Abstract
Objectives
Geriatric depression is difficult to treat and frequently accompanied by cognitive complaints that increase risk for dementia. New treatment strategies targeting both depression and cognition are urgently needed.
Methods
We conducted a 6-month double-blind placebo-controlled trial to assess the efficacy and tolerability of escitalopram + memantine (ESC/MEM) compared to escitalopram + placebo (ESC/PBO) for improving mood and cognitive functioning in depressed older adults with subjective memory complaints (). Primary outcome was change in depression as assessed by the HAM-D post-treatment (at 6 months). Remission was defined as HAM-D <6; naturalistic follow-up continued until 12 months.
Results
Of the 95 randomized participants, 62 completed the 6-month assessment. Dropout and tolerability did not differ between groups. Mean daily escitalopram dose was 11.1 mg (SD=3.7; range: 5–20 mg). Mean daily memantine dose was 19.3 mg (SD=2.6; range 10–20 mg). Remission rate within ESC/MEM was 45.8% and 47.9%, compared to 38.3% and 31.9% in ESC/PBO, at 3 and 6 months respectively (χ2(1)=2.0, p=0.15). Both groups improved significantly on the HAM-D at 3, 6 and 12 months, with no observed between-group differences. ESC/MEM demonstrated greater improvement in delayed recall (F(2,82)=4.3, p=.02) and executive functioning (F(2,82)=5.1, p=.01) at 12 months compared to ESC/PBO.
Conclusions
The combination of memantine with escitalopram was well-tolerated and as effective as escitalopram and placebo in improving depression using HAM-D. Combination memantine and escitalopram was significantly more effective than escitalopram and placebo in improving cognitive outcomes at 12 months. Future reports will address the role of biomarkers of aging in treatment response.
Geriatric depression is a common and debilitating disorder, with 1–5% of the general population and 10–15% of primary care patients meeting criteria for major depressive disorder (MDD) (1, 2). Geriatric depression has a poorer prognosis compared to depression experienced earlier in life (3–9), with over 60% of geriatric patients failing to achieve remission in response to first-line antidepressant treatment (10, 11). In addition, depression in late life is often accompanied by cognitive impairment (12) that often persists beyond successful treatment of the depression (13). Both depression and cognitive impairment are known risk factors for dementia (14), and among older adults with mild cognitive impairment (MCI), those with comorbid depression are 28% more likely to develop dementia than those without depression (15). Likewise, endorsement of subjective memory complaints, even with normal performance on neuropsychological tests, is associated with Alzheimer disease (AD) biomarkers and increased risk for MCI and dementia (16). Therefore, treatment strategies for geriatric depression that target both depressive symptoms and cognitive functioning are urgently needed. One prior study investigated antidepressant therapy augmentation with donepezil for treatment of geriatric depression, but found no reduction in conversion to dementia or depression recurrence in a placebo-controlled trial (17).
Accumulating research evidence points to the role of glutamate in the pathophysiology of MDD (18). N-methyl-D-aspartate (NMDA) is a postsynaptic ionotropic receptor that modulates glutamate transmission and has demonstrated involvement in synaptic plasticity and memory function. NMDA antagonists have been shown to have antidepressant effects in both animal (19–22) and human studies (23–25). Memantine is a low-to-moderate affinity noncompetitive NMDA receptor antagonist that reduces excessive glutamate receptor signaling and is used to slow cognitive decline in Alzheimer’s Disease (AD) (26). A systematic review and meta-analysis including data from 6 RCTs conducted with older adults with dementia found that memantine significantly decreased overall behavioral and psychological symptoms compared to placebo (27). Although the antidepressant effects of memantine have been well-documented in animal models, evidence of an antidepressant effect in humans has been mixed (28–34).
Escitalopram is a selective serotonin reuptake inhibitor (SSRI) with documented efficacy, tolerability, and safety for treatment of geriatric depression. In studies of older adults with depression, escitalopram has been compared favorably to citalopram (35) as well as serotonin-norepinephrine reuptake inhibitors (SNRIs) including duloxetine and venlafaxine (36). In an open-label trial conducted with older depressed adults with cognitive impairment, 12 months of combined escitalopram and memantine was associated with improved cognition and a low rate of conversion to dementia (37). One prior 8-week pilot study of depressed older adults receiving citalopram reported no significant between-group difference in depressive symptoms following treatment with add-on memantine vs. placebo (38), but a longer follow-up may be required in order to detect potential benefits for mood and cognition.
We performed a 6-month randomized double-blind placebo-controlled trial of escitalopram combined with either memantine or placebo in older depressed adults who endorsed subjective memory complaints (a group at high risk for conversion to MCI and AD). We report the primary outcome of depression severity measured by change in 24-item HAM-D score at mid-treatment (3 months), post-treatment (6 months), and at naturalistic follow-up (12 months). Secondary outcomes included depressive symptoms as assessed by the Montgomery-Åsberg Depression Rating Scale (MADRS) (52), clinical global impression (CGI), anxiety symptoms, apathy, and psychological resilience. We assessed domains of cognition frequently impaired in geriatric depression: learning (39), memory (delayed recall) (40), and executive functioning (41–43) at 6 and 12 months. We hypothesized that depression and memory would improve more with the combination treatment of memantine and escitalopram compared to escitalopram and placebo at 6 and 12 months.
Methods
Participants
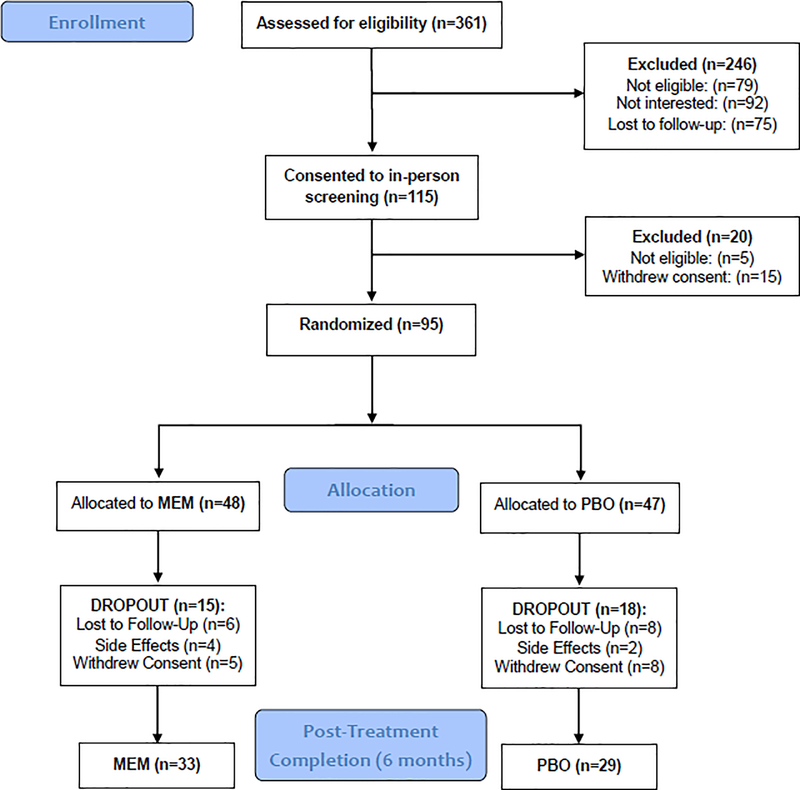
The UCLA Institutional Review Board approved all study procedures (). Participants were recruited from the UCLA Neuropsychiatric Hospital inpatient and outpatient service and from community advertising between October 2013 and May 2018. Three hundred and sixty-one individuals were assessed via phone screening, yielding 115 participants for in-person diagnostic interview. Of these, 95 met inclusion criteria and were randomized to receive either escitalopram with placebo (n= 47) or escitalopram with memantine (n = 48) (please see CONSORT diagram; Figure 1). Participants provided written informed consent prior to study enrollment.
Figure 1.
Consort diagram.
Inclusion and Exclusion Criteria
The Structured Clinical Interview for DSM-IVR/DSM-5 (SCID) was administered by HL or a trained, masters-level research associate to diagnose MDD and rule out other diagnoses (e.g., psychosis). Inclusion criteria were: 1) presence of MDD according to DSM-IVR/DSM-5 criteria; 2) score of ≥ 16 on the 24-item Hamilton Rating Scale for Depression (HAM-D)(44); 3) subjective memory complaints (affirmative response to the question, “Have you experienced memory problems over the past 6 months?” during phone screening); 4) absence of dementia (see “Screening for Dementia) below); and 4) age ≥ 60 years. Exclusion criteria were: 1) lifetime history of any psychiatric disorder except MDD, co-morbid anxiety, or insomnia; 2) recent and/or current unstable medical or neurological disorders; 3) diagnosis of moderate or severe neurocognitive impairment; or 4) known allergic reaction to escitalopram or memantine. Participants were free of psychotropic medications for at least two weeks before starting the trial (four weeks in the case of fluoxetine). No participants were currently taking a cognitive enhancer at study entry.
Screening for Dementia
The evaluation for dementia included: 1) An interview by a study psychiatrist who also administered the Clinical Dementia Rating Scale) (47) to identify physical and cognitive limitations; 2) a standard battery of hematologic studies, blood chemistries, liver and thyroid function tests, B12 and folate levels, and RPR test; 3) neurological examination (UPDRS); 4) neuropsychological examination (detailed below); and 5) psychiatric evaluation (SCID-DSM-IVR/5). In addition, individuals with an established dementia diagnosis or who scored ≤ 23 on the Mini-Mental State Examination (MMSE) (45, 46) were considered to have dementia and were excluded.
Diagnosis of MCI
Whether or not eligible participants met criteria for MCI was determined using established guidelines (48, 49). MCI was defined as: 1) a stage between normal cognition and dementia (Clinical Dementia Rating Scale (CDR) score of 0.5 (47)); 2) patient-reported decline in cognition; 3) objective impairment on neurocognitive testing; 4) no significant functional impairment. Objective impairment on neurocognitive testing was defined as scoring one standard deviation (SD) below age- and education-specific norms on at least two screening memory tests (Hopkins Verbal Learning Test, Revised, [either Total or Delayed scores] and Wechsler Memory Scale Third Edition, WMS-III, verbal paired associates, [either Total or Delayed scores]). Participants who met this criterion and had a CDR score of 0.5 were classified as having amnestic MCI (either single or multiple domains) (50).
Randomization
Eligible participants were randomized in a 1:1 ratio to escitalopram/placebo or escitalopram/memantine using a computer-generated random assignment scheme. A block randomization strategy (with randomly selected blocks of length 4 and 6) was used to maintain balance throughout the trial.
Intervention Procedures
All study participants received a 14-day supply of the study medications that were prepared and dispensed by the UCLA Pharmacy. All participants received 10–20 mg of escitalopram daily open-label throughout the trial. Matching capsules containing memantine (MEM) or placebo were given and titrated from 5 mg/day up to 10 mg twice daily (i.e., 20 mg per day) during the first four weeks. Participants with insufficient improvement (as indicated by a CGI improvement rating ≥3) at the completion of week 4 were titrated upward on the escitalopram dose to 20 mg per day. Participants with a CGI improvement rating of 1 or 2 were instructed to continue with the same dose. Participants reporting side effects attributed to the study medications were instructed to decrease their dosage. The minimum allowed dosages were 5 mg once daily for MEM and 10 mg once daily for escitalopram. The expected effective dose of MEM was 20 mg daily. All in-person assessments were done weekly for the first month, then bi-weekly for additional 3 months, and monthly thereafter. Measures of depression and safety were administered at each visit; secondary clinical measures were assessed at 3, 6, and 12 months, while cognitive outcomes were assessed at 6 and 12 months.
Safety Assessments
All participants received an initial baseline medical assessment including a complete physical examination with neurological and neuropsychiatric examinations, electrocardiogram (ECG), and laboratory testing.
Clinical Measures
Depression
The primary outcome measure was change on the 24-item Hamilton Depression Rating Scale (HAM-D-) at post treatment (6 months) (44). Remission of depressive symptoms was defined as a HAM-D score ≤6. The clinician-administered Clinical Global Impressions (CGI) Severity and Improvement scales (51) were used to quantify overall severity of depression and improvement over time. Higher scores indicate greater severity and lesser improvement. The Montgomery-Åsberg Depression Rating Scale (MADRS)(52) was used as a secondary measure of depressive symptoms.
Additional secondary outcomes included symptoms that frequently co-occur with geriatric depression and predict poorer treatment response, such as apathy (53–56) and anxiety (57). We also assessed psychological resilience (a protective factor shown to improve treatment response in geriatric depression) (58), comorbid medical burden (a predictor of outcomes in geriatric depression)(59, 60), and vascular risk factors associated with both geriatric depression and cognitive impairment (61, 62).
Apathy
Comorbid apathy was evaluated using the clinician-rated Apathy Evaluation Scale (AES)(63). AES total scores range from 18–72 (lower scores indicate greater apathy).
Anxiety
Anxiety symptoms were assessed via the 14-item clinician-rated Hamilton Anxiety Rating Scale (HAM-A) (64, 65).
Resilience
Psychological resilience (including items reflecting grit, coping self-efficacy, and spirituality) (66) was assessed via the 25-item Connor-Davidson Resilience Scale (CD-RISC) (67). Higher scores indicate greater resilience.
Physical health
Medical comorbidity was quantified at baseline using the clinician-rated Cumulative Illness Rating Scale for Geriatrics (CIRS-G (68)); higher scores indicate greater illness severity. Cerebrovascular risk (CVRF) was assessed via the ‘Stroke Risk Factor Prediction Chart’ from the Framingham Study (62).
Cognitive assessment
Participants completed a comprehensive neuropsychological test battery at baseline, 6 months and 12 months to assess the following cognitive domains: learning (California Verbal Learning Test-II [Trial 1 through 5 Total] (69), Rey-Osterrieth Complex Figure Test [3-minute recall] (70), Verbal Pairs Associate [immediate recall]) (71); delayed recall (California Verbal Learning Test-II [long delayed free recall](69), Rey-Osterrieth Complex Figure Test [30-minute delayed recall] (70), Verbal Paired Associates (delayed recall) (71)), and executive functioning (Trail Making Test B(72), Stroop interference [Golden version](73), Controlled Oral Word Association test [FAS] (74, 75)). We transformed raw scores to z-scores for each test score for each participant. Z-scores were reversed when necessary so that higher z-scores represent higher performance for all measures. These z-scores were averaged within each neuropsychological domain to produce composite scores and then averaged over all tests to calculate a global cognitive performance score.
Subjective memory complaints
Subjective memory complaints were assessed via the 64-item Memory Functioning Questionnaire (MFQ) scale (76). Higher scores indicate higher self-reported memory functioning (i.e., fewer memory complaints).
Statistical analysis
Data were entered at the time of collection and analyzed after completion of the trial. All data were inspected for outliers, homogeneity of variance and other assumptions to ensure their appropriateness for parametric statistical tests. Safety analyses were performed using descriptive statistics and frequency distribution of dropouts. The treatment groups were compared using t-tests (for continuous variables) or chi-squared tests (for categorical variables) on all demographic and clinical measures at baseline. Subjective memory reports were compared between the study sample at baseline and a normative sample (52) using t-tests. Intent-to-treat analyses were used for all outcomes. Continuous outcomes (e.g., depression, cognitive scores) were analyzed using a mixed effects general linear model, as implemented in SAS PROC MIXED, including treatment group as the between-subject factor, time as the within-subject factor, and the interaction term between time and treatment group. Age, sex, education, and any variables that differed between treatment groups at baseline (see Results) were used as additional covariates (education was controlled for in analyses of cognitive outcomes only). The proportion of participants who achieved remission was analyzed using a chi-squared test. MADRS and CGI - Severity scales were used as secondary measures of depression outcome. Anxiety, apathy, and psychological resilience were analyzed using ANCOVAs, with group, age, sex and baseline scores as predictors. Post hoc analyses determined the significance of specific pair-wise group differences and within-group changes. We present test scores and statistics as well as effect sizes (Cohen’s d) for group differences. Effect size magnitude was interpreted using Cohen’s(77) guidelines for standardized mean difference (SMD) of 0.2, 0.5, and 0.8, as representing small, medium, and large effect sizes, respectively. Given the novel and preliminary nature of the study, we present results of analyses conducted on a range of secondary outcome measures and set the level of significance at the alpha level of p ≤ 0.05, two-tailed, without accounting for multiple comparisons. As such, results for secondary outcome measures should be interpreted with caution.
Results
Sample Characteristics
Baseline demographic and clinical characteristics of the randomized sample (n = 95) by treatment group are summarized in Table 1. The average age of participants at baseline was 71.9 (SD=6.8) years, the mean depression severity was 17.8 (SD=2.3) on the HAM-D, and the average MMSE score was 27.9 (SD=1.7). At baseline, treatment groups did not differ significantly in age, sex ratio, education, race, MMSE, HAM-D, CD-RISC, CIRS and CVRF scores. Treatment groups did differ significantly in baseline MADRS, HAM-A, AES, and MFQ (Seriousness of Forgetting) scores. Therefore, all analyses of the primary outcome measures were conducted with these variables as covariates. Scores on the MFQ factors indicated that memory complaints were significantly greater in our sample (Table 2) compared with normative samples aged 60–89 years (52). Thirteen participants (13.7% of the sample) met criteria for MCI at baseline (7 in the ESC/MEM and 6 in the ESC/PBO groups).
Table 1.
Comparison of baseline clinical and demographic characteristics.
| ESC/MEM (n=48) M(SD) | ESC/PBO (n=47) M(SD) | Statistic | |
|---|---|---|---|
| Demographics | |||
| Age | 71.0 (6.9) | 72.8 (6.8) | t(93)=1.3, p=2 |
| Women (%) | 26 (54.2%) | 25 (53.2%) | χ2(1)=0.0, p=9 |
| Education | 15.7 (2.4) | 16.2 (2.6) | t(93)=1.0, p=.3 |
| Race (%) | 37 (77.1%) | 33 (73.3%) | Fisher’s exact p = 0.3 |
| Caucasian | 37 (77.1%) | 33 (73.3%) | |
| African American | 4 (8.3%) | 2 (4.4%) | |
| Other | 4 (8.3%) | 2 (4.4%) | |
| Hispanic | 3 (6.3%) | 8 (17.8%) | |
| Age at depression onset | 45.7 (22.2) | 44.2 (23.5) | t(90) = 0.3, p = 0.8 |
| Number of episodes | 4.6 (3.9) | 5.3 (5.2) | t(90) = 0.7, p = 0.5 |
| Chronic course >24 month | 37 (77.1%) | 34 (73.9%) | χ2(1)=0.1, p=.7 |
| MMSE | 28.1 (1.7) | 27.6 (1.7) | t(92) = 1.5, p = .1 |
| MCI | 7 (15%) | 6 (13%) | Fisher’s exact p = 1.0 |
| Clinical Measures | |||
| HAM-D | 17.8 (2.3) | 17.7 (2.4) | t(93)=0.2, p = 0.8 |
| MADRS | 16.7 (3.2) | 14.8 (3.5) | t(93)=2.8, p = 0.01 |
| HAM-A | 10.0 (3.5) | 8.4 (4.0) | t(93)=2.0, p = 0.04 |
| AES | 46.1 (9.0) | 50.0 (8.8) | t(93)=2.2, p = 0.03 |
| CDRISC | 56.5 (14.6) | 62.0 (18.1) | t(93)=1.6, p = 0.1 |
| CGI - Severity | 3.3 (0.7) | 3.5 (0.6) | t(77) = 1.1, p = 0.3 |
| CIRS-G | 5.1 (3.2) | 4.6 (3.6) | t(93)=0.8, p = 0.4 |
| CVRF | 10.5(4.7) | 11.3(4.3) | t(93)=0.9, p = 0.4 |
| Memory Functioning Questionnaire | |||
| Frequency of forgetting | 138.1 (32.2) | 149.8 (30.5) | t(91) = 1.8, p = .08 |
| Seriousness of forgetting | 66.8 (25.1) | 80.6 (19.7) | t(91) = 3.0, p = .004 |
| Retrospective functioning | 14.2 (5.4) | 14.9 (6.2) | t(91)=0.6, p = 0.6 |
| Mnemonics usage | 25.0 (9.4) | 21.9 (10.7) | t(91) = 1.5, p = .1 |
Note: M=Mean; SD= standard deviation. HAM-D = Hamilton Depression Rating Scale; MADRS = Montgomery Asberg Depression Rating Scale; HAM-A = Hamilton Anxiety Rating Scale; AES = Apathy Evaluation Scale; CDRISC = Connor-Davidson Resilience Scale; CGI = Clinical Global Impressions (missing for 8 Namenda and 8 Placebo subjects); CIRS-G = Cumulative Illness Rating Scale for Geriatrics; CVRF = Cerebrovascular risk factors.
Table 2.
Memory Functioning Questionnaire (MFQ) factor means and standard deviations (SDs) for study participants compared to a normative sample.
| MFQ Factor* | Study Participants | Normative Sample (Gilewski et al., 1990) | Normative (Pooled)# | Statistics$ | ||
|---|---|---|---|---|---|---|
| Age 60–86 | Age 60–69 | Age 70–79 | Age 80–89 | Age 60–89 | ||
| Frequency of Forgetting | 143.9 (31.8) | 152.1 (28.1) | 148.7 (29.1) | 148.5 (26.9) | 150.3 (28.4) | t(602) = 1.97, p= .05 |
| Seriousness of Forgetting | 73.6 (23.5) | 85.5 (20.1) | 83.6 (20.9) | 82.3 (19.6) | 84.3 (20.4) | t(602) = 4.56, p<.0001 |
| Retrospective Functioning | 14.6 (5.8) | 18.3 (5.7) | 18.2 (5.7) | 17.9 (5.6) | 18.2 (5.7) | t(602) = 5.59, p<.0001 |
| Mnemonics Usage | 23.4 (10.2) | 30.9 (9.4) | 29.7 (10.3) | 28.3 (8.7) | 30.1 (9.7) | t(602) = 6.04, p<.0001 |
Lower scores indicate lower self-reported memory performance
Pooled mean and SD calculated from normative samp I e ages 60–69 (N=242)
70–79 (N=210), and 80–89 (N=59)
Two-sample t-statistics comparing study participants (N=95) and normative sample ages 60–89 (N=511)
Escitalopram (ESC) daily doses ranged between 5–20 mg; memantine (MEM) daily doses ranged between 10–20 mg. Mean escitalopram dose did not significantly differ between groups (ESC/MEM: 10.5 (3.9) mg; ESC/PBO: 11.7 (3.5) mg). Mean memantine dose was 19.3 (2.6) mg in the ESC/MEM group. Thirty-five ESC/MEM and 32 ESC/PBO participants completed the midpoint assessment at 3 months; 33 ESC/MEM and 29 ESC/PBO participants completed the post-treatment assessment at 6 months (Figure 1). Twenty-three ESC/MEM and 21 ESC/PBO participants completed the naturalistic follow-up assessment at 12 months. Dropout rates did not significantly differ between the two arms (3 months: 27.1% ESC/MEM and 31.9% ESC/PBO; 6 months: 31.3% ESC/ MEM and 38.3% ESC/PBO; 12 months: 52.1% ESC/MEM and 55.3% ESC/PBO (Fisher’s exact p-value = 0.6 (3 months), 0.5 (6 months) and 0.8 (12 months)). Tolerability as assessed by number of side effects did not differ between two arms (ESC/MEM: median number of side effects = 1, range 0–11; ESC/PBO: median number of side effects = 1, range 0–11; Kruskal-Wallis χ2(1) = 1.1, p = 0.3). Details of all side effects reported by participants are included in theSupplementary Table.
Changes in Outcome Measures
Depression
Primary outcome
HAM-D
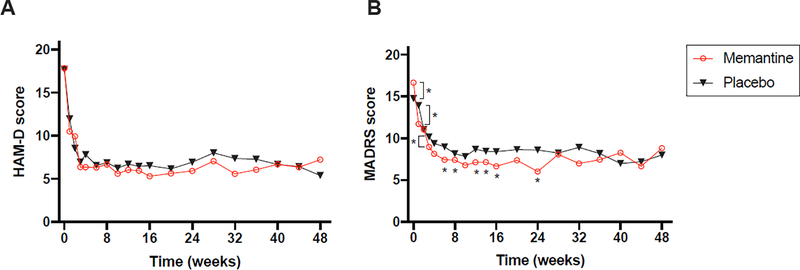
HAM-D scores at baseline, 3 months, 6 months, and 12 months as well as estimated effect sizes (Cohen’s ds) with associated 95% confidence intervals for the two study arms are presented in Table 3. Treatment groups did not significantly differ in change on HAM-D score from baseline to 3 months (F(8,84) = 1.38, p = 0.2), 6 months (F(12,84) = 1.0, p = 0.5) or 12 months (F(18,84) = 0.8, p = 0.7). Both groups demonstrated significant improvement in depressive symptoms from baseline to 3 months (ESC/MEM: change in HAM-D = −11.7 (4.7), t(34)=−14.7, p < .0001; ESC/PBO: change in HAM-D = −11.3 (4.4), t(31)=−14.5, p < .0001), 6 months (ESC/MEM: change in HAM-D = −11.8 (4.9), t(32)=−13.8, p < .0001; ESC/PBO: change in HAM-D = −11.1 (4.9), t(28)=−12.1, p < .0001) and 12 months (ESC/MEM: change in HAM-D = −9.9 (6.1), t(22)=−7.7, p < .0001; ESC/PBO: change in HAM-D = −12.2 (5.7), t(20)=−9.8, p < .0001). Changes in HAM-D over time are depicted graphically in Figure 2.
Table 3.
Means and standard deviations of ESC/MEM and ESC/PBO groups on all clinical variables.
| Baseline (N=95) | 3 months (n=67) | 6 months (n=62) | 12 months (n=44) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ESC/MEM | ESC/PBO | ESC/MEM | ESC/PBO | ES* (95% CI) | ESC/MEM | ESC/PBO | ES* (95% CI) | ESC/MEM | ESC/PBO | ES* (95% CI) | |
| Depression | |||||||||||
| HAM-D | 17.8 (2.3) | 17.7 (2.4) | 6.0 (4.5) | 6.7 (4.7) | 0.09 (−0.39, 0.57) | 5.9 (5.2) | 6.9 (5.1) | 0.16 (−0.33, 0.64) | 7.2 (5.8) | 5.4 (5.3) | 0.39 (−0.21, 0.98) |
| MADRS | 16.7 (3.2) | 14.8 (3.5) | 7.1 (5.9) | 8.7 (5.5) | 0.57 (0.08, 1.06) | 6.0 (5.5) | 8.6 (4.5) | 0.86 (0.33, 1.35) | 8.8 (7.3) | 8.0 (6.5) | 0.02 (−0.57, 0.61) |
| CGI | 3.3 (0.7) | 3.5 (0.6) | 2.3 (1.0) | 2.7 (0.8) | 0.17 (−0.31, 0.65) | 2.2 (1.0) | 2.6 (1.0) | 0.21 (−0.29, 0.69) | 2.0 (1.0) | 2.3 (1.0) | 0.19 (−0.40 0.78) |
| Anxiety (HAM-A) | 10.0 (3.5) | 8.4 (4.0) | 4.5 (4.1) | 5.0 (3.9) | 0.33 (−0.16, 0.81) | 4.7 (3.9) | 6.1 (5.4) | 0.60 (0.08, 1.08) | 5.3 (4.3) | 4.3 (2.9) | 0.10 (−0.49, 0.69) |
| Apathy (AES) | 46.0 (9.0) | 50.0 (8.8) | 55.7 (7.5) | 53.9 (7.0) | 0.68 (0.18, 1.17) | 56.6 (8.8) | 56.3 (6.2) | 0.64 (0.12, 1.12) | 56.0 (7.6) | 58.2 (7.3) | 0.25 (−0.35, 0.84) |
| Resilience (CD-RISC) | 56.5 (14.6) | 61.9 (18.1) | 64.8 (16.4) | 66.9 (13.7) | 0.11 (−0.37, 0.58) | 65.9 (14.6) | 65.4 (11.6) | 0.40 (−0.10, 0.88) | 66.3 (15.5) | 66.4 (13.8) | 0.27 (−0.32, 0.86) |
| Cognition (Global) | 0.02 (0.62) | −0.04 (0.70) | N/A | N/A | N/A | 0.03 (0.53) | −0.10 (0.67) | 0.42 (−0.08, 0.93) | 0.15 (0.67) | −0.26 (0.71) | 0.94 (0.32, 1.55) |
| Learning | 0.09 (0.79) | −0.03 (0.89) | N/A | N/A | N/A | 0.07 (0.75) | −0.10 (0.81) | 0.25 (−0.25, 0.75) | 0.20 (0.81) | −0.26 (0.90) | 0.41 (−0.18, 1.00) |
| Delayed Recall | 0.07 (0.74) | −0.02 (0.86) | N/A | N/A | N/A | 0.08 (0.69) | −0.09 (0.83) | 0.28 (−0.22, 0.78) | 0.23 (0.80) | −0.28 (0.82) | 0.75 (0.14, 1.35) |
| Executive Function | −0.13 (0.57) | −0.08 (0.61) | N/A | N/A | N/A | −0.07 (0.48) | −0.12 (0.65) | 0.39 (−0.11, 0.90) | 0.01 (0.54) | −0.23 (0.64) | 0.96 (0.34, 1.57) |
Note.
ES = Cohen’s d effect size (standardized mean difference in change between groups). N/A = not assessed. Between-group differences in mean changes from baseline to time-point indicated were estimated by a mixed effects general linear model, including treatment group, time, and time by treatment group interaction, controlling for age, sex and baseline scores. Data were available for 35 ESC/MEM and 32 ESC/PBO participants at 3 months, 33 ESC/MEM and 29 ESC/PBO participants at 6 months and 23 ESC/MEM and 21 ESC/PBO participants at 12 months.
Figure 2. Change in depressive symptoms over time.
Note: Asterisks indicate significant (p<.05) between-group differences in MADRS mean changes from baseline to timepoint indicated, as estimated by a mixed effects general linear model, including treatment group, time, and time by treatment group interaction, controlling for age, sex and baseline scores. Week 1: t(91) = 3.77, p = .0003. Week 3: t(91) = 2.23, p = .03. Week 4: t(91) = 2.50, p = .01. Week 6: t(91) = 2.86, p = .005. Week 8: t(91) = 2.20, p = .03. Week 10: t(91) = 2.05, p = .04. Week 12: t(91) = 2.29, p = .02. Week 14: t(91) = 2.22, p = .03. Week 16: t(91) = 2.65, p = .01. Week 24: t(91) = 3.27, p = .002
Secondary outcome measures
MADRS
Controlling for age, sex and baseline scores, the group difference in change in MADRS score did not reach significance at 3 months (F(1,62) = 3.1, p=.08) or at 12 months (F(1,39) = 0.1, p=.9). However, there was a significant group difference in change in MADRS score from baseline to 6 months (F(1,57) = 6.5, p=.01) (Table 3, Figure 2). While both groups demonstrated significant improvement in MADRS scores at 6 months (ESC/MEM: change in MADRS = −10.2 (5.4), t(32)=−10.9, p < .0001; ESC/PBO: change in MADRS = −5.8 (4.8), t(28)=−6.5, p < .0001), the change in ESC/MEM group was significantly greater.
CGI
Changes in clinician-rated global impression of depression severity as assessed by the CGI did not significantly differ between groups at 3 months (F(7,72) = 1.69, p = 0.2) or 6 months (F(11,72) = 1.4, p = 0.2) but were significantly different at 12 months (F(17,72) = 2.2, p = 0.01). To compare the groups in the proportion of participants who experienced clinically significant improvement, we combined those with CGI Improvement score of 1 or 2 (very much or much improved). In the ESC/MEM group, twenty-five participants (52.1%) improved much or very much compared to 15 participants (31.9%) of ESC/PBO group (χ2(1)=3.9; p= 0.06) at the 3-month assessment. Twenty participants (41.7%) improved much or very much compared to 14 participants (29.8%) of ESC/PBO group (χ2(1)=1.5; p= 0.2) at the 6-month assessment. At the 12-month assessment, 16 of the remaining 23 participants (69.6%) in the ESC/MEM group and 14 of the remaining 21 participants (66.7%) in the ESC/PBO group improved.
Remitter analyses
Twenty-two of 48 ESC/MEM participants (45.8%) met remission criteria (HAM-D ≤ 6) at 3 months, and 18 of 47 ESC/PBO participants (38.3%) remitted. This difference was not statistically significant (χ2(1)=0.6, p=0.5). At 6 months, 23 ESC/MEM participants (47.9%) and 15 ESC/PBO participants (31.9%) continued in remission. This difference was not statistically significant (χ2(1)=2.5, p=0.1). The Number Needed to Treat (NNT), defined as the number of patients that need to be treated with ESC/MEM in order for one patient to remit compared to ESC/PBO was 7. In the analyses of predictors of remission at 3 and 6 months, only lower anxiety scores at baseline (HAM-A) were significantly associated with treatment remission, χ2(1)= 6.2, p = .01 at 3 months, and χ2(1) = 5.3, p = .02 at 6 months. Remitters had lower anxiety at baseline (mean(sd) = 8.1(3.3)) compared to non-remitters (mean(SD) = 10.7(4.1)).
Cognition
Z-scores for each of the cognitive domains (learning, delayed recall and executive functioning) as well as global cognitive performance at baseline, 6 months, and 12 months are presented in Table 3. No significant between-group differences were observed at baseline or 6 months. However, significant between-group differences favoring the ESC/MEM group were observed at 12 months in delayed recall (F(2,82)=4.3, p=.02) and executive functioning (F(2,82)=5.1, p=.01) as well as the global performance score (F(2,82)=7.2, p=.001). Controlling for change in HAM-D did not change these findings.
Apathy
There were no significant between-group differences in apathy at either the 3-month (F(1,61) = 3.5, p = 0.06) or 6-month (F(1,56) = 2.1, p = 0.15) assessment. At the 3-month assessment, the ESC/MEM group improved significantly in apathy (change in AES = 9.2 (7.7), t(34)=7.0,p < 0.0001) and the ESC/PBO group did not (change in AES = 3.9 (10.4), t(31)=1.6, p = 0.1) and at the 6-month assessment, both groups improved in apathy (ESC/MEM group change in AES = 10.0 (8.7), t(32)=6.6, p < 0.0001; ESC/PBO group change in AES = 4.5 (8.1), t(28)=2.9, p = 0.007).
Anxiety
The between-group difference in change in anxiety was significant at 6 months (F(1,57) = 4.2, p = 0.04), but not at 3 or 12 months. Both groups improved in anxiety at 6 months (ESC/MEM: change in HAM-A = −4.8 (4.0), t(32)=−6.9, p < 0.0001; ESC/PBO: change in HAM-A = −2.5 (3.8), t(28)=−3.5, p = 0.002 at 6 months) but the improvement for the ESC/MEM group was greater. However, the 6-month between-group difference was no longer significant after controlling for change in HAM-D (F(1,56) = 3.21, p = 0.08).
Resilience
The between-group difference in change in resilience was not significant (F(1,62) = 0.0, p = 0.9 at 3 months; F(1,57) = 1.0, p = 0.3 at 6 months). While the ESC/MEM group improved in resilience at both 3 and 6 month assessments (change in CD-RISC = 9.2 (12.2), t(34)=4.5, p < 0.0001 at 3 months; 11.1 (8.5), t(32)=7.5, p < 0.0001 at 6 months), the improvement in the ESC/PBO group was significant only at 3 months (change in CD-RISC = 7.6 (17.6), t(31)=2.5, p =0.02 at 3 months; 5.7 (17.6), t(28)=1.7, p =0.1 at 6 months).
Discussion
Our study is the first placebo-controlled RCT to evaluate the effect of combination memantine and escitalopram on mood and cognitive function in older adults with major depression and subjective memory complaints. Although we failed to find group differences on the primary outcome of depression symptoms as assessed by change in HAM-D, the combination of memantine and escitalopram resulted in significantly improved mood as assessed by MADRS and anxiety (HAM-A) at 6 months, and cognitive function at 12 months, compared to escitalopram and placebo. The magnitude of these effects ranged from medium to large on the MADRS and anxiety at 3 and 6 months. The magnitude of the between-group difference in global cognitive function at 12 months was large. These results are consistent with those of a prior open-label study demonstrating a significant effect of combined escitalopram and memantine on cognitive performance at 12 months in depressed older adults with cognitive impairment (37). Although the combination of escitalopram and memantine also appeared beneficial for apathy and resilience that achieved moderate effect sizes at 6 months, the between-group differences failed to reach statistical significance. After we controlled for change in HAM-D, the change in HAM-A was no longer significant, which highlights the inter-related nature of depression and anxiety symptoms. By contrast, all cognitive outcomes remained significantly different between the groups after controlling for change in HAM-D. This suggests that improvement in domains of cognitive function occurred independently from improvement in depression and highlights the importance of identifying treatment approaches for this population that target both depression and cognition.
In our study, between-group differences in mood improvement were statistically significant only for the MADRS post-treatment and not the HAM-D. This is consistent with reports showing the MADRS to be more sensitive to treatment-related changes in depression severity compared to the HAM-D (79). The significant change in MADRS scores is further supported by the clinician ratings of overall depression severity, which decreased significantly by 12 months in the ESC/MEM compared to ESC/PBO group (80–82).
Two previous RCTs of memantine treatment in geriatric depression reported no significant effects using the HAM-D. An RCT of citalopram plus memantine vs. citalopram vs. placebo found no significant effect of 20 mg daily memantine on depression in older adults with MDD as assessed by either the HAM-D or the Geriatric Depression Scale (38). Another RCT examined the effect of memantine vs. placebo among older adults who had recently been admitted to a skilled nursing facility and were experiencing depressive symptoms (31). They reported no significant effect of 20 mg memantine daily on depressive symptoms as assessed by the HAM-D. Each of these authors posited that the dose of memantine (20 mg per day; the maximum dose approved for human use and the same used in the current study) may have been insufficient to elicit significantly improved treatment response. The results of our study also support prior reports of good tolerability of 20 mg of memantine in depressed older adults (31, 38), as drop-out rates and reported side effects were similar across treatment groups.
Several limitations of the current study should be noted. First, we used a convenience sample of outpatients with moderate MDD and subjective memory complaints. Our sample was relatively demographically homogenous, with the majority being Caucasian and college-educated. Although we enrolled participants with subjective memory complaints, relatively few met criteria for MCI, and participants with psychiatric comorbidity, suicidality, or moderate-to-severe neurocognitive impairment were excluded. As such, our results may not generalize to patients with acute medical illness, severe depression, more severe cognitive impairment, or more demographically diverse samples. Second, we observed significant differences in several measures between treatment groups at baseline. Third, there was relatively high dropout rate at 12 months and further the missingness at 12-month may not be at random, but rather affected by tolerability and perceived improvement; thus the 12-month results, especially those related to cognition, are likely underpowered and should be regarded as preliminary and will need to be confirmed in larger samples. Finally, we note that due to the novel nature of the study and out of concern that multiplicity adjustment might obscure possibly important findings,–we did not correct for multiple comparisons in our analyses. As such, there is a distinct possibility of Type I error, and analyses of secondary outcomes should be interpreted with caution.
Despite these limitations, our study is the first placebo-controlled RCT to address the potential of memantine combination with escitalopram to enhance clinical and cognitive outcomes in geriatric depression presenting with subjective memory complaints over the course of 6–12 months. Our results suggest that this combination is safe and may improve symptoms of depression, anxiety and cognitive outcomes in this difficult-to-treat population. Detection of improvement in cognitive function may require follow-up of 12 months or longer. This is consistent with in vitro and in vivo research suggesting that memantine may improve cognition through both synaptic and extrasynaptic NMDA inhibition as well glutamate/NMDA-mediated neurodegeneration. Cognitive benefits follow molecular alterations and tissue remodeling that may require at least 12 months before detection of significant change on cognitive outcomes. We are currently analyzing the role of change in biomarkers of aging in the heterogeneity of treatment response that could shed light on the mechanisms of observed cognitive effects. Future studies with longer follow-up period should address the potential of combination escitalopram and memantine to prevent or reverse cognitive decline in older adults with major depression.
Supplementary Material
Highlights.
New treatment strategies targeting both depression and cognitive impairment in older adults are urgently needed.
In our randomized controlled trial of escitalopram + memantine (ESC/MEM) vs. escitalopram + placebo (ESC/PBO), ESC/MEM for geriatric major depression with subjective memory complaints was as effective in reducing severity of depression post-treatment, but it was more effective in improving cognitive outcomes at 12 month follow-up compared to ESC/PBO.
Our results indicate that the combination of memantine with escitalopram is safe and effective for improving clinical and cognitive outcomes in the difficult-to-treat population.
Acknowledgements
This work was supported by NIH grants MH097892, AT009198, and the National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881. The authors thank the Semel Institute Biostatistics Core (SIStat) for database management and support.
Disclosures: Dr. Lavretsky received research support from Allergan/ Forest Laboratories. All other authors report no financial relationships with commercial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blazer DG. Depression in late life: review and commentary. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2003;58(3):M249–M65. [DOI] [PubMed] [Google Scholar]
- 2.Haigh EAP, Bogucki OE, Sigmon ST, Blazer DG. Depression among Older Adults: a 20-Year Update on Five Common Myths and Misconceptions. The American Journal of Geriatric Psychiatry. 2018. [DOI] [PubMed] [Google Scholar]
- 3.Alexopoulos GS, Young RC, Abrams RC, Meyers B, Shamoian CA. Chronicity and relapse in geriatric depression. Biological Psychiatry. 1989;26(6):551–64. [DOI] [PubMed] [Google Scholar]
- 4.Reynolds CFr, Dew MA, Pollock BG, Mulsant BH, Frank E, Miller MD, et al. Maintenance treatment of major depression in old age. New England Journal of Medicine. 2006;354(11):1130–8. [DOI] [PubMed] [Google Scholar]
- 5.Nelson JC, Delucchi K, Schneider LS. Efficacy of second generation antidepressants in late-life depression: a meta-analysis of the evidence. The American Journal of Geriatric Psychiatry. 2008;16(7):558–67. [DOI] [PubMed] [Google Scholar]
- 6.Nelson JC, Delucchi KL, Schneider LS. Moderators of outcome in late-life depression: a patient-level meta-analysis. American Journal of Psychiatry. 2013;170(6):651–9. [DOI] [PubMed] [Google Scholar]
- 7.Rutherford BR, Roose SP. A model of placebo response in antidepressant clinical trials. American Journal of Psychiatry. 2013;170(7):723–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sackeim HA, Roose SP, Burt T. Optimal length of antidepressant trials in late-life depression. Journal of clinical psychopharmacology. 2005;25(4):S34–S7. [DOI] [PubMed] [Google Scholar]
- 9.Entsuah AR, Huang H, Thase ME. Response and remission rates in different subpopulations with major depressive disorder administered venlafaxine, selective serotonin reuptake inhibitors, or placebo. The Journal of clinical psychiatry. 2001. [DOI] [PubMed] [Google Scholar]
- 10.Charney DS, Nemeroff CB, Lewis L, Laden SK, Gorman JM, Laska EM, et al. National Depressive and Manic-Depressive Association consensus statement on the use of placebo in clinical trials of mood disorders. Arch Gen Psychiatry. 2002;59(3):262–70. [DOI] [PubMed] [Google Scholar]
- 11.Thase ME. Achieving remission and managing relapse in depression. J Clin Psychiatry. 2003;64 Suppl 18:3–7. [PubMed] [Google Scholar]
- 12.Morimoto SS, Kanellopoulos D, Manning KJ, Alexopoulos GS. Diagnosis and treatment of depression and cognitive impairment in late life. Annals of the New York Academy of Sciences. 2015;1345(1):36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nebes RD, Pollock BG, Houck PR, Butters MA, Mulsant BH, Zmuda MD, et al. Persistence of cognitive impairment in geriatric patients following antidepressant treatment: a randomized, double-blind clinical trial with nortriptyline and paroxetine. Journal of psychiatric research. 2003;37(2):99–108. [DOI] [PubMed] [Google Scholar]
- 14.Tan EYL, Köhler S, Hamel REG, Munoz-Sânchez JL, Verhey FRJ, Ramakers IHGB. Depressive Symptoms in Mild Cognitive Impairment and the Risk of Dementia: A Systematic Review and Comparative Meta-Analysis of Clinical and Community-Based Studies. Journal of Alzheimer’s Disease. 2019(Preprint):1–11. [DOI] [PubMed] [Google Scholar]
- 15.Mourao RJ, Mansur G, Malloy-Diniz LF, Castro Costa E, Diniz BS. Depressive symptoms increase the risk of progression to dementia in subjects with mild cognitive impairment: systematic review and meta-analysis. International journal of geriatric psychiatry. 2016;31(8):905–11. [DOI] [PubMed] [Google Scholar]
- 16.Vega JN, Zurkovsky L, Albert K, Melo A, Boyd B, Dumas J, et al. Altered brain connectivity in early postmenopausal women with subjective cognitive impairment. Frontiers in neuroscience. 2016;10:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reynolds CF, Butters MA, Lopez O, Pollock BG, Dew MA, Mulsant BH, et al. Maintenance treatment of depression in old age: a randomized, double-blind, placebo-controlled evaluation of the efficacy and safety of donepezil combined with antidepressant pharmacotherapy. Archives of general psychiatry. 2011;68(1):51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerhard DM, Wohleb ES, Duman RS. Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity. Drug discovery today. 2016;21(3):454–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiss JP, Szasz BK, Fodor L, Mike A, Lenkey N, Kurko D, et al. GluN2B-containing NMDA receptors as possible targets for the neuroprotective and antidepressant effects of fluoxetine. Neurochemistry international. 2012;60(2):170–6. [DOI] [PubMed] [Google Scholar]
- 20.Ates-Alagoz Z, Adejare A. NMDA receptor antagonists for treatment of depression. Pharmaceuticals. 2013;6(4):480–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quan MN, Zhang N, Wang YY, Zhang T, Yang Z. Possible antidepressant effects and mechanisms of memantine in behaviors and synaptic plasticity of a depression rat model. Neuroscience. 2011;182:88–97. [DOI] [PubMed] [Google Scholar]
- 22.Réus GZ, Stringari RB, Kirsch TR, Fries GR, Kapczinski F, Roesler R, et al. Neurochemical and behavioural effects of acute and chronic memantine administration in rats: further support for NMDA as a new pharmacological target for the treatment of depression? Brain research bulletin. 2010;81(6):585–9. [DOI] [PubMed] [Google Scholar]
- 23.aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biological psychiatry. 2010;67(2):139–45. [DOI] [PubMed] [Google Scholar]
- 24.Rasmussen KG, Lineberry TW, Galardy CW, Kung S, Lapid MI, Palmer BA, et al. Serial infusions of low-dose ketamine for major depression. Journal of Psychopharmacology. 2013;27(5):444–50. [DOI] [PubMed] [Google Scholar]
- 25.Mathew SJ, Shah A, Lapidus K, Clark C, Jarun N, Ostermeyer B, et al. Ketamine for treatment-resistant unipolar depression. CNS drugs. 2012;26(3):189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gellis ZD, McClive-Reed KP, Brown E. Treatments for depression in older persons with dementia. The annals of long-term care: the official journal of the American Medical Directors Association. 2009;17(2):29. [PMC free article] [PubMed] [Google Scholar]
- 27.Maidment ID, Fox CG, Boustani M, Rodriguez J, Brown RC, Katona CL. Efficacy of memantine on behavioral and psychological symptoms related to dementia: a systematic metaanalysis. Annals of Pharmacotherapy. 2008;42(1):32–8. [DOI] [PubMed] [Google Scholar]
- 28.Zarate CA Jr, Singh JB, Quiroz JA, De Jesus G, Denicoff KK, Luckenbaugh DA, et al. A double-blind, placebo-controlled study of memantine in the treatment of major depression. American Journal of Psychiatry. 2006;163(1):153–5. [DOI] [PubMed] [Google Scholar]
- 29.Ferguson JM, Shingleton RN. An open-label, flexible-dose study of memantine in major depressive disorder. Clinical neuropharmacology. 2007;30(3):136–44. [DOI] [PubMed] [Google Scholar]
- 30.Koukopoulos A, Serra G, Koukopoulos AE, Reginaldi D, Serra G. The sustained mood-stabilizing effect of memantine in the management of treatment resistant bipolar disorders: findings from a 12-month naturalistic trial. Journal of Affective Disorders. 2012;136(1–2):163–6. [DOI] [PubMed] [Google Scholar]
- 31.Lenze EJ, Skidmore ER, Begley AE, Newcomer JW, Butters MA, Whyte EM. Memantine for late-life depression and apathy after a disabling medical event: a 12-week, double-blind placebo-controlled pilot study. International journal of geriatric psychiatry. 2012;27(9):974–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anand IS, Florea VG, Solomon SD, Konstam MA, Udelson JE. Noninvasive assessment of left ventricular remodeling: concepts, techniques, and implications for clinical trials. Journal of cardiac failure. 2002;8(6 Suppl):S452–64. [DOI] [PubMed] [Google Scholar]
- 33.Muhonen LH, Lonnqvist J, Juva K, Alho H. Double-blind, randomized comparison of memantine and escitalopram for the treatment of major depressive disorder comorbid with alcohol dependence. J Clin Psychiatry. 2008;69(3):392–9. [DOI] [PubMed] [Google Scholar]
- 34.Kishi T, Matsunaga S, Iwata N. A meta-analysis of memantine for depression. Journal of Alzheimer’s Disease. 2017;57(1):113–21. [DOI] [PubMed] [Google Scholar]
- 35.Wu E, Greenberg PE, Yang E, Yu A, Haim Erder M. Comparison of escitalopram versus citalopram for the treatment of major depressive disorder in a geriatric population. Current medical research and opinion. 2008;24(9):2587–95. [DOI] [PubMed] [Google Scholar]
- 36.Cipriani A, Santilli C, Furukawa TA, Signoretti A, Nakagawa A, McGuire H, et al. Escitalopram versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2009(2):CD006532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pelton GH, Harper OL, Roose SP, Marder K, D’Antonio K, Devanand DP. Combined treatment with memantine/es-citalopram for older depressed patients with cognitive impairment: a pilot study. International journal of geriatric psychiatry. 2016;31(6):648–55. [DOI] [PubMed] [Google Scholar]
- 38.Omranifard V, Shirzadi E, Samandari S, Afshar H, Maracy MR. Memantine add on to citalopram in elderly patients with depression: a double-blind placebo-controlled study. Journal of research in medical sciences: the official journal of Isfahan University of Medical Sciences. 2014;19(6):525. [PMC free article] [PubMed] [Google Scholar]
- 39.Lamar M, Charlton R, Zhang A, Kumar A. Differential associations between types of verbal memory and prefrontal brain structure in healthy aging and late life depression. Neuropsychologia. 2012;50(8):1823–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elderkin-Thompson V, Kumar A, Bilker WB, Dunkin JJ, Mintz J, Moberg PJ, et al. Neuropsychological deficits among patients with late-onset minor and major depression. Archives of Clinical Neuropsychology. 2003;18(5):529–49. [DOI] [PubMed] [Google Scholar]
- 41.Baudic S, Tzortzis C, Barba GD, Traykov L. Executive deficits in elderly patients with major unipolar depression. Journal of geriatric psychiatry and neurology. 2004;17(4):195–201. [DOI] [PubMed] [Google Scholar]
- 42.Lockwood KA, Alexopoulos GS, van Gorp WG. Executive Dysfunction in Geriatric Depression. American Journal of Psychiatry. 2002;159(7):1119–26. [DOI] [PubMed] [Google Scholar]
- 43.Rapp MA, Dahlman K, Sano M, Grossman HT, Haroutunian V, Gorman JM. Neuropsychological differences between late-onset and recurrent geriatric major depression. American Journal of Psychiatry. 2005;162(4):691–8. [DOI] [PubMed] [Google Scholar]
- 44.Hamilton M A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry. 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. [DOI] [PubMed] [Google Scholar]
- 46.Folstein MF, Anthony JC, Parhad I, Duffy B, Gruenberg EM. The Meaning of Cognitive Impairment in the Elderly. Journal of the American Geriatrics Society. 1985;33(4):228–35. [DOI] [PubMed] [Google Scholar]
- 47.Hughes CP, Berg L, Danziger W, Coben LA, Martin RL. A new clinical scale for the staging of dementia. The British journal of psychiatry. 1982;140(6):566–72. [DOI] [PubMed] [Google Scholar]
- 48.Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. Jama. 2014;312(23):2551–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of internal medicine. 2004;256(3):183–94. [DOI] [PubMed] [Google Scholar]
- 50.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment-beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. Journal of internal medicine. 2004;256(3):240–6. [DOI] [PubMed] [Google Scholar]
- 51.Busner J, Targum SD, Miller DS. The Clinical Global Impressions scale: errors in understanding and use. Comprehensive Psychiatry. 2009;50(3):257–62. [DOI] [PubMed] [Google Scholar]
- 52.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. The British journal of psychiatry. 1979;134(4):382–9. [DOI] [PubMed] [Google Scholar]
- 53.Eyre HA, Siddarth P, van Dyk K, St. Cyr N, Baune BT, Barrio JR, et al. Neural correlates of apathy in late-life depression: a pilot [18F] FDDNP positron emission tomography study. Psychogeriatrics. 2017;17(3):186–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yuen GS, Bhutani S, Lucas BJ, Gunning FM, AbdelMalak B, Seirup JK, et al. Apathy in late-life depression: common, persistent, and disabling. The American Journal of Geriatric Psychiatry. 2015;23(5):488–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuen GS, Gunning FM, Woods E, Klimstra SA, Hoptman MJ, Alexopoulos GS. Neuroanatomical correlates of apathy in late-life depression and antidepressant treatment response. Journal of affective disorders. 2014;166:179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chaturvedi SK, Sarmukaddam SB. Prediction of outcome in depression by negative symptoms. Acta psychiatrica Scandinavica. 1986;74(2):183–6. [DOI] [PubMed] [Google Scholar]
- 57.Mohlman J, Bryant C, Lenze EJ, Stanley MA, Gum A, Flint A, et al. Improving recognition of late life anxiety disorders in Diagnostic and Statistical Manual of Mental Disorders: observations and recommendations of the Advisory Committee to the Lifespan Disorders Work Group. International journal of geriatric psychiatry. 2012;27(6):549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laird KT, Lavretsky H, St Cyr N, Siddarth P. Resilience predicts remission in antidepressant treatment of geriatric depression. International journal of geriatric psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wells KB, Stewart A, Hays RD, Burnam MA, Rogers W, Daniels M, et al. The functioning and well-being of depressed patients: results from the Medical Outcomes Study. Jama. 1989;262(7):914–9. [PubMed] [Google Scholar]
- 60.Lavretsky H, Kitchen C, Mintz J, Kim M-D, Estanol L, Kumar A. Medical burden, cerebrovascular disease, and cognitive impairment in geriatric depression: modeling the relationships with the CART analysis. CNS spectrums. 2002;7(10):716–22. [DOI] [PubMed] [Google Scholar]
- 61.Schneider B, Ercoli L, Siddarth P, Lavretsky H. Vascular burden and cognitive functioning in depressed older adults. The American Journal of Geriatric Psychiatry. 2012;20(8):673–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22(3):312–8. [DOI] [PubMed] [Google Scholar]
- 63.Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res. 1991;38(2):143–62. [DOI] [PubMed] [Google Scholar]
- 64.Hamilton M The assessment of anxiety states by rating. The British journal of medical psychology. 1959;32(1):50–5. [DOI] [PubMed] [Google Scholar]
- 65.Maier W, Buller R, Philipp M, Heuser I. The Hamilton Anxiety Scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. J Affect Disord. 1988;14(1):61–8. [DOI] [PubMed] [Google Scholar]
- 66.Laird KT, Lavretsky H, Paholpak P, Vlasova RM, Roman M, St Cyr N, et al. Clinical correlates of resilience factors in geriatric depression. Int Psychogeriatr. 2018:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Connor KM, Davidson JRT. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC). Depression and anxiety. 2003;18:76–82. [DOI] [PubMed] [Google Scholar]
- 68.Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry research. 1992;41(3):237–48. [DOI] [PubMed] [Google Scholar]
- 69.Delis DC. California verbal learning test: Adult version Manual San Anotnio, TX: Psychological Corporation; 2000. [Google Scholar]
- 70.Meyers JE, Meyers KR. Rey Complex Figure Test under four different administration procedures. Clinical Neuropsychologist. 1995;9(1):63–7. [Google Scholar]
- 71.Wechsler D The Wechsler Adult Intelligence Scale, III Manual. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 72.Arnett JA, Labovitz SS. Effect of physical layout in performance of the Trail Making Test. Psychological Assessment. 1995;7(2):220. [Google Scholar]
- 73.Golden CJ, Marsella AJ, Golden EE. Cognitive relationships of resistance to interference. Journal of Consulting and Clinical Psychology. 1975;43(3):432. [DOI] [PubMed] [Google Scholar]
- 74.Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery and REHABIT: A model for integrating evaluation and remediation of cognitive impairment. Cognitive Rehabilitation. 1988;6(3):10–7. [Google Scholar]
- 75.Benton A, Hamsher K, Varney RN, Spreen O. Contribution to neuropsychological assessment: a clinical manual. New York, NY: Oxford University Press; 1983. [Google Scholar]
- 76.Gilewski MJ, Zelinski EM, Schaie KW. The Memory Functioning Questionnaire for assessment of memory complaints in adulthood and old age. Psychology and aging. 1990;5(4):482. [DOI] [PubMed] [Google Scholar]
- 77.Cohen J Statistical power analysis for the behavioral sciences: Lawrence Erlbaum Associates, Inc; 1977. [Google Scholar]
- 78.Rothman KJ, Greenland S, Lash TL. Modern epidemiology: Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia; 2008. [Google Scholar]
- 79.Iannuzzo RW, Jaeger J, Goldberg JF, Kafantaris V, Sublette ME. Development and reliability of the HAM-D/MADRS interview: an integrated depression symptom rating scale. Psychiatry research. 2006;145(1):21–37. [DOI] [PubMed] [Google Scholar]
- 80.Davidson J, Turnbull CD, Strickland R, Miller R, Graves K. The Montgomery-Åsberg Depression Scale: reliability and validity. Acta Psychiatrica Scandinavica. 1986;73(5):544–8. [DOI] [PubMed] [Google Scholar]
- 81.Senra C Evaluation and monitoring of symptom severity and change in depressed outpatients. Journal of clinical psychology. 1996;52(3):317–24. [DOI] [PubMed] [Google Scholar]
- 82.Mulder RT, Joyce PR, Frampton C. Relationships among measures of treatment outcome in depressed patients. Journal of Affective Disorders. 2003;76(1–3):127–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.