Abstract
Objectives
To investigate the rates of frailty and frailty characteristics and examine the clinical and neuropsychological correlates of frailty in adults with late life depression (LLD).
Methods
Data were used from the evaluation of 134 individuals over the age of 60 years (45 men, 89 women) with a depressive diagnosis who enrolled in studies for the treatment of their depression. Depression, neuropsychological functioning, white matter hyperintensity (WMH) burden via magnetic resonance imaging, and characteristics of frailty were assessed.
Results
Fried frailty burden (≥ 3 characteristics) was present in 25% of the sample, with this rate increasing to 45.5% when using clinically meaningful cut-scores for gait speed (< 1 m/s) and physical activity levels (< 1000 kcal/week). Moreover, 62% of the sample exhibited gait slowing (< 1 m/s) or weakness (grip strength), with 29% demonstrating both. Greater frailty burden was associated with greater HRSD severity in covariate adjusted linear regression models (t127=2.41, p = .02). Greater frailty burden was not associated with neuropsychological dysfunction, nor was it associated with greater WMH burden.
Conclusions
Findings from this study show that frailty, specifically physical frailty deficits in mobility and strength, is highly comorbid in adults with LLD, associated with greater depressive symptom severity, and does not appear to be associated with the vascular depression subtype of LLD. Future research should investigate the relationship between frailty and antidepressant treatment response as well as test whether there are age-related biological processes that result in the manifestation of the frail-depressed subtype of LLD.
Depressive illness affects as many as 25% of adults over the age of 60, the most rapidly growing demographic group in the US.1 Adults with late life depression (LLD) respond poorly to available treatments2,3 and thus incur massive personal, social, and economic cost. Treatments may fail in older adults because diagnostic criteria and pathophysiological models for depression are based primarily on studies of younger adults, even though the symptoms and pathophysiology of LLD change with age.4 Thus, an understanding of how aging interacts with LLD may identify different subgroups with unique clinical manifestations and underlying mechanisms that can then be targeted with more personalized approaches to treatment. For instance, age-related cognitive changes such as the development of white matter hyperintensities (WMH) and executive dysfunction are associated with incident LLD and poorer outcomes (the Vascular Depression [VD] hypothesis of LLD).5,6 It is imperative to identify additional clinical/pathophysiology-based subgroups of LLD to develop interventions to improve health trajectories for adults with LLD.
One such subgroup may be the frail-depressed. The biological syndrome of frailty (Fried phenotype) is a syndrome of bioenergetic deficits defined clinically by the presence of decreases in strength (hand-grip strength), self-reported energy, and physical activity, slowed motor performance, and unintentional weight loss. Frailty is associated with but distinct from disability, disease,7 and depression.8 It is bidirectionally related to LLD,9 and, when comorbid with LLD, associated with increased mortality risk in later life.10 One hypothesis is that frailty in the context of LLD may represent accelerated biological aging, and mechanisms, including inflammatory processes, dopamine deficiencies, and mitochondrial bioenergetics, have been postulated that may explain this intersection.11 Alternatively, frailty could merely represent another clinical manifestation of VD, as increased frailty is associated with greater WMH burden and greater executive dysfunction.12
Information on the relationship between frailty and LLD has been garnered through secondary analyses of epidemiological studies. These studies were not originally designed to deconstruct this relationship, however, and therefore have methodological limitations, specifically with respect to their assessment of depression. The aims of this investigation were threefold: 1) To examine the rates of total frailty burden and the individual frailty characteristics in adults with LLD, 2) To assess the clinical and cognitive correlates of frailty in adults with LLD, and 3) To test whether frailty is distinct from VD, a known age-related subtype of LLD To our knowledge, this is the first study designed to characterize frailty in the context of LLD.
Methods
Participants
Individuals included in this study presented at the Clinic for Aging, Anxiety, and Mood Disorders (CAAM) at the New York State Psychiatric Institute (NYSPI) for evaluation between June 2013 and December 2018. and entered into ongoing antidepressant treatment protocols in CAAM. To enter these protocols, patients were ≥ 60 years of age with a diagnosis of either a Major Depressive Episode (MDE) or Persistent Depressive Disorder (PDD), with a rater-administered Hamilton Depression Rating Scale (HRSD) ≥ 16. Patients were excluded from participation if they suffered from an acute, unstable, or severe medical illness, had significant cognitive impairment (30-item Mini Mental State Exam [MMSE] < 24) or a diagnosis of dementia, history of psychosis or bipolar disorder, or a diagnosis of substance abuse or dependence in the last 12-months prior to evaluation. Data were obtained from their evaluation and/or baseline visits prior to initiation of new treatment in CAAM. The total sample consists of 134 adults with LLD who underwent both depression and frailty assessments, with sample size differences noted where applicable.
Assessments
DEPRESSION
Diagnosis of a depressive disorder was obtained from the rater-administered Structured Clinical Interview Diagnostic for DSM 5 (SCID). As part of the SCID, self-reported number of depressive episodes, length of current episode, and age of first onset of a depressive episode were obtained. Depression severity is assessed by the rater-administered 24-item HRSD.13
COGNITION
The 30-item MMSE14 was used to assess global cognition. Episodic memory was assessed using Logical Memory Test I & II.15 Executive function was assessed via the Stroop Color-Word Interference Test16, and Trail-Making Test Part B.17 Attention and psychomotor speed were assessed with the Trail-Making Test Part A17 as well as Digit Symbol Substitution Task.15
FRAILTY CHARACTERISTICS
Frailty characteristics were assessed at either evaluation or baseline visits. Individuals were coded as having a frailty characteristic based on previously established cut-points.7 The original Fried criteria, specifically for grip strength, gait speed, and physical activity levels, was based on the lowest quintile observed from the sample in the Cardiovascular Health Study. Since that time, less severe levels for characteristics like gait speed (< 1 m/s) and physical activity (< 1000 kcal/week) have been identified as clinically significant and may be more appropriate for use in an outpatient setting such as the one utilized in this study. As such, both Fried cut-offs and clinically significant cut-offs will be used to delineate frailty categories (Non-frail [0 deficits], Intermediate Frail [1–2 deficits], and Frail [≥ 3 deficits]) in this study. Patients’ average gait speed (m/s) was derived from two trials of subjects’ usual walking speed within the clinic space. A Fried-level cut-score (< .8 m/s) and a clinically significant cut-score (< 1 m/s) are reported.18 A dynamometer was used to assess grip strength (two trials in dominant hand unless dominant hand was compromised), with average grip strength (kgf) recorded and gender-specific cut-scores (≤ 32 for males, ≤ 21 for females). Self-report of significant unintentional weight loss in the last year (≥ 10 lbs or 5% of their body weight) was recorded. Physical activity was measured using the Minnesota Leisure Time Physical Activity Questionnaire, with a Fried cut-score (< 270 kcal/week) and a clinically meaningful cut-score (< 1000 kcal/week) reported (the latter is the minimum threshold recommended by the Center for Disease Control). Fatigue was measured by two exhaustion-items from the Center for Epidemiologic Studies Depression Scale (CES-D19). These items are scored 0–3, with a response of ≥ 2 on either item denoting frailty level fatigue.
IMAGING
Scanning was conducted with a research-dedicated 3T GE MR750 magnetic resonance imaging (MRI) Scanner. Total white matter hyperintensity (WMH) volumes were derived from T2-weighted fluid-attenuated inversion recovery (FLAIR) images with in-house developed software. Briefly, each participant’s FLAIR image was corrected for intensity normalization,20 then skull-stripped21 and intensity normalized again. The skull-stripped images were sent through a high pass filter at the mode of the distribution of the image voxel intensity values. A half Gaussian mixture model was fit to the log-transformed histogram of the intensity values of each image. The Gaussian distribution that encapsulated the highest intensity values defined the hyperintense voxels and were labeled. Any cluster of labeled voxels that comprised fewer than 5 voxels was removed from the mask. The labeled images were visually inspected and false positives removed. The number of labeled voxels was summed and multiplied by voxel dimensions to yield a total volume in cm3.
Statistical Analysis
For descriptive analyses, study participants were grouped according to their Fried frailty burden. Continuous variables were summarized with means and standard deviations within each group and in the entire sample. Frailty group differences were assessed using ANOVA. Categorical variables were summarized with frequencies and percentages within each group and in the entire sample. Differences in categorical percentages were assessed using Fisher’s Exact test.
Depression severity (HRSD) was the primary outcome of interest. To assess the association between HRSD and frailty burden (Fried and clinically meaningful frailty were each treated as an ordinal variable) or individual frailty characteristics, we fit separate linear regression models with HRSD as the response and each one of the aforementioned frailty measures as the primary predictor, adjusting for age, sex, education, and number of medical comorbidities. We also fit a separate covariate adjusted simultaneous model with the five individual frailty characteristics entered as predictors. For this model, we computed the variance inflation factor (VIF) for each estimated coefficient and found all VIFs < 1.74, indicating little effect of the possible linear relationships among the predictors on the estimated standard errors. The modelling strategy was repeated for the secondary depression variables (age of onset for first MDE, length of current MDE [log transformed], and number of lifetime MDEs) and neuropsychological measures, treating each of them as the response in the models. Finally, in the subset of subjects with imaging data available, the same regression modelling strategy was repeated with WMH as the response in the linear models, additionally adjusting for intracranial volume.
Results
A sample of 134 individuals were evaluated in the CAAM at NYSPI and recruited into ongoing treatment studies. The sample was well-educated (16 years of education), primarily White (63%) and female (66%) with mild/moderate levels of medical comorbidity who were cognitively intact (MMSE 28±1.48). They presented primarily with a MDE (92%) that was moderately severe (HRSD 23.1± 5.84), recurrent and persistent, with the average age of onset of their first MDE being roughly 40 years of age (Table 1).
Table 1.
Baseline characteristics for adults with late life depression by frailty burden
| Variable | Total sample (n = 134) | Fried Non-frail (n = 10) | Fried Intermediate Frail (n = 91) | Fried Frail (n = 33) | Statistics |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, years | 69.9 (7.43) | 68.0 (5.01) | 69.0 (6.91) | 72.8 (8.73) | F2, 131 = 3.64, P = .029 |
| Gender, # Female (% Female) | 89 (66.4%) | 7 (70.0%) | 59 (64.8%) | 23 (69.7) | P = .912 |
| Education, years | 16.2 (2.79) | 15.7 (2.12) | 16.4 (2.94) | 15.8 (2.52) | F2, 116 = .64, p = .528 |
| Ethnicity, # Hispanic (% Hispanic) | 17 (12.7%) | 2 (22%) | 11 (12%) | 4 (12%) | P = .254 |
| Race, # Caucasian/Black (% Black) | 85/28 (20.9%) | 6/2 (22.2%) | 56/21 (24.1%) | 23/5 (17.2%) | P = .759 |
| Medical burden | 4.4 (2.54) | 4.2 (3.03) | 3.9 (2.39) | 5.8 (2.38) | F2, 123=6.57, P = .002 |
| Depression | |||||
| HRSD | 23.1 (5.84) | 19.6 (3.44) | 23.3 (5.76) | 23.7 (6.38) | F2, 130 = 2.05, p = .132 |
| Diagnosis, # MDE/PDD (% MDE) | 119/11 (91.5%) | 7/2 (77.8%) | 83/7 (92.2%) | 29/2 (93.5%) | P = .254 |
| # of episodes | 2.1 (2.08) | 2.1 (1.46) | 1.8 (1.42) | 2.9 (3.56) | F2, 83 = 1.95, P = .149 |
| Length of current, years | 6.9 (13.8) | 3.7 (3.56) | 7.0 (14.42) | 7.8 (14.49) | F2, 107 = .437, P = .65 a |
| Age of first onset, years | 39.7 (23.3) | 44.1 (21.71) | 39.8 (23.28) | 37.9 (24.6) | F2, 112 = .243, P = .785 |
| Frailty | |||||
| Gait, m/s | 1.02 (0.25) | 1.23 (.21) | 1.09 (.19) | .75 (.21) | F2, 131 = 42.03, P < .001 |
| # (%) Gait < .8 m/s | 27 (20.1%) | 0 | 5 (5.5%) | 22 (66.7%) | P < .001 |
| # (%) Gait < 1 m/s | 57 (42.5%) | 1 (10%) | 29 (31.9%) | 27 (81.8%) | P < .001 |
| Grip ave strength, kg force | 25.2 (9.59) | 31.3 (9.42) | 26.7 (9.42) | 19.3 (7.68) | F2, 131 = 10.77, P < .001 |
| Frailty grip, # Frail (%) | 65 (48.5%) | 0 | 37 (40.7%) | 28 (84.8%) | P < .001 |
| Physical Activity, kcal/wk | 1730 (2000) | 2190.4 (2024.74) | 2090.8 (2174.49) | 583.2 (660.17) | F2, 131=7.89, P < .001 |
| Activity < 270 kcal/wk | 24 (17.9%) | 0 | 10 (11%) | 14 (42.4%) | P < .001 |
| Activity < 1000 kcal=/wk | 64 (47.8%) | 5 (50%) | 33 (36.3%) | 26 (78.8%) | P < .001 |
| Weight loss, # (% WL) | 39 (29.1%) | 0 (0%) | 18 (19.8%) | 21 (63.6%) | P < .001 |
| Fatigue, No. (% Fatigue) | 98 (73.1%) | 0 | 70 (76.9) | 28 (84.8%) | P < .001 |
| Clinically Significant Frailty | P < .001 | ||||
| Intermediate, # (%) | 68 (50.7%) | 5 (50%) | 63 (69.2%) | 0 | |
| Frail, # (%) | 61 (45.5%) | 0 | 28 (30.8%) | 33 (100%) | |
| Neuropsychological function | |||||
| MMSE | 28.4 (1.48) | 28.0 (2.11) | 28.5 (1.49) | 28.1 (1.2) | F2, 128=.85, P = .428 |
| Digit Symbol | 40.2 (10.9) | 44.9 (12.53) | 41.0 (10.69) | 36.4 (10.33) | F2, 106 = 2.74, P = .069 |
| Trailmaking Test A, s | 46.5 (17.3) | 47.5 (21.81) | 45.5 (17.15) | 49.5 (15.91) | F2, 74 = .33, P = .72 |
| Trailmaking Test B, s | 123 (61.0) | 129.8 (68.59) | 126.5 (67.38) | 112.5 (46.05) | F2, 39 = .23, P = .79 |
| Stroop Interference | 43.5 (9.56) | 47.0 (13.04) | 43.9 (9.11) | 41.6 (9.44) | F2, 98=1.22, P = .301 |
| Logical Memory Immediate | 14.0 (4.50) | 17.4 (3.66) | 13.9 (3.98) | 13.3 (5.63) | F2, 109=2.73, P = .069 |
| Logical Memory Delay | 12.5 (4.33) | 14.3 (4.2) | 12.6 (4.43) | 11.8 (4.05) | F2, 108 = 1.04, P =.357 |
| White matter hyperintensity volume, cm3 | 2.02 (2.04) | 4.8 (4.27) | 1.9 (1.92) | 1.8 (1.56) | F2, 60=3.29, P = .044 |
Note. P-values for categorical variables are based on Fisher’s Exact Test, so no test statistic is reported. Abbreviations: Fried Non-Frail (0 characteristics). Fried Intermediate Frail (1–2 characteristics). and Fried Frail (≥ 3 characteristics); HRSD, Total score on the 24-item Hamilton Rating Scale for Depression; MDE. Major Depressive Episode; PDD, Persistent Depressive Disorder; m/s, meters per second; kg force, kilograms of force; kcal/wk, kilocalories per week; WL, weight loss; MMSE, 30-item Mini-Mental State Exam; s, seconds; cm3 cubic centimeters.
ANOVA performed after log-transforming the values, but mean (sd) are presented on original scale.
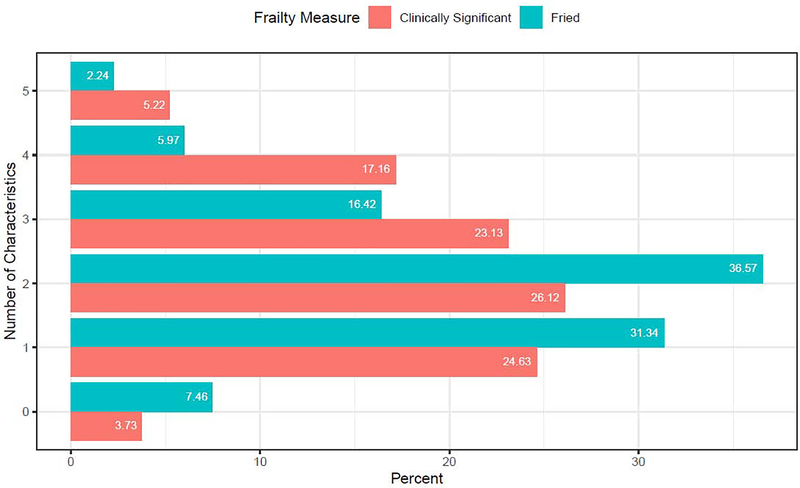
Fried Frailty (≥ 3 characteristics) was present in 25% of the sample (Figure 1), with individuals presenting in the clinic with 1.89±1.10 characteristics. Individuals who were Frail according to Fried criteria were older and had greater medical burden, but did not differ on other demographic categories from the Non-Frail or Intermediate Frail (Table 1). Using the clinically meaningful cut-scores for gait speed and activity levels, the sample exhibited 2.4±1.28 frailty characteristics, with 45.5% of the sample presenting as Frail and 51% presenting as Intermediate Frail (Figure 1).
Figure 1. Breakdown of frailty burden in adults with late life depression.
Note. The figure displays the number of frailty characteristics and the prevalence of that level of frailty burden that was observed in this sample of adults with LLD using both the cut scores for the Fried Frailty phenotype as well as for clinically significant frailty which utilizes different cut scores for gait speed (< 1 m/s) and physical activity levels (< 1000 kcal/week).
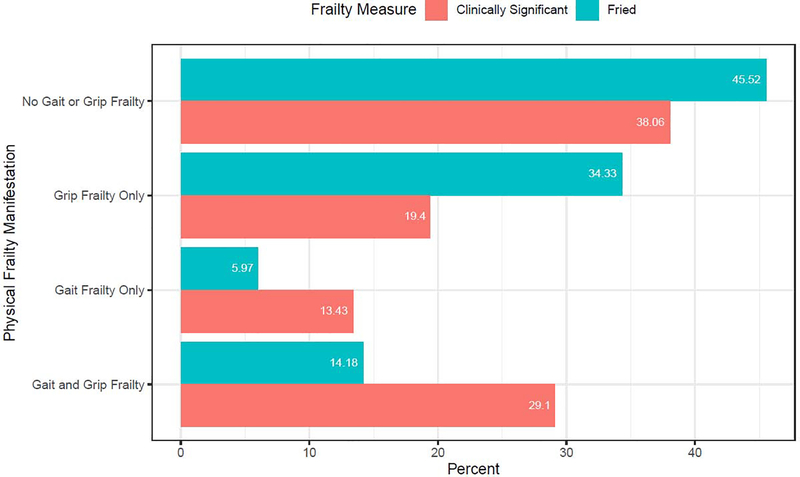
Given the phenomenological overlap between symptoms of depression and characteristics of frailty, the proportion of individuals who exhibited physical frailty level deficits (gait speed, grip strength) that do not overlap with symptoms of LLD was examined. As shown in Figure 2, the majority of adults with LLD exhibit physical deficits in mobility and/or strength, with 54% demonstrating a Fried criteria physical deficit (weak grip strength and/or gait speed < .8 m/s) and 62% demonstrating a clinically significant physical deficit (weak grip strength and/or gait speed < 1 m/s). Almost one third of the overall sample (29%) demonstrated clinically significant deficits in both gait and grip strength during their evaluation, with 20% demonstrating a gait speed < .8 m/s, 42.5% < 1 m/s, and 48.5% exhibiting frailty level deficits in grip strength. Furthermore, we used an ANOVA to examine the relationship between rater-administered psychomotor retardation (HRSD item 8) with gait speed to assess whether gait speed is in itself a measure of a known symptom of depression. We observed no mean differences in gait speed by retardation group (item scored 0, 1, or 2), F2,130 = 1.68, p = 0.19.
Figure 2. Prevalence of physical frailty deficits including slow gait speed and weak grip strength in adults with late life depression.
Note. The figure displays the manifestations of frailty based on the presence or absence of the physical characteristics of frailty burden observed in this sample of adults with LLD. Two cut scores were used for the definition of slow gait: the Fried Frailty definition which utilizes cut score of < .8 m/s and a clinically significant cut score which utilizes a gait speed of < 1 m/s.
FRAILTY AND DEPRESSION
Covariate adjusted linear regression models were fit to test the association between HRSD and both total frailty burden and individual frailty characteristics. Clinically significant frailty burden (t127=2.41, p = .02) rather than Fried frailty burden (t127=1.86, p = .06) was associated with greater HRSD severity. Using the clinically meaningful cut-scores, individuals with ≥ 3 frailty characteristics had HRSD scores that were, on average, 2.18 points greater than individuals with Intermediate frailty (1–2 characteristics), and 4.36 HRSD points greater than Non-frail individuals (Table 2).
Table 2.
Covariate adjusted linear regression models testing the association between depression severity, frailty burden, and individual frailty characteristics
| HRSD |
||||
| Individual Models | β-weight (Standardized β-weight) | SE | t-statistic (df) | p-value |
| Fried frailty total score | 1.80 (0.97) | 0.96 | 1.86 (127) | .065 |
| Clin. Sign. frailty total score | 2.18 (1.24) | 0.91 | 2.41 (127) | .017 |
| Gait speed | −4.49 (−1.13) | 2.28 | −1.97 (127) | .051 |
| Grip strength | 0.02 (0.18) | 0.07 | 0.28 (127) | .779 |
| Fatigue | 3.15 | 1.12 | 2.82 (127) | .006 |
| Weight loss | 2.44 | 1.12 | 2.18 (127) | .031 |
| Physical activity levels | 4.14×10−4 (0.88) | 2.61×10−4 | 1.58 (127) | .116 |
| Simultaneous Model | β-weight (Standardized β-weight) | SE | t-statistic | p-value |
| Gait speed | −3.55 (−0.89) | 2.39 | −1.46 (123) | .140 |
| Grip strength | 0.02 (0.16) | 0.07 | 0.25 (123) | .805 |
| Fatigue | 2.89 | 1.12 | 2.59 (123) | .011 |
| Weight loss | 1.72 | 1.12 | 1.54 (123) | .127 |
| Physical activity levels | 5.06×10−4 (1.01) | 2.60×10−4 | 1.95 (123) | .054 |
Note. All models adjusting for age, sex, education and number of medical comorbidities. Standardized β-weights (or B-weights) are provided for each continuous predictor. Individual Models are bivariate regressions with covariates (one predictor), as compared to the Simultaneous model which included all five frailty characteristics as continuous variables. Values in the table show the average change in HRSD for a 1-SD increase in the predictor. Abbreviations: HRSD, Total score on the 24-item Hamilton Rating Scale for Depression; Clin. Sign. Frailty total score, total score of frailty characteristics using clinically significant cut scores for gait (< 1 meter/second) and physical activity levels (< 1000 kilocalories/week).
Of the five frailty characteristics, three were associated with greater HRSD severity in individual models: gait speed (t127=−1.97, p = .05), fatigue (t127=2.82, p = .01), and significant weight loss (t127=2.18, p = .03; Table 2). Translated as above for instance, having frailty-level fatigue at evaluation corresponds with a HRSD score that is 3.15 points higher compared with individuals without fatigue. When the five individual frailty characteristics were entered simultaneously into a covariate-adjusted linear regression model, only fatigue (t123= 2.59, p = .011) was associated with HRSD severity (Table 2).
Similar linear models were fit for the secondary depression variables (age of onset for first MDE, length of current MDE, and number of lifetime MDE), but neither overall frailty burden nor individual frailty characteristics were associated with these secondary depression variables.
FRAILTY AND NEUROPSYCHOLOGICAL FUNCTION
Covariate adjusted linear models tested the association between each neuropsychological test scores and both frailty burden and individual frailty characteristics. Neither total frailty burden nor individual frailty characteristics with the exception of gait speed (t125 = 2.09, p = .04) was associated with MMSE. In fact, only two frailty characteristics showed any relationship with neuropsychological test scores: weight loss (associated with digit symbol [t103= −2.23, p = .03], and Logical Memory immediate recall [t106 = 1.95, p = .05]), and gait speed (associated with both Logical Memory immediate and delayed recall [t106 = 3.42, p < .001 and t105=2.74, p = .01, respectively], and borderline associated with both digit symbol [t103 = 1.83, p = .07] and Stroop Color-Word Interference [t95 = 1.76, p = .08]).
WHITE MATTER HYPERTENSIVE BURDEN
Similar covariate adjusted linear models were fit in a subset of the sample to test the association between frailty and total WMH burden, with intracranial volume entered as an additional covariate (Table 3). Only total Fried frailty burden was associated with total WMH burden (t55 = −2.22, p = .03) showing that greater frailty burden was associated with less WMH. This association appears driven by a few extreme values in the Non-frail sample (as noted in Table 1).
Table 3.
Covariate adjusted linear regression models testing association between white matter hyperintensity burden and both frailty burden and individual frailty characteristics
| White Matter Hyperintensity Burden |
||||
|---|---|---|---|---|
| Individual Models | β-weight (Standardized β-weight) | SE | t-statistic (df) | p-value |
| Fried frailty burden | −1.16 (−0.63) | 0.52 | −2.22 (55) | .031 |
| Clin. Sign. frailty burden | −0.40 (−0.22) | 0.50 | −0.79 (55) | .435 |
| Gait speed | 0.48 (0.12) | 1.21 | 0.40 (55) | .691 |
| Grip strength | 0.03 (0.33) | 0.03 | 1.06 (55) | .295 |
| Fatigue | −0.42 | 0.56 | −0.75 (55) | .455 |
| Weight loss | 0.06 | 0.58 | 0.10 (55) | .921 |
| Physical activity levels | −1.65×10−5 (−0.03) | 1.57×10−4 | −0.10 (55) | .918 |
Note. All models adjusting for age, sex, education and number of medical comorbidities. Standardized β-weights (or B-weights) are provided for each continuous predictor. These values show the average change in HRSD for a 1-SD increase in the predictor. Abbreviations: Clin. Sign. Frailty total score, total score of frailty characteristics using clinically significant cut scores for gait (< 1 meter/second) and physical activity levels (< 1000 kilocalories/week).
Discussion
Frailty in later life is associated with increased morbidity and mortality. Failty and LLD appear to be bi-directionally related to one another and their intersection doubles mortality rates in older individuals,9,10 yet these findings were based on epidemiological samples which poorly characterized the diagnosis and severity of depression. To address these limitations, we examined the intersection between frailty and depression using data from adults with LLD who were comprehensively assessed in a clinic setting and entered into depression treatment studies. We observed that frailty, including physical deficits such as slow gait and weak grip-strength, is 1) highly comorbid in adults with LLD, 2) associated with greater depressive symptom severity, and 3) appears distinct from VD, a known age-related subtype of LLD that is associated with poor outcomes in later life.
One concern regarding the study of frailty in the context of LLD was the potential phenomenological overlap between symptoms of LLD (weight loss, decreased physical activities, low energy) and characteristics of frailty (fatigue, decreased leisure activities, weight loss).22 Although this overlap may indeed exist, the presence of the objectively-measured, physical deficits of frailty observed in this study (49% exhibiting frailty-level grip strength, 20% exhibiting gait speeds < .8 m/s and 43% exhibiting gait speeds < 1 m/s) demonstrates that the frailty-depression relationship is not the result of mere symptom overlap. This assertion was supported by the lack of an association observed between gait speed and psychomotor retardation. Thus, adults with LLD display deficits in strength and mobility that expand beyond classic depression symptoms. In fact, the proportion of individuals in this study presenting with these physical deficits is far greater than has been observed in epidemiological samples of older individuals. For instance, in this study 43% presented with gait speeds < 1 m/s compared with 26% of older individuals in the Health Aging and Body Composition Study.9 Deficits in strength and mobility observed in this study could contribute to the increased fall risk and decreased independence that is observed in adults with LLD and their assessment may identify new clinical and pathophysiological targets for intervention implementation to drastically improve the quality of life of adults with LLD.
Surprisingly, we observed minimal association between overall frailty burden and neuropsychological functioning. Prior research showed that frailty is associated with greater overall cognitive impairment and deficits in both processing speed and executive functioning (similar to VD). These findings led to the recognition of a “cognitive frailty” syndrome.23,24 Similarly, Fried frailty is associated with both cognitive decline as well as biological variables (inflammation, low Brain-Derived Natriuretic Factor) in older adults that are themselves predictors of cognitive decline.25–27 Additionally, in the few studies that have investigated a neural signature of frailty in older adults, there was a mild to moderate association between frailty and increased WMH burden.12,28 We observed no such association between frailty and total WMH burden. Furthermore, we observed no association between total frailty burden and cognitive impairment, including deficits in executive function. As such, these findings support the frail-depressed as a potential subtype of LLD that is not associated with VD, which is marked by both increased WMH burden and executive dysfunction.
Of the individual frailty deficits, slow gait speed was associated with poorer performance on tests of episodic memory function, processing speed, and executive functioning. These findings are consistent with prior research that identified the presence of a Motor Cognitive Risk Syndrome (MCR) in later life, a syndrome defined by slow gait speed and subjective cognitive complaints. MCR is associated with increased dementia risk, specifically risk for vascular dementia.29 Based on normative data, the gait abnormality in MCR is typically defined as a gait speed < 1 m/s30 which is consistent with the cut-off used to identify clinically significant frailty in this study. Relatedly, frailty (defined by the Frailty Index, not the Fried phenotype) is a risk factor for the development of MCR in later life, even after adjusting for mobility deficits and medical comorbidity, suggesting potential shared mechanisms.31 Although cardiovascular disease and inflammatory processes are associated with MCR, increased WMH burden is not, which is also consistent with the lack of an association between WMH and gait speed in this current study.32,33 Given these findings, it may be possible that although the frail-depressed may be a separable entity from adults with VD, frailty in the context of LLD may in part represent the presence of MCR in adults with LLD.
The findings from this study which appear to be both clinically meaningful (> 4 point difference in HRSD between Non-frail and Frail individuals) and theoretically interesting support what has been observed in epidemiological studies: that frailty and depression are highly comorbid, and that greater frailty burden is associated with greater depressive symptom severity. Still unknown, however, is whether frailty and depression either share some underlying biological mechanism or are simply frequent co-travelers in later life without a biological link. One hypothesized relationship between LLD and frailty is that recurrent MDE’s experienced over time causes a “biological scar” that would increase the risk of developing frailty. This hypothesis was not supported by the findings from this study that demonstrated no relationship between number of depressive episodes and age of first onset of the depressive episodes and frailty burden or individual frailty characteristics. To better understand the nature of the relationship between frailty and depression, future studies are needed to test whether different mechanisms of biological aging (inflammatory processes, mitochondrial bioenergetics) are associated with the frail-depressed, and whether frailty or specific manifestations of frailty are associated with decreased antidepressant medication response in adults with LLD. If indeed LLD shares potential mechanisms with frailty and the LLD is itself reversible, it may also mean that some degree of the frailty itself may be reversible thereby potentially altering deleterious trajectories for these at risk older individuals.
There are important limitations to this study. This sample consisted of individuals who 1) responded to an advertisement or were referred from local physicians and 2) were eligible for ongoing depression treatment studies on LLD. As such, the findings reported here may not be generalizable to the greater population. Additionally, a more rigorous inclusion/exclusion criteria was applied to the sample as these individuals enrolled in ongoing treatment protocols in the CAAM, and thus individuals with acute or unstable medical illnesses or comorbid illnesses such as substance abuse disorders who may present with characteristics of frailty as well depression were not included in this study. Similarly, the CAAM is an outpatient clinic and therefore patients who are unable to travel to such a clinic (individuals in assisted living or nursing homes) may have been excluded. These two points are noteworthy as both rates of depressive illness and frailty are higher in individuals with greater medical comorbidity and in individuals who live in assisted living or nursing home settings. As such, this study potentially underestimates the rates of frailty characteristics observed in adults with LLD. These limitations are offset by specific strengths including the recruitment of a large sample of older adults who were diagnosed with MDE or PDD using the gold standard measures for both diagnosis and symptom severity characterization. Furthermore, these patients were assessed on the most widely used measures of neuropsychological functioning and quantitative methods for classifying WMH burden.
In conclusion, findings from this study showed that frailty, including deficits in mobility and strength, is highly comorbid in adults with LLD and associated with greater depressive symptom severity,. Furthermore, we did not observe any evidence of an association between frailty and characteristics of VD, a known subtype of LLD. Future research should investigate the relationship between frailty and frailty manifestation and antidepressant treatment response as well as whether there are shared mechanisms that result in the frail-depressed manifestation of LLD.
Highlights.
- What is the primary question addressed in this study?
- Recent studies have shown an association between the biological syndrome of of frailty and late life depression. These studies, however, were primarily epidemiological and not constructed to deconstruct the frailty-depression relationship. As such, the primary focus of this study was to investigate the rates of frailty and frailty characteristics and examine the clinical and neuropsychological correlates of frailty in adults with late life depression.
- What is the main finding of this study?
- The main findings from this study are that frailty, specifically physical frailty deficits in mobility and strength, is highly comorbid in adults with late life depression and associated with greater depressive symptom severity. Furthermore, we saw no evidence of a relationship between frailty and the vascular depression subtype of LLD.
- What is the meaning of the finding?
- This study provides evidence that frailty, and in particular physical deficits in mobility and strength, is prevalent in adults with late life depression and may differ from known subtypes of late life depression such as vascular depression. As such, these deficits and their biological correlates may prove useful as targets for future intervention studies.
Acknowledgements
This research was supported by grants from the National Institute of Mental Health (K23 MH099097, K01 MH113850, and R01 MH102293). Drs. Brown, Roose, Ciarleglio, Brickman, and Rutherford, as well as Ms. O’Boyle, Chung, Igwe, Gomez and Naqvi and Mr. Maas have nothing to disclose. Drs. Brown and Ciarleglio had full access to all of the data and take responsibility for the integrity of the data and the accuracy of the data analysis.
This research was supported by grants from the National Institute of Mental Health (K23 MH099097, K01 MH113850, and R01 MH102293). No Disclosures to Report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Meeks TW, Vahia IV, Lavretsky H, Kulkarni G, Jeste DV. A tune in “a minor” can “b major”: a review of epidemiology, illness course, and public health implications of subthreshold depression in older adults. J Affect Disord. 2011;129(1–3):126–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sneed JR, Culang ME, Keilp JG, Rutherford BR, Devanand DP, Roose SP. Antidepressant medication and executive dysfunction: a deleterious interaction in late-life depression. Am J Geriatr Psychiatry. 2010;18(2):128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sneed JR, Rutherford BR, Rindskopf D, Lane DT, Sackeim HA, Roose SP. Design makes a difference: a meta-analysis of antidepressant response rates in placebo-controlled versus comparator trials in late-life depression. Am J Geriatr Psychiatry. 2008;16(1):65–73. [DOI] [PubMed] [Google Scholar]
- 4.Brown PJ, Wall MM, Chen C, et al. Biological Age, Not Chronological Age, is Associated with Late Life Depression. J Gerontol A Biol Sci Med Sci. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheline YI, Pieper CF, Barch DM, et al. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010;67(3):277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sneed JR, Rindskopf D, Steffens DC, Krishnan KR, Roose SP. The vascular depression subtype: evidence of internal validity. Biol Psychiatry. 2008;64(6):491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. [DOI] [PubMed] [Google Scholar]
- 8.Mezuk B, Lohman M, Dumenci L, Lapane KL. Are Depression and Frailty Overlapping Syndromes in Mid- and Late-life? A Latent Variable Analysis. Am J Geriatr Psychiatry 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown PJ, Roose SP, Zhang J, et al. Inflammation, Depression, and Slow Gait: A High Mortality Phenotype in Later Life. J Gerontol A Biol Sci Med Sci. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown PJ, Roose SP, Fieo R, et al. Frailty and depression in older adults: a high-risk clinical population. Am J Geriatr Psychiatry. 2014;22(11):1083–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown PJ, Rutherford BR, Yaffe K, et al. The Depressed Frail Phenotype: The Clinical Manifestation of Increased Biological Aging. Am J Geriatr Psychiatry. 2016;24(11):1084–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung HW, Kim SW, Yoon SJ, Choi JY, Kim KI, Kim CH. Associations between frailty, retinal microvascular changes, and cerebral white matter abnormalities in Korean older adults. J Am Geriatr Soc. 2014;62(11):2209–2210. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton M. Development of a rating scale for primary depressive illness. British Journal of Social and Clinical Psychology. 1967;6:278–296. [DOI] [PubMed] [Google Scholar]
- 14.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 15.D.A. W. Manual for the Wechsler Adult Intelligence Scale-Revised. New York: Psychological Corporation; 1981. [Google Scholar]
- 16.Golden CJ. Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Chicago, IL: Skoelting; 1978. [Google Scholar]
- 17.Reitan R. Validity of the Trail-Making Test as an indication of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 18.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- 20.Tustison NJ, Avants BB, Cook PA, et al. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 2010;29(6):1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mezuk B, Edwards L, Lohman M, Choi M, Lapane K. Depression and frailty in later life: a synthetic review. Int J Geriatr Psychiatry. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gross AL, Xue QL, Bandeen-Roche K, et al. Declines and impairment in Executive Function Predict Onset of Physical Frailty. J Gerontol A Biol Sci Med Sci. 2016;71(12):1624–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelaiditi E, Cesari M, Canevelli M, et al. Cognitive frailty: rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. J Nutr Health Aging. 2013;17(9):726–734. [DOI] [PubMed] [Google Scholar]
- 25.Buchman AS, Boyle PA, Wilson RS, Tang Y, Bennett DA. Frailty is associated with incident Alzheimer’s disease and cognitive decline in the elderly. Psychosom Med. 2007;69(5)483–489. [DOI] [PubMed] [Google Scholar]
- 26.Hsu FC, Kritchevsky SB, Liu Y, et al. Association between inflammatory components and physical function in the health, aging, and body composition study: a principal component analysis approach. J Gerontol A Biol Sci Med Sci. 2009;64(5):581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scalzo P, Kummer A, Bretas TL, Cardoso F, Teixeira AL. Serum levels of brain-derived neurotrophic factor correlate with motor impairment in Parkinson’s disease. J Neurol. 2010;257(4):540–545. [DOI] [PubMed] [Google Scholar]
- 28.Chen WT , Chou KH, Liu LK, et al. Reduced cerebellar gray matter is a neural signature of physical frailty. Hum Brain Mapp. 2015;36(9):3666–3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verghese J, Wang C, Lipton RB, Holtzer R. Motoric cognitive risk syndrome and the risk of dementia. J Gerontol A Biol Sci Med Sci. 2013;68(4):412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oh-Park M, Holtzer R, Xue X, Verghese J. Conventional and robust quantitative gait norms in community-dwelling older adults. J Am Geriatr Soc. 2010;58(8):1512–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sathyan S, Ayers E, Gao T, et al. Frailty and Risk of Incident Motoric Cognitive Risk Syndrome. J Alzheimers Dis. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mergeche JL, Verghese J, Allali G, et al. White Matter Hyperintensities in Older Adults and Motoric Cognitive Risk Syndrome. J Neuroimaging Psychiatry Neurol. 2016;1(2):73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang N, Allali G, Kesavadas C, et al. Cerebral Small Vessel Disease and Motoric Cognitive Risk Syndrome: Results from the Kerala-Einstein Study. J Alzheimers Dis. 2016;50(3):699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]