Abstract
The synthesis, physical properties and calculated performances of six stereo- and regioisomeric cyclobutane nitric ester materials is described. While the calculated performances of these isomers, as expected, were similar, their physical properties were found to be extremely different. By altering the stereo- and regiochemistry, complete tunability in the form of low-or high-melting solids, standalone melt-castable explosives, melt-castable explosive eutectic compounds, and liquid propellant materials were obtained. This demonstrates that theoretical calculations should not be the main factor in driving the design of new materials, and that stereo- and regiochemistry matter when designing compounds of potential relevance to energetic formulators.
Graphical Abstract
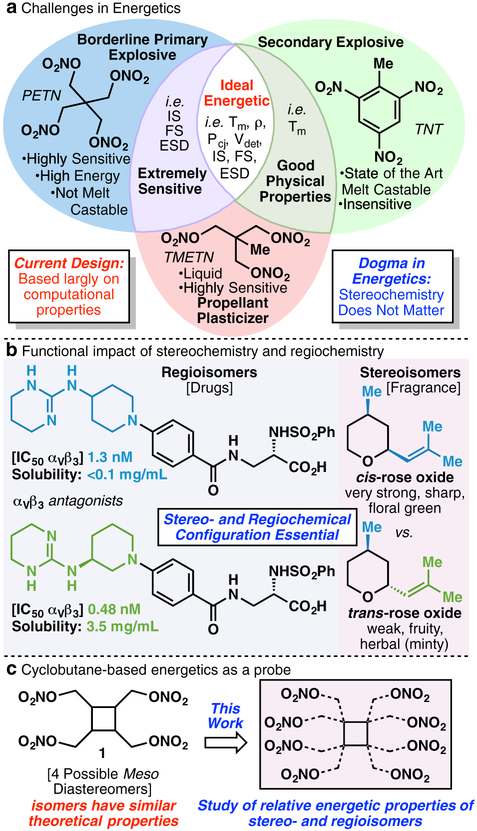
In pursuit of designing high-performing energetic materials,1 the main criteria today are based on theoretically predicted performance properties.2 While a given material’s synthetic accessibility and oxygen balance are factors in the design of new energetics, historically these molecules have been prepared based largely on computer calculated properties (density, heat of formation (ΔH), detonation pressure (Pcj), detonation velocity (Vdet), and specific impulse (Isp)). Hence, many nitrated derivatives based on various nitrogen- and oxygen-rich heterocyclic systems have been synthesized to-date.3–10 Unfortunately, while many of these legacy materials are high-performing based on computational calculations, they are plagued by issues such as very high sensitivity, thermal instability, moisture sensitivity, and in the case of nitrogen-rich energetic salts, an incompatibility with many common ingredients in an explosive or propellant formulation mixture. For this reason, classic energetics such as the highly sensitive primary explosive pentaerythritol tetranitrate (PETN), the current state-of-the-art melt-castable explosive trinitrotoluene (TNT), and the sensitive liquid plasticizer trimethylolethane trinitrate (TMETN) still find common use today (Figure 1A). Herein a long-overlooked strategy for the design of useful energetic materials is presented in an effort to develop highly energetic melt-castable and propellant plasticizing materials.
Figure 1.
(a) Challenges and variables in the design of modern energetics; (b) Impacts of stereo- and regiochemistry in medicine and fragrances; (c) Platform to interrogate stereo- and regiochemistry in energetics.
In designing energetic materials, exploration of a class of materials based on systematic stereo- and regiochemical permutations is without precedent. This has largely stemmed from the perception that stereo- or regioisomers are not advantageous to pursue since the predicted performance properties between the isomers will be minimal. Yet the critical role of stereo- and regiochemistry is documented in nearly all other areas of the chemical enterprise.11 For example, as shown in Figure 1B, regioisomers can exhibit drastically different physical properties and when recognition events take place with chiral receptors, the stereochemistry of a small-molecule binder matters.12–13
Herein, the long-held assumption that stereo- and regiochemistry are of little influence in the energetics field is challenged with a systematic analysis of a set of cyclobutane-based nitric esters, differing only in stereo- and regiochemistry (Figure 1C). Despite having similar theoretical performance properties, this set of isomeric strained molecules exhibits remarkable differences in physical properties, tunable by virtue of simple stereo- and regiochemical changes.
Azetidine and cyclobutane moieties tolerate various nitration conditions. Due to their ring strain, which further increases performance by raising the heat of formation of the system, nitro group-bearing materials containing these cores have been designed in the past. Two of the most energetic materials containing these strained rings are 1,1,3,3-tetranitro cyclobutane (TNCB)14 and the melt-castable explosive trinitroazetidine (TNAZ).15 TNCB was calculated to possess an explosive power in excess of HMX, but unfortunately decomposes at 165 °C, and is hence of little practical value. Like TNCB, TNAZ was also calculated to possess an explosive performance in excess of HMX, possessing a melting point of 101–103 °C and a decomposition temperature of 216 °C, which classifies it as a potential melt-castable explosive material. Despite successful efforts to scale TNAZ,16 its vapor pressure in the molten state was found to be significantly higher than that of the benchmark melt-castable explosive TNT. This presented a significant safety and processing hazard, all but ending TNAZ’s potential applications as a melt-castable explosive material. It was noticed in searching the literature that tetra-(nitroxymethyl) cyclobutane, nor any of its isomers, had been made and thus the physical and energetic properties stemming from their stereo- and regiochemistries had not been studied. As a result, the tetrasubstituted cyclobutane nitric ester scaffold 1 was chosen based on its nitrated structure and its ring strain, coupled with its ability to introduce stereo- and regiochemistry into the structure.
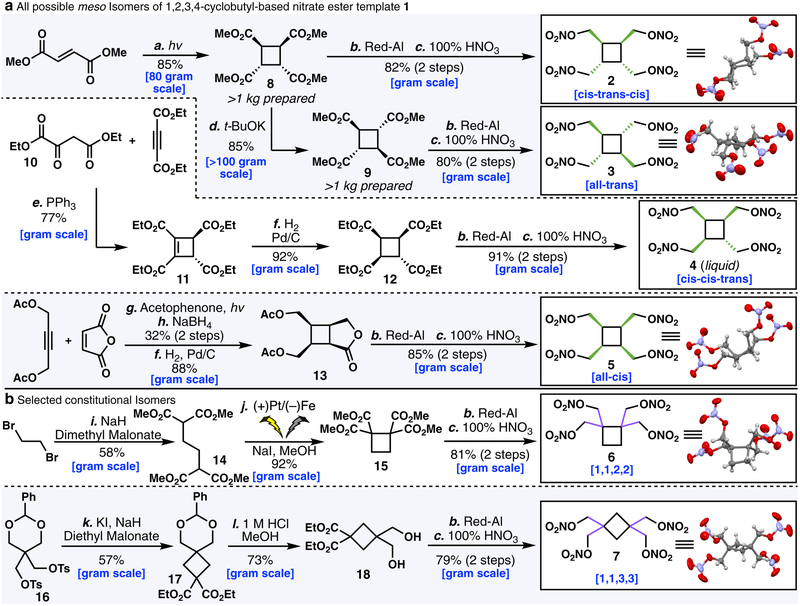
Scheme 1A outlines the scalable synthesis of all four possible meso stereochemical isomers of compound 1 (2–5), as well as two constitutional isomers (6–7). To begin, both compounds 2 and 3 can be accessed by the photochemical dimerization of dimethyl fumarate.17 This yields the head-to-head dimer 8, in which the ester substituents around the central cyclobutane ring have a cis-trans-cis relationship to one another. Direct reduction of 8 gave the tetraol, which was immediately subjected to nitration to provide 2. Conversely, 8 could be epimerized under basic conditions18 to afford its all-trans stereoisomer 9. A similar sequence of reduction and nitration furnished the all-trans nitric ester 3.
Scheme 1.
Conditions: (a) hv, H2O, rt. (b) 60% Red-Al (5 equiv.), Toluene, 0 to 80 °C, 16 h. (c) 100% HNO3, 0 °C to rt (d) t-BuOK (0.3 equiv.), MeOH, 80 °C, 24 h. (e) 10 (1 equiv.), Diethyl acetylenedicarboxylate (1 equiv.), PPh3 (1 equiv.), DCM, −15 °C to rt, 48 h. (f) Pd/C (5 mol%), H2, EtOAc, rt, overnight. (g) Maleic anhydride (1 equiv.), 2-butyne-1,4-diol diacetate (1.2 equiv.), acetophenone (0.2 equiv.), hv, MeCN, rt, 9 days. (h) NaBH4 (1.03 equiv.), THF, −65 °C to rt. (i) Dibromoethane (1 equiv.), Dimethyl malonate (5 equiv.), NaH (4 equiv.), DMF, 80 °C, 24 h. (j) NaI (0.67 equiv.), MeOH, Pt anode, Fe cathode, 100 mA, 6 F/mol. (k) Diethyl malonate (2.04 equiv.), NaH (2 equiv.), KI (0.1 equiv.), DMF, 70 to 140 °C, overnight. (l) 1 M HCl/MeOH (1:6), overnight.
To obtain the cis-cis-trans meso stereoisomer, 1019 was employed in a 1,4-addition/intramolecular Wittig cyclization20 cascade to afford 11 as the sole diastereomer. Hydrogenation of the cyclobutene set the remaining two stereocenters, which after reduction and nitration yielded 4.
Synthesis of the all-cis stereoisomer 5 proved to be more challenging as the route needed to be both highly scalable and stereoselective for our purposes. Previous reports for the synthesis of the all-cis stereoisomer of 1 commenced from acenaphthylene21 (6% overall yield), cyclooctatetraene22 (5% overall yield), or 2(5H)-furanone23 (3% overall yield, solid-state dimerization at −78 °C). It was reasoned that intercepting a cyclic intermediate of different oxidation state may eliminate some of the pitfalls that plagued previous syntheses. After much experimentation, acetate-protected 2-butyne-1,4-diol and maleic anhydride were found to undergo [2+2] cycloaddition. Direct reduction of the crude anhydride to the corresponding cyclobutene-lactone to aid in isolation and hydrogenation gave the all-cis product 13 as the sole diastereomer (convex approach of H2). Lactone 13 was then directly subjected to reduction and nitration to give the final meso stereoisomer 5.
Two constitutional isomers of 1 were also targeted as a control to gauge the influence of regiochemistry on the physical and energetic properties within the same cyclobutane series (Scheme 1B). To that end, dimethyl malonate was alkylated with dibromoethane to give 14, in which the major byproduct arose from the intermediate mono-addition adduct intramolecularly cyclizing to give 1,1-diester cyclopropane. At this point, all initial attempts to cyclize 14 under reported bromination/thermal cyclization conditions24 proved entirely irreproducible in our hands. Therefore, we instead turned to electrochemical methods. Gratifyingly, we found that a slight modification to the procedure reported by Elinson et al.25 cleanly gave the desired 15 in excellent yield under anodic conditions (see SI). Two additional steps then gave access to the 1,1,2,2-tetrasubstituted nitric ester 6. Finally, the 1,1,3,3 constitutional isomer 7 could be accessed via the known compound 1626 after alkylation, acetonide deprotection, reduction of the diester, and nitration.
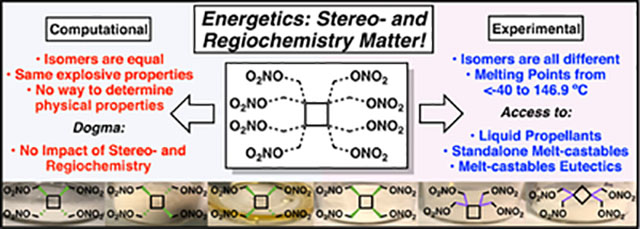
With cyclobutane isomers 2–7 in hand, each isomer’s physical properties and sensitivities to impact, friction and electrostatic discharge was determined. These values, along with the theoretical energetic properties27, are given in Table 1. As expected, based off the experimentally derived densities, the calculated detonation velocities, detonation pressures, heats of formation and specific impulses of isomers 2–7 were in close agreement with one another. It is for this reason that the pursuit of stereo- and regioisomeric structures in designing energetic materials has historically been dismissed. An examination of the physical properties, however, tells a much different story.
Table 1.
Physical properties, sensitivities and theoretical performance of isomers 2–7, TNT and TMETN.
[Blue] = Desirable Melt Castable Properties [Purple] = Desirable Propellant Properties [Red] = Undesirable Properties
| Data category | TNT | TMETN | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|---|
| Tm [°C][a] | 80.4 | −3 | 106.0 | 47.5 | <−40 | 100.8 | 85.9 | 146.9 |
| Tdec [°C][b] | 295.0 | 182.0 | 198.5 | 199.7 | 186.8 | 194.3 | 192.8 | 196.2 |
| ΩCO2 [%][c] | −74 | −34.5 | −44.9 | −44.9 | −44.9 | −44.9 | −44.9 | −44.9 |
| ΩCO [%][d] | −24.7 | −3.1 | −9.0 | −9.0 | −9.0 | −9.0 | −9.0 | −9.0 |
| ρ [gcm−3][e] | 1.65 | 1.47 | 1.64 | 1.605 | 1.543 | 1.66 | 1.683 | 1.651 |
| Pcj [GPa][f] | 19.3 | 23.7 | 24.5 | 22.9 | 24.5 | 24.5 | 24.6 | 24.4 |
| Vdet [ms−1][g] | 6950 | 7050 | 7438 | 7544 | 7577 | 7504 | 7604 | 7472 |
| Isp [s][h] | - | 247.0 | 240.5 | 238.7 | 240.4 | 242.5 | 243.6 | 240.6 |
| ΔfH° [kJ mol–1][i] | −59.3 | −425.0 | −510.1 | −535.9 | −512.0 | −480.8 | −465.5 | −509.2 |
| IS[j] [J] | 15 | 0.2028 | 6.2 | 6.2 | 9.0 | 6.2 | 4.7 | 6.2 |
| FS[k] [N] | 240 | - | 240 | 240 | >360 | 240 | >360 | >360 |
| ESD[l] [J] | 0.25 | - | 0.125 | 0.125 | 0.125 | 0.125 | >0.25 | >0.25 |
Tm = onset temperature of melting;
Tdec = onset temperature of decomposition;
ΩCO2 = CO2 oxygen balance;
ΩCO = CO oxygen balance;
ρ = derived density from X-ray data;
Pcj = detonation pressure;
Vdet = detonation velocity;
Isp = specific impulse;
ΔfH° = molar enthalpy of formation;
IS = impact sensitivity;
FS = friction sensitivity;
ESD = electrostatic discharge sensitivity
While melt-castable explosive candidates typically need to exhibit a minimum temperature difference of 75 °C between melting and decomposition temperatures and melt between 80–125 °C, the all-trans cyclobutane isomer 3 is a low-melting energetic solid. Thus, its 47.5 °C melting point is too low to be considered a met-castable explosive. On the other hand, the all-cis and cis-trans-cis cyclobutane isomers 5 and 2 both possess melting and decomposition temperatures that allow them to find potential use in melt-castable explosive eutectic formulations. While 5 and 2 possess melting points slightly above 100 °C, these materials can be formulated with other high-energy energetic compounds to form a melting point between 80–95 °C. This melting point range is ideal for melt-castable explosive operations because it allows for steam heating to be employed at ambient pressure during a casting operation, reducing operating costs. In examining the cyclobutane regioisomers, 7 possesses too high of a melting point to be of practical value in the melt-castable explosive arena. However, 6 exhibits a melting point that fits well in the standalone melt-castable range. Like TNT, 6 does not need to be mixed with anything to further depress the melting point for casting operations to occur. It is worth noting that melt-castable candidates 2, 5 and 6 possess detonation pressures that are ca. 25% more powerful than TNT, exceeding the explosive power of the state-of-the-art melt-castable ingredient by a wide margin. Furthermore, 2, 5 and 6 possess equivalent or lower friction sensitivities than TNT. Although these three tetranitric ester materials have higher impact sensitivities than TNT, 6 is still less sensitive to impact than the commonly manufactured PETN (3 J), while 2 and 5 possess identical impact sensitivities to the ubiquitous explosive RDX (6.2 J). The 0.125 J electrostatic discharge sensitivity of 2, 5 and 6 is also equal to that of RDX. Thus, possessing sensitivities that are equal to or lower than commonly handled explosive materials demonstrates that these melt-castable tetranitric ester candidates are safe to handle.
Surprisingly, cis-cis-trans isomer 4 is distinct from the other cyclobutane tetranitric ester isomers in that it is a liquid, and can thus be classified as a potential energetic plasticizer ingredient for potential use in propellant formulations. As presented in Table 1, 4 was found to possess a significantly lower sensitivity to impact, yet possesses a higher density than the propellant plasticizer TMETN.28 Although TMETN possesses a slightly higher specific impulse compared to 4, it has a relatively high freezing point of −3 °C. Strikingly 4, which differs from 2 (m.p. 106 °C) by a single stereocenter, did not freeze even at −40 °C. The low freezing point of 4 offers significant potential benefits with regard to propellant formulation capabilities, such as forming high-energy freezing point eutectic materials that are not currently possible with TMETN. It thus appears that by taking advantage of the stereo- and regiochemistry of a given CHNO molecule, it is possible to develop an entirely tunable energetic system that can potentially serve energetics formulators in both the explosives and propellants field.
In summary, it has been demonstrated that stereo- and regiochemistry should play a significant role in the design of new energetic materials and should not be dismissed on theoretical grounds. Depending on the stereochemistry and regiochemistry employed, tunable molecules were designed that gave rise to low-melting solids, standalone melt-castable explosives, melt-castable explosive eutectic compounds, high-melting solid materials, and extreme low-melting liquid materials. Such tunability has the potential to cater to both the explosives and propellants community.
Supplementary Material
ACKNOWLEDGMENT
Financial support for this work was provided by the Defense Advanced Research Projects Agency (DARPA, Agreement No. HR0011–18–9–0021). We are thankful to Dr. Anne Fischer for insightful discussions, the NSF GRFP (predoctoral fellowship to L.M.B.), the Department of Defense (NDSEG fellowship to J.T.E.), and Bristol-Myers Squibb (Graduate Fellowship to J.T.E.). We are grateful to Dr. Dee-Hua Huang and Dr. Laura Pasternack (Scripps Research) for assistance with NMR spectroscopy.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Detailed experimental procedures and analytical data (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).(a) Klapötke TM Chemistry of High-Energy Materials, 3rd ed; De Gruyter: Berlin, 2015; [Google Scholar]; (b) Agrawal JP; Hodgson RD Organic Chemistry of Explosives; Wiley: Hoboken, NJ, 2007; [Google Scholar]; (c) Dobratz BM Properties of Chemical Explosives and Explosive Simulants; UCRL-52997; Lawrence Livermore National Labratory: CA, 1972. [Google Scholar]
- (2).(a) Politzer P; Murray JS; Seminario JM; Lanes P; Grice ME; Concha MC Computational characterization of energetic materials. J. Mol. Struct 2001, 573, 1–10 [Google Scholar]; (b) Rice BM; Hare JJ; Byrd EFC Accurate Predictions of Crystal Densities Using Quantum Mechanical Molecular Volumes. J. Phys. Chem 2007, 111, 10874–10879 [DOI] [PubMed] [Google Scholar]; (c) Keshavarz MH Prediction of heats of sublimation of nitroaromatic compounds via their molecular structure. J. Hazard. Mater 2008, 151, 499–506. [DOI] [PubMed] [Google Scholar]
- (3).Huang H; Zhou Z; Liang L; Song J; Wang K; Cao D; Sun W; Bian C; Xue M Nitrogen-Rich Energetic Monoanionic Salts of 3,4-Bis(1H-5-tetrazolyl)furoxan. Chem. Asian J 2012, 7, 707–714. [DOI] [PubMed] [Google Scholar]
- (4).Wei H; Zhang J; He C; Shreeve JM Energetic Salts Based on Furazan-Functionalized Tetrazoles: Routes to Boost Energy. Chem. Eur. J 2015, 21, 8607–8612. [DOI] [PubMed] [Google Scholar]
- (5).Gao H; Wang R; Twamley B; Hiskey MA; Shreeve JM 3-Amino-6-nitroamino-tetrazine (ANAT)-based energetic salts. Chem. Commun 2006, 38, 4007–4009. [DOI] [PubMed] [Google Scholar]
- (6).Klenov MS; Guskov AA; Anikin OV; Churakov AM; Strelenko YA; Fedyanin IV; Lyssenko KA; Tartakovsky VA Angew. Chem. Int. Ed 2016, 55, 11472–11475. [DOI] [PubMed] [Google Scholar]
- (7).Zhang C; Sun C; Hu B; Yu C; Lu M Synthesis and characterization of the pentazole anion cyclo-N5- in (N5)6(H3O)3(NH4)4Cl. Science 2017, 355, 374–376. [DOI] [PubMed] [Google Scholar]
- (8).Lin Q–H; Li Y–C; Liu W; Wang Y; Pang S–P Nitrogen-rich salts based on 5-hydrazino-1H-tetrazole: A new family of high-density energetic materials. J. Mater. Chem. A 2013, 1, 6776–6785. [Google Scholar]
- (9).Xu Z; Cheng G; Zhu S; Lin Q; Yang H Nitrogen-rich salts based on the combination of 1,2,4-triazole and 1,2,3-triazole rings: A facile strategy for fine tuning energetic properties. J. Mater. Chem. A 2018, 6, 2239–2248. [Google Scholar]
- (10).Thottempudi V; Zhang J; He C; Shreeve JM Azo substituted 1,2,4-oxadiazoles as insensitive energetic materials. RSC Adv. 2014, 4, 50361–50364. [Google Scholar]
- (11).Eliel EL; Wilen SH Stereochemistry of Organic Compounds, 1st ed; Wiley-Interscience: New York, 1994. [Google Scholar]
- (12).Ishikawa M; Hiraiwa Y; Kubota D; Tsushima M; Watanabe T; Murakami S; Ouchi S; Ajito K Tricyclic pharmacophore-based molecules as novel integrin αVβ3 antagonists. Part III: Synthesis of potent antagonists with αVβ3/αIIbβ3 dual activity and improved water solubility. Bioorg. Med. Chem 2006, 14, 2131–2150. [DOI] [PubMed] [Google Scholar]
- (13).Yamamoto T; Matsuda H; Utsumi Y Hagiwara T Kanisawa T Synthesis and odor of optically active rose oxide. Tetrahedron Lett. 2002, 43, 9077–9080. [Google Scholar]
- (14).Archibald TG; Garver LC; Baum K; Cohen MC Synthesis of polynitrocyclobutane derivatives. J. Org. Chem 1989, 54, 2869–2873. [Google Scholar]
- (15).Archibald TG; Gilardi R; Baum K; George C Synthesis and x-ray crystal structure of 1,3,3-trinitroazetidine. J. Org. Chem 1990, 55, 2920–2924. [Google Scholar]
- (16).Coburn MD; Hiskey MA; Archibald TG Scale-up and waste-minimization of the Los Alamos process for 1,1,3-trinitroazetidine (TNAZ). Waste Management, 1998, 17, 143–146. [Google Scholar]
- (17).Lai MC; Chang CW; Ong CW Cyclobutanetetracarboxylate Compound and Preparation Method Thereof. US Patent US20060089505A1, 2006.
- (18).Suzuki H; Tamura T Cage-Shaped Cyclobutanoic Dianhydrides and Process for Production Thereof. European Patent EP1813592A1, 2005.
- (19).Barr KJ; Scott ME; Thompson CF; Anthony N; Cammarano CM; Bakshi RK; Mohanty SK; Korapala CS; Latthe PR; Kambam VN; Sarkar SK; Thatai JT Pyrrolidine derived beta 3 adrenegic receptor agonists. US Patent US9486448B2, 2016.
- (20).Yavari I; Nourmohammadian F Stereoselective Synthesis of Tetraalkyl Cyclobutene-1,2,3,4-tetracarboxylates. Synthesis of Tetraalkyl (Z,Z)-Buta-1,3-diene-1,2,3,4-tetracarboxylates. J. Chem. Research 1999, 512–513. [Google Scholar]
- (21).Griffin GW; Veber DF cis,cis,cis-1,2,3,4-Tetracarbomethoxycyclobutane; structure of β-heptacyclene. J. Am. Chem. Soc 1960, 82, 6417. [Google Scholar]
- (22).Schroder G; Martin W Structure of the Cyclooctatetraene Dimer of Melting Point 53 °C. Angew. Chem. Int. Ed 1966, 5, 130. [Google Scholar]
- (23).Ohga K; Matsuo T A Study on the Photochemistry of α,β-Unsaturated γ-Lactones. I. The Structures of the Photodimers of 4-Hydroxycrotonic Acid γ-Lactone. Bull. Chem. Soc. Jpn 1970, 43, 3505–3510. [Google Scholar]
- (24).(a) Kostyanovsky RG; Krutius ON; El’natanov YI Reactions of alkylenebisbromomalonates with Nucleophiles. Russ. Chem. Bull 1994, 43, 2065–2069 [Google Scholar]; (b) Gol’mov VP; Malevannaya ZP A New Preparative Method for Cis-cyclobutane-1,2-dicarboxylic Acid. Zhurnal Obshchei Khimii, 1961, 31, 665–669 [Google Scholar]; (c) Vogel I Syntheses of Cyclic Compounds. Part I. Ethyl βγ-Dimethylbutane-ααδδ-tetracarboxylate and Some Cyclobutane Compounds Derived Therefrom. J. Chem. Soc 1927, 1985–1994. [Google Scholar]
- (25).Elinson MI; Fedukovich SK; Ugrak BI; Nikishin GI Electrochemical Oxidation of Tetramethyl Esters of α,α,ω,ω-Alcanetetracarboxylic Acids. Russ. Chem. Bull 1992, 41, 1827–1833. [Google Scholar]
- (26).Pecquet P; Huet F; Legraverend M; Bisagni E Synthesis of New Carbocyclic Analogues of Oxetanocin A and Oxetanocin G. Heterocycles 1992, 34, 739–745. [Google Scholar]
- (27).(a) Byrd EFC; Rice BM Improved Heats of Formation of Energetic Materials Using Quantum Mechanical Calculations. J. Phys. Chem. A 2006, 110, 1005–1013; [DOI] [PubMed] [Google Scholar]; (b) Byrd EFC; Rice BM A Comparison of Methods to Predict Solid Phase Heats of Formation of Molecular Energetic Salts. J. Phys. Chem. A 2009, 113, 345–352; [DOI] [PubMed] [Google Scholar]; (c) Rice BM; Hare JJ; Byrd EFC Accurate Predictions of Crystal Densities Using Quantum Mechanical Molecular Volumes. J. Phys. Chem. A 2007, 111, 10874–10879; [DOI] [PubMed] [Google Scholar]; (d) Rice BM; Byrd EFC Evaluation of electrostatic descriptors for predicting crystalline density.J. Comput. Chem 2013, 34, 2146–2151. [DOI] [PubMed] [Google Scholar]; (e) Bastea S; Fried LE; Glaesman KR; Howard WM; Kuo IFW; Souers PC; Vitello PA Cheetah 7.0 Thermochemical Code; Energetic Materials Center, Lawrence Livermore National Laboratory: Livermore, CA, 2012. [Google Scholar]
- (28).Meyer R; Köhler J; Homburg A Explosives, 6th Edition, 2007, 227. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.