Abstract
Growing evidence indicates that parental smoking is associated with risk of offspring obesity. The purpose of this study was to identify whether parental tobacco smoking during gestation was associated with risk of diabetes mellitus. This is a prospective study of 44- to 54-year-old daughters (n = 1801) born in the Child Health and Development Studies pregnancy cohort between 1959 and 1967. Their mothers resided near Oakland California, were members of the Kaiser Foundation Health Plan and reported parental tobacco smoking during an early pregnancy interview. Daughters reported physician diagnoses of diabetes mellitus and provided blood samples for hemoglobin A1C measurement. Prenatal maternal smoking had a stronger association with daughters’ diabetes mellitus risk than prenatal paternal smoking, and the former persisted after adjustment for parental race, diabetes and employment (aRR = 2.4 [95% confidence intervals 1.4–4.1] P < 0.01 and aRR = 1.7 [95% confidence intervals 1.0–3.0] P = 0.05, respectively). Estimates of the effect of parental smoking were unchanged when further adjusted by daughters’ birth weight or current body mass index (BMI). Maternal smoking was also significantly associated with self-reported type 2 diabetes diagnosis (2.3 [95% confidence intervals 1.0–5.0] P < 0.05). Having parents who smoked during pregnancy was associated with an increased risk of diabetes mellitus among adult daughters, independent of known risk factors, providing further evidence that prenatal environmental chemical exposures independent of birth weight and current BMI may contribute to adult diabetes mellitus. While other studies seek to confirm our results, caution toward tobacco smoking by or proximal to pregnant women is warranted in diabetes mellitus prevention efforts.
Keywords: birth weight, diabetes, obesity, smoking, tobacco
Introduction
Environmental tobacco smoke (ETS) remains a common modifiable exposure worldwide, with over 126 million non-smoking American adults and children being exposed.1 Both adult and childhood ETS exposures are associated with increased risk of insulin resistance, glucose intolerance and type 2 diabetes.2–6 These findings raise the possibility that fetal ETS exposure, either through active or passive maternal smoking during pregnancy, may also increase the risk of diabetes.
Both active and passive maternal tobacco exposure are known to increase the risk of numerous pregnancy complications including low birth weight.7–9 Further, emerging yet extensive human and experimental evidence indicates that maternal tobacco exposure during pregnancy also increases risk of obesity in off-spring.10–12 Given that low birth weight and obesity are also associated with increased risk of diabetes in adulthood,13–16 it is plausible that fetal ETS could lead to increased risk of diabetes in adulthood through low birth weight and/or elevated body mass index (BMI).17,18 Although the direction of the association of active maternal smoking with offspring diabetes varies across studies,10,18 it is unclear whether these discrepancies result from exposure bias due to variability in women’s willingness to report smoking during pregnancy.19–21
Whether paternal smoking can contribute to fetal response to ETS exposure is seldom considered in observational studies. We know of two human studies that demonstrated protective associations between offspring type 1 diabetes and paternal smoking,22,23 and one study that demonstrated paternal smoking was associated with increased risk of offspring type 2 diabetes.18 Despite these sparse discrepancies, the positive association between paternal smoking and offspring obesity12,16,18,24–26 suggests that paternal smoking may at least be associated with increased risk offspring diabetes risk indirectly via its association with obesity.
There is insufficient evidence to conclude whether fetal ETS exposure via parental smoking contributes to risk of diabetes mellitus. Indeed, the assessment of maternal smoking and risk of offspring type 2 diabetes was identified as a research need in a recent review.10 In this study, we hypothesize that parental tobacco smoking is associated with increased risk of diabetes mellitus in middle-aged offspring. We tested our hypothesis in a prospective birth cohort, the Child Health and Development Studies (CHDS), with parental tobacco smoking identified by self-report that has been validated by serum cotinine levels.27
Method
Population
The CHDS is a pregnancy cohort designed to evaluate the associations between prenatal exposures and health outcomes in the parents and offspring. The CHDS recruited women who were members of the Kaiser Foundation Health Plan based in Oakland, California, between 1959 and 1967.28 The CHDS participants gave verbal consent for an in-person interview, which was generally conducted at the first prenatal visit, and gave permission for access to their medical records and their children’s medical records.
The present study evaluates the adult daughters who were born to the mothers of the CHDS cohort and participated in a recent follow-up study from 2010 to 2013, the ‘Three Generations (3Gs) Study.’ Women were eligible for the 3Gs Study if they did not have a severe congenital illness that would preclude participation, were willing to receive invitations to new studies and were not incarcerated or too ill to participate. The eligible pool (n = 8401) constituted 92% of all live-born CHDS daughters, and the 82% (n = 6905) who were address-locatable were mailed an invitation to participate in the study. After the initial invitation was mailed to the address-locatable eligible pool, 43 refused, another 55 were identified as deceased or ineligible and 590 were identified with an incorrect address. Of the remaining 6217 address-locatable eligible pool, 80% (n = 5003) were phone-locatable and attempted for telephone contact, among which 60% completed a telephone interview (n = 3003). Just over a third of the telephone interview participants also participated in a home visit study (n = 1195), during which a certified phlebotomist collected non-fasting blood samples. The number of home visits and the number of A1C assays that were performed (n = 557 randomly selected from the pool of daughters with available whole-blood samples) were determined by availability of resources. The home visit sample targeted the following three groups: daughters of mothers with breast cancer, daughters who had participated in an earlier (2005–2008) breast density study and a random sample of daughters with an over-sample of African Americans.
The present analysis sample is based on 1801 daughters with available data on parental tobacco smoking during pregnancy, race, occupation, report of parental diabetes and self-report of body weight. Of the 1801 daughters, 370 had A1C measures. The analysis sample includes 48 sister pairs and one set of three sisters. There were proportionately fewer African Americans and fewer daughters born preterm with low birth weight in the analysis sample compared with the entire CHDS cohort. Parents of daughters included in the analysis were more educated, had higher incomes, lower parity and smoked less at study entry.
Verbal consent was required for the telephone interview and signed written consent was required before initiation of the home visit. The 3Gs Study was approved by the institutional review board of the Public Health Institute.
Parental tobacco use
Parental tobacco use during the index pregnancy was defined by maternal self-report. Only mothers who reported that they smoked at least one cigarette a day were considered as tobacco smokers. Paternal tobacco smoking was ascertained from pregnant women who reported that their husbands currently smoked at least one cigarette a day, or currently smoked a pipe or cigar at least once weekly. We analyzed household smoking simultaneously in four separate categories where (1) only mothers smoked, (2) only husbands smoked, (3) both mothers and husbands smoked and (4) neither mothers nor husbands smoked. Smoking data were collected before the impact of the 1964 Surgeon General’s Report. This report is widely considered the turning point that initiated anti-smoking attitudes in America.29,30 Therefore, we suggest that most women were willing to accurately report smoking behavior. The validity of maternal self-report is further supported by concordance with measured serum cotinine levels, previously demonstrated in the CHDS.27
Potential covariates
During the prenatal interview, mothers reported their race/ ethnicity, height, pre-pregnancy weight and occupation. They also reported race/ethnicity, height, weight and occupation for their husbands. Height and weight were used to calculate BMI (kg/m2) for each parent, from which BMI was dichotomized as BMI ⩾ 25 (obese or overweight) v. BMI < 25 (normal).31 Report of husbands with professional, technical or managerial occupations were categorized as professional. Report of husbands in occupations with clerical, sales or operative duties or working as craftsmen, foremen, service workers, laborers or members of the armed services were grouped into an ‘other occupation’ category. Mothers who reported professional or managerial jobs were categorized as professional, whereas mothers reporting their work as homemaking, secretarial, clerical, servicing or industrial were grouped into an ‘other occupation’ category.
Mothers’ dates of last menstrual period were reported by mothers during their in-person prenatal interview. Birth dates of daughters were obtained from medical records. These were used to calculate daughters’ gestational age to the nearest completed week. Birth weight was also extracted from obstetric records. Because birth weight tends to have a non-linear association with diabetes risk later in life, it was categorized as low birth weight (<2500 g), healthy birth weight and macrosomia (>4000 g).13,32–34
Adult daughters’ interview and blood-draw dates were used with birth dates to calculate age (in years) at interview and blood draw. Tobacco use (ever v. never), current height and weight and daughters’ report of a parent having ever been diagnosed with diabetes mellitus were determined from telephone interviews of the daughters. Daughters’ self-reported height and weight were used to calculate BMI, where BMI < 25 was considered normal, 25 ⩽ BMI < 30 was considered overweight and BMI ⩾ 30 was considered obese.31
Diabetes mellitus
During their adult interview, daughters reported whether a doctor ever told them that they have diabetes. Daughters were asked to exclude diabetes that only occurred during pregnancy. Daughters were also asked how old they were when they were first diagnosed with diabetes, and what type of diabetes they were told they had. Whole-blood hemoglobin A1C was measured from a subset of daughters who participated in the home visit by the Clinical and Epidemiologic Research Laboratory of the Boston Children’s Hospital. We principally defined cases of diabetes mellitus as those daughters whose doctors ever told them they had diabetes while not pregnant and/or whose A1C values were ⩽6.5% (47.5 mmol/mol).35 In four secondary analyses, we (1) restricted case status to only those daughters who reported diagnosed diabetes, which ignored the A1C status, (2) restricted case status to only those daughters who reported a doctor telling them they had type 2 diabetes, which ignored report of type 1 diabetes and A1C status, (3) restricted cases to the subset of non-siblings and (4) restricted cases to the subset of non-siblings plus one randomly selected sister from each family. These secondary analyses were performed to exclude the potential impact of the home visit sampling criteria and of the sibling sampling, as well as to facilitate comparison with other studies.
Statistical analysis
Prevalences were calculated in PROC FREQ (SAS 9.4, Cary, NC, USA). We modeled the effect of the four parental smoking categories on continuous outcomes to calculate least square means, their standard errors and their significance while accounting for random effects of potential correlations among siblings in PROC MIXED. We modeled the effect of the parental smoking categories on dichotomous outcomes to calculate risk ratios, their confidence intervals (CI) and their significance while accounting for random effects of potential correlations among siblings in PROC GENMOD. To further examine the potential impact of including siblings on the estimation of smoking effects, we ran models on subsets including only non-siblings and including non-siblings plus one randomly selected sister per family. Variables that changed the size of the parameter estimated for any parental smoking category by 10% or more, or were found to be statistically significant predictors in the saturated model, were included as covariates in the multivariable models (GENMOD).7 The prevalences and risks determined in PROC FREQ and PROC GENMOND were used for post-hoc power calculations (PROC POWER). We evaluated the onset of type 2 diabetes as a time-dependent function of parental smoking categories by testing the significance of cross-product terms between type 2 diabetes onset (age at diagnosis) and parental smoking (categories described above) while accounting for the main effect of parental smoking and random effects of potential correlations among siblings in PROC PHREG. PROC LIFETEST was used to generate data graphed in GraphPad Prism 6 (La Jolla, CA, USA).
Results
The distribution of parental tobacco smoking during pregnancy across the variables we considered in our analyses is shown in Table 1. As expected, daughters’ mean birth weight was significantly reduced in households with maternal tobacco smoking. We further found that maternal, but not paternal, BMI was also negatively associated with parental tobacco smoking status. Conversely, paternal, but not maternal, diabetes was associated with parental tobacco smoking.
Table 1.
Distribution of parental, early-life and adult characteristics by parental tobacco use status in 1801 women [n (row %) or least squared means (S.E.)]
| No parental tobacco [606 (33.6)] | Maternal tobacco [116 (6.4)] | Paternal tobacco [619 (34.4)] | Parental tobacco [460 (25.5)] | |
|---|---|---|---|---|
| Maternal | ||||
| Race/ethnicity | ||||
| African American | 75 (26.4%) | 13 (4.6%) | 125 (44.0%)** | 71 (25.0%) |
| White, Asian, Hispanic, other | 531 (35.0%) | 103 (6.8%) | 494 (32.6%) | 389 (25.6%) |
| BMI (kg/m2)a | 22.9 (0.2) | 22.7 (0.3) | 23.2 (0.2) | 22.2 (0.2)# |
| Diabetes | ||||
| Never | 518 (34.5%) | 95 (6.3%) | 506 (33.7%) | 381 (25.4%) |
| Ever | 88 (29.2%) | 21 (7.0%) | 113 (37.5%) | 79 (26.2%) |
| Occupation | ||||
| Professional | 97 (35.5%) | 21 (7.7%) | 104 (38.1%) | 51 (18.7%)❡ |
| Other | 509 (33.3%) | 95 (6.2%) | 515 (33.7%) | 409 (26.8%) |
| Paternal | ||||
| Race/ethnicity | ||||
| African American | 78 (25.9%) | 17 (5.6%) | 131 (43.5%)** | 75 (24.9%) |
| White, Asian, Hispanic, other | 528 (35.2%) | 99 (6.6%) | 488 (32.5%) | 385 (25.7%) |
| BMI (kg/m2)b | 24.3 (0.2) | 24.5 (0.4) | 24.4 (0.1) | 24.5 (0.2) |
| Diabetes | ||||
| Never | 492 (34.7%) | 94 (6.6%) | 480 (33.9%) | 351 (24.8%) |
| Ever | 114 (29.7%) | 22 (5.7%) | 139 (36.2%) | 109 (28.4%)❡ |
| Occupation | ||||
| Professional | 343 (41.9%) | 54 (6.6%) | 256 (31.3%) | 166 (20.3%) |
| Other | 263 (26.8%) | 62 (6.3%) | 363 (37.0%) | 294 (29.9%)❡ |
| Daughters | ||||
| Gestational age (weeks)c | 39.9 (0.1) | 40.1 (0.2) | 40.0 (0.1) | 39.7 (0.1) |
| Birth weight (g) | 3350.9 (19.4) | 3149.3 (44.1)** | 3324.0 (19.3) | 3114.5 (22.4)** |
| Age (years) | 48.7 (0.1) | 49.2 (0.2)❡ | 48.8 (0.1) | 49.2 (0.1)** |
| Tobacco smokingd | ||||
| Never | 384 (37.5%) | 69 (6.7%) | 356 (34.7%)❡ | 216 (21.1%)** |
| Ever | 221 (28.5%) | 47 (6.1%) | 262 (33.9%) | 243 (31.4%) |
| BMI (kg/m2) | 26.7 (0.3) | 27.7 (0.6) | 27.7 (0.3)# | 27.7 (0.3)❡ |
| Hb A1C (%)e | 5.8 (0.1) | 5.8 (0.2) | 5.7 (0.1) | 5.9 (0.1) |
| Hb A1C (mmol/mol)e | 39.9 (0.7) | 39.9 (1.4) | 38.8 (0.7) | 41.0 (0.7) |
| Hb A1Ce | ||||
| Unmeasured | 488 (34.1%) | 91 (6.4%) | 495 (34.6%) | 357 (25.0%) |
| Measured | 118 (31.9%) | 25 (6.8%) | 124 (33.5%) | 103 (27.8%) |
| Type 2 diabetes | ||||
| Absent | 589 (34.4%) | 107 (6.2%)❡ | 590 (34.5%) | 425 (24.8%)# |
| Present | 17 (18.9%) | 9 (10.0%) | 29 (32.2%) | 35 (38.9%) |
| Type 1 diabetes❡ | ||||
| Absent | 606 (33.8%) | 115 (6.4%) | 612 (34.2%) | 459 (25.6%) |
| Present | 0 (0%) | 1 (11.1%) | 7 (77.8%) | 1 (11.1%) |
BMI, body mass index; Hb, hemoglobin.
n = 1748.
n = 1208.
n = 1777.
n = 1798.
n = 370.
Analysis of significant differences in least square means or counts were preformed in PROC MIXED or PROC GENMOD (type 1 diabetes in PROC FREQ Fishers Exact), respectively, where ❡P < 0.05, #P < 0.01, ✶✶P < 0.0001 compared with no tobacco use during pregnancy.
Numerous aspects of daughters’ adult metabolic health were also associated with parental tobacco smoking during gestation (Table 1). Paternal tobacco smoking was significantly associated with a 1 kg/m2 increase in the mean of daughters’ BMI (maternal only tobacco use was non-significant, P = 0.16). In contrast, maternal smoking during pregnancy was associated with increased risk of daughters’ self-reported type 2 diabetes (paternal only tobacco use was non-significant, P = 0.08). The number of cases defined by type 1 diabetes report and A1C status were far less than those defined by type 2 diabetes report in this sample. Despite small numbers, there was a significant association of parental smoking on type 1 diabetes, with an absence of type 1 diabetes cases among non-smoking households and the highest prevalence of type 1 diabetes cases occurring among daughters with in utero exposure to paternal tobacco smoke. The A1C measurement status and mean percent A1C among daughters did not differ across parental tobacco use categories. Although missing information about parental smoking during pregnancy was the main source of sample attrition from the 3003 telephone interviews, the absence of parental smoking data was not significantly associated with diabetes mellitus (P = 0.8).
To maximize the accuracy of case ascertainment, we defined diabetes mellitus from both self-report of doctor-diagnosed diabetes for all 1801 women and from measured A1C for a random subset of 370 women, as shown in Table 2. The inclusion of diabetes mellitus cases defined by A1C ⩾ 6.5% (47.5 mmol/mol) led to the identification of an additional 10 diabetes mellitus cases that were not identified by self-report. Some of these 10 cases identified exclusively by A1C are consistent with a recent development of diabetes, as four of these ten women had A1C ⩾ 6.5% (47.5 mmol/mol) during a home visit which occurred 1–2 years after they reported their diabetes status. Because we did not measure auto-antibodies to distinguish type 1 diabetes from type 2 diabetes, and did not identify what clinical parameters physicians utilized to diagnose diabetes, we conservatively describe our primary outcome of interest as diabetes mellitus. However, we also provide results for self-reported type 2 diabetes mellitus alone to facilitate comparisons with other studies.
Table 2.
Diabetes mellitus cases (n = 109) according to self-reported (ever v. never, n = 1801) and clinically defined (A1C n = 370) diabetes mellitus in the analysis population (n = 1801)
| Self-reported type 1 diabetes | Self-reported type 2 diabetes | A1C categories | Diabetes mellitus | Frequency | Percent |
|---|---|---|---|---|---|
| Never | Never | Unmeasured | Never | 1355 | 75.2 |
| Never | Never | Normal | Never | 197 | 10.9 |
| Never | Never | Pre-diabetes | Never | 140 | 7.8 |
| Never | Never | Diabetes | Ever | 10 | 0.6 |
| Never | Ever | Unmeasured | Ever | 69 | 3.8 |
| Never | Ever | Normal | Ever | 1 | 0.1 |
| Never | Ever | Pre-diabetes | Ever | 6 | 0.3 |
| Never | Ever | Diabetes | Ever | 14 | 0.8 |
| Ever | Never | Unmeasured | Ever | 7 | 0.4 |
| Ever | Never | Normal | Ever | 1 | 0.1 |
| Ever | Never | Diabetes | Ever | 1 | 0.1 |
Women prenatally exposed to only maternal smoking had an unadjusted three-fold increased risk of diabetes mellitus (Table 3). Women exposed to only paternal smoking while in utero had an unadjusted two-fold increased risk of diabetes mellitus (Table 3). Our analyses included 48 sister pairs and one set of three sisters among the 1801 daughters. To evaluate the potential contribution of siblings to our analyses, we also restricted analyses to the subset of non-siblings and the subset of non-siblings plus one randomly selected sister from each family. These associations were the same (data not shown) as those reported in Table 3. In order to determine whether these unadjusted associations were robust to confounding and known risk factors, variables in Table 1 were considered for inclusion in the adjusted multivariable models presented in Table 3. After adjustment by other significant variables (parental race, parental diabetes, parental professional employment), both maternal and paternal tobacco smoking continued to be associated with increased risk of daughters’ diabetes mellitus (Table 3). Paternal smoking had a marginally significant association with daughters’ diabetes mellitus risk. However, there was no evidence of a further increase in daughters’ risk of diabetes mellitus when both parents smoked compared with the risk when only mothers smoked.
Table 3.
Association between parental tobacco use during gestation and diabetes mellitus in 1801 women
| No parental tobacco | Maternal tobacco | Paternal tobacco | Parental tobacco | |
|---|---|---|---|---|
| Number of diabetics | 18 | 11 | 39 | 41 |
| Number at risk | 588 | 105 | 580 | 419 |
| RR (95% CI) | 1.0 (Reference) | 3.2 (1.5–6.6) | 2.1 (1.2–3.7) | 3.0 (1.8–5.2) |
| P-value | <0.01 | <0.01 | <0.0001 | |
| aRR (95% CI)a | 1.0 (Reference) | 2.7 (1.3–5.4) | 1.7 (1.0–3.0) | 2.4 (1.4–4.1) |
| P-value | <0.01 | 0.05 | <0.01 | |
| aRR (95% CI)b | 1.0 (Reference) | 2.6 (1.2–5.3) | 1.7 (1.0–3.0) | 2.4 (1.4–4.1) |
| P-value | <0.05 | <0.05 | <0.01 | |
| aRR (95% CI)c | 1.0 (Reference) | 2.7 (1.4–5.3) | 1.7 (1.0–2.8) | 2.2 (1.3–3.7) |
| P-value | <0.01 | 0.06 | <0.01 |
Adjusted for parental African American race, parental diabetes and parental professional employment.
Adjusted for parental African American race, parental diabetes, parental professional employment and index case birth weight categories.
Adjusted for parental African American race, parental diabetes, parental professional employment and index case body mass index categories.
The associations between parental tobacco smoking and daughters’ diabetes risk were unchanged when birth weight was added to the final adjusted models, suggesting that the effect of maternal tobacco smoking on birth weight (Table 1) did not mediate the association of maternal tobacco use and daughters’ diabetes mellitus (Table 3). We observed an increase in adult daughters’ BMI associated with paternal smoking (Table 1); however, associations between parental smoking and daughters’ diabetes were unaltered after adjusting for daughters’ BMI (Table 3). This suggests that the effect of paternal tobacco use on daughters’ diabetes risk was not mediated by daughters’ BMI. In all adjusted models, paternal smoking was associated with a 70% increased risk of daughters’ diabetes mellitus that hovered around significance, whereas maternal smoking was associated with a significant two- to three-fold risk of diabetes mellitus among daughters. A post-hoc power calculation for our sample scenario estimated a 43% probability to detect an effect size of 70% or greater at a significance threshold of α = 0.05.
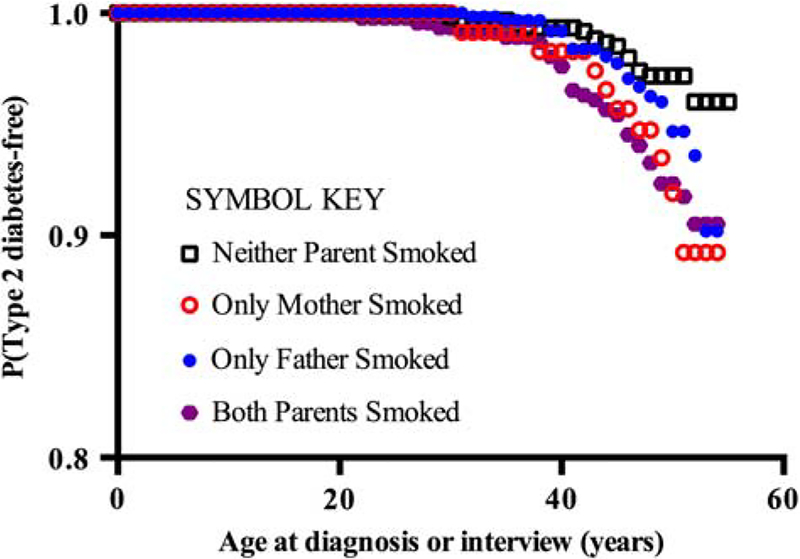
To confirm that the results shown in Table 3 were not an artifact of case definition, the adjusted model of Table 3 was applied to two alternative definitions of diabetes. When only cases of self-reported diabetes were included in our case definition [misclassifying 10 cases where A1C ⩾ 6.5% (47.5 mmol/mol), Table 2], the aRRs were essentially unchanged (aRR = 2.6 [95% CI 1.2–5.5] P < 0.05, 1.7 [95% CI 1.0–3.0] P = 0.07 and 2.2 [95% CI 1.2–3.8] P < 0.01 for maternal, paternal and parental tobacco use, respectively). Similarly, when only cases of self-reported type 2 diabetes were included in our case definition (ignoring self-reported type 1 diabetes and A1C), the aRRs were essentially the same as reported in Table 3 (aRR = 2.3 [95% CI 1.0–5.0] P < 0.05, 1.3 [95% CI 0.7–2.4] P = 0.3 and 2.1 [95% CI 1.2–3.7] P <0.05 for maternal, paternal and parental tobacco use, respectively). There was no evidence of time dependence among the association of parental tobacco smoking and the age of onset of self-reported type 2 diabetes (Fig. 1). There were too few cases of self-reported type 1 diabetes to assess a potential time-dependent association with parental tobacco smoking.
Fig. 1.
Probability of reporting physician-diagnosed type 2 diabetes as a function of age and parental tobacco smoking during gestation.
Discussion
We prospectively assessed over 1800 women to evaluate the association between diabetes mellitus and parental tobacco smoking during gestation. Daughters’ risk of diabetes mellitus was increased in association with either both parents smoking or only the mother smoking during gestation. This association supports the hypothesis that parental tobacco smoking is associated with increased risk of diabetes mellitus in adult offspring, and is consistent with the hypothesis that prenatal environmental chemical exposures independent of birth weight may contribute to the developmental origins of health and disease.13,36 Further, this is the first study to demonstrate that parental smoking during gestation is associated with increased risk of diabetes mellitus independent of BMI.17,18 This novel finding is biologically plausible, as rodents prenatally exposed to nicotine have a permanent loss of pancreatic β cells commencing at birth,37 before the occurrence of increased body and/or fat weight in rodents with prenatal nicotine or cigarette exposure.10
There are limited number of studies that have evaluated the association of maternal tobacco smoking during gestation with risk of offspring diabetes, and none of these studies isolated the sole effects of maternal smoking separately from the co-occurrence of paternal smoking or other prenatal ETS exposures.10,18 Further, the majority (85%) of these studies5,18,38–48 evaluated births occurring after the 1964 Surgeon General’s Report, which was the pivotal report that warned against smoking and initiated the change in smoking attitudes.29,30 Thus, the negative findings of these studies may be due to misclassification bias associated with under-reporting of parental smoking exposure during gestation.19–21 In contrast, the CHDS enrollment period largely predated public health anti-smoking campaigns for women, and, as expected, self-reported tobacco smoking is consistent with serum cotinine levels in the CHDS cohort.27 We suggest that our study is more robust to misclassification bias associated with response to anti-smoking public health campaigns.29,30
All other known studies of prenatal smoking associations with offspring diabetes that were also conducted before the Surgeon General Report were conducted in the United Kingdom. The majority of these studies also found an increased risk of offspring diabetes mellitus associated with maternal smoking during gestation.17,22,49,50 Similar to the results reported here, the National Child Development Study of the United Kingdom found an increased risk of type 2 diabetes among offspring of mothers who smoked.49 Another study predating the Surgeon General warning reported that the significant association between prenatal maternal smoking and type 2 diabetes was mediated by the birth weight and BMI of offspring;17 however, birth weight and BMI did not appear to be mediators of the association between prenatal maternal smoking and diabetes risk in this study. These discrepancies may reflect our inclusion of paternal smoking and all cases of diabetes mellitus.
The effect of paternal smoking (risk increased by 70%) was smaller than that seen for maternal smoking (risk increased by 170%). Although we were underpowered to find a modest paternal effect, we observed one with marginal statistical significance. Thus, we cannot exclude the possibility that paternal smoking has a small effect on daughters’ diabetes risk. One larger study ascertaining paternal smoking status in 2001 found a smaller association between paternal smoking and diabetes risk, which is consistent with the 95% CI we observed in this study.18 In contrast, two European studies spanning the release of the Surgeon General’s 1964 Report found a decreased risk of type 1 diabetes associated with paternal smoking.22,23 Although we were also underpowered to evaluate small associations of parental smoking and offspring type 1 diabetes, it is notable that the majority of our type 1 diabetes cases were born in households where only fathers smoked. Further, we do not have information on postnatal smoke exposure, and therefore cannot rule out a contribution of postnatal smoking to associations reported in this study. Although few human cohorts are positioned to confirm our results, larger birth cohorts that collected data on paternal tobacco smoking during gestation before the Surgeon General warning should seek to confirm the association of prenatal paternal tobacco use and offspring diabetes that we observed, and should attempt to distinguish whether such an association applies to all sources of ETS exposure during prenatal and postnatal periods.
We were limited in our ability to detect all diabetes mellitus cases, given that we did not measure A1C in all participants, and were unable to measure levels of auto-antibodies. Consistent with misclassification bias, only 6.1% of the women of this study had diabetes mellitus, compared with 15% physician-diagnosed diabetes between 2007 and 2010 among 45- to 64-year-old women of the US population. Although our lower diabetes prevalence could also be explained by our lower obesity prevalence (26.4 here v. 38.3% obesity among 45- to 54-year-old women of the United States in 2009–2012),51 any bias in the classification of our diabetes mellitus cases would likely cause the risk estimates reported here to be smaller than their true size. Although the analysis sample was different from the CHDS cohort as a whole because it included fewer daughters with high-risk characteristics, this weakness would likely bias toward not finding an association with tobacco use.
From a public health perspective, reduced fetal ETS exposure appears to be an important modifiable risk factor for diabetes mellitus in offspring. Medical doctors should consider advising pregnant smokers that emerging research suggests that tobacco smoking cessation at home may benefit offspring by reducing their risk of developing diabetes mellitus, independent of the effects of adult BMI or birth weight on diabetes risk. Given that we were underpowered to significantly resolve a paternal smoking association with offspring diabetes risk and few studies conducted before the Surgeon General warning have designs capable of confirming our marginal paternal smoking association with risk of offspring diabetes mellitus, caution toward smoking near pregnant women is also warranted in order to prevent diabetes mellitus.
Acknowledgments
The authors thank Mr Gary Bradwin of the Clinical and Epidemiological Research Laboratory of the Boston Children’s Hospital for quantitating hemoglobin A1C. The authors also thank the members of the CHDS and 3Gs Study for their participation and the CHDS staff, the CHDS Participant Advisory Council and the 3Gs External Advisory Council for their support and work on this study.
Financial Support
This research was supported through funding by the National Institute of Environmental Health Sciences (M.L., R00 ES019919), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (B.C., HHSN275 201100020C) and The California Breast Cancer Research Program Special Research Initiative (B.C., 15ZB - 0186).
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation (i.e. the Belmont Report), the federal regulations found at 45 CFR part 46 et seq and has been approved by the institutional committee (i.e. the institutional review board of the Public Health Institute).
References
- 1.United States. Public Health Service. Office of the Surgeon General. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. 2006. US Department of Health and Human Services, Public Health Service, Office of the Surgeon General: Rockville, MD. [Google Scholar]
- 2.Henkin L, Zaccaro D, Haffner S, et al. Cigarette smoking, environmental tobacco smoke exposure and insulin sensitivity: the Insulin Resistance Atherosclerosis Study. Ann Epidemiol. 1999; 9, 290–296. [DOI] [PubMed] [Google Scholar]
- 3.Kowall B, Rathmann W, Strassburger K, et al. Association of passive and active smoking with incident type 2 diabetes mellitus in the elderly population: the KORA S4/F4 cohort study. Eur J Epidemiol. 2010; 25, 393–402. [DOI] [PubMed] [Google Scholar]
- 4.Weitzman M, Cook S, Auinger P, et al. Tobacco smoke exposure is associated with the metabolic syndrome in adolescents. Circulation. 2005; 112, 862–869. [DOI] [PubMed] [Google Scholar]
- 5.Thiering E, Bruske I, Kratzsch J, et al. Prenatal and postnatal tobacco smoke exposure and development of insulin resistance in 10 year old children. Int J Hyg Environ Health. 2011; 214, 361–368. [DOI] [PubMed] [Google Scholar]
- 6.Houston TK, Person SD, Pletcher MJ, et al. Active and passive smoking and development of glucose intolerance among young adults in a prospective cohort: CARDIA study. BMJ. 2006; 332, 1064–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.La Merrill M, Stein CR, Landrigan P, Engel SM, Savitz DA. Prepregnancy body mass index, smoking during pregnancy, and infant birth weight. Ann Epidemiol. 2011; 21, 413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leonardi-Bee J, Smyth A, Britton J, Coleman T. Environmental tobacco smoke and fetal health: systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2008; 93, F351–F361. [DOI] [PubMed] [Google Scholar]
- 9.El-Mohandes AA, Kiely M, Blake SM, Gantz MG, El-Khorazaty MN. An intervention to reduce environmental tobacco smoke exposure improves pregnancy outcomes. Pediatrics. 2010; 125, 721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behl M, Rao D, Aagaard K, et al. Evaluation of the association between maternal smoking, childhood obesity, and metabolic disorders: a national toxicology program workshop review. Env Health Perspect. 2013; 121, 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattsson K, Kallen K, Longnecker MP, Rignell-Hydbom A, Rylander L. Maternal smoking during pregnancy and daughters’ risk of gestational diabetes and obesity. Diabetologia. 2013; 56, 1689–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris HR, Willett WC, Michels KB. Parental smoking during pregnancy and risk of overweight and obesity in the daughter. Int J Obes (Lond). 2013; 37, 1356–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hales CN, Barker DJ, Clark PM, et al. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991; 303, 1019–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poulsen P, Vaag AA, Kyvik KO, Moller Jensen D, Beck-Nielsen H. Low birth weight is associated with NIDDM in discordant monozygotic and dizygotic twin pairs. Diabetologia. 1997; 40, 439–446. [DOI] [PubMed] [Google Scholar]
- 15.Lindsay RS, Dabelea D, Roumain J, et al. Type 2 diabetes and low birth weight: the role of paternal inheritance in the association of low birth weight and diabetes. Diabetes. 2000; 49, 445–449. [DOI] [PubMed] [Google Scholar]
- 16.Narayan KM, Boyle JP, Thompson TJ, Gregg EW, Williamson DF. Effect of BMI on lifetime risk for diabetes in the U.S. Diabetes Care. 2007; 30, 1562–1566. [DOI] [PubMed] [Google Scholar]
- 17.Thomas C, Hypponen E, Power C. Prenatal exposures and glucose metabolism in adulthood: are effects mediated through birth weight and adiposity? Diabetes Care. 2007; 30, 918–924. [DOI] [PubMed] [Google Scholar]
- 18.Jaddoe VW, de Jonge LL, van Dam RM, et al. Fetal exposure to parental smoking and the risk of type 2 diabetes in adult women. Diabetes Care. 2014; 37, 2966–2973. [DOI] [PubMed] [Google Scholar]
- 19.Webb DA, Boyd NR, Messina D, Windsor RA. The discrepancy between self-reported smoking status and urine cotinine levels among women enrolled in prenatal care at four publicly funded clinical sites. J Public Health Manag Pract. 2003; 9, 322–325. [DOI] [PubMed] [Google Scholar]
- 20.Britton GR, Brinthaupt J, Stehle JM, James GD. Comparison of self-reported smoking and urinary cotinine levels in a rural pregnant population. J Obstet, Gynecol Neonatal Nurs. 2004; 33, 306–311. [DOI] [PubMed] [Google Scholar]
- 21.Shipton D, Tappin DM, Vadiveloo T, et al. Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: a retrospective, cross sectional study. BMJ. 2009; 339, b4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toschke AM, Ehlin A, Koletzko B, Montgomery SM. Paternal smoking is associated with a decreased prevalence of type 1 diabetes mellitus among offspring in two national British birth cohort studies (NCDS and BCS70). J Perinat Med. 2007; 35, 43–47. [DOI] [PubMed] [Google Scholar]
- 23.Rasouli B, Grill V, Midthjell K, et al. Risk of autoimmune diabetes in adults contrasting with increased risk in overweight men with type 2 diabetes: a 22-year follow-up of the Hunt study. Diabetes Care. 2013; 36, 604–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwok MK, Schooling CM, Lam TH, Leung GM. Paternal smoking and childhood overweight: evidence from the Hong Kong ‘Children of 1997’. Pediatrics. 2010; 126, e46–e56. [DOI] [PubMed] [Google Scholar]
- 25.von Kries R, Bolte G, Baghi L, Toschke AM. Parental smoking and childhood obesity – is maternal smoking in pregnancy the critical exposure? Int J Epidemiol. 2008; 37, 210–216. [DOI] [PubMed] [Google Scholar]
- 26.Leary SD, Smith GD, Rogers IS, et al. Smoking during pregnancy and offspring fat and lean mass in childhood. Obesity (Silver Spring). 2006; 14, 2284–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.English PB, Eskenazi B, Christianson RE. Black-white differences in serum cotinine levels among pregnant women and subsequent effects on infant birthweight. Am J Public Health. 1994; 84, 1439–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van den Berg BJ, Christianson RE, Oechsli FW. The California Child Health and Development Studies of the School of Public Health, University of California at Berkeley. Paediatr Perinat Epidemiol. 1988; 2, 265–282. [DOI] [PubMed] [Google Scholar]
- 29.Housman MG. Smoking and health: the 1964 Surgeon General’s report as a turning point in the anti-smoking movement. Harv Health Policy Rev. 2001; 2, 118–126. [Google Scholar]
- 30.Proctor RN. The history of the discovery of the cigarette-lung cancer link: evidentiary traditions, corporate denial, global toll. Tob Control. 2012; 21, 87–91. [DOI] [PubMed] [Google Scholar]
- 31.National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults – the evidence report. Obes Res. 1998; 6(Suppl. 2), 51S–209S. [PubMed] [Google Scholar]
- 32.Boulet SL, Alexander GR, Salihu HM, Pass M. Macrosomic births in the United States: determinants, outcomes, and proposed grades of risk. Am J Obstet Gynecol. 2003; 188, 1372–1378. [DOI] [PubMed] [Google Scholar]
- 33.Wardlaw TM, World Health Organization, UNICEF. Low Birthweight: Country, Regional and Global Estimates. 2004. WHO and UNICEF: Geneva and New York, NY. [Google Scholar]
- 34.Chiavaroli V, Giannini C, D’Adamo E, et al. Insulin resistance and oxidative stress in children born small and large for gestational age. Pediatrics. 2009; 124, 695–702. [DOI] [PubMed] [Google Scholar]
- 35.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012; 35(Suppl. 1), S64–S71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freinkel N Banting lecture 1980. Of pregnancy and progeny. Diabetes. 1980; 29, 1023–1035. [DOI] [PubMed] [Google Scholar]
- 37.Bruin JE, Kellenberger LD, Gerstein HC, Morrison KM, Holloway AC. Fetal and neonatal nicotine exposure and postnatal glucose homeostasis: identifying critical windows of exposure. J Endocrinol. 2007; 194, 171–178. [DOI] [PubMed] [Google Scholar]
- 38.Johansson A, Hermansson G, Ludvigsson J, ABIS Study Group. Tobacco exposure and diabetes-related autoantibodies in children: results from the ABIS Study. Ann N Y Acad Sci. 2008; 1150, 197–199. [DOI] [PubMed] [Google Scholar]
- 39.Wahlberg J, Vaarala O, Ludvigsson J. Group as Asthma and allergic symptoms and type 1 diabetes-related autoantibodies in 2.5-yr-old children. Pediatr Diabetes. 2011; 12, 604–610. [DOI] [PubMed] [Google Scholar]
- 40.Hummel M, Schenker M, Ziegler AG. Influence of perinatal factors on the appearance of islet autoantibodies in offspring of parents with type 1 diabetes. Pediatr Diabetes. 2001; 2, 40–42. [DOI] [PubMed] [Google Scholar]
- 41.Dahlquist G, Kallen B. Maternal-child blood group incompatibility and other perinatal events increase the risk for early-onset type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1992; 35, 671–675. [DOI] [PubMed] [Google Scholar]
- 42.Marshall AL, Chetwynd A, Morris JA, et al. Type 1 diabetes mellitus in childhood: a matched case control study in Lancashire and Cumbria, UK. Diabet Med. 2004; 21, 1035–1040. [DOI] [PubMed] [Google Scholar]
- 43.Robertson L, Harrild K. Maternal and neonatal risk factors for childhood type 1 diabetes: a matched case-control study. BMC Public Health. 2010; 10, 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenbauer J, Herzig P, Kaiser P, Giani G. Early nutrition and risk of type 1 diabetes mellitus – a nationwide case-control study in preschool children. Exp Clin Endocrinol Diabetes. 2007; 115, 502–508. [DOI] [PubMed] [Google Scholar]
- 45.Sipetic SB, Vlajinac HD, Kocev NI, et al. The Belgrade Childhood Diabetes Study: a multivariate analysis of risk determinants for diabetes. Eur J Public Health. 2005; 15, 117–122. [DOI] [PubMed] [Google Scholar]
- 46.Svensson J, Carstensen B, Mortensen HB, Borch-Johnsen K, Danish Study Group of Childhood Diabetes. Early childhood risk factors associated with type 1 diabetes – is gender important? Eur J Epidemiol. 2005; 20, 429–434. [DOI] [PubMed] [Google Scholar]
- 47.Horta BL, Gigante DP, Nazmi A, et al. Maternal smoking during pregnancy and risk factors for cardiovascular disease in adulthood. Atherosclerosis. 2011; 219, 815–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cupul-Uicab LA, Skjaerven R, Haug K, et al. in utero exposure to maternal tobacco smoke and subsequent obesity, hypertension, and gestational diabetes among women in the MoBa cohort. Env Health Perspect. 2012; 120, 355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montgomery SM, Ekbom A. Smoking during pregnancy and diabetes mellitus in a British longitudinal birth cohort. BMJ. 2002; 324, 26–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Power C, Atherton K, Thomas C. Maternal smoking in pregnancy, adult adiposity and other risk factors for cardiovascular disease. Atherosclerosis. 2010; 211, 643–648. [DOI] [PubMed] [Google Scholar]
- 51.Health, United States. Special feature on prescription drugs, 2014. Statistics NCfH, Hyattsville, MD. [PubMed] [Google Scholar]