Abstract
Objectives To present our method of median anterior skull base (ASB) reconstruction using a subcranial approach with a free flap in cases of naso-fronto-orbital (NFO) bony segment failure and in patients at high risk for future failure of the bony frontal segment.
Design This study presents as a retrospective case series.
Setting Tertiary university-affiliated medical center.
Participants Adult and pediatric patients who underwent median ASB resection via the subcranial approach with a free flap reconstruction were participated in this study.
Main Outcome Measures Pathologic outcome and postoperative quality of life (QoL) as assessed by a validated Hebrew version of the “Anterior Skull Base Quality-of-Life Questionnaire.”
Results The departmental database yielded 13 suitable patients aged between 15 and 70 years. The main indication ( n = 7) for ASB surgery was osteoradionecrosis (ORN) of the NFO bony segment which was first detected at an average of 3.6 years (range: 2–32 years) postradiation therapy. High-risk patients for future ORN of the NFO segment ( n = 3) were primarily reconstructed using a vascularized free flap. Nine patients had malignant disease, and four of them were alive without evidence of disease during the follow-up period (average, 48 months). Their QoL was comparable to that of patients who had undergone subcranial ASB resection without free flap reconstruction.
Conclusions The ASB median free flap method of reconstruction is a safe and reliable in cases of large complex median ASB defects without orbital resection or maxillectomy. This approach is suitable for patients who had undergone previous surgery, radiotherapy and/or those who present with osteoradionecrosis, as well as for patients with high risk of NFO segment ORN.
Keywords: anterior skull base, osteoradionecrosis, subcranial approach, skull base reconstruction, free flap reconstruction of skull base defect
Introduction
The anterior skull base (ASB) is a complex three-dimensional (3D) anatomical compartment situated superiorly to the nasal cavity, paranasal sinuses and orbits, and inferiorly to the intracranial compartment. The ASB provides isolation of the sterile intracranial content from the hostile contaminated nasal cavity and paranasal sinuses. It also provides mechanical support to the intracranial structures, resisting gravitation, and intracranial pressure (ICP). Resection of the ASB is the primary therapeutic modality of various malignant conditions, including those of the nasal cavity, paranasal sinuses, the orbits and anterior fossa, 1 as well as of few benign conditions. The extent of the open resection is a function of the size and location of the disease and it can be performed by means of a craniofacial approach, a subcranial approach, or combinations and modifications of these two. 2 3
Failure to adequately reconstruct the ASB might result in a devastating outcome. 4 After ASB resection and reconstruction via the subcranial approach, the bony naso-fronto-orbital (NFO) segment is at risk for ischemic necrosis (osteonecrosis). High risk of NFO osteonecrosis exists in postoperative irradiated patients, usually due to osteoradionecrosis (ORN). 5 Although pericranial wrapping of the NFO segment has greatly reduced the incidence of ORN, 6 7 the consequences are devastating when it does occur ( Fig. 1 ) and a revision surgery is usually necessary.
Fig. 1.

Clinical appearance of a 42-year-old woman, 24 months after surgery through the subcranial approach followed by radiotherapy. The ORN process involved the skin of the forehead as well as an exposed bony segment superior to the right medial canthus (arrow). Axial and 3D CT scan reconstruction of the skull in the same patient. Note the devastating effect of the ORN on the NFO bony segment. ORN, osteoradionecrosis; NFO, naso-fronto-orbital; 3D CT, three-dimensional computed tomography.
In the search for an option for the management of these cases and to prevent future ORN in high-risk patients, a new solution emerged in the form of vascularized free flap reconstruction of the ASB which replaces FNO segment (in the primary operation of high-risk patients) or the necrotic NFO segment (in a secondary revision operation once ORN has been developed). Here, we describe the challenges and difficulties as well as the surgical technique and solutions for patients who underwent free flap reconstruction of the ASB either as a primary reconstruction of the frontocribriform area, or as a replacement of the ORN NFO bony segment (secondary reconstruction).
Methods
This retrospective study was approved by the institutional review board of our medical center. A medical record review of all patients operated via the subcranial approach to the ASB in our referral tertiary skull base center from January 1999 to June 2017 was conducted. Inclusion criteria were primary or secondary reconstruction of the subcranial bony and soft tissue defect, namely, an NFO bony and soft tissue segment or the frontocribriform area and using a vascular free flap. Data on demographics, clinical records, and radiological, pathological, surgical, and postoperative reports were retrieved and analyzed. Quality of life (QoL) was evaluated by a validated version of the “Anterior Skull Base Quality-of-Life Questionnaire,” 8 developed in our institution. This questionnaire includes six life domains (vitality, pain, emotions, etc.), each can be grade from 0 to 3 (the more, the better). We compared their QoL to our previously published data, 8 of an age- and gender-matched patients underwent ASB resection using the subcranial approach, without a free flap. The data was compared using one-sample Wilcoxon's sign-rank test.
Preoperative Evaluation
A multidisciplinary assessment was performed for each surgical candidate. The assessment is fundamental to ensure surgical success and patient safety 9 and the team includes a head and neck surgeon, a reconstructive plastic surgeon, a neurosurgeon, a head and neck oncologist, a head and neck pathologist, a head and neck radiologist, and an anesthesiologist. All available data on the patient, including a comprehensive medical history, a meticulous physical examination, and careful computerized tomographic (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET-CT) scans were performed. Broad-spectrum antibiotics consisting of a combination of cefuroxime and metronidazole were routinely administered perioperatively. 10
Operative Technique
The basic operative technical problem is the result of shortage of space, bordered by the periorbit laterally, frontal lobe dura superiorly, and basisphenoid posteriorly. This small “anatomical corridor” limits the volume of free flap and the posterior anchoring of the flap to the skull base.
General Preparations
All patients were operated under general anesthesia and in the supine position. No tracheostomy or hair shaving was performed. A continuous lumbar catheter had been used in the past to facilitate frontal lobe retraction and to reduce the risk of postoperative cerebrospinal fluid (CSF) leak; however, we abandoned this technique 6 years ago 11 and no lumbar catheter is routinely inserted.
Dural Reconstruction
Dural reconstruction is performed in a meticulous manner to prevent CSF leak and tension pneumocephalus. The dimensions of the dural defect dictated the material and technique of reconstruction, ranging from a minimal dural defect managed with primary closure, to a small dural defect sealed with temporalis fascia to moderate and large dural defects necessitating fascia lata reconstruction. The fascia lata was tailored according to the size and shape of the dural defect and it could extend up to 20 × 10 cm. 12 The methods of dural reconstruction in the primary surgery of future high-risk patients for ORN ( n = 3) or in the secondary reconstruction of the necrotic NFO segment ( n = 10) are listed in Table 1 .
Table 1. Patient's demographics, pathology and reconstruction mode of the cohort.
| Gender | Age (y) | Pathology | Primary reconstruction | Y to complication | Complication | Secondary reconstruction | Dural reconstruction | Vascular anastomosis |
|---|---|---|---|---|---|---|---|---|
| F | 70 | Esthesioneuroblastoma | Fascia lata | 2 | ORN | VRAM | Primary | Superficial temporal |
| M | 14 | Meningioma | Fascia lata | 32 | ON | ALT | – | Superficial temporal |
| M | 32 | Adenocarcinoma | Fascia lata | 7 | ORN | ALT | – | Facial vessels |
| F | 42 | Adenocarcinoma | Fascia lata | 2 | ORN | ALT | Primary | Superficial temporal |
| F | 61 | Meningioma | Fascia lata | 6 | ON | RF | – | Facial vessels |
| F | 15 | Osteosarcoma | Fascia lata | 5 | ORN | ALT | – | Superficial temporal |
| M | 50 | SCC in IP | Fascia lata | 4 | ORN | ALT | – | Superficial temporal |
| M | 22 | Chondrosarcoma | Fascia lata | 2 | ORN | ARF | – | Superficial temporal |
| F | 66 | Esthesioneuroblastoma | Fascia lata | 3 | ORN | ALT | – | Superficial temporal |
| F | 36 | Sinusitis | Fascia lata | 7 | ON | ALT | – | Facial vessels |
| F | 42 | Angiosarcoma | VRAM | – | – | – | Primary | Superficial temporal |
| F | 42 | Esthesioneuroblastoma | ALT | – | – | – | Temporalis m. fascia | Superficial temporal |
| M | 58 | Meningioma | ALT | – | – | – | Primary | Facial vessels |
Abbreviations: ALT, anterior lateral thigh; IP, inverted papilloma; ON, osteonecrosis; ORN, osteoradionecrosis; RF, radial forearm; SCC, squamous cell carcinoma; VRAM, vebtral rectus abdominis muscle.
Free Flap Reconstruction
The free flap was designed and harvested simultaneously with the resection process and according to the preoperative assessment and intraoperative findings. In cases of secondary resection due to bony NFO segment ORN, the necrotic bone was resected and the anterior cranium was reconstructed using soft tissue alone. After a primary watertight dural seal had been established, as described above, the final desired size and shape of the free flap was defined by using a silicone template. Anterolateral thigh (ALT) flap was used in 9/13 patients and is an excellent flap for this purpose. Debulking of the ALT was performed when needed (four of nine cases) but pedicle length did not limit the reconstruction method. Basically, flap type was chosen according to the defect size and shape. The main difficulty of free flap reconstruction of the ASB in this setting. when there is no orbital exenteration or maxillectomy. is the shortage of space in this restricted anatomical corridor. The posterior anchorage to the bony contour of the defect might extend as posteriorly as the basisphenoid and lesser sphenoidal wings ( Fig. 2 ) becoming highly problematic. We overcame this difficulty by stitches that had been locted prior to final free flap positioning through holes drilled in advance through the posterior and lateral bony defect ( Fig. 2 ). The free flap was then “parachuted” to its final position ( Fig. 2 ) and after the posterior profile of the defect was reconstructed, the lateral contour was connected bilaterally in a posterior–anterior fashion using stitches previously placed through the soft and bony tissue of the surrounding ASB. The superficial temporal (nine cases) and facial (four cases) vessels were used and were end-to-end anastomosed to the free flap's vessels ( Table 1 , Fig. 3 ). The necrotic bony NFO segment was not anchored back to the cranium. In all cases, the NFO segment was not reused. No other plates or rigid reconstruction was used whatsoever.
Fig. 2.

Stitches are located at the posterior bony margins of the skull base defect prior to free flap (ALT) insertion. Then, the free flap is “parachuted” to its final position at the skull base. ALT, anterolateral thigh.
Fig. 3.

Vascular anastomosis of the free flap to the superficial temporal vessels in a 21-year-old female who operated due to osteonecrosis of the NFO segment as the result of multiple subcranial approach operations for complicated sinusitis during childhood. NFO, naso-fronto-orbital.
In four cases, the ORN process of the NFO segment also included fistulas to the overlying forehead skin ( Fig. 1 ). In these cases, a patch of non-de-epithelialized ALT ( Fig. 4 ) was used to replace the resected skin. A 150 cm Vaseline gauze was applied to the subcranial cavity to provide support to the flap against the pulsations of the dura and the effect of gravity.
Fig. 4.

The free fasciocutaneous ALT flap for the reconstruction of the defect. The triangular skin paddle will face the intranasal cavity while the small epidermal island (*) will close the forehead skin defect. ALT, anterolateral thigh.
Postoperative Care
Postoperatively, the patients underwent a routine CT scan to rule out tension pneumocephalus or hemorrhage and were then transferred to the critical care unit for monitoring. All flaps were monitored using implantable Doppler's devices. Skin monitoring was used as an additional method for flaps viability, when indicated. A combination of cefuroxime and metronidazole was administered prophylactically until the postoperative day (POD) 5. Enoxaparin was used routinely for the early postoperative period (24–48 hours postoperatively) until patient's ambulation occurs.
Oral diets were provided as early as the first POD. The Vaseline gauze tamponade was pulled out between the POD 5 to 7. Stool softeners, anticough medications and elevated head positions were used in the early postoperative period to reduce pressure on the free flap that could result from valsalva maneuvers. Oncologic follow-up consisted of physical medical history, physical examination, and MRI and PET-CT imaging studies when needed ( Fig. 5 ). The lack of osseous anterior cranium was not associated with additional challenges regarding imaging for malignant recurrence. Cosmetic outcome is shown in Fig. 6 .
Fig. 5.

An axial, coronal, and midsagittal T1 MRI views of an anterolateral thigh free flap used for anterior skull base reconstruction, at 1-year follow-up. Note the absence of a NFO bony segment, as well as the posterior anchorage of the flap to the basisphenoid. NFO, naso-fronto-orbital.
Fig. 6.
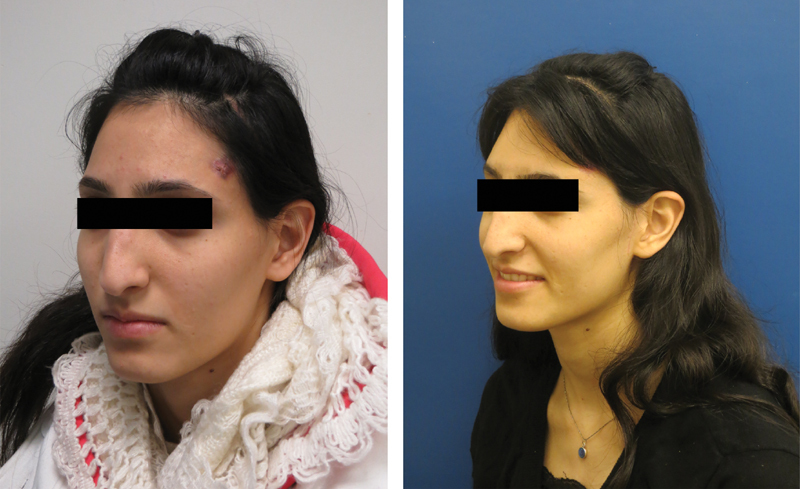
Cosmetic outcome of the patient described in Fig. 3 .
Statistical Analyses
The data were collected, recorded and analyzed using R , a language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria).
The one-sample Wilcoxon's sign-rank test was used to compare our data to those published in the literature. Means, medians, and ranges were used to describe our data. All statistical tests were two-tailed and significance was defined as p < 0.05.
Results
Thirteen patients met the inclusion criteria during the study period and their demographics and clinical characteristics are listed in Table 1 . It should be emphasized that in all cases ( n = 13) the NFO segment was not reused for reconstruction, either due to ORN or due to high risk for future necrosis. The male-to-female ratio was 5:8, with an average age at surgery of 42 years (range: 15–70 years). The overall cohort includes 13 patients. In 10, ASB reconstruction with free flap (FF) was chosen as a result of previous NFO graft failure. This revision surgery (secondary reconstruction) was indicated for reconstructive and not oncological issue. In the additional three cases, the resection and prophylactic reconstruction were performed at the same surgery (primary reconstruction). The average time from primary surgery to complication was 7 years, ranging from 2 to 32 years. In the three primary reconstructed patients, the osseous defect size (the craniotomy window) ranged from 3 × 8 cm (24 cm 2 ) to 4 × 9.5 cm (38 cm 2 ). Resection of the previously operated ostero-radio-necrotized NFO segment is associated with dural tear due to adhesions. Dural defect in primary operation can occur as a result of malignant invasion. In any case, dural tear was observed in 9/13 cases. Five of them sealed primarily, three sealed with temporalis facia, and one with fascia lata grafting. In nine patiens, a lumbar drainage was used routinely. In all four cases operated since 2012, no lumbar drainage was used.
Three patients whose dimensions of the planned defect and the planned adjuvant radiotherapy carried a high risk of ORN underwent the procedure as a primary intervention. Median ASB reconstruction was performed by an ALT free flap in nine cases, and a radial forearm and a ventral rectus abdominis free flap were used in two cases each. Surgery was performed for reconstruction purposes and not for the extirpation of recurrent tumors in all cases of secondary reconstruction. Among our 13 patients series, we had no major complication. We faced some minor donor site complication (i.e., seroma) or selflimiting flap dehiscence which were cured with conservative treatment.
ASB questionnaires were filled in at least 1 year (range: 1–12 years) following the free flap ASB median reconstruction and the responses were comparable to those reported for patients who underwent subcranial ASB resection without free flap. This questionnaire includes several domains (performance, physical function, vitality, pain, specific symptoms, and impact on emotions). The QoL is reflected by addition of all domains state, with higher grade represents better quality. The average results in each domain are summarized in Table 2 and they showed no differences between the two groups.
Table 2. Patient's quality of life by specific domains, with and without free flap reconstruction of the ASB.
| Category | Subcranial for ASB | p -Value | |||
|---|---|---|---|---|---|
| With FF | Without FF | ||||
| Mean | Median | Mean | Median | ||
| Performance | 3.17 | 3 | 2.97 | 3 | 0.527 |
| Physical | 3.17 | 3 | 2.78 | 3.11 | 0.289 |
| Vitality | 3 | 3 | 3 | 2.93 | 0.832 |
| Pain | 3.17 | 2.5 | 2.83 | 2.71 | 0.783 |
| Specific | 2.33 | 2 | 3.03 | 3.15 | 0.13 |
| Impact | 2.83 | 2.5 | 2.86 | 3.02 | 0.832 |
Abbreviations: ASB, anterior skull base; FF, free flap.
Discussion
The aim of a reconstruction of the median ASB is the preservation of function and the provision of an acceptable quality of life and aesthetics. The size, shape and tissue of the reconstruction are a function of the site and extent of the resection. In limited ASB resections without the need for an orbital exenteration or a maxillectomy, reconstruction can usually be performed with preservation of the NFO bony segment combined with fascia lata or pericranium wrapping. 6 7 11 In cases of NFO failure due to ORN or in cases of high-risk for future NFO failure (e.g., irradiated patients, previous operations, etc.), the reconstruction is performed by means of vascularized soft tissue alone, without NFO bony segment preservation. That technique preserves the aim of the reconstruction, specifically, isolation of the oronasal cavities from the intracranial cavity, with good cosmetic outcome, and well-preserved QoL. The QoL of patients who had undergone the surgical technique, in the long-term, is comparable to that of patients who had undergone endoscopic ASB resection.
An extensive skull base defect is inevitable following an open ASB resection. The free conduit between the paranasal sinuses through the soft and bony tissue defect and the intracranial space must be meticulously obliterated to restore the function of the ASB. This involves the establishment of a barrier between the contaminated sinonasal cavity and the sterile intracranial compartment with the prevention of CSF leak by a watertight dural seal and of airflow into the intracranial compartment. Also imperative are the maintenance of a functional sinonasal system and a good cosmetic outcome.
A protocol 11 was developed in our institution to guide the optimal reconstruction modality for each defect, from minimal dural tear, which requires only primary closure to massive orbitomaxillary resection, which necessitates bulky free flap reconstruction. According to that protocol and based on our experience, the subcranial approach using a type B osteotomy (NFO bony segment, which includes the frontal sinus, is removed) should be chosen when a median ASB resection is needed without orbital exenteration or maxillectomy. Even though the QoL was similar between FF and none-FF reconstruction of the ASB in the long term, FF is associated with longer operative time and hospitalization period. Furthermore, it exposes the patient to donor site morbidity and is technically difficult, especially regarding the posterior anchoring as far as the basisphenoid, as it was addressed in the manuscript. If ORN has already been developed, or the frontal skull is invaded by malignant tumor, there is no other choice but to use FF reconstruction. The important issue is when to perform this reconstruction mode “prophylactically.” According to our experiences, in cases associated with neoadjuvant or postoperative radiotherapy, large frontal bony defect or a revision surgery, the high probability of future ORN justifies ASB reconstruction with FF, with its associated risks.
It is important to bear in mind that the NFO segment is vulnerable to an intraoperative thermal injury during the osteotomy process, as well as to intra- and postoperative ischemic injury. 4 5 When the supporting tissue functions adequately, the NFO segment usually tolerates those hazards well, providing mechanical support and forehead contour delineation. However, when the ASB has been irradiated, or when adjuvant radiotherapy is planned, as well as in revision open surgeries for recurrence of malignancies, the resistance of the NFO segment to ischemic injury is greatly reduced, whereupon it would need to be wrapped by pericranium in an attempt to reduce the risk for ORN. In severe cases, in which the chances of NFO preservation are negligible, a free flap is used as an alternative.
Conclusion
The median ASB free flap reconstruction is a safe and reliable method for a long-sized, complex median ASB defect following open resection without orbital exenteration or maxillectomy. It is most suitable for patients who had undergone previous operations, preoperative radiotherapy, and/or ORN of the bony frontal segment.
Conflict of Interest None declared.
Financial Disclosure
There are no financial interests, arrangements, or payments to disclose.
References
- 1.Bentz B G, Bilsky M H, Shah J P, Kraus D. Anterior skull base surgery for malignant tumors: a multivariate analysis of 27 years of experience. Head Neck. 2003;25(07):515–520. doi: 10.1002/hed.10250. [DOI] [PubMed] [Google Scholar]
- 2.Fliss D M, Abergel A, Cavel O, Margalit N, Gil Z. Combined subcranial approaches for excision of complex anterior skull base tumors. Arch Otolaryngol Head Neck Surg. 2007;133(09):888–896. doi: 10.1001/archotol.133.9.888. [DOI] [PubMed] [Google Scholar]
- 3.Fliss D M, Zucker G, Amir A, Gatot A. The combined subcranial and midfacial degloving technique for tumor resection: report of three cases. J Oral Maxillofac Surg. 2000;58(01):106–110. doi: 10.1016/s0278-2391(00)80027-0. [DOI] [PubMed] [Google Scholar]
- 4.Dias F L, Sá G M, Kligerman J et al. Complications of anterior craniofacial resection. Head Neck. 1999;21(01):12–20. doi: 10.1002/(sici)1097-0347(199901)21:1<12::aid-hed2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 5.Ness J A, Chang H S, Grabowski C M, Marentette L J. Osteoradionecrosis of the anterior cranium. Skull Base Surg. 1996;6(04):259–266. doi: 10.1055/s-2008-1058635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gil Z, Fliss D M.Pericranial wrapping of the frontal bone after anterior skull base tumor resection Plast Reconstr Surg 200511602395–398., discussion 399 [DOI] [PubMed] [Google Scholar]
- 7.Hao S P, Chen H C, Wei F C, Chen C Y, Yeh A R, Su J L.Systematic management of osteoradionecrosis in the head and neck Laryngoscope 1999109081324–1327., discussion 1327–1328 [DOI] [PubMed] [Google Scholar]
- 8.Gil Z, Abergel A, Spektor S, Shabtai E, Khafif A, Fliss D M. Development of a cancer-specific anterior skull base quality-of-life questionnaire. J Neurosurg. 2004;100(05):813–819. doi: 10.3171/jns.2004.100.5.0813. [DOI] [PubMed] [Google Scholar]
- 9.Gil Z, Patel S G, Bilsky M, Shah J P, Kraus D H. Complications after craniofacial resection for malignant tumors: are complication trends changing? Otolaryngol Head Neck Surg. 2009;140(02):218–223. doi: 10.1016/j.otohns.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 10.Schade R P, Schinkel J, Roelandse F W et al. Lack of value of routine analysis of cerebrospinal fluid for prediction and diagnosis of external drainage-related bacterial meningitis. J Neurosurg. 2006;104(01):101–108. doi: 10.3171/jns.2006.104.1.101. [DOI] [PubMed] [Google Scholar]
- 11.Gil Z, Abergel A, Leider-Trejo L et al. A comprehensive algorithm for anterior skull base reconstruction after oncological resections. Skull Base. 2007;17(01):25–37. doi: 10.1055/s-2006-959333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gök A, Erkutlu I, Alptekin M, Kanlikama M.Three-layer reconstruction with fascia lata and vascularized pericranium for anterior skull base defects Acta Neurochir (Wien) 20041460153–56., discussion 56–57 [DOI] [PubMed] [Google Scholar]


