Abstract
Purpose
Alzheimer’s disease is a neurodegenerative disorder, and most common form of dementia afflicting over 35 million people worldwide. Rivastigmine is a widely used therapeutic for ameliorating clinical manifestations of Alzheimer’s disease. However, current treatments require frequent dosing either orally or via transdermal patch that lead to compliance issues and administration errors risking serious adverse effects. Our objective was to develop a smart polymer based delivery system for controlled release of rivastigmine over an extended period following a single subcutaneous injection.
Methods
Rivastigmine release was optimized by tailoring critical factors including polymer concentration, polymer composition, drug concentration, solvent composition, and drug hydrophobicity (rivastigmine tartrate vs base). Optimized in vitro formulation was evaluated in vivo for safety and efficacy.
Results
Formulation prepared using PLGA (50:50) at 5% w/v in 95:5 benzyl benzoate: benzoic acid demonstrated desirable controlled drug release characteristics in vitro. The formulation demonstrated sustained release of rivastigmine tartrate for 7 days in vivo with promising biocompatibility and acetylcholinesterase inhibition efficacy for 14 days.
Conclusion
The results exemplify an easily injectable controlled release formulation of rivastigmine prepared using phase-sensitive smart polymer. The optimized formulation significantly increases the dosing interval, and can potentially improve patient compliance as well as quality of life of patients living with Alzheimer’s disease.
Keywords: alzheimer’s disease, controlled release, phase sensitive, rivastigmine, smart polymers
INTRODUCTION
Alzheimer’s disease (AD) is the most common form of dementia associated with aging. It is estimated that more than 5 million Americans have AD that pose a financial burden of over $200 million annually (1). AD significantly reduces life span and quality of living of an affected individual and is considered to be a leading cause of death in the United States, just behind heart disease and cancer (2). Yet, AD has significantly less money going towards research and hasn’t had as much breakthrough research towards finding treatments or cures in the last few decades (3,4).
AD is typically diagnosed upon development of memory loss and cognitive impairment, but the damage to the brain that cause these symptoms usually begins several years before any indication (5). Damage to the brain is caused when proteins aggregate and form amyloid plaques and neurofibrillary tangles. The plaques and tangles lead to neuronal cell death and reduction in levels of key neurotransmitter, acetylcholine. As the disease progresses, the neurons within the hippocampus responsible for memory can no longer communicate efficiently and neurocognitive impairment becomes apparent (6). More damage leads to worsening symptoms that eventually result in institutionalization in most cases, which accounts for the majority of the cost associated with this disease (7). The patient will lose their ability to perform daily tasks and usually end up bed ridden prior to death as the body shuts down (1).
Rivastigmine is a cholinesterase inhibitor that improves symptoms in AD patients by preventing breakdown of acetylcholine in the central nervous system. The increased availability of acetylcholine allows for improved neurotransmission and hence, an improvement in memory and cognitive function. Its main mechanism of action is the prevention of acetylcholine metabolism by binding to the esteratic and ionic sites on acetylcholinesterase. Acetylcholinesterase is found as three isoforms throughout the body, AChET, AChEH, and AChER.
The binding of rivastigmine is preferential for the AChEx isoform, or G1 isoform of acetylcholinesterase, which is the dominant form in patients with Alzheimer’s (8,9). In addition, rivastigmine is unique in that it is the only treatment option that also inhibits butyrylcholinesterase which has predominant expression in AD, especially as the disease progresses (10). Rivastigmine largely acts on cholinesterase within the central nervous system, thus preventing increased acetylcholine concentrations throughout the rest of the body that would otherwise cause many complications since acetylcholine is an extremely common neurotransmitter. Treatment in the early stages of AD allow the patient to remain independent longer, delay institutionalization as well as relieve or at least delay caregiver burden (7,11–13). This is significant since the majority of the cost of the disease arise due to institutionalization. As for advanced AD, there is evidence that rivastigmine can help with eating problems (14). The current dosage forms for rivastigmine have been approved for treatment of mild to severe AD and include oral solution, oral capsule, and transdermal patch. Transdermal patches allow for increased dosing interval, better patient compliance, and decreased caregiver burden (11,12,15–17). Despite the advantage, the patch formulation is required to be applied daily, which may be an impediment to patient independence. Other controlled release formulations of rivastigmine such as those involving smart polymers that do not require daily administrations may decrease dependency on caregivers.
Smart polymers utilizing amphiphilic block copolymers have been studied extensively as controlled delivery systems for many therapeutics. The use of amphiphilic block copolymers for drug delivery was first proposed in early 1980s (18). Some of their merits include ease of manufacture and incorporation of drug, protection of therapeutic within the gel depot, optimization potential, and biocompatibility. Amongst smart polymers, phase sensitive polymers are soluble in biocompatible organic solvents which allows for homogeneous mixing of drugs and easy subcutaneous injection of the free- flowing solution. In situ gel depot formation takes place at the injection site, upon diffusion of organic solvent in which the polymer is dissolved. The depot takes the shape of the area in which it is injected and has a jelly like consistency that allows it to be flexible enough for movement as needed with the movement of the body. The therapeutic becomes entrapped and protected within the gel and its release becomes dependent upon diffusion through the polymer matrix and slow breakdown of the polymer. The release of therapeutic through such copolymeric depot-based delivery systems can be modified by tailoring polymer concentration, composition, drug concentration, solvent composition, and drug hydrophobicity (rivastigmine tartrate vs base) to achieve required therapeutic levels over an extended period. Sustained release over prolonged period would potentially help increase the dosing interval, patient compliance, and quality of life of people living with AD (19).
Current limitations of other controlled release injectable delivery systems include toxicity, burst release, inability to attain zero order release kinetics, and loss of efficacy due to degradation of therapeutic. Rivastigmine therapy in current clinical practice is burdened with limitations such as skin irritation upon daily application of patches, poor oral bioavailability, and frequent injections at short intervals owing to short half-life post parenteral administration, clearly demonstrating the need for a better controlled delivery system with minimal toxicity (19). The aim of the current work was to develop a novel, smart polymer based controlled release delivery system of rivastigmine for efficient management of AD. Various formulation parameters such as polymer composition, solvent composition, and polymer concentration were first optimized in vitro before evaluation in vivo. Polymer characteristics, polymer concentration, solvent composition, drug properties as well as drug loading were thoroughly examined in an in vitro release model, followed by investigation in Sprague Dawley rats for efficacy and biocompatibility. The overall aim of this research was to investigate phase sensitive polymer depot-based subcutaneously injectable delivery system as a safe, biocompatible, therapeutically relevant and patient compliant sustained release formulation for AD management.
MATERIALS AND METHODS
Materials
Poly(lactic-co-glycolic acid) (PLGA) 50:50 was purchased from Absorbable Polymers International (Cupertino, CA, USA). Poly lactic acid (PLA) was purchased from Polyscitech (West Lafayette, IN, USA). Phosphate buffered saline (PBS) was purchased from American Type Culture Collection (ATCC, Rockville, MD, USA). Rivastigmine tartrate (RT) and rivastigmine base (RB) were obtained from TCI America (Portland, OR, USA) and Xi’an Health Biomedical Technology Co. (China), respectively. DNTB (5,5′-dithiobis-(2-nitrobenzoic acid) was purchased from Sigma-Aldrich (St. Louis, MO, USA). S-Acetylthiocholine iodide (98%) was purchased from Alfa Aesar (Ward Hill, MA, USA). Micro bicinchoninic acid (BCA) kit was purchased from Thermo Fisher Scientific (Waltham, MA, USA). All other chemicals were of analytical grade and used without further modification.
Phase Sensitive Formulation Preparation and In Vitro Drug Release Comparing Polymer Concentration
Phase sensitive samples were prepared with PLA (109 kDa) at polymer concentrations of 2.5, 5, and 10% (w/v) in 100% benzyl benzoate. Homogenous mixture was obtained upon vigorous stirring and sonication. To this mixture, rivastigmine base was incorporated at a concentration of 60 mg/mL. Using a 25 G syringe, 0.5 mL of the formulation was injected in glass tubes and placed in a water bath at 37°C. Pre-warmed PBS (10 mM, pH 7.4) containing 0.01% w/v sodium azide was then added to each tube as release medium (3 mL per tube). The tubes were capped to prevent evaporation and incubated at 37°C under constant shaking at 35 rpm. Sample aliquots (1 mL) were taken at 30min, 1 h, 6 h, 1, 3, 7, 14, 21, 28, 35,42, 49, 63, and 77 days and replaced with 1 mL fresh pre-warmed release medium. Released rivastigmine base was quantified using reversed phase - high performance liquid chromatography (RP-HPLC) using conditions as shown in Table I (20). Six replicates were analyzed per concentration and analyzed for burst release and cumulative percent drug released.
Table I.
Chromatographic Conditions for Rivastigmine Quantification
| Column | Thermo Scientific™ Hypersil GOLD™ C18 column (250 × 4.6 mm, 5 μm) |
|---|---|
| Column temperature | 30°C |
| Elution | Isocratic |
| Mobile phase composition | 0.01 M sodium-1-heptane sulphonate (pH: 3.0 with dilute phosphoric acid)–acetonitrile (72:28, v/v) |
| Flow rate | 1.0 ml/min |
| Injection volume | 10 μl |
| Run time | 20 min |
| Detector, Detection wavelength | UV, 217 nm |
In Vitro Release Comparing Drug Concentration
Phase sensitive samples were prepared with PLA (109 kDa) at polymer concentration of 5% (w/v) in 100% benzyl benzoate. Homogenous mixture was obtained upon vigorous mixing and sonication and to it rivastigmine base was added at concentrations of 60, 120, and 180 mg/ml. Release study was conducted as described before with samples collection time points of 30 min, 1 h, 6 h, 1, 3, 5, 7, 14, 21, 28, 35, 42, 49, 56, 63, 70, 77, 84, and 98 days and released rivastigmine base was quantified using RP-HPLC. Six replicates were analyzed per concentration and analyzed for burst release and cumulative percent drug released.
In Vitro Drug Release Comparing Polymer Composition
Four polymers of varying molecular weight and/or composition: PLA (109 kDa), PLA (40 kDa), PLGA (PLA:PGA, 85:15), and PLGA (PLA:PGA, 50:50) were evaluated. Phase sensitive samples were prepared at polymer concentration of 5% (w/v) in 100% benzyl benzoate. After vigorous mixing and sonication, 216 mg/mL rivastigmine base was added to the mixture. Using a 25 G syringe, 0.5 mL of the formulation was injected in glass tubes and placed in a water bath. Pre-warmed PBS (10 mM, pH 7.4) containing 0.01% w/v sodium azide was then added to each tube as release medium (3 mL per tube). The tubes were capped to prevent evaporation and incubated at 37°C under constant shaking at 35 rpm. Sample aliquots (1 mL) were taken at 1, 7, 14, 21, 28, 35, 42, and 49 days and replaced with 1 mL fresh pre-warmed release medium. Released rivastigmine base was quantified using RP-HPLC. Six replicates were analyzed per concentration and analyzed for burst release and cumulative percent drug released.
In Vitro Drug Release Comparing Solvent Composition
Solvent composition was examined for all the aforementioned polymers (PLA (109 kDa), PLA (40 kDa), PLGA (PLA:PGA, 85:15), and PLGA (PLA:PGA, 50:50) by varying the ratio of benzyl benzoate (BB) to benzyl alcohol (BA). Phase sensitive samples were prepared at polymer concentration of 5% (w/v) in each of the following benzyl benzoate to benzyl alcohol (BB:BA) solvent compositions: 100:0, 95:5, 90:10, and 85:15. After vigorous mixing and sonication, 216 mg/mL of rivastigmine base was added to the mixture. Using a 25 G syringe, 0.5 mL of the formulation was injected in glass tubes and placed in a water bath. Pre-warmed PBS (10 mM, pH 7.4) containing 0.01% w/v sodium azide was then added to each tube as release medium (3 mL per tube). The tubes were capped to prevent evaporation and incubated at 37°C under constant shaking at 35 rpm. Sample aliquots (1 mL) were taken at 1, 7, 14, 21, 28, 35, 42, 49, and 56 days and replaced with 1 mL fresh pre-warmed release medium. Released rivastigmine base was quantified using RP-HPLC. Six replicates were analyzed per concentration and analyzed for burst release and cumulative percent drug released.
In Vitro Release Comparing Drug Hydrophobicity
Phase sensitive samples were prepared with PLGA (50:50) at polymer concentration of 5% (w/v) in 95:5 benzyl benzoate: benzyl alcohol solvent. After vigorous mixing and sonication, 33.6 mg/mL of rivastigmine tartrate or 216 mg/mL of rivastigmine base was added to the mixture. Using a 25 G syringe, 0.5 mL of the formulation was injected in glass tubes and placed in a water bath. Pre-warmed PBS (10 mM, pH 7.4) containing 0.01% w/v sodium azide was then added to each tube as release medium (3 mL per tube). The tubes were capped to prevent evaporation and incubated at 37°C under constant shaking at 35 rpm. Sample aliquots (1 mL) were taken at 6 h, 1, 3, 5, 7, 9, 12, 14, 21, 28, 35, and 42 days and replaced with 1 mL fresh pre-warmed release medium. Released rivastigmine tartrate was quantified using RP-HPLC. Six replicates were analyzed per concentration and analyzed for burst release and cumulative percent drug released.
In Vivo Phase Sensitive Formulation Preparation and Experimental Set up
PLGA (50:50) was dissolved in 95:5 BB:BA at 5% (w/v). Rivastigmine tartrate was suspended via thorough mixing at a concentration of 4.2 mg/mL (0.3 mg per day / 0.5 mL per injection * 7 days). Conversion from human dose to animal equivalent dose (AED) was calculated using the following equation: AED (mg / kg) = Human dose (mg / kg) × Km ratio, where human dose is 0.2 mg/kg (12 mg/ 60 kg) and the correction factor (Km) ratio for a rat is 6.2, giving an AED of 1.24 mg/kg. Average rat body weight was 0.25 kg which gives a daily dose of 0.3 mg (21). Rivastigmine tartrate solution was prepared at 0.6 mg/mL in PBS (0.3 mg per day / 0.5 mL per injection). Adult Sprague Dawley (SD) rats, approximately 8 weeks old, were used to evaluate the release profiles and biocompatibility of phase sensitive formulations. All experiments were conducted in accordance with the North Dakota State University animal care committee guidelines (approved protocol # A16079) and to the Guide for the Care and Use of Animals of the Institute of Laboratory Animal Resources, National Research Council. The animals were housed under controlled temperature conditions with 12 h light and dark cycles and given access to food and water ad libitum. Animals were divided into groups of 6 and subcutaneously injected with the following formulations: (i) blank control (PBS injection), (ii) rivastigmine tartrate solution containing 4.2 mg/mL drug, and (iii) phase sensitive formulation containing 4.2 mg/mL rivastigmine tartrate. Three additional groups of 6 animals were used to further determine rivastigmine tartrate plasma concentration at certain time points (15 min, 3 days, and 7 days) post administration of phase sensitive formulation to investigate effects of the drug on acetylcholinesterase inhibition over various time periods. The formulations were injected as a single dose in the dorsal neck region by tightly securing the scruff of the animal and injecting 0.5 mL ofthe formulation in the subcutaneous region to form a single depot. Care was taken to keep all formulation characteristics, needle angle, and administration site consistent in order to form reproducible injection site depots. Moreover, PLA/PEG copolymers show pliability and mucomimetic characteristics which helps in securing the gel depot within the subcutaneous tissue region while minimizing mechanical irritation from such controlled release depots (22,23).
In Vivo Release Profile of Rivastigmine Tartrate from Phase Sensitive Formulation
Blood samples of 0.4 ml were drawn from the tail vein of the rats at 15 min, 7 days, and 14 days for the blank control, 15 min, 30 min, 1, 3, and 24 h for the solution group, and 15 min, 30 min, 1 h, 3 h, 1, 3, 7, 10, and 14 days for the phase sensitive group. Thereafter, rivastigmine tartrate was extracted from the blood samples using 100% acetonitrile. Extracted drug was quantified using RP-HPLC. Release profile was plotted as drug amount versus time. Time points for sampling are outlined in Table II.
Table II.
Blood Withdrawal Schedule from Blank Control, RT Solution, and Phase Sensitive Formulation (n = 6/group)
| Experimental Groups | Blood Sampling Time Post Administration |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.25 h | 0.5 h | 1 h | 3 h | 1 day | 3 days | 7 days | 10 days | 14 days | |
| Blank Control | x | x | x | ||||||
| RT Solution | x | x | x | x | x | ||||
| Phase Sensitive Formulation | x | x | x | x | x | x | x | x | x |
Evaluation of Acetylcholinesterase Inhibition In Vivo
Animals were sacrificed at specific time points post single administration of smart copolymer formulation as outlined in Table III. Animal brains were harvested and homogenized with PBS (10 mL per brain). The homogenate was centrifuged at 18,000 rpm, at 4°C for 30 min to collect the tissue lysate. Acetylcholinesterase inhibition was evaluated in tissue lysate using Ellman method (24). A standard curve was plotted using a serial dilution of acetylthiocholine iodide. Normalization was performed on all samples by determining protein content in assayed brain samples using micro BCA protein assay kit.
Table III.
Time Points for Evaluation of Acetylcholinesterase Inhibition (n = 6/group/time point)
| Experimental Groups | Time points |
||||
|---|---|---|---|---|---|
| 15 min | 1 day | 3 days | 7 days | 14 days | |
| Blank Control | x | ||||
| RT Solution | x | ||||
| Phase Sensitive Formulation | x | x | x | x | |
In Vivo Biocompatibility
Biocompatibility of the formulation at the injection site was visually evaluated at the end of each study. Injection site was dissected and evaluated for signs of inflammation such as vasodilation, redness, and swelling.
Statistical Analysis
All data are expressed as mean ± standard deviation (SD). Statistical analyses were performed using ANOVA. A p value of less than 0.05 was considered significant.
RESULTS
In Vitro Drug Release Comparing Polymer Concentration
All formulations of PLA (109 kDa) were prepared in the concentration range of 2.5 to 10% (w/v). Drug release profiles did not follow an expected trend of increased release duration with increase in polymer concentration as shown in Fig. 1. Instead, 5% w/v polymer concentration demonstrated the best sustained release profile. It is postulated that hydrophobic-hydrophilic balance can influence release profile. Hydrophobic effect is driven by reduction in entropy. At certain polymer concentrations, the monomers of the polymer will be able to better arrange themselves in an effort to reduce entropy, forming a more energetically favorable state. This is evident in this study, where the best performing concentration of polymer was 5% (w/v) with a release duration of 77 days and minimal burst release. All formulations had commendable control over burst release at 3.5–6% and completely released the drug in 42 or 49 days for 2.5 and 10% (w/v) formulation respectively.
Fig. 1.
In vitro release profiles of rivastlgmlne base comparing effect of increasing PLA (109 kDa) concentration. PLA (109 kDa) was formulated in solvent composed of 100:0 BB:BA (benzyl benzoate: benzoic acid).
In Vitro Release Comparing Drug Concentration
Rivastigmine base readily dissolved at concentrations of 60, 120, and 180 mg/ml in 5% (w/v) PLA (109 kDa) in 100% benzyl benzoate to benzyl alcohol. Duration of complete release of the drug was 49, 77, and 98 days for the 60, 120, and 180 mg/ml formulations, respectively as depicted in Fig. 2. Length of release was not proportional to drug concentration but a trend of increase in drug concentration resulting in an increased duration ofrelease was observed. Burst release was minimal in all formulations.
Fig. 2.
In vitro release profiles of rivastigmine base (RB) comparing effect of drug concentrations in PLA (109 kDa) formulations (n = 6). PLA (109 kDa) was formulated in 100:0 BB:BA (benzyl benzoate: benzoic acid).
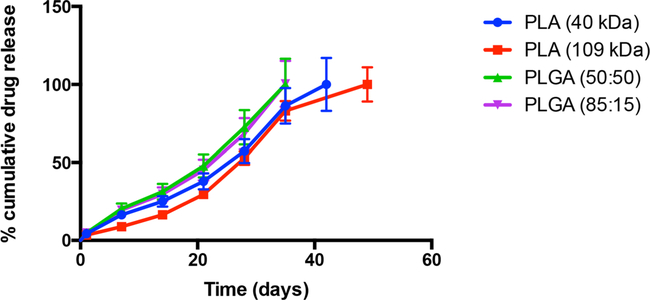
In Vitro Drug Release Comparing Polymer Composition
Polymer composition was investigated by comparing four polymers: PLA (109 kDa), PLA (40 kDa), PLGA (85:15), and PLGA (50:50). Formulations easily dissolved 216 mg/mL rivastigmine base after 5% (w/v) of polymer being studied was dissolved in 100% benzyl benzoate. Release duration was 35 days for both PLGA formulations, 42 days for the PLA (40 kDa) formulation, and 49 days for the PLA (109 kDa) formulation as shown in Fig. 3. While the duration of release was longer for the PLA formulations, it also followed a more biphasic release pattern when compared to the profiles of PLGA. Burst release was well controlled for all formulations.
Fig. 3.
In vitro release profiles of rivastlgmlne base comparing effect of polymer composition (n = 6). Solvent used for formulations was 100:0 BB:BA (benzyl benzoate: benzoic acid).
In Vitro Drug Release Comparing Solvent Composition
PLA (109 kDa), PLA (40 kDa), PLGA (85:15), and PLGA (50:50) were dissolved at 5% (w/v) in solvents composed of benzyl benzoate and benzyl alcohol in ratios of 100:0, 95:5, 90:10, and 85:15. All ratios easily dissolved the four polymers along with rivastigmine base at a concentration of 216 mg/ml. Release duration was between 42 and 56 days for all formulations and all solvent ratios as shown in Fig. 4. While the duration of release was the longest at 56 days for the PLA (40 kDa) formulation, it still followed a more biphasic release pattern when compared to the drug release profiles of PLGA. Burst release was well controlled for all formulations but a trend was observed for increased burst release as benzyl alcohol composition increased from 0 to 15%.
Fig. 4.
In vitro release profiles of rivastigmine base comparing effect of solvent composition benzyl benzoate: benzoic acid (BB:BA) in formulations prepared using (a) PLA (40 kDa) at 5% (w/v); (b) PLA (109 kDa) at 5% (w/v); (c) PLGA (50:50) at 5% (w/v) and (d) PLGA (85:15) at 5% (w/v) (n = 6).
It is worthy to note that the PLGA (50:50) in 95:5 BB:BA formulation could potentially be best suited for controlled delivery of rivastigmine base, given the factors of low burst release (3%), duration of complete release (42 days), zero order release profile, amount of drug release per day (4.4 mg/day), and low variance among formulation samples.
In Vitro Release Comparing Drug Hydrophobicity
Phase sensitive formulations of rivastigmine base or tartrate were prepared at a PLGA (50:50) polymer concentration of 5% (w/v) in 95:5 benzyl benzoate: benzyl alcohol solvent. Rivastigmine tartrate (RT) was completely released in 14 days while rivastigmine base (RB) was released in 42 days, Fig. 5. Burst release of rivastigmine tartrate was 26% which is more than 8 times higher than that observed for formulations with hydrophobic rivastigmine base (3%). The amount of rivastigmine tartrate that could be incorporated into this formulation may be a limiting factor due to its hydrophilicity. However, 33.6 mg/mL rivastigmine tartrate still adequately maintained drug concentrations for 14 days for therapeutic effectiveness at ~2.4 mg/day which would avoid first pass metabolism and act as the equivalent of approximately 4 mg/day dose. At this release rate, the therapeutic window of 1–400 ng/ml can be maintained.
Fig. 5.
In vitro release profiles of rivastlgmlne tartrate (RT) and rivastlgmlne base (RB) for comparing effect of drug hydrophobicity (n = 6).
In Vivo Release Profile of Rivastigmine Tartrate
Under in vivo conditions, a controlled release of rivastigmine base from optimized phase sensitive formulation was not observed, resulting in drug related toxicity. Therefore, rivastigmine tartrate was utilized for all further in vivo studies. Blood samples were collected from the tail vein of rats at predetermined time points and RT was extracted using acetonitrile followed by centrifugation. RT was released through day 7 for the phase sensitive formulation, Fig. 6 A and a burst release amounting to 0.5 mg was reached in 15 mins, Fig. 6 B. On the other hand, RT from solution showed a peak plasma level in 15 min amounting to 0.3 mg but was rapidly eliminated from circulation within 3 h post administration, Fig. 7.
Fig. 6.
(a) In vivo release profile of RT from phase sensitive formulation over 14 days. (b) In vivo burst release of RT in 3 h post administration of phase sensitive formulation. (n = 6). * indicates a significant difference from solution group (p ≤ 0.05).
Fig. 7.
In vivo release profile of RT from solution (n = 6). * Indicates a significant difference from phase sensitive formulation group (p ≤ 0.05).
Evaluation of Acetylcholinesterase Inhibition In Vivo
Acetylcholinesterase inhibition was evaluated using Ellman’s assay which quantifies products of acetylcholine hydrolysis by acetylcholinesterase. Specifically, Ellman’s assay measures the amount of thiol compounds present in a solution. Inhibition of acetylcholinesterase will result in lower quantities of acetylcholine breakdown products which have the thiol moiety that is detected using Ellman’s assay. Protein assay was performed to normalize protein content in all samples. Post normalization, thiol products in the samples were measured to gauge extent of acetylcholinesterase inhibition. The average thiocholine concentration in blank control animals was found to be 339 mM. In comparison, the average amount of thiocholine in the solution group was 406 mM at 1-day post administration. The increase in thiocholine for the solution group is likely due to an over compensation of acetylcholinesterase expression and activity in response to the temporary inhibition (25). The animal groups that were sacrificed at 15 min, day 3, day 7, and day 14 had thiocholine concentrations of 242, 208, 195, and 265 mM respectively. These results are summarized in Table IV and Fig. 8.
Table IV.
Acetylcholinesterase Activity in Different Experimental Groups (n = 6)
| Experimental Group | Time of Blood collection | Average Concentration of Thiocholine (mM) | Percent AChE Activity |
|---|---|---|---|
| Blank Control | Day 14 | 339 ± 1 | 100% |
| RT Solution | Day 1 | 406 ± 3 | 120% |
| Phase Sensitive Formulation | Cmax: 15 min | 242 ± 15 | 71% |
| Phase Sensitive Formulation | Day 3 | 208 ± 23 | 61% |
| Phase Sensitive Formulation | Day 7 | 195 ± 12 | 58% |
| Phase Sensitive Formulation | Day 14 | 265 ± 17 | 78% |
Fig. 8.
Percent in vivo acetylcholinesterase activity upon treatment with RT encapsulating phase sensitive formulation (denoted as formulation) at 15 min, day 3, day 7, and day 14; blank control at day 14; and RT solution at day 1.
In Vivo Biocompatibility
For visual biocompatibility inspection, the injection site was dissected and observed for signs of inflammation such as redness, swelling, and vasodilation. Slight redness and vasodilation at the injection site of RT solution and phase sensitive formulation groups at day 1 and 15 min post administration, respectively were observed. However, by day 3 and 7 the redness was mostly resolved, and only slight vasodilation was noticeable. At day 14, signs of inflammation were completely resolved for phase sensitive and blank control groups, indicating an overall good biocompatibility of formulations as depicted in Fig. 9.
Fig. 9.
Visual inspection of subcutaneous injection site for biocompatibility determination. Injection site post administration of phase sensitive formulation conta ning (a) rivastigmine base at 15 min; (b) rivastigmine tartrate at 15 min; (c) rivastigmine tartrate at day 3; (d) rivastigmine tartrate at day 7; and (e) rivastigmine tartrate at day 14. The injection site post administration of other controls is shown as (f) PBS control at day 14, and (g) rivastigmine solution at day 1.
DISCUSSION
Phase sensitive smart polymers are a safe and suitable delivery system for prolonged delivery of therapeutics. The ease of drug incorporation and formulation manipulation to obtain the desired release kinetics is extremely valuable. For the preparation of phase sensitive biodegradable implant, a combination of water miscible solvent and water insoluble polymer was utilized. When this blend is injected into an aqueous environment, diffusion of water soluble solvent followed by precipitation of water insoluble polymer occurs that results in formation of a solid implant (26). Thereafter, drug is released from this polymer depot in a controlled way through combination of several mechanisms. Three confirmed mechanisms include i) Fickian diffusion of drug through polymer matrix, ii) formation of aqueous channels due to penetration of water in the matrix and diffusion of drug through these pores and iii) release of drug due to erosion of the matrix (26). In this study, we investigated factors such as polymer concentration, drug concentration, polymer composition, solvent composition, and hydrophobicity of the drug molecule can guide the development of the best suited formulation for a specific drug. Furthermore, testing these variables can uncover characteristics that might otherwise be counter intuitive or go unnoticed.
Polymer concentration can be easily varied to explore drug release from a phase sensitive release system (26,27). In this study, drug release profiles were evaluated by increasing the weight to volume ratio of polymer to solvent. PLA formulation prepared using 5% w/v polymer concentration demonstrated longer controlled release duration compared to 2.5 and 10% w/v formulations, owing most likely to differences in polymer hydrophobicity. In a hydrophobic environment, the primary driving force is entropy, wherein arrangement of molecules in a more energetically favorable orientation will allow reduction in entropy. However, in the case of amphiphilic polymers, the orientation that each monomer is driven to take is typically to form micelles to reduce free energy of the system. Following the formation of micelles, they can further arrange among each other to again reduce free energy. However, problem arises when there isn’t the correct balance of polymer needed to form optimal micelle structure and subsequent organization. This likely leads to the results that we observed when the balance of polymer for optimal reduction in free energy is either met or disturbed. When the correct balance is achieved, and organization is optimal, the release profile reflects this in the improved controlled release of drug molecule which can be attributed to a couple of effects that rely on this organization structure such as ability to diffuse out and polymer hydrolysis rate.
Drug concentration can influence release profile and used as a means to obtain a desired release rate (28). As seen with the studies performed, increasing drug concentration did not proportionately increase duration of drug release. However, if there is a desired amount of drug release per unit of time, increasing or decreasing drug concentration may influence the result.
For smart polymer based formulations, the polymer composition can play a major role on the release profile of incorporated drug (26,28–33). We examined four polymer compositions: PLA (109 kDa), PLA (40 kDa), PLGA (85:15), and PLGA (50:50) which were selected based on varying hydrophobicity of each polymer. An increase in molecular weight of PLA from 40 to 109 kDa increases the hydrophobicity of the formulation. Similarly, an increase in lactide to glycolide ratio in PLGA formulations from 50:50 to 85:15 increases hydrophobicity of the formulation, since lactide is more hydrophobic than glycolide. Conversely, addition of glycolide to polymer composition will decrease hydrophobicity which in turn can influence drug release profile. Overall, an increase in hydrophobicity plays a major role in decreasing the rate of polymer degradation by repelling water that drive polymer hydrolysis. Decreased rate of hydrolysis will slow the release of drug molecules and result in the observed drug release profiles where the most hydrophobic polymer, PLA (109 kDa), demonstrated the longest duration of release. However, the biphasic nature of drug release from PLA based formulation also demonstrates the negative impact of slow hydrolysis, wherein the initial release is due to drug diffusion and subsequent release is due to polymer degradation. In the case of PLGA copolymers, the addition of the more hydrophilic component glycolide allows for a balance between diffusion driven release and polymer degradation driven release.
Solvent composition is unique to phase sensitive smart polymers and very influential on drug release profile since the formation of polymer depot is impacted by the hydrophobicity of the solvent and the nature in which it is displaced from the polymer solution (19,26). As expected, the more hydrophobic solvent will take longer to undergo phase transition to produce a polymer depot. This can impact drug release profile by influencing the formation of depot and its physical characteristics such as depot surface, matrix uniformity, and formation of pores and channels throughout the matrix. These characteristics will have a direct effect on hydrolysis of polymer and influence polymer degradation driven release. We observed this in our study when comparing benzyl benzoate and benzyl alcohol ratios as the solvent. In the study, increase of benzyl alcohol led to increase in burst release and subsequent shorter duration of complete release, in most cases (Fig. 4). This result is supported by the fact that benzyl alcohol is more hydrophilic compared to benzyl benzoate. The increased hydrophilicity of the solvent drives faster phase transition for depot formation as solvent is displaced rapidly into the aqueous environment. We also observed that solvent composition of 95:5 BB:BA for PLGA (50:50) is more favorable for controlled drug release than 100:0 BB:BA, as may be expected. This again demonstrates the delicate balance that hydrophobic effect has on drug release profile (19,26,27).
Altering the hydrophobic nature ofthe drug can also greatly influence its release from phase sensitive polymers (34). Rivastigmine base is hydrophobic while rivastigmine tartrate is hydrophilic. Naturally, incorporating a hydrophobic drug in a hydrophobic release system is easy and as observed in our study, can provide better controlled release in vitro compared to hydrophilic drug. However, since the phase transition of these smart polymers relies on the displacement of hydrophobic organic solvent, it is possible that the hydrophobic drug is also displaced along with the solvent. Therefore, having a formulation that offers opposing characteristics can be of value when continuing to develop a delivery system. Rivastigmine tartrate, being hydrophilic, would not be expected to displace in similar way as rivastigmine base during phase transition, but instead could be driven to slow diffusion-controlled release after depot formation. We observed controlled release of rivastigmine tartrate from our phase sensitive delivery system in vivo which was in contrast to the release profile of rivastigmine base in vitro. For example, rivastigmine base had a lower burst release and a longer duration of release in vitro. However in vivo, the rivastigmine base formulation did not show adequate control of burst release compared to rivastigmine tartrate, leading to toxicity due to high rivastigmine base levels. The inability to minimize burst release is attributed to hydrophobic rivastigmine base displacing with the hydrophobic solvent during phase transition after injection into the aqueous environment in the subcutaneous space. In light of these observations, rivastigmine tartrate was utilized for in vivo studies. The formulation of rivastigmine tartrate in a phase sensitive smart polymer composed of PLGA (50:50) at 5% (w/v) in 95:5 BB:BA was accomplished by suspending the drug in the smart polymer. Solution of rivastigmine tartrate and a blank control of PBS were also used as formulation controls.
The acetylcholinesterase activity in blank controls that were injected with PBS, was considered as baseline level for the study. The group administered rivastigmine tartrate solution demonstrated an increase in acetylcholinesterase activity at 24 h post administration. This result although counterintuitive, was expected and is supported by work from other researchers suggesting that acute inhibition of acetylcholinesterase leads to over compensation of acetylcholinesterase activity by increasing expression of this enzyme and its subsequent activity (25). Phase sensitive formulation of rivastigmine tartrate provided a continuous drug release at therapeutic level over 7 days with minimal burst release. A total circulating drug amount of 0.5 mg was the maximum drug amount observed during burst release at 15 min post administration. There onwards, release followed zero-order kinetics and provided drug amounts in the range of about 0.1–0.2 mg. Furthermore, rivastigmine tartrate was shown to be bioactive as evidenced by its capability of inhibiting acetylcholinesterase. The inhibition of acetylcholinesterase continued even after levels of drug could no longer be detected, for up to day 14 post administration. This is likely due to a combination of factors such as prolonged exposure to rivastigmine tartrate and low levels of drug (below the limit of detection) that is continually released as the depot’s core polymer degrades and hence continuing to exert an inhibitory effect.
In addition, the formulation was shown to be biocompatible as evidenced by inspection of the injection site post administration. In vivo biocompatibility of phase sensitive smart polymers has been previously studied in depth (27,28,30,31,34,35). Our lab has looked at breakdown of these polymers and found that PEG is broken into smaller segments and lactide and glycolide from PLGA are broken into their respective acids, which are consumed by the citric acid cycle and are easily eliminated from the body. Degradation studies have been done weighing the remaining gel depot over time to examine breakdown as it progresses. In vivo studies conducted in our lab, demonstrate exceptional biocompatibility of such copolymeric formulations upon histological examination. In this study, we wanted to assess the local vasodilation, damage to vasculature, necrosis, or tissue damage due to rivastigmine release by visual examination. Inflammation is a side effect typically noticed immediately after any injection. The ability to resolve signs of inflammation and vasodilation, as shown in our study, support the biocompatibility of the formulation. Vasodilation has been well documented as a side effect of benzyl benzoate and therefore, vasodilation at the injection site was expected (36). The vasodilation could also increase blood circulation at the injection site to further aid displacement of the organic solvent from the polymer depot and increase the rate of therapeutic entering the blood system. This would also corroborate the unexpected toxicity caused by the rapid release of hydrophobic rivastigmine base along with the organic solvent from the formulation. Likewise, it also supports the controlled release and minimal burst release of rivastigmine tartrate. Since rivastigmine tartrate is hydrophilic, it neither partitions into the organic solvent nor gets displaced upon phase transition, thus entrapped within the polymer depot and subsequently released in a controlled fashion.
Furthermore, to develop a pharmaceutically acceptable polymeric injectable suspension, physicochemical properties such as syringeability, injectability, needle clogging, uniformity, re-suspendability, and viscosity are critical parameters (37). In this study, we evaluated syringeability of the final formulation by injecting it through a 25G needle for both in vitro and in vivo experiments. The formulation was syringeable and injectable without causing needle clogging. Viscosity of the phase-sensitive copolymer increases with gradual diffusion of water miscible solvent leaving behind a precipitate, which swells in water forming a gel-like consistency (26). In our study, we found drug release from this delivery system within appreciable standard deviation limit, and reproducible between experiments by keeping all formulation, injection, in vitro release conditions (37°C, 35 rpm), and in vivo administration consistent between replicates.
Taken together, the study illustrates influence of various formulation parameters in the design of phase sensitive polymer-based sustained release system for rivastigmine. Rivastigmine tartrate formulation made using PLGA (50:50) at 5% w/v concentration in 95:5 BB:BA was the optimized formulation that demonstrated desirable efficacy and biocompatibility in vivo.
CONCLUSIONS
Phase sensitive smart polymers are composed of biocompatible polymers and organic solvents. Following injection, the biocompatible organic solvent is displaced, leaving any incorporated therapeutic entrapped and protected within the polymer depot. Release of the therapeutic is dependent upon controlled diffusion and slow breakdown of the polymer over an extended period. The ability to adjust the polymer composition as well as the solvent composition allows this smart polymer to be versatile with the ability to be easily manipulated to provide the best release profile for the therapeutic of interest. In addition, polymer concentration, drug concentration, and drug hydrophobicity can also be altered to reach the desired release profile.
By examining the aforementioned factors, we have observed the influence these parameters have on drug release. Modulating polymer characteristics, polymer-drug concentrations, solvent compositions, and drug properties (salt vs base), the drug release profile can be significantly modified. For example, while PLA polymers offer lengthened release, PLGA copolymers may be better suited given they follow zero order release kinetics more closely. Upon analysis of all factors, PLGA (50:50) at 5% w/v concentration in 95:5 BB:BA, offered the best drug release profile. Optimized phase sensitive formulation containing hydrophobic rivastigmine base gave longer duration of release in vitro, but under in vivo conditions, the formulation containing hydrophilic rivastigmine tartrate demonstrated superior controlled release over the course of 7 days and was biocompatible as well as biodegradable.
The currently marketed rivastigmine formulations for the treatment of Alzheimer’s disease have 24 h dosing interval or less. Our formulation would significantly increase the dosing interval to 7 days and allow for less fluctuations in drug levels to better maintain therapeutic concentrations and symptom relief. The phase sensitive formulation of rivastigmine tartrate is a promising advancement in the treatment of Alzheimer’s disease and should be further developed.
ACKNOWLEGEMENTS AND DISCLOSURES
This research was supported by the National Institutes of Health (NIH) grant# R15GM114701. The authors declare no conflict of interest regarding the publication of this article.
REFERENCES
- 1.National Institute of Aging. Alzheimer’s Disease Fact Sheet.; Accessed: 2019 12/20 Available from: https://www.nia.nih.gov/health/alzheimers-disease-fact-sheet.
- 2.Weuve J, Hebert LE, Scherr PA, Evans DA. Deaths in the United States among persons with Alzheimer’s disease (2010–2050). Alzheimers Dement. 2014;10(2):e40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alzheimer’s association. Generation alzheimer’s the defining disease of the baby boomers.; Accessed: 2019 12/20 Available from: https://act.alz.org/site/DocServer/ALZ_BoomersReport.pdf?docID=521.
- 4.National Cancer Institute. NCI Budget and Appropriations.; Accessed: 2019 12/20 Available from: https://www.cancer.gov/about-nci/budget.
- 5.Mather M, Harley CW. The locus Coeruleus: essential for maintaining cognitive function and the aging brain. Trends Cogn Sci. 2016;20(3):214–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar A, Singh A. Ekavali. A review on Alzheimer’s disease pathophysiology and its management: an update. Pharmacol Rep. 2015;67(2): 195–203. [DOI] [PubMed] [Google Scholar]
- 7.Nagy B, Brennan A, Brandtmuller A, Thomas SK, Sullivan SD, Akehurst R. Assessing the cost-effectiveness of the rivastigmine transdermal patch for Alzheimer’s disease in the UK using MMSE- and ADL-based models. Int J Geriatr Psychiatry. 2011;26(5):483–94. [DOI] [PubMed] [Google Scholar]
- 8.Kracmarova A, Drtinova L, Pohanka M . Possibility of Acetylcholinesterase overexpression in Alzheimer disease patients after therapy with Acetylcholinesterase inhibitors. Acta Med (Hradec Kralove). 2015;58(2):37–42. [DOI] [PubMed] [Google Scholar]
- 9.Williams BR, Nazarians A, Gill MA. A review of rivastigmine: a reversible cholinesterase inhibitor. Clin Ther. 2003;25(6):1634–53. [DOI] [PubMed] [Google Scholar]
- 10.Eskander MF, Nagykery NG, Leung EY, Khelghati B, Geula C. Rivastigmine is a potent inhibitor ofacetyl- and butyrylcholinesterase in Alzheimer’s plaques and tangles. Brain Res. 2005;1060(1–2): 144–52. [DOI] [PubMed] [Google Scholar]
- 11.Cotrell V, Wild K, Bader T. Medication management and adherence among cognitively impaired older adults. J Gerontol Soc Work. 2006;47(3–4):31–46. [DOI] [PubMed] [Google Scholar]
- 12.Dhillon S Spotlight on rivastigmine transdermal patch: in dementia of the Alzheimer’s type. Drugs Aging. 2011;28(11):927–30. [DOI] [PubMed] [Google Scholar]
- 13.Alva G, Grossberg GT, Schmitt FA, Meng X, Olin JT. Efficacy of rivastigmine transdermal patch on activities of daily living: item responder analyses. IntJ Geriatr Psychiatry. 2011;26(4):356–63. [DOI] [PubMed] [Google Scholar]
- 14.Uwano C, Suzuki M, Aikawa T, Ebihara T, Une K, Tomita N, et al. Rivastigmine dermal patch solves eating problems in an individual with advanced Alzheimer’s disease. J Am Geriatr Soc. 2012;60(10): 1979–80. [DOI] [PubMed] [Google Scholar]
- 15.Adler G, Mueller B, Articus K. The transdermal formulation of rivastigmine improves caregiver burden and treatment adherence ofpatients with Alzheimer’s disease under daily practice conditions. IntJ Clin Pract. 2014;68(4):465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cortez Pinto L, Martinho Pimenta AJ, Figueira ML, Fernandes JM. More Patients Show Reduced Agitation/aggression with Rivastigmine Transdermal Monotherapy Than with Oral Monotherapies for Alzheimer’s Disease – Results From the Exept Study in Portugal. European Psychiatry 2015 28–31 March 2015;30:1445. [Google Scholar]
- 17.Dhillon S Rivastigmine Transdermal Patch Drugs 2011;71(9). [DOI] [PubMed] [Google Scholar]
- 18.Pratten MK, Lloyd JB, Horpel G, Ringsdorf H. Micelle-forming block copolymers : pinocytosis by macrophages and interaction with model membranes. Die Makromol Chemie. 2003;186(4): 725–33. [Google Scholar]
- 19.Chen S, Singh J. Controlled delivery of testosterone from smart polymer solution based systems: in vitro evaluation. IntJ Pharm. 2005;295(1–2):183–90. [DOI] [PubMed] [Google Scholar]
- 20.Rao BM, Srinivasu MK, Kumar KP, Bhradwaj N, Ravi R, Mohakhud PK, et al. A stability indicating LC method for Rivastigmine hydrogen tartrate. Journal of Pharmaceutical and Biomedical Analysis 2005 7 February 2005;37(1):57–63. [DOI] [PubMed] [Google Scholar]
- 21.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wake MC, Gupta PK, Mikos AG. Fabrication of pliable biodegradable polymer foams to engineer soft tissues. Cell Transplant. 1996;5(4):465–73. [DOI] [PubMed] [Google Scholar]
- 23.Bonacucina G, Cespi M, Mencarelli G, Giorgioni G, Filippo PG. Thermosensitive self-assembling block copolymers as drug delivery systems. Polymers. 2011;3(2):779–811. [Google Scholar]
- 24.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–7. [DOI] [PubMed] [Google Scholar]
- 25.Yamada K, Nabeshima T. Animal models of Alzheimer’s disease and evaluation of anti-dementia drugs. Pharmacol Ther. 2000;88(2):93–113. [DOI] [PubMed] [Google Scholar]
- 26.Chen S, Singh J. In vitro release of levonorgestrel from phase sensitive and thermosensitive smart polymer delivery systems. Pharm Dev Technol. 2005;10(2):319–25. [DOI] [PubMed] [Google Scholar]
- 27.Chen S, Pederson D, Oak M, Singh J. In vivo absorption of steroidal hormones from smart polymer based delivery systems. J Pharm Sci. 2010;99(8):3381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang Y, Singh J. Thermosensitive drug delivery system of salmon calcitonin: in vitro release, in vivo absorption, bioactivity and therapeutic efficacies. Pharm Res. 2010;27(2):272–84. [DOI] [PubMed] [Google Scholar]
- 29.Tang Y, Singh J. Biodegradable and biocompatible thermosensitive polymer based injectable implant for controlled release of protein. Int J Pharm. 2009;365(1–2):34–43. [DOI] [PubMed] [Google Scholar]
- 30.Chen S, Pieper R, Webster DC, Singh J. Triblock copolymers: synthesis, characterization, and delivery of a model protein. Int J Pharm. 2005;288(2):207–18. [DOI] [PubMed] [Google Scholar]
- 31.Al-Tahami K, Oak M, Mandke R, Singh J. Basal level insulin delivery: in vitro release, stability, biocompatibility, and in vivo absorption from thermosensitive triblock copolymers. J Pharm Sci. 2011; 100(11):4790–803. [DOI] [PubMed] [Google Scholar]
- 32.Oak M, Singh J. Controlled delivery of basal level of insulin from chitosan-zinc-insulin-complex-loaded thermosensitive copolymer. J Pharm Sci. 2012;101(3):1079–96. [DOI] [PubMed] [Google Scholar]
- 33.Oak M, Singh J. Chitosan-zinc-insulin complex incorporated thermosensitive polymer for controlled delivery of basal insulin in vivo. J Control Release. 2012; 163(2):145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oak M, Mandke R, Lakkadwala S, Lipp L, Singh J. Effect ofmolar mass and water solubility of incorporated molecules on the degradation profile ofthe triblock copolymer delivery system. Polymers. 2015;7(8): 1510–21. [Google Scholar]
- 35.Sharma D, Arora S, Singh J. Smart thermosensitive copolymer incorporating chitosan–zinc–insulin electrostatic complexes for controlled delivery of insulin: effect of chitosan chain length. Int J Polym Mater Polym Biomater. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaikh J Benzyl Benzoate In: Wexler P, editor. Encyclopedia of Toxicology. 2nd ed.: Elsevier; 2005. p. 264–265. [Google Scholar]
- 37.Schwendeman SP, Shah RB, Bailey BA, Schwendeman AS. Injectable controlled release depots for large molecules. J Control Release. 2014;190:240–53. [DOI] [PMC free article] [PubMed] [Google Scholar]