Abstract
Background Management of anterior cranial base malignancies requires multidisciplinary care. Radiation therapy remains a mainstay of definitive or adjuvant treatment. Apart from primary hypothyroidism, the effects of radiation on the hypothalamic–pituitary axis after high-dose treatment of head and neck malignancies remain poorly described. We describe a comprehensive screening protocol for surveillance and characterize the incidence of pituitary dysfunction after radiation for anterior cranial base malignancies.
Methods A review of patients prospectively enrolled in a skull base registry at an academic center was performed. Included patients had a history of anterior skull base malignancy and external beam radiation to the primary site, with comprehensive post-treatment pituitary serologies and at least 1 year of post-radiation follow-up. Routine hormonal screening was initiated during the study period for all patients with anterior skull base irradiation.
Results Eighty-one patients met inclusion. Fifty-eight patients (71%) demonstrated some laboratory abnormality. Thirty patients (37%) demonstrated evidence of hypopituitarism. Twenty-four (29%) demonstrated central hypogonadism, and 16% of patients showed central hypothyroidism. Ten patients (12%) displayed central adrenal insufficiency with six patients demonstrating panhypopituitarism. Primary tumor location and maximum dose of radiation to the gland appeared to correlate with incidence of hypopituitarism.
Conclusion Radiation for malignancies of the anterior skull base resulted in a 37% incidence of hypopituitarism in our study. Given the potential morbidity of hypopituitarism, we recommend annual post-treatment screening in these patients. We describe a comprehensive set of serologies that can be utilized, and recommend updating clinical guidelines to reflect the necessity of this screening.
Keywords: sinonasal malignancies, radiation side effects, radiation-induced hypopituitarism, hypopituitarism screening
Introduction
Sinonasal malignancies are rare diseases, accounting for 3 to 5% of all head and neck cancers. 1 Comprehensive treatment paradigms involve a combination of evolving open and endoscopic surgical approaches, a variety of radiation modalities, and an array of chemotherapy regimens. 2 3 Multidisciplinary management is beneficial in tailoring treatment plans, particularly to the wide variety of histologies that can present at the anterior cranial base and frequent complications that can arise as a result of treatment. 2 4
In the last two decades, advances in delivery of radiation therapy have yielded improvements in treatment outcomes. The development of intensity-modulated radiation therapy (IMRT) and proton beam therapy has helped to minimize complications, particularly at the skull base. 5 6 7 However, adequate radiation coverage of the gross tumor and high-risk areas of the skull base to achieve locoregional control often require delivery of high RT dose to the sella. Due to the aggressive nature of many of these malignancies, most radiation oncologists prioritize aggressive tumor dosing over contouring around the sella.
Common regional side effects and complications of external beam radiation to the head and neck can include mucositis, xerostomia, dysgeusia, dental caries, and skin irritation among others. 4 8 Less frequent, more morbid delayed complications include pharyngeal stricture, sinocutaneous fistulae, osteoradionecrosis, and radiation-related sarcomas. 9 10 The toxicity of radiation to the thyroid gland is also well described in the head and neck literature. Various reports highlight a 20 to 50% incidence of post-radiation primary hypothyroidism after neck irradiation, 11 12 likely dependent on the dose of radiation delivered to the thyroid bed. 13 The incidence and sequelae of radiation-induced hypothyroidism in the head and neck cancer survivor are significant, and routine screening of thyroid function is recommended in the National Comprehensive Cancer Network (NCCN) guidelines. 14
The effects of radiation on the pituitary gland are also well described, with evidence of dose-dependent pituitary insufficiency. 15 However, most of the data on radiation-induced hypopituitarism was derived from traditional conformal radiation modalities for diseases requiring lower doses than sinonasal malignancies (pituitary adenomas, whole brain irradiation, etc.). Furthermore, hypopituitarism is associated with significant morbidity and 55% increase in mortality, particularly in nonreplaced patients with a history of radiation. 16 Specific patterns of dose-dependence have been observed, with derangement in growth hormone and prolactin at lower radiation doses, followed by gonadotrophs, adrenocorticotropic hormone (ACTH), and thyroid-stimulating hormone (TSH) at increasing doses. 15 Furthermore, patterns of radiation-induced hypopituitarism are often delayed, with incidences up to 60% after 10 years. 15
Ipekci et al reported a high incidence of hypopituitarism in patients with nasopharynx carcinoma receiving therapeutic radiation, 17 which was similarly reported by Ratnasignam et al in a subsequent study. 18 In each study, somatotroph derangements were seen with highest frequency, followed by gonadotrophs, ACTH, and very low incidence of central hypothyroidism. In contrast, Huang et al describe a similar cohort of nasopharyngeal carcinoma survivors with a much higher incidence of TSH dysfunction, contrary to traditional literature. 19 These data remain, to our knowledge, the only contemporary data on radiation-induced hypopituitarism for head and neck malignancies requiring high-dose irradiation at the skull base. There are no recent data evaluating the incidence of pituitary insufficiency after radiation for sinonasal malignancies, and no consensus recommendations on screening algorithms. We aim to evaluate the implementation of a routine screening protocol to characterize patterns of radiation-induced hypopituitarism in patients with sinonasal malignancies seen at a tertiary care center.
Methods
A retrospective review of patients prospectively enrolled in an Institutional Review Board (IRB)-approved anterior skull base registry (IRB HUM00036763) at a tertiary care center from June 2012 to June 2015 was performed. Patients enrolled in the University of Michigan anterior skull base registry with any sinonasal or anterior skull base malignancy, a history of external beam radiation, and at least 1 year of post-radiation follow-up, were eligible. A complete list of inclusion and exclusion criteria are highlighted in Table 1 . In addition to the above demographics, tumor location for each patient was characterized into one of six anterior cranial base subsites: nasal cavity/ethmoid (often indistinguishable), sphenoid, clivus, nasopharynx, maxillary sinus/infratemporal fossa, and orbit. Comprehensive retrospective data collection included patient demographics, tumor type and location, date of initial and subsequent irradiation, dose profile to the pituitary gland, location where patient underwent radiation, history of chemotherapy, whether the patient underwent surgical resection with curative intent, and the detailed results of screening pituitary serologies. Observational statistics on serologies and clinical data were recorded in Excel, and two-tailed Z-test was used to compare for significance where applicable.
Table 1. Inclusion and exclusion criteria for study analysis.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Anterior skull base (sinonasal) malignancy | Absence of comprehensive screening serologies |
| History of external beam radiation to primary | Less than 1 year of post-radiation follow-up |
| Enrolled in anterior skull base registry June 2012–June 2015 | Evidence of hypopituitarism prior to radiation therapy |
| Radiation completed by June 2015 |
Evaluation of radiation fields : For 47 patients treated with IMRT at the University of Michigan, all patients were treated definitively or in an adjuvant setting with fractionated radiation to total doses of 60 to 70 Gy over 30 to 35 fractions prescribed so that 99% of high-risk planning target volume (PTV) was encompassed by 99% prescribed dose. No prospective dose constraints were placed on the sella or pituitary, but standard 54 Gy max point dose was accepted to optic chiasm/optic nerve planning organs at-risk volume (PRV). The sella was retrospectively contoured into Eclipse planning system, and a dose volume histogram (DVH) analysis was performed, and maximum point dose as well as mean dose to the sella was calculated. The DVH is a histogram that graphs the radiation dose over a defined volume (such as the pituitary gland, as outlined retrospectively) and can define the maximum and average dosing over this volume. The PTV is the defined area of planned radiation dosing; it encompasses the full tumor volume (or clinical target volume [CTV]) with a small margin around the tumor to ensure the entire tumor is effectively treated. Thus, the PTV is typically larger than the CTV. Lastly, organs at risk are critical structures that have limitations in dosing before causing significant dysfunction. In the skull base, this includes the optic chiasm and optic nerves. The PRV is the planning volume encompassing these critical structures (with necessary margin) that must avoid high-dose irradiation.
Surveillance protocol : At our institution, prior to the study period, skull base patients with a history of radiation were rarely screened for pituitary dysfunction, and only prompted partial serologic evaluation when presenting with severe symptoms of pituitary dysfunction. During the study period, with the development of the anterior skull base registry, increasing awareness of the endocrine effects of radiation led to the establishment of a routine screening protocol for patients with a history of anterior cranial base irradiation for malignancy, even if asymptomatic. A comprehensive set of anterior pituitary serologies was performed in all patients, with the comprehensive serology profile detailed in Table 2 . Screening profiles were performed ∼1 year following radiation, and yearly thereafter.
Table 2. Comprehensive list of yearly pituitary screening serologies.
| Yearly screening serologies | |
|---|---|
| 8AM cortisol | Prolactin |
| GH | IGF-1 |
| Free T4 | TSH |
| LH | FSH |
| Total testosterone | Bioavailable testosterone |
Abbreviations: FSH, follicle-stimulating hormone; GH, growth hormone; IGF-1, insulin-like growth factor 1; LH, luteinizing hormone; TSH, thyroid-stimulating hormone.
Evaluation of endocrinopathy : Many patients' abnormal serologies prompted a comprehensive evaluation in our Pituitary Endocrine Clinic, where the patients were evaluated for clinical pathology. For these patients, the diagnosis from clinic evaluation was used to assign an endocrinopathy. For patients who had not been evaluated clinically, the serologies were reviewed in detail with the senior pituitary endocrinologist (AB) to assign a clinical diagnosis, where applicable. Clinical hypopituitarism fell into one of three categories, which included central hypogonadism, central hypothyroidism, and secondary adrenal insufficiency. Secondary hypogonadism was documented in younger women by the finding of amenorrhea with low luteinizing hormone (LH) and follicle-stimulating hormone (FSH) concentrations, and in postmenopausal women as inappropriately low LH and FSH. In men, this diagnosis was made by the finding of low serum testosterone with concomitant nonelevated LH and FSH. Secondary hypothyroidism was established by low freeT4 concentrations with nonelevated TSH. Secondary adrenal failure was documented by the 8 to 9 am serum cortisol < 5µg/dL. 20 Primary hypothyroidism was diagnosed by elevated TSH, and primary gonadal failure in men and younger women by elevated LH and FSH concentrations.
Patients with serologic evidence of all three central clinical entities were characterized as having panhypopituitarism. At our institution, aberrancies in growth hormone are rarely treated as isolated clinical entities and thus growth hormone deficiency was not utilized as a separate clinical diagnosis.
Results
Five-hundred eight patients were enrolled in the anterior skull base registry during the eligibility period, with 136 patients with malignancy. One-hundred three patients met full inclusion criteria, and 22 patients were excluded due to inadequate follow-up or lack of screening serologies, for a total of 81 patients evaluated. Age ranged from 15 to 79 years, and 63% of patients were male. Seventy-five percent of patients underwent surgical resection with curative intent as part of their treatment regimen, and 45% of patients were treated with chemotherapy during their treatment course. Sixty-two percent of patients were treated at the study institution with IMRT, while the remaining 38% were treated locally or at another referral center, with five patients referred for proton beam therapy ( Table 3 ). A wide variety of histopathologies were represented, as are typical for skull base malignancies ( Table 4 ). Seventy-three percent of patients demonstrated at least one abnormal serology on screening profiles, meaning that the majority of patients have at least one laboratory value that falls outside of the normal range after radiation (though does not necessarily indicate clinical hypopituitarism). After endocrinologist review, 37% of patients screened showed evidence of clinical hypopituitarism, with 29% of screened patients demonstrating central hypogonadism, 16% with central hypothyroidism, 12% with central adrenal insufficiency, and 7% with panhypopituitarism ( Fig. 1 ). Thirteen percent showed hyperprolactinemia, and 23% showed aberrancies in somatotrophs. Subgroup analysis by primary tumor site showed an incidence of hypopituitarism in 50% of patients with sphenoid primaries and 47% of patients with nasal cavity/ethmoid primary tumors, while 0% of patients with maxillary sinus or orbital primaries demonstrated hypopituitarism ( p < 0.05) ( Fig. 2 ). Radiation at the study institution with IMRT versus those treated elsewhere did not affect the incidence of hypopituitarism, with 42 and 32% incidence, respectively ( p = 0.39). Fifty percent of patients with a history of chemotherapy demonstrated hypopituitarism, while only 29% of those without chemotherapy demonstrated hypopituitarism ( p = 0.06).
Table 3. Demographics of the patient population.
| Demographics | |
|---|---|
| Age (range) | 15–79 years |
| Male | 63% |
| Primary surgery | 75% |
| Chemotherapy | 45% |
| IMRT at UM | 62% |
Abbreviations: IMRT, intensity-modulated radiation therapy; UM, University of Michigan.
Table 4. Histopathologies represented in the study.
| Tumor type | Patients |
|---|---|
| Adenocarcinoma | 4 |
| Adenoid cystic | 3 |
| Chondrosarcoma | 2 |
| Chordoma | 7 |
| Olfactory neuroblastoma | 21 |
| Melanoma | 3 |
| Metastasis | 3 |
| Neuroendocrine carcinoma | 3 |
| Nasopharyngeal carcinoma | 2 |
| Other | 3 |
| Sarcoma | 5 |
| Squamous cell carcinoma | 17 |
| Sinonasal undifferentiated Carcinoma | 8 |
Fig. 1.
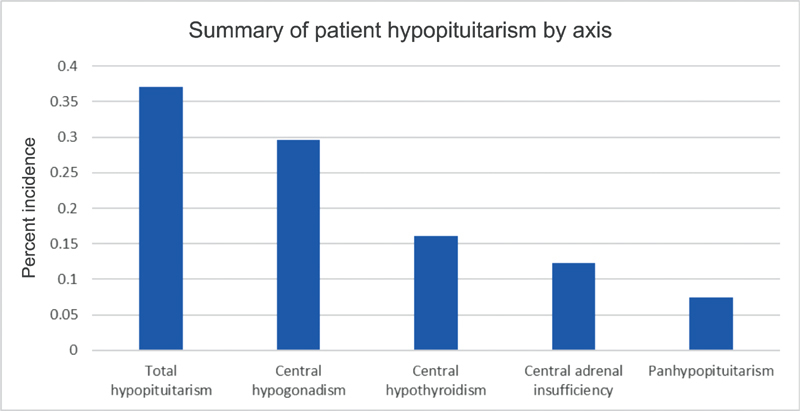
Breakdown of radiation-induced hypopituitarism by axis. Total incidence was 37%, with many patients demonstrating multiple axes influenced. Seven percent of patients in our series were panhypopituitary.
Fig. 2.
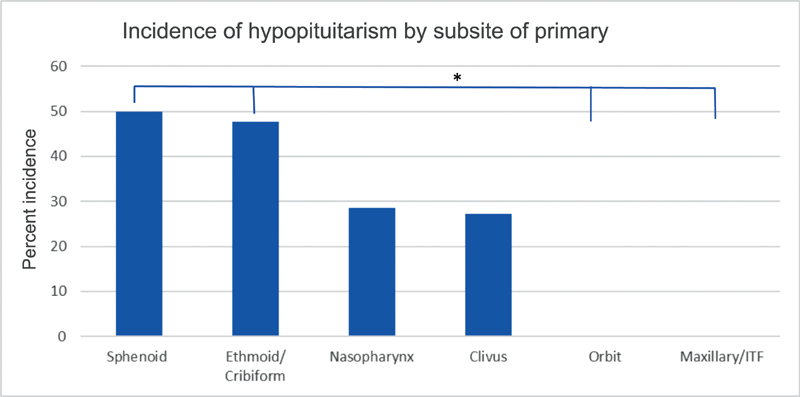
Hypopituitarism based on subsite of primary tumor. Sphenoid and ethmoid/nasal cavity tumors were significantly more likely than tumors of the orbit or maxillary sinus/infratemporal fossa to develop hypopituitarism.
Notably, many of our patients underwent radiation several years prior to the initiation of the skull base registry, and average follow-up from initial radiation is 40 months (median 47 months). For patients with last screening under 40 months from completion of radiation, hypopituitarism incidence was 32%, while for patients over 40 months from completion of radiation was 42% ( p = 0.32). When divided into 2-year increments from completion of radiation to last serologies, there was no statistical difference at any time-point in our patients.
When stratifying patients by maximum point dose of radiation to the sella, there was a strong trend of increasing incidence of hypopituitarism with increasing dose of radiation ( Fig. 3 ). There was a trend for statistical significance depending on the dose to the hypothalamic–pituitary area: patients who sustained at least 50 Gy (maximum dose) to the sella had a 46% incidence of hypopituitarism versus 21% in those whose max point dose was less than 50 Gy ( p = 0.075).
Fig. 3.
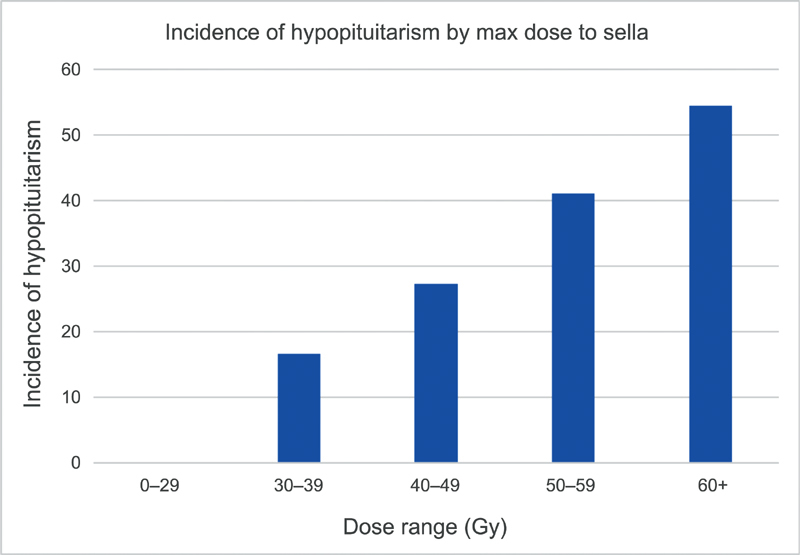
Incidence of hypopituitarism in patients treated with intensity-modulated radiation therapy at University of Michigan stratified by maximum dose to the sella. There is a trend toward increasing dysfunction with increased radiation dose, though with smaller numbers, none of the groups reached statistical significance.
Discussion
Malignancies involving the cranial base are rare, and long-term follow-up guidelines for survivors are essentially nonexistent. There is great variability in the United States and internationally in who follows these survivors, duration of follow-up, follow-up imaging modalities and timing, and any additional testing. The NCCN guidelines specify follow-up recommendations for head and neck cancers and include a recommendation for evaluations of TSH every 6 to 12 months if the neck is irradiated. 21 Notably, screening of these patients with serum TSH alone, as suggested by NCCN guidelines, could mislead the physician to believe the patient is hyper thyroid due to suppressed TSH levels, when in fact, the patient may have central hypothyroidism. Measurement of freeT4 is mandatory in these circumstances as only this parameter can document central hypothyroidism. Similarly, as many patients receive chemotherapy that is known to affect gonadal function measurement of gonadotropins only may obscure the diagnosis of combined primary (gonadal) and secondary (radiation-related) hypogonadism. 22 Ideally, guidelines would eventually include specific pituitary function screening recommendations for irradiated sinonasal malignancies or skull base tumors.
The impact of radiation on the hypothalamic–pituitary axis is well described. Dose-dependent responses have long been characterized, and Darzy highlights a progressive incidence of dysfunction first in growth hormone, followed by gonadotrophs, ACTH, and finally TSH only at higher doses. 23 Furthermore, the delayed impacts are well described, with significant dysfunction 5 to 10 years following radiation. 18 23 24 While many skull base surgeons and radiation oncologists recognize a risk of hypopituitarism in cranial base irradiation, we believe the incidence and impact of this dysfunction are significantly underestimated. This study demonstrates a high prevalence of endocrine abnormalities in patients with cranial base radiation, and highlights a comprehensive yet simple algorithm for endocrinologic evaluation for these patients. Notably, post-radiation hypopituitarism has also been attributed to vascular damage to the hypothalamus. However, hypothalamic radiation dose is largely limited to less than 40 Gy due to proximity to the optic chiasm, while stratification of dose to the sella allows broader stratification.
There are several important findings to note from our study. First, the described incidence of central hypogonadism, hypothyroidism, and adrenal insufficiency all fall within the ranges that have been highlighted by the recent data on nasopharynx carcinoma. 17 18 19
Our data suggests patients at highest risk are, not surprisingly, those who sustain higher doses of radiation to the sella. Both primary tumor subsite and the trends based on the Dmax to the sella correlate to the well-described patterns of dose dependence. Utilizing the subsite of the primary tumor, a statistically significant difference was observed, and this may be a clinically valuable way to distinguish patients at highest risk ( Fig. 2 ). Sphenoid and ethmoid/nasal cavity tumors appear to carry the highest risk, followed closely by tumors located in the nasopharynx or clivus. Importantly, there was a significantly lower incidence of pituitary dysfunction in patients whose primary tumors were in the orbit or maxillary sinus, suggesting that these patients with sinonasal or skull base tumors may not carry a significant risk. Furthermore, there was a clear trend in patients treated at our institution with IMRT in the incidence of hypopituitarism compared with the Dmax to the sella. Notably, due to the wide variability in radiation delivery techniques and poor access to detailed radiation records, patients treated at outside institutions were not included in the radiation dose analysis. However, with only 47 patients in the detailed dose analysis, we were unable to reach statistical significance. Additionally, patients with a history of chemotherapy trended toward higher incidence of hypopituitarism, and this has been similarly demonstrated in prior studies. 25 Chemotherapy is known to potentiate the effects of radiation, but further studies would be required to better refine those patients at highest risk.
This study also highlights the critical importance of multidisciplinary care for these complex patients. In addition to the otolaryngologist and neurosurgeon, these patients are often followed up for long-term by their treating radiation oncologist and possibly in an oncology survivors' clinic. Understanding the importance and incidence of hypopituitarism in these patients is of critical importance for the clinicians performing their follow-up surveillance. Furthermore, an endocrinologist with pituitary disorder experience is an essential part of the treatment team, helping to navigate the complex serologies, differentiate clinical from subclinical disease, and drive supplementation and treatment recommendations. For example, in our clinical endocrine practice, patients with somatotroph abnormalities are not treated as a distinct clinical entity (such as growth hormone deficiency) but rather the somatotroph laboratory studies are used as an overall marker of pituitary gland function. Additionally, posterior pituitary dysfunction is an extremely rare complication of radiation, and has not been observed in our patients.
There are several limitations in our study. First, the patient cohort spans a wide range of disease processes, with pathologies that are highly variable. This is inherent in virtually all skull base registries. Multi-institutional studies would likely be required to further refine the dataset and patient populations. Limited numbers of patients included in detailed radiation dose analysis limited our ability to definitively highlight dose dependence, as many of our patients were treated at outside institutions. Furthermore, we did not exclude patients who underwent surgical interventions at the sella. In theory, surgical devascularization could have contributed to dysfunction. However, these patients would have likely been identified prior to initiating adjuvant radiation and would have been excluded.
Another limitation to the study is an inability to clearly elucidate a temporal relationship between radiation treatment and increasing hypopituitarism. The progressive increase in pituitary dysfunction after radiation is well described, 15 23 24 26 and we would anticipate more of our patients develop dysfunction with continued surveillance. However, our demographics were unique in that several our patients were long term survivors who were enrolled in the registry years after treatment. 27 None of these patients underwent screening serologies prior to the registry was initiated. Still, extended follow-up was not significantly associated with hypopituitarism, as has been classically reported in the literature. Although our study was not designed to identify when after treatment patients develop hypopituitarism, and did not clearly demonstrate increased hypopituitarism with longer follow-up, we believe yearly surveillance is important due to the abundant evidence of increasing incidence with time.
Conclusion
Hypopituitarism is a well-described complication of pituitary irradiation; however, the incidence and impact are likely underestimated in clinical practice. We describe a comprehensive pituitary screening protocol that was initiated at our institution, and highlight a significant incidence of pituitary dysfunction, with 37% of patients demonstrating some component of hypopituitarism after irradiation for skull base malignancies. Based on this data, we recommend pituitary screening serologies in patients undergoing high-dose irradiation for skull base malignancies, such as the protocol detailed. Clinical guidelines for following patients with these rare tumors are sparse, and we recommend revising these guidelines to reflect this data.
Funding Statement
Funding There was no funding for this research. The authors have no relevant conflicts of interest to disclose. This review was conducted under IRB approval HUM00036763. All patients were appropriately consented for enrollment and participation.
This article demonstrates a significant incidence (37%) of radiation-induced hypopituitarism after treatment of anterior cranial base malignancies. This incidence is likely underestimated by many clinicians, and we describe a yearly screening protocol that could be utilized for surveillance.
Author Contributions
Kyle VanKoevering: Data acquisition, analysis, statistics, drafting, reviewing, and editing. Katayoon Sabetsarvestani: Data acquisition, reviewing, and editing. Stephen E. Sullivan: Conceptualization, reviewing, and editing. Ariel Barkan: Data analysis, critical review, and editing. Michelle Mierzwa: Data acquisition, analysis, critical review, and editing. Erin L. McKean: Conceptualization, data analysis, critical review, and editing.
References
- 1.Lund V J, Stammberger H, Nicolai P et al. European position paper on endoscopic management of tumours of the nose, paranasal sinuses and skull base. Rhinol Suppl. 2010;22(22):1–143. [PubMed] [Google Scholar]
- 2.Castelnuovo P, Turri-Zanoni M, Battaglia P, Antognoni P, Bossi P, Locatelli D. Sinonasal malignancies of anterior skull base: histology-driven treatment strategies. Otolaryngol Clin North Am. 2016;49(01):183–200. doi: 10.1016/j.otc.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Banuchi V, Mallen J, Kraus D. Cancers of the nose, sinus, and skull base. Surg Oncol Clin N Am. 2015;24(03):563–577. doi: 10.1016/j.soc.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Jang J W, Chan A W. Prevention and management of complications after radiotherapy for skull base tumors: a multidisciplinary approach. Adv Otorhinolaryngol. 2013;74:163–173. doi: 10.1159/000342293. [DOI] [PubMed] [Google Scholar]
- 5.Fraass B A, Kessler M L, McShan D L et al. Optimization and clinical use of multisegment intensity-modulated radiation therapy for high-dose conformal therapy. Semin Radiat Oncol. 1999;9(01):60–77. doi: 10.1016/s1053-4296(99)80055-1. [DOI] [PubMed] [Google Scholar]
- 6.Tsien C, Eisbruch A, McShan D, Kessler M, Marsh R, Fraass B. Intensity-modulated radiation therapy (IMRT) for locally advanced paranasal sinus tumors: incorporating clinical decisions in the optimization process. Int J Radiat Oncol Biol Phys. 2003;55(03):776–784. doi: 10.1016/s0360-3016(02)04274-8. [DOI] [PubMed] [Google Scholar]
- 7.Fossati P, Vavassori A, Deantonio L, Ferrara E, Krengli M, Orecchia R. Review of photon and proton radiotherapy for skull base tumours. Rep Pract Oncol Radiother. 2016;21(04):336–355. doi: 10.1016/j.rpor.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engelmeier R L, King G E. Complications of head and neck radiation therapy and their management. J Prosthet Dent. 1983;49(04):514–522. doi: 10.1016/0022-3913(83)90314-1. [DOI] [PubMed] [Google Scholar]
- 9.Hammerlid E, Silander E, Hörnestam L, Sullivan M. Health-related quality of life three years after diagnosis of head and neck cancer--a longitudinal study. Head Neck. 2001;23(02):113–125. doi: 10.1002/1097-0347(200102)23:2<113::aid-hed1006>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 10.Alon E E, Lipschitz N, Bedrin L et al. Delayed sino-nasal complications of radiotherapy for nasopharyngeal carcinoma. Otolaryngol Head Neck Surg. 2014;151(02):354–358. doi: 10.1177/0194599814530858. [DOI] [PubMed] [Google Scholar]
- 11.Boomsma M J, Bijl H P, Langendijk J A. Radiation-induced hypothyroidism in head and neck cancer patients: a systematic review. Radiother Oncol. 2011;99(01):1–5. doi: 10.1016/j.radonc.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Jereczek-Fossa B A, Alterio D, Jassem J, Gibelli B, Tradati N, Orecchia R. Radiotherapy-induced thyroid disorders. Cancer Treat Rev. 2004;30(04):369–384. doi: 10.1016/j.ctrv.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Ling S, Bhatt A D, Brown N Vet al. Correlative study of dose to thyroid and incidence of subsequent dysfunction after head and neck radiationHead Neck 2016 [DOI] [PubMed]
- 14.Pfister D G, Ang K K, Brizel D M et al. Head and neck cancers, version 2.2013. Featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2013;11(08):917–923. doi: 10.6004/jnccn.2013.0113. [DOI] [PubMed] [Google Scholar]
- 15.Darzy K H. Radiation-induced hypopituitarism after cancer therapy: who, how and when to test. Nat Clin Pract Endocrinol Metab. 2009;5(02):88–99. doi: 10.1038/ncpendmet1051. [DOI] [PubMed] [Google Scholar]
- 16.Jasim S, Alahdab F, Ahmed A T et al. Mortality in adults with hypopituitarism: a systematic review and meta-analysis. Endocrine. 2017;56(01):33–42. doi: 10.1007/s12020-016-1159-3. [DOI] [PubMed] [Google Scholar]
- 17.Ipekci S H, Cakir M, Kiyici A, Koc O, Artac M. Radiotherapy-induced hypopituitarism in nasopharyngeal carcinoma: the tip of an iceberg. Exp Clin Endocrinol Diabetes. 2015;123(07):411–418. doi: 10.1055/s-0035-1549963. [DOI] [PubMed] [Google Scholar]
- 18.Ratnasingam J, Karim N, Paramasivam S S et al. Hypothalamic pituitary dysfunction amongst nasopharyngeal cancer survivors. Pituitary. 2015;18(04):448–455. doi: 10.1007/s11102-014-0593-6. [DOI] [PubMed] [Google Scholar]
- 19.Huang S, Wang X, Hu C, Ying H. Hypothalamic-pituitary-thyroid dysfunction induced by intensity-modulated radiotherapy (IMRT) for adult patients with nasopharyngeal carcinoma. Med Oncol. 2013;30(04):710. doi: 10.1007/s12032-013-0710-9. [DOI] [PubMed] [Google Scholar]
- 20.Erturk E, Jaffe C A, Barkan A L. Evaluation of the integrity of the hypothalamic-pituitary-adrenal axis by insulin hypoglycemia test. J Clin Endocrinol Metab. 1998;83(07):2350–2354. doi: 10.1210/jcem.83.7.4980. [DOI] [PubMed] [Google Scholar]
- 21.Pfister D G, Spencer S, Brizel D M et al. Head and neck cancers, Version 2.2014. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2014;12(10):1454–1487. doi: 10.6004/jnccn.2014.0142. [DOI] [PubMed] [Google Scholar]
- 22.Isaksson S, Bogefors K, Ståhl O et al. High risk of hypogonadism in young male cancer survivors. Clin Endocrinol (Oxf) 2018;88(03):432–441. doi: 10.1111/cen.13534. [DOI] [PubMed] [Google Scholar]
- 23.Darzy K H, Shalet S M. Hypopituitarism following radiotherapy. Pituitary. 2009;12(01):40–50. doi: 10.1007/s11102-008-0088-4. [DOI] [PubMed] [Google Scholar]
- 24.Darzy K H. Radiation-induced hypopituitarism. Curr Opin Endocrinol Diabetes Obes. 2013;20(04):342–353. doi: 10.1097/MED.0b013e3283631820. [DOI] [PubMed] [Google Scholar]
- 25.Bhandare N, Kennedy L, Malyapa R S, Morris C G, Mendenhall W M. Hypopituitarism after radiotherapy for extracranial head and neck cancers. Head Neck. 2008;30(09):1182–1192. doi: 10.1002/hed.20847. [DOI] [PubMed] [Google Scholar]
- 26.Taku N, Gurnell M, Burnet N, Jena R. Time dependence of radiation-induced hypothalamic-pituitary axis dysfunction in adults treated for non-pituitary, intracranial neoplasms. Clin Oncol (R Coll Radiol) 2017;29(01):34–41. doi: 10.1016/j.clon.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Ward P D, Heth J A, Thompson B G, Marentette L J. Esthesioneuroblastoma: results and outcomes of a single institution's experience. Skull Base. 2009;19(02):133–140. doi: 10.1055/s-0028-1096195. [DOI] [PMC free article] [PubMed] [Google Scholar]


