Abstract
Objective
Globally, grass pollens (GP) are major aeroallergen triggers of allergic rhinitis (AR) and asthma. However, patterns of allergic sensitisation to pollen of temperate (Pooideae: Lolium perenne) and subtropical (Chloridoideae: Cynodon dactylon and Panicoideae: Paspalum notatum) subfamilies in diverse climates remain unclear. This study aims to evaluate the level of allergic sensitisation and IgE specificity for major GP allergens representing the three subfamilies in biogeographically distinct regions.
Methods
Participants (GP‐allergic with AR, 330; non‐atopic, 29; other allergies, 54) were recruited in subtropical: Queensland, and temperate: New South Wales, Western and South Australia, regions. Clinical history, skin prick test (SPT), total and specific IgE to GP and purified allergens (ImmunoCAP) were evaluated. Cross‐inhibition of sIgE with Pas n 1, Cyn d 1 and Lol p 1 by GP extracts was investigated.
Results
Queensland participants showed higher sensitisation to P. notatum and C. dactylon than L. perenne GP. sIgE was higher to Pas n 1 and Cyn d 1, and sIgE to Pas n 1 and Cyn d 1 was inhibited more by Panicoideae and Chloridoideae, respectively, than Pooideae GP. Conversely, participants from temperate regions showed highest sensitisation levels to L. perenne GP and Lol p 1, and sIgE to Lol p 1 was inhibited more by Pooideae than other GP.
Conclusion
Levels and patterns of sensitisation to subtropical and temperate GP in AR patients depended on biogeography. Knowledge of the specificity of sensitisation to local allergens is important for optimal diagnosis and choice of allergen‐specific immunotherapy to maximise benefit.
Keywords: allergic rhinitis, allergy, cross‐inhibition, grass pollen, IgE
Global distributions of grass subfamilies vary with climate and biogeography. Allergic rhinitis patients with grass pollen allergy, exemplified by four Australian climatic regions, showed higher levels of allergic sensitisation and specific IgE cross‐reactivity to pollen allergens of temperate (e.g. Lolium perenne: Lol p 1) or subtropical (e.g. Paspalum notatum: Pas n 1) grasses, depending upon biogeography. Understanding patterns of patient sensitisation to temperate and subtropical grass pollens is critical for optimal diagnosis and formulation of allergen immunotherapy.

Introduction
Grass pollen (GP) is the most clinically important outdoor aeroallergen source involved in eliciting allergic rhinitis (AR) and asthma in sensitised patients.1 GP has been identified as the leading trigger of the most severe thunderstorm‐related asthma outbreak globally,2 including the most recent and fatal occurring in Melbourne, Australia, in 2016, with 10 related deaths.3, 4, 5 Both temperate and subtropical grasses are found in places with diverse climatic regions such as parts of Australia, Africa, India and America6 (Supplementary figure 1). Research has indicated differences in allergen composition and immune recognition between the pollen of grasses from different subfamilies.7, 8, 9 Given an increase in the global population living in subtropical climates,10 the competitive advantage of subtropical grasses at elevated carbon dioxide concentrations11 and the expansion of subtropical zones because of climate change,12, 13 subtropical grasses of Panicoideae (Paspalum notatum and Sorghum halepense) and Chloridoideae (Cynodon dactylon), subfamilies are likely to become more important allergen sources in the future.
Early research from southern subtropical states of the United States of America (USA) indicated that P. notatum GP was the most frequently positive GP amongst 429 AR patients from Louisiana and of atopic children of military personnel in Texas.14 In the subtropical region of Queensland (QLD), Australia, patients with AR showed higher levels of allergic sensitisation to pollen of subtropical grasses P. notatum and C. dactylon than temperate L. perenne by skin prick test (SPT), serum‐specific IgE15 and cross‐inhibition of specific IgE (spIgE) reactivity with whole GP extracts.16 In contrast, patients from the temperate region of Melbourne (Australia) showed higher serum spIgE reactivity with L. perenne GP extract and its major allergen, Lol p 1, than with P. notatum and C. dactylon GP and their group 1 allergens, Pas n 1 and Cyn d 1, respectively.17
A subsequent cross‐inhibition study of AR patients from Queensland (QLD) showed that spIgE to subtropical P. notatum, C. dactylon and S. halepense GP was inhibited more by these subtropical GP than a mixture of five temperate GP.18 Elsewhere using pooled sera of five patients highly allergic to temperate GP in Minnesota, USA, Bermuda GP was unable to achieve 50% inhibition of sIgE reactivity with temperate GP Koeleria macrantha, Dactylis glomerata, Festuca pratensis or L. perenne. Conversely, in the same study, four orders of magnitude more than P. pratense GP extract were required to achieve 50% inhibition of IgE reactivity with C. dactylon GP.19 Collectively, these studies indicate that GP allergens of subtropical species have distinct immunological reactivity from temperate GP. However, research to date has not integrated clinical history with sensitisation studies of patients from diverse climates. Most cross‐inhibition studies have used small numbers of subjects or serum pools to examine relationships between GP extracts rather than major allergen components.
This study aimed to comprehensively investigate regional differences in levels of allergic sensitisation to subtropical and temperate GP in different biogeographical locations separated by thousands of kilometres. This is the first study to evaluate the variation in levels of spIgE recognition of major group 1 pollen allergens representing subtropical grasses Panicoideae (P. notatum and S. halepense) and Chloridoideae (C. dactylon) subfamilies, as well as temperate grasses Pooideae (L. perenne and P. pratense), using a semi‐automated microscale IgE cross‐inhibition format exemplified across four Australian regions in distinct climatic zones.
Results
A total of 330 GP‐allergic participants with AR were recruited based on clinical history and positive SPT to at least one of four GP tested (Table 1). The age distributions of the GP‐allergic AR group were similar to both control groups, non‐atopic (NA) participants and those with other allergies,20 frequently house dust mite and/or cat dander, who showed negative SPT and serum spIgE to the four GP tested (Table 1). There was a higher proportion (65.5%) of females in the GP‐allergic group than the NA group (44.8%) (P < 0.05). The other allergy (OA) and GP‐allergic participants showed higher levels of total IgE than the NA group. Amongst the GP‐allergic group, there was no difference in gender nor total IgE between participants from each state (Table 2), QLD with a subtropical climate, NSW with mixed temperate and subtropical climates, and WA and SA with temperate climates (Supplementary figure 1).21 GP‐allergic participants from NSW were older than other regions and had lower level of grass pollen sensitisation (Table 2).
Table 1.
Demographics and clinical history of participants recruited in the Grass Pollen Allergy Study (GPAS)
| Non‐atopic | Other allergy | Allergic rhinitis with Grass pollen allergy | |
|---|---|---|---|
| Number | 29 | 54 | 330 |
| Age[Link] | 45.4 (37.5–57.4) | 32.6 (19.2–36.8) | 41.2 (35.3–46.6) |
| Number of females (%)[Link] | 13 (44.8%) | 27 (50%) | 216 (65.5%)* |
| Number with asthma (%)[Link] | 1 (3.4%) | 14 (25.9%)* | 126 (38.1%)**** |
| Sum of SPT (mm)[Link] | 5 (4–12) | 13.5 (8–20)**** | 39 (29–54)**** |
| Total IgE (IQR)[Link] | 31.5 (10.7–67.5) | 142 (49.7–346)**** | 126 (52.5–290)**** |
Values expressed as medians with interquartile range in parentheses.
IQR, interquartile range; n.s., not significant; SPT, skin prick test.
Fisher's exact test for difference in frequencies relative to the NA group.
Kruskal–Wallis test for difference between medians between groups with Dunn's pairwise comparisons.
P ≤ 0.05.
P ≤ 0.0001.
Table 2.
Age, gender, total IgE, grass pollen‐specific and allergen‐specific IgE of grass pollen allergic patients from different regions
| Region | QLD | WA | NSW | SA | Statistical differences |
|---|---|---|---|---|---|
| Number | 138 | 73 | 39 | 80 | |
| Age[Link] | 44 (36–51.8) | 39 (28–49.5) | 52 (41–66) | 39 (30–50) |
NSW > QLD** NSW > WA*** NSW > SA*** |
| Number of females (%)[Link] | 98 (71.0) | 42 (57.5) | 26 (66.7) | 51 (63.0) | n.s. |
| Total IgE, kU L−1 [Link] | 128.5 (73–252.8) | 154 (45.5–318.5) | 137 (62.8–302.5) | 68 (41–189) | n.s. |
| Sum of SPT to GP (mmD)[Link] | 22 (18–27) | 30 (22–48.8) | 18 (9–27) | 25 (19–34) |
WA > NSW**** WA > QLD** SA > NSW*** |
| Sum of GP‐spIgE (kU L−1)[Link] | 29.38 (8.0–70.3) | 20.69 (3.1–51.6) | 9.63 (2.5–24.3) | 22.92 (7.4–88.0) |
QLD > NSW* SA > NSW* |
Data shown as median (interquartile range).
n.s., not significant; NSW, New South Wales; QLD, Queensland; SA, South Australia; SPT, skin prick test; WA, Western Australia.
Kruskal–Wallis test for difference between medians in groups with Dunn's pairwise comparisons.
Fisher's exact test for difference in frequencies.
P ≤ 0.05.
P ≤ 0.01.
P ≤ 0.001.
P ≤ 0.0001.
The patterns of sensitisation to four common allergic GP amongst GP‐allergic participants from each state were compared within each region (Figure 1). GP‐allergic participants from QLD showed significantly higher levels of sensitisation to pollen of P. notatum and C. dactylon than L. perenne by SPT and serum spIgE (Figure 1a and b). QLD participants also showed higher serum spIgE with group 1 allergen components Pas n 1 (P ≤ 0.0001) and Cyn d 1 (P ≤ 0.01) than with Lol p 1 (Figure 1c).
Figure 1.
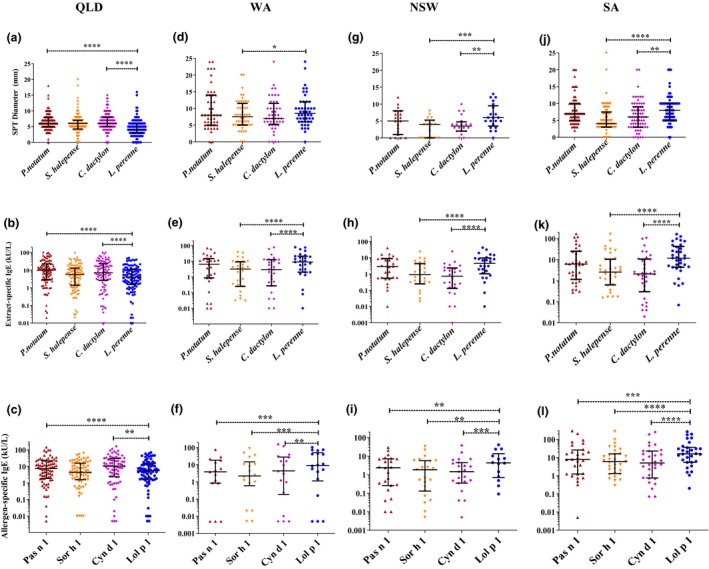
Levels of allergic sensitivity for patients with allergic rhinitis to four grass pollen extracts in different regions measured by skin prick test diameters [(a) QLD n = 127–130; (d) WA n = 48; (g) NSW n = 18–21; (j) SA n = 69–72], serum‐specific IgE concentrations to grass pollen extract [(b) QLD n = 98; (e) WA n = 27; (h) NSW n = 26; (k) SA n = 34] and serum‐specific IgE concentrations to purified group 1 allergen components [(c) QLD n = 76 or 77; (f) WA n = 15–18; (i) NSW n = 14–23; (l) SA n = 28–31]. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 and ****P ≤ 0.0001.
In Western Australia (WA), GP‐allergic participants showed a higher level of sensitisation L. perenne GP than S. halepense GP by SPT diameter (P ≤ 0.05; Figure 1d) and serum spIgE (P ≤ 0.0001; Figure 1e). L. perenne GP‐spIgE concentrations were significantly higher than C. dactylon GP‐spIgE (P ≤ 0.0001), but no difference was observed in SPT (Figure 1d and e). The serum Lol p 1 spIgE concentrations were significantly higher than spIgE with Cyn d 1 (P ≤ 0.01), Sor h 1 (P ≤ 0.001) and Pas n 1 (P ≤ 0.01; Figure 1f).
In New South Wales (NSW), GP‐allergic participants showed a higher level of sensitisation to L. perenne than both S. halepense (P ≤ 0.01) and C. dactylon (P ≤ 0.001) by SPT (Figure 1g). This was more marked for serum spIgE concentrations to these GP extracts (P ≤ 0.0001; Figure 1h). The serum spIgE concentrations were significantly higher for Lol p 1 than any of the subtropical GP allergen, Cyn d 1 (P ≤ 0.001), Sor h 1 (P ≤ 0.01) and Pas n 1 (P ≤ 0.01; Figure 1i).
In South Australia (SA), GP‐allergic participants showed higher SPT diameters to L. perenne than to either S. halepense (P ≤ 0.01) or C. dactylon (P ≤ 0.001) and higher serum spIgE concentrations to the extracts (P ≤ 0.0001; Figure 1j and k). GP‐allergic participants from SA showed significantly higher concentrations of spIgE to Lol p 1 than any of the subtropical GP allergen, Cyn d 1 (P ≤ 0.001), Sor h 1 (P ≤ 0.0001) and Pas n 1 (P ≤ 0.0001; Figure 1l).
Cross‐inhibition of sIgE to purified allergen by GP extracts
Individual sera were subjected to microscale spIgE cross‐inhibition assays to examine further the specificity and avidity of IgE binding to purified pollen allergens of subtropical, Pas n 1 and Cyn d 1, as well as temperate, Lol p 1. The ages, gender, total IgE and sum of GP‐spIgE were similar amongst the subset of participants who were selected for spIgE cross‐inhibition assays for each region (Supplementary table 1). Differences were observed in Cyn d 1 spIgE levels in patient sera from QLD with NSW and SA (P > 0.05). Exemplary inhibition curves for one patient from QLD and one patient from SA for inhibition of spIgE reactivity with Pas n 1, Cyn d 1 and Lol p 1 by subtropical Panicoideae: P. notatum, S. halepense; Chloridoideae: C. dactylon; and Pooideae: L. perenne and P. pratense GP extracts in comparison with raw peanut extract (RPN) are shown in Supplementary figures 3b, c and d, and 4b, c and d.
In QLD, self‐inhibition by the GP extract from which the allergen was purified was significantly higher than the RPN control for Pas n 1, Cyn d 1 and Lol p 1 (Figures 2a, b and c, and 3a, b and c). Maximum inhibition of spIgE reactivity with Pas n 1 by C. dactylon (P ≤ 0.05) and L. perenne GP (P ≤ 0.05) was significantly lower than self‐inhibition by P. notatum GP (Figure 2a). The area under the curve for spIgE of reactivity with Pas n 1 was significantly different than self‐inhibition by P. notatum GP, and L. perenne (P ≤ 0.01), P. pratense (P ≤ 0.001), C. dactylon (P ≤ 0.01) GP, but not S. halepense GP, indicating specific and avid IgE binding to Pas n 1 (Figure 3a). Maximum inhibition of spIgE reactivity with Cyn d 1 by L. perenne (P ≤ 0.001) and P. pratense (P ≤ 0.01) GP was significantly lower than self‐inhibition by C. dactylon GP (Figure 2b). Similarly, the area under the inhibition curves for spIgE reactivity with Cyn d 1 was significant different than self‐inhibition by C. dactylon GP for L. perenne (P ≤ 0.01) and P. pratense (P ≤ 0.01), indicating specific and avid IgE reactivity with Cyn d 1 (Figure 3b). Maximum inhibition of spIgE reactivity with Lol p 1 by C. dactylon was significantly lower (P ≤ 0.05) than self‐inhibition by L. perenne GP (Figure 2c). However, no difference in the area under the inhibition curve of spIgE reactivity to Lol p 1 was observed for any GP (P ≤ 0.0001; Figure 3c).
Figure 2.
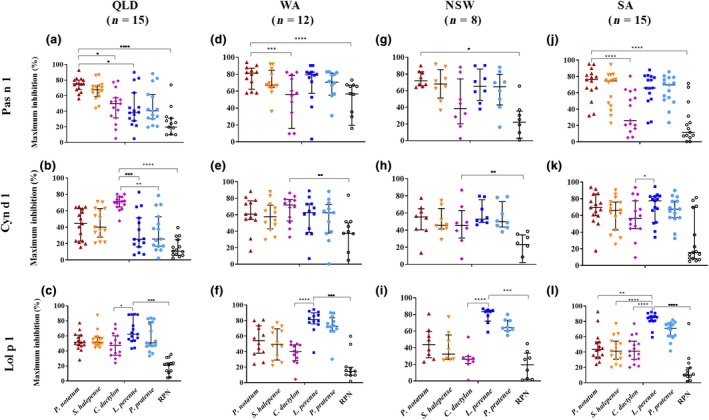
Maximum cross‐inhibition of serum IgE reactivity with purified allergen components Pas n 1, Cyn d 1 and Lol p 1 by inhibitor extracts; P. notatum ( ), S. halepense (
), S. halepense ( ), C. dactylon (
), C. dactylon ( ), L. perenne (
), L. perenne ( ), P. pratense (
), P. pratense ( ) and raw peanut control (
) and raw peanut control ( ) for a subset of patients from Queensland (QLD), Western Australia (WA), New South Wales (NSW) and South Australia (SA). Maximum inhibition expressed as median and interquartile range. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 and ****P ≤ 0.0001.
) for a subset of patients from Queensland (QLD), Western Australia (WA), New South Wales (NSW) and South Australia (SA). Maximum inhibition expressed as median and interquartile range. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 and ****P ≤ 0.0001.
Figure 3.
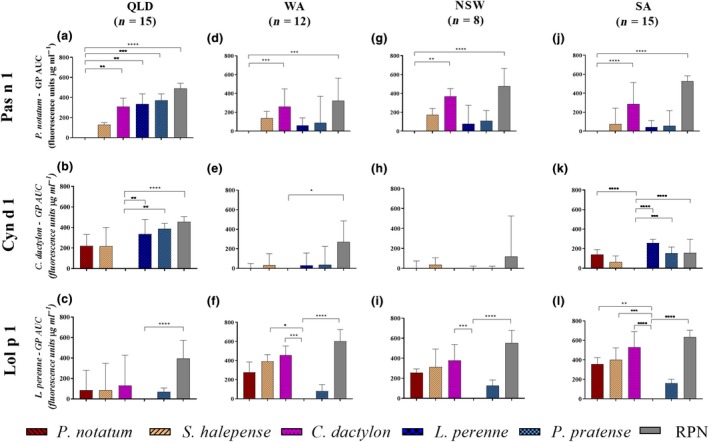
Difference in area under the cross‐inhibition curve between inhibition of specific IgE reactivity with purified allergen components Pas n 1, Cyn d 1 and Lol p 1 by inhibitor extracts; P. notatum, S. halepense, C. dactylon, L. perenne, P. pratense and raw peanut negative control, for a subset of patients in Queensland (QLD), Western Australia (WA), New South Wales (NSW) and South Australia (SA). Data expressed as median and upper quartile (whiskers) difference in area under the curve (AUC) relative to the self‐inhibitor grass pollen (GP) extract and other inhibitor extracts. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 and ****P ≤ 0.0001.
In WA, significant self‐inhibition was observed for Pas n 1 (P ≤ 0.0001), Cyn d 1 (P ≤ 0.01) and Lol p 1 (P ≤ 0.0001) (Figures 2d, e and f, and 3d, e and f). spIgE reactivity with Pas n 1 by C. dactylon (P ≤ 0.001) was significantly lower than self‐inhibition by P. notatum by maximum inhibition and area under the inhibition curve, exemplifying the difference in IgE specificity between Cyn d 1 and Pas n 1 (Figures 2d and 3d). No significant difference in the area under the inhibition curve of IgE reactivity with Cyn d 1 between self‐inhibition by C. dactylon and other GP was observed (Figure 3e). Maximum inhibition of spIgE reactivity with Lol p 1 by C. dactylon was significantly lower (P ≤ 0.0001) than self‐inhibition by L. perenne GP (Figure 2f). Large differences in the area under the inhibition curves of spIgE reactivity to Lol p 1 between self‐inhibition by L. perenne and C. dactylon (P ≤ 0.0001) and S. halepense (P ≤ 0.05) were observed (Figure 3f).
In NSW, self‐inhibition compared with RPN was observed for Pas n 1 (P ≤ 0.01), Cyn d 1 (P ≤ 0.001) and Lol p 1 (P ≤ 0.001) based on maximum inhibition (Figure 2g, h and i) and for Pas n 1 (P ≤ 0.0001) and Lol p 1(P ≤ 0.0001) by difference in the area under the inhibition curve (Figure 3g and i). However, the area under the curve between self‐inhibition of Cyn d 1 spIgE by C. dactylon GP did not differ from RPN indicating that IgE binding to Cyn d 1 in these subjects may not have been specific for Cyn d 1 (Figure 3h). spIgE reactivity to Pas n 1 was inhibited less by C. dactylon GP than P. notatum GP based on the area under the inhibition curve (P ≤ 0.001) but the maximum inhibition did not differ (Figures 2g and 3g). spIgE reactivity with Lol p 1 was inhibited less by C. dactylon GP than self‐inhibition by L. perenne based on maximum inhibition (P ≤ 0.0001) and difference in area under the inhibition curve (P < 0.001; Figures 2i and 3i).
In SA, significant self‐inhibition compared to RPN was observed for spIgE reactivity with Pas n 1 (P < 0.0001) and Lol p 1 (P ≤ 0.0001 (Figures 2j, k and l, and 3j, k and l). Inhibition of Pas n 1 spIgE reactivity by C. dactylon GP was significantly lower than self‐inhibition by P. notatum GP by maximum inhibition (P ≤ 0.0001) and difference in area under the inhibition curve (P ≤ 0.0001; Figures 2j and 3j). The maximum inhibition of spIgE reactivity of Cyn d 1 by RPN did not differ (Figure 2k), but significant differences from self‐inhibition by C. dactylon GP based on area under the inhibition curve were observed with P. notatum GP (P ≤ 0.001), L. perenne GP (P ≤ 0.0001) and P. pratense GP (P ≤ 0.001; Figure 3k). Maximum inhibition of Lol p 1 spIgE reactivity by C. dactylon GP (P<0.0001), S. halepense (P<0.0001) and P. notatum (P<0.01) were significantly lower than self‐inhibition by L. perenne GP (Figure 2l). The area under the inhibition curve of spIgE reactivity with Lol p 1 was lower than self‐inhibition by L. perenne GP for C. dactylon GP (P ≤ 0.0001), P. notatum GP (P ≤ 0.01) and S. halepense GP (P ≤ 0.001), whilst that for P. pratense GP was similar to L. perenne GP (Figure 3l).
Discussion
Whilst GP is the major outdoor allergen trigger for allergic rhinitis worldwide, there is considerable biological diversity amongst Poaceae subfamilies that influences the allergen composition and degree of similarity between GP allergens.8, 22 Biogeographical variation over four regions in Australia with different latitudes and climates, QLD, WA, NSW and SA, appeared to affect the level of sensitisation to Panicoideae, Chloridoideae and Pooideae GP determined by skin prick test diameter as well as serum spIgE concentration to GP extracts and purified group 1 allergen components. Overall, participants from the subtropical region of QLD showed higher sensitivity to Panicoideae and Chloridoideae GP, whilst those from temperate climates of SA showed higher sensitivity to the Pooideae GP.
The observed difference in concentrations of spIgE to GP allergen components appeared to be more pronounced than differences between concentrations of spIgE to whole GP extracts, for example spIgE to Lol p 1 compared to L. perenne GP extract in WA, NSW and SA, suggesting that measuring spIgE to allergen components may offer better diagnostic precision than GP extracts.22 However, diagnostic tests for spIgE to Pas n 1 and Sor h 1 are not available commercially and Cyn d 1 is only available as a natural purified allergen component, which may show false positives because of cross‐reactive carbohydrate determinants (CCD) in populations where grass is not an important aeroallergen source.
This study is one of the first to compare GP allergen sensitisation profiles from multiple states across a wide geographical range separated by 4300 km east to west and 1100 km north to south. The IgE cross‐inhibition assays revealed regional variation in the avidity of spIgE for purified group 1 allergen components subtropical and temperate GP. Specific and unique recognition of Pas n 1 and Cyn d 1 by participant spIgE was observed for individuals from the subtropical region of QLD that could not be blocked by L. perenne or P. pratense GP. Conversely, in the temperate region of SA, spIgE reactivity with Lol p 1 was inhibited less by Panicoideae and Chloridoideae GP.
The use of microscale cross‐inhibition assays allowed for resolution of specificity and avidity of spIgE reactivity for the three group 1 allergens by comparing both maximum inhibition and the area under the inhibition curve comparisons for GP from separate subfamilies. The comparisons of difference in the area under the inhibition curves between other GP and self‐inhibitor GP extracts indicated differences in spIgE avidity. In previous studies, investigators have compared the inhibitor concentration at which 50% inhibition (IC50) is reached.18 In this study, cases where GP extracts and the irrelevant plant inhibitor control RPN extract did not reach the IC50, the area under the curve was more useful than reliance on IC50 for quantitative analysis. For example, in patients from QLD, only C. dactylon and L. perenne showed significantly different maximum inhibition to P. notatum self‐inhibition of spIgE to Pas n 1, whereas analysis of the area under inhibition curves revealed differences between C. dactylon, L. perenne and P. pratense GP and P. notatum GP. Similarly, for participants from WA, only C. dactylon GP showed a significant difference in maximum inhibition of spIgE with Lol p 1, whereas analysis of area under the curve indicated significant differences from self‐inhibition of spIgE with Lol p 1 by L. perenne GP and C. dactylon as well as S. halepense GP.
Across all regions, GP from the same subfamily showed comparable IgE reactivity to each other, as exemplified by the Panicoideae P. notatum and S. halepense and Pooideae L. perenne and P. pratense GP. C. dactylon GP showed unique spIgE reactivity indicating the presence of some distinct Cyn d 1 epitopes compared to allergens of either Panicoideae or Pooideae GP. The phylogenetic relationship between grasses within subfamilies is closer than between subfamilies,16 and consequently, there is higher primary sequence similarities of major allergens within subfamilies.23 Structural differences between epitopes of allergens of separate grass subfamilies are likely to account for the observed region‐dependent, species‐specific IgE recognition of GP allergens in sensitised GP‐allergic participants, although there has been little comparative B‐cell epitope analysis of GP allergens to date.15, 24
A limitation of this study was the small number of NSW participants, reducing the sample size for the cross‐inhibition analysis (n = 8), because of low serum availability. Also, of the 15 participants living in QLD who were selected for the cross‐inhibition assays based on sum of GP‐specific IgE and serum availability, four were born outside QLD, in regions with a predominance of L. perenne (i.e. NSW, WA and the United Kingdom), contributing to variation in the range of inhibition of spIgE with Pas n 1 and Lol p 1 by L. perenne and P. pratense GP. This suggests the place of origin where sensitisation occurred and migration, may diversify the allergen sensitisation profiles of individuals within a population. Regional differences in sensitisation rates, even for closely related taxa, based on analysis of large Japanese IgE data sets have been recently reported.25 These findings reinforce that clinical interpretation of spIgE responses needs to be correlated with symptoms upon exposure and the allergens likely to be encountered by the patient within their usual environment.
Allergen‐specific immunotherapy (AIT) offers effective reduction in symptoms and reduces new sensitisations and the risk of progression to asthma in children.26 Assessment of patients receiving temperate GP sublingual immunotherapy (SLIT) tablets showed lowered mean AR medication prescriptions and lower proportions of new onset of asthma than the non‐AIT‐treated groups.27 However, currently available AIT is not standardised for subtropical GP, which may be important for efficacious treatment of people who are primarily allergic to Panicoideae and/or Chloridoideae GP, given that only one of three T‐cell epitopes is shared between major allergens Pas n 1, Cyn d 1 and Lol p 1.28, 29, 30 More specific assessment of GP sensitisation profiles, particularly in countries with multiple climatic zones, may serve to precisely identify the relevant GP to achieve the most efficacious treatment for an individual patient.22 More broadly, understanding current patterns of allergic sensitisation is important for being prepared to respond to changing floristic zones and allergen distributions in future.
Conclusions
The outcomes of this study demonstrate that sensitisation to GP in AR patients varies according to the biogeographical region of residence. Patients showed more specific and avid IgE reactivity to GP of the most biogeographically abundant grasses within their respective climatic regions. GP of the same subfamily showed similar levels of spIgE cross‐inhibition to each other indicating subfamily‐specific allergic sensitisation. Allergens of the two subtropical Panicoideae and Chloridoideae grass subfamilies show separate IgE reactivity patterns. Regional variation in allergic sensitivity is clinically important worldwide for optimal diagnosis and targeted AIT for patients with AR because of GP allergy, especially in places where patients may be co‐exposed to multiple types of GP from separate subfamilies.
Methods
Participants
Participants for the Grass Pollen Allergy Study (GPAS; HREC/2009/QPAH/296)20 were recruited with informed consent. Adult participants [NA n = 29, OA n = 54] and GP‐allergic participants with AR (n = 330) were recruited at clinical allergy and immunology specialist clinics in QLD: Cairns, Townsville, Brisbane, Toowoomba and the Gold Coast (latitudes −21 to −27°S); Western Australia (WA): Perth and Fremantle (latitude −32°S); New South Wales (NSW): Sydney (latitude −33.9°S); and South Australia (SA): Adelaide (latitude −35°S) (Table 1). Patients who had prior GP immunotherapy were excluded. Participant demographics, clinical history and SPT to ten environmental aeroallergen extracts including four GP, P. notatum, S. halepense, C. dactylon and L. perenne, Southern GP mix (Poa pratensis, Dactylis glomerata, Agrostis gigantea, Phleum pratense, Anthoxanthum odoratum), as well as house dust mite, cat dander, Alternaria, Aspergillus moulds and ragweed pollen (GreerLabs, Lenoir, NC, USA), were assessed.20 Serum total and spIgE to four GP extracts (g17, g10, g2 and g5 ImmunoCAPs, Thermo Fisher Scientific, Uppsala, Sweden) were measured. spIgE concentrations to purified natural allergen components, Pas n 1, Cyn d 1 and Lol p 1, that were biotinylated were measured using streptavidin ImmunoCAPs as previously described.20
Cross‐inhibition assay by DELFIA time‐resolved fluorescence
A subset of sera from 8 to 15 GP‐allergic participants were selected from each region based on availability of serum and sum of spIgE concentrations to four GP. Individual sera were firstly titrated against three purified allergens representing each of the Poaceae subfamilies, Pas n 1, Cyn d 1 and Lol p 1, coated at 1 μg mL−1,20 from a serum dilution of 1/10 with fourfold serial dilutions (exemplified in Supplementary figures 2 and 3). The mid‐point of the linear phase of the titration curve was chosen for each individual as the serum dilution for the cross‐inhibition assay. For each serum, the same dilution was used for testing inhibition of spIgE with for each of the three allergens. Cross‐inhibition immunoassays of spIgE reactivity with Pas n 1, Cyn d 1 and Lol p 1 were then performed as previously described17 with the following modifications. Five GP extracts, and RPN extract as an irrelevant plant allergen control, were tested as inhibitors at concentrations of 0.016–100 μg mL−1. Assays were performed in quadruplicate in a 10 μL volume in 384‐well format assisted by semi‐automated liquid handling. Allergen spIgE binding was assessed by incubation with polyclonal rabbit anti‐human IgE (Dako, Glostrup, Denmark) at 1/2000 dilution and then Europium‐conjugated goat anti‐rabbit IgG (PerkinElmer, Waltham, MA, USA) at 1/1000 dilution. Reactivity was detected by DELFIA Time‐Resolved Fluorescence (PerkinElmer) after 20‐min incubation at room temperature.
Data analysis
Participant gender and percentage with asthma were assessed by Fisher's exact test for difference in frequencies between groups. Outcomes of continuous variables were tested for normality of distribution by the Kolmogorov–Smirnov test. Participant age, sum of SPT and total IgE between groups were then compared by Kruskal–Wallis ANOVA for difference between medians in groups with Dunn's pairwise comparisons.
Responses of GP‐allergic participants within each state for SPT, serum spIgE to GP extracts and purified group 1 allergen components were expressed as scatterplots with median and interquartile range. Data were compared by Friedman ANOVA with Dunn's multiple comparison for paired data of participants within each state.
For spIgE cross‐inhibition data, the maximum inhibition and area under the curve were calculated. Self‐inhibition was defined as inhibition of IgE reactivity with a purified allergen by the GP extract the allergen was derived from, such as the inhibition of Pas n 1 spIgE reactivity by P. notatum GP, Cyn d 1 by C. dactylon GP and Lol p 1 by L. perenne GP. Significant self‐inhibition was judged to have been achieved when self‐inhibition is significantly higher than the inhibition of spIgE reactivity by the irrelevant plant allergen inhibition control of RPN extract. The differences between the maximum level of self‐inhibition and maximum level of inhibition by the other GP extracts were compared. The difference in area under the inhibition curve for each inhibitor extract relative to self‐inhibition was calculated by subtraction from the area under the curve for the self‐inhibitor extract. Area under the curve data were expressed as graph bars of the median difference in area under the curve with upper interquartile range as upper whisker.
Conflict of interest
Professor Davies reports grants from the Australian National Health and Medical Research Council (NHMRC) (Development 1017441) and Asthma Foundation of Queensland, during the conduct of the study; grants from National Foundation of Medical Research Innovation (NFMRI), Allergy and Immunology Foundation of Australasia and Emergency Medicine Foundation, outside the submitted work; Professor Davies is an inventor of patents owned by QUT; US PTO 14/311944 issued, a patent PCT/AU2014/000630/WO2014_201499, a patent PCT/AU2015/050348 pending and a patent AU2008/316301 issued; Professor Davies leads the NHMRC AusPollen Partnership Project (GNT 1116107) with matching cash and in‐kind co‐sponsorship from The Australasian Society for Clinical Immunology and Allergy, Asthma Australia, Bureau of Meteorology, Commonwealth Scientific and Industrial Research Organisation, Stallergenes Australia, Federal Office of Meteorology and Climatology MeteoSwiss, Switzerland. Professor Davies is CI of current NFMRI grant with co‐sponsorship from Abionic, Switzerland. She has had a contracted research grant from Stallergenes (France), in‐kind provision of materials from Thermo Fisher (Sweden) and services from Sullivan Nicolaides Pathology (QLD, Australia). Professor Davies's institute has received honorarium payments and travel expenses for education sessions and conference presentations from Stallergenes Australia and WyMedical. Mr Kailaivasan reports grants from QUT‐Malaysia Australia Colombo Plan Commemoration Scholarship. Dr P. Smith reports grants from Mylan, outside the submitted work; Dr P Smith speaking fees from GlaxoSmithKline, Novartis, Astra Zeneca, Mundipharma and Mylan and he has served on an advisory board for Seqirus in the last 3 years. Dr Langguth is employed by Sullivan Nicolaides Pathology. Dr Upham reports grants from NHMRC during the conduct of the study; personal fees from Astra Zeneca, Novartis, GSK and Boehringer Ingelheim, outside the submitted work. Mrs Timbrell, Dr van Nunen, Dr Solley, Dr W Smith and Dr McLean‐Tooke have nothing to disclose.
Supporting information
Acknowledgments
We thank clinical research nurses Michelle Towers and Tina Collins (Princess Alexandra Hospital, Brisbane) and Melanie Burk (Royal North Shore Hospital, Sydney) for assistance with recruitment and phlebotomy. We thank Thermo Fisher, Sweden, for providing in‐kind ImmunoCAP materials and Sullivan Nicolaides Pathology (Australia) for providing in‐kind pathology services for this project. We are grateful for the technical assistance of Mr Lindsay Reibelt and Ms Claire Simmonds (Sullivan Nicolaides Pathology) for facilitating the testing of specific IgE to purified allergen components. This research was supported by Asthma Foundation of Queensland and the (Australian) National Health and Medical Research Council Development Grant Number 1017441 with in‐kind contribution of materials from Thermo Fisher Scientific (Sweden) and immunopathology services from Sullivan Nicolaides Pathology (Queensland, Australia). Mr Kailaivasan is supported by a QUT‐Malaysia Australia Colombo Plan Commemoration Scholarship.
References
- 1. Bauchau V, Durham SR. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J 2004; 24: 758–764. [DOI] [PubMed] [Google Scholar]
- 2. Davies JM, Erbas B, Simunovic M, Al Kouba J, Milic A. Literature review on thunderstorm asthma and its implications for public health advice: final report. Brisbane, QLD: Queensland University of Technology; Contracted by Department of Health and Human Services, Victorian State Government; 2017. Available from: https://www2.health.vic.gov.au/about/publications/researchandreports/thunderstorm-asthma-literature-review-may-2107 (accessed August 12, 2019). [Google Scholar]
- 3. Hew M, Sutherland M, Thien F, O'Hehir R. The Melbourne thunderstorm asthma event: can we avert another strike? Intern Med J 2017; 47: 485–487. [DOI] [PubMed] [Google Scholar]
- 4. Lee J, Kronborg C, O'Hehir RE, Hew M. Who's at risk of thunderstorm asthma? The ryegrass pollen trifecta and lessons learnt from the Melbourne thunderstorm epidemic. Respir Med 2017; 132: 146–148. [DOI] [PubMed] [Google Scholar]
- 5. Thien F, Beggs PJ, Csutoros D et al The Melbourne epidemic thunderstorm asthma event 2016: an investigation of environmental triggers, effect on health services, and patient risk factors. Lancet Planet Health 2018; 2: e255–e263. [DOI] [PubMed] [Google Scholar]
- 6. Medek DE, Beggs PJ, Erbas B et al Regional and seasonal variation in airborne grass pollen levels between cities of Australia and New Zealand. Aerobiologia 2016; 32: 289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Andersson K, Lidholm J. Characteristics and immunobiology of grass pollen allergens. Int Arch Allergy Immunol 2003; 130: 87–107. [DOI] [PubMed] [Google Scholar]
- 8. Davies JM. Grass pollen allergens globally: the contribution of subtropical grasses to burden of allergic respiratory diseases. Clin Exp Allergy. 2014; 44: 790–801. [DOI] [PubMed] [Google Scholar]
- 9. Johansen N, Weber RW, Ipsen H, Barber D, Broge L, Hejl C. Extensive IgE cross‐reactivity towards the Pooideae grasses substantiated for a large number of grass‐pollen‐sensitized subjects. Int Arch Allergy Immunol 2009; 150: 325–334. [DOI] [PubMed] [Google Scholar]
- 10. Staten P, Lu J, Grise KM, Davis SM, Birner T. Re‐examining tropical expansion. Nat Clim Change 2018; 8: 768–775. [Google Scholar]
- 11. Morgan JA, LeCain DR, Pendall E et al C4 grasses prosper as carbon dioxide eliminates desiccation in warmed semi‐arid grassland. Nature 2011; 476: 202–205. [DOI] [PubMed] [Google Scholar]
- 12. Norris JR, Allen RJ, Evan AT, Zelinka MD, O'Dell CW, Klein SA. Evidence for climate change in the satellite cloud record. Nature 2016; 536: 72. [DOI] [PubMed] [Google Scholar]
- 13. Turton SM. Expansion of the tropics: revisiting frontiers of geographical knowledge. Geogr Res 2017; 55: 3–12. [Google Scholar]
- 14. Calabria CW, Dice J. Aeroallergen sensitization rates in military children with rhinitis symptoms. Ann Allergy Asthma Immunol 2007; 99: 161–169. [DOI] [PubMed] [Google Scholar]
- 15. Ball T, Fuchs T, Sperr WR et al B cell epitopes of the major timothy grass pollen allergen, phl p 1, revealed by gene fragmentation as candidates for immunotherapy. FASEB J 1999; 13: 1277–1290. [DOI] [PubMed] [Google Scholar]
- 16. Davies JM, Li H, Green M, Towers M, Upham JW. Subtropical grass pollen allergens are important for allergic respiratory diseases in subtropical regions. Clin Transl Allergy 2012; 2: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Davies JM, Dang TD, Voskamp A et al Functional immunoglobulin E cross‐reactivity between Pas n 1 of Bahia grass pollen and other group 1 grass pollen allergens. Clin Exp Allergy 2011; 41: 281–291. [DOI] [PubMed] [Google Scholar]
- 18. Nony E, Timbrell V, Hrabina M et al Specific IgE recognition of pollen allergens from subtropic grasses in patients from the subtropics. Ann Allergy Asthma Immunol 2015; 114: 214–220.e2. [DOI] [PubMed] [Google Scholar]
- 19. Leiferman KM, Gleich GJ. The cross‐reactivity of IgE antibodies with pollen allergens. I. Analyses of various species of grass pollens. J Allergy Clin Immunol 1976; 58: 129–139. [DOI] [PubMed] [Google Scholar]
- 20. Timbrell VL, Riebelt L, Simmonds C et al An immunodiagnostic assay for quantitation of specific IgE to the major pollen allergen component, Pas n 1, of the subtropical Bahia grass. Int Arch Allergy Immunol 2014; 165: 219–228. [DOI] [PubMed] [Google Scholar]
- 21. Bureau of Meteorology . Climate classification of Australia 2005. Available from: http://www.bom.gov.au/jsp/ncc/climate_averages/climate-classifications/index.jsp?maptype=kpngrp#maps.
- 22. Matricardi PM, Kleine‐Tebbe J, Hoffmann HJ et al EAACI molecular allergology user's guide. Pediatr Allergy Immunol 2016; 27(Suppl 23): 1–250. [DOI] [PubMed] [Google Scholar]
- 23. Davies JM, Mittag D, Dang TD et al Molecular cloning, expression and immunological characterisation of Pas n 1, the major allergen of Bahia grass Paspalum notatum pollen. Mol Immunol 2008; 46: 286–293. [DOI] [PubMed] [Google Scholar]
- 24. Yuan HC, Wu KG, Chen CJ et al Mapping of IgE and IgG4 antibody‐binding epitopes in Cyn d 1, the major allergen of Bermuda grass pollen. Int Arch Allergy Immunol 2012; 157: 125–135. [DOI] [PubMed] [Google Scholar]
- 25. Minami T, Fukutomi Y, Inada R et al Regional differences in the prevalence of sensitization to environmental allergens: analysis on IgE antibody testing conducted at major clinical testing laboratories throughout Japan from 2002 to 2011. Allergol Int 2019; 68: 440–449. [DOI] [PubMed] [Google Scholar]
- 26. Dhami S, Kakourou A, Asamoah F et al Allergen immunotherapy for allergic asthma: a systematic review and meta‐analysis. Allergy 2017; 72: 1825–1848. [DOI] [PubMed] [Google Scholar]
- 27. Devillier P, Wahn U, Zielen S, Heinrich J. Grass pollen sublingual immunotherapy tablets provide long‐term relief of grass pollen‐associated allergic rhinitis and reduce the risk of asthma: findings from a retrospective, real‐world database subanalysis. Expert Rev Clin Immunol 2017; 13: 1199–1206. [DOI] [PubMed] [Google Scholar]
- 28. Burton MD, Papalia L, Eusebius NP, O'Hehir RE, Rolland JM. Characterization of the human T cell response to rye grass pollen allergens Lol p 1 and Lol p 5. Allergy 2002; 57: 1136–1144. [DOI] [PubMed] [Google Scholar]
- 29. Etto T, de Boer C, Prickett S et al Unique and cross‐reactive T cell epitope peptides of the major Bahia grass pollen allergen, Pas n 1. Int Arch Allergy Immunol 2012; 159: 355–366. [DOI] [PubMed] [Google Scholar]
- 30. Eusebius NP, Papalia L, Suphioglu C et al Oligoclonal analysis of the atopic T cell response to the group 1 allergen of Cynodon dactylon (bermuda grass) pollen: pre‐ and post‐allergen‐specific immunotherapy. Int Arch Allergy Immunol 2002; 127: 234–244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


