Abstract
Background
Chemoradiotherapy (CRT) is the standard treatment for patients with inoperable stage III non‐small cell lung cancer (NSCLC) stage III. With a median OS beyond 30 months, adequate pulmonary function (PF) is essential to ensure acceptable quality of life after treatment. Forced expiratory volume in 1 second (FEV1) and diffusing capacity of the lung for carbon monoxide (DLCO) are the most widely used parameters to assess lung function. The aim of the current study was to evaluate dose‐volume effects of accelerated high‐dose radiation on PF.
Methods
A total of 72 patients were eligible for the current analysis. After induction chemotherapy, all patients received dose‐differentiated accelerated radiotherapy with intensity‐modulated radiotherapy (IMRT‐DART). PF tests were performed six weeks, three and six months after the end of radiotherapy.
Results
The median total dose to the tumor was 73.8 Gy (1.8 Gy bid) with a size dependent range between 61.2 and 90 Gy. In the whole cohort, 321 pulmonary function tests were performed. At six months, the median FEV1 relative to baseline was 0.95 (range: 0.56–1.36), and the relative median DLCO decreased to 0.98 (range: 0.64–1.50). The correlation between V20total lung and FEV1 decline was statistically significant (P = 0.023). A total of 13 of 34 (38%) COPD patients had a 4%–21% FEV1 decrease.
Conclusion
Patients with a V20total lung < 21% are at a low risk for PF decrease after high dose irradiation treatment. Although overall short term FEV1 and DLCO differ only moderately from baseline these changes may be clinically important, especially in patients with COPD.
Key points
Significant findings:
Pulmonary function after high dose irradiation decreases only moderately.
FEV1 and DLCO decrease depend on V20total lung.
Small differences in lung function may be clinically important for COPD patients.
KPS predicts minimal clinically important differences (MCID).
What this study adds:
This study shows that high‐dose irradiation delivered with intensity‐modulated techniques does not impair short‐term lung function even in patients with compromised respiratory capacity before treatment. This is a pre‐requisite for adequate quality of life after thoraco‐oncological therapy.
Keywords: DLCO, FEV1, minimal clinically important difference (MCID), NSCLC, radiotherapy
Introduction
Chemoradiotherapy (CRT) is the standard treatment for patients with inoperable stage III non‐small cell lung cancer (NSCLC). In the past two decades, concomitant regimens1, 2, 3, 4, 5 achieved local control (LC) rates of 55–70% and a median overall survival (OS) beyond 30 months.5 Some single center studies on dose escalation strategies presented similar results with respect to LC6 and OS.7 With improved outcome, the maintenance of an adequate pulmonary function (PF) is essential to ensure acceptable quality of life after treatment.
The limited number of analyses on post‐radiotherapy (RT) PF reveals inconclusive results. While one would expect a diminishing of lung function after RT, some study groups report an improvement.8 This somewhat counterintuitive observation might be caused by tumor and atelectasis retraction after RT, which allows for better unfolding of physiologically active lung tissue.8 In contrast to most reports on PF after RT which focus on breast cancer, lymphoma9, 10 and esophageal cancer,11 in lung cancer patients the disease itself may impair lung function. Thus, the differentiation between post‐treatment effects and respiratory symptoms caused by cancer and pulmonary comorbidities may be difficult. It seems that especially patients with impaired lung function before treatment are at higher risk for decreased PF thereafter.12 Since radiographic changes do not always correlate with clinical symptoms,11, 13 pulmonary function tests (PFT) are a better surrogate to assess residual lung function than CT scans.
It is still a matter of debate which of the parameters from the panel of PFTs is the best to estimate post‐RT lung function. Forced expiratory volume in 1 second (FEV1) and diffusing capacity of the lung for carbon monoxide (DLCO) are most widely used. Radiation causes inflammatory response and pneumocyte desquamation, followed by hyaline membrane formation and endothelial cell damage in the pulmonary vessels.12 The next step is deposition of collagen14 leading to chronic fibrotic changes. Since post‐RT effects in lung tissue mainly occur in the alveolar compartment and less in the airway system, DLCO may be the more appropriate PF marker after RT.15, 16
Additionally, pulmonary comorbidity such as COPD plays an important role since early inflammatory reactions could be more severe in these patients.12 Therefore, decreases of a few percent in FEV1 may constitute a minimal clinically important difference (MCID) for COPD patients.11, 17, 18, 19 Hence, minimizing the radiation dose to normal lung tissue by advanced irradiation techniques such as intensity‐modulated radiotherapy (IMRT) is crucial for patients who already present with impaired lung function before treatment.20 The risk for radiation induced lung injury (RILI) increases with concomitant chemoradiotherapy (cCRT),21 inclusion of taxanes in the systemic treatment22 and tumor location in the lower lobes.23, 24
The aim of the current study was to quantify and correlate the effect of high dose RT on pulmonary function measured by FEV1 and DLCO within six months after the end of treatment.
Methods
Patients
Between January 2015 and December 2018, 138 patients were treated for stage III NSCLC (eighth edition of the TNM staging system) after discussion in the interdisciplinary tumor board comprising pulmonologists, medical oncologists, radiologists, thoracic surgeons and radiation‐oncologists. Patient data were collected within a prospective study setting, which was approved by the local ethics committee. A total of 72 patients were eligible for the current analysis, which was approved by the local ethics committee. A total of 62 patients were excluded for one of the following reasons: comorbidities without a curative treatment option (19), previous cancer within five years (nine), unclear pathology (three) or pleural effusion (five), age > 85 years (two), impaired lung function (four), no induction chemotherapy (10), and delayed referral by peripheral hospital (10). One patient refused twice daily treatment with accelerated radiotherapy, and another who was referred by an external hospital, asked to be followed‐up there. Two patients died shortly after diagnosis before start of any treatment. The diagnostic work‐up for each patient required 18F‐FDG‐PET‐CT, cranial MRT, bronchoscopy and endobronchial ultrasound‐guided transbronchial needle aspiration (EBUS‐TBNA) in the mediastinum. Pulmonary function was assessed before radiation treatment by body plethysmography, blood gas analysis and DLCO. Similar to Schytte et al.8 we observed a large variation in PF before RT. Hence, follow‐up PFT values were normalized to each patient's baseline values. This means that if the ratio was exactly 1.00 the patient had precisely the same PFT at follow‐up compared to baseline. PFTs were repeated at the end of the treatment course and at each follow‐up visit; that is, at six weeks, three and six months after completion of RT.
Chemoradiotherapy
After induction chemotherapy, all patients received dose‐differentiated accelerated radiotherapy with intensity modulated radiotherapy (IMRT‐DART). The details of this treatment approach were described elsewhere.6 In brief, the cornerstones of this regimen are the sequential chemoradiation mode, the dose increments from 73.8 to 90 Gy depending on tumor size and twice daily treatment with 1.8 Gy per fraction. In the current study, however, the former conventional 3D‐target splitting technique was replaced by IMRT. As of 2017, ultracentrally located tumors with invasion of the central vessels or airways in pretreatment contrast enhanced thoracic CT scan were treated ‐ regardless of size ‐ with a total dose of 61.2 or 73.8 Gy in order to reduce the risk of lethal hemorrhage due to rapid tumor regression.24 Involved lymph nodes received 54–61.2 Gy in twice daily fractions of 1.8 Gy each. The nodes next to the involved area were treated with 1.4 Gy bid to a total dose of 47.6 Gy. As for dose constraints, the following limits were applied24, 25, 26, 27, 28: mean lung dose (MLD) < 20 Gy, V25total lung < 30%, V20ipsilateral lung < 50%, mean esophageal dose (MED) < 34 Gy, maximum dose to the spinal cord 45 Gy (maximum dose of 1.3 Gy per fraction), V25heart < 10%. In order to mitigate potential esophageal toxicity, all patients received local antimycotic prophylaxis.29 Radiotherapy planning was performed when patients received the second cycle of systemic treatment. Induction chemotherapy consisted of two cycles of either Cisplatinum or Carboplatinum combined with Pemetrexed, Gemcitabine or Vinorelbine according to histology. As of September 2017, patients received Durvalumab maintenance therapy for one year after the end of radiotherapy.5, 30
Follow‐up
Follow‐up was performed six weeks, three and six months after the end of radiotherapy including clinical investigation, contrast enhanced thoracic CT scan and PFTs as described above. In cases of suspicious local relapse a PET‐CT scan combined with a rebiopsy was performed. PFTs were stopped when the patient developed pulmonary progression, which could potentially impair lung function, such as pleural effusion, multiple metastases or lymphangiosis. Toxicity was reported using CTCAE 4.03. Hence, pneumonitis grade 2 was defined as pulmonary symptoms (eg, cough, dyspnea) limiting instrumental activity in daily life (IADL) that required the administration of steroids. Pneumonitis grade 1, which corresponded to radiographic changes in the follow‐up CT scan only, was not assessed. Dysphagia and odynophagia that required the administration of nonsteroidal analgesics and opioids were scored as grade 2 and 3 respectively. The endpoint of the current analysis was pulmonary function six months after the end of radiotherapy.
Statistical analysis
Clinical outcome was calculated with the Kaplan‐Meier method as of the end of radiotherapy. For comparison of subgroups, the log‐rank test was used. Multivariate analysis (MVA) was performed with the Cox‐regression (forward stepwise). The Pearson test was applied in order to detect correlations. Statistics were calculated in SPSS version 24.
Results
Patient and treatment characteristics
A total of 72 patients with histologically or cytologically proven stages IIIa to IIIc NSCLC were eligible for the current analysis. The median age was 66 years (range 29–82 years) including 50 (69%) men and 22 (31%) women. Nine patients (12%) had a weight loss of more than 5% before treatment. The median KPS was 90 (range 50–100) and the median Charlson comorbidity index (CCI) was five (range 2–9). For baseline patient characteristics see Table 1.
Table 1.
Patient and treatment characteristics
| Patient and treatment characteristics N = 72 | ||
|---|---|---|
| Gender | ||
| Male | 50 | 69% |
| Female | 22 | 31% |
| Age (years) | ||
| Median | 66 | x |
| Range | 29–82 | |
| Weight loss | ||
| <5% | 63 | 88% |
| >5% | 9 | 12% |
| KPS | ||
| Median | 90 | x |
| Range | 50–100 | |
| T‐stage | ||
| Tx | 1 | 1% |
| T1 | 20 | 28% |
| T2 | 19 | 26% |
| T3 | 14 | 20% |
| T4 | 18 | 25% |
| N‐stage | ||
| N0 | 1 | 1% |
| N1 | 8 | 11% |
| N2 | 49 | 68% |
| N3 | 14 | 20% |
| UICC | ||
| IIIa | 51 | 71% |
| IIIb | 20 | 28% |
| IIIc | 1 | 1% |
| Tumor volume (mL) | ||
| Median | 15 | x |
| Range | 1–183 | |
| Tumor location | ||
| Upper lobe | 48 | 67% |
| Middle lobe | 6 | 8% |
| Lower lobe | 18 | 25% |
| Peripheral | 43 | 60% |
| Central | 29 | 40% |
| Smoking status | ||
| Unknown | 2 | 2% |
| Current smoker | 45 | 63% |
| Ex smoker | 22 | 31% |
| Never smoker | 3 | 4% |
| CCI | ||
| Median | 5 | x |
| Range | 2–9 | |
| Induction chemotherapy with platinum doublet | n | 72 |
| Cycles | 2 | |
| Radiation therapy | ||
| Radiation technique | IMRT | 67 |
| VMAT | 5 | |
| Tumor dose (Gy) | Median | 73.8 |
| Range | 61.2–90 | |
| Lymph node dose (Gy) | Median | 61.2 |
| Range | 54–61.2 | |
| ENI (Gy) | Median | 47.6 |
| Range | 0–47.6 | |
| V20 ipsilateral lung (%) | Median | 36.5 |
| Range | 18–53 | |
| V20 total lung (%) | Median | 21 |
| Range | 11–35 | |
| V25 total lung (%) | Median | 16 |
| Range | 8–25 | |
| Mean lung dose (Gy) | Median | 12.3 |
| Range | 7–18 | |
| Maximum esophageal dose (Gy) | Median | 63 |
| Range | 26–81 | |
| Mean esophageal dose (Gy) | Median | 23 |
| Range | 6–34 | |
KPS, Karnofsky performance score; CCI, Charlson comorbidity index: ENI, elective nodal irradiation.
All patients received two cycles of platinum‐based induction chemotherapy before accelerated radiation treatment, which was delivered by step‐and‐shoot IMRT (ss‐IMRT) or VMAT. The median tumor dose was 73.8 Gy (range 61.2–90 Gy). Four patients received 61.2 Gy due to tumor invasion of central bronchi or vessels. Involved lymph nodes received a median dose of 61.2 Gy (54–61.2 Gy), while elective lymph nodes were irradiated with a median total dose of 47.6 (range 0–47.6 Gy). The median values for V20total lung, V20ipsilateral lung, V25total lung and MLD were 21% (range 11%–35%), 37% (18%–53%), 16% (8%–25%) and 12.3 Gy (7–18 Gy) respectively. The median MED was 23 Gy (6–34 Gy). Treatment details are summarized in Table 1.
Clinical outcome
With a median follow‐up of 15.8 months (range 0.3–50.5 months) 46/72 (64%) patients were still alive. The one‐year OS and the LC rates were 77% and 70%, respectively (Fig 1a,b). The actuarial median OS was 42.1 months (95% CI: 23.0–61.2 months). A total of 20 patients (27%) died from tumor progression and three (5%) from other causes (cardiac failure and multiorgan failure). One patient (1%) was lost to follow‐up. Two patients died within three months after the end of radiation treatment. The CT scan showed progressive fibrotic changes suspicious of acute pneumonitis. In one patient with a middle lobe tumor, the maximum of the structural changes on the follow‐up CT occurred in the lower lobe of the contralateral lung. This patient had a history of diabetes and cardiac disorders, which were stable when CRT started. The pretreatment FEV1 was 2.5 L corresponding to 74% of the expected value. MLD and V25heart was 16 Gy and 31%, respectively. The heart constraint was transgressed because of the unfavorable tumor location. The second patient was admitted to the clinic for acute cardiac syndrome six weeks after the end of the radiotherapy course. At the same time thrombosis of the pulmonary arteries in both lower lobes was diagnosed. The pretreatment FEV1 in this patient was 2.7 L (88% of the expected value), the MLD and V25heart were 15.8 Gy and 8%, respectively. In both cases radiation induced pneumonitis as a cause of death could not be entirely excluded, hence they were scored as having grade 5 toxicity (Table 2). Grade 2 pneumonitis requiring cortisone treatment occurred in 3/72 patients (4.2%), clinically relevant esophagitis (grade 2 and 3) was diagnosed in 28/72 patients (38.9%).
Figure 1.
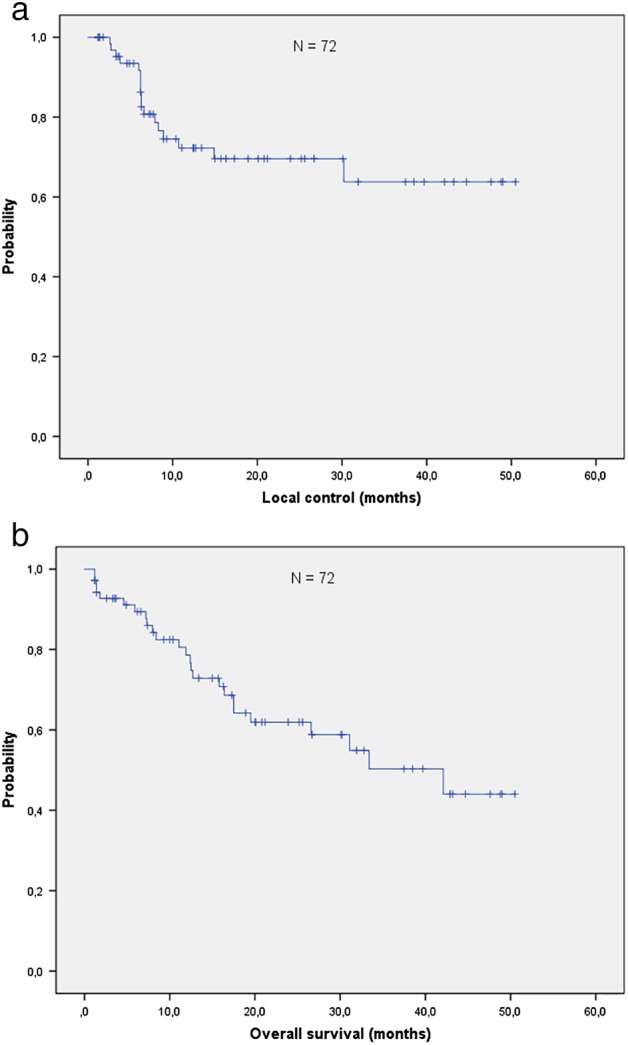
(a) Local control. (b) Overall survival.
Table 2.
Clinical outcome in patients with pneumonitis and esophagitis
| Treatment related toxicity | |||
|---|---|---|---|
| Grade | N | % | |
| Pneumonitis | 2 | 3 | 4.2% |
| 3 | 3 | 0.0% | |
| 4 | 0 | 0.0% | |
| 5 | 2 | 2.8% | |
| Esophagitis | 2 | 20 | 27.8% |
| 3 | 8 | 11.1% | |
| 4 | 0 | 0.0% | |
| 5 | 0 | 0.0% | |
Decreases in pulmonary function are moderate
Within a period of six months, 321 PFTs were performed in 72 patients. A total of 55 of the 72 (76%) patients completed PFTs at six months because 10 patients had died intercurrently, and seven had a shorter follow‐up. The box plot in Figure 2a shows that the median FEV1 at six months relative to baseline was 0.95 (range: 0.56–1.36) in the whole cohort. At the same point of time, the relative median DLCO decreased to 0.98 (range: 0.64–1.50) (Fig 3a). FEV1 measurements differed significantly between six months and the end of RT (two‐sided Pearson correlation, P‐value = 0.000; Fig 2a). A comparison by quartiles of FEV1 decrease revealed that individuals with the best PFTs at six months (= quartile 4) had a significantly lower V20total lung compared to the rest of the study population (Mann‐Whitney‐U test, P‐value = 0.000; Fig 2b). The median V20total lung in this group was 18% (range 11%–29%), while the overall median in quartiles 1 to 3 was 22% (range 13%–35%). As for DLCO, the difference between six months and the end of RT showed a trend (two‐sided Pearson correlation P‐value = 0.069). The comparison by quartiles DLCO decrease however revealed no statistically significant difference (Mann‐Whitney‐U test P‐value = 0.687; Fig 3b). The three patients with grade 2 pneumonitis had diverging changes in FEV1 at six months. In two patients, the decrease relative to baseline was 17% and 3%, respectively, while the third patient showed a relative increase of 28%.
Figure 2.
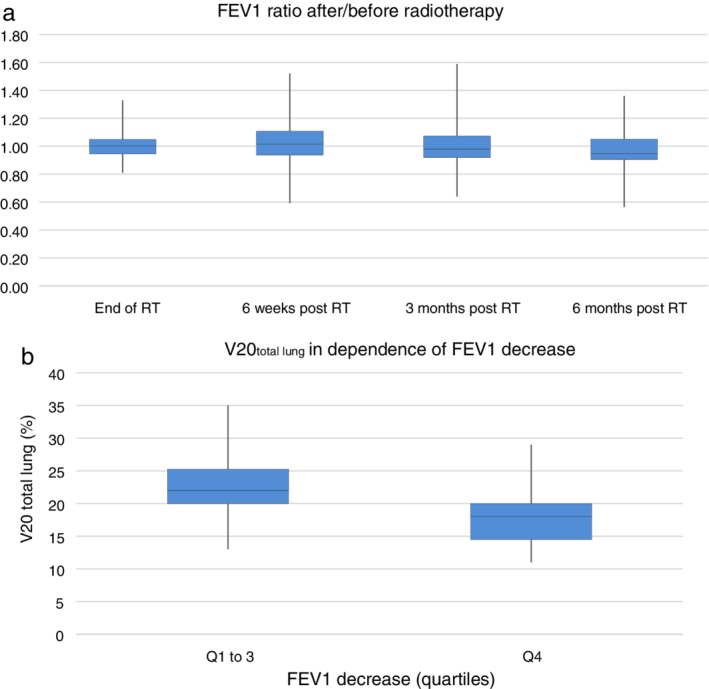
(a) Forced expiratory volume in 1 second (FEV1) six months after radiotherapy (RT) in relation to baseline: the median FEV1 declines to 0.95 (range: 0.56–1.36) relative to baseline. The measurements at the end of RT differed significantly from the values at six months (two‐sided Pearson correlation P‐value = 0.000). (b) V20total lung in dependence of FEV1 decrease: Patients with the best FEV1 (quartile 4) six months after the end of RT compared to the rest of the study population (quartile 1 to 3) have a significantly lower V20total lung (Mann‐Whitney‐U test, P‐value = 0.000).
Figure 3.
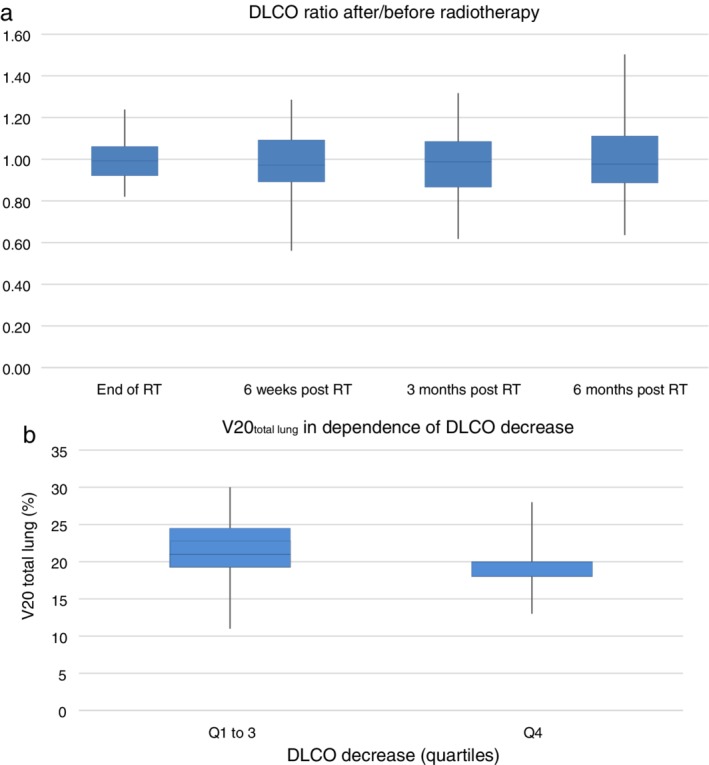
(a) Diffusing capacity of the lung for carbon monoxide (DLCO) six months after radiotherapy (RT) in relation to baseline: the median DLCO decreases to 0.98 (range: 0.64–1.50) relative to baseline. The differences in the measurements at the end of RT at six months showed a trend (two‐sided Pearson correlation P‐value = 0.069). (b) V20total lung in dependence of DLCO decrease. Patients with the best DLCO (quartile 4) six months after the end of RT compared to the rest of the study population (quartile 1 to 3) did not have a significantly different V20total lung (Mann‐Whitney‐U test, P‐value = 0.687).
FEV1 and DLCO decrease depend on V20total lung
Of the 55 patients with a follow‐up at six months, 32 (58%) had a decrease in FEV1. In these 32 patients, the median FEV1 reduction was 9% (range 1%–44%). In 21/55 (38%) patients, the decline was <10%, and in 11/55 (20%) patients it was above. Among the latter, 2/55 (4%) patients had a decrease of more than 20%. In order to derive a dose‐effect correlation, patients were divided in four groups by V20total lung (11%–15%, 16%–20%, 21%–25%, 26%–30%). The probability of any FEV1 decrease per group was as follows: 33% (2/6 patients), 38% (8/21patients), 88% (15/17 patients) and 64% (7/11), respectively (Fig S1). In the highest dose group (V20: 26%–30%) one would expect a higher probability of FEV1 decrease than in the previous group, but this result might be biased by the small total number of patients (Fig S1). The obvious correlation between increased V20total lung and FEV1 decline was statistically significant (one‐sided Pearson correlation 0.023). In the whole cohort, the median V20total lung amounted to 21%. Under the assumption that a FEV1 reduction of >3% could be clinically relevant,17, 19 we compared patients with a V20 above and below median. The patients in the second group had a significantly lower probability of FEV1 decrease >3% than those in the first group (log‐rank P‐value = 0.029, Fig S2).
In 46/72 (64%) of the patients a DLCO measurement at six months could be obtained, while this information was missing in 26/72 (36%) patients for one of the following reasons: treatment finished less than six months before (seven), death (10) or technical reasons (nine). Among the 46 patients with a DLCO ratio available at six months, 25 (54%) had a median decrease of 11% (range 1%–36%). In 12/46 (26%) patients it declined less than 10%, whereas in 8/46 (17%) patients >10%. Among the latter, five patients had a decrease >20%. Again, the same V20total lung dose groups were used as for FEV1. The probability of any DLCO decrease per group was as follows: 40% (2/5 patients), 50% (8/19 patients), 69% (11/16 patients) and 66% (4/6 patients) respectively (Fig S3). Again, the slightly lower than expected DLCO decline in the highest dose group (V20: 26%–30%) might be caused by the limited number of respective patients (Fig S3). The obvious correlation between increased V20total lung and DLCO decrease showed a strong trend (one‐sided Pearson correlation 0.082).
FEV1 and MCID in COPD patients
Considering the moderate FEV1 and DLCO decrease, the question of clinical relevance arises. For COPD patients, it is widely acknowledged by international pulmonary societies that minimal FEV1 decreases of >3% constitute so‐called minimal clinically important differences (MCID).17, 19 In order to address this issue we analyzed the subset of 34/72 (47.2%) patients presenting with COPD.
A total of 13 of 34 (38%) patients had a 4%–21% decrease in FEV1 within six months after the end of RT. Similar to the whole group, the probability of FEV1 reduction was higher in those patients with a V20total lung above the median (= 21%, log rank P‐value = 0.102, Fig S4). In order to detect factors that potentially influence MCID we performed a multivariate analysis (Cox regression, forward stepwise) including patient (gender, age, weight loss, KPS, tumor volume, tumor location, CCI) as well as dosimetric parameters (V20ipsilateral lung, V20total lung, V25total lung, MLD). Surprisingly, KPS was the only variable that remained a significantly predictive factor for MCID (P = 0.048; HR 0.966; 95% CI 0.933–1.000; Table 3).
Table 3.
Univariate (UVA) and multivariate (MVA) analysis (Cox regression, forward stepwise) of clinical and dosimetric variables that potentially influence FEV1 decrease >3% in COPD patients
| COPD patients (N = 34) | ||
|---|---|---|
| Parameter | UVA | MVA |
| Gender | 0.382 | n.s. |
| Age | 0.179 | n.s. |
| Weight loss | 0.354 | n.s. |
| Karnofsky performance score | 0.043 | 0.048 |
| Tumor volume | 0.778 | n.s. |
| Tumor location | ||
| Lobe | 0.190 | n.s. |
| Peripheral or central | 0.653 | n.s. |
| Charlson comorbidity index | 0.264 | n.s. |
| Lung dose constraints | ||
| V20 ipsilateral lung | 0.369 | n.s. |
| V20 total lung | 0.349 | n.s. |
| V25 total lung | 0.955 | n.s. |
| Mean lung dose | 0.248 | n.s. |
CCI, Charlson comorbidity index; KPS, Karnofsky performance score; n.s., not significant.
Discussion
This analysis shows that overall PF declines are moderate within six months after the end of therapy in NSCLC stage III patients treated with IMRT‐DART. The median decrease in FEV1 and DLCO relative to baseline was 0.95 (range: 0.56–1.36) and 0.98 (range: 0.64–1.50), respectively. Nonetheless, in the subset of COPD patients, even small changes may be clinically relevant. MVA revealed that this minimal clinically important difference (MCID) depends primarily on KPS.
In accordance with a previous study8 proposing six months after the end of RT as appropriate to assess short‐term PF changes, we also chose this point of time as the endpoint of the current analysis. Given the relatively high radiation doses, overall FEV1 and DLCO declines were unexpectedly low. A possible reason for this finding could be that patients presented with good PF before treatment. In this respect, the current study is comparable to a recent prospectively randomized control trial: 90% (65/72) of our patients had a pre‐RT FEV1 > 1.2 L which was the minimum required by the RTOG 0617.4 A second reason is the advanced treatment technique with IMRT that allows for better sparing of organs at risk, that is, normal lung tissue. This is in line with a reanalysis of the RTOG 0617 data, which showed less pulmonary toxicity in patients treated with IMRT compared to conventional 3D radiotherapy.20 In a long‐term study including 556 patients treated over 15 years, Schytte et al. observed a PF loss in dependence of the year of treatment due to advances in irradiation technology. With highly conformal RT techniques, declines in FEV1 and DLCO were less pronounced.8
A recent systematic review11 focused on FEV1 and DLCO as the most commonly used measures for post RT lung function, and revealed inconclusive data. Although some studies reported significant decreases in both PF parameters,23, 31 three of seven studies described a loss in FEV1 only (two of the three merely in pneumonitis patients).23, 32 Except for one,33 most of these studies could not detect any risk factors for PF changes in MVA, which made the authors conclude that the number of individuals per trial is too small to serve as a basis for modeling post RT pulmonary function.11 In fact, four of the seven studies quoted by Niezink et al. are smaller than the current one. With 82 patients, one has approximately the same size, and the remaining two include 100 and 250 patients, respectively.11 Since this review comprised patients with both lung and esophageal cancer, its results are not entirely comparable to the current study. From a clinical point of view, there is a difference between PF after thoracic irradiation for esophageal and lung cancer. In the latter, the lung itself is the site of disease, which may cause pulmonary symptoms and consecutive PF changes. Additionally, the fractionation schedules include both stereotactic ablative body radiotherapy (SABR) and conventional RT. Both techniques may have different dose dependent effects on PF.
The decreases in FEV1 and DLCO in our cohort correlate inversely to V20total lung, which becomes evident by a comparison based on the quartiles of PF loss (Figs 2b and 3b) and by differences between dose groups according to V20total lung (Figs S1 and S3). This is partially in line with Gopal et al. who found a decrease of 1.3% DLCO with an increase of 1% in V20.32 Since radiation primarily induces microvasculature damages, DLCO may serve as a more appropriate surrogate marker for post RT lung function changes than FEV1.11, 34 On the other hand, Weinreich et al. demonstrated in a cohort of 50 COPD patients that DLCO is more associated with general condition than with measurable gas exchange since it is potentially biased by BMI and diabetes,35 which is, to some extent, corroborated by the current study (Fig 3b). Of note, while FEV1 decrease showed significant differences at various time points, DLCO measurement did not reveal comparable alterations. Obviously the time dependent changes of these two parameters do not correlate with each other. This finding questions the suitability of DLCO for post RT lung function estimation, meaning that the ideal surrogate marker is yet to be found.
As stated above, the overall absolute PF changes are moderate and therefore potentially not important for all individuals. Yet, for COPD patients,17 who present with impaired lung function before treatment, even marginal declines in PF may be of clinical relevance. Based on interventional studies with bronchodilators, a “minimal clinically important difference” (MCID), which is the smallest difference perceivable by the patient,36 has been defined for FEV1.18 The American Thoracic Society/European Respiratory Society task force considers a FEV1 change of >3% from baseline as clinically relevant in COPD patients.17, 18, 19 In the context of thoracic irradiation, data on MCID have not been published thus far. In the current study, approximately 40% of the patient population with COPD developed a dose‐dependent FEV1 decrease >3% after RT (Fig S4). MVA including clinical parameters showed that baseline KPS was a predictive factor that significantly influenced MCID (Table 3).
Although being larger than the majority of the studies reviewed by Niezink et al.11 the current analysis is limited by the small number of patients which makes it difficult to draw definitive conclusions. Since post‐RT PF is influenced by clinically apparent pneumonitis which occurs up to six months after the end of radiotherapy, FEV1 and DLCO values may be blurred. The grading of side effects depends on the physician's subjective judgment as well as the scoring system. The study by Park et al. may serve as an example in this respect.23 The authors report a grade 2 pneumonitis rate of 37%, whereas in the current study this side‐effect – clinically defined by the administration of cortisone – occurred in 4.2% of the patients. Apart from that, a differentiation between pneumonitis and locoregional tumor relapse can be challenging. Moreover, as lung cancer is an age‐associated disease, the inclusion of elderly patients deserves further consideration when discussing the clinical impact of lung function impairment in daily life. A total of 22 (31%) patients in the present cohort were 70 years or older. Due to reduced activity, this age group may not notice small lung function declines post RT as long as the pretreatment PF was not impaired by COPD. Finally, the median follow‐up of 15.8 months is too short to realistically assess OS and long‐term toxicity. Therefore, it remains open what short‐term PF changes mean in the long run and in how far COPD progression influences follow‐up beyond six months.8
Our study is strengthened by the large number of PFTs. Furthermore, the obligatory inclusion of 18F‐FDG‐PET in the diagnostic work‐up as well as the advanced radiation technique allow for best possible estimation of post RT effects on FEV1 and DLCO.
In conclusion, patients with a V20total lung < 21% are at a low risk for PF decrease after high dose irradiation treatment. Although overall short‐term FEV1 and DLCO differ only moderately from baseline these changes may be clinically important, especially in patients with COPD.
Disclosure
All authors declare that they have no conflict of interest.
Supporting information
Figure S1 In order to derive a dose‐effect correlation, patients were binned in four groups by V20 (11%–15%, 16%–20%, 21%–25%, 26%–30%). The probability of any FEV1 decrease per group was as follows: 33% (2/6 patients), 38% (8/21patients), 88% (15/17 patients) and 64% (7/11), respectively. In the highest dose bin (V20: 26%–30%) one would expect a higher probability of FEV1 decrease than in the previous groups. As the number of patients was small, this data point should be taken with caution. The obvious correlation between increased V20total lung and FEV1 decline was statistically significant (one‐sided Pearson correlation 0.023).
Figure S2 In the whole cohort the median V20total lung was 21%. We compared the group of patients with a V20 above median (blue) to those below (green). The patients in the second group had a significantly lower probability of FEV1 decrease >3% than those in the first group (log‐rank P‐value = 0.029).
Figure S3 The same dose bins as for the estimation of FEV1 decrease were used. The probability of any DLCO decline per group was as follows: 40% (2/5 patients), 50% (8/19 patients), 69% (11/16 patients) and 66% (4/6 patients), respectively. In the highest dose bin (V20: 26%–30%) one would expect a higher probability of DLCO decline than in the previous groups which is due to the limited number of patients in this group, not the case. The obvious correlation between increased V20total lung and DLCO decrease showed a strong trend (one‐sided Pearson correlation 0.082).
Figure S4 A total of 13 of the 34 (38%) patients with COPD had a 3–21% decrease in FEV1 within 6 months after the end of RT. Similar to the whole group, the probability of FEV1 reduction was higher in those patients with a V20total lung above the median (=21%, logrank P‐value = 0.102).
References
- 1. Furuse K, Fukuoka M, Kawahara M et al Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non‐small‐cell lung cancer. J Clin Oncol 1999; 17 (9): 2692–9. [DOI] [PubMed] [Google Scholar]
- 2. Fournel P, Robinet G, Thomas P et al Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non‐small‐cell lung cancer: Groupe Lyon‐Saint‐Etienne d'Oncologie Thoracique‐Groupe Francais de Pneumo‐Cancerologie NPC 95‐01 study. J Clin Oncol 2005; 23 (25): 5910–7. 10.1200/JCO.2005.03.070. [DOI] [PubMed] [Google Scholar]
- 3. Curran WJ Jr, Paulus R, Langer CJ et al Sequential vs. concurrent chemoradiation for stage III non‐small cell lung cancer: Randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011; 103 (19): 1452–60. 10.1093/jnci/djr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bradley JD, Paulus R, Komaki R et al Standard‐dose versus high‐dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non‐small‐cell lung cancer (RTOG 0617): A randomised, two‐by‐two factorial phase 3 study. Lancet Oncol 2015; 16 (2): 187–99. 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Antonia SJ, Villegas A, Daniel D et al Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018; 379 (24): 2342–50. 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 6. Wurstbauer K, Deutschmann H, Dagn K et al DART‐bid (Dose‐differentiated accelerated radiation therapy, 1.8 Gy twice daily)–a novel approach for non‐resected NSCLC: Final results of a prospective study, correlating radiation dose to tumor volume. Radiat Oncol 2013; 8: 49 10.1186/1748-717X-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walraven I, van den Heuvel M, van Diessen J et al Long‐term follow‐up of patients with locally advanced non‐small cell lung cancer receiving concurrent hypofractionated chemoradiotherapy with or without cetuximab. Radiother Oncol 2016; 118 (3): 442–6. 10.1016/j.radonc.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 8. Schytte T, Bentzen SM, Brink C, Hansen O. Changes in pulmonary function after definitive radiotherapy for NSCLC. Radiother Oncol 2015; 117 (1): 23–8. 10.1016/j.radonc.2015.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jaen J, Vazquez G, Alonso E et al Long‐term changes in pulmonary function after incidental lung irradiation for breast cancer: A prospective study with 7‐year follow‐up. Int J Radiat Oncol Biol Phys 2012; 84 (5): e565–70. 10.1016/j.ijrobp.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 10. Krengli M, Sacco M, Loi G et al Pulmonary changes after radiotherapy for conservative treatment of breast cancer: A prospective study. Int J Radiat Oncol Biol Phys 2008; 70 (5): 1460–7. 10.1016/j.ijrobp.2007.08.050. [DOI] [PubMed] [Google Scholar]
- 11. Niezink AGH, de Jong RA, Muijs CT, Langendijk JA, Widder J. Pulmonary function changes after radiotherapy for lung or esophageal cancer: A systematic review focusing on dose‐volume parameters. Oncologist 2017; 22 (10): 1257–64. 10.1634/theoncologist.2016-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Borst GR, De Jaeger K, Belderbos JS, Burgers SA, Lebesque JV. Pulmonary function changes after radiotherapy in non‐small‐cell lung cancer patients with long‐term disease‐free survival. Int J Radiat Oncol Biol Phys 2005; 62 (3): 639–44. 10.1016/j.ijrobp.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 13. Marks LB, Fan M, Clough R et al Radiation‐induced pulmonary injury: Symptomatic versus subclinical endpoints. Int J Radiat Biol 2000; 76 (4): 469–75. [DOI] [PubMed] [Google Scholar]
- 14. Theise ND, Henegariu O, Grove J et al Radiation pneumonitis in mice: A severe injury model for pneumocyte engraftment from bone marrow. Exp Hematol 2002; 30 (11): 1333–8. 10.1016/S0301-472x(02)00931-1. [DOI] [PubMed] [Google Scholar]
- 15. Sorichter S. Lung function. Radiologe 2009; 49 (8): 676–86. 10.1007/s00117-009-1877-0. [DOI] [PubMed] [Google Scholar]
- 16. Brunelli A, Charloux A, Bolliger CT et al ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo‐radiotherapy) . Eur Respir J 2009; 34 (1): 17–41. 10.1183/09031936.00184308. [DOI] [PubMed] [Google Scholar]
- 17. Jones PW, Beeh KM, Chapman KR, Decramer M, Mahler DA, Wedzicha JA. Minimal clinically important differences in pharmacological trials. Am J Respir Crit Care Med 2014; 189 (3): 250–5. 10.1164/rccm.201310-1863PP. [DOI] [PubMed] [Google Scholar]
- 18. Donohue JF. Minimal clinically important differences in COPD lung function. COPD 2005; 2 (1): 111–24. [DOI] [PubMed] [Google Scholar]
- 19. Cazzola M, MacNee W, Martinez FJ et al Outcomes for COPD pharmacological trials: From lung function to biomarkers. Eur Respir J 2008; 31 (2): 416–69. 10.1183/09031936.00099306. [DOI] [PubMed] [Google Scholar]
- 20. Chun SG, Hu C, Choy H et al Impact of intensity‐modulated radiation therapy technique for locally advanced non‐small‐cell lung cancer: A secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. J Clin Oncol 2017; 35 (1): 56–62. 10.1200/JCO.2016.69.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vogelius IR, Bentzen SM. A literature‐based meta‐analysis of clinical risk factors for development of radiation induced pneumonitis. Acta Oncol 2012; 51 (8): 975–83. 10.3109/0284186x.2012.718093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Palma DA, Senan S, Tsujino K et al Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: An international individual patient data meta‐analysis. Int J Radiat Oncol Biol Phys 2013; 85 (2): 444–50. 10.1016/j.ijrobp.2012.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Park YH, Kim JS. Predictors of radiation pneumonitis and pulmonary function changes after concurrent chemoradiotherapy of non‐small cell lung cancer. Radiat Oncol J 2013; 31 (1): 34–40. 10.3857/roj.2013.31.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wurstbauer K, Zehentmayr F, Deutschmann H et al DART‐bid for loco‐regionally advanced NSCLC: Summary of acute and late toxicity with long‐term follow‐up; experiences with pulmonary dose constraints. Strahlenther Onkol 2017; 193 (4): 315–23. 10.1007/s00066-016-1095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zehentmayr F, Sohn M, Exeli AK et al Normal tissue complication models for clinically relevant acute esophagitis (>/= grade 2) in patients treated with dose differentiated accelerated radiotherapy (DART‐bid). Radiat Oncol 2015; 10: 121 10.1186/s13014-015-0429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Graham MV, Purdy JA, Emami B et al Clinical dose‐volume histogram analysis for pneumonitis after 3D treatment for non‐small cell lung cancer (NSCLC). Int J Radiat Oncol 1999; 45 (2): 323–9. 10.1016/S0360-3016(99)00183-2. [DOI] [PubMed] [Google Scholar]
- 27. Marks LB, Bentzen SM, Deasy JO et al Radiation dose‐volume effects in the lung. Int J Radiat Oncol Biol Phys 2010; 76 (3 Suppl): S70–6. 10.1016/j.ijrobp.2009.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Armstrong JG, Zelefsky MJ, Leibel SA et al Strategy for dose escalation using 3‐dimensional conformal radiation therapy for lung cancer. Ann Oncol 1995; 6 (7): 693–7. 10.1093/oxfordjournals.annonc.a059286. [DOI] [PubMed] [Google Scholar]
- 29. Wurstbauer K, Merz F, Sedlmayer F. Amphotericin B lozengers: Prophylaxis for esophagitis in thoracic radiotherapy: A prospective study. Strahlenther Onkol 2009; 185 (8): 512–6. 10.1007/s00066-009-1938-3. [DOI] [PubMed] [Google Scholar]
- 30. Antonia SJ, Villegas A, Daniel D et al Durvalumab after chemoradiotherapy in stage III non‐small‐cell lung cancer. N Engl J Med 2017; 377 (20): 1919–29. 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 31. Enache I, Noel G, Jeung MY et al Impact of 3D conformal radiotherapy on lung function of patients with lung cancer: A prospective study. Respiration 2013; 86 (2): 100–8. 10.1159/000342371. [DOI] [PubMed] [Google Scholar]
- 32. Gopal R, Starkschall G, Tucker SL et al Effects of radiotherapy and chemotherapy on lung function in patients with non‐small‐cell lung cancer. Int J Radiat Oncol Biol Phys 2003; 56 (1): 114–20. [DOI] [PubMed] [Google Scholar]
- 33. Lopez Guerra JL, Gomez DR, Zhuang Y et al Changes in pulmonary function after three‐dimensional conformal radiotherapy, intensity‐modulated radiotherapy, or proton beam therapy for non‐small‐cell lung cancer. Int J Radiat Oncol Biol Phys 2012; 83 (4): e537–43. 10.1016/j.ijrobp.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abratt RP, Morgan GW. Lung toxicity following chest irradiation in patients with lung cancer. Lung Cancer 2002; 35 (2): 103–9. [DOI] [PubMed] [Google Scholar]
- 35. Weinreich UM, Thomsen LP, Brock C, Karbing DS, Rees SE. Diffusion capacity of the lung for carbon monoxide ‐ a potential marker of impaired gas exchange or of systemic deconditioning in chronic obstructive lung disease? Chron Respir Dis 2015; 12 (4): 357–64. 10.1177/1479972315601946. [DOI] [PubMed] [Google Scholar]
- 36. Jaeschke R, Singer J, Guyatt GH. Measurement of health‐status ‐ ascertaining the minimal clinically important difference. Control Clin Trials 1989; 10 (4): 407–15. 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 In order to derive a dose‐effect correlation, patients were binned in four groups by V20 (11%–15%, 16%–20%, 21%–25%, 26%–30%). The probability of any FEV1 decrease per group was as follows: 33% (2/6 patients), 38% (8/21patients), 88% (15/17 patients) and 64% (7/11), respectively. In the highest dose bin (V20: 26%–30%) one would expect a higher probability of FEV1 decrease than in the previous groups. As the number of patients was small, this data point should be taken with caution. The obvious correlation between increased V20total lung and FEV1 decline was statistically significant (one‐sided Pearson correlation 0.023).
Figure S2 In the whole cohort the median V20total lung was 21%. We compared the group of patients with a V20 above median (blue) to those below (green). The patients in the second group had a significantly lower probability of FEV1 decrease >3% than those in the first group (log‐rank P‐value = 0.029).
Figure S3 The same dose bins as for the estimation of FEV1 decrease were used. The probability of any DLCO decline per group was as follows: 40% (2/5 patients), 50% (8/19 patients), 69% (11/16 patients) and 66% (4/6 patients), respectively. In the highest dose bin (V20: 26%–30%) one would expect a higher probability of DLCO decline than in the previous groups which is due to the limited number of patients in this group, not the case. The obvious correlation between increased V20total lung and DLCO decrease showed a strong trend (one‐sided Pearson correlation 0.082).
Figure S4 A total of 13 of the 34 (38%) patients with COPD had a 3–21% decrease in FEV1 within 6 months after the end of RT. Similar to the whole group, the probability of FEV1 reduction was higher in those patients with a V20total lung above the median (=21%, logrank P‐value = 0.102).


