Abstract
Importance:
Phosphoinositide 3-kinase (PI3K) inhibitors are a class of small-molecule inhibitors approved for the treatment of certain leukemias and lymphomas. Their dermatologic side effect profile is poorly described. A detailed understanding of the cutaneous adverse events from PI3K inhibitors will help dermatologists and oncologists identify and effectively manage these eruptions.
Objective:
To describe a specific cutaneous side effect reaction pattern from PI3K inhibitors.
Design:
Retrospective analysis.
Setting:
Academic medical center.
Participants:
Patients receiving PI3K inhibitors referred to the Skin Toxicities Program in The Center for Cutaneous Oncology for dermatologic reactions while on therapy.
Main Outcomes and Measures:
Clinical, histopathologic, and laboratory history was obtained and reviewed.
Results:
Three patients on PI3K inhibitors for treatment of malignancy developed diffuse erythroderma and keratoderma within 4 days to 5 months of initiating therapy. Clinical and histopathologic findings were consistent with pityriasis rubra pilaris-like reactions. All patients improved with topical and oral corticosteroids, oral acitretin, and drug discontinuation.
Conclusion and Relevance:
Pityriasis rubra pilaris-like cutaneous eruptions may develop secondary to PI3K inhibition. These reactions may be treated based on their specific morphology. Further understanding of the pathophysiology of dermatologic adverse events secondary to small molecule inhibitors may help elucidate the immunologic mechanism of primary, idiopathic dermatoses.
Introduction
Phosphoinositide 3-kinase (PI3K) inhibitors are a new class of molecular targeted therapies effective against and being studied in wide range of malignancies.1 The PI3K pathway functions in cell proliferation and survival, promoting cellular differentiation.2 Class I PI3K, the most important for signal transduction, is a heterodimer with both a p85 regulatory and a p110 catalytic subunit, the latter of which exists in 4 distinct isoforms (α, β, γ, and δ).3 The p110α and p110β isoforms are ubitquitously expressed, but p110γ and p110δ isoform expression is limited to hematopoietic cells.3 PI3K inhibition is available in the form of pan-class I PI3K inhibitors, isoform-selective class I PI3K inhibitors, and dual PI3K-mTOR inhibitors.2 Given their recent development, the adverse effect profile, including cutaneous toxicities, are still being observed and described. We present 3 cases of patients receiving PI3K inhibition who developed pityriasis rubra pilaris (PRP)-like cutaneous reactions.
Reports of Cases
Case 1
A woman in her 40’s with Grade III anaplastic oligodendroglioma was treated with GDC-0084 (dual pan-class I PI3K/mTOR inhibitor) after developing intractable nausea on temozolamide. Within 4 days of her first cycle she developed pruritus and photo-distributed erythematous patches on her trunk, which subsequently generalized. She also developed oral ulcers and worsening of pre-existing essential tremor.
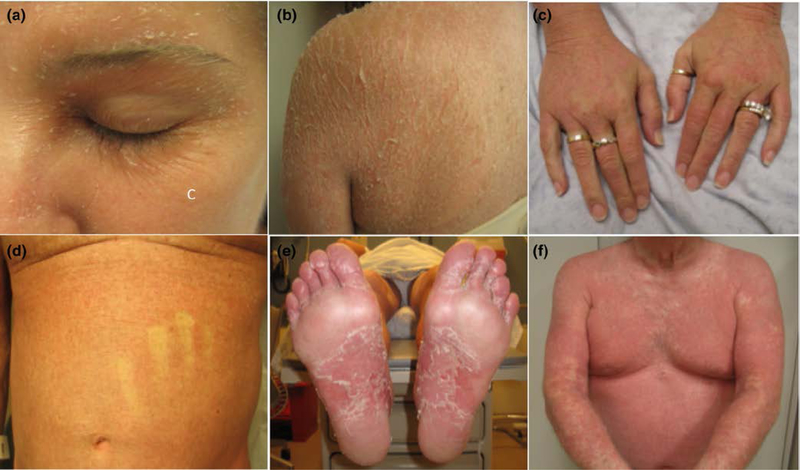
On physical exam, she had diffuse orange/pink erythroderma with islands of sparing on the trunk and extremities, with overlying fine and desquamative scale. (Figure 1A). The palms and soles were involved with erythema and mild keratoderma (Figure 1B). There was diffuse nonscarring alopecia, scalp erythema, and perifollicular scale. A punch biopsy showed pityriasiform chanegs with alternating ortho and parakeratosis, spongiotic dermatitis and numerous eosinophils. Her presentation was thought most consistent with a PRP-like reaction to the trial drug. High potency topical steroids and oral antihistamines provided mild improvement. A short course of systemic prednisone temporarily improved her eruption. She was started on acitretin and gradually increased to 25mg daily. Acitretin controlled her cutaneous symptoms with decreased erythema and scaling and some hair regrowth; while not resolved, her improvement was sufficient to remain on the PI3K inhibitor. She ultimately discontinued the trial after 6 months of therapy due to intolerable worsening of her essential tremor. She continued acitretin 25 mg daily and had resolution of cutaneous symptoms 3 months after stopping GDC-0084.
Figure 1.
Clinical images at presentation. A,B; Patient 1. C,D; Patient 2. E,F; Patient 3.
Case 2
A man in his 60’s with a 10-year history of previously untreated chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) was initiated on ofatumumab (monoclonal anti-CD20 antibody) and idelalisib (selective PI3K p110δ inhibitor).
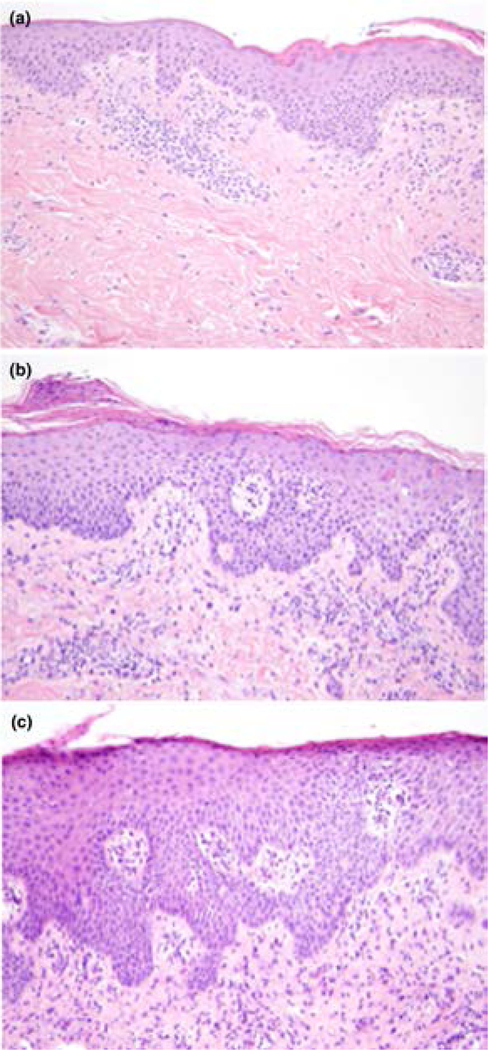
Approximately 5 months after starting therapy, he developed a diffuse, erythematous, pruritic eruption. Systemic corticosteroids improved the eruption and pruritus, but his symptoms recurred upon taper. Due to increasing body surface area involvement, he was referred to dermatology. Upon examination, the eruption had evolved to orange-red erythroderma with small islands of sparing. There was diffuse fine scaling and exfoliation along with nonscarring alopecia of the scalp and eyebrows (Figure 1C,D). His palms and soles had mild keratoderma and there was bilateral ectropion. A punch biopsy was performed and revealed pityriasiform and spongiotic dermatitis with eosinophils (Figure 2A). Laboratory evaluation showed normal CD4+ count (430/uL), mild leukopenia (3.07 k/uL), mild anemia (11.1 g/dL), and mild thrombocytopenia (134 k/uL). Liver function tests were normal.
Figure 2.
Punch biopsy specimens. A, Patient 2. Acanthosis and spongiosis with pityriasiform scale (hematoxylin-eosin, original magnification × 40). B, Patient 3. Subacute spongiotic dermatitis with pityriasiform scale and superficial perivascular lymphocytic infiltrate (hematoxylin-eosin, original magnification × 40).
The clinical impression was a PRP-like eruption secondary to idelalisib. He was started on a prednisone taper and acitretin 10mg every other day, the dose of which was limited by hypertriglyceridemia. The patient also applied mid-potency topical corticosteroids to his body and tazarotene cream to his soles. He initially showed considerable improvement of his skin findings, although cutaneous involvement continued to wax and wane. Despite partial response of the CLL/SLL, he was removed from trial due to continued severity of the cutaneous reaction as well as the development of suppurative adenitis.
Case 3
A man in his 60’s with history of SLL was initiated on idelalisib after developing progressive disease on rituximab/fludarabine as well as fludarabine/cyclophosphamide/rituximab. Six weeks after starting idelalsib, the patient developed an erythematous, pruritic eruption on the trunk that spread to include his extremities over 1–2 weeks. He denied oral ulcerations, ocular symptoms, or skin tenderness. The patient was afebrile and there were no other new medications. Idelalsib was discontinued, but the eruption continued to worsen. Skin examination was significant for diffuse orange-red patches with follicular prominence and islands of sparing over the trunk and extremities (Figure 1E,F). His palms and soles were spared and nails were normal. Laboatory evaluation was significant for mild eosinophilia and new transaminitis (AST 190 U/L, ALT 310 U/L). Punch biopsy was performed and revealed subacute spongiotic dermatitis with pityriasiform scale and superficial perivascular lymphocytic infiltrate (Figure 2B).
Due to concern for PRP-like hypersensitivity to idelalsib, the drug was discontinued and the patient was started on a 2 week oral prednisone taper along with mid-potency topical steroids. His eruption cleared and laboratories normalized. The patient was subsequently started on venetoclax.
Discussion
The PI3K inhibitors are a novel class of molecular targeted therapies that hold considerable promise in the treatment of multiple malignancies.1 In clinical trials involving the selective PI3K p110δ inhibitor idelalisib, skin reactions were noted in 23–58% of patients.4,5 The cutaneous eruptions have been described as “maculopapular” in one Phase I study of a pan-isoform PI3K inhibitor, but most trials lack further descriptive specificity.6 The effect of isoform subtype or underlying immune dysregulation on development of adverse reactions is unknown. Studies of mTOR inhibitors have shown mucocutaneous side effects such as stomatitis and xerosis, but no PRP-like eruptions have been described.7
PRP is a distinct and very rare papulosquamous disorder of unknown etiology. It has been associated with impaired cell-mediated immunity and familial variants have been linked to genetic mutations relevant to other autoinflammatory skin disorders.8–10 Given that the PI3K/mTOR pathway is upregulated locally in psoriasis and that there are reports of mTOR inhibitors treating psoriasis, it may initially appear suprising that these patients developed an inflammatory dermatosis.11,12 However, the fact that all 3 patients were on a drug that inhibited p110δ (either specifically or due to broad suppression of all p110 isoforms) is likely significant. P110δ blockade in mice and humans results in decreased regulatory T cell (Treg) function.13,14 An autoimmune hepatitis secondary to PI3K inhibitor therapy has also been previously described and attributed to this finding.14 The effects of p110δ blockade at the level of the keratinocyte may therefore be out-weighed by global Treg inhibition.14 While we cannot definitively prove that PI3K inhibition caused the cutaneous reaction pattern in these patients, the distinct morphology, temporal association with a common target and improvement after discontinuation supports this explanation. We therefore hypothesize that PI3K inhibition may provide enough immune dysregulation in a vulnerable host to result in a PRP-like eruption.
The dermatologic findings in our patients responded to oral acitretin, short courses of oral prednisone, topical steroids, and topical retinoids. Given reports of efficacy in idiopathic PRP, topical vitamin D analogs, methotrexate, and phototherapy could also be considered.15 While the understanding of treatments for cutaneous side effects secondary to all subtypes of PI3K inhibitors is limited, these cases emphasize the importance of early dermatologic evaluation with clinical and histologic classification. This is essential to effectively characterize these toxicities and initiate targeted, timely and appropriate interventions in a effort to keep patients on their lifesaving anti-cancer therapies.
Acknowledgement Section
Nicole LeBoeuf had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Dewan, Sowerby, LeBoeuf.
Acquisition, analysis, and interpretation of data: Dewan, Sowerby, Jadeja, Lian, LeBoeuf.
Drafting of manuscript: Dewan, Sowerby, LeBoeuf.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: N/A.
Adminsitrative, technical, or material support: All authors.
Study supervision: LeBoeuf.
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4(12):988–1004. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Ding J, Meng LH. PI3K isoform-selective inhibitors: next-generation targeted cancer therapies. Acta Pharmacol Sin. 2015;36(10):1170–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11(5):329–341. [DOI] [PubMed] [Google Scholar]
- 4.Kahl BS, Spurgeon SE, Furman RR, et al. A phase 1 study of the PI3Kδ inhibitor idelalisib in patients with relapsed/refractory mantle cell lymphoma (MCL). Blood. 2014;123(22):3398–3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Brien SM, Lamanna N, Kipps TJ, et al. A phase 2 study of idelalisib plus rituximab in treatment-naïve older patients with chronic lymphocytic leukemia. Blood. 2015;126(25):2686–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bendell JC, Rodon J, Burris HA, et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012;30(3):282–290. [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372(9637):449–456. [DOI] [PubMed] [Google Scholar]
- 8.Misery I, Faure M, Claidy A. Pityriasis rubra pilaris and human immunodeficiency virus infection--type 6 pityriasis rubra pilaris? Br J Dermatol. 1996;135(6):1008–1009. [DOI] [PubMed] [Google Scholar]
- 9.Shvili D, David M, Mimouni M. Childhood-onset pityriasis rubra pilaris with immunologic abnormalities. Pediatr Dermatol. 1987;4(1):21–23. [DOI] [PubMed] [Google Scholar]
- 10.Takeichi T, Sugiura K, Nomura T, et al. Pityriasis Rubra Pilaris Type V as an Autoinflammatory Disease by CARD14 Mutations. JAMA Dermatol. 2016. [DOI] [PubMed] [Google Scholar]
- 11.Chamcheu JC, Chaves-Rodriquez MI, Adhami VM, et al. Upregulation of PI3K/AKT/mTOR, FABP5 and PPARβ/δ in Human Psoriasis and Imiquimod-induced Murine Psoriasiform Dermatitis Model. Acta Derm Venereol. 2016;96(6):854–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frigerio E, Colombo MD, Franchi C, Altomare A, Garutti C, Altomare GF. Severe psoriasis treated with a new macrolide: everolimus. Br J Dermatol. 2007;156(2):372–374. [DOI] [PubMed] [Google Scholar]
- 13.Patton DT, Garden OA, Pearce WP, et al. Cutting edge: the phosphoinositide 3-kinase p110 delta is critical for the function of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2006;177(10):6598–6602. [DOI] [PubMed] [Google Scholar]
- 14.Lampson BL, Kasar SN, Matos TR, et al. Idelalisib given front-line for treatment of chronic lymphocytic leukemia causes frequent immune-mediated hepatotoxicity. Blood. 2016;128(2):195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein A, Landthaler M, Karrer S. Pityriasis rubra pilaris: a review of diagnosis and treatment. Am J Clin Dermatol. 2010;11(3):157–170. [DOI] [PubMed] [Google Scholar]