Abstract
Background
Amrubicin chemotherapy is a treatment option for patients with non‐small cell lung cancer (NSCLC) after third‐line treatment in Japan. Although topoisomerase‐II (Topo‐II), a target of amrubicin, has been reported to be a prognostic or predictive marker for chemosensitivity and clinical outcomes in various types of malignancies, its effects in the Japanese population remain unknown.
Methods
Data regarding 44 patients with advanced NSCLC treated with amrubicin between April 2004 and May 2014 were retrospectively analyzed. We evaluated the expression levels of Topo‐II by immunohistochemical staining of tumor specimens obtained via biopsy or surgical resection.
Results
The majority of enrolled patients were men (68%) with a median age of 67 (range, 43–78) years. The most common histological type was adenocarcinoma (70%). High Topo‐II expression was observed in 13 (30%) of the 44 patients. The median progression‐free survival and overall survival (OS) durations were 1.8 and 8.8 months, respectively. While there was no significant association between Topo‐II expression and progression‐free survival, patients with low Topo‐II expression had significantly longer OS than did those with high Topo‐II expression. Good performance status and low expression of Topo‐II were all significantly associated with a favorable OS.
Conclusion
Low expression of Topo‐II was identified as an independent prognostic factor for longer survival in patients with NSCLC receiving amrubicin, a Topo‐II inhibitor.
Key points
Significant findings of the study
The median progression‐free survival and overall survival (OS) durations were 1.8 and 8.8 months, respectively.
While there was no significant association between Topo‐II expression and progression‐free survival, patients with low Topo‐II expression had significantly longer OS than did those with high Topo‐II expression.
Good performance status and low expression of Topo‐II were all significantly associated with a favorable OS.
What this study adds
This study is the first to assess the effects of topoisomerase‐II (Topo‐II), a target of amrubicin, as a prognostic or predictive marker for chemosensitivity and clinical outcomes in the Japanese population.
Keywords: Amrubicin, non‐small cell lung cancer, prognostication, topoisomerase‐II
Introduction
Lung cancer is the most common cancer worldwide, death from which accounted for 27% of all cancer‐related deaths in 2016.1 Non‐small cell lung cancer (NSCLC) accounts for 85% of all primary lung cancers.1 Most patients with advanced NSCLC experience disease relapse and require subsequent therapies. Docetaxel, pemetrexed, gemcitabine, ramucirumab plus docetaxel, nivolumab, pembrolizumab, or atezolizumab is recommended by the National Comprehensive Cancer Network guidelines as subsequent chemotherapy for patients with advanced NSCLC.2
Amrubicin hydrochloride (completely synthetic 9‐amino‐anthracycline) is converted to its active metabolite (amrubicinol) through the reduction of a C‐13 ketone group to a hydroxyl group by carbonyl reductase.3 Amrubicin and amrubicinol are inhibitors of DNA topoisomerase‐II (Topo‐II) and exert cytotoxic effects by stabilizing a Topo‐II‐mediated cleavable complex rather than acting as DNA intercalators. Amrubicinol is 5–100‐fold more active than amrubicin.4 Amrubicin has been approved for the treatment of small cell lung cancer (SCLC) and NSCLC in Japan,5 and is a treatment option for patients with advanced NSCLC after third‐line treatment. The response rates have been shown to be more than 75% and approximately 20% among patients with SCLC and NSCLC, respectively, in previous phase II studies of 45 mg/m2 amrubicin as first‐line treatment.5, 6 We reported a phase II study of amrubicin administered at 35 mg/m2 for three consecutive days to patients with previously treated lung cancer. Dose reduction resulted in a comparable response with fewer adverse events.7 To assess the efficacy of amrubicin in previously treated NSCLC, randomized, open‐label, phase III trials for amrubicin and docetaxel were conducted recently in Japan. The median progression‐free survival (PFS) durations of the amrubicin and docetaxel groups were 3.6 and 3.0 months, respectively; the overall survival (OS) durations were 14.6 and 13.5 months, respectively; and the overall response rates were 14.4% and 19.6%, respectively.8 Although amrubicin did not significantly improve the PFS, which was the primary endpoint compared with that reported with docetaxel, it appears to be a suitable regimen for patients with recurrent advanced NSCLC.
Topoisomerase II alpha (Topo‐IIα) is a nuclear enzyme that is upregulated in proliferating cells. It catalyzes the ATP‐dependent breakage and rejoining of double‐stranded DNA and serves as the molecular target of Topo‐II inhibitors including etoposide.9 Topo‐II, a target of anthracycline drugs, has been reported as a prognostic or predictive marker for chemosensitivity and clinical outcomes in various types of malignancies.10, 11, 12, 13 However, the relationship between Topo‐II expression and response to amrubicin remains unclear. In our previous study, SCLC patients with a high expression of Topo‐II receiving amrubicin had significantly longer PFS and OS than did those with a low expression of Topo‐II.14 Patients with refractory relapse showed significantly higher Topo‐II expression levels than did those with sensitive relapse.14 Against these backgrounds, we conducted a clinicopathological study to investigate the relationship between Topo‐II expression and amrubicin efficacy in patients with previously treated advanced NSCLC.
Ki‐67 is mainly expressed during the active phase of the cell cycle, such as G1, S, G2, and mitosis.15, 16 A high expression of Ki‐67, a cellular proliferation marker, has been shown to be correlated with prognosis in malignant tumors.15, 16 A review of the prognostic and predictive value of Ki‐67 labeling index in NSCLC showed that though it has some prognostic influence, there was no consensus in this regard according to disease stage and histology.17 In an in vitro study, the expression levels of Topo‐IIα were found to be closely related to tumor cell proliferation and aggression.18, 19 The relationship of expression of Topo‐II and Ki‐67 has also been reported in malignant tumors.20, 21, 22
Against these backgrounds, we conducted a clinicopathological study to investigate the relationship between Topo‐II expression and amrubicin efficacy, and Topo‐II expression and Ki‐67 in patients with previously treated advanced NSCLC.
Methods
Patient eligibility
We screened 63 patients with advanced and previously treated NSCLC who had received amrubicin between April 2004 and May 2014 at two Japanese institutions (Shibukawa Medical Center and Gunma University Hospital). A total of 19 patients were excluded because they had received amrubicin as part of a doublet chemotherapy (n = 14), had insufficient specimens (n = 4), or had only cytological diagnosis (n = 1). Thus, the data of 44 patients were retrospectively analyzed in this study (Figure S1). The patients received 30–35 mg/m2 of amrubicin on days one, two, and three every three to four weeks which was continued until disease progression, the appearance of intolerable toxicity, or withdrawal of consent. Epidermal growth factor receptor (EGFR) mutations were examined using the peptide nucleic acid‐locked nucleic acid polymerase chain reaction clamp method.
Data collection
We retrospectively collected clinical data including those regarding histological type, antitumor effects, and survival from patients' medical records. Radiographic tumor responses were evaluated according to the Response Evaluation Criteria In Solid Tumors, version 1.1. PFS was calculated from the initiation of amrubicin treatment to the date of disease progression or death from any cause. OS was recorded from the start of amrubicin treatment until death or censoring on the date of the last follow‐up consultation. This study was conducted in compliance with the tenets of the Declaration of Helsinki. The study protocol was approved by the institutional review boards of Gunma University Hospital (ref. 1287) and National Hospital Organization Shibukawa Medical Center (ref. 15‐03‐05).
Immunohistochemical staining
We evaluated the expression levels of Topo‐II by immunohistochemical staining of archival tumor specimens obtained via surgical resection (17 samples, 39%) and biopsy (27 samples, 61%) before first‐line chemotherapy. Immunohistochemical staining was performed as previously described23 using anti‐Topo‐II, an affinity purified rabbit polyclonal antibody (ab180393; Abcam, Cambridge, UK; 1:100 dilution). Cells with stained nuclei were defined as having tested positive for Topo‐II. The proportion of Topo‐II‐positive cells was assessed by a semi‐quantitative scoring method in which samples were assigned a score based on the proportion of positively‐stained cells: 1, <10%; 2, 10% to <25%; 3, 25% to <50%; 4, 50% to <75%; and 5, ≥75%. According to previous study, Scores of 1–4 were considered to indicate low expression, while a score of five was considered indicative of high expression.14 Immunohistochemical staining for Ki‐67 was performed as described previously24 using a murine monoclonal antibody against Ki‐67 (Dako, Glostrup, Denmark; 1:40 dilution). A highly cellular area of the immunostained section was assessed to evaluate Ki‐67 staining. Staining of the nuclei of epithelial cells at any level was defined as indicative of high expression. Approximately 1000 nuclei were counted on each slide. The Ki‐67 labeling index was defined as the proportion of Ki‐67‐stained nuclei, which was considered to reflect proliferative activity. In a review that investigated the prognostic value of Ki‐67 in NSCLC, cutoff levels were defined as ranging from 5% to 30% of Ki‐67 expressing cells.25 In another study, the commonly used cutoff values in NSCLC and other human cancers ranged from 20%–40%.22 The median labeling index of Ki‐67 in our data was 20% (range, 0–91). Therefore, in reference to cutoff values used in this study, high expression was defined as a labeling index of 20% or higher. Sections were evaluated via light microscopy by at least two investigators who were blinded to the sample data. Both investigators evaluated the slides at the same time until a consensus was reached. Both investigators were not informed of patient outcomes.
Statistical analysis
The correlations between immunohistochemical expression and clinicopathological characteristics were assessed using Fisher's exact test. The correlation between different variables was analyzed using the nonparametric Spearman's rank test. Survival curves were generated using the Kaplan‐Meier method, and differences in survival were compared using the log‐rank test. Multivariate analyses were performed using the Cox proportional hazards model to assess the independence of Topo‐II expression. The statistical analyses were performed using Graphpad Prism version 6 (GraphPad Software, La Jolla, CA, USA) and EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan).26 Analyses items with P < 0.05 were considered statistically significant.
Results
Patient characteristics according to Topo‐II expression
A total of 44 patients were included in the analyses, 40 of whom died. The median follow‐up time was 269 (range, 7–2092) days. The patients' characteristics are shown in Table 1. The histological types consisted of adenocarcinoma (70.5%), squamous cell carcinoma (22.7%), large cell carcinoma (4.5%), and pleomorphic carcinoma (2.3%). Nine patients had EGFR mutations. EGFR mutation status was not evaluated in 15 patients.
Table 1.
Baseline patient characteristics
| Characteristic | No. of patients (n = 44) |
|---|---|
| Gender | |
| Male/female | 30/14 |
| Median age at treatment (years) | 67 (43–78) |
| Performance status (PS) | |
| 0/1/2 | 22/19/3 |
| Histology | |
| Ad/Sqcc/Lcc/pleomorphic carcinoma | 31/10/2/1 |
| Stage | |
| IIIA/IIIB/IV | 2/5/37 |
| EGFR mutation status | |
| Mutation/wild‐type/unknown | 9/20/15 |
| Smoking status | |
| Smoker/non‐smoker | 28/16 |
| Number of regimens | |
| 2/3/4/5/6/7/≧8 | 13/5/8/10/4/3/1 |
| Median (range) | 3 (2–12) |
| AMR, number of cycles | |
| Median (range) | 2 (1–12) |
| Response to AMR | |
| CR/PR/SD/PD | 0/4/28/12 |
Ad, adenocarcinoma; AMR, amrubicin; CR, complete response; EGFR, epidermal growth factor receptor; Lcc, large cell carcinoma; PD, progressive disease; PR, partial response; PS, performance status; SD, stable disease; Sqcc, squamous cell carcinoma.
The median number of cycles of amrubicin was two (range, 1–12) and associated adverse events are shown in Table S1. After amrubicin chemotherapy, 36 patients (82%) received other chemotherapy agents. The median number of follow‐up therapeutic regimens was 2 (range, 0–6 regimens). The chemotherapy regimens administered after amrubicin are shown in Table S2. S‐1 was the most commonly administered agent. A total of 27 patients with low Topo‐II expression received post‐chemotherapy treatment after amrubicin.
Immunohistochemical findings
Fig 1 shows the immunohistochemical staining of Topo‐II predominantly in the nuclei of tumor cells with no cytoplasmic staining. A total of 30% of specimens (13/44) showed high expression, and the average score for Topo‐II was 4.0 ± 1.2. Scores of one, two, three, four, and five were observed in 18% (8/44), 6% (3/44), 16% (7/44), 30% (13/44), and 30% (13/44) of patients, respectively. Table 2 summarizes the patients’ characteristics according to the level of Topo‐II expression. The median age was used as the cutoff point for age. Good performance status was defined as a performance status of 0 which was considered as the cutoff point for performance status. Smokers were defined as all smokers, and non‐smokers were defined as participants who had never smoked. The proportion of tumors with high Ki‐67 labeling index was 52% (23/44), and the median Ki‐67 labeling index was 20 (range, 0–91). There was no significant difference in the Ki‐67 labeling index between patients with EGFR mutation and wild‐type (P = 0.69) (Figure S2a). Similarly, no significant difference in the Ki‐67 labeling index was observed between patients with low and high expressions of Topo‐II (P > 0.99) (Figure S2b). There were no significant differences in other cutoff values of Ki‐67 (labeling indexes of 25%, 30%, 35%, and 40%) between patients with low and high expressions of Topo‐II (P > 0.99).
Figure 1.
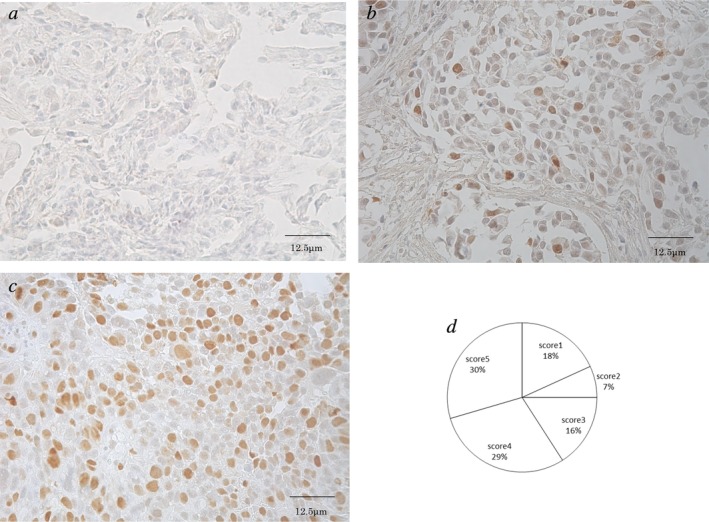
Immunohistochemical staining of topoisomerase‐II in NSCLC patients (400× magnification). (a,b) Low expression of topoisomerase‐II is shown in nuclei (score 1 and 3, respectively). (c) High expressions of topoisomerase‐II is shown in nuclei (score 5). (d) The score according to immunohistochemical staining of topoisomerase‐II.
Table 2.
The characteristics according to the expression of topoisomerase‐II
| Topoisomerase‐II | |||
|---|---|---|---|
| Characteristic | High (n = 13) | Low (n = 31) | P‐value |
| Age | |||
| ≤66 | 5 | 17 | 0.51 |
| ≥67 | 8 | 14 | |
| Gender | |||
| Male | 9 | 21 | >0.99 |
| Female | 4 | 10 | |
| Smoking status | |||
| Smoker | 8 | 20 | >0.99 |
| Non‐smoker | 5 | 11 | |
| Stage | |||
| IIIA, IIIB | 0 | 7 | 0.09 |
| IV | 13 | 24 | |
| Histology | |||
| Adenocarcinoma | 9 | 22 | >0.99 |
| Nonadenocarcinoma | 4 | 9 | |
| Histology | |||
| Squamous cell carcinoma | 1 | 9 | 0.24 |
| Nonsquamous cell carcinoma | 12 | 22 | |
| PS | |||
| 0 | 6 | 16 | >0.99 |
| 1, 2 | 7 | 15 | |
| EGFR mutation status | |||
| mutation | 4 | 5 | 0.41 |
| wild‐type, unknown | 9 | 26 | |
| Response to first‐line treatment | |||
| CR, PR | 6 | 14 | >0.99 |
| SD, PD | 7 | 17 | |
| Number of regimens | |||
| <3 | 4 | 14 | 0.51 |
| ≥3 | 9 | 17 | |
| AMR, Number of cycles | |||
| <2 | 6 | 14 | >0.99 |
| ≥2 | 7 | 17 | |
| Response to AMR | |||
| PR | 2 | 2 | 0.57 |
| SD, PD | 11 | 29 | |
| Ki‐67 labeling index | |||
| <20 LI | 7 | 16 | >0.99 |
| ≥20 LI | 6 | 15 | |
AMR, amrubicin; CR, complete response; EGFR, epidermal growth factor receptor; LI, labeling index; PD, progressive disease; PR, partial response; PS, performance status; SD, stable disease.
Treatment efficacy
Among the 44 patients, the response rate to amrubicin treatment were 9.1%. The overall response rates to amrubicin treatment in patients with high and low Topo‐II expression were 15.4% and 6.5%, respectively. There was no significant difference in the overall response to amrubicin treatment between patients with low and high expression of Topo‐II, respectively (P = 0.57).
Survival analysis according to level of Topo‐II expression
The median PFS and OS were 1.8 and 8.8 months, respectively. There was no significant difference in PFS between patients with low and high expressions of Topo‐II (Fig 2a). Patients with a low expression of Topo‐II had a significantly longer OS than did those with a high expression of Topo‐II (Fig 2b). Patients with an EGFR mutation showed no significant differences in PFS and OS compared to those with wild‐type EGFR or an unknown EGFR mutation status (PFS 0.8 vs. 1.8 months, HR = 1.96, P = 0.05; OS, 7.2 vs. 10.9 months, HR = 0.99, P = 0.97, respectively) (Fig 2c,d).
Figure 2.
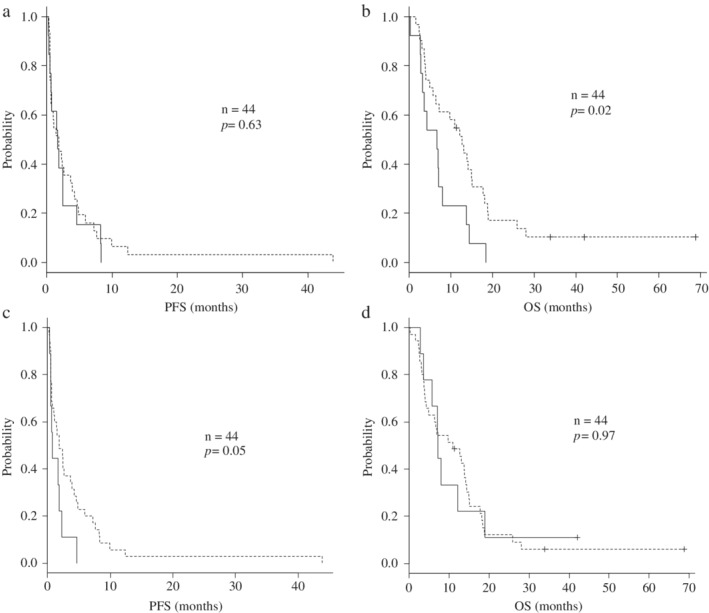
(a) Kaplan‐Meier curves for progression‐free survival (PFS) with amrubicin according to the expression of topoisomerase‐II. Patients with decreased expression of topoisomerase‐II had no significantly difference PFS than those with increased expression of topoisomerase‐II (1.7 and 1.8 months, HR 0.86, P = 0.63). (b) Kaplan‐Meier curves for overall survival (OS) with amrubicin according to the expression of topoisomerase‐II. Patients with decreased expression of topoisomerase‐II had a significantly longer OS than those with increased expression of topoisomerase‐II (12.7 and 6.6 months, HR 0.47, P = 0.02). Topo‐II:  high,
high,  low. (c) Kaplan‐Meier curves for progression‐free survival (PFS) with amrubicin according to EGFR mutation status. Patients with an EGFR mutation had no significantly difference PFS than those with wild‐type EGFR or with an unknown EGFR mutation status (0.8 months and 1.8 months, HR 1.96, P = 0.05). (d) Kaplan‐Meier curves for overall survival (OS) with amrubicin according to EGFR mutation status. Patients with an EGFR mutation had no significantly difference OS than those with wild‐type EGFR or with an unknown EGFR mutation status (7.2 months and 10.9 months, HR 0.99, P = 0.97).
low. (c) Kaplan‐Meier curves for progression‐free survival (PFS) with amrubicin according to EGFR mutation status. Patients with an EGFR mutation had no significantly difference PFS than those with wild‐type EGFR or with an unknown EGFR mutation status (0.8 months and 1.8 months, HR 1.96, P = 0.05). (d) Kaplan‐Meier curves for overall survival (OS) with amrubicin according to EGFR mutation status. Patients with an EGFR mutation had no significantly difference OS than those with wild‐type EGFR or with an unknown EGFR mutation status (7.2 months and 10.9 months, HR 0.99, P = 0.97).  EGFR mutation,
EGFR mutation,  wild‐type, unknown.
wild‐type, unknown.
Univariate and multivariate analyses of PFS and OS
Univariate analysis showed that a good performance status (defined as a performance status of 0), higher number of regimens before amrubicin, and response to amrubicin were all significantly associated with prolonged PFS (Table 3). Univariate analysis also showed that good performance status, stage IIIA/IIIB disease, and low expression of Topo‐II were all significantly associated with prolonged OS (Table 4). According to the results of the univariate log‐rank test, we screened variables with a cutoff of P < 0.05 in the multivariate analysis. Multivariate analysis confirmed that higher number of regimens before amrubicin, and response to amrubicin were independent prognostic factors associated with a prolonged PFS (Table 5). Good performance status and low expression of Topo‐II were identified as independent factors associated with prolonged OS in the multivariate analysis (Table 6).
Table 3.
Univariate analysis of progression‐free survival from the initiation of AMR therapy
| Progression‐free survival | |||
|---|---|---|---|
| Variables | Hazard ratio | 95% CI | P‐value |
| Age (≤ 66 vs. ≥ 67 years) | 1.27 | 0.71–2.34 | 0.42 |
| Gender (male vs. female) | 0.97 | 0.51–1.83 | 0.93 |
| PS (0 vs.1,2) | 0.56 | 0.28–0.95 | 0.04 |
| Stage (IIIA, IIIB vs. IV) | 0.53 | 0.28–1.07 | 0.10 |
| EGFR mutation status (mutation vs. wild‐type, unknown) | 2.58 | 0.99–6.76 | 0.053 |
| Histology (adenocarcinoma vs. nonadenocarcinoma) | 1.20 | 0.65–2.27 | 0.56 |
| Smoking status (smoker vs. non‐smoker) | 1.38 | 0.77–2.53 | 0.29 |
| Number of regimens before AMR (<3 vs. ≥ 3) | 0.53 | 0.26–0.87 | 0.02 |
| Response to AMR (PR vs. SD, PD) | 0.29 | 0.17–0.66 | <0.01 |
| topoisomerase‐II (low vs. high) | 0.86 | 0.43–1.65 | 0.63 |
| Ki‐67 labeling index (<20 LI vs. ≥20 LI) | 1.04 | 0.58–1.89 | 0.90 |
AMR, amrubicin; CI, confidence interval; CR, complete response; EGFR, epidermal growth factor receptor; HR, hazard ratio; LI, labeling index; PD, progressive disease; PFS, progression‐free survival; PR, partial response; PS, performance status; SD, stable disease.
Table 4.
Univariate analysis of overall survival (OS) from the initiation of AMR therapy
| Overall survival (OS) | |||
|---|---|---|---|
| Variables | Hazard ratio | 95% CI | P‐value |
| Age (≤ 66 vs. ≥ 67 years) | 1.58 | 0.87–3.08 | 0.13 |
| Gender (male vs. female) | 1.24 | 0.64–2.37 | 0.53 |
| PS (0 vs.1,2) | 0.37 | 0.15–0.58 | <0.01 |
| Stage (IIIA, IIIB vs. IV) | 0.22 | 0.15–0.57 | <0.01 |
| EGFR mutation status (mutation vs. wild‐type, unknown) | 0.99 | 0.46–2.13 | 0.97 |
| Histology (adenocarcinoma vs. nonadenocarcinoma) | 1.10 | 0.56–2.13 | 0.79 |
| Smoking status (smoker vs. non‐smoker) | 1.25 | 0.66–2.36 | 0.51 |
| Number of regimens before AMR (<3 vs. ≥ 3) | 0.59 | 0.31–1.08 | 0.09 |
| Response to AMR (PR vs. SD, PD) | 0.43 | 0.23–1.25 | 0.15 |
| topoisomerase‐II (low vs. high) | 0.47 | 0.16–0.84 | 0.02 |
| Ki‐67 labeling index (<20 LI vs. ≥20 LI) | 1.09 | 0.59–2.02 | 0.79 |
AMR, amrubicin; CI, confidence interval; CR, complete response; EGFR, epidermal growth factor receptor; HR, hazard ratio; LI, labeling index; OS, overall survival; PD, progressive disease; PR, partial response; PS, performance status; SD, stable disease.
Table 5.
Multivariate analysis of progression‐free survival from the initiation of AMR therapy
| PFS | |||
|---|---|---|---|
| Variables | Hazard ratio | 95% CI | P‐value |
| PS (0 vs.1,2) | 1.57 | 0.83–2.94 | 0.16 |
| Stage (IIIA, IIIB vs. IV) | 0.95 | 0.36–2.52 | 0.91 |
| EGFR mutation status (mutation vs. wild‐type, unknown) | 0.56 | 0.25–1.24 | 0.15 |
| Number of regimens before AMR (<3 vs. ≥ 3) | 2.17 | 1.04–4.53 | 0.04 |
| Response to AMR (PR vs. SD, PD) | 5.63 | 1.46–21.80 | 0.01 |
| topoisomerase‐II (low vs. high) | 1.19 | 0.55–2.58 | 0.67 |
AMR, amrubicin; CI, confidence interval; CR, complete response; EGFR, epidermal growth factor receptor; HR, hazard ratio; PD, progressive disease; PFS, progression‐free survival; PR, partial response; PS, performance status; SD, stable disease.
Table 6.
Multivariate analysis of overall survival from the initiation of AMR therapy
| OS | |||
|---|---|---|---|
| Variables | Hazard ratio | 95% CI | P‐value |
| PS (0 vs.1,2) | 2.57 | 1.23–5.36 | 0.01 |
| Stage (IIIA, IIIB vs. IV) | 3.42 | 0.95–12.28 | 0.06 |
| EGFR mutation status (mutation vs. wild‐type, unknown) | 1.44 | 0.63–3.31 | 0.39 |
| Number of regimens before AMR (<3 vs. ≥ 3) | 1.07 | 0.51–2.25 | 0.86 |
| Response to AMR (PR vs. SD, PD) | 3.58 | 0.94–13.54 | 0.06 |
| topoisomerase‐II (low vs. high) | 2.88 | 1.23–6.76 | 0.01 |
AMR, amrubicin; HR, hazard ratio; OS, overall survival; CI, confidence interval; CR, complete response; EGFR, epidermal growth factor receptor; PD, progressive disease; PR, partial response; PS, performance status; SD, stable disease.
Discussion
To the best of our knowledge, this is the first study to assess the relationship between Topo‐II expression and the efficacy of amrubicin in NSCLC. We found that high Topo‐II expression was an independent factor for reduced OS in patients with advanced NSCLC receiving amrubicin. Consistent with our observation, high Topo‐II expression has previously been shown to be significantly related to reduced OS in various cancers including urinary bladder carcinoma, breast cancer, gallbladder carcinoma, ovarian cancer, hepatocellular carcinoma, Hodgkin's lymphoma and prostate cancer.11, 27, 28, 29, 30, 31, 32 In an in vitro study, the expression levels of Topo‐IIα were found to be closely related to tumor cell proliferation and aggression.18, 19 Furthermore, previous studies showed that Topo‐IIα overexpression was correlated with an aggressive form of breast cancer in aspects such as tumor grade, advanced stage, and lymph node metastasis.11, 33, 34 Biesaga et al. reported that Topo‐II overexpression was correlated with hormone receptor negativity, CK5/6 expression, higher proliferation rate, and was most frequently found in basal‐like tumors, and thus a high stage of tumor aggressiveness.13 Therefore, a high Topo‐II expression may contribute to a tumor‐aggressive phenotype, resulting in an unfavorable clinical outcome in NSCLC.
In our previous study, SCLC patients with a high expression of Topo‐II receiving amrubicin had significantly longer PFS and OS than did those with a low expression of Topo‐II.14 In agreement with this observation, previous studies reported that high Topo‐II activity was associated with sensitivity to Topo‐II inhibitors, including doxorubicin and etoposide, in human lung cancer cell lines.35, 36 A relationship between elevated Topo‐II expression and increased sensitivity to anthracycline‐based chemotherapy agents such as etoposide, doxorubicin, and mitoxantrone was also observed in breast cancer.37, 38, 39 Thus, it is likely that Topo‐II overexpression predicts an increased sensitivity to Topo‐II inhibitors. However, there was no significant association between Topo‐II expression and chemosensitivity to amrubicin in NSCLC in this study. Notably, several lines of evidence indicate lower activity of Topo‐II in NSCLC compared with that in SCLC.35, 40, 41 In fact, the frequency of undetectable or weak Topo‐II expression (a score < 3) was higher in NSCLC tumors than in SCLC tumors in our previous study.14 Therefore, chemosensitivity to amrubicin may be less dependent on Topo‐II expression in NSCLC compared with SCLC. Considering that elevated Topo‐II expression has been associated with an aggressive tumor phenotype and worse clinical outcomes in several human cancers, Topo‐II expression may serve as a prognostic marker, rather than a predictive marker, for amrubicin therapy in NSCLC.
On the other hand, Topo‐II may play opposite roles in NSCLC and SCLC. Topo‐II has multiple roles in diverse biological contexts and the biological functions are modulated by a variety of protein‐protein interactions,42, 43 suggesting that the function of Topo‐II may change depending on the cellular context. The difference in the function of Topo‐II may result in discrepancies in the efficacy of amrubicin according to Topo‐II expression in NSCLC and SCLC. Further studies are needed to clarify the functional roles of Topo‐II in NSCLC and SCLC.
In our study, patients with high and low expression of Ki‐67 showed no significant differences in overall response rates (8.7% vs. 9.5%, P > 0.99) or disease control rates (69.6% vs. 76.2%, P = 0.74) of amrubicin treatment. In our study, no significant difference was observed in the Ki‐67 labeling index between patients with low and high expression of Topo‐II (Table 2, Figure S2b). No significant difference was found even when we modified the various cutoff values to those used in previous studies. Further studies are needed to clarify the association between Ki‐67 and Topo‐II in NSCLC.
Our study had some limitations. First, the sample size was small which may have introduced bias into our results. Second, the EGFR mutation statuses of tumors may have influenced our results. A marginally significant difference in the PFS was detected between patients with different EGFR mutation statuses; thus, the EGFR mutation status may be a prognostic factor. However, there was no significant difference in the EGFR mutation status between patients with low and high expression of Topo‐II (Fig 2c). Moreover, no significant difference in the OS was detected between patients with different EGFR mutation statuses (Fig 2d). Therefore, EGFR mutation status did not appear to influence our results. Third, the post‐chemotherapy treatment after amrubicin therapy may have influenced our results. A total of 27 patients (87%) with low Topo‐II expression and 10 patients (77%) with high Topo‐II expression received post‐chemotherapy agents after amrubicin therapy. As shown in Table S2, the number of regimens trend with more the group of low Topo‐II expression than high Topo‐II expression, but no significant difference was detected according to the level of expression of Topo‐II. Fourth, there is a possibility that the expression of Topo‐II varies according to chemotherapy before amrubicin. However, rebiopsy at the time of the recurrence is difficult in the case of lung cancer. Future studies using rebiopsy specimens will be necessary if possible. Finally, the overall response rate was too low to assess the relationship between Topo‐II expression and the efficacy of amrubicin in NSCLC. Therefore, a larger sample size will be required for further analysis.
In conclusion, low Topo‐II expression is an independent prognostic factor for longer survival among patients with advanced NSCLC treated with amrubicin. Topo‐II may be a promising marker for predicting patients' outcomes following the administration of amrubicin as a Topo‐II inhibitor.
Disclosure
The authors report no conflict of interest.
Supporting information
Figure S1. Flow chart showing patient selection.
Figure S2. (a) The relationship between EGFR mutation status and Ki‐67 was shown. There was no significant difference in the Ki‐67 labeling index between patients with EGFR mutation and wild type (P = 0.69). (b) The relationship between the expression of topoisomerase‐II and Ki‐67 was shown. There was no significant difference in the Ki‐67 labeling index between patients with a low and those with a high expression of topoisomerase‐II (p > 0.99).
Table S1. Adverse events.
Table S2. The post‐chemotherapy after amrubicin according to the expression of topoisomerase‐II.
Acknowledgments
There was no funding for this study. We thank Dr Akira Mogi, Dr Kimihiro Shimizu, Department of General Thoracic Surgery, and Dr Yusuke Tsukagoshi, Tomomi Masuda, Norimistu Kasahara, Masataka Maeno, Department of Respiratory Medicine, Gunma University Graduate of Medicine, Gunma, Japan, for their help with collection of tumor samples and clinical data.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Ettinger DS, Aisner DL, Wood DE et al NCCN guidelines insights: Non‐small cell lung cancer, version 5.2018. J Natl Compr Canc Netw 2018; 16: 807–21. [DOI] [PubMed] [Google Scholar]
- 3. Tani N, Yabuki M, Komuro S, Kanamaru H. Characterization of the enzymes involved in the vitro metabolism of amrubicin hydrochloride. Xenobiotica 2005; 35: 1121–33. [DOI] [PubMed] [Google Scholar]
- 4. Yamaoka T, Hanada M, Ichii S, Morisada S, Noguchi T, Yanagi Y. Cytotoxicity of amrubicin, a novel 9‐aminoanthracycline, and its active metabolite amrubicinol on human tumor cells. Jpn J Cancer Res 1998; 89: 1067–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kurata T, Okamoto I, Tamura K, Fukuoka M. Amrubicin for non‐small‐cell lung cancer and small‐cell lung cancer. Invest New Drugs 2007; 25: 499–504. [DOI] [PubMed] [Google Scholar]
- 6. Ogawa M. Novel anticancer drugs in Japan. J Cancer Res Clin Oncol 1999; 125: 134–40. [DOI] [PubMed] [Google Scholar]
- 7. Kaira K, Sunaga N, Tomizawa Y et al A phase II study of amrubicin, a synthetic 9‐aminoanthracycline, in patients with previously treated lung cancer. Lung Cancer 2010; 69: 99–104. [DOI] [PubMed] [Google Scholar]
- 8. Yoshioka H, Katakami N, Okamoto H et al A randomized, open‐label, phase III trial comparing amrubicin versus docetaxel in patients with previously treated non‐small‐cell lung cancer. Ann Oncol 2017; 28: 285–91. [DOI] [PubMed] [Google Scholar]
- 9. Barret JM, Calsou P, Larsen AK, Salles B. A cisplatin‐resistant murine leukemia cell line exhibits increased topoisomerase II activity. Mol Pharmacol 1994; 46: 431–6. [PubMed] [Google Scholar]
- 10. Murphy AJ, Hughes CA, Barrett C et al Low‐level TOPO2A amplification in prostate cancer is associated with HER2 duplication, androgen resistance, and decreased survival. Cancer Res 2007; 67: 2893–8. [DOI] [PubMed] [Google Scholar]
- 11. Fritz P, Cabrera CM, Dippon J et al c‐erbB2 and topoisomerase IIalpha protein expression independently predict poor survival in primary human breast cancer: A retrospective study. Breast Cancer Res 2005; 7: R374–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaufmann SH, Karp JE, Jones RJ et al Topoisomerase II levels and drug sensitivity in adult acute myelogenous leukemia. Blood 1994; 83: 517–30. [PubMed] [Google Scholar]
- 13. Biesaga B, Niemiec J, Ziobro M, Wysocka J, Kruczak A. Prognostic potential of topoisomerase IIα and HER2 in a retrospective analysis of early advanced breast cancer patients treated with adjuvant anthracycline chemotherapy. Breast 2011; 20: 338–50. [DOI] [PubMed] [Google Scholar]
- 14. Miura Y, Kaira K, Sakurai R et al High expression of topoisomerase‐II predicts favorable clinical outcomes in patients with relapsed small cell lung cancers receiving amrubicin. Lung Cancer 2018; 115: 42–8. [DOI] [PubMed] [Google Scholar]
- 15. Cattoretti G, Becker MH, Key G et al Monoclonal antibodies against recombinant parts of the Ki‐67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave‐processed formalin‐fixed paraffin sections. J Pathol 1992; 168: 357–63. [DOI] [PubMed] [Google Scholar]
- 16. Gerdes J, Li L, Schlueter C et al Immunobiochemical and molecular biologic characterization of the cell proliferation‐associated nuclear antigen that is defined by monoclonal antibody Ki‐67. Am J Pathol 1991; 138: 867–73. [PMC free article] [PubMed] [Google Scholar]
- 17. Jakobsen JN, Sørensen JB. Clinical impact of ki‐67 labeling index in non‐small cell lung cancer. Lung Cancer 2013; 79: 1–7. [DOI] [PubMed] [Google Scholar]
- 18. Giaccone G, van Ark‐Otte J, Scagliotti G et al Differential expression of DNA topoisomerases in non‐small cell lung cancer and normal lung. Biochim Biophys Acta 1995; 1264: 337–46. [DOI] [PubMed] [Google Scholar]
- 19. Kreipe H, Alm P, Olsson H, Hauberg M, Fischer L, Parwaresch R. Prognostic significance of a formalin‐resistant nuclear proliferation antigen in mammary carcinomas as determined by the monoclonal antibody Ki‐S1. Am J Pathol 1993; 142: 651–7. [PMC free article] [PubMed] [Google Scholar]
- 20. Sun G, Wang S, Wang Y. Expressions of Topo IIα and Ki67 in breast cancer and its clinicopathologic features and prognosis. Pak J Med Sci 2019; 35: 715–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Iżycka‐Świeszewska E, Lipska‐Ziętkiewicz BS, Adamkiewicz‐Drożyńska E et al Proliferation index revisited in neuroblastic tumors. Folia Neuropathol 2014; 52: 243–52. [DOI] [PubMed] [Google Scholar]
- 22. Sundov D, Caric A, Mrklic I et al P53, MAPK, topoisomerase II alpha and Ki67 immunohistochemical expression and KRAS/BRAF mutation in ovarian serous carcinomas. Diagn Pathol 2013; 8: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shimizu K, Kaira K, Tomizawa Y et al ASC amino‐acid transporter 2 (ASCT2) as a novel prognostic marker in non‐small cell lung cancer. Br J Cancer 2014; 110: 2030–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaira K, Oriuchi N, Imai H et al Prognostic significance of L‐type amino acid transporter1 exession in resectable stage I‐III nonsmall cell lung cancer. Br J Cancer 2008; 98: 742–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matin B, Paesmans M, Mascaux C et al Ki‐67 expression and patients survival in lung cancer: Systematic review of the literature with meta‐analysis. Br J Cancer 2004; 91: 2018–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013; 48: 452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Simon R, Atefy R, Wagner U et al HER‐2 and TOP2A coamplification in urinary bladder cancer. Int J Cancer 2003; 107: 764–72. [DOI] [PubMed] [Google Scholar]
- 28. Washiro M, Ohtsuka M, Kimura F et al Upregulation of topoisomerase IIalpha expression in advanced gallbladder carcinoma: A potential chemotherapeutic target. J Cancer Res Clin Oncol 2008; 134: 793–801. [DOI] [PubMed] [Google Scholar]
- 29. Ferrandina G, Petrillo M, Carbone A et al Prognostic role of topoisomerase‐IIalpha in advanced ovarian cancer patients. Br J Cancer 2008; 98: 1910–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wong N, Yeo W, Wong WL et al TOP2A overexpression in hepatocellular carcinoma correlates with early age onset, shorter patients survival and chemoresistance. Int J Cancer 2009; 124: 644–52. [DOI] [PubMed] [Google Scholar]
- 31. Doussis‐Anagnostopoulou IA, Vassilakopoulos TP, Thymara I et al Topoisomerase IIalpha expression as an independent prognostic factor in Hodgkin's lymphoma. Clin Cancer Res 2008; 14: 1759–66. [DOI] [PubMed] [Google Scholar]
- 32. Li X, Liu Y, Chen W et al TOP2Ahigh is the phenotype of recurrence and metastasis whereas TOP2Aneg cells represent cancer stem cells in prostate cancer. Oncotarget 2014; 5: 9498–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nakopoulou L, Lazaris AC, Kavantzas N et al DNA topoisomerase II‐alpha immunoreactivity as a marker of tumor aggressiveness in invasive breast cancer. Pathobiology 2000; 68: 137–43. [DOI] [PubMed] [Google Scholar]
- 34. Depowski PL, Rosenthal S, Brien TP, Stylos S, Johnson RL, Ross JS. Topoisomerase IIalpha expression in breast cancer: Correlation with outcome variables. Mod Pathol 2000; 13: 542–7. [DOI] [PubMed] [Google Scholar]
- 35. Kasahara K, Fujiwara Y, Sugimoto Y et al Determinants of response to the DNA topoisomerase II inhibitors doxorubicin and etoposide in human lung cancer cell lines. J Natl Cancer Inst 1992; 84: 113–8. [DOI] [PubMed] [Google Scholar]
- 36. Giaccone G, Gazdar AF, Beck H, Zunino F, Capranico G. Multidrug sensitivity phenotype of human lung cancer cells associated with topoisomerase II expression. Cancer Res 1992; 52: 1666–74. [PubMed] [Google Scholar]
- 37. Coon JS, Marcus E, Gupta‐Burt S et al Amplification and overexpression of topoisomerase IIalpha predict response to anthracycline‐based therapy in locally advanced breast cancer. Clin Cancer Res 2002; 8: 1061–7. [PubMed] [Google Scholar]
- 38. Mukherjee A, Shehata M, Moseley P, Rakha E, Ellis I, Chan S. Topo2alpha protein expression predicts response to anthracycline combination neo‐adjuvant chemotherapy in locally advanced primary breast cancer. Br J Cancer 2010; 103: 1794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fry AM, Chresta CM, Davies SM et al Relationship between topoisomerase II level and chemosensitivity in human tumor cell line. Cancer Res 1991; 51: 6592–5. [PubMed] [Google Scholar]
- 40. Kreisholt J, Sorensen M, Jensen PB, Nielsen BS, Andersen CB, Sehested M. Immunohischemical detection of DNA topoisomerase IIalpha, P‐glycoprotein and multidrug resistance protein (MRP) in small‐cell and non‐small‐cell lung cancer. Br J Cancer 1998; 77: 1469–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guinee DG Jr, Holden JA, Benfield JR et al Comparison of DNA topoisomerase II alpha expression in small cell and nonsmall cell carcinoma of the lung. In search of a mechanism of chemotherapeutic response. Cancer 1996; 78: 729–35. [DOI] [PubMed] [Google Scholar]
- 42. Nitiss JL. DNA topoisomerase II and its growing repertoire of biological functions. Nat Rev Cancer 2009; 9: 327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bollimpelli VS, Dholaniya PS, Kondapi AK. Topoisomerase IIβand its role in different biological contexts. Arch Biochem Biophys 2017; 633: 78–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow chart showing patient selection.
Figure S2. (a) The relationship between EGFR mutation status and Ki‐67 was shown. There was no significant difference in the Ki‐67 labeling index between patients with EGFR mutation and wild type (P = 0.69). (b) The relationship between the expression of topoisomerase‐II and Ki‐67 was shown. There was no significant difference in the Ki‐67 labeling index between patients with a low and those with a high expression of topoisomerase‐II (p > 0.99).
Table S1. Adverse events.
Table S2. The post‐chemotherapy after amrubicin according to the expression of topoisomerase‐II.


