Abstract
There are limited data on the clinical efficiency of afatinib in non‐small cell lung cancer (NSCLC) patients with uncommon epidermal growth factor receptor (EGFR) mutations. Moreover, the efficacy and safety of afatinib in elderly patients with these mutations has not been established. Here, we describe a case of successful treatment of a patient aged >80 years with lung adenocarcinoma positive for the uncommon EGFR L861Q mutation with low‐dose afatinib. An 83‐year‐old woman presented with cough and dyspnea. A chest computed tomography (CT) scan revealed tumors in the left upper lobe, left pleural effusion, and multiple lung metastases in both lungs. The patient was diagnosed with lung adenocarcinoma with an EGFR L861Q mutation based on cytological findings. The patient received 30 mg/day of afatinib and experienced no severe adverse events. Two weeks later, partial response was observed based on a CT scan. The results of the present case support the effectiveness and safety of low‐dose afatinib in elderly patients with EGFR L861Q mutation‐positive NSCLC.
Keywords: Afatinib, L861Q, low‐dose, non‐small cell lung cancer, uncommon epidermal growth factor receptor mutation
Introduction
Epidermal growth factor receptor (EGFR) mutations occur in about 45% of Japanese patients with non‐small cell lung cancer (NSCLC) and mostly in those with adenocarcinoma.1 Exon 19 deletion and exon 21 L858R are the most common mutations accounting for about 90% of EGFR mutation‐positive NSCLC.2 Uncommon EGFR mutations include exon 20 insertions, G919X in exon 18, L861Q in exon 21, S768I in exon 20, and complex mutations.3
Exon 19 deletion and exon 21 L858R show high sensitivity to tyrosine kinase inhibitor (TKI) treatment. However, the efficacy of TKI treatment in uncommon EGFR mutation‐positive NSCLC, especially elderly patients aged >80 years, has not been established.
This report describes the case of an 83‐year‐old patient with uncommon EGFR L861Q mutation‐positive NSCLC who was successfully treated with low‐dose afatinib.
Case report
An 83‐year‐old Japanese woman with a history of a cough for one month and dyspnea presented to the hospital. She was a non‐smoker with no family history of lung cancer. Her performance status was one. Upon examination, her body temperature, respiratory rate, pulse rate, blood pressure and oxygen saturation (room air) were 36.8°C, 28 per minute, 158/96 mmHg, 88 per minute, and 95%, respectively. Chest auscultation revealed decreased respiratory sounds in her lower left lung due to pleural effusion. The levels of tumor markers carcinoembryonic antigen (CEA) and sialyl SSEA‐1 (SLX) were elevated (5.3 ng/mL and 103 U/mL).
On admission, chest radiograph and computed tomography (CT) scans revealed an atypically shaped tumor in the left upper lung, left pleural effusion with dissemination, and multiple lung metastases in both her lungs (Figs 1a and 2a). The cytology tests identified adenocarcinoma of the lung with an EGFR L861Q mutation in the bloody pleural effusion. Although bronchoscopy findings did not indicate malignancy, we determined that the left upper lung tumor was the primary lesion because we did not detect any other lesions. The left pleural effusion was drained several times to obtain a definitive diagnosis. After confirmation of the diagnosis (lung adenocarcinoma cT2aN2M1a with an EGFR L861Q mutation), afatinib was administered at a dose of 30 mg/day. Grade I adverse events (AEs) such as diarrhea, stomatitis, and dry skin were noted, but these symptoms resolved with the use of loperamide, azulene, and topical steroids. No severe AEs due to afatinib were observed.
Figure 1.
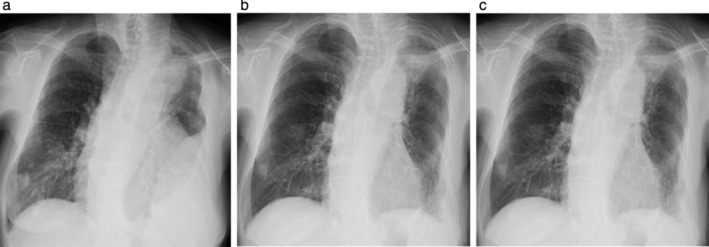
Chest radiographs (a) on admission, (b) day 17, and (c) day 42 after initiating low‐dose afatinib therapy. The results showed a 40 mm‐sized lung tumor in the left upper lobe, left pleural effusion, and multiple lung metastases in both lungs (a). The lung tumor, left pleural effusion, and multiple lung metastases showed a remarkable decrease at day 17 (b) and continued to decrease 42 days after low‐dose afatinib treatment (c).
Figure 2.
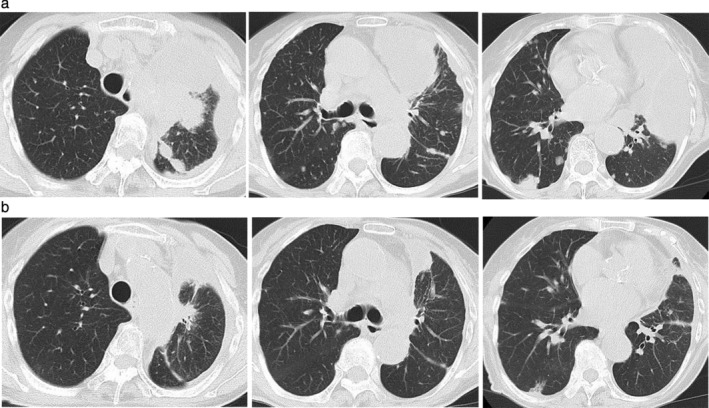
Chest computed tomography scan (a) on admission in the upper column and (b) on day 42 after initiating low‐dose afatinib treatment in the lower column. There was a significant decrease in the size of the lung tumor in the left upper lobe, left pleural effusion, and multiple lung metastases in both lungs.
After two weeks of afatinib therapy, the primary tumor, pleural effusion, and multiple lung metastases significantly decreased (Fig 1b). In addition, the levels of SLX and CEA (especially the level of SLX) decreased (Fig 3). The patient maintained partial response (PR) for two months as observed by chest CT scans at the time of writing this report (Figs 1c and 2b).4
Figure 3.
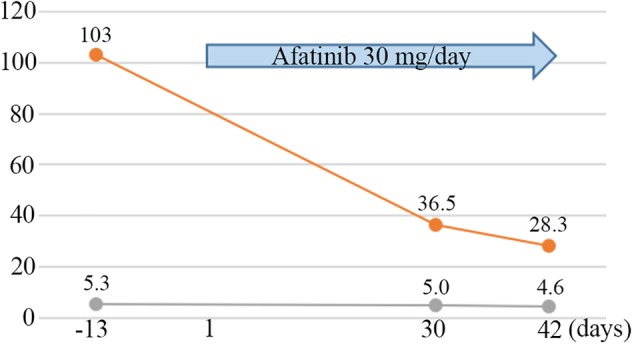
Clinical course and changes in the levels of carcinoembryonic antigen (CEA) and sialyl SSEA‐1 (SLX). ( ) SLX (U/mL) and (
) SLX (U/mL) and ( ) CEA (ng/mL).
) CEA (ng/mL).
Discussion
Here, we report the clinical activity and safety of low‐dose afatinib treatment in an elderly patient aged >80 years with lung adenocarcinoma positive for the uncommon EGFR L861Q mutation.
L861Q in exon 21 is an uncommon EGFR mutation that represents almost 2% of all EGFR mutations.5 In general, uncommon EGFR mutations including L861Q are resistant to gefitinib, a first‐generation EGFR‐TKI.6 However, previous in vitro data showed that L861Q mutations are sensitive to afatinib compared to first‐generation EGFR‐TKIs (gefitinib and erlotinib).7 Afatinib is an irreversible inhibitor that covalently binds to EGFR kinase, whereas gefitinib and erlotinib are reversible EGFR kinase inhibitors. It has been reported that these different effects of EGFR‐TKI on uncommon EGFR mutation‐positive NSCLC might be due to the different structural‐based interactions.8 Moreover, the LUX‐Lung trials reported that afatinib had good clinical activity in NSCLC with uncommon EGFR L861Q mutation, with an objective response rate of 56.3%, median progression‐free survival of 8.2 months, and median overall survival of 17.1 months.2 However, the analysis included only a few cases with uncommon mutations, and the effectiveness of afatinib in elderly patients aged >80 years with uncommon EGFR mutation‐positive NSCLC remains unknown.
An important clinical problem is that afatinib has high rates of severe AEs including grade 3–4 diarrhea, skin rash, and paronychia that led to treatment discontinuation.9, 10 Recently, a dose reduction of afatinib to <40 mg was found to have no effect on its efficacy in EGFR 19del and L858R mutation‐positive NSCLC.11, 12 Afatinib plasma concentrations were not significantly different between patients with a dose reduction to 30 mg/day and with 40 mg/day. Furthermore, a dose reduction to 30 mg/day reduced the incidence of grade > 3 AEs.13 In elderly patients (age ≥ 70 years) with EGFR 19del and L858R mutation‐positive NSCLC, the efficacy and feasibility of afatinib treatment starting at 30 mg/day have been reported.14 Since these previous reports focused on EGFR 19del and L858R mutations, the efficiency and safety of afatinib dose reduction in other uncommon EGFR mutations has not been known.
Based on the above data, we administered a low‐dose afatinib (30 mg daily) therapy. The patient had only grade 1 AEs including diarrhea, stomatitis, and dry skin. After two weeks of afatinib treatment, PR was observed. The results of the present case support the effective clinical activity and safety of low‐dose afatinib in elderly patients with EGFR L861Q mutation‐positive NSCLC.
Disclosure
The authors have no conflicts of interest to declare.
Acknowledgments
None.
References
- 1. Midha A, Dearden S, McCormack R. EGFR mutation incidence in non‐small‐cell lung cancer of adenocarcinoma histology: A systematic review and global map by ethnicity (mutMapII). Am J Cancer Res 2015; 5 (9): 2892–911. [PMC free article] [PubMed] [Google Scholar]
- 2. Yang JC, Sequist LV, Geater SL e a. Clinical activity of afatinib in patients with advanced non‐small‐cell lung cancer harbouring uncommon EGFR mutations: A combined post‐hoc analysis of LUX‐Lung 2, LUX‐Lung 3, and LUX‐Lung 6. Lancet Oncol 2015; 16 (7): 830–8. [DOI] [PubMed] [Google Scholar]
- 3. Pilotto S, Rossi A, Vavala T e a. Outcomes of first‐generation EGFR‐TKIs against non‐small‐cell lung cancer harboring uncommon EGFR mutations: A post hoc analysis of the BE‐POSITIVE study. Clin Lung Cancer 2018; 19 (1): 93–104. [DOI] [PubMed] [Google Scholar]
- 4. Eisenhauer EA, Therasse P, Bogaerts J e a. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45 (2): 228–47. [DOI] [PubMed] [Google Scholar]
- 5. Zhang T, Wan B, Zhao Y e a. Treatment of uncommon EGFR mutations in non‐small cell lung cancer: New evidence and treatment. Transl Lung Cancer Res 2019; 8 (3): 302–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Watanabe S, Minegishi Y, Yoshizawa H e a. Effectiveness of gefitinib against non‐small‐cell lung cancer with the uncommon EGFR mutations G719X and L861Q. J Thorac Oncol 2014; 9 (2): 189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kobayashi Y, Mitsudomi T. Not all epidermal growth factor receptor mutations in lung cancer are created equal: Perspectives for individualized treatment strategy. Cancer Sci 2016; 107 (9): 1179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Banno E, Togashi Y, Nakamura Y e a. Sensitivities to various epidermal growth factor receptor‐tyrosine kinase inhibitors of uncommon epidermal growth factor receptor mutations L861Q and S768I: What is the optimal epidermal growth factor receptor‐tyrosine kinase inhibitor? Cancer Sci 2016; 107 (8): 1134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sequist LV, Yang JC, Yamamoto N e a. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013; 31 (27): 3327–34. [DOI] [PubMed] [Google Scholar]
- 10. Wu YL, Zhou C, Hu CP e a. Afatinib versus cisplatin plus gemcitabine for first‐line treatment of Asian patients with advanced non‐small‐cell lung cancer harbouring EGFR mutations (LUX‐Lung 6): An open‐label, randomised phase 3 trial. Lancet Oncol 2014; 15 (2): 213–22. [DOI] [PubMed] [Google Scholar]
- 11. Liang SK, Lee MR, Liao WY, Ho CC, Ko JC, Shih JY. Prognostic factors of afatinib as a first‐line therapy for advanced EGFR mutation‐positive lung adenocarcinoma: A real‐world, large cohort study. Oncotarget 2018; 9 (34): 23749–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang CJ, Tsai MJ, Hung JY e a. The clinical efficacy of Afatinib 30 mg daily as starting dose may not be inferior to Afatinib 40 mg daily in patients with stage IV lung Adenocarcinoma harboring exon 19 or exon 21 mutations. BMC Pharmacol Toxicol 2017; 18 (1): 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang JC, Sequist LV, Zhou C et al Effect of dose adjustment on the safety and efficacy of afatinib for EGFR mutation‐positive lung adenocarcinoma: Post hoc analyses of the randomized LUX‐Lung 3 and 6 trials. Ann Oncol 2016;27(11):2103–10. [DOI] [PubMed] [Google Scholar]
- 14. Imai H, Kaira K, Suzuki K e a. A phase II study of afatinib treatment for elderly patients with previously untreated advanced non‐small‐cell lung cancer harboring EGFR mutations. Lung Cancer 2018; 126: 41–7. [DOI] [PubMed] [Google Scholar]


