Abstract
G1 cyclins are considered essential for DNA replication and cell division. A recent report now shows that some cells can cycle in the absence of G1 cyclins. In embryonic stem cells and cancer cells, G1 cyclins are required to activate cyclin-dependent kinases to phosphorylate core pluripotency factors and maintain pluripotency.
Progression through the cell cycle is commonly thought to require a series of coordinated events under the control of key cell cycle regulators that include cyclins and cyclin-dependent kinases (CDKs). The prevailing view is that specific cyclin-CDK complexes must be active to allow progression at each step of the cell cycle from G1 to S to G2 and M. Thus, complete inhibition of cyclin-CDK complexes that are normally active in one phase would prevent progression to the next phase of the cell cycle. A study by Liu et al.1 now challenges this dogma and demonstrates that G1 cyclins are not absolutely required for cell cycle progression in some cell types, including embryonic stem cells (ESCs). Instead, the authors identify a new function for G1 cyclin-CDK complexes in the stabilization of pluripotency factors required for the maintenance of pluripotency.
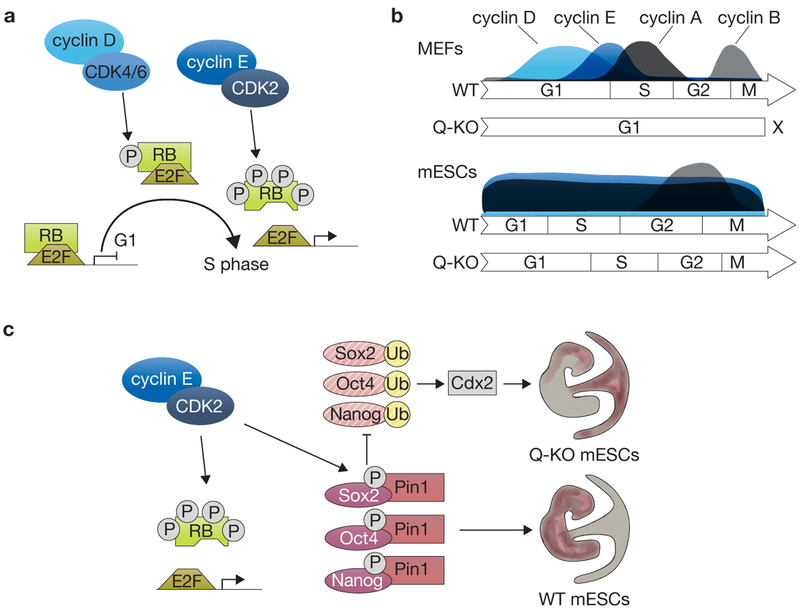
G1 cyclins consist of two families, the cyclin D (cyclin D1, D2 and D3) and the cyclin E (cyclin E1 and E2) families (reviewed in ref. 2). In a simplified model, cyclin D activates CDK4/6 and phosphorylates the retinoblastoma (RB) family of proteins (RB, p107 and p130) in early/mid-G1. In late G1, cyclin E activates CDK2 and hyperphosphorylates RB family proteins, leading to their inhibition and the release of E2F transcription factors, resulting in the activation of factors necessary for S-phase entry and progression (Fig. 1a; ref. 2). Thus, inactivation of G1 cyclins would be expected to completely block cells in G1. However, many cell types, including mouse embryonic fibroblasts (MEFs), can cycle nearly normally in the absence of D-type cyclins3. Similarly, deletion of E-type cyclins only affects the proliferation of specific lineages4. Furthermore, most mouse cells can proliferate in the combined absence of the three G1 CDKs, CDK2, CDK4, and CDK6. In this context, G1 cyclins activate CDK1, which compensates for the deleted CDKs5. In a real genetic tour de force, Liu et al.1 analysed the necessity of G1 cyclins for cell proliferation by generating mammalian cells with the deletion of all five G1 cyclins (quintuple-knockout, Q-KO).
Figure 1.
G1 cyclins and cyclin-dependent kinases in cell cycle progression and maintenance of pluripotency in mESCs. (a) In the canonical model cyclin D activates CDK4/6 and phosphorylates RB in early/mid-Gl and cyclin E hyperphosphorylates RB in late Gl. This leads to the release of E2F transcription factors, the expression of cell cycle genes, and progression through S phase, (b) The proportional length of cell cycle phases and expression levels of cyclins in wild-type (WT) and Gl cyclin Q-KO MEFs and mESCs. MEFs are characterized by a cell-cycle-specific expression profile of cyclins and a long G1 phase, whereas mESCs have a short G1 phase and express cyclins in a cell-cycle-independent fashion, except for cyclin B. The Q-KO of G1 cyclins stalls the cell cycle in MEFs, while it prolongs G1 phase without having a significant impact on cell proliferation in mESCs. (c) Cyclin E-CDK2 protects core pluripotency factors from degradation in mESCs. Cyclin E activates CDK2 and leads to phosphorylation of RB and the pluripotency factors Oct4, Sox2 and Nanog. Phosphorylated pluripotency factors are bound by Pin1, which protects them from ubiquitination (Ub) and proteasomal degradation. Degradation of pluripotency factors in G1 cyclin Q-KO mESCs leads to upregulation of the trophectoderm marker Cdx2, which is usually repressed by Oct4. WT mESCs normally contribute to the embryo proper in chimaera assays, but G1 cyclin Q-KO mESCs contribute primarily to the placenta and neural lineages. RB proteins exist almost exclusively in the hyperphosphorylated state in mESCs due to high CDK2 activity.
Surprisingly, Liu et al. found that Q-KO mouse ESCs (mESCs) have only mild cell cycle phenotypes, with a doubling of the time spent in G1 (from 3 h to 6 h). In contrast, Q-KO MEFs rapidly arrest in G1, as expected. The difference between the two cell types may be explained by the different expression patterns of cyclins: in MEFs, only the G1 cyclins are expressed in G1 and the progression through the G1 phase is rather long (Fig. 1b); in mESCs, most cyclins (with the exception of cyclin B) are expressed throughout the cell cycle and the G1 phase is comparably short (reviewed in ref. 6). These differences in the cell cycle machinery, including the presence of cyclin A in the G1 phase7, could be responsible for CDK2 (or CDK1) activation in G1/S phase and the partial rescue of the cell cycle arrest in mESCs. It is also possible that differences downstream of cyclin-CDK complexes, including low levels of RB, render mESCs less dependent on the activity of G1 cyclins8. It would be interesting to determine if overexpression of a stable form of cyclin A in G1 could rescue the G1 arrest observed in Q-KO MEFs. It would also be interesting to examine other cell types that can cycle independently of G1 cyclins to investigate other possible mechanisms that allow cell cycle progression without D- or E-type cyclins in mESCs.
On further analysis of the phenotype of Q-KO mESCs, Liu et al. made a second seminal observation. Q-KO mESCs lose their undifferentiated appearance and are characterized by substantially decreased protein levels of the core pluripotency factors Oct4, Nanog and Sox2. This phenotype was not observed in mESCs with either D- or E-type cyclins knocked out. As levels of cyclin D are very low in mESCs and are induced in cyclin E mutant mESCs, the authors propose that cyclin E is the main cyclin responsible for maintaining pluripotency, with cyclin D being able to compensate. In a series of molecular and biochemical experiments, Liu et al. show that pluripotency factors are hypophosphorylated and exhibit a shorter half-life in Q-KO mESCs. Although it is known that phosphorylation of Nanog, Sox2 and Oct4 stabilizes these proteins via protection from ubiquitination and proteasomal degradation mediated by Pin1 (refs 9,10), Liu et al. demonstrate that the kinase responsible for these phosphorylation events in mESCs is mainly CDK2 activated by cyclin E (Fig. 1c). Additionally, the authors identify the phosphorylation sites and elegantly show that overexpression of an isoform of Nanog with phospho-mimicking substitutions leads to a reduced loss of pluripotency in G1 cyclin Q-KO mESCs.
An additional interesting phenotype of Q-KO mESCs is the expression of genes that are usually detected in the trophectoderm lineage (for example Cdx2, Ehox and Eomes) and not in mESCs, which are derived from a developmental stage after formation of the trophectoderm. However, this reprogramming step might be explained by the fact that Oct4 is less stable and less abundant in Q-KO mESCs, and Oct4 usually represses the expression of Cdx2, a transcription factor whose elevated expression is known to induce a trophoblast stem-cell-like status11. Analysis of markers in single Q-KO mESCs shows that Q-KO ESCs are a heterogeneous population with mostly early trophectodermal progenitors and a few undifferentiated or partially differentiated ESCs.
To further examine the relationship between G1 cyclins, stemness, and differentiation in vivo rather than in culture, the authors generated chimeric embryos with Q-KO mESCs and performed teratoma formation assays, in which mESCs are injected into immunocompromised mice. These studies demonstrate conclusively that a number of mammalian cell types in chimeric embryos and teratomas can divide in the total absence of G1 cyclins. These experiments also show that Q-KO mESCs efficiently contribute to the formation of the placenta and neural tissues in chimeric embryos and have a bias towards neural differentiation in teratomas in this genetic background. Liu et al. hypothesize that Sox2 downregulation as a result of the loss of the five G1 cyclins is not as pronounced as the downregulation of other pluripotency factors, and these remaining levels of Sox2 may prime the mutant mESCs towards the neural lineage12. Future experiments with tissue-specific inducible deletion of the five G1 cyclins will help to determine the role of these cyclins in specific lineages in vivo in a context more defined than in chimeric embryos and teratomas, in which multiple cell types are mixed.
The observations by Liu et al. provide another striking example of the multiple connections between the core cell-cycle machinery and pluripotency networks, including transcriptional, post-transcriptional, and post-translational mechanisms (recently reviewed in refs 13,14). In addition to offering new insights into fundamental mechanisms of cell cycle progression and pluripotency, this work also has translational implications. Acute inhibition of CDK2 kinase activity in cancer cells inhibits cell cycle progression, similar to the effects of deleting G1 cyclins in many somatic cell types. Furthermore, CDK2 inhibition may contribute to differentiated features in tumorigenic stemcell-like cells by lengthening the G1 phase and lowering levels of pluripotency factors such as Sox2 and/or Oct4, as shown in mESCs1,15. This strategy might prevent the long-term expansion of cancer cells by decreasing their selfrenewal and pluripotency. Liu et al. provide preliminary evidence to support the idea that CDK2 inhibition could affect pluripotency in glioblastoma and breast cancer cells. However, more work is needed to test and validate this promising possibility further in order to use inhibition of CDK2 in new ways to treat cancer.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Contributor Information
Julia Arand, Stanford University Medical Center, Departments of Pediatrics and Genetics, Stanford Medical School, SIM1 Building, 265 Campus Drive, Stanford, California 94305, USA..
Julien Sage, Stanford University Medical Center, Departments of Pediatrics and Genetics, Stanford Medical School, SIM1 Building, 265 Campus Drive, Stanford, California 94305, USA..
References
- 1.Liu L et al. Nat. Cell Biol 19, 177–188 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sherr CJ Cell 73, 1059–1065 (1993). [DOI] [PubMed] [Google Scholar]
- 3.Kozar K et al. Cell 118, 477–491 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Geng Y et al. Cell 114, 431–443 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Santamaria D et al. Nature 448, 811–815 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Conklin JF & Sage J J. Cell Biochem 108, 1023–1030 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stead E et al. Oncogene 21, 8320–8333 (2002). [DOI] [PubMed] [Google Scholar]
- 8.Savatier P, Huang S, Szekely L, Wiman KG & Samarut J Oncogene 9, 809–818 (1994). [PubMed] [Google Scholar]
- 9.Nishi M et al. J. Biol. Chem 286, 11593–11603 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moretto-Zita M et al. Proc. Natl Acad. Sci. USA 107, 13312–13317 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niwa H et al. Cell 123, 917–929 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Thomson M et al. Cell 145, 875–889 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kareta MS, Sage J & Wernig M Curr. Opin. Cell Biol 37, 68–74 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soufi A & Dalton S Development 143, 4301–4311 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koledova Z et al. Stem Cells Dev. 19, 181–194 (2010). [DOI] [PubMed] [Google Scholar]