal transition (EndMT), depending on the conditions.76,77 The TGF-β family has been implicated to some extent in all animal models of heart disease involving fibrosis. This includes MI,78,79 pressure overload,80 Ang II-induced cardiomyopathy,81 and diabetic cardiomyopathy.82 With respect to MI, although TGF-β is considered to negatively affect cardiomyocyte physiology and promote fibrosis, it has been shown to be cardioprotective in ischemia/reperfusion injury.83,84 It is likely that such differences are due to low levels of TGF-β being necessary for proper tissue homeostasis, while high levels lead to cardiomyopathy and fibrosis, as has been postulated previously by other investigators.60 In addition, signaling differences between cell types can affect outcome,85 and latent vs active TGF-β forms are frequently not distinguished, thus confounding interpretation. Interestingly, both p38α MAPK86 and Smad387 signaling have been implicated in myofibroblast conversion in fibrotic animal hearts, suggesting the involvement of both noncanonical and canonical TGF-β signaling.
Examples of other mediators of cardiac fibrosis.
Angiotensin II.
Acting mainly via its type I receptor (AT1R), Ang II is known to induce profibrotic responses from cardiac fibroblasts, including increased ECM synthesis.88-91 At least some, if not all of these effects are believed to occur indirectly via expression of TGF-β181,92,93 and/or transient receptor potential cation channel subfamily C member 6 (TRPC6), the latter of which activates calcineurin/nuclear factor of activated T-cells (NFAT) signaling.94
Endothelin-1.
Via endothelin receptor type A, endothelin-1 (ET-1) has been shown to increase the collagen production of cultured human cardiac fibroblasts.95 ET-1 has been implicated in the cardiac fibrosis observed with aging,96 streptozotocin-induced experimental diabetes,97 and Ang II infusion.98 ET-1 has also been shown to be mitogenic to cultured neonatal rat cardiac fibroblasts, a process that was dependent on the production of intracellular reactive oxygen species.99
Connective tissue growth factor.
Connective tissue growth factor (CTGF, also called CCN2) expression is associated with fibrosis in human heart failure patients100 and experimental animal models.101 CTGF expression can be induced by stimulation of cardiac fibroblasts and cardiomyocytes with TGF-β and its upregulation has been implicated in the profibrotic responses to TGF-β.102 In 2015, Accornero et al published a rather thorough investigation of the effects of CTGF on cardiac fibrosis using multiple mouse models and concluded that CTGF was of minimal importance.103 However, more recently, Ang II-induced cardiac fibrosis was shown to be dependent on the autocrine production of CTGF from fibroblasts, but not myocytes,104 and intraperitoneal injection of an anti-CTGF monoclonal antibody was able to improve post-MI left ventricular remodeling, including remote-site interstitial fibrosis, in a mouse model.105 The reasons for these discrepancies are unknown, but certainly deserve more study.
Catecholamines.
Chronic adrenergic stimulation of the heart can lead to myocyte hypertrophy and cardiac fibrosis. Mouse and human cardiac fibroblasts are known to express β2-adrenergic receptors,106,107 which typically activate adenylate cyclase with the resulting cyclic AMP being inhibitory to profibrotic fibroblast activity.108 However, chronic β2-adrenergic receptor stimulation leads to G protein-coupled receptor kinase 2 (GRK2)-β-arrestin-dependent uncoupling of β-adrenergic signaling, which enhances the profibrotic phenotype. This β-arrestin-dependent process also appears to be active in fibrotic, diseased hearts.109,110
Growth on stiff matrices.
Growth on stiff substrates (eg, tissue culture plastic) is well known to activate fibroblasts. In addition to the activation of AT1R111 and TGF-β, the latter necessitating the involvement of integrins,68 Rho-dependent formation of F-actin stress fibers on stiff matrices dissociates monomeric G-actin-myocardin-related transcription factor-A (MRTF-A) complexes, allowing MRTF-A to remain in the nucleus. Nuclear MRTF-A can then act in concert with serum response factor and/or TGF-β-activated Smads to upregulate the transcription of profibrotic markers, such as α-smooth muscle actin (α-SMA) and collagen genes.112-114
Source of collagen-secreting cells.
The cell types that have been implicated as responsible for collagen secretion in the diseased heart are numerous, and include resident interstitial fibroblasts (and myofibroblasts), cells derived from EndMT (or epithelial-to-mesenchymal transition (EMT)), inflammatory cells (see below), glioma-associated oncogene (Gli)+ pericytes, and infiltrating fibrocytes (typically circulating CD34+, CD45+, Col I+ bone marrow progenitor cells, but see Pilling et al115 for more selective markers). Fibrocytes were shown to be involved in fibrosis of ischemia/reperfusion cardiomyopathy in mice,116 and ablation of pericytes ameliorated cardiac fibrosis induced by ascending aortic constriction in mice.117 EndMT has been implicated in pressure overload-induced (transverse aortic constriction [TAC]) cardiac fibrosis in mice, as assessed by Tie1Cre and FSP1-GFP cell lineage tracking.118 However, Tie1, which was previously thought to be endothelial-specific, was shown to be expressed also by subsets of hematopoietic cells,119,120 and FSP1, which was believed to be fibroblast-specific, was subsequently shown to be expressed in hematopoietic, endothelial, and vascular smooth muscle cells.121 Indeed, a thorough analysis of COL1A1-expressing cells after aortic banding in mice, avoiding conclusions based on Tie1 and FSP1, did not find a contribution of EndMT to fibrosis.122 In addition, more recent reports in mice using in-depth lineage tracing have found that myofibroblasts in injured hearts and COL1A1-expressing fibroblasts in infarcted hearts are derived from transcription factor 21+ (Tcf21+) tissue-resident fibroblasts123 and resident fibroblasts of epicardial origin,124 respectively. Although all lineage tracing experiments have limitations, these latter 2 studies used models that are considered to be among the best currently available. This strongly suggests that most, if not all, collagen-producing cells in diseased hearts arise from resident fibroblasts. However, in the latter report,124 fibrocytes were noted on the epicardial surface of the hearts near the ligation suture, indicating that other cell types could be involved in certain remodeling situations.
Inflammatory cell infiltration.
Inflammatory cells are known to be involved in cardiac fibrosis that is associated with MI. These cells can be resident tissue macrophages125 as well as infiltrating inflammatory cells, such as neutrophils, monocytes, and macrophages.126 This inflammation can be broadly divided into 2 phases: the initial proinflammatory phase involved in inflammatory cell recruitment and removal of dead tissue, and the reparative phase involved in tissue healing.127 Disruption of either of these phases (by augmentation or inhibition) can be detrimental, leading to excessive fibrosis, chamber dilatation, or even infarct rupture. Inflammation has also been shown to be involved in cardiac fibrosis induced by other pathological states. For example, CXCR2-expressing monocytes, macrophages, and neutrophils were involved in the cardiac remodeling (including fibrosis) observed in Ang II-treated (1μg/kg/min) hypertensive mice.128 Using lineage tracking, Ivey et al recently showed that resident fibroblast proliferation and inflammatory cell increases both peaked at one week, regardless of fibrotic insult (ie, TAC, isoproterenol injection, or coronary artery ligation), suggesting a connection between them.129 We have previously shown that inflammatory cytokines, such as tumor necrosis factor-α, can upregulate AT1R,130,131 making cultured rat cardiac fibroblasts more responsive to Ang II stimulation,89 so the potential for crosstalk between cell types exists. Overall, inflammatory cells are certainly involved in cardiac fibrosis that is associated with myocardial damage (ie, reparative fibrosis). Their influence on reactive fibrosis is still uncertain. However, immunoinflammatory dysfunction has been implicated in the increased reactive cardiac fibrosis and diastolic dysfunction associated with aging in mice.132
Activated fibroblasts and myofibroblasts.
Fibroblasts in the normal adult heart are considered quiescent, although as noted recently by Mouton et al, they are not truly quiescent, expressing genes necessary for ECM homeostasis.133 Fibroblasts in diseased hearts become “activated” to various degrees, a term that has broad meaning. Experimental MI is one of the best understood models of cardiac fibrosis because it produces overt, well-defined collagen deposition. Using this model, both Fu et al134 and Mouton et al133 have drawn similar conclusions in that “activation” of cardiac fibroblasts involves proliferation/migration that precedes, and overlaps with, ECM production and maturation. However, the latter investigators noted that proinflammatory genes were upregulated at earlier time points (ie, <3 days), while the former group reported that infarct-resident cells at later time points (ie, 2—4 weeks) gained a unique phenotype, which the investigators termed “matrifibrocyte,” to help maintain scar integrity. These “matrifibrocytes” had lost α-SMA expression, while retaining elevated COL1A1 and COL3A1 expression. How fibroblasts “activate” in other models of cardiac fibrosis, especially those involving reactive fibrosis, is less clear, but ultimately it is the accumulation of ECM proteins that is critical.
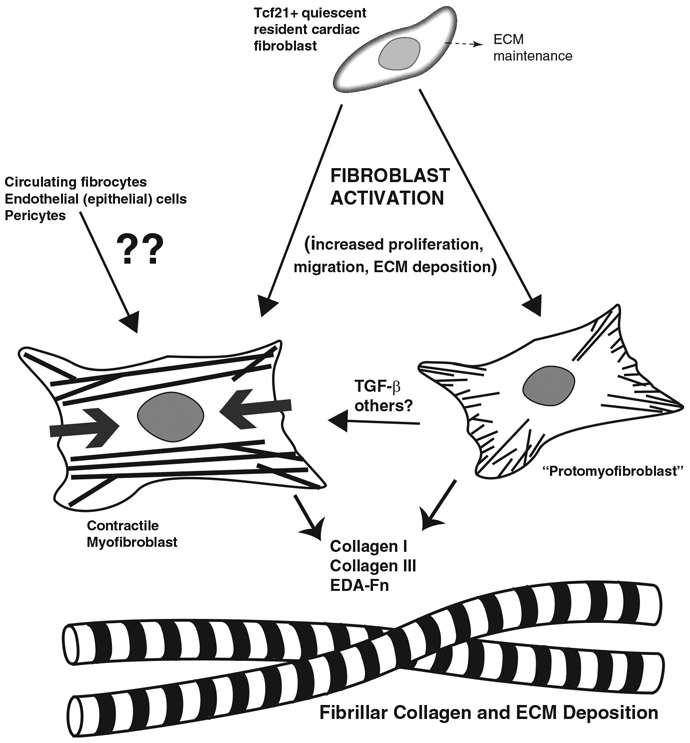
Overall, myofibroblasts are considered to be strongly activated fibroblasts and the major collagen producers in many fibrotic tissues. Indeed, myofibroblasts have been found in many animal models of cardiac fibrosis, including coronary artery ligation,86,129,135 TAC,87 and isoproterenol injection.129 By definition, these cells have acquired the capacity to synthesize the contractile α-SMA. However, not all Col I-expressing cells are positive for α-SMA in fibrotic heart tissue, estimated at 89% 5 days after permanent coronary artery ligation,129 61% after 7 days of isoproterenol,129 and 15% at both 7 and 28 days after TAC.122 There could be many reasons for this lack of overlap. For example, fibroblasts can convert to protomyofibroblasts, which have actin stress fibers, enhanced collagen expression, and extra domain A (EDA)-fibronectin expression, but they do not express α-SMA.136 Platelet-derived growth factor is one factor that has been shown to initiate the conversion of fibroblasts to protomyofibroblasts137; active TGF-β is then necessary to convert protomyofibroblasts to myofibroblasts.68,136 In addition, as indicated with the “matrifibrocyte” experiments above, a myofibroblast could downregulate α-SMA expression while still maintaining elevated collagen secretion. Therefore, fibroblasts that have been activated to increase collagen synthesis do not necessarily need to be myofibroblasts, and a mix of cell phenotypes could be responsible for collagen secretion in fibrotic regions of the heart, depending on the animal model or human disease state. A simplified summary of fibroblast activation, taking into consideration some of the mechanisms mentioned above, is shown in Fig 1.
Fig 1.
Simplified pictorial representation of cardiac fibroblast activation. Actin stress fibers are depicted in the protomyofibroblast as thin lines and contractile α-smooth muscle actin-containing fibers are depicted in the myofibroblast as thick lines (with thick gray arrows denoting contractile tension). Fibroblast activation in the heart involves a complex mix of pathways, but the most important result is the increased deposition of ECM. EDA-Fn, extra domain A fibronectin; ??, questionable contribution, limited contribution, or contribution only in certain contexts.
MicroRNAs.
MicroRNAs (miRNAs) have also been implicated in affecting cardiac fibrosis. For example, miR-21 has been shown to promote TGF-β1-mediated fibroblast to myofibroblast transition in rat cardiac fibroblasts138 and potentially to regulate fibrosis at the MI zone by its expression in cardiac macrophages.139 miR-22 has been shown to be upregulated in the border zone of MI mice and to increase fibroblast functions via downregulating caveolin-3 expression in cultured neonatal rat cardiac fibroblasts.140 miR-130a was shown to be upregulated in the hearts of Ang II-infused mice and its upregulation promoted profibrotic gene expression and myofibroblast transformation, possibly by targeting peroxisome proliferator-activated receptor-γ (PPARγ).141 miR-155 was shown to be involved in cultured fibroblast activation and Ang II-induced cardiac fibrosis in mice; the investigators concluded that miR-155-dependent downregulation of suppressor of cytokine signaling 1, which augmented TGF-β signaling, was responsible.142 Zhao et al demonstrated that paracrine transfer of miR-328 from cardiomyocytes to cardiac fibroblasts could activate the TGF-β pathway and increase fibrosis in mice.143 In addition, miR-133a downregulation was associated with fibrosis after TAC in mice, possibly increasing serum response factor, CTGF, and COL1A1 expression.43 It seems that any miRNA that can target critical factors during fibroblast activation has the potential to influence fibrotic outcomes.
Other considerations.
Fibroblast activation plays a major role in cardiac fibrosis, but other processes can certainly modulate outcomes. Lysyl oxidase (LOX)-mediated collagen crosslinking, a normal process to strengthen collagen fibrils and fibers, can lead to excessive stiffening of fibrotic tissue, impeding cardiac function.144,145 Tissue transglutaminase, another secreted enzyme that can crosslink collagen albeit differently than LOX, has been associated with diastolic dysfunction in the hearts of mice subjected to TAC. These effects were reportedly due to both enzymatic and non-enzymatic functions of tissue transglutaminase.146 Our group has previously shown that cardiac fibroblasts display a different expression pattern of endoplasmic reticulum-localized, single-stranded procollagen-modifying enzymes when they are ascorbate-starved vs when they are ascorbate-replete.147 Given that ascorbate may be limiting in ischemic and/or oxidative environments, this could have implications for their functioning in vivo, although the complexities of this system make study and predictions difficult.
Animal model limitations.
Although much information has been garnered from experimental models of cardiac fibrosis, there are still limitations to extrapolating the conclusions derived from these models to human patients. In addition to questions of species variations as well as physiological discrepancies due to heart size differences, the models themselves can have limitations. For example, the frequently used TAC model of pressure overload may be more similar to aortic coarctation in adults than the more common aortic stenosis or hypertension that it is presumed to represent. Where experimental models are most limiting is arguably in reflecting human reactive fibrosis. Diffuse interstitial fibrosis could develop over many years in humans, remaining subclinical throughout most of that time. Such passage of time is difficult to mimic in animal models due to time and budget constraints as well as species longevity. It is imperative in this situation to gain a better understanding of how fibrosis progresses in human patients and how those changes correlate to symptoms.
Various methods have been used to assess myocardial fibrosis in humans. The gold standard is direct evaluation of myocardial tissue by endomyocardial biopsy and histopathologic assessment.148 This method is invasive, carries risks to patients, and has the additional drawback of potential sampling errors.149 The use of noninvasive and/or minimally invasive techniques for monitoring cardiac fibrosis in humans would be greatly beneficial both as a research tool to further understanding and as a clinical tool to assess patients. Although the technologies are still arguably in their infancy, the last sections of this review will deal with the current state of these critical detection systems, namely serum cardiovascular fibrosis markers and imaging.
CARDIOVASCULAR FIBROSIS MARKERS IN HUMANS
Biomarkers of cardiovascular fibrosis.
The most recent American Heart Association/American College of Cardiology/Heart Failure Society of America guidelines for the management of heart failure give a class IIb recommendation for measurement of biomarkers of myocardial fibrosis for additive risk stratification in patients with either acute decompensated or chronic heart failure.150 Specifically, the document notes that biomarkers of myocardial fibrosis, including soluble suppression of tumorigenicity 2 (ST2) receptor, galectin-3 (Gal-3), and highly sensitive cardiac troponin (hsTn) are predictive of outcomes in heart failure patients, and are additive to the established natriuretic peptides in their prognostic value. The adoption of markers of fibrosis into the guidelines reflects the findings of a large body of translational and clinical studies, while also acknowledging that large-scale, prospective studies validating their clinical utility are still lacking. Below is a concise overview of these 3 clinically relevant serum markers and some preclinical markers of cardiac fibrosis.
Galectin-3.
Gal-3 is a β-galactoside-binding lectin and a matricellular protein with important roles in cell adhesion, inflammation, and tissue fibrosis.151 Gal-3 is expressed by fibroblasts and inflammatory cells, including activated macrophages, and is involved in myofibroblast activation via the TGF-β signaling pathway.152-154 It is also linked to collagen production, macrophage infiltration, and cardiac hypertrophy.153 Upregulation of Gal-3 has been demonstrated in animal models of myocardial,155 vascular,156 renal,157 and hepatic154 fibrosis.
Gal-3 expression is low in normal myocardium in humans, but is upregulated in various pathologic conditions.158 In a rat model of hypertensive heart failure, Gal-3 expression was increased at an early stage of hypertrophy, before clinical heart failure, specifically in the rats that eventually developed heart failure.153 In addition, infusion of Gal-3 into the pericardial space of normal rats induced fibrosis and heart failure,153 while inhibition of Gal-3 may protect against fibrosis, adverse cardiac remodeling, and the development of heart failure.155,159
Some analyses have suggested that circulating levels of Gal-3 may not correlate with human myocardial levels of Gal-3 and tissue fibrosis,160 while other clinical studies have suggested a link. In patients with giant coronary aneurysms due to Kawasaki disease, both circulating Gal-3 levels and myocardial expression of Gal-3 (in densely fibrotic areas of the myocardium and arterial media) are elevated.161 Regardless of its association with tissue fibrosis, higher circulating Gal-3 levels are associated with increased risk of death or readmission for heart failure in patients with either acute or chronic heart failure.162 Higher Gal-3 levels are also associated with incident heart failure risk and mortality among individuals with acute coronary syndrome,163 and among apparently healthy community-dwelling individuals.164-166 The clinical uptake of Gal-3 testing is still low; however, Gal-3 remains an important research tool for evaluating links between myocardial dysfunction and fibrosis and inflammation.
Soluble ST2.
ST2 is a member of the interleukin (IL)-1 receptor-like family of proteins. It is expressed as both a transmembrane form (ST2L) and a soluble receptor (sST2). Expression of both isoforms of ST2 is upregulated in cardiomyocytes and fibroblasts in response to mechanical stress. The ligand for ST2 is IL-33, which is also upregulated in response to mechanical stress. The IL-33/ST2L interaction triggers a cascade via NF-κB that protects against inflammation, myocardial fibrosis, and cardiac hypertrophy. However, sST2 acts as a decoy receptor; when sST2, which lacks transmembrane and intracellular components, binds to IL-33, it does not initiate the beneficial signaling cascade.167 Thus, high levels of sST2 are associated with increased tissue fibrosis and organ dysfunction.168
In animal models, blocking IL-33/ST2L interactions results in excess tissue fibrosis and myocardial hypertrophy after exposure to TAC-induced cardiac strain. In contrast, treatment with recombinant IL-33 reduced hypertrophy and fibrosis and improved survival after TAC in wild type but not ST2 null mice.167
In acute and chronic heart failure, and in individuals at risk for heart failure, increased sST2 levels are associated with a worse prognosis.168 A recent meta-analysis with patient-level data showed that this prognostic ability is independent of natriuretic peptide and hsTn levels.169 Unlike Gal-3, sST2 is gaining some traction in clinical use as several clinical cohorts have provided some degree of validation for its prognostic use, albeit primarily with retrospective analyses. Like Gal-3, the specific clinical response warranted by an elevated sST2 level remains to be elucidated and confirmed with prospective trials.
hsTn as an indirect indicator.
Although cardiac troponin is known primarily as a marker of myocyte injury and necrosis, myocyte necrosis ultimately leads to replacement fibrosis and several lines of evidence have shown an association between cardiac troponin and myocardial fibrosis. Troponin is a regulatory protein within the contractile apparatus of striated muscles, which helps modulate calcium-mediated actin-myosin interactions. Two of the 3 subunits of the troponin complex, T and I, have cardiac isoforms that are distinct from the skeletal isoforms. Thus, measurement of either cTnT or cTnI is highly specific for cardiomyocyte injury.170
Cardiac troponin is released from cells in the setting of cardiomyocyte necrosis and cell membrane degradation, but low levels of release are also seen in the absence of cell necrosis. Although the precise mechanisms of these elevations in the absence of necrosis remain unclear, inflammatory factors leading to degradation and fragmentation of troponin combined with increased membrane permeability may be one explanation.171
With the evolution of hsTn assays that can now detect circulating cardiac troponin in the majority of healthy individuals, it has become clear that even within the “normal” range, higher hsTn is associated with incident heart failure and mortality.162,172 In a large multiethnic cohort of individuals initially free of cardiovascular disease, baseline levels of hsTnT were associated with longitudinal changes in left ventricular structure that were consistent with adverse remodeling. In addition, hsTn levels were associated with replacement myocardial fibrosis, as imaged by late gadolinium enhancement (LGE) on cardiac magnetic resonance imaging.173 The pattern of enhancement was not typical of an ischemic pattern. hsTn levels have also been associated with fibrosis in patients with severe aortic stenosis174 and with hypertrophic cardiomyopathy.175,176
Clinically, the use of hsTn is now entrenched in medicine for diagnosis of MI as well as prognosis in acute coronary syndromes and in heart failure.177 Its association with fibrosis in subclinical presentations of disease is a more recent discovery, and whether the identification of this association may lead to early targeted preventative therapies remains an area of interest.
Preclinical markers of collagen metabolism.
While not currently in clinical use, a large number of markers associated with collagen synthesis and degradation have been evaluated for an association with myocardial fibrosis.178 HFpEF is characterized by increased interstitial deposition and crosslinking of Col I, with a small increase in the Col I/Col III ratio. Three classes of proteins reflecting the metabolism of collagen have received significant attention, in particular MMPs, tissue inhibitors of metalloproteinases (TIMPs), and procollagen terminal peptides/telopeptides (markers of collagen turnover). These markers have been associated with various cardiovascular disease risk factors,179-181 left ventricular structural changes (TIMP-1),182 left ventricular remodeling after MI (type I collagen C-terminal telopeptide [CITP]),183 hypertension-induced HFpEF and heart failure with reduced ejection fraction (HFrEF) (MMP-1:TIMP-1 ratio),184 and incident cardiovascular events and mortality (TIMP-1 and N-terminal peptide of procollagen type III).185
Despite these associations, it is unknown whether any of these markers will come to have significant clinical impact, but studies are beginning to evaluate this possibility. Recently, the CITP:MMP-1 ratio, a marker inversely associated with the degree of myocardial collagen crosslinking, was studied as an effect modifier for response to spironolactone. Among 381 patients with stable NYHA class II or III HFpEF who were randomized to daily spironolactone or placebo, a low CITP: MMP-1 ratio (suggesting a high degree of myocardial collagen crosslinking) identified a population who were resistant to the beneficial effects of spironolactone on left ventricular diastolic dysfunction as assessed by E/e′.186 In contrast, subjects with a high CITP:MMP-1 ratio showed improvement in diastolic function with spironolactone treatment. This study suggests that biomarker phenotyping of patients will assist with the search for therapeutic options in HFpEF, and that the degree of fibrosis is a critical factor to consider.
Other markers associated with regulation of collagen turnover, including TGF-β1,60 growth differentiation factor-15, osteopontin,187 and others,178 have been associated with myocardial fibrosis in animal models and preliminary studies, but they lack clear and consistent clinical applications.
DETECTION OF MYOCARDIAL FIBROSIS IN HUMAN PATIENTS BY IMAGING
Echocardiography.
Echocardiography is perhaps the oldest noninvasive imaging technique for assessment of myocardial fibrosis. The altered acoustic impedance by fibrosis can be quantified by backscatter techniques188 and has been validated by direct biopsy in patients with aortic stenosis and dilated cardiomyopathy.189,190 This method is reliable and widely available, but limited by image quality, and hence is currently typically not used. More recently, strain imaging by speckle tracking has been used in a variety of diseases in which left ventricular functional abnormalities may be present. Strain provides a local measure of left ventricular function, which may be adversely affected by fibrosis. However, because strain imaging is only functional, it does not provide a direct assessment of the tissue. In patients with hypertrophic cardiomyopathy, it has been demonstrated that a reduction in strain is associated with an increase in fibrosis as determined by quantification of delayed gadolinium enhancement with magnetic resonance imaging (MRI), but this has not been directly validated against biopsy.191
Positron emission tomography.
Utilizing positron emission tomography with 15O-labeled water and carbon monoxide (C15O) allows quantification of perfusion and can indirectly assess fibrosis through a perfusable tissue index. This index assesses the amount of myocardium perfused by water, with fibrotic myocardium exchanging water less rapidly. This method has been compared to patients with known dilated cardiomyopathy and presumed fibrosis, but has not been validated by direct biopsy. A reduction in perfusable tissue index is considered representative of a higher degree of fibrosis.192,193
Cardiac MRI.
For defining cardiac anatomy, cardiac MRI (also abbreviated as CMR) provides high levels of accuracy and has been shown to have significant utility in noninvasive characterization of myocardial tissue and etiologies for heart failure.194 LGE is one of the most widely utilized modalities for assessment of focal myocardial scar and fibrosis. LGE imaging is able to depict differences in signal intensity in T1-weighted images obtained approximately 10 minutes after the administration of a gadolinium-based contrast agent. In fibrotic tissue, T1 recovery times are shortened due to higher concentrations of gadolinium in the extracellular space while in normal myocardium gadolinium washes out more quickly. As a result of this difference, an inversion time can be chosen so that normal myocardium appears dark and fibrotic myocardial tissue appears bright on inversion recovery images.195 This technique has been well validated and correlated to the severity and extent of myocardial fibrosis.196
There is a great deal of clinical experience with LGE and it is widely available on clinical cardiac MRI systems. However, this technique has several important limitations. LGE only provides a relative signal that measures fibrosis compared to other regions of myocardium. Hence, it is most useful when there are regions of myocardium that are known or expected to be normal and importantly, it is less useful in detecting myocardial fibrosis that is diffuse (ie, reactive fibrosis). Moreover, there is a lack of correlation with collagen volume assessed by biopsy samples in diffuse fibrosis.194 LGE is also not specific for fibrosis as increased signal can be seen in regions with edematous or inflamed tissue.197 Finally, LGE is largely a qualitative process with evaluation performed by visual assessment of images obtained (although tools do exist for quantitative characterization of LGE). This results in inter- and intraobserver variability and challenges in comparing results to follow-up examinations or between individual patients.198
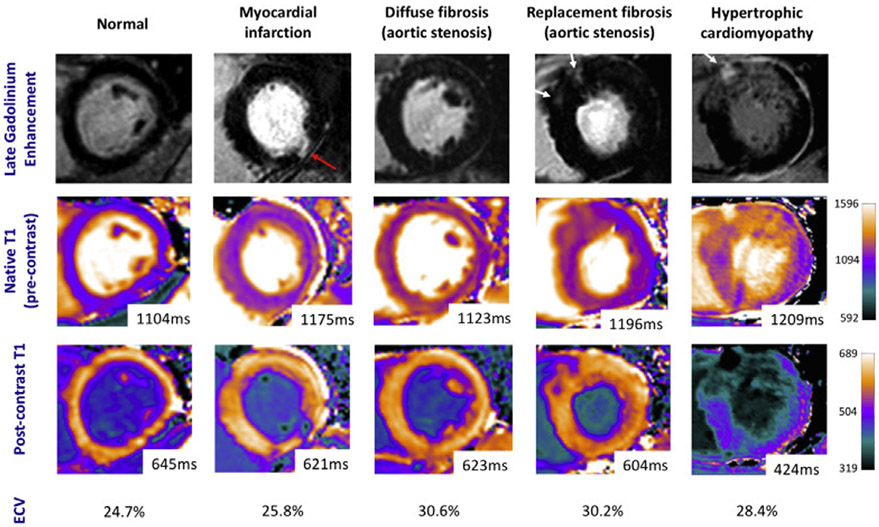
Quantitative evaluation of myocardial fibrosis can be assessed by MRI with T1 mapping techniques. Various sequences have been developed to directly measure the longitudinal relaxation time, which measures how quickly proton spins within myocardial tissue re-equilibrate due to interactions with the surrounding tissue after being excited with a radiofrequency pulse. Areas of fibrosis cause T1 shortening compared to that of normal myocardium and the results can be displayed using parametric pixel illustration maps of relaxation times (Fig 2).199,200 This method can acquire a set of interpretable images with a single breath hold acquisition; it is reproducible and with reportedly high levels of intraobserver agreement.201 T1 mapping has been validated by comparisons with biopsies in patients with cardiomyopathy, valvular disease (aortic stenosis, aortic regurgitation, and mitral regurgitation), and myocarditis. Postcontrast enhanced T1 mapping in combination with native (noncontrast) T1 mapping and the hematocrit level allows calculation of the extracellular volume (ECV) fraction. As elevated quantities of ECV are associated with increased fibrosis and collagen deposition, this is helpful in further quantifying the degree of fibrosis.202 Measurements of ECV have been validated with histologic analyses of biopsy specimens for several different diseases.203
Fig 2.
Example cardiac magnetic resonance images in common cardiac conditions showing late gadolinium enhancement, native (precontrast) and postcontrast T1 mapping, and calculated extracellular volume (ECV) fractions. Red arrows identify areas of subendocardial delayed enhancement. White arrows identify midwall delayed enhancement. Reproduced, with permission, from Everett et al.204 (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
T1 mapping in specific disease states.
T1 mapping has been shown to have prognostic utility in a number of diseases. In patients with dilated cardiomyopathy, native T1 values and ECV have been shown to be predictive of composite all-cause mortality and heart failure events.205 Elevated native T1 mapping values have also been shown to be an independent predictor of adverse outcomes in patients with moderate or severe aortic stenosis.206 Surveillance with T1 mapping in adult cancer patients 3 years after treatment with anthracycline-based chemotherapies showed elevated proportions of ECV when adjusted for other known risk factors. The longterm implications of elevations in ECV on left ventricular function in this patient population are not yet known.207 The relationship between cardiovascular risk and fibrosis in men and women has also been investigated using T1 mapping. There is evidence that a greater degree of fibrosis is associated with higher cardiovascular disease risk scores in men. However, this finding was not seen in women, and the exact role that T1 mapping may play in risk assessment is still unclear.208
CONCLUSIONS AND PERSPECTIVES
We have reviewed the current understanding of cardiac fibrosis in human disease, experimental models of cardiac fibrosis, and the mechanisms involved, as well as the current techniques available to assess cardiac fibrosis in patients. Many gaps in knowledge still remain, with the greatest deficiencies existing in the jump from animal models to human patients and the development of techniques to adequately assess fibrotic regions without biopsy. Myocardial fibrosis is almost universally seen in all forms of heart disease and the degree of cardiac dysfunction is generally proportional to the degree of ECM deposition.209 However, in later stages of heart disease and/or when collagen deposition becomes symptomatic, the resulting fibrosis may no longer be reversible.210,211 With continued understanding, the hope one day is to be able to intervene and halt or slow aberrant ECM deposition before symptoms appear and this point of no return is reached.
ACKNOWLEDGMENTS
We thank Sophia Tesluk for proofreading the final version of the manuscript. All authors have read the journal’s policy on disclosure of potential conflicts of interest. L.B.D. has received speaking fees from Roche Diagnostics, and consulting fees from Siemens Healthineers and Abbott Laboratories. She also has received research support (supplies only) from Critical Diagnostics. R.T.C., D.K., A.M.K., and B.H.G. have no disclosures. All authors have read the journal’s authorship agreement. The manuscript has been reviewed and approved by all authors.
Abbreviations:
- Ang II
angiotensin II
- AT1R
angiotensin II type 1 receptor
- CITP
type I collagen C-terminal telopeptide
- CMR
cardiac MRI
- Col I
collagen I
- Col III
collagen III
- COL1A1
collagen type I alpha 1 chain
- COL3A1
collagen type III alpha 1 chain
- CTGF
connective tissue growth factor
- ECM
extracellular matrix
- ECV
extracellular volume
- EDA
extra domain A
- EMT
epithelial-to-mesenchymal transition
- EndMT
endothelial-to-mesenchymal transition
- ET-1
endothelin-1
- Gal-3
galectin-3
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- hsTn
highly sensitive cardiac troponin
- IL
interleukin
- LAP
TGF-β latency associated protein
- LGE
late gadolinium enhancement
- LOX
lysyl oxidase
- MAPK
mitogen-activated protein kinase
- MI
myocardial infarction
- miRNA
microRNA
- MMP
matrix metalloproteinase
- MRI
magnetic resonance imaging
- MRTF-A
myocardin-related transcription factor-A
- ST2
suppression of tumorigenicity 2
- ST2L
transmembrane ST2
- sST2
soluble ST2
- TAC
transverse aortic constriction
- Tcf21
transcription factor 21
- TGF-β
transforming growth factor-β
- TIMP
tissue inhibitor of metalloproteinases
- α-SMA
α-smooth muscle actin
REFERENCES
- 1.Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol Heart Circ Physiol 2007;293:H1883–91. [DOI] [PubMed] [Google Scholar]
- 2.Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: the renaissance cell. Circ Res 2009;105:1164–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res 2005;65:40–51. [DOI] [PubMed] [Google Scholar]
- 4.Borer JS, Truter S, Herrold EM, et al. Myocardial fibrosis in chronic aortic regurgitation: molecular and cellular responses to volume overload. Circulation 2002;105:1837–42. [DOI] [PubMed] [Google Scholar]
- 5.Frangogiannis NG. Cardiac fibrosis: cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol Aspects Med 2019;65:70–99. [DOI] [PubMed] [Google Scholar]
- 6.Chin CWL, Everett RJ, Kwiecinski J, et al. Myocardial fibrosis and cardiac decompensation in aortic stenosis. JACC Cardiovasc Imaging 2017;10:1320–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dusenbery SM, Jerosch-Herold M, Rickers C, et al. Myocardial extracellular remodeling is associated with ventricular diastolic dysfunction in children and young adults with congenital aortic stenosis. J Am Coll Cardiol 2014;63:1778–85. [DOI] [PubMed] [Google Scholar]
- 8.Schnee JM, Hsueh WA. Angiotensin II, adhesion, and cardiac fibrosis. Cardiovasc Res 2000;46:264–8. [DOI] [PubMed] [Google Scholar]
- 9.Leask A Getting to the heart of the matter: new insights into cardiac fibrosis. Circ Res 2015;116:1269–76. [DOI] [PubMed] [Google Scholar]
- 10.Strauer BE. Development of cardiac failure by coronary small vessel disease in hypertensive heart disease? J Hypertens Suppl 1991;9:S11–20. [DOI] [PubMed] [Google Scholar]
- 11.Maron BJ, Wolfson JK, Epstein SE, Roberts WC. Intramural (“small vessel”) coronary artery disease in hypertrophic cardiomyopathy. J Am Coll Cardiol 1986;8:545–57. [DOI] [PubMed] [Google Scholar]
- 12.Beltrami CA, Finato N, Rocco M, et al. Structural basis of end-stage failure in ischemic cardiomyopathy in humans. Circulation 1994;89:151–63. [DOI] [PubMed] [Google Scholar]
- 13.Coronel R, Casini S, Koopmann TT, et al. Right ventricular fibrosis and conduction delay in a patient with clinical signs of Brugada syndrome: a combined electrophysiological, genetic, histopathologic, and computational study. Circulation 2005;112:2769–77. [DOI] [PubMed] [Google Scholar]
- 14.Brower GL, Gardner JD, Forman MF, et al. The relationship between myocardial extracellular matrix remodeling and ventricular function. Eur J Cardiothorac Surg 2006;30:604–10. [DOI] [PubMed] [Google Scholar]
- 15.Burlew BS. Diastolic dysfunction in the elderly—the interstitial issue. Am J Geriatr Cardiol 2004;13:29–38. [DOI] [PubMed] [Google Scholar]
- 16.Maragiannis D, Alvarez PA, Ghosn MG, et al. Left ventricular function in patients with hypertrophic cardiomyopathy and its relation to myocardial fibrosis and exercise tolerance. Int J Cardiovasc Imaging 2018;34:121–9. [DOI] [PubMed] [Google Scholar]
- 17.Zile MR, Baicu CF, Ikonomidis JS, et al. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation 2015;131:1247–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gulati A, Jabbour A, Ismail TF, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA 2013;309:896–908. [DOI] [PubMed] [Google Scholar]
- 19.Ahmad T, Fiuzat M, Neely B, et al. Biomarkers of myocardial stress and fibrosis as predictors of mode of death in patients with chronic heart failure. JACC Heart Fail 2014;2:260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Briasoulis A, Mallikethi-Reddy S, Palla M, Alesh I, Afonso L. Myocardial fibrosis on cardiac magnetic resonance and cardiac outcomes in hypertrophic cardiomyopathy: a meta-analysis. Heart 2015;101:1406–11. [DOI] [PubMed] [Google Scholar]
- 21.Zorzi A, Perazzolo MM, Rigato I, et al. Nonischemic left ventricular scar as a substrate of life-threatening ventricular arrhythmias and sudden cardiac death in competitive athletes. Circ Arrhythm Electrophysiol 2016;9:e004229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gal P, Marrouche NF. Magnetic resonance imaging of atrial fibrosis: redefining atrial fibrillation to a syndrome. Eur Heart J 2017;38:14–9. [DOI] [PubMed] [Google Scholar]
- 23.Lau DH, Linz D, Schotten U, Mahajan R, Sanders P, Kalman JM. Pathophysiology of paroxysmal and persistent atrial fibrillation: rotors, foci and fibrosis. Heart Lung Circ 2017;26:887–93. [DOI] [PubMed] [Google Scholar]
- 24.Zhang S, Wilson J, Madani M, Feld G, Greenberg B. Atrial arrhythmias and extensive left atrial fibrosis as the initial presentation of MYH7 Gene Mutation. JACC Clin Electrophysiol 2018;4:1488–90. [DOI] [PubMed] [Google Scholar]
- 25.Horn MA, Trafford AW. Aging and the cardiac collagen matrix: Novel mediators of fibrotic remodelling. J Mol Cell Cardiol 2016;93:175–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meschiari CA, Ero OK, Pan H, Finkel T, Lindsey ML. The impact of aging on cardiac extracellular matrix. Geroscience 2017;39:7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suthahar N, Meijers WC, Sillje HHW, de Boer RA. From inflammation to fibrosis-molecular and cellular mechanisms of myocardial tissue remodelling and perspectives on differential treatment opportunities. Curr Heart Fail Rep 2017;14:235–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russo I, Frangogiannis NG. Diabetes-associated cardiac fibrosis: cellular effectors, molecular mechanisms and therapeutic opportunities. J Mol Cell Cardiol 2016;90:84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kosmala W, Przewlocka-Kosmala M, Wojnalowicz A, Mysiak A, Marwick TH. Integrated backscatter as a fibrosis marker in the metabolic syndrome: association with biochemical evidence of fibrosis and left ventricular dysfunction. Eur Heart J Cardiovasc Imaging 2012;13:459–67. [DOI] [PubMed] [Google Scholar]
- 30.Mahajan R, Lau DH, Sanders P. Impact of obesity on cardiac metabolism, fibrosis, and function. Trends Cardiovasc Med 2015;25:119–26. [DOI] [PubMed] [Google Scholar]
- 31.Lusetti M, Licata M, Silingardi E, Reggiani BL, Palmiere C. Cardiac toxicity in selective serotonin reuptake inhibitor users. Am J Forensic Med Pathol 2015;36:293–7. [DOI] [PubMed] [Google Scholar]
- 32.Andersohn F, Garbe E. Cardiac and noncardiac fibrotic reactions caused by ergot-and nonergot-derived dopamine agonists. Mov Disord 2009;24:129–33. [DOI] [PubMed] [Google Scholar]
- 33.Mota JM, Sousa LG, Riechelmann RP. Complications from carcinoid syndrome: review of the current evidence. Ecancermedicalscience 2016;10:662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu JP, Chang-Lee SN, Day CH, et al. Secondhand Smoke exposure enhances cardiac fibrosis effects on the aging rat hearts. Acta Cardiol Sin 2016;32:594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van de Schoor FR, Aengevaeren VL, Hopman MT, et al. Myocardial fibrosis in athletes. Mayo Clin Proc 2016;91:1617–31. [DOI] [PubMed] [Google Scholar]
- 36.Bovelli D, Plataniotis G, Roila F. Cardiotoxicity of chemotherapeutic agents and radiotherapy-related heart disease: ESMO clinical practice guidelines. Ann Oncol 2010;21(Suppl 5): v277–82. [DOI] [PubMed] [Google Scholar]
- 37.Kim MA, Yang D, Kida K, et al. Effects of ACE2 inhibition in the post-myocardial infarction heart. J Card Fail 2010;16:777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDonald H, Peart J, Kurniawan N, et al. Hexarelin treatment preserves myocardial function and reduces cardiac fibrosis in a mouse model of acute myocardial infarction. Physiol Rep 2018;6:e13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santos-Gallego CG, Vahl TP, Goliasch G, et al. Sphingosine-1-phosphate receptor agonist fingolimod increases myocardial salvage and decreases adverse postinfarction left ventricular remodeling in a porcine model of ischemia/reperfusion. Circulation 2016;133:954–66. [DOI] [PubMed] [Google Scholar]
- 40.Ishida N, Iwata H, Shimabukuro K, et al. Effects of omentopexy combined with granulocyte colony-stimulating factor in a rabbit heart model. Eur J Cardiothorac Surg 2011;39:375–80. [DOI] [PubMed] [Google Scholar]
- 41.Weber KT, Janicki JS, Shroff SG, Pick R, Chen RM, Bashey RI. Collagen remodeling of the pressure-overloaded, hypertrophied nonhuman primate myocardium. Circ Res 1988;62:757–65. [DOI] [PubMed] [Google Scholar]
- 42.Xia Y, Lee K, Li N, Corbett D, Mendoza L, Frangogiannis NG. Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload. Histochem Cell Biol 2009;131:471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Renaud L, Harris LG, Mani SK, et al. HDACs regulate miR-133a expression in pressure overload-induced cardiac fibrosis. Circ Heart Fail 2015;8:1094–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klein G, Schaefer A, Hilfiker-Kleiner D, et al. Increased collagen deposition and diastolic dysfunction but preserved myocardial hypertrophy after pressure overload in mice lacking PKCepsilon. Circ Res 2005;96:748–55. [DOI] [PubMed] [Google Scholar]
- 45.Morgan LA, Olzinski AR, Upson JJ, et al. Soluble epoxide hydrolase inhibition does not prevent cardiac remodeling and dysfunction after aortic constriction in rats and mice. J Cardiovasc Pharmacol 2013;61:291–301. [DOI] [PubMed] [Google Scholar]
- 46.Varagic J, Ahmad S, Voncannon JL, et al. Nebivolol reduces cardiac angiotensin II, associated oxidative stress and fibrosis but not arterial pressure in salt-loaded spontaneously hypertensive rats. J Hypertens 2012;30:1766–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coelho-Filho OR, Shah RV, Neilan TG, et al. Cardiac magnetic resonance assessment of interstitial myocardial fibrosis and cardiomyocyte hypertrophy in hypertensive mice treated with spironolactone. J Am Heart Assoc 2014;3:e000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hlavackova L, Vrankova S, Janega P, Pechanova O, Babal P. The effect of indapamide on development of myocardial hypertrophy and fibrosis in L-NAME-induced hypertension in rat. Physiol Res 2011;60:845–52. [DOI] [PubMed] [Google Scholar]
- 49.Verboven M, Deluyker D, Ferferieva V, et al. Western diet given to healthy rats mimics the human phenotype of diabetic cardiomyopathy. J Nutr Biochem 2018;61:140–6. [DOI] [PubMed] [Google Scholar]
- 50.Chen S, Evans T, Mukherjee K, Karmazyn M, Chakrabarti S. Diabetes-induced myocardial structural changes: role of endothelin-1 and its receptors. J Mol Cell Cardiol 2000;32:1621–9. [DOI] [PubMed] [Google Scholar]
- 51.Liu W, Gong W, He M, et al. Spironolactone protects against diabetic cardiomyopathy in streptozotocin-induced diabetic rats. J Diabetes Res 2018;2018:9232065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Law BA, Levick SP, Carver WE. Alterations in cardiac structure and function in a murine model of chronic alcohol consumption. Microsc Microanal 2012;18:453–61. [DOI] [PubMed] [Google Scholar]
- 53.Levick SP, Soto-Pantoja DR, Bi J, et al. Doxorubicin-induced myocardial fibrosis involves the neurokinin-1 receptor and direct effects on cardiac fibroblasts. Heart Lung Circ 2018:in press. doi: 10.1016/j.hlc.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knuepfer MM, Branch CA, Gan Q, Fischer VW. Cocaine-induced myocardial ultrastructural alterations and cardiac output responses in rats. Exp Mol Pathol 1993;59:155–68. [DOI] [PubMed] [Google Scholar]
- 55.van der Pol A, Gil A, Tromp J, et al. OPLAH ablation leads to accumulation of 5-oxoproline, oxidative stress, fibrosis, and elevated fillings pressures: a murine model for heart failure with a preserved ejection fraction. Cardiovasc Res 2018;114:1871–82. [DOI] [PubMed] [Google Scholar]
- 56.Robert V, Van TN, Cheav SL, Mouas C, Swynghedauw B, Delcayre C. Increased cardiac types I and III collagen mRNAs in aldosterone-salt hypertension. Hypertension 1994;24:30–6. [DOI] [PubMed] [Google Scholar]
- 57.Alex L, Russo I, Holoborodko V, Frangogiannis NG. Characterization of a mouse model of obesity-related fibrotic cardiomyopathy that recapitulates features of human heart failure with preserved ejection fraction. Am J Physiol Heart Circ Physiol 2018;315:H934–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Strungs EG, Ongstad EL, O’Quinn MP, Palatinus JA, Jourdan LJ, Gourdie RG. Cryoinjury models of the adult and neonatal mouse heart for studies of scarring and regeneration. Methods Mol Biol 2013;1037:343–53. [DOI] [PubMed] [Google Scholar]
- 59.Sigusch HH, Campbell SE, Weber KT. Angiotensin II-induced myocardial fibrosis in rats: role of nitric oxide, prostaglandins and bradykinin. Cardiovasc Res 1996;31:546–54. [PubMed] [Google Scholar]
- 60.Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J Mol Cell Cardiol 2011;51:600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim KK, Sheppard D, Chapman HA. TGF-beta1 signaling and tissue fibrosis. Cold Spring Harb Perspect Biol 2018;10: a022293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koli K, Saharinen J, Hyytiainen M, Penttinen C, Keski-Oja J. Latency, activation, and binding proteins of TGF-beta. Microsc Res Tech 2001;52:354–62. [DOI] [PubMed] [Google Scholar]
- 63.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev 2000;14:163–76. [PMC free article] [PubMed] [Google Scholar]
- 64.Murphy-Ullrich JE, Poczatek M. Activation of latent TGF-beta by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev 2000;11:59–69. [DOI] [PubMed] [Google Scholar]
- 65.Barcellos-Hoff MH, Dix TA. Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol 1996;10:1077–83. [DOI] [PubMed] [Google Scholar]
- 66.Lyons RM, Keski-Oja J, Moses HL. Proteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. J Cell Biol 1988;106:1659–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Munger JS, Huang X, Kawakatsu H, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 1999;96:319–28. [DOI] [PubMed] [Google Scholar]
- 68.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol 2007;179:1311–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J 2002;21:1743–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003;425:577–84. [DOI] [PubMed] [Google Scholar]
- 71.Petrov VV, Fagard RH, Lijnen PJ. Stimulation of collagen production by transforming growth factor-beta1 during differentiation of cardiac fibroblasts to myofibroblasts. Hypertension 2002;39:258–63. [DOI] [PubMed] [Google Scholar]
- 72.Seeland U, Haeuseler C, Hinrichs R, et al. Myocardial fibrosis in transforming growth factor-beta(1) (TGF-beta(1)) transgenic mice is associated with inhibition of interstitial collagenase. Eur J Clin Invest 2002;32:295–303. [DOI] [PubMed] [Google Scholar]
- 73.Mauviel A Transforming growth factor-beta: a key mediator of fibrosis. Methods Mol Med 2005;117:69–80. [DOI] [PubMed] [Google Scholar]
- 74.Gray MO, Long CS, Kalinyak JE, Li HT, Karliner JS. Angiotensin II stimulates cardiac myocyte hypertrophy via paracrine release of TGF-beta 1 and endothelin-1 from fibroblasts. Cardiovasc Res 1998;40:352–63. [DOI] [PubMed] [Google Scholar]
- 75.Schultz JJ, Witt SA, Glascock BJ, et al. TGF-beta1 mediates the hypertrophic cardiomyocyte growth induced by angiotensin II. J Clin Invest 2002;109:787–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lebrin F, Deckers M, Bertolino P, ten Dijke P. TGF-beta receptor function in the endothelium. Cardiovasc Res 2005;65: 599–608. [DOI] [PubMed] [Google Scholar]
- 77.Yoshimatsu Y, Watabe T. Roles of TGF-beta signals in endothelial-mesenchymal transition during cardiac fibrosis. Int J Inflam 2011;2011:724080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thompson NL, Bazoberry F, Speir EH, et al. Transforming growth factor beta-1 in acute myocardial infarction in rats. Growth Factors 1988;1:91–9. [DOI] [PubMed] [Google Scholar]
- 79.Hao J, Ju H, Zhao S, Junaid A, Scammell-La Fleur T, Dixon IM. Elevation of expression of Smads 2, 3, and 4, decorin and TGF-beta in the chronic phase of myocardial infarct scar healing. J Mol Cell Cardiol 1999;31:667–78. [DOI] [PubMed] [Google Scholar]
- 80.Koitabashi N, Danner T, Zaiman AL, et al. Pivotal role of cardiomyocyte TGF-beta signaling in the murine pathological response to sustained pressure overload. J Clin Invest 2011;121:2301–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rosenkranz S TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res 2004;63:423–32. [DOI] [PubMed] [Google Scholar]
- 82.Yue Y, Meng K, Pu Y, Zhang X. Transforming growth factor beta (TGF-beta) mediates cardiac fibrosis and induces diabetic cardiomyopathy. Diabetes Res Clin Pract 2017;133:124–30. [DOI] [PubMed] [Google Scholar]
- 83.Lefer AM, Ma XL, Weyrich AS, Scalia R. Mechanism of the cardioprotective effect of transforming growth factor beta 1 in feline myocardial ischemia and reperfusion. Proc Natl Acad Sci USA 1993;90:1018–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen H, Li D, Saldeen T, Mehta JL. TGF-beta 1 attenuates myocardial ischemia-reperfusion injury via inhibition of upregulation of MMP-1. Am J Physiol Heart Circ Physiol 2003;284:H1612–7. [DOI] [PubMed] [Google Scholar]
- 85.Kong P, Shinde AV, Su Y, et al. Opposing actions of fibroblast and cardiomyocyte Smad3 signaling in the infarcted myocardium. Circulation 2018;137:707–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Molkentin JD, Bugg D, Ghearing N, et al. Fibroblast-specific genetic manipulation of p38 mitogen-activated protein kinase in vivo reveals its central regulatory role in fibrosis. Circulation 2017;136:549–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khalil H, Kanisicak O, Prasad V, et al. Fibroblast-specific TGF-beta-Smad2/3 signaling underlies cardiac fibrosis. J Clin Invest 2017;127:3770–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Villarreal FJ, Kim NN, Ungab GD, Printz MP, Dillmann WH. Identification of functional angiotensin II receptors on rat cardiac fibroblasts. Circulation 1993;88:2849–61. [DOI] [PubMed] [Google Scholar]
- 89.Peng J, Gurantz D, Tran V, Cowling RT, Greenberg BH. Tumor necrosis factor-alpha-induced AT1 receptor upregulation enhances angiotensin II-mediated cardiac fibroblast responses that favor fibrosis. Circ Res 2002;91:1119–26. [DOI] [PubMed] [Google Scholar]
- 90.Iwata M, Cowling RT, Gurantz D, et al. Angiotensin-(1–7) binds to specific receptors on cardiac fibroblasts to initiate antifibrotic and antitrophic effects. Am J Physiol Heart Circ Physiol 2005;289:H2356–63. [DOI] [PubMed] [Google Scholar]
- 91.Hafizi S, Wharton J, Morgan K, et al. Expression of functional angiotensin-converting enzyme and AT1 receptors in cultured human cardiac fibroblasts. Circulation 1998;98:2553–9. [DOI] [PubMed] [Google Scholar]
- 92.Lee AA, Dillmann WH, McCulloch AD, Villarreal FJ. Angiotensin II stimulates the autocrine production of transforming growth factor-beta 1 in adult rat cardiac fibroblasts. J Mol Cell Cardiol. 1995;27:2347–57. [DOI] [PubMed] [Google Scholar]
- 93.Campbell SE, Katwa LC. Angiotensin II stimulated expression of transforming growth factor-beta1 in cardiac fibroblasts and myofibroblasts. J Mol Cell Cardiol 1997;29:1947–58. [DOI] [PubMed] [Google Scholar]
- 94.Davis J, Burr AR, Davis GF, Birnbaumer L, Molkentin JD. A TRPC6-dependent pathway for myofibroblast transdifferentiation and wound healing in vivo. Dev Cell 2012;23:705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hafizi S, Wharton J, Chester AH, Yacoub MH. Profibrotic effects of endothelin-1 via the ETA receptor in cultured human cardiac fibroblasts. Cell Physiol Biochem 2004;14:285–92. [DOI] [PubMed] [Google Scholar]
- 96.Wang X, Guo Z, Ding Z, et al. Endothelin-1 upregulation mediates aging-related cardiac fibrosis. J Mol Cell Cardiol 2015;80:101–9. [DOI] [PubMed] [Google Scholar]
- 97.Widyantoro B, Emoto N, Nakayama K, et al. Endothelial cell-derived endothelin-1 promotes cardiac fibrosis in diabetic hearts through stimulation of endothelial-to-mesenchymal transition. Circulation 2010;121:2407–18. [DOI] [PubMed] [Google Scholar]
- 98.Adiarto S, Heiden S, Vignon-Zellweger N, et al. ET-1 from endothelial cells is required for complete angiotensin II-induced cardiac fibrosis and hypertrophy. Life Sci 2012;91:651–7. [DOI] [PubMed] [Google Scholar]
- 99.Cheng CM, Hong HJ, Liu JC, et al. Crucial role of extracellular signal-regulated kinase pathway in reactive oxygen species-mediated endothelin-1 gene expression induced by endothelin-1 in rat cardiac fibroblasts. Mol Pharmacol 2003;63:1002–11. [DOI] [PubMed] [Google Scholar]
- 100.Koshman YE, Patel N, Chu M, et al. Regulation of connective tissue growth factor gene expression and fibrosis in human heart failure. J Card Fail 2013;19:283–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chuva de Sousa Lopes SM, Feijen A, Korving J, et al. Connective tissue growth factor expression and Smad signaling during mouse heart development and myocardial infarction. Dev Dyn 2004;231:542–50. [DOI] [PubMed] [Google Scholar]
- 102.Chen MM, Lam A, Abraham JA, Schreiner GF, Joly AH. CTGF expression is induced by TGF- beta in cardiac fibroblasts and cardiac myocytes: a potential role in heart fibrosis. J Mol Cell Cardiol 2000;32:1805–19. [DOI] [PubMed] [Google Scholar]
- 103.Accornero F, van Berlo JH, Correll RN, et al. Genetic analysis of connective tissue growth factor as an effector of transforming growth factor beta signaling and cardiac remodeling. Mol Cell Biol 2015;35:2154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dorn LE, Petrosino JM, Wright P, Accornero F. CTGF/CCN2 is an autocrine regulator of cardiac fibrosis. J Mol Cell Cardiol 2018;121:205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vainio LE, Szabo Z, Lin R, et al. Connective tissue growth factor inhibition enhances cardiac repair and limits fibrosis after myocardial infarction. JACC Basic Transl Sci 2019;4:83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Meszaros JG, Gonzalez AM, Endo-Mochizuki Y, Villegas S, Villarreal F, Brunton LL. Identification of G protein-coupled signaling pathways in cardiac fibroblasts: cross talk between G (q) and G(s). Am J Physiol Cell Physiol 2000;278:C154–62. [DOI] [PubMed] [Google Scholar]
- 107.Turner NA, Porter KE, Smith WH, White HL, Ball SG, Balmforth AJ. Chronic beta2-adrenergic receptor stimulation increases proliferation of human cardiac fibroblasts via an autocrine mechanism. Cardiovasc Res 2003;57:784–92. [DOI] [PubMed] [Google Scholar]
- 108.Swaney JS, Roth DM, Olson ER, Naugle JE, Meszaros JG, Insel PA. Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase. Proc Natl Acad Sci USA 2005;102:437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li J, Philip JL, Xu X, Theccanat T, Abdur RM, Akhter SA. Beta-arrestins regulate human cardiac fibroblast transformation and collagen synthesis in adverse ventricular remodeling. J Mol Cell Cardiol 2014;76:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Woodall MC, Woodall BP, Gao E, Yuan A, Koch WJ. Cardiac fibroblast GRK2 deletion enhances contractility and remodeling following ischemia/reperfusion injury. Circ Res 2016;119:1116–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zou Y, Akazawa H, Qin Y, et al. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol 2004;6:499–506. [DOI] [PubMed] [Google Scholar]
- 112.Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell 2003;113:329–42. [DOI] [PubMed] [Google Scholar]
- 113.Small EM, Thatcher JE, Sutherland LB, et al. Myocardin-related transcription factor-a controls myofibroblast activation and fibrosis in response to myocardial infarction. Circ Res 2010;107:294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Muehlich S, Hermanns C, Meier MA, Kircher P, Gudermann T. Unravelling a new mechanism linking actin polymerization and gene transcription. Nucleus 2016;7:121–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One 2009;4: e7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Haudek SB, Xia Y, Huebener P, et al. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci USA 2006;103:18284–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kramann R, Schneider RK, DiRocco DP, et al. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 2015;16:51–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zeisberg EM, Tarnavski O, Zeisberg M, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 2007;13:952–61. [DOI] [PubMed] [Google Scholar]
- 119.Payne S, De Val S, Neal A. Endothelial-specific cre mouse models. Arterioscler Thromb Vasc Biol 2018;38:2550–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Carlson TR, Hu H, Braren R, Kim YH, Wang RA. Cell-autonomous requirement for beta1 integrin in endothelial cell adhesion, migration and survival during angiogenesis in mice. Development 2008;135:2193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kong P, Christia P, Saxena A, Su Y, Frangogiannis NG. Lack of specificity of fibroblast-specific protein 1 in cardiac remodeling and fibrosis. Am J Physiol Heart Circ Physiol 2013;305: H1363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Moore-Morris T, Guimaraes-Camboa N, Banerjee I, et al. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J Clin Invest 2014;124:2921–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kanisicak O, Khalil H, Ivey MJ, et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat Commun 2016;7:12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Moore-Morris T, Cattaneo P, Guimaraes-Camboa N, et al. Infarct fibroblasts do not derive from bone marrow lineages. Circ Res 2018;122:583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pinto AR, Paolicelli R, Salimova E, et al. An abundant tissue macrophage population in the adult murine heart with a distinct alternatively-activated macrophage profile. PLoS One 2012;7:e36814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Frantz S, Nahrendorf M. Cardiac macrophages and their role in ischaemic heart disease. Cardiovasc Res 2014;102:240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nahrendorf M, Swirski FK, Aikawa E, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 2007;204:3037–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang L, Zhang YL, Lin QY, et al. CXCL1-CXCR2 axis mediates angiotensin II-induced cardiac hypertrophy and remodelling through regulation of monocyte infiltration. Eur Heart J 2018;39:1818–31. [DOI] [PubMed] [Google Scholar]
- 129.Ivey MJ, Kuwabara JT, Pai JT, Moore RE, Sun Z, Tallquist MD. Resident fibroblast expansion during cardiac growth and remodeling. J Mol Cell Cardiol 2018;114:161–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gurantz D, Cowling RT, Villarreal FJ, Greenberg BH. Tumor necrosis factor-alpha upregulates angiotensin II type 1 receptors on cardiac fibroblasts. Circ Res 1999;85:272–9. [DOI] [PubMed] [Google Scholar]
- 131.Cowling RT, Gurantz D, Peng J, Dillmann WH, Greenberg BH. Transcription factor NF-kappa B is necessary for up-regulation of type 1 angiotensin II receptor mRNA in rat cardiac fibroblasts treated with tumor necrosis factor-alpha or interleukin-1 beta. J Biol Chem 2002;277:5719–24. [DOI] [PubMed] [Google Scholar]
- 132.Cieslik KA, Taffet GE, Carlson S, Hermosillo J, Trial J, Entman ML. Immune-inflammatory dysregulation modulates the incidence of progressive fibrosis and diastolic stiffness in the aging heart. J Mol Cell Cardiol 2011;50:248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mouton AJ, Ma Y, Rivera Gonzalez OJ, et al. Fibroblast polarization over the myocardial infarction time continuum shifts roles from inflammation to angiogenesis. Basic Res Cardiol 2019;114:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fu X, Khalil H, Kanisicak O, et al. Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart. J Clin Invest 2018;128:2127–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bogatyryov Y, Tomanek RJ, Dedkov EI. Structural composition of myocardial infarction scar in middle-aged male and female rats: does sex matter? J Histochem Cytochem 2013;61:833–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Elson EL, Qian H, Fee JA, Wakatsuki T. A model for positive feedback control of the transformation of fibroblasts to myofibroblasts. Prog Biophys Mol Biol 2018:in press. doi: 10.1016/j.pbiomolbio.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 2002;3:349–63. [DOI] [PubMed] [Google Scholar]
- 138.Zhou XL, Xu H, Liu ZB, Wu QC, Zhu RR, Liu JC. miR-21 promotes cardiac fibroblast-to-myofibroblast transformation and myocardial fibrosis by targeting Jagged1. J Cell Mol Med 2018;22:3816–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bejerano T, Etzion S, Elyagon S, Etzion Y, Cohen S. Nanoparticle delivery of miRNA-21 mimic to cardiac macrophages improves myocardial remodeling after myocardial infarction. Nano Lett 2018;18:5885–91. [DOI] [PubMed] [Google Scholar]
- 140.Zhang L, Yin H, Jiao L, et al. Abnormal downregulation of caveolin-3 mediates the pro-fibrotic action of microRNA-22 in a model of myocardial infarction. Cell Physiol Biochem 2018;45:1641–53. [DOI] [PubMed] [Google Scholar]
- 141.Li L, Bounds KR, Chatterjee P, Gupta S. MicroRNA-130a, a potential antifibrotic target in cardiac fibrosis. J Am Heart Assoc 2017;6:e006763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wei Y, Yan X, Yan L, et al. Inhibition of microRNA155 ameliorates cardiac fibrosis in the process of angiotensin IIinduced cardiac remodeling. Mol Med Rep 2017;16:7287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhao D, Li C, Yan H, et al. Cardiomyocyte derived miR-328 promotes cardiac fibrosis by paracrinely regulating adjacent fibroblasts. Cell Physiol Biochem 2018;46:1555–65. [DOI] [PubMed] [Google Scholar]
- 144.Lopez B, Gonzalez A, Hermida N, Valencia F, de Teresa E, Diez J. Role of lysyl oxidase in myocardial fibrosis: from basic science to clinical aspects. Am J Physiol Heart Circ Physiol 2010;299:H1–9. [DOI] [PubMed] [Google Scholar]
- 145.El Hajj EC, El Hajj MC, Ninh VK, Bradley JM, Claudino MA, Gardner JD. Detrimental role of lysyl oxidase in cardiac remodeling. J Mol Cell Cardiol 2017;109:17–26. [DOI] [PubMed] [Google Scholar]
- 146.Shinde AV, Su Y, Palanski BA, Fujikura K, Garcia MJ, Frangogiannis NG. Pharmacologic inhibition of the enzymatic effects of tissue transglutaminase reduces cardiac fibrosis and attenuates cardiomyocyte hypertrophy following pressure overload. J Mol Cell Cardiol 2018;117:36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cowling RT, Park JI, Sotimehin AE, Greenberg BH. Ascorbate starvation alters endoplasmic reticulum-resident enzymes in cardiac fibroblasts, priming them for increased procollagen secretion. J Mol Cell Cardiol 2017;113:1–8. [DOI] [PubMed] [Google Scholar]
- 148.Roberts WC, Siegel RJ, McManus BM. Idiopathic dilated cardiomyopathy: analysis of 152 necropsy patients. Am J Cardiol 1987;60:1340–55. [DOI] [PubMed] [Google Scholar]
- 149.Holzmann M, Nicko A, Kuhl U, et al. Complication rate of right ventricular endomyocardial biopsy via the femoral approach: a retrospective and prospective study analyzing 3048 diagnostic procedures over an 11-year period. Circulation 2008;118:1722–8. [DOI] [PubMed] [Google Scholar]
- 150.Yancy CW, Jessup M, Bozkurt B, et al. ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017;136:e137–61. [DOI] [PubMed] [Google Scholar]
- 151.Dumic J, Dabelic S, Flogel M. Galectin-3: an open-ended story. Biochim Biophys Acta 2006;1760:616–35. [DOI] [PubMed] [Google Scholar]
- 152.Mackinnon AC, Gibbons MA, Farnworth SL, et al. Regulation of transforming growth factor-beta1-driven lung fibrosis by galectin-3. Am J Respir Crit Care Med 2012;185:537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sharma UC, Pokharel S, van Brakel TJ, et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 2004;110:3121–8. [DOI] [PubMed] [Google Scholar]
- 154.Henderson NC, Mackinnon AC, Farnworth SL, et al. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc Natl Acad Sci USA 2006;103:5060–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yu L, Ruifrok WP, Meissner M, et al. Genetic and pharmacological inhibition of galectin-3 prevents cardiac remodeling by interfering with myocardial fibrogenesis. Circ Heart Fail 2013;6:107–17. [DOI] [PubMed] [Google Scholar]
- 156.Calvier L, Miana M, Reboul P, et al. Galectin-3 mediates aldosterone-induced vascular fibrosis. Arterioscler Thromb Vasc Biol 2013;33:67–75. [DOI] [PubMed] [Google Scholar]
- 157.Henderson NC, Mackinnon AC, Farnworth SL, et al. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol 2008;172:288–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.de Boer RA, Voors AA, Muntendam P, van Gilst WH, van Veldhuisen DJ. Galectin-3: a novel mediator of heart failure development and progression. Eur J Heart Fail 2009;11:811–7. [DOI] [PubMed] [Google Scholar]
- 159.Liu YH, D’Ambrosio M, Liao TD, et al. N-acetyl-seryl-aspartyllysyl-proline prevents cardiac remodeling and dysfunction induced by galectin-3, a mammalian adhesion/growth-regulatory lectin. Am J Physiol Heart Circ Physiol 2009;296:H404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Besler C, Lang D, Urban D, et al. Plasma and cardiac galectin-3 in patients with heart failure reflects both inflammation and fibrosis: implications for its use as a biomarker. Circ Heart Fail 2017;10:e003804. [DOI] [PubMed] [Google Scholar]
- 161.Numano F, Shimizu C, Jimenez-Fernandez S, et al. Galectin-3 is a marker of myocardial and vascular fibrosis in Kawasaki disease patients with giant aneurysms. Int J Cardiol 2015;201:429–37. [DOI] [PubMed] [Google Scholar]
- 162.de Boer RA, Daniels LB, Maisel AS, Januzzi JL Jr.. State of the art: newer biomarkers in heart failure. Eur J Heart Fail. 2015;17:559–69. [DOI] [PubMed] [Google Scholar]
- 163.Grandin EW, Jarolim P, Murphy SA, et al. Galectin-3 and the development of heart failure after acute coronary syndrome: pilot experience from PROVE IT-TIMI 22. Clin Chem 2012;58:267–73. [DOI] [PubMed] [Google Scholar]
- 164.Daniels LB, Clopton P, Laughlin GA, Maisel AS, Barrett-Connor E. Galectin-3 is independently associated with cardiovascular mortality in community-dwelling older adults without known cardiovascular disease: The Rancho Bernardo Study. Am Heart J 2014;167:674–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.de Boer RA, van Veldhuisen DJ, Gansevoort RT, et al. The fibrosis marker galectin-3 and outcome in the general population. J Intern Med 2012;272:55–64. [DOI] [PubMed] [Google Scholar]
- 166.Ho JE, Liu C, Lyass A, et al. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol 2012;60:1249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest 2007;117:1538–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Daniels LB, Bayes-Genis A. Using ST2 in cardiovascular patients: a review. Future Cardiol 2014;10:525–39. [DOI] [PubMed] [Google Scholar]
- 169.Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. JAMA 2017;318:1925–6. [DOI] [PubMed] [Google Scholar]
- 170.Apple FS. Tissue specificity of cardiac troponin I, cardiac troponin T and creatine kinase-MB. Clin Chim Acta 1999;284:151–9. [DOI] [PubMed] [Google Scholar]
- 171.Wu AH. Increased troponin in patients with sepsis and septic shock: myocardial necrosis or reversible myocardial depression? Intensive Care Med 2001;27:959–61. [DOI] [PubMed] [Google Scholar]
- 172.Saunders JT, Nambi V, de Lemos JA, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the atherosclerosis risk in communities study. Circulation 2011;123:1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Seliger SL, Hong SN, Christenson RH, et al. High-sensitive cardiac troponin t as an early biochemical signature for clinical and subclinical heart failure: MESA (Multi-Ethnic Study of Atherosclerosis). Circulation 2017;135:1494–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chin CW, Messika-Zeitoun D, Shah AS, et al. A clinical risk score of myocardial fibrosis predicts adverse outcomes in aortic stenosis. Eur Heart J 2016;37:713–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hasler S, Manka R, Greutmann M, et al. Elevated high-sensitivity troponin T levels are associated with adverse cardiac remodelling and myocardial fibrosis in hypertrophic cardiomyopathy. Swiss Med Wkly 2016;146:w14285. [DOI] [PubMed] [Google Scholar]
- 176.Gawor M, Spiewak M, Kubik A, et al. Circulating biomarkers of hypertrophy and fibrosis in patients with hypertrophic cardiomyopathy assessed by cardiac magnetic resonance. Biomarkers 2018;23:676–82. [DOI] [PubMed] [Google Scholar]
- 177.Aimo A, Januzzi JL Jr., Vergaro G, et al. Prognostic value of high-sensitivity troponin t in chronic heart failure: an individual patient data meta-analysis. Circulation 2018;137:286–97. [DOI] [PubMed] [Google Scholar]
- 178.Lopez B, Gonzalez A, Ravassa S, et al. Circulating biomarkers of myocardial fibrosis: the need for a reappraisal. J Am Coll Cardiol 2015;65:2449–56. [DOI] [PubMed] [Google Scholar]
- 179.Tayebjee MH, Nadar S, Blann AD, Gareth BD, MacFadyen RJ, Lip GY. Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in hypertension and their relationship to cardiovascular risk and treatment: a substudy of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT). Am J Hypertens 2004;17:764–9. [DOI] [PubMed] [Google Scholar]
- 180.Sundstrom J, Evans JC, Benjamin EJ, et al. Relations of plasma total TIMP-1 levels to cardiovascular risk factors and echocardiographic measures: the Framingham heart study. Eur Heart J 2004;25:1509–16. [DOI] [PubMed] [Google Scholar]
- 181.Dhingra R, Pencina MJ, Schrader P, et al. Relations of matrix remodeling biomarkers to blood pressure progression and incidence of hypertension in the community. Circulation 2009;119:1101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hansson J, Lind L, Hulthe J, Sundstrom J. Relations of serum MMP-9 and TIMP-1 levels to left ventricular measures and cardiovascular risk factors: a population-based study. Eur J Cardiovasc Prev Rehabil 2009;16:297–303. [DOI] [PubMed] [Google Scholar]
- 183.Iraqi W, Rossignol P, Angioi M, et al. Extracellular cardiac matrix biomarkers in patients with acute myocardial infarction complicated by left ventricular dysfunction and heart failure: insights from the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) study. Circulation 2009;119:2471–9. [DOI] [PubMed] [Google Scholar]
- 184.Lopez B, Gonzalez A, Querejeta R, Larman M, Diez J. Alterations in the pattern of collagen deposition may contribute to the deterioration of systolic function in hypertensive patients with heart failure. J Am Coll Cardiol 2006;48:89–96. [DOI] [PubMed] [Google Scholar]
- 185.Velagaleti RS, Gona P, Sundstrom J, et al. Relations of biomarkers of extracellular matrix remodeling to incident cardiovascular events and mortality. Arterioscler Thromb Vasc Biol 2010;30:2283–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ravassa S, Trippel T, Bach D, et al. Biomarker-based phenotyping of myocardial fibrosis identifies patients with heart failure with preserved ejection fraction resistant to the beneficial effects of spironolactone: results from the Aldo-DHF trial. Eur J Heart Fail 2018;20:1290–9. [DOI] [PubMed] [Google Scholar]
- 187.Matsui Y, Jia N, Okamoto H, et al. Role of osteopontin in cardiac fibrosis and remodeling in angiotensin II-induced cardiac hypertrophy. Hypertension 2004;43:1195–201. [DOI] [PubMed] [Google Scholar]
- 188.Picano E, Pelosi G, Marzilli M, et al. In vivo quantitative ultrasonic evaluation of myocardial fibrosis in humans. Circulation 1990;81:58–64. [DOI] [PubMed] [Google Scholar]
- 189.Di Bello V, Giorgi D, Viacava P, et al. Severe aortic stenosis and myocardial function: diagnostic and prognostic usefulness of ultrasonic integrated backscatter analysis. Circulation 2004;110:849–55. [DOI] [PubMed] [Google Scholar]
- 190.Mizuno R, Fujimoto S, Saito Y, Nakamura S. Non-invasive quantitation of myocardial fibrosis using combined tissue harmonic imaging and integrated backscatter analysis in dilated cardiomyopathy. Cardiology 2007;108:11–7. [DOI] [PubMed] [Google Scholar]
- 191.Popovic ZB, Kwon DH, Mishra M, et al. Association between regional ventricular function and myocardial fibrosis in hypertrophic cardiomyopathy assessed by speckle tracking echocardiography and delayed hyperenhancement magnetic resonance imaging. J Am Soc Echocardiogr 2008;21:1299–305. [DOI] [PubMed] [Google Scholar]
- 192.Knaapen P, Boellaard R, Gotte MJ, et al. Perfusable tissue index as a potential marker of fibrosis in patients with idiopathic dilated cardiomyopathy. J Nucl Med 2004;45:1299–304. [PubMed] [Google Scholar]
- 193.Knaapen P, Gotte MJ, Paulus WJ, et al. Does myocardial fibrosis hinder contractile function and perfusion in idiopathic dilated cardiomyopathy? PET and MR imaging study. Radiology 2006;240:380–8. [DOI] [PubMed] [Google Scholar]
- 194.Parsai C, O’Hanlon R, Prasad SK, Mohiaddin RH. Diagnostic and prognostic value of cardiovascular magnetic resonance in non-ischaemic cardiomyopathies. J Cardiovasc Magn Reson 2012;14:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Croisille P, Revel D, Saeed M. Contrast agents and cardiac MR imaging of myocardial ischemia: from bench to bedside. Eur Radiol 2006;16:1951–63. [DOI] [PubMed] [Google Scholar]
- 196.Kim RJ, Fieno DS, Parrish TB, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 1999;100:1992–2002. [DOI] [PubMed] [Google Scholar]
- 197.Mewton N, Liu CY, Croisille P, Bluemke D, Lima JA. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol 2011;57:891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Haaf P, Garg P, Messroghli DR, Broadbent DA, Greenwood JP, Plein S. Cardiac T1 mapping and extracellular volume (ECV) in clinical practice: a comprehensive review. J Cardiovasc Magn Reson 2016;18:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified look-locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med 2004;52:141–6. [DOI] [PubMed] [Google Scholar]
- 200.Piechnik SK, Ferreira VM, Dall’Armellina E, et al. Shortened Modified Look-Locker Inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J Cardiovasc Magn Reson 2010;12:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Messroghli DR, Plein S, Higgins DM, et al. Human myocardium: single-breath-hold MR T1 mapping with high spatial resolution—reproducibility study. Radiology 2006;238: 1004–12. [DOI] [PubMed] [Google Scholar]
- 202.Diao KY, Yang ZG, Xu HY, et al. Histologic validation of myocardial fibrosis measured by T1 mapping: a systematic review and meta-analysis. J Cardiovasc Magn Reson 2016;18:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sibley CT, Noureldin RA, Gai N, et al. T1 mapping in cardiomyopathy at cardiac MR: comparison with endomyocardial biopsy. Radiology 2012;265:724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Everett RJ, Stirrat CG, Semple SI, Newby DE, Dweck MR, Mirsadraee S. Assessment of myocardial fibrosis with T1 mapping MRI. Clin Radiol 2016;71:768–78. [DOI] [PubMed] [Google Scholar]
- 205.Puntmann VO, Carr-White G, Jabbour A, et al. T1-mapping and outcome in nonischemic cardiomyopathy: all-cause mortality and heart failure. JACC Cardiovasc Imaging 2016;9:40–50. [DOI] [PubMed] [Google Scholar]
- 206.Lee H, Park JB, Yoon YE, et al. Noncontrast myocardial T1 mapping by cardiac magnetic resonance predicts outcome in patients with aortic stenosis. JACC Cardiovasc Imaging 2018;11:974–83. [DOI] [PubMed] [Google Scholar]
- 207.Jordan JH, Vasu S, Morgan TM, et al. Anthracycline-associated T1 mapping characteristics are elevated independent of the presence of cardiovascular comorbidities in cancer survivors. Circ Cardiovasc Imaging 2016;9:e004325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yi CJ, Wu CO, Tee M, et al. The association between cardiovascular risk and cardiovascular magnetic resonance measures of fibrosis: the Multi-Ethnic Study of Atherosclerosis (MESA). J Cardiovasc Magn Reson 2015;17:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci 2014;71:549–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.McLenachan JM, Dargie HJ. Ventricular arrhythmias in hypertensive left ventricular hypertrophy. Relationship to coronary artery disease, left ventricular dysfunction, and myocardial fibrosis. Am J Hypertens 1990;3:735–40. [DOI] [PubMed] [Google Scholar]
- 211.Segura AM, Frazier OH, Buja LM. Fibrosis and heart failure. Heart Fail Rev 2014;19:173–85. [DOI] [PubMed] [Google Scholar]