Abstract
Over the last two decades, the discovery of ketamine’s antidepressant properties has galvanized research into the neurobiology of treatment-resistant depression. Nevertheless, the mechanism of action underlying antidepressant response to ketamine remains unclear. This study reviews electrophysiological studies of ketamine’s effects in individuals with depression as well as healthy controls, with a focus on two putative markers of synaptic potentiation: gamma oscillations and long-term potentiation. The review focuses on: 1) measures of gamma oscillations and power and their relationship to both acute, psychotomimetic drug effects as well as delayed antidepressant response in mood disorders; 2) changes in long-term potentiation as a promising measure of synaptic potentiation following ketamine administration; and 3) recent efforts to model antidepressant response to ketamine using novel computational modeling techniques, in particular the application of dynamic causal modeling to electrophysiological data. The latter promises to better characterize the mechanisms underlying ketamine’s antidepressant effects.
Keywords: ketamine, electrophysiology, gamma, long-term potentiation, dynamic causal modeling
1. Introduction
It has been three decades since seminal work implicated the NMDA subtype of glutamate receptors in the pathophysiology of depression (Trullas and Skolnick, 1990). A decade later, ketamine’s antidepressant effects were first reported in a controlled trial (Berman et al., 2000), galvanizing research into the neurobiology underpinning mood disorders and leading to increased focus on the glutamatergic system’s putative role in both major depressive disorder (MDD) (Bernard et al., 2011; Choudary et al., 2005) and bipolar depression (Eastwood and Harrison, 2010). A wealth of studies shave since explored the efficacy of ketamine and its metabolites as a clinical treatment option for mood disorders (Diazgranados et al., 2010; Zanos et al., 2019; Zarate et al., 2006), and interest in targeting the glutamatergic system has grown exponentially (Ohgi et al., 2015).
A growing body of evidence now demonstrates that a single infusion of subanesthetic-dose ketamine rapidly (within hours) relieves depressive symptoms in individuals with both MDD (Fava et al., 2018; Murrough et al., 2013a; Murrough et al., 2013b; Zarate et al., 2006) and bipolar depression (Diazgranados et al., 2010; Zarate et al., 2012), including those who are treatment-resistant. Studies have also observed rapid antidepressant effects following intranasal ketamine administration (Lapidus et al., 2014), which led the FDA to approve esketamine (the intranasally-administered S-enantiomer of ketamine) as a conjunctive treatment for treatment-resistant depression in 2019. Repeat infusions of ketamine have been shown to maintain ketamine’s antidepressant effects far beyond that of a single administration (Murrough et al., 2013b; Phillips et al., 2019). Additional studies have also explored ketamine’s effectiveness in other clinical domains, including obsessive-compulsive disorder (Rodriguez et al., 2013), post-traumatic stress disorder (Feder et al., 2014), suicidal ideation (Murrough et al., 2015), and social anxiety disorder (Taylor et al., 2018). Given the broad range of its therapeutic effects, understanding the mechanism of action underlying rapid antidepressant response to ketamine could ultimately help expedite the development of novel, rapid-acting therapeutics to more effectively treat depressive symptoms without the associated psychotomimetic side effects and abuse potential of ketamine (Krystal et al., 1994).
Ketamine is a noncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist (Hirota and Lambert, 1996). Early work examining the link between glutamatergic system dysfunction and depression focused on the role of NMDA receptor activation in long-term potentiation (LTP) in the hippocampus (Trullas and Skolnick, 1990), while more recent work has focused on ketamine’s specific role in enhancing LTP outside the hippocampus (Kirk et al., 2010; Sumner et al., 2019). However, the pharmacological profile of ketamine and its underlying mechanism of action appear to extend beyond simply modulating glutamate transmission and include direct and indirect high affinity antagonistic binding properties at the NMDA receptor as well as α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) throughput modulation (Maeng et al., 2008; Zanos et al., 2016). Subanesthetic-dose ketamine administration leads to immediate presynaptic disinhibition of glutamatergic neurons, producing a glutamate surge (Moghaddam et al., 1997). This surge is thought to result from NMDA receptor blockade by ketamine of fast-spiking γ-aminobutyric acid (GABA)-ergic interneurons, leading to local inhibition of interneuron tonic firing and subsequent disinhibition of pyramidal neurons (Homayoun and Moghaddam, 2007; Widman and McMahon, 2018). Due to NMDA receptor blockade on post-synaptic excitatory neurons, excess synaptic glutamate is primarily taken up by AMPA receptors (Duman et al., 2019; Moghaddam et al., 1997), thereby activating neuroplasticity-related signaling pathways including mammalian target of rapamycin complex 1 (Li et al., 2010; Li et al., 2011) and brain-derived neurotrophic factor (Liu et al., 2012a), both of which result in increased synaptic potentiation and synaptogenesis. This synaptic potentiation and synaptogenesis has been posited to ultimately form the foundation of ketamine’s antidepressant effectiveness (Autry et al., 2011; Duman and Aghajanian, 2012; Nosyreva et al., 2013).
This paper will focus on two promising neuroimaging markers of ketamine response in electrophysiology: gamma oscillations (approximately 30–60 Hz) and LTP, both of which are putative measures of synaptic potentiation (Table 1). The manuscript will also briefly discuss how new computational modeling methods can be applied to better characterize ketamine’s effects in electrophysiological data (Table 2). Here, an attempt has been made to synthesize the disparate literature on these electrophysiological markers, including providing hypotheses about ketamine’s specific role in generating these markers and their relationship to antidepressant response. The literature is summarized in three sections: ketamine and gamma oscillations and power, ketamine and LTP, and computational modeling of ketamine effects. Key limitations and areas where further research is needed are noted wherever appropriate.
Table 1.
Gamma Oscillations/Power, Long-Term Potentiation, and Ketamine Response
| Authors | Ketamine Dosage | Time of measurement (acute or delayed) | Study Sample | Study Design/Task | Modality | Placebo controlled | Ketamine Findings |
|---|---|---|---|---|---|---|---|
| Cornwell et al., 2012 | Subanesthetic dose infusion | Delayed effects measured 6 to 7 hours post-infusion | MDD, N=20 | Somatosensory stimulation | MEG | No | Increased gamma band (30–50 Hz) power in responders compared with non-responders |
| de la Salle et al., 2016 | Subanesthetic dose infusion | Acute effects measured during infusion | HV, N=21 | Resting state, eyes closed | EEG | Yes | Increased gamma band (30–60 Hz) current density estimates across the scalp and in regions of the default mode and salience network |
| Gilbert et al., 2018 | Subanesthetic dose infusion | Delayed effects measured 6 to 9 hours post-infusion | HV, N=18 and MDD, N=18 | Somatosensory stimulation | MEG | Yes | Increased gamma band (30–58 Hz) evoked responses in bilateral somatosensory cortex and frontal cortex |
| Hong et al., 2010 | Subanesthetic dose infusion | Acute effects measured immediately following infusion | HV, N=10 | Paired click auditory task | EEG | Yes | Increased gamma band (40–85 Hz) power spectrum density at CZ electrode site |
| Lazarewicz et al., 2010 | Anesthetic dose infusion | Acute effects measured during infusion | Mice, N=20 | Paired click auditory task | Implanted EEG | No | Increased background gamma band (30–80 Hz) power, increased gamma band evoked power, and increased induced gamma band responses |
| Maksimow et al., 2006 | Subanesthetic followed by anesthetic dose infusion | Acute effects measured during infusion | HV, N=8 | Resting state and following sedation | EEG | Active propofol anesthesia control | Increased gamma band (30.2–48 Hz) activity and gamma spindles during anesthetic dosing |
| Moran et al., 2015 | Anesthetic dose infusion | Acute effects measured during infusion | Rats, N=10 | Locomotor activity in open-field arena | EEG | Yes | Increased gamma band (40–75 Hz) power, particularly in medial prefrontal cortex |
| Muthukumaraswamy et al., 2015 | Subanesthetic dose infusion | Acute effects measured during infusion | HV, N=19 | Resting state, eyes open | MEG | Yes | Increased low (30–49 Hz) and high (51–99 Hz) gamma band activity |
| Nugent et al., 2019a | Subanesthetic dose infusion | Delayed effects measured 6 to 9 hours post-infusion | HV, N=25 and MDD, N=35 | Resting state, eyes closed | MEG | Yes | Increased gamma band (30–50 Hz) power in both groups; for MDDs, higher post-ketamine gamma band power was associated with better response |
| Nugent et al., 2019b | Subanesthetic dose infusion | Delayed effects measured 6 to 9 hours post-infusion | HV, N=25 and MDD, N=31 | Somatosensory stimulation | MEG | Yes | Difference in gamma band (30–50 Hz) power between ketamine and placebo sessions significantly correlated with antidepressant response |
| Shaw et al., 2015 | Subanesthetic dose infusion | Acute effects measured during infusion | HV, N=20 | Visuomotor paradigm | MEG | Yes | Increased gamma band amplitude in primary motor (60–90 Hz) and visual (30–80 Hz) cortices with reduced peak gamma frequency in visual cortex |
| Sumner et al., 2019 | Subanesthetic dose infusion | Delayed effects measured 3 to 4 hours post-infusion | MDD, N=30 | Visual grating stimulation | EEG | Active remifentanil hydrochloride control | Increased visual P2 component long-term potentiation compared with placebo |
| Zacharias et al., 2020 | Subanesthetic dose infusion | Acute effects measured during infusion | HV, N=17 | Resting state, eyes closed | EEG | Yes | Marginally increased gamma band (30–50 Hz) activity |
MDD: individuals with major depressive disorder; HV: healthy volunteers; MEG: magnetoencephalography; EEG: electroencephalography
Table 2.
Dynamic Causal Modeling of Ketamine Effects
| Authors | Ketamine Dosage | Time of measurement (acute or delayed) | Study Sample | Study Design/Task | Modality | Regions Included | Modeled Ketamine Effects |
|---|---|---|---|---|---|---|---|
| Gilbert et al., 2018 | Subanesthetic dose infusion | Delayed effects measured 6 to 9 hours post-infusion | HV, N=18 and MDD, N=18 | Somatosensory stimulation | MEG | Primary somatosensory cortex and frontal cortex | Increased top-down NMDA receptor drive between frontal cortex and primary somatosensory cortex; reduced AMPA receptor drive associated with antidepressant response two weeks following ketamine administration |
| Moran et al., 2015 | Anesthetic dose infusion | Acute effects measured during infusion | Rats, N=10 | Locomotor activity in open-field arena | EEG | Hippocampus and medial prefrontal cortex | Increased bottom-up AMPA receptor drive and reduced NMDA receptor drive from hippocampus to medial prefrontal cortex coupled with reduced top-down NMDA receptor drive; decreased intrinsic NMDA estimates in hippocampus and increased local feedback inhibition onto hippocampal pyramidal cells |
| Muthukumaraswamy et al., 2015 | Subanesthetic dose infusion | Acute effects measured during infusion | HV, N=19 | Resting state, eyes open | MEG | Parietal and frontal cortices | Reduced top-down AMPA receptor and NMDA receptor drive between frontal and parietal cortices; decreased gain on parietal superficial pyramidal cells associated with self-reported blissful state |
| Rosch et al., 2019 | Subanesthetic dose infusion | Acute effects measured during infusion | HV, N=18 | Auditory oddball paradigm | EEG | Primary auditory cortex, superior temporal gyrus, and inferior frontal gyrus | Increased inhibitory drive in primary auditory cortex and superior temporal gyrus; decreased inhibitory drive in inferior frontal gyrus |
| Schmidt et al., 2012 | Subanesthetic dose infusion | Acute effects measured during infusion | HV, N=19 | Auditory oddball paradigm | EEG | Primary auditory cortex, superior temporal gyrus, and inferior frontal gyrus | Increased bottom-up drive between primary auditory cortex and superior temporal gyrus for deviant tones; bottom-up drive associated with self-reported impaired control and cognition |
| Shaw et al., 2019 | Subanesthetic dose infusion | Acute effects measured during infusion | HV, N=20 | Visual grating stimulation | MEG | Visual cortex | Reduced gain on superficial pyramidal cells coupled with increased gain on inhibitory interneurons; shortened decay time of NMDA receptors, increased decay time on GABA receptors, and increased cortico-thalamic projection from layer IV into the thalamus relay populations |
| Sumner et al., 2019 | Subanesthetic dose infusion | Delayed effects measured 3 to 4 hours post-infusion | MDD, N=30 | Visual grating stimulation | EEG | Middle occipital gyrus, inferior temporal cortex, and superior parietal cortex | Increased bottom-up drive between middle occipital gyrus and both inferior temporal cortex and superior parietal cortex; asymmetrical increased and decreased top-down drive between these same regions |
MDD: individuals with major depressive disorder; HV: healthy volunteers; MEG: magnetoencephalography; EEG: electroencephalography
2. Ketamine and Gamma: Altering Excitation/Inhibition Balance
Within psychiatry, a growing body of evidence suggests that alterations in the ratio of cortical excitation/inhibition balance could underlie a host of disorders, including depression (Fogaça and Duman, 2019; Fuchs et al., 2017; Godfrey et al., 2018; Kato et al., 2019). Short-lived gamma frequency oscillations have been shown to emerge from the coordinated interaction of excitation and inhibition in modeling work (Brunel and Wang, 2003; Buzsáki and Wang, 2012; Economo and White, 2012; Ray and Maunsell, 2015) as well as in animal work (Csicsvari et al., 2003; Whittington et al., 1995). In addition, the magnitude of gamma oscillations has been shown to be modulated by slower rhythms, including delta (approximately 1–4 Hz), theta (approximately 5–8 Hz), and alpha (approximately 9–14 Hz) frequencies (Buzsáki and Wang, 2012). Recent empirical work has explored the link between depression and gamma oscillations more directly, with several studies demonstrating alterations in gamma oscillations in both MDD and bipolar disorder (Bi et al., 2018; Lee et al., 2010; Liu et al., 2012b; Liu et al., 2014). These changes potentially reflect alterations in glutamatergic excitation-GABAergic inhibition concomitant with depression (Fee et al., 2017; Lloyd et al., 1989), suggesting that aberrant gamma oscillations might be a putative biomarker of mood disorders, including MDD (for a review see (Fitzgerald and Watson, 2018)). As an extension of this work, gamma rhythms have been found to be correlated with the generation of action potentials (Nir et al., 2007; Watson et al., 2018), suggesting that pharmacological interventions that increase gamma via synaptic potentiation might serve to alleviate depressive symptoms by increasing cortical excitation.
Acute ketamine administration, which leads to pyramidal cell disinhibition downstream of NMDA receptor antagonism (Homayoun and Moghaddam, 2007; Widman and McMahon, 2018), should lead to robust changes in gamma oscillations. In fact, this finding has been well-replicated across species, perhaps driven by reduced NMDA receptor-mediated input to fast-spiking parvalbumin-expressing GABAergic interneurons (Anver et al., 2011; Carlén et al., 2012; Hong et al., 2010; Lazarewicz et al., 2010; Maksimow et al., 2006; Sohal et al., 2009). In addition, acute ketamine administration has been found to decrease lower-frequency oscillations, including delta (Hong et al., 2010) and theta (Lazarewicz et al., 2010) oscillations, in line with predictions from modeling work. While it is outside the scope of this review to focus on lower-frequency oscillations and ketamine-mediated antidepressant response, this is certainly an area for further evaluation. Instead, the present review examines changes in gamma oscillations in response to acute (effects around the drug’s half-life) and delayed (effects occurring after the drug’s half-life as well as psychotomimetic side effects) ketamine administration, with a focus on potential mechanistic explanations for these effects drawn from electrophysiological studies of ketamine administration in both healthy individuals and those with mood disorders.
In both humans and animals, recent electrophysiological work found robust increases in gamma oscillations and measures of gamma power following acute ketamine administration at therapeutic, subanesthetic levels (de la Salle et al., 2016; Hong et al., 2010; Maksimow et al., 2006; Muthukumaraswamy et al., 2015; Shaw et al., 2015; Zacharias et al., 2020) as well as at higher doses where schizophrenia-like effects become more manifest (Lazarewicz et al., 2010; Moran et al., 2015), though these effects may not translate to increases in gamma coherence (Witkin et al., 2017). In healthy human controls receiving subanesthetic-dose ketamine, increased gamma oscillations/power were found in resting-state networks (de la Salle et al., 2016; Maksimow et al., 2006; Muthukumaraswamy et al., 2015; Zacharias et al., 2020) and in motor and visual areas during a visuomotor task (Shaw et al., 2015). Given that acute ketamine administration, even at subanesthetic doses, leads to transient dissociative and psychotomimetic effects that resemble the positive and negative symptoms of schizophrenia (Krystal et al., 1994), one potential hypothesis for this acute increase in gamma is that it reflects dysfunctions of conscious integration, that is, the dissociative and psychotomimetic effects that occur during ketamine administration (Pinault, 2008). A second hypothesis is that sustained and elevated gamma power post-ketamine might constitute pyramidal cell noise, disrupting information processing within pyramidal cell assemblies (Moran et al., 2015). Evidence from animal work supports this second assertion, as theta-gamma coupling within the hippocampus was enhanced at a low ketamine dose (25 mg/kg, used to test a schizophrenia model), but disrupted at higher doses (Caixeta et al., 2013). These findings point to acute increases in gamma synchrony that reflect both a drug-induced dissociative state and associated changes in pyramidal cell information processing. What has yet to be determined, however, is how these acute gamma oscillations relate to antidepressant response in specific mood disorders, or how they might directly relate to the dissociative clinical symptoms experienced during ketamine administration.
To date, no studies have assessed ketamine’s acute effects on gamma oscillations in mood disorders. However, several studies have assessed its delayed effects on both gamma power and antidepressant response (Cornwell et al., 2012; Gilbert et al., 2018; Nugent et al., 2019a; Nugent et al., 2019b); such studies have measured electrophysiological changes in gamma power during an antidepressant response window outside of ketamine’s acute, dissociative drug effects, which dissipate by about one hour post-infusion in most individuals (Luckenbaugh et al., 2014). These studies all found enhanced gamma power several hours (approximately six to nine hours) post-ketamine administration, including within large-scale brain networks during rest (Nugent et al., 2019a) and within primary and secondary somatosensory cortices following tactile stimulation of the index finger (Cornwell et al., 2012; Gilbert et al., 2018; Nugent et al., 2019b).
With regard to antidepressant response, Cornwell and colleagues found that ketamine responders (defined as those experiencing a greater than 50% reduction in depressive symptoms post-ketamine) showed a uniform increase in gamma power (a putative metric of cortical excitability and synaptic potentiation) post-ketamine infusion compared to baseline (Cornwell et al., 2012), but that non-responders showed no such changes. The same study also found a positive correlation between gamma power and plasma levels of norketamine, a major active metabolite of ketamine with a relatively long half-life (Cornwell et al., 2012). One limitation of the study, however, was that ketamine was administered open-label, meaning that there was no control for expectancy effects. To address this short-coming, Nugent and colleagues replicated these same findings of increased gamma power using a double-blind, placebo-controlled study design, providing further evidence that delayed electrophysiological measures of gamma power might be an important biomarker of antidepressant response to ketamine (Nugent et al., 2019b). Building on this work, Nugent and colleagues subsequently found that baseline levels of resting-state gamma power within large-scale brain networks in individuals with MDD moderated the relationship between post-ketamine changes in gamma power and antidepressant response. In particular, they found that depressed participants with lower baseline gamma power levels who experienced large increases in gamma power post-ketamine had better antidepressant response than those with higher baseline gamma levels who also experienced large increases in gamma power post-ketamine (Nugent et al., 2019a).
Taken together, these findings suggest that there might be some optimal level (a “sweet spot”) of gamma power that is key to mediating antidepressant response following ketamine administration. Within the field of psychiatry, these findings also provide a potential bridge between gamma oscillations, ketamine’s antidepressant effects, depression, and schizophrenia – the idea of an optimal homeostatic balance of excitation/inhibition. This notion would be consistent with the idea that increases (e.g., in schizophrenia) and decreases (e.g., in depression) in gamma power beyond a homeostatic ideal could be pathological, with ketamine-mediated upregulation of gamma power providing antidepressant relief only in depressed individuals who have reduced gamma power levels at baseline (reflecting lower levels of excitation-inhibition). Further work is needed to examine the robustness of these findings.
3. Ketamine and Long-Term Potentiation
The pivotal role that NMDA receptors play in LTP and synaptic plasticity was first described almost 40 years ago, focusing on LTP within the hippocampus (Collingridge et al., 1983; Harris et al., 1984). Subsequently, the first experimental test of NMDA antagonists used an animal model of depression (inescapable stress) to examine how reduced NMDA neurotransmission within an NMDA receptor-rich area of the hippocampus could ameliorate depressive symptomatology, thus providing the first evidence that NMDA receptor antagonism was associated with effective antidepressant response (Trullas and Skolnick, 1990). Since then, the role that NMDA receptors play in LTP was found to extend outside of the hippocampus to anatomically early areas within the visual (Artola and Singer, 1987; Kirk et al., 2010) and auditory cortices (Kirk et al., 2010) using paradigms that include rapid, repetitive presentations of sensory stimuli (e.g., flashing visual checkerboards, trains of auditory tones). In addition, several studies have demonstrated reduced LTP in the visual and auditory cortices of individuals with mood disorders (Normann et al., 2007; Ostermann et al., 2012; Yeap et al., 2009). Taken together, these studies suggest that up-regulation of LTP via ketamine administration might serve as an important mechanistic process mediating ketamine’s antidepressant effects.
While the importance of NMDA receptors in LTP is well known, only a few electrophysiological studies have explored ketamine-mediated effects on LTP in humans, and only one has focused on antidepressant response in MDD (Sumner et al., 2019). Sumner and colleagues examined LTP as a marker of neural plasticity within a delayed time window of three to four hours post-ketamine infusion and found enhanced LTP in the visual system following ketamine administration; specifically, the P2 event-related potential (ERP) amplitude was increased post-ketamine compared to an active placebo, remifentanil (Sumner et al., 2019). However, the authors found no evidence of a relationship between ERP changes and antidepressant response, leaving open the question of whether ERP effects generally track with antidepressant response. Given that this is the only study to date demonstrating ketamine-mediated effects on LTP in humans with depression, more work is needed to determine whether measures of LTP in primary visual and auditory cortices could serve as effective biomarkers of ketamine-mediated antidepressant response.
4. Computational Modeling of Ketamine’s Effects
Recent advances in computational modeling have shifted focus onto translational efforts to bridge neuroscience and psychiatry. These novel computational methods attempt to characterize abnormalities in brain systems that underlie psychiatric diseases, including depression. Though in its infancy, such work could potentially enable the identification, categorization, and prediction of dimensional processes in depression and other psychiatric conditions (Ferrante et al., 2019). Progress in this field has led to recent attempts to use computational methods to characterize ketamine’s effects in animal models (Moran et al., 2015) as well as in both healthy controls (Muthukumaraswamy et al., 2015; Schmidt et al., 2012; Shaw et al., 2019) and individuals with mood disorders (Gilbert et al., 2018; Sumner et al., 2019). These studies have all used dynamic causal modeling (DCM), which fits a biologically-plausible model of neural dynamics to measured electrophysiological signals. While it is outside the scope of this review to describe the sets of coupled differential equations that govern dynamics in DCM, model inversion—the fitting of parameterized mean-field neuronal models to electrophysiological data features—results in parameter estimates that govern unobservable neuronal states such as receptor-mediated connectivity between cell populations and decay times of specific receptor types. These models have been used to measure parameter effects using ketamine dosing levels that exacerbate schizophrenia symptoms (Moran et al., 2015) as well as lower, antidepressant-level doses (Gilbert et al., 2018; Muthukumaraswamy et al., 2015; Rosch et al., 2019; Schmidt et al., 2012; Shaw et al., 2019; Sumner et al., 2019). Below, we focus first on ketamine’s effects in animals and healthy humans, then discuss ketamine’s antidepressant effects.
In animal models of schizophrenia, ketamine was found to amplify bottom-up AMPA receptor drive and attenuate NMDA receptor drive between the hippocampus and medial prefrontal cortex (the two regions modeled in the task) in a dose-dependent fashion; ketamine also attenuated top-down NMDA receptor drive from medial prefrontal cortex to hippocampus in a dose-dependent fashion (Moran et al., 2015) (Figure 1). In addition, ketamine decreased intrinsic estimates of NMDA in the hippocampus in a dose-dependent fashion, while also increasing local feedback inhibition onto hippocampal pyramidal cells (Moran et al., 2015). In humans, acute, subanesthetic-dose ketamine attenuated top-down AMPA and NMDA receptor drive between the frontal and parietal cortices in healthy individuals at rest, and also decreased the gain on parietal superficial pyramidal cells (Muthukumaraswamy et al., 2015). During a visuomotor task, ketamine decreased the gain on superficial pyramidal cells while increasing the gain on inhibitory interneurons within the visual cortex (Shaw et al., 2019). Ketamine administration was also found to shorten the decay time of NMDA receptors in the visual cortex, lengthen the decay time of GABAA receptors in the visual cortex, and increase the cortico-thalamic projection from layer VI in the visual cortex into the thalamus relay populations (Shaw et al., 2019). Finally, using auditory mismatch negativity tasks, ketamine was found to amplify the bottom-up drive between the primary auditory cortex and the superior temporal gyrus for deviant tones (Schmidt et al., 2012) and to increase the inhibitory drive within both these regions while decreasing inhibitory drive in the inferior frontal gyrus (Rosch et al., 2019).
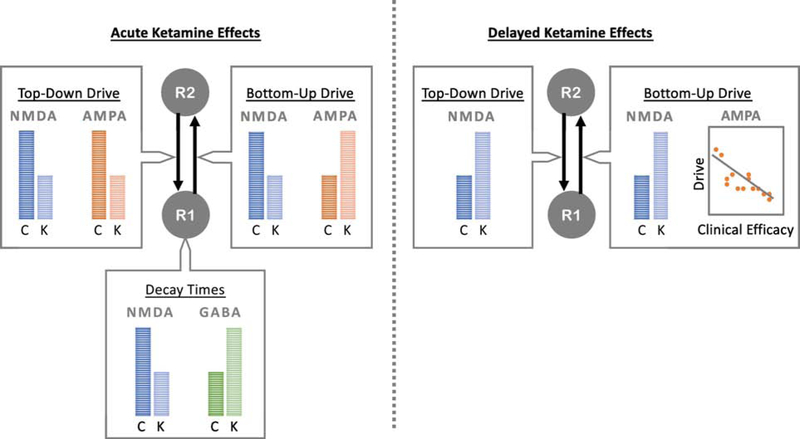
Figure 1.
Dynamic Causal Modeling of Ketamine Effects on AMPA, NMDA, and GABA Parameters. Dynamic Causal Modeling (DCM) has been used to model bottom-up and top-down drive in brain regions important for somatosensory, locomotor, auditory, and visual tasks, in addition to resting state network regions. Using models that include parameters for AMPA, NMDA, and GABA receptor signaling, acute ketamine administration has been found to attenuate bottom-up and top-down NMDA receptor drive, in addition to shortening the decay time of NMDA receptors in lower-level regions of the processing hierarchy. In addition, acute ketamine has been found to increase the bottom-up drive of AMPA receptors, decrease the top-down drive of AMPA receptors, and lengthen the decay time of GABA receptors in lower-level regions. In contrast, delayed measures of receptor-mediated signaling suggest that ketamine increases both bottom-up and top-down NMDA receptor drive, perhaps related to upregulation and sensitization effects. In addition, while both NMDA and AMPA receptor drive are associated with antidepressant response on the day of infusion, only changes in AMPA receptor drive were associated with longer-term changes in antidepressant response at two weeks following ketamine administration. R1: region 1; R2: region 2; C: control; K: ketamine.
Interestingly, of these parameters, only two have been associated with clinical symptoms: the pyramidal gain parameter and the bottom-up drive from primary auditory cortex to superior temporal gyrus. Muthukumaraswamy and colleagues found that the pyramidal gain parameter was associated with self-reported blissful state following ketamine administration (Muthukumaraswamy et al., 2015), strongly suggesting that this parameter is associated with the drug’s psychotomimetic effects. Schmidt and colleagues found that the bottom-up drive between the primary auditory cortex and the superior temporal gyrus was associated with individuals’ subjective ratings of impaired control and cognition, again suggesting that this parameter is associated with psychotomimetic effects. As an overarching summary, ketamine administration seems to alter NMDA and AMPA receptor drive/connectivity in key regions important for both resting networks and task-specific networks, and some of these parameters are associated with dissociative and psychotomimetic effects during acute administration.
Modeling delayed ketamine-mediated effects in individuals with MDD, ketamine was found to amplify the top-down NMDA receptor drive between frontal cortex and primary somatosensory cortex during a somatosensory stimulation task (Gilbert et al., 2018). Given that ketamine is an NMDA antagonist, this unexpected result is perhaps attributable to upregulation and sensitization effects. In terms of antidepressant response, both reduced NMDA and AMPA receptor drive have been associated with better antidepressant response, though only changes in AMPA receptor drive were associated with longer-term changes in antidepressant response two weeks following ketamine administration (Gilbert et al., 2018). This second finding is in keeping with the hypothesis that changes in AMPA throughput mediate antidepressant response. Using a visual grating task to measure changes in LTP, ketamine was also found to broadly amplify bottom-up drive between the middle occipital gyrus and both the inferior temporal cortex and superior parietal cortex, while asymmetrically attenuating and amplifying top-down drive between these same regions (Sumner et al., 2019). This latter study used a second-level Bayesian modeling extension of DCM to assess ketamine’s effects and did not directly measure associations between modeled parameter estimates and antidepressant response.
In summary, recent advances in computational modeling offer promise for uncovering how ketamine alters receptor-mediated connectivity between cell populations and even decay times of specific receptor types. However, much research is still needed in this area, particularly efforts to explore ketamine-mediated effects in depression, which could help better characterize differences between those who respond to ketamine versus those who do not. More work is also needed to model acute drug effects in mood disorders, which could potentially help differentiate parameters that govern psychotomimetic effects versus those that mediate antidepressant response.
5. Conclusions
Electrophysiological measures of both gamma oscillations/power and LTP hold considerable promise for uncovering the potential mechanistic processes mediating ketamine’s antidepressant effects. As reviewed above, acute increases in gamma oscillations have been identified following ketamine administration, although additional work is needed to determine whether this marker is associated with antidepressant response in addition to dissociative and psychotomimetic drug effects. However, delayed estimates of gamma power are potentially promising biomarkers of ketamine response and synaptic potentiation that highlight important differences between responders and non-responders. Nevertheless, more recent findings showing that baseline gamma might moderate the relationship between responders and non-responders suggests that future studies are needed to better characterize baseline gamma in depression. While this review focused on gamma in particular, additional work is also needed to better characterize ketamine’s effects on other frequencies and on cross-frequency coupling, examining how these measures correlate with antidepressant response.
As described above, studies of LTP similarly suggest that it is a strong marker of neural plasticity, but limited work has focused on how it is altered following ketamine administration. Future studies are needed to better characterize the role of LTP in depression and to measure how it changes following ketamine administration. In this context, novel computational methods are particularly promising tools to better characterize brain networks and connectivity metrics in mood disorders, in addition to providing stronger mechanistic accounts of how these are altered post-ketamine administration. Additional research is needed to better assess modeled parameter changes associated with direct antidepressant response to ketamine. Such knowledge could ultimately help develop next-generation antidepressants by providing a more complete, mechanistic understanding of ketamine’s antidepressant effects.
Highlights.
Electrophysiological studies of ketamine’s effects are reviewed
Review includes ketamine response in depression and healthy subjects
Markers of synaptic potentiation reviewed include gamma and long-term potentiation
Dynamic causal modeling of ketamine response is discussed
Acknowledgements
The authors thank the 7SE research unit and staff for their support. Ioline Henter (NIMH) provided invaluable editorial assistance.
Funding and Role of Funding Source.
Funding for this work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIA MH002857), by a NARSAD Independent Investigator Award to Dr. Zarate, and by a Brain and Behavior Mood Disorders Research Award to Dr. Zarate. These organizations had no further role in study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
For submission to Pharmacology, Biochemistry, and Behavior for the Special Issue Recent Advances in the Antidepressant Actions of Ketamine and Agents with Ketamine-like Antidepressant Profiles, December 2019
Declaration of Interest: Dr. Zarate is listed as a coinventor on a patent for the use of ketamine and its metabolites in major depression and suicidal ideation. Dr. Zarate is listed as a co-inventor on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydro and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain. Dr. Zarate is listed as co-inventor on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders; he has assigned his patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government. Dr. Gilbert has no conflict of interest to disclose, financial or otherwise.
References
- Anver H, Ward PD, Magony A, Vreugdenhil M, 2011. NMDA Receptor Hypofunction Phase Couples Independent γ-Oscillations in the Rat Visual Cortex. Neuropsychopharmacology 36, 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artola A, Singer W, 1987. Long-term potentiation and NMDA receptors in rat visual cortex. Nature 330, 649–652. [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng P. f., Kavalali ET, Monteggia LM, 2011. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475, 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH, 2000. Antidepressant effects of ketamine in depressed patients. Biological Psychiatry 47, 351–354. [DOI] [PubMed] [Google Scholar]
- Bernard R, Kerman IA, Thompson RC, Jones EG, Bunney WE, Barchas JD, Schatzberg AF, Myers RM, Akil H, Watson SJ, 2011. Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression. Mol Psychiatry 16, 634–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi K, Chattun MR, Liu X, Wang Q, Tian S, Zhang S, Lu Q, Yao Z, 2018. Abnormal early dynamic individual patterns of functional networks in low gamma band for depression recognition. Journal of Affective Disorders 238, 366–374. [DOI] [PubMed] [Google Scholar]
- Brunel N, Wang X-J, 2003. What Determines the Frequency of Fast Network Oscillations With Irregular Neural Discharges? I. Synaptic Dynamics and Excitation-Inhibition Balance. Journal of Neurophysiology 90, 415–430. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Wang X-J, 2012. Mechanisms of Gamma Oscillations. Annual Review of Neuroscience 35, 203–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caixeta FV, Cornélio AM, Scheffer-Teixeira R, Ribeiro S, Tort ABL, 2013. Ketamine alters oscillatory coupling in the hippocampus. Scientific Reports 3, 2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlén M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, Rühlmann C, Jones SR, Deisseroth K, Sheng M, Moore CI, Tsai LH, 2012. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry 17, 537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudary PV, Molnar M, Evans SJ, Tomita H, Li JZ, Vawter MP, Myers RM, Bunney WE Jr., Akil H, Watson SJ, Jones EG, 2005. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci U S A 102, 15653–15658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Kehl SJ, McLennan H, 1983. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. The Journal of Physiology 334, 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell BR, Salvadore G, Furey M, Marquardt CA, Brutsche NE, Grillon C, Zarate CA, 2012. Synaptic Potentiation Is Critical for Rapid Antidepressant Response to Ketamine in Treatment-Resistant Major Depression. Biological Psychiatry 72, 555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Jamieson B, Wise KD, Buzsáki G, 2003. Mechanisms of Gamma Oscillations in the Hippocampus of the Behaving Rat. Neuron 37, 311–322. [DOI] [PubMed] [Google Scholar]
- de la Salle S, Choueiry J, Shah D, Bowers H, McIntosh J, Ilivitsky V, Knott V, 2016. Effects of Ketamine on Resting-State EEG Activity and Their Relationship to Perceptual/Dissociative Symptoms in Healthy Humans. Frontiers in Pharmacology 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA Jr., 2010. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry 67, 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK, 2012. Synaptic Dysfunction in Depression: Potential Therapeutic Targets. Science 338, 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Sanacora G, Krystal JH, 2019. Altered Connectivity in Depression: GABA and Glutamate Neurotransmitter Deficits and Reversal by Novel Treatments. Neuron 102, 75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ, 2010. Markers of glutamate synaptic transmission and plasticity are increased in the anterior cingulate cortex in bipolar disorder. Biol Psychiatry 67, 1010–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economo MN, White JA, 2012. Membrane Properties and the Balance between Excitation and Inhibition Control Gamma-Frequency Oscillations Arising from Feedback Inhibition. PLOS Computational Biology 8, e1002354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Freeman MP, Flynn M, Judge H, Hoeppner BB, Cusin C, Ionescu DF, Mathew SJ, Chang LC, Iosifescu DV, Murrough J, Debattista C, Schatzberg AF, Trivedi MH, Jha MK, Sanacora G, Wilkinson ST, Papakostas GI, 2018. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder A, Parides MK, Murrough JW, Perez AM, Morgan JE, Saxena S, Kirkwood K, aan het Rot M, Lapidus KAB, Wan L-B, Iosifescu D, Charney DS, 2014. Efficacy of Intravenous Ketamine for Treatment of Chronic Posttraumatic Stress Disorder: A Randomized Clinical Trial. JAMA Psychiatry 71, 681–688. [DOI] [PubMed] [Google Scholar]
- Fee C, Banasr M, Sibille E, 2017. Somatostatin-Positive Gamma-Aminobutyric Acid Interneuron Deficits in Depression: Cortical Microcircuit and Therapeutic Perspectives. Biological Psychiatry 82, 549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante M, Redish AD, Oquendo MA, Averbeck BB, Kinnane ME, Gordon JA, 2019. Computational psychiatry: a report from the 2017 NIMH workshop on opportunities and challenges. Mol Psychiatry 24, 479–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PJ, Watson BO, 2018. Gamma oscillations as a biomarker for major depression: an emerging topic. Translational Psychiatry 8, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogaça MV, Duman RS, 2019. Cortical GABAergic Dysfunction in Stress and Depression: New Insights for Therapeutic Interventions. Frontiers in Cellular Neuroscience 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs T, Jefferson SJ, Hooper A, Yee PH, Maguire J, Luscher B, 2017. Disinhibition of somatostatin-positive GABAergic interneurons results in an anxiolytic and antidepressant-like brain state. Mol Psychiatry 22, 920–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert JR, Yarrington JS, Wills KE, Nugent AC, Zarate CA Jr, 2018. Glutamatergic Signaling Drives Ketamine-Mediated Response in Depression: Evidence from Dynamic Causal Modeling. International Journal of Neuropsychopharmacology 21, 740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey KEM, Gardner AC, Kwon S, Chea W, Muthukumaraswamy SD, 2018. Differences in excitatory and inhibitory neurotransmitter levels between depressed patients and healthy controls: A systematic review and meta-analysis. Journal of Psychiatric Research 105, 33–44. [DOI] [PubMed] [Google Scholar]
- Harris EW, Ganong AH, Cotman CW, 1984. Long-term potentiation in the hippocampus involves activation of N-methyl-D-aspartate receptors. Brain Research 323, 132–137. [DOI] [PubMed] [Google Scholar]
- Hirota K, Lambert DG, 1996. Ketamine: its mechanism(s) of action and unusual clinical uses. British Journal of Anaesthesia 77, 441–444. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B, 2007. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci 27, 11496–11500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, Buchanan RW, O’Donnell P, Thaker GK, Weiler MA, Lahti AC, 2010. Gamma and Delta Neural Oscillations and Association with Clinical Symptoms under Subanesthetic Ketamine. Neuropsychopharmacology 35, 632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato TM, Fujimori-Tonou N, Mizukami H, Ozawa K, Fujisawa S, Kato T, 2019. Presynaptic dysregulation of the paraventricular thalamic nucleus causes depression-like behavior. Scientific Reports 9, 16506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk IJ, McNair NA, Hamm JP, Clapp WC, Mathalon DH, Cavus I, Teyler TJ, 2010. Long-term potentiation (LTP) of human sensory-evoked potentials. WIREs Cognitive Science 1, 766–773. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr, Charney DS, 1994. Subanesthetic Effects of the Noncompetitive NMDA Antagonist, Ketamine, in Humans: Psychotomimetic, Perceptual, Cognitive, and Neuroendocrine Responses. Arch Gen Psychiatry 51, 199–214. [DOI] [PubMed] [Google Scholar]
- Lapidus KAB, Levitch CF, Perez AM, Brallier JW, Parides MK, Soleimani L, Feder A, Iosifescu DV, Charney DS, Murrough JW, 2014. A Randomized Controlled Trial of Intranasal Ketamine in Major Depressive Disorder. Biological Psychiatry 76, 970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarewicz MT, Ehrlichman RS, Maxwell CR, Gandal MJ, Finkel LH, Siegel SJ, 2010. Ketamine Modulates Theta and Gamma Oscillations. Journal of Cognitive Neuroscience 22, 1452–1464. [DOI] [PubMed] [Google Scholar]
- Lee P-S, Chen Y-S, Hsieh J-C, Su T-P, Chen L-F, 2010. Distinct neuronal oscillatory responses between patients with bipolar and unipolar disorders: A magnetoencephalographic study. Journal of Affective Disorders 123, 270–275. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS, 2010. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329, 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS, 2011. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 69, 754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK, 2012a. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry 71, 996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T-Y, Hsieh J-C, Chen Y-S, Tu P-C, Su T-P, Chen L-F, 2012b. Different patterns of abnormal gamma oscillatory activity in unipolar and bipolar disorder patients during an implicit emotion task. Neuropsychologia 50, 1514–1520. [DOI] [PubMed] [Google Scholar]
- Liu TY, Chen YS, Su TP, Hsieh JC, Chen LF, 2014. Abnormal Early Gamma Responses to Emotional Faces Differentiate Unipolar from Bipolar Disorder Patients. BioMed Research International 2014, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd KG, Zivkovic B, Scatton B, Morselli PL, Bartholimi G, 1989. The gabaergic hypothesis of depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry 13, 341–351. [DOI] [PubMed] [Google Scholar]
- Luckenbaugh DA, Niciu MJ, Ionescu DF, Nolan NM, Richards EM, Brutsche NE, Guevara S, Zarate CA, 2014. Do the dissociative side effects of ketamine mediate its antidepressant effects? Journal of Affective Disorders 159, 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK, 2008. Cellular Mechanisms Underlying the Antidepressant Effects of Ketamine: Role of α-Amino-3-Hydroxy-5-Methylisoxazole-4-Propionic Acid Receptors. Biological Psychiatry 63, 349–352. [DOI] [PubMed] [Google Scholar]
- Maksimow A, Särkelä M, Långsjö JW, Salmi E, Kaisti KK, Yli-Hankala A, Hinkka-Yli-Salomäki S, Scheinin H, Jääskeläinen SK, 2006. Increase in high frequency EEG activity explains the poor performance of EEG spectral entropy monitor during S-ketamine anesthesia. Clinical Neurophysiology 117, 1660–1668. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D, 1997. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 17, 2921–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran RJ, Jones MW, Blockeel AJ, Adams RA, Stephan KE, Friston KJ, 2015. Losing Control Under Ketamine: Suppressed Cortico-Hippocampal Drive Following Acute Ketamine in Rats. Neuropsychopharmacology 40, 268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ, 2013a. Antidepressant Efficacy of Ketamine in Treatment-Resistant Major Depression: A Two-Site Randomized Controlled Trial. American Journal of Psychiatry 170, 1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV, 2013b. Rapid and Longer-Term Antidepressant Effects of Repeated Ketamine Infusions in Treatment-Resistant Major Depression. Biological Psychiatry 74, 250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Soleimani L, DeWilde KE, Collins KA, Lapidus KA, Iacoviello BM, Lener M, Kautz M, Kim J, Stern JB, Price RB, Perez AM, Brallier JW, Rodriguez GJ, Goodman WK, Iosifescu DV, Charney DS, 2015. Ketamine for rapid reduction of suicidal ideation: a randomized controlled trial. Psychological Medicine 45, 3571–3580. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Shaw AD, Jackson LE, Hall J, Moran R, Saxena N, 2015. Evidence that Subanesthetic Doses of Ketamine Cause Sustained Disruptions of NMDA and AMPA-Mediated Frontoparietal Connectivity in Humans. The Journal of Neuroscience 35, 11694–11706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir Y, Fisch L, Mukamel R, Gelbard-Sagiv H, Arieli A, Fried I, Malach R, 2007. Coupling between Neuronal Firing Rate, Gamma LFP, and BOLD fMRI Is Related to Interneuronal Correlations. Current Biology 17, 1275–1285. [DOI] [PubMed] [Google Scholar]
- Normann C, Schmitz D, Fürmaier A, Döing C, Bach M, 2007. Long-Term Plasticity of Visually Evoked Potentials in Humans is Altered in Major Depression. Biological Psychiatry 62, 373–380. [DOI] [PubMed] [Google Scholar]
- Nosyreva E, Szabla K, Autry AE, Ryazanov AG, Monteggia LM, Kavalali ET, 2013. Acute Suppression of Spontaneous Neurotransmission Drives Synaptic Potentiation. The Journal of Neuroscience 33, 6990–7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent AC, Ballard ED, Gould TD, Park LT, Moaddel R, Brutsche NE, Zarate CA, 2019a. Ketamine has distinct electrophysiological and behavioral effects in depressed and healthy subjects. Mol Psychiatry 24, 1040–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent AC, Wills KE, Gilbert JR, Zarate CA, 2019b. Synaptic potentiation and rapid antidepressant response to ketamine in treatment-resistant major depression: A replication study. Psychiatry Research: Neuroimaging 283, 64–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgi Y, Futamura T, Hashimoto K, 2015. Glutamate Signaling in Synaptogenesis and NMDA Receptors as Potential Therapeutic Targets for Psychiatric Disorders. Curr Mol Med 15, 206–221. [DOI] [PubMed] [Google Scholar]
- Ostermann J, Uhl I, Köhler E, Juckel G, Norra C, 2012. The loudness dependence of auditory evoked potentials and effects of psychopathology and psychopharmacotherapy in psychiatric inpatients. Human Psychopharmacology: Clinical and Experimental 27, 595–604. [DOI] [PubMed] [Google Scholar]
- Phillips JL, Norris S, Talbot J, Birmingham M, Hatchard T, Ortiz A, Owoeye O, Batten LA, Blier P, 2019. Single, Repeated, and Maintenance Ketamine Infusions for Treatment-Resistant Depression: A Randomized Controlled Trial. American Journal of Psychiatry 176, 401–409. [DOI] [PubMed] [Google Scholar]
- Pinault D, 2008. N-Methyl d-Aspartate Receptor Antagonists Ketamine and MK-801 Induce Wake-Related Aberrant γ Oscillations in the Rat Neocortex. Biological Psychiatry 63, 730–735. [DOI] [PubMed] [Google Scholar]
- Ray S, Maunsell JHR, 2015. Do gamma oscillations play a role in cerebral cortex? Trends in Cognitive Sciences 19, 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez CI, Kegeles LS, Levinson A, Feng T, Marcus SM, Vermes D, Flood P, Simpson HB, 2013. Randomized Controlled Crossover Trial of Ketamine in Obsessive-Compulsive Disorder: Proof-of-Concept. Neuropsychopharmacology 38, 2475–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosch RE, Auksztulewicz R, Leung PD, Friston KJ, Baldeweg T, 2019. Selective Prefrontal Disinhibition in a Roving Auditory Oddball Paradigm Under N-Methyl-D-Aspartate Receptor Blockade. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 4, 140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Diaconescu AO, Kometer M, Friston KJ, Stephan KE, Vollenweider FX, 2012. Modeling Ketamine Effects on Synaptic Plasticity During the Mismatch Negativity. Cerebral Cortex 23, 2394–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw AD, Muthukumaraswamy SD, Saxena N, Adams N, Moran RJ, Singh KD, 2019. In-silico evidence that increased cortico-thalamic connectivity and superficial pyramidal disinhibition underlie broadband task-related spectral changes induced by ketamine. bioRxiv, 688044. [Google Scholar]
- Shaw AD, Saxena N, Jackson, L. E, Hall JE, Singh KD, Muthukumaraswamy SD, 2015. Ketamine amplifies induced gamma frequency oscillations in the human cerebral cortex. European Neuropsychopharmacology 25, 1136–1146. [DOI] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K, 2009. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459, 698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner RL, McMillan R, Spriggs MJ, Campbell D, Malpas G, Maxwell E, Deng C, Hay J, Ponton R, Kirk IJ, Sundram F, Muthukumaraswamy SD, 2019. Ketamine Enhances Visual Sensory Evoked Potential Long-term Potentiation in Patients With Major Depressive Disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. [DOI] [PubMed] [Google Scholar]
- Taylor JH, Landeros-Weisenberger A, Coughlin C, Mulqueen J, Johnson JA, Gabriel D, Reed MO, Jakubovski E, Bloch MH, 2018. Ketamine for Social Anxiety Disorder: A Randomized, Placebo-Controlled Crossover Trial. Neuropsychopharmacology 43, 325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trullas R, Skolnick P, 1990. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. European Journal of Pharmacology 185, 1–10. [DOI] [PubMed] [Google Scholar]
- Watson BO, Ding M, Buzsáki G, 2018. Temporal coupling of field potentials and action potentials in the neocortex. European Journal of Neuroscience 48, 2482–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JGR, 1995. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature 373, 612–615. [DOI] [PubMed] [Google Scholar]
- Widman AJ, McMahon LL, 2018. Disinhibition of CA1 pyramidal cells by low-dose ketamine and other antagonists with rapid antidepressant efficacy. Proceedings of the National Academy of Sciences 115, E3007–E3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin JM, Monn JA, Li J, Johnson B, McKinzie DL, Wang XS, Heinz BA, Li R, Ornstein PL, Smith SC, Mitch CH, Calligaro DO, Swanson S, Allen D, Phillips K, Gilmour G, 2017. Preclinical predictors that the orthosteric mGlu2/3 receptor antagonist LY3020371 will not engender ketamine-associated neurotoxic, motor, cognitive, subjective, or abuse-liability-related effects. Pharmacology Biochemistry and Behavior 155, 43–55. [DOI] [PubMed] [Google Scholar]
- Yeap S, Kelly SP, Reilly RB, Thakore JH, Foxe JJ, 2009. Visual sensory processing deficits in patients with bipolar disorder revealed through high-density electrical mapping. J Psychiatry Neurosci 34, 459–464. [PMC free article] [PubMed] [Google Scholar]
- Zacharias N, Musso F, Müller F, Lammers F, Saleh A, London M, de Boer P, Winterer G, 2020. Ketamine effects on default mode network activity and vigilance: A randomized, placebo-controlled crossover simultaneous fMRI/EEG study. Human Brain Mapping n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Highland JN, Stewart BW, Georgiou P, Jenne CE, Lovett J, Morris PJ, Thomas CJ, Moaddel R, Zarate CA, Gould TD, 2019. (2R,6R)-hydroxynorketamine exerts mGlu2 receptor-dependent antidepressant actions. Proceedings of the National Academy of Sciences 116, 6441–6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA Jr., Gould TD, 2016. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533, 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr., Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V, Luckenbaugh DA, 2012. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry 71, 939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK, 2006. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63, 856–864. [DOI] [PubMed] [Google Scholar]