Abstract
Purpose
To investigate the ameliorative effects of Vitellaria paradoxa (VP) nut extract for an anterior cruciate ligament transection with medial meniscectomy (ACLT+MMx)-induced osteoarthritis (OA) in high-fat diet (HFD)-induced obese rats.
Methods
The rats were fed by HFD for 5 weeks before surgery-induced OA. Rats were treated orally with three different doses of VP nut extract (111.6, 223.2, and 446.4 mg/kg) for 8 weeks.
Results
The VP nut triterpene-rich extract decreased the level of triglycerides and increased high-density lipoprotein-cholesterol. The level of nitric oxide, interleukin (IL)-1β, IL-6, and tumor necrosis factor-α decreased after treatment with VP nut triterpene-rich extract, especially in high-doses. The VP nut triterpene-rich extracts also alleviated swelling in the knee OA, weight-bearing difference, and suppressed cartilage degradation.
Conclusion
The Vitellaria paradoxa nut triterpene-rich extract suppressed proinflammatory mediators and attenuated the cartilage degradation and pain in osteoarthritis with an obesity rat model. As such, Vitellaria paradoxa nut triterpene-rich extract can be used as an alternative for osteoarthritis treatment.
Keywords: medial meniscectomy, obesity, osteoarthritis, triterpene, Vitellaria paradoxa
Introduction
Osteoarthritis (OA) is the most common form of arthritis disease and also known as degenerative joint disease.1 It occurs due to several risk factors, such as age, sex, injury or surgery, and overuse of joints.2 The role of articular cartilage in the body includes slowing external impact and reducing the friction of the articular surface.3 However, with increasing age, physical activity and repeated external pressure and stimulation will result in the gradual loss of articular cartilage proteoglycan and articular cartilage damage,4,5 and finally, the cartilage gradually loses its elasticity and the ability to repair it.6 Joint biomechanical properties change causing damage to articular cartilage, subchondral bone, and synovial inflammation structural damage, including remodeling, sclerosis, and bone spurs generation.7
The post-traumatic-induced OA models have been widely used for experimental studies including either with medial meniscectomy, anterior cruciate ligament, or both.8,9 Surgical-induced instability in the knee mimics joint injury in humans.10,11 Both pharmacological and non-pharmacological treatments have been used for OA management.12 Oral analgesics or anti-inflammatory drugs seem to be useful for alleviating OA symptoms. However, they do not seem effective for long-term application and are also accompanied by some side effects such as an increased risk of gastrointestinal and cardiovascular diseases.13,14 According to these conditions, functional foods have become an interesting thing to evaluate them as anti-arthritis agents.
Vitellaria paradoxa nut extracts mainly consist of the unsaponifiable material parts containing triterpene with hydrocarbon and other minor compounds. Previous studies reported that triterpene possesses anti-inflammatory, anti-oxidation, anti-allergies, and antipruritic effects as well as improved diabetic nephropathy.15–17 Documented studies of triterpene have shown reduced inflammation in patients with osteoarthritis, joint pain, cartilage disintegration and bone loss.18 A previous study reported that in histological evidence, the triterpene from shea nut extract attenuated knee OA development in the standard-fed (normal) diet rat model.8 Additionally, a recent study also reported that oral supplementation of shea nut oil triterpene also reduced OA symptoms and triglycerides level without changes in total cholesterol, high-density lipoprotein-cholesterol, body urea nitrogen, and aspartate transaminase in a normal diet rat model.19 In this present study, we developed the model under obesity conditions and observed the biochemical properties, such as the proinflammatory cytokines, lipid properties, and enzymatic antioxidant activities as well as evaluating cartilage damage.
Obesity has positively correlated with knee osteoarthritis, which will accelerate the deterioration of the knee joint.20 Obesity affects osteoarthritis in joints as the weight of the load increases, which leads to damage to the cartilage and metabolic factors such as adipokines.21 The adipokines (ie, leptin and adiponectin) and proinflammatory cytokines (ie, tumor necrosis factor-α, interleukin-1β, and interleukin-6) were highly expressed in adipose tissues in obesity conditions.22 As such, we hypothesized that triterpene from Vitellaria paradoxa also had ameliorative effects on osteoarthritis symptoms in an obese rats model. Therefore, this study aimed to investigate the beneficial effects of triterpene-rich extract from Vitellaria paradoxa nut on anterior cruciate ligament transection and medial meniscectomy to induce osteoarthritis with high-fat diet-induced obese rats.
Materials and Methods
Materials
The shea (Vitellaria paradoxa) nut extract (FlexNow Plus, SheaFlex 75) was provided by Universal Integrated Corporation (Taipei, Taiwan). The shea nut extract chemical compounds have been analyzed by Cantox Health Sciences International (Ontario, Canada). The major compounds of this extract are composed of esterified triterpene alcohols and sterols (5055%), diglycerides and triglycerides (40–55%), followed by some minor compounds, such as cinnamic acid and hydrogenated cinnamic acid (1–4%); kariten (1–3%); free sterols, free triterpene alcohols, other minor components (0–1%); and free fatty acids (0.05–0.20%). The standard laboratory chow-fed diet (Laboratory Rodent Diet 5001) was purchased from PMI Nutrition International, Inc. (Brentwood, MO, USA). Lard was purchased from MP Biomedicals (Cat. No. 902140, Santa Ana, CA, USA). The plasma total cholesterol (TC) and triglyceride (TG) concentration were detected through commercial kits (Randox, CO, USA). The proinflammatory cytokines including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 were measured with commercial ELISA kits (R&D Systems, MN, USA).
Animal Experiment
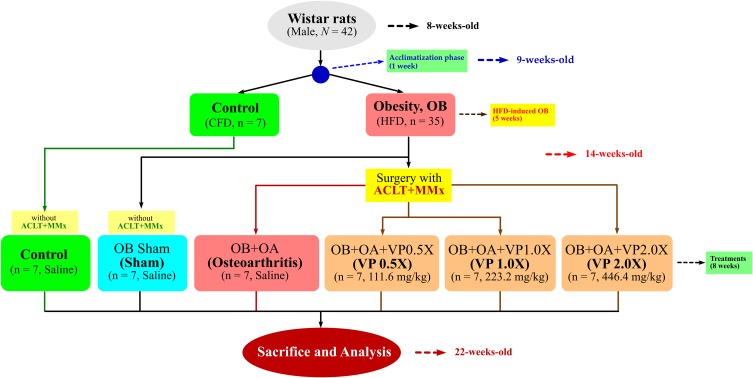
Forty-two Male Wistar rats (8 weeks old) were purchased from the BioLASCO Taiwan Co., Ltd. (Yilan, Taiwan). The rats were housed individually on a 12 hour dark/light cycle. All of the procedures were carried out according to the Animal Protection Act (Act/APC) and the Experimental Animal Ethics Committee of the Council of Agriculture (CoA) of the Executive Yuan, Taiwan. The Institutional Animal Care and Use Committee (IACUC Approval No. 104028) of the National Taiwan Ocean University reviewed and approved all protocols. Briefly, rats were fed with a standard chow-fed diet (CFD) for a week to acclimatize. The control group (seven rats per group, n = 7) were also fed with a chow-fed diet, and 35 diet-induced obesity rats were placed on the high-fat diet (HFD) for 5 weeks (Figure 1). The HFD was composed of ~20% of fat in the total diet or ~40% calories from fat by adding lard according to the previous method. Whereas, the CFD was composed of ~5% of fat or ~13.5% calories from fat.23
Figure 1.
The flowchart of surgical-induced osteoarthritis in an obesity rat model.
Abbreviations: CFD, chow-fed diet; HFD, high-fat diet; VP, Vitellaria paradoxa.
After a 5-week obesity induction phase, the anterior cruciate ligament transection (ACLT) with medial meniscectomy (MMx) were performed to induce osteoarthritis (OA). The Obese+OA groups were fed by daily oral gavage with three different doses of VP triterpene-rich extract (0.5VP, 111.6 mg/kg of body weight, 223.2 mg/kg, and 446.4 mg/kg) according to the previous method.8 The rats were sacrificed after treatment for 8 weeks. The rats were fasted for 12 hours before sacrifice. The rats were placed in an empty chamber and the CO2 was delivered to the chamber for euthanasia. The operated-knee joints were fixed in 10% formalin for histopathological examinations and grading according to OARSI osteoarthritis cartilage histopathology assessment as reported from the previous study.24 Briefly, Grade 0, Normal cartilage, hyaline articular cartilage uninvolved with OA; Grade 1, Threshold in cartilage for OA and characterized by the retention of the articular cartilage surface layer; Grade 2, Focal discontinuity of the cartilage superficial zone; Grade 3, The extension of matrix cracks into the mid zone to form vertical fissures (clefts); Grade 4, Cartilage erosion; Grade 5, Denudation, the complete erosion of the hyaline cartilage to a level of mineralized cartilage or bone; and Grade 6, Changes in the contour of the cartilage surface (deformation).
Surgical-Induced Knee Osteoarthritis
Rats were anesthetized with Xylazine 13 mg/kg (Rompun, Bayer, Pittsburgh, PA, USA) and Tiletamine-Zolazepam 20 mg/kg (Zoletil 50, Virbac Lab., Carros, France) were injected intraperitoneally.25 For the OA models, after shaving the hairs on their right knee, the right stifle joint was approached through a medial parapatellar arthrotomy. Then, the anterior cruciate ligament transection and a partial medial meniscus were removed (ACLT+MMx). Whereas sham surgery was conducted by entering the joint via a lateral incision, spraying the joint with saline, and closing the joint capsule without ACLT+MMx. After surgery, the wound was washed with sterile saline and, both capsule and skin were sutured using Vicryl 4–0 (Ethicon, Edinburgh, UK) absorbable suture and monofilament 4–0 Nylon threads (Ethicon, Edinburgh, UK), respectively. Intraperitoneal injection was performed twice a day for 3 days with Cefazolin (20 mg/kg) to prevent infection.
Weight-Bearing Difference and Knee Width Measurement
Incapacitance tester for measuring pain behavior can be observed in the animal joint pain. Under normal circumstances, the average weight of the rat hind limb will be assigned; if one side has a knee injury, the limb on the uninjured side will bear more weight. The rats were placed in boxes on their feet and balance tested. The rats stood on their hind legs while a bipedal balance tester measured the weight distribution of the two hind legs, each measurement time was 5 seconds, repeated 5 times for the mean value. Hind limb weight distribution is expressed as the difference between the weight of the ipsilateral and contralateral limbs.26 Before the induction of obesity and surgery, the difference in knee swelling t between the operated-knee and unoperated-knee were measured by electronic digital caliper every week until the end of the experiment.27
Plasma Biochemical Analysis
Blood samples were collected by heparinized-syringe into collection tubes and centrifuged at 1000× g at 4°C for 15 minutes to obtain the blood plasma. The plasma total cholesterol, triglyceride, superoxide dismutase activities, and proinflammatory cytokines including the tumor necrosis factor-α, interleukin (IL)-1β, and IL-6 were measured with commercial ELISA kits. All the methods conducted by following the manufacturer’s protocols. Reduced glutathione (GSH) level was estimated by following the earlier method of Ellman.28 Briefly, the plasma (500 μL) were mixed with 10% trichloroacetic acid (500 μL). The contents were mixed well for complete precipitation of proteins and centrifuged at 20,000× g for 5 minutes. An aliquot of clear supernatant (10 μL) was taken and mixed with 85 μL of PBS. Ellman’s reagent (5 μL) was added. After 5 minutes, the optical density was measured at 412 nm against a blank. Whereas, nitric oxide (NO) release was measured by using Griess reagent.29
Statistical Analysis
All the values were shown as the mean ± standard error of the mean (SEM). To determine the significant (p < 0.05) differences in all groups in all parameters we performed one-way analyses of variance (ANOVA) with Duncan post hoc test using SPSS software (v.22.0; IBM Corp., NY, USA).
Results
Effects of VP Nut Triterpene-Rich Extract on Body Weight Compositions
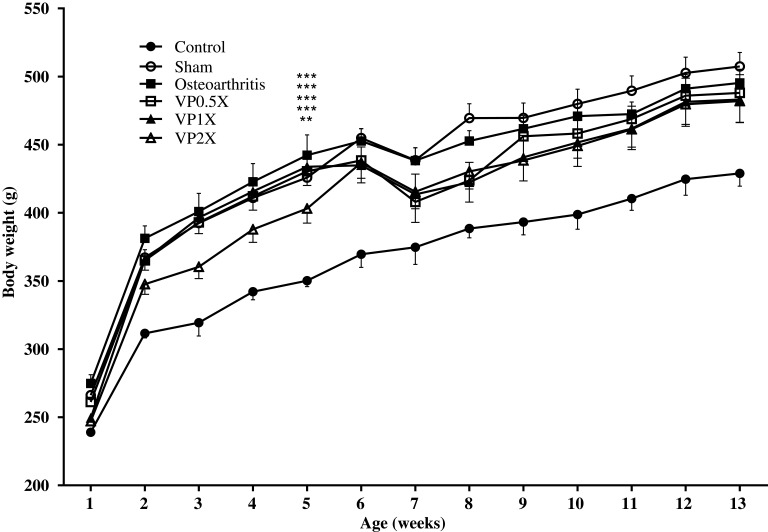
At 5 weeks of age after purchasing from the BioLASCO company, the mean weight of the high-fat diet group was significantly higher than the control group (Figure 2). After feeding with VP nut triterpene-rich extract, the results showed that the body weight of the Sham group was higher than the other groups. The amount of fatty tissues was also significantly increased in the obesity groups. and it reduced after feeding with VP nut triterpene-rich extract in the treated-groups, however, the values were not significant when compared to the untreated-group. This extract also did not cause any effects on weight of heart, liver, kidney, and spleen between the groups (Table 1).
Figure 2.
Effects of VP nut triterpene-rich extracts on body weight.
Notes: Data are shown as the mean ± SEM. Differences were considered significant at **p < 0.01 and ***p < 0.001 versus Control group.
Abbreviation: VP, Vitellaria paradoxa.
Table 1.
Effects of VP Nut Triterpene-Rich Extracts on Organ and Fat Tissue Weight in OA Rats After Treatment for 8 Weeks
| Organ Weight (% Body Weight) | Control | Sham | OA | VP0.5X | VP1X | VP2X |
|---|---|---|---|---|---|---|
| Heart | 0.31±0.01a | 0.30±0.01a | 0.31±0.01a | 0.33±0.01a | 0.32±0.01a | 0.31±0.01a |
| Liver | 3.00±0.07a | 2.88±0.10a | 2.95±0.09a | 2.98±0.11a | 2.92±0.08a | 2.90±0.08a |
| Spleen | 0.18±0.008a | 0.17±0.02a | 0.17±0.01a | 0.17±0.01a | 0.17±0.01a | 0.17±0.003a |
| Kidney | 0.74±0.02a | 0.69±0.02a | 0.74±0.02a | 0.70±0.02a | 0.73±0.01a | 0.70±0.02a |
| Abdominal fat | 0.95±0.04b | 2.36±0.22a | 2.21±0.19a | 2.12±0.17a | 2.14±0.13a | 2.15±0.18a |
| Epididymal fat | 0.94±0.14b | 2.29±0.23a | 2.25±0.09a | 2.19±0.07a | 2.15±0.10a | 2.11±0.12a |
Notes: Data are shown as the mean ± SEM. The values with different letters in the same row (a and b) represent a significant difference (p < 0.05) as analyzed by Duncan’s multiple range test.
Abbreviations: OA, osteoarthritis; VP, Vitellaria paradoxa.
Effects of VP Nut Triterpene-Rich Extract on Plasma Biochemistry
By detecting the plasma lipid metabolism-related indicators, we found that total cholesterol (TC) level was significantly higher in the Sham and OA groups when compared to the Control group (Table 2). However, after treatment with VP nut triterpene-rich extract, the TC level of the VP-treated groups was significantly lower than the OA group especially for the high-dose of VP nut triterpene-rich extract group. High levels of triglycerides (TG) were seen in the Sham and OA groups, however, there was no significant difference after treatment with VP nut triterpene-rich extract. Additionally, VP-treated groups also significantly increased the high-density lipoprotein-cholesterol (HDL-C) levels and there was no change in low-density lipoprotein-cholesterol (LDL-C) levels.
Table 2.
Effects of VP Triterpene-Rich Extracts on Plasma Total Cholesterol, Triglycerides, Low-Density Lipoprotein-Cholesterol, and High-Density Lipoprotein-Cholesterol in OA Rats After Treatment for 8 Weeks
| Plasma Lipid (mg/dL) | Control | Sham | OA | VP0.5X | VP1X | VP2X |
|---|---|---|---|---|---|---|
| TG | 338.87±14.89b | 383.98±14.11ab | 443.10±12.06a | 372.14±15.98ab | 395.35±19.92ab | 359.95±11.68b |
| TC | 82.64±3.26b | 95.29±8.49a | 95.39±7.90a | 88.55±11.94ab | 88.38±13.54ab | 88.97±11.21ab |
| LDL-C | 14.79±0.30a | 14.93±0.27a | 15.63±0.31a | 15.84±0.38a | 15.41±0.49a | 15.56±0.38a |
| HDL-C | 54.92±0.99ab | 49.19±1.64c | 53.47±0.77bc | 54.58±1.54bc | 57.98±2.52ab | 60.27±2.49a |
Notes: Data are shown as the mean ± SEM. The values with different letters in the same row (a–c) represent a significant difference (p < 0.05) as analyzed by Duncan’s multiple range test.
Abbreviations: HDL-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol; TC, total cholesterol; TG, triglycerides; OA, osteoarthritis; VP, Vitellaria paradoxa.
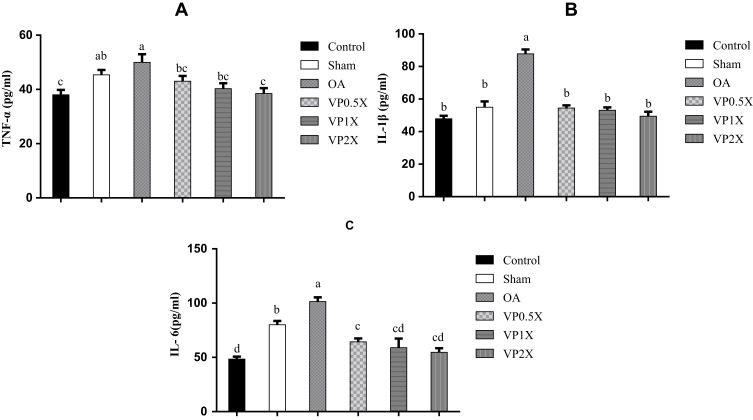
VP Nut Triterpene-Rich Extract Downregulates Proinflammatory Cytokines
The proinflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 were significantly increased in the Sham and OA groups when compared to the Control group (Figure 3A–C). After feeding with a high-dose of VP nut triterpene-rich extracts, the proinflammatory cytokines level was significantly decreased when compared to the OA group. The TNF-α and IL-6 levels in the high-dose of VP nut triterpene-rich extract groups also were significantly decreased when compared to both Sham and OA groups.
Figure 3.
Effects of VP nut triterpene-rich extracts on (A) TNF-α, (B) IL-1β, and (C) IL-6 levels.
Notes: Data are shown as the mean ± SEM. The values with different letters (a–d) represent a significant (p < 0.05) difference as analyzed by Duncan’s multiple range test.
Abbreviations: IL, interleukin; OA, osteoarthritis; TNF, tumor necrosis factor; VP, Vitellaria paradoxa.
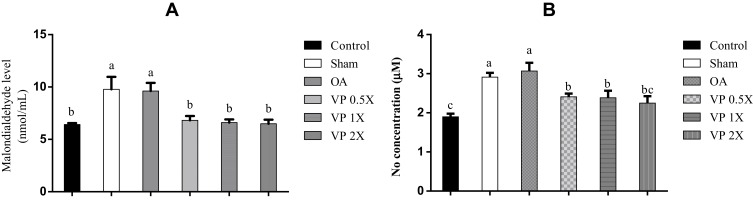
VP Nut Triterpene-Rich Extract Enhances Enzymatic Antioxidant Activity
The superoxide dismutase (SOD) and glutathione (GSH) levels after being fed with VP nut triterpene-rich extract do not show any significant differences when compared to the OA group, however they do show higher activities (Table 3). Additionally, lipid peroxidation (malondialdehyde, MDA) results indicated that, feeding with VP nut extracts significantly reduced the MDA value. It was confirmed that the number of free radical production was significantly reduced (Figure 4A). Our results also showed that nitric oxide (NO) release after being fed with the triterpene-rich extract from VP nut, was significantly lower than in the untreated-groups (Sham and OA) (Figure 4B).
Table 3.
Effects of VP Nut Triterpene-Rich Extract on Enzymatic Antioxidants Activity in OA Rats After Treatment for 8 Weeks
| Antioxidant Properties | Control | Sham | OA | VP 0.5X | VP 1X | VP 2X |
|---|---|---|---|---|---|---|
| SOD (U/mL) | 155.58±12.47a | 149.74±7.41a | 131.37±10.24a | 138.75±4.91a | 137.99±3.92a | 140.40±6.37a |
| GSH (mg/dL) | 0.22±0.006a | 0.23±0.007a | 0.22±0.006a | 0.24±0.004a | 0.25±0.008a | 0.23±0.009a |
Notes: The values with the same letters in the same row represent are not significantly different (p < 0.05)as analyzed by Duncan’s multiple range test.
Abbreviations: GSH, glutathione; OA, osteoarthritis; SOD, superoxide dismutase; VP, Vitellaria paradoxa.
Figure 4.
Effects of VP nut triterpene-rich extracts on (A) malondialdehyde level and (B) NO release.
Notes: Data are shown as the mean ± SEM. The values with different letters (a-c) represent a significant (p < 0.05) difference as analyzed by Duncan’s multiple range test.
Abbreviations: NO, nitric oxide; OA, osteoarthritis; VP, Vitellaria paradoxa.
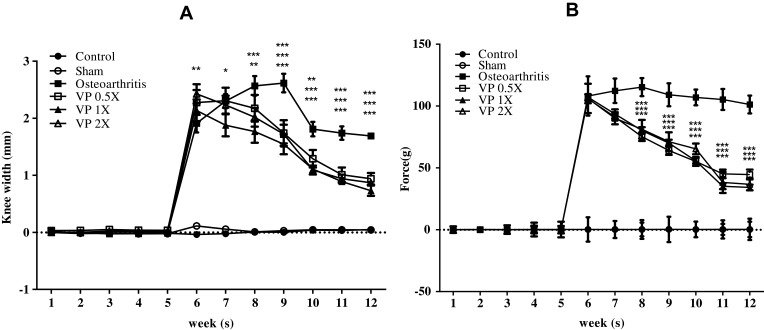
VP Nut Triterpene-Rich Extract Suppresses Knee Width and Weight-Bearing Distribution
The Control and Sham groups did not induce knee swelling, whereas, obesity with ACLT+MMx surgery-induced OA group showed an increase in knee swelling (Figure 5A). On the other hand, after feeding with triterpene-rich extract from VP nut for 8 weeks, the knee swelling significantly reduced when compared to the untreated osteoarthritis group. Further, using an Incapacitance tester, we assessed the status of rat knee pain and discomfort. The results showed that the hindlimb weight-bearing alteration on the contralateral and the ipsilateral limb of the untreated OA group was significantly higher than triterpene-rich from VP nut-treated groups. Whereas, the sham operation did not see any changes in hind limb weight-bearing (Figure 5B).
Figure 5.
The rats knee properties: (A) knee width and (B) weight-bearing distribution of the knee joint.
Notes: Data are shown as the mean ± SEM. Differences were considered significant at *p < 0.05; **p < 0.01; and ***p < 0.001 versus OA group.
Abbreviations: OA, osteoarthritis; VP, Vitellaria paradoxa.
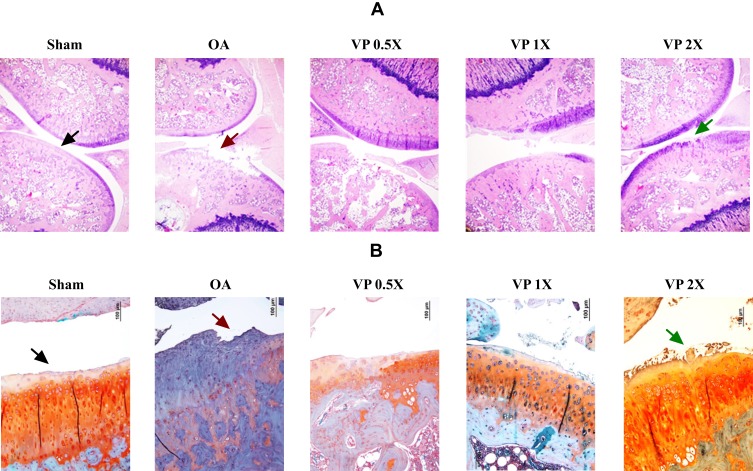
Triterpene-Rich Extract Ameliorates Knee-Joint Histopathology
The hematoxylin and eosin (H&E) and Safranin-O staining confirmed that the Sham group was not involved with OA (Grade 0, normal cartilage), whereas the untreated OA group (OA) showed the superficial zone degradation and hypotrophy (Grade 3) as shown in Figure 6A and B. The cartilage damage was reduced to Grade 2 after treatment with VP nut triterpene-rich extract especially in the high-dose (VP 2X) groups.
Figure 6.
Knee joint histopathology: (A) Hematoxylin and eosin (H&E) and (B) Safranin-O staining. The Safranin-O interpretation: Cartilage (orange to red), cytoplasm (bluish or grey-green) and nuclei (black).
Abbreviations: OA, osteoarthritis; VP, Vitellaria paradoxa.
Discussion
A previous study reported that Vitellaria paradoxa (VP) nut extract is composed of unsaponifiable matter and the major compound consists of triterpene alcohol fractions, such as lupeol, α-amyrin, β-amyrin, butyrospermol, and other triterpene alcohols.30 Additionally, gas-liquid chromatography (GLC) also proved that shea nut extracts are composed of triterpene alcohol fractions, such as α-amyrin, β-amyrin, lupeol, and butyrospermol.31 According to a previous study, the shea (Vitellaria paradoxa) nut oil triterpene attenuated knee OA development in standard or normal diet rat model based on the histological evidence.8 In this present study, we used surgical-induced osteoarthritis in a high-fat diet-induced obesity rat model, then treated the rats with triterpene-rich extract from VP nut extract for 8 weeks. It has been reported that obesity is associated with OA progression, especially on knee OA due to increased body weight resulting in increased joint loading. A previous study also reported that feeding with HFD regulates the pain threshold by altered motor coordination in a mouse model.32 Although, there were not any significant effects on the reduction of body weight, body weight after treatment with VP nut triterpene-rich extract was lower than the untreated OA group (Figure 2). By reducing the body weight, it also reduced the mechanical stress on the knee joint and became an alternative non-pharmacological treatment to suppress OA development.33
Increasing the body weight in obesity condition is also accompanied by increasing the fat tissues, such as abdominal fat and epididymal adipose tissues (Table 1). As shown in Table 2, feeding with HFD also significantly increased the total cholesterol level. The untreated-OA group also showed a significantly high level of triglyceride when compared to the control group, whereas this was not significant with the sham group. A cohort study reported that serum triglyceride showed a significant trend between the normal population and osteoarthritis.34 Additionally, high serum triglyceride also was observed in OA patients when compared to non-OA.35
The adipokines, such as leptin and adiponectin as well as proinflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1, and IL-6 were highly expressed in adipose tissues in obesity conditions.22 Previous studies have also reported that feeding with a high-fat diet increases the fat mass and also TNF-α, IL-6, leptin, as well as adiponectin expression.36,37 These proinflammatory cytokines have been reported to be associated with the development of OA by induction with some degradation enzymes including matrix metalloproteinases (MMPs), such as MMP-1, MMP-3, and MMP-13 through activation of the nuclear factor (NF)-kβ signaling pathway.38,39 High expression of TNF-α, IL-1β, and IL-6 showed on untreated (Sham and OA) groups (Figure 3). Treatment with VP nut triterpene-rich extracts successfully decreased the expression of these proinflammatory cytokines. According to this condition, we hypothesized that triterpene-rich extract from VP nut suppressed the expression of MMPs at the articular surface and inhibited cartilage degeneration. A previous study reported that triterpene from shea extracts was shown to have anti-inflammatory properties by suppressing NF-κB activation and also antioxidant activity.15 Additionally, triterpene-rich extract also decreased the TC level, whereas the HDL-C level was increased. The ability of triterpene to regulate lipid metabolism has been reported by the previous study.40 This previous study reported that the triterpene which is isolated from the stem bark of Protorhus longifolia caused a significant lowering of the levels of TC and LDL-C with an increase in HDL-C in HFD-induced hyperlipidemia rats. A previous study also showed that triterpene (Antcins) from Antrodia cinnamomea possess an ability as an agonist for peroxisome proliferator-activated receptor-α (PPAR-α). PPAR-α has been used for treating dyslipidemia and metabolic disorders.41 A recent study reported that shea nut oil triterpene also reduced HDL-C in the normal diet of rats.19
In this present study we also showed that triterpene-rich extract from VP nut extract enhanced the enzymatic antioxidant activities especially in superoxide dismutase (SOD) activity when compared to the untreated osteoarthritis group (Table 3). This extract also reduced oxidative stress as indicated by lower lipid peroxidation and nitric oxide release compared to the untreated OA group (Figure 4). The lower activities of SOD and glutathione peroxidase (GPx) showed in obesity compared to a healthy condition and was accompanied by higher malondialdehyde (MDA, as a lipid peroxidation marker) level.42–44 Additionally, high expression of nitric oxide (NO) showed in OA patients and as a proinflammatory mediator, it positively correlated with OA development.45
In many cases, osteoarthritis is accompanied by joint swelling and pain.46 A previous study reported that significant knee swelling was observed in ACLT with the saline-treated group.27 In this present study, the untreated OA group showed an increase in knee swelling and imbalance of hind limb weight-bearing (Figure 5A and B). After treatment with VP nut triterpene-rich extract especially in high-doses, both knee swelling and weight-bearing were reduced. Pain is caused by weight-bearing imbalance resulting in instability on their posture and reduced mobility as well as their quality of life.46,47 In the cartilage with OA, the signaling of proinflammatory cytokines, such as TNF-α and IL-1β could induce the expression of other inflammatory cytokines and various chondrolytic mediators including prostaglandin (PG)-E2. Additionally, in vitro study of various triterpenes, such as taraxasterol and betulinic acid suppressed NF-κB activation and protected human chondrocytes by inhibiting MMPs, NO, and PG-E2 production.48,49
Under H&E and Safranin-O staining observation (Figure 6), the Sham group not involved with OA is characterized by smooth cartilage surfaces, the matrix and chondrocytes are organized into superficial, mid and deep zones (Grade 0, normal cartilage).24 Whereas, the untreated-OA group (Osteoarthritis group) showed OA progression by degradation on the superficial zone and hypocellularity (Grade 3). It was also accompanied by extension of matrix cracks into the mid zone. Treatment with VP nut extract especially in high-dose (VP 2X) successfully suppressed OA development with a small number of matrix cracks extending completely through the superficial zone (Grade 2). Grade 2 OA was also characterized by the focal discontinuity of the cartilage superficial zone.24 A previous study reported that triterpene extract from shea nut oil reduced the imbalanced weight-bearing and attenuated knee OA development.8,19 Additionally, lupeol acetate also showed the anti-arthritic effects on complete Freund’s adjuvant (CFA)‐induced arthritic rats.50
Overall, this present study showed the beneficial effects of triterpene-rich extract from Vitellaria paradoxa nut extract on surgically-induced OA with a high-fat diet-induced obese rat model. In this study, we also found that some measurements showed a similar ameliorative effect on the level of expressions when treated with VP nut extracts. Previous literature reported that in the case of the search for an optimum concentration, one of the critical roles is the tolerance mechanism. The organism learns slowly to decrease the distributing effect of the drug, when it is repeatedly disturbed by a specific drug by opposing the disturbance at the moment it occurs.51 Therefore, we hypothesized that in some cases of measurements, a low-dose of VP nut extract is the most suitable concentration to regulate its expression, when the dose increases (high-dose), the rats show a tolerance mechanism to VP nut extract and provide similar effects between low- and high-dose of VP nut extract.
Conclusion
Oral administration of a triterpene-rich extract from Vitellaria paradoxa nut ameliorated some conditions in post-traumatic-induced osteoarthritis in obese rats, such as reduced body weight, total cholesterol, oxidative stress, and suppressed proinflammatory cytokine expressions. This extract also ameliorated joint swelling and pain. Therefore, it also attenuated the cartilage degradation. According to these findings, triterpene-rich extract from Vitellaria paradoxa nut can be used as a supplement for osteoarthritis improvement. This result also supported that functional foods can be used as an alternative for osteoarthritis treatment.
Acknowledgments
We are very grateful that Universal Integrated Corporation (Taipei, Taiwan) provided the SheaFlex75 for this study.
Disclosure
The authors report no conflicts of interest in this work
References
- 1.Di Rosa M, Szychlinska MA, Tibullo D, Malaguarnera L, Musumeci G. Expression of CHI3L1 and CHIT1 in osteoarthritic rat cartilage model. A morphological study. Eur J Histochem. 2014;58:3. doi: 10.4081/ejh.2014.2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaganti RK, Lane NE. Risk factors for incident osteoarthritis of the hip and knee. Curr Rev Musculoskelet Med. 2011;4(3):99–104. doi: 10.1007/s12178-011-9088-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musumeci G, Trovato FM, Loreto C, et al. Lubricin expression in human osteoarthritic knee meniscus and synovial fluid: a morphological, immunohistochemical and biochemical study. Acta Histochem. 2014;116(5):965–972. doi: 10.1016/j.acthis.2014.03.011 [DOI] [PubMed] [Google Scholar]
- 4.Szychlinska MA, Imbesi R, Castrogiovanni P, et al. Assessment of vitamin D supplementation on articular cartilage morphology in a young healthy sedentary rat model. Nutrients. 2019;11:6. doi: 10.3390/nu11061260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szychlinska M, Trovato F, Di Rosa M, et al. Co-expression and co-localization of cartilage glycoproteins CHI3L1 and lubricin in osteoarthritic cartilage: morphological, immunohistochemical and gene expression profiles. Int J Mol Sci. 2016;17:3. doi: 10.3390/ijms17030359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felson DT. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133(8):635–646. doi: 10.7326/0003-4819-133-8-200010170-00016 [DOI] [PubMed] [Google Scholar]
- 7.Gelse K, Mühle C, Franke O, et al. Cell-based resurfacing of large cartilage defects: long-term evaluation of grafts from autologous transgene-activated periosteal cells in a porcine model of osteoarthritis. Arthritis Rheumatism. 2008;58(2):475–488. doi: 10.1002/(ISSN)1529-0131 [DOI] [PubMed] [Google Scholar]
- 8.Kao J-H, Lin S-H, Lai C-F, Lin Y-C, Kong Z-L, Wong C-S. Shea nut oil triterpene concentrate attenuates knee osteoarthritis development in rats: evidence from knee joint histology. PLoS One. 2016;11(9):e0162022. doi: 10.1371/journal.pone.0162022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szychlinska MA, Castrogiovanni P, Trovato FM, et al. Physical activity and Mediterranean diet based on olive tree phenolic compounds from two different geographical areas have protective effects on early osteoarthritis, muscle atrophy and hepatic steatosis. Eur J Nutr. 2018;58(2):565–581. doi: 10.1007/s00394-018-1632-2 [DOI] [PubMed] [Google Scholar]
- 10.Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20(10):739–744. doi: 10.1097/01.bot.0000246468.80635.ef [DOI] [PubMed] [Google Scholar]
- 11.Janusz MJ, Bendele AM, Brown KK, Taiwo YO, Hsieh L, Heitmeyer SA. Induction of osteoarthritis in the rat by surgical tear of the meniscus: inhibition of joint damage by a matrix metalloproteinase inhibitor. Osteoarthritis Cartilage. 2002;10(10):785–791. doi: 10.1053/joca.2002.0823 [DOI] [PubMed] [Google Scholar]
- 12.Castrogiovanni P, Di Rosa M, Ravalli S, et al. Moderate physical activity as a prevention method for knee osteoarthritis and the role of synoviocytes as biological key. Int J Mol Sci. 2019;20:3. doi: 10.3390/ijms20030511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fibel KH, Howard JH, Brian CH. State-of-the-art management of knee osteoarthritis. World J Clin Cases. 2015;3(2):89–101. doi: 10.12998/wjcc.v3.i2.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ Clin Res. 2011;342(jan11 1):c7086. doi: 10.1136/bmj.c7086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akihisa T, Kojima N, Kikuchi T, et al. Anti-inflammatory and chemopreventive effects of triterpene cinnamates and acetates from shea fat. J Oleo Sci. 2010;59(6):273–280. doi: 10.5650/jos.59.273 [DOI] [PubMed] [Google Scholar]
- 16.Fodouop SPC, Gatsing D, Tangue BT, et al. Effect of Salmonella typhimurium infection on rat’s cell oxidation and in vivo antioxidant activity of Vitellaria paradoxa and Ludwigia abyssinica aqueous extract. Asian Pacific J Trop Dis. 2015;5(1):38–46. doi: 10.1016/S2222-1808(14)60624-1 [DOI] [Google Scholar]
- 17.Oliveira FA, Lima-Junior RCP, Cordeiro WM, et al. Pentacyclic triterpenoids, α,β-amyrins, suppress the scratching behavior in a mouse model of pruritus. Pharmacol Biochem Behav. 2004;78(4):719–725. doi: 10.1016/j.pbb.2004.05.013 [DOI] [PubMed] [Google Scholar]
- 18.Cheras PA, Myers SP, Paul-Brent P-A, Outerbridge KH, Nielsen GVL. Randomized double-blind placebo-controlled trial on the potential modes of action of sheaflex70tm in osteoarthritis. Phytother Res. 2009;24(8):1126–1131. [DOI] [PubMed] [Google Scholar]
- 19.Chen I-J, Lin S-H, Wong C-S. Oral shea nut oil triterpene concentrate supplement ameliorates pain and histological assessment of articular cartilage deterioration in an ACLT injured rat knee osteoarthritis model. PLoS One. 2019;14:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Felson DT. Osteoarthritis: new insights. Part 2: treatment approaches. Ann Intern Med. 2000;133:9. [DOI] [PubMed] [Google Scholar]
- 21.Arden N, Nevitt M. Osteoarthritis: epidemiology. Best Pract Res Clin Rheumatol. 2006;20(1):3–25. doi: 10.1016/j.berh.2005.09.007 [DOI] [PubMed] [Google Scholar]
- 22.Chandel NS, Schumacker PT, Arch RH. Reactive oxygen species are downstream products of TRAF-mediated signal transduction. J Biol Chem. 2001;276(46):42728–42736. doi: 10.1074/jbc.M103074200 [DOI] [PubMed] [Google Scholar]
- 23.Woods SC, Seeley RJ, Rushing PA, D’Alessio D, Tso P. A controlled high-fat diet induces an obese syndrome in rats. J Nutr. 2003;133(4):1081–1087. doi: 10.1093/jn/133.4.1081 [DOI] [PubMed] [Google Scholar]
- 24.Pritzker KPH, Gay S, Jimenez SA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14(1):13–29. doi: 10.1016/j.joca.2005.07.014 [DOI] [PubMed] [Google Scholar]
- 25.Hayami T, Pickarski M, Zhuo Y, Wesolowski GA, Rodan GA, Duong LT. Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone. 2006;38(2):234–243. doi: 10.1016/j.bone.2005.08.007 [DOI] [PubMed] [Google Scholar]
- 26.Mapp P, Walsh D, Bowyer J, Maciewicz R. Effects of a metalloproteinase inhibitor on osteochondral angiogenesis, chondropathy and pain behavior in a rat model of osteoarthritis. Osteoarthritis Cartilage. 2010;18(4):593–600. doi: 10.1016/j.joca.2009.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsai W-Y, Wu J-L, Liu -C-C, et al. Early intraarticular injection of hyaluronic acid attenuates osteoarthritis progression in anterior cruciate ligament-transected rats. Connect Tissue Res. 2013;54(1):49–54. doi: 10.3109/03008207.2012.734877 [DOI] [PubMed] [Google Scholar]
- 28.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6 [DOI] [PubMed] [Google Scholar]
- 29.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of Nitrate, Nitrite, and [15N]nitrate in Biological Fluids. Anal Biochem. 1982;126(1):131–138. doi: 10.1016/0003-2697(82)90118-X [DOI] [PubMed] [Google Scholar]
- 30.Peers KE. The non-glyceride saponifiables of shea butter. J Sci Food Agric. 1977;28(11):1000–1009. doi: 10.1002/(ISSN)1097-0010 [DOI] [Google Scholar]
- 31.Akihisa T, Kojima N, Katoh N, et al. Triterpene alcohol and fatty acid composition of shea nuts from seven African countries. J Oleo Sci. 2010;59(7):351–360. doi: 10.5650/jos.59.351 [DOI] [PubMed] [Google Scholar]
- 32.Micheli L, Lucarini E, Trallori E, et al. Phaseolus vulgaris L. Extract: alpha-amylase inhibition against metabolic syndrome in mice. Nutrients. 2019;11:8. doi: 10.3390/nu11081778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puett DW. Published trials of nonmedicinal and noninvasive therapies for hip and knee osteoarthritis. Ann Intern Med. 1994;121(2):133–140. doi: 10.7326/0003-4819-121-2-199407150-00010 [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Gil M, Reyes C, Ramos R, et al. Serum lipid levels and risk of hand osteoarthritis: the chingford prospective cohort study. Sci Rep. 2017;7:1. doi: 10.1038/s41598-017-03317-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Philbin EF, Ries MD, Groff GD, Sheesley KA, French TS, Pearson TA. Osteoarthritis as a determinant of an adverse coronary heart disease risk profile. Eur J Cardiovasc Prev Rehabi. 1996;3(6):529–533. doi: 10.1177/174182679600300608 [DOI] [PubMed] [Google Scholar]
- 36.Chien M-Y, Ku Y-H, Chang J-M, Yang C-M, Chen C-H. Effects of herbal mixture extracts on obesity in rats fed a high-fat diet. J Food Drug Anal. 2016;24(3):594–601. doi: 10.1016/j.jfda.2016.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng C-H, Lin H-T, Chung D-J, Huang C-N, Wang C-J. Mulberry leaf extracts prevent obesity-induced NAFLD with regulating adipocytokines, inflammation and oxidative stress. J Food Drug Anal. 2018;26(2):778–787. doi: 10.1016/j.jfda.2017.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahn S-J, Rhim E-M, Kim J-Y, et al. Tumor necrosis factor-α induces matrix metalloproteinases-3, −10, and −13 in human periodontal ligament cells. J Periodontol. 2014;85(3):490–497. doi: 10.1902/jop.2013.130063 [DOI] [PubMed] [Google Scholar]
- 39.Rigoglou S, Papavassiliou AG. The NF-κB signalling pathway in osteoarthritis. Int J Biochem Cell Biol. 2013;45(11):2580–2584. doi: 10.1016/j.biocel.2013.08.018 [DOI] [PubMed] [Google Scholar]
- 40.Machaba KE, Cobongela SZZ, Mosa RA, Oladipupo LA, Djarova TG, Opoku AR. In vivo anti-hyperlipidemic activity of the triterpene from the stem bark of Protorhus longifolia (Benrh) Engl. Lipids Health Dis. 2014;13:1. doi: 10.1186/1476-511X-13-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y-J, Lee S-C, Hsu C-H, Kuo Y-H, Yang -C-C, Lin F-J. Antcins, triterpenoids from Antrodia cinnamomea, as new agonists for peroxisome proliferator-activated receptor α. J Food Drug Anal. 2019;27(1):295–304. doi: 10.1016/j.jfda.2018.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ozata M, Mergen M, Oktenli C, et al. Increased oxidative stress and hypozincemia in male obesity. Clin Biochem. 2002;35(8):627–631. doi: 10.1016/S0009-9120(02)00363-6 [DOI] [PubMed] [Google Scholar]
- 43.Patel MD, Kishore K, Patel DJ. Evaluation of oxidative stress and serum magnesium levels in South Indian obese males. Int J Sci Res. 2014;3(3):229–230. doi: 10.15373/22778179/MARCH2014/77 [DOI] [Google Scholar]
- 44.Gujjala S, Putakala M, Nukala S, Bangeppagari M, Ramaswamy R, Desireddy S. Renoprotective effect of Caralluma fimbriata against high-fat diet-induced oxidative stress in Wistar rats. J Food Drug Anal. 2016;24(3):586–593. doi: 10.1016/j.jfda.2016.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vuolteenaho K, Moilanen T, Knowles RG, Moilanen E. The role of nitric oxide in osteoarthritis. Scand J Rheumatol. 2009;36(4):247–258. doi: 10.1080/03009740701483014 [DOI] [PubMed] [Google Scholar]
- 46.Krasnokutsky S, Attur M, Palmer G, Samuels J, Abramson SB. Current concepts in the pathogenesis of osteoarthritis. Osteoarthritis Cartilage. 2008;16:S1–S3. doi: 10.1016/j.joca.2008.06.025 [DOI] [PubMed] [Google Scholar]
- 47.Lee D-G, Park S-Y, Chung W-S, et al. Fucoidan prevents the progression of osteoarthritis in rats. J Med Food. 2015;18(9):1032–1041. doi: 10.1089/jmf.2014.3334 [DOI] [PubMed] [Google Scholar]
- 48.Piao T, Ma Z, Li X, Liu J. Taraxasterol inhibits IL-1β-induced inflammatory response in human osteoarthritic chondrocytes. Eur J Pharmacol. 2015;756:38–42. doi: 10.1016/j.ejphar.2015.03.012 [DOI] [PubMed] [Google Scholar]
- 49.Jingbo W, Aimin C, Qi W, Xin L, Huaining L. Betulinic acid inhibits IL-1β-induced inflammation by activating PPAR-γ in human osteoarthritis chondrocytes. Int Immunopharmacol. 2015;29(2):687–692. doi: 10.1016/j.intimp.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 50.Kweifio-Okai G, Carroll AR. Antiarthritic effect of lupeol acetate. Phytother Res. 1993;7(2):213–215. doi: 10.1002/(ISSN)1099-1573 [DOI] [Google Scholar]
- 51.Peper A. Aspects of the relationship between drug dose and drug effect. Dose-Response. 2009;7:2. doi: 10.2203/dose-response.08-019.Peper [DOI] [PMC free article] [PubMed] [Google Scholar]