ABSTRACT
Background
Maternal metabolic abnormalities have been related to offspring obesity especially during childhood.
Objectives
We analyzed whether the gestational diabetes mellitus (GDM)-associated melatonin receptor 1B (MTNR1B) genotype of mothers modified the relation between maternal gestational weight gain and childhood obesity.
Methods
A total of 1114 Chinese mother-child pairs (mothers with or without prior GDM) were included. Mothers’ MTNR1B rs10830962 genotype and gestational weight gain were assessed. Indicators of childhood obesity included BMI-for-age z-score, weight-for-age z-score, waist circumference, and body fat. Childhood overweight and obesity were also analyzed.
Results
We found that the maternal MTNR1B genotype significantly interacted with gestational weight gain on indicators of offspring's obesity (all P for interaction < 0.05). After multivariable adjustment, BMI-for-age z-scores associated with 1-kg gestational weight gain were 0.009 (SE 0.018), 0.026 (SE 0.010), and 0.061 (SE 0.010) in children with the maternal MTNR1B genotype CC, CG, and GG, respectively (P-interaction = 0.012). Similar interactions were observed for weight-for-age z-score, waist circumference, and body fat (P-interaction = 0.001, 0.003, and 0.012, respectively). The associations remained consistently significant in women with and without GDM. We also found significant interactions between the maternal MTNR1B genotype and gestational weight gain on the offspring's childhood overweight and obesity (P-interaction = 0.005 and 0.026, respectively).
Conclusions
The maternal MTNR1B genotype might interact with gestational weight gain on offspring's obesity risk during childhood.
Keywords: MTNR1B, gestational weight gain, childhood obesity, gestational diabetes mellitus, gene–environment interaction
Introduction
Childhood obesity has become a major public health problem globally. In 2015, 107.7 million children were obese worldwide, corresponding to a worldwide prevalence of childhood overweight and obesity of 23% (1). Moreover, 70% of obese adolescents become obese adults (2). Childhood obesity is associated with markedly increased risks for a variety of cardiometabolic disorders during both childhood and adulthood (1, 3).
Prenatal exposure to maternal metabolic abnormalities contributes to the vicious intergenerational cycle of metabolic disorders, and is among the leading risk factors for obesity in offspring especially during childhood (4). Excessive weight gain during pregnancy has been associated with offspring obesity (5). Women with gestational diabetes mellitus (GDM) are more likely to gain excess body weight during pregnancy (6, 7). A recent genome-wide association study identified a variant in the melatonin receptor 1B (MTNR1B) gene associated with GDM in East Asian women (8). In our previous analysis, we found that the MTNR1B genotype interacted with gestational weight gain on postpartum glycemic changes in Chinese women with a history of GDM (9). We hypothesized that the association of gestational weight gain with offspring's adiposity measures including weight, BMI, body fat, waist circumference, and obesity risk might differ according to the maternal MTNR1B genotype.
In the current study, by taking advantage of comprehensive information collected from 1114 mother-child pairs, we investigated the interactions between weight gain during pregnancy and maternal MTNR1B rs10830962 genotype on indicators of offspring's obesity during childhood, including weight, BMI, body fat, and waist circumference.
Methods
Study population
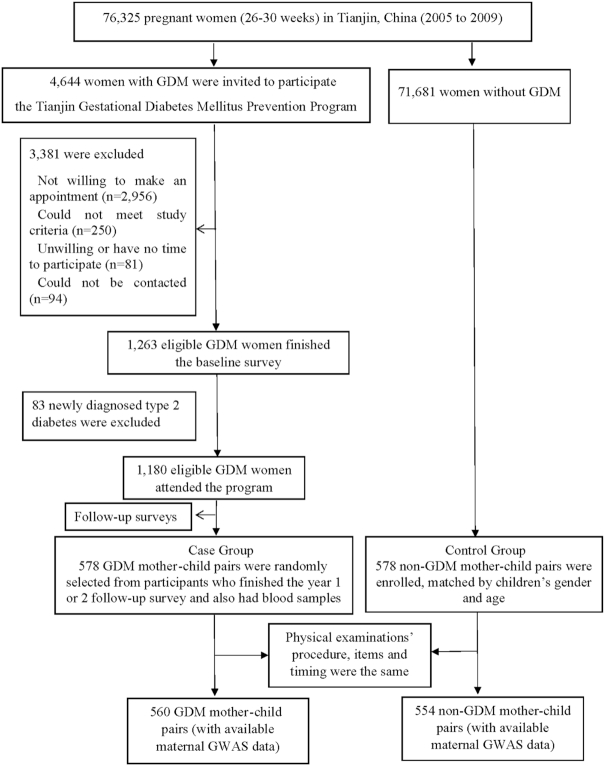
We conducted the study in 1114 mother-child pairs (560 GDM and 554 non-GDM mother-child pairs) in Tianjin, China. The GDM mothers came from the Tianjin Gestational Diabetes Mellitus Prevention Program (10, 11), screening for GDM women in 6 urban districts of Tianjin, China, launched by the Tianjin Women's and Children's Health Center from 1999. A total of 76,325 pregnant women were screened from 2005 to 2009, among whom 4644 women were diagnosed with GDM according to the 1999 WHO criteria (12). In the GDM diagnosis, we used a 2-step-approach. First, all pregnant women at 26–30 gestational weeks participated in a 1-h 50-g glucose screening test. Second, women with a glucose concentration ≥7.8 mmol/L were referred to take a 75-g 2-h oral-glucose-tolerance test (OGTT) at the Tianjin Women's and Children's Health Center. Women with either diabetes (fasting glucose ≥7mmol/L or 2-h glucose ≥11.1mmol/L) or impaired glucose tolerance (2-h glucose ≥7.8 and <11.1mmol/L) in the 75-g glucose 2-h OGTT result were regarded as having GDM. Then, all 4,644 women with GDM were invited to participate in the Tianjin Gestational Diabetes Mellitus Prevention Program. During August 2009 to July 2011, 1,263 women with prior GDM returned and finished the baseline survey. Between the returned and unreturned GDM women, there were no differences at 26–30 gestational weeks’ OGTT in age (28.9 compared with 28.7 y), 2-h glucose (9.23 compared with 9.16 mmol/L), fasting glucose (5.34 compared with 5.34 mmolLl), or the prevalence of impaired glucose tolerance (90.9% compared with 91.8%), and diabetes (9.1% compared with 8.2%) (13). After excluding 83 newly diagnosed women with type 2 diabetes, the remaining 1180 women with prior GDM attended the Tianjin Gestational Diabetes Mellitus Prevention Program. The details have been described previously (13, 14). We randomly selected 578 GDM mother-child pairs who finished the year 1 or 2 follow-up survey and also had blood samples as the GDM case group. No differences at baseline age (32.3 compared with 32.4 y), BMI (23.9 compared with 24.0 kg/m2), fasting glucose (5.21 compared with 5.23 mmol/L), and 2-h glucose (6.57 compared with 6.59 mmol/L) were found between GDM women who were selected and those not selected as the case group. We simultaneously and randomly recruited 578 non-GDM mother–child pairs from 71,681 non-GDM women who finished the GDM screening at the same period with age and sex frequency-matched to 578 children of GDM mothers. The clinical examination's procedure, items, and timing for non-GDM mother-child pairs were almost the same as the GDM mother-child pairs (Figure 1). In total, 1114 mother-child pairs (560 GDM and 554 non-GDM) with gene data available were included in the final analysis (Figure 1). This study was approved by the Human Subjects Committee of Tianjin Women's and Children's Health Center, and written informed consent from each participant was obtained. The study protocol was also approved by the Institutional Review Board of Pennington Biomedical Research Center.
FIGURE 1.
Flow chart. GDM, gestational diabetes mellitus; GWAS, genome-wide association studies.
Assessment of mother's weight changes and covariates
At the baseline survey, all mothers filled in the questionnaire. The questionnaire inquired about sociodemographic characteristics including age, education, family income, marital status, and occupation; pregnancy outcomes [gestational age, number of childbirth, prepregnancy weight, weight gain during pregnancy, self-reported hypertensive disorders of pregnancy, history of GDM (values of fasting and 2-h glucose in the 26–30 gestational weeks’ OGTT), and the treatment of GDM during the pregnancy]; and lifestyle in the past year (smoking habits and physical activity).
Using the standardized protocol, GDM and non-GDM mothers’ height at baseline was measured without shoes by specially trained research doctors. All mothers’ prepregnancy BMIs were calculated by their self-reported prepregnancy weight and measured height. Gestational weight gain was categorized as inadequate, adequate, and excessive by 2009 Institute of Medicine guidelines (15). Adequate gestational weight gain was defined according to prepregnancy BMI as follows: 12.5–18 kg if prepregnancy BMI <18.5; 11.5–16 kg if prepregnancy BMI = 18.5–24.9; 7–11.5 kg if prepregnancy BMI = 25.0–29.9; and 5–9 kg if prepregnancy BMI ≥30. Gestational weight gain below or above the recommendation was defined as inadequate or excessive, respectively.
Assessment of children's indicators of obesity and covariates
Children's information was obtained by another questionnaire completed by their mothers, such as children's general information, including age, sex, birth date, birth length, birth weight, infant feeding patterns, and lactation duration; routine activities (physical activities and sleep duration); dietary habits and history of diseases.
Children's body weight and height were examined in the study visit. Body weight was measured with a beam balance scale to the nearest 0.1 kg, and height by a stadiometer to the nearest 0.1 cm. BMI was calculated the same as for mothers. In addition, we measured the waist circumference and body fat. Waist circumference was measured midway between the 10th rib and the top of the iliac crest to the nearest 0.1 cm. Body fat was measured by a body composition analyzer (InBody Co, Ltd) to the nearest 0.1%.
We used WHO growth standards to calculate children's weight-for-age z-scores and BMI-for-age z-scores, which are gender-independent classification systems, representing equivalent weight per BMI-for-age percentile based on the WHO standards (16). The indicators of childhood obesity, including weight-for-age z-score, BMI-for-age z-score, waist circumference, and body fat percentage, were our primary outcomes. We defined children's overweight and obesity according to the WHO age- and gender-specific growth standards (16): normal weight was defined as a BMI <85th percentile (z-score <1.035), overweight as a BMI between the 85th and 95th percentiles (1.035 ≤ z-score < 1.645), and obesity as a BMI >95th percentile (z-score ≥1.645). Children's overweight and obesity status were our secondary outcomes.
Genotyping
Genomic DNA was extracted from the buffy coat fraction of centrifuged blood using 4ºC, 3000rpm for 15 min,a QIAamp Blood Maxi Kit (Qiagen). The MTNR1B single nucleotide polymorphism (SNP) rs10830962 was genotyped by quantitative real-time TaqMan PCR (Applied Biosystems). The genotyping success rate was >98%. For quality control, 10% of the samples were genotyped and the concordance rate was >99%. The allele frequency of the SNPs was in Hardy–Weinberg equilibrium (P > 0.05).
Statistical analysis
We used chi-square test for categorical variables to compare proportions. General linear models were applied for continuous variables to compare means of characteristics by gestational weight gain categories (inadequate, adequate, and excessive). Indicators of offspring's childhood obesity associated with gestational weight gain by each additional copy of the MTNR1B rs10830962 G allele were estimated by using general linear models, and offspring's overweight and obesity status were examined by using multivariate logistic regression models. We included the following covariates in the multivariate models: 1) Model 1: children's age, sex, birth weight, maternal age at pregnancy, history of GDM, gestational age at delivery, maternal prepregnancy BMI. For weight-for-age z-score and BMI-for-age z-score, which were calculated based on sex- and age-specific standards, children's age and sex were excluded in the adjustment. 2) Model 2: Model 1 plus maternal lifestyle, socioeconomic and other related factors: smoking status (no, past, current), marital status, education (secondary school, senior high school, bachelor, master), family monthly income (<5000, 5000–8000, ≥8000 yuan), occupation of mother (farmer/worker, office worker, service professional worker, unemployed person, and other), hypertensive disorders of pregnancy, number of fetal childbirths, treatment of GDM (none, insulin, lifestyle control). 3) Model 3: Model 2 plus children's variables: feeding patterns (exclusive breast feeding, mixed breast and formula feeding, and exclusive formula feeding), lactation duration, outdoor physical activity time, sleeping time, vegetable intake frequency, fruit intake frequency, history of disease in recent 3 mo (no, yes).
The interaction between gestational weight gain and MTNR1B rs10830962 genotype was tested by the introduction of a product term for these variables in the model. We examined the multivariable-adjusted mean values of indicators of children's obesity according to gestational weight gain and the MTNR1B rs10830962 genotype by using general linear models. We also tested the multicolinearity between variables included in the multivariate models, and results showed that all the variance inflation factors were <2, indicating no issue of multicolinearity. In addition, we performed stratified analyses by GDM status to explore the modification effect of maternal GDM status on such associations. In sensitivity analysis, we further adjusted for children's BMI to assess whether the genetic associations with childhood waist circumference and body fat were independent of childhood BMI. The missing rates of covariates in this study were low, ranging from 0.1% to 1.4%. So our analyses were conducted using the complete data, consistent with our previous studies. P values were 2-sided and P < 0.05 was considered statistically significant. Statistical analyses were performed in SAS version 9.4 (SAS Institute).
Results
Baseline characteristics about mother–child pairs
The characteristics of mother–child pairs in the Tianjin Study according to maternal gestational weight gain are presented in Table 1. Compared with women with inadequate gestational weight gain, women with excessive gestational weight gain were younger, and had a higher prepregnancy BMI and lower education level after adjusting for age (all P < 0.05). Compared with children born to women who had adequate gestational weight gain, children born to women who had excessive gestational weight gain exhibited higher birth weight and higher body weight, height, BMI, body fat, waist circumference, BMI-for-age z-scores, and weight-for-age z-scores, after adjustment for children's age; whereas children born to women with inadequate gestational weight gain showed lower values in these measures (all P < 0.0001). The frequency of MTNR1B rs10830962 genotype in the children was not different among the 3 categories of maternal gestational weight gain. No other differences in characteristics across the categories of maternal gestational weight gain were observed.
TABLE 1.
Characteristics of mother–child pairs in Tianjin Study1
| Gestational weight gain | ||||
|---|---|---|---|---|
| Inadequate (n = 153) | Adequate (n = 370) | Excessive (n = 591) | P value2 | |
| Maternal characteristics | ||||
| Maternal age at pregnancy, y | 30.6 ± 3.4 | 30.4 ± 3.2 | 29.8 ± 3.3 | 0.002 |
| Gestational age at delivery, wk | 39.1 ± 1.4 | 39.1 ± 1.4 | 39.1 ± 1.5 | 0.75 |
| Prepregnancy BMI, kg/m2 | 21.2 ± 2.2 | 21.6 ± 2.5 | 22.7 ± 3.5 | <0.001 |
| Current smokers, n (%) | 5 (3.3) | 8 (2.2) | 18 (3.0) | 0.86 |
| Treatment of GDM | 0.055 | |||
| None | 86 (56.2) | 193 (52.2) | 348 (58.9) | |
| Insulin | 1 (0.7) | 9 (2.4) | 4 (0.7) | |
| Lifestyle control | 66 (43.1) | 168 (45.4) | 239 (40.4) | |
| Maternal education | 0.004 | |||
| Secondary school | 1 (0.7) | 7 (1.9) | 6 (1.0) | |
| Senior high school | 18 (11.8) | 36 (9.7) | 103 (17.4) | |
| Bachelor | 117 (76.5) | 276 (74.6) | 433 (73.3) | |
| Master | 17 (11.1) | 51 (13.8) | 49 (8.3) | |
| Family income, yuan/mo | 0.09 | |||
| <5000 | 27 (17.8) | 41 (11.4) | 100 (17.1) | |
| 5000–7999 | 32 (21.1) | 99 (27.4) | 151 (25.8) | |
| ≥8000 | 93 (61.2) | 221 (61.2) | 334 (57.1) | |
| History of GDM, n (%) | 77 (50.3) | 194 (52.4) | 289 (48.9) | 0.57 |
| Married, n (%) | 151 (98.7) | 368 (99.5) | 585 (99.0) | 0.63 |
| Single pregnancy, n (%) | 153 (100.0) | 367 (99.2) | 578 (97.8) | 0.06 |
| Hypertensive disorders of pregnancy, n (%) | 3 (2.0) | 11 (3.0) | 32 (5.4) | 0.06 |
| Child characteristics | ||||
| Age, y | 5.9 ± 1.3 | 5.8 ± 1.2 | 5.9 ± 1.2 | 0.71 |
| Female, n (%) | 70 (45.8) | 182 (49.2) | 278 (47.0) | 0.72 |
| Birth weight, kg | 3.3 ± 0.4 | 3.4 ± 0.4 | 3.6 ± 0.5 | <0.001 |
| Feeding patterns, n (%) | 0.92 | |||
| Exclusive breast feeding | 65 (42.5) | 165 (44.6) | 251 (42.5) | |
| Mixed breast and formula feeding | 65 (42.5) | 156 (42.2) | 262 (44.4) | |
| Exclusive formula feeding | 23 (15.0) | 49 (13.2) | 77 (13.1) | |
| Lactation duration, mo | 4.1 ± 3.6 | 4.6 ± 3.6 | 4.4 ± 5.3 | 0.80 |
| Physical activity, h/d | 2.0 ± 0.8 | 2.2 ± 0.9 | 2.1 ± 0.9 | 0.42 |
| Vegetable intake frequency, n (%) | 0.38 | |||
| ≤1 time/d | 15 (9.8) | 24 (6.5) | 51 (8.6) | |
| 2 times/d | 131 (85.6) | 333 (90.0) | 508 (86.0) | |
| ≥3 times/d | 7 (4.6) | 13 (3.5) | 32 (5.4) | |
| Fruit intake frequency, n (%) | 0.83 | |||
| <1 time/d | 4 (2.6) | 13 (3.5) | 21 (3.6) | |
| 1 time/d | 49 (32.0) | 133 (35.9) | 214 (36.2) | |
| >3 times/d | 100 (65.4) | 224 (60.5) | 356 (60.2) | |
| Sleeping time, h (%) | 3.6 (1.0) | 3.6 (0.9) | 3.6 (1.0) | 0.76 |
| History of disease in recent 3 mo, n (%) | 61 (39.9) | 144 (38.9) | 209 (35.4) | 0.41 |
| Weight, kg | 20.9 ± 5.0 | 22.0 ± 6.4 | 23.5 ± 6.8 | <0.001 |
| Height, cm | 117.0 ± 9.0 | 117.5 ± 9.7 | 119.0 ± 9.6 | <0.001 |
| BMI, kg/m2 | 15.1 ± 1.9 | 15.7 ± 2.3 | 16.3 ± 2.6 | <0.001 |
| Body fat, % | 17.2 ± 6.3 | 19.3 ± 7.3 | 21.1 ± 8.2 | <0.001 |
| Waist circumference, cm | 53.7 ± 5.0 | 54.9 ± 6.0 | 56.4 ± 7.0 | <0.001 |
| BMI-for-age z-score | −0.3 ± 1.2 | 0.01 ± 1.2 | 0.4 ± 1.4 | <0.001 |
| Weight-for-age z-score | 0.07 ± 1.1 | 0.4 ± 1.1 | 0.8 ± 1.3 | <0.001 |
| Risk allele frequency (GG), % | 31.4 | 30.5 | 35.0 | 0.34 |
1Data are mean ± SD or n (%) as appropriate. GDM, gestational diabetes mellitus.
2 P values were calculated by chi-square test for categorical variables and general linear models for continuous variables after adjusting for children's age (except age, birth weight, and lactation duration) for children's characteristics, or maternal age (except maternal age) for maternal characteristics.
Associations of maternal MTNR1B genotype with offspring childhood obesity-related outcomes
We examined the association of maternal gestational weight gain with indicators of offspring's childhood obesity according to the MTNR1B genotype using 3 models (Table 2). We found that maternal gestational weight gain significantly interacted with the maternal MTNR1B genotype on indicators of offspring's obesity (all P-interaction <0 0.05). After full adjustment (Model 3), childhood weight-for-age z-scores associated with 1-kg maternal gestational weight gain were 0.004 (SE 0.016), 0.021 (SE 0.009), and 0.063 (SE 0.009) in mothers with the CC, CG, and GG genotypes, respectively (P-interaction = 0.001). Each 1-kg maternal gestational weight gain was associated with childhood BMI-for-age z-scores of 0.009 (SE 0.018), 0.026 (SE 0.010), and 0.061 (SE 0.010) across the CC, CG, and GG genotypes, respectively (P-interaction = 0.012). Other indicators of waist circumference (centimeters) and body fat percentage were 0.017 (0.077), 0.106 (SE 0.044), and 0.263 (SE 0.047), and 0.064 (SE 0.117), 0.134 (SE 0.060), and 0.373 (SE 0.059) across rs10830962 genotypes CC, CG, and GG, respectively (P-interaction = 0.003 and 0.012, respectively). In addition, we found significant interactions between the maternal MTNR1B genotype and gestational weight gain on childhood overweight (P-interaction = 0.005), and obesity (P-interaction = 0.026) (Supplementary Figure 1). After further adjustment for children's BMI, childhood waist circumference associated with each1-kg maternal gestational weight gain were −0.02 (SE 0.04), 0.02 (SE 0.02), and 0.05 (SE 0.03) in mothers with the CC, CG and GG genotypes, respectively (P-interaction = 0.016). The corresponding body fat associated with each 1-kg maternal gestational weight gain were 0.009 (SE 0.065), 0.021 (SE 0.029), and 0.113 (SE 0.035) (P-interaction = 0.157) (data not reported in the table).
TABLE 2.
Indicators of offspring's childhood obesity with gestational weight gain by MTNR1B rs10830962 genotype1
| CC (n = 203) | CG (n = 543) | GG (n = 368) | |||||
|---|---|---|---|---|---|---|---|
| β (SE) | P | β (SE) | P | β (SE) | P | P for interaction | |
| Model 12 | |||||||
| Weight-for-age z-score | −0.002 (0.015) | 0.874 | 0.026 (0.009) | 0.003 | 0.053 (0.009) | <0.0001 | 0.006 |
| BMI-for-age z-score | −0.0009 (0.016) | 0.956 | 0.029 (0.009) | 0.003 | 0.055 (0.009) | <0.0001 | 0.018 |
| Waist circumference, cm | −0.026 (0.068) | 0.704 | 0.125 (0.041) | 0.003 | 0.237 (0.043) | <0.0001 | 0.005 |
| Body fat percentage | 0.003 (0.104) | 0.973 | 0.153 (0.055) | 0.006 | 0.312 (0.055) | <0.0001 | 0.022 |
| Model 23 | |||||||
| Weight-for-age z-score | 0.001 (0.015) | 0.940 | 0.024 (0.009) | 0.009 | 0.059 (0.009) | <0.0001 | 0.004 |
| BMI-for-age z-score | 0.004 (0.016) | 0.801 | 0.027 (0.010) | 0.006 | 0.061 (0.010) | <0.0001 | 0.017 |
| Waist circumference, cm | −0.004 (0.072) | 0.952 | 0.108 (0.043) | 0.013 | 0.259 (0.044) | <0.0001 | 0.005 |
| Body fat percentage | 0.039 (0.108) | 0.719 | 0.140 (0.058) | 0.016 | 0.355 (0.016) | <0.0001 | 0.021 |
| Model 34 | |||||||
| Weight-for-age z-score | 0.004 (0.016) | 0.828 | 0.021 (0.009) | 0.023 | 0.063 (0.009) | <0.0001 | 0.001 |
| BMI-for-age z-score | 0.009 (0.018) | 0.601 | 0.026 (0.010) | 0.011 | 0.061 (0.010) | <0.0001 | 0.012 |
| Waist circumference, cm | 0.017 (0.077) | 0.825 | 0.106 (0.044) | 0.018 | 0.263 (0.047) | <0.0001 | 0.003 |
| Body fat percentage | 0.064 (0.117) | 0.583 | 0.134 (0.060) | 0.025 | 0.373 (0.059) | <0.0001 | 0.012 |
1The β coefficient (SE)represents children's traits in obesity trait per 1-kg increment of gestational weight gain. C, cytosine; G, guanine; GDM, gestational diabetes mellitus; MTNR1B, melatonin receptor 1B; SE standard error.
Model 1: adjusted for children's age, sex, birth weight, maternal age at pregnancy, history of GDM, gestational age at delivery, maternal prepregnancy BMI. For weight-for-age z-score and BMI-for-age z-score, which were calculated based on sex- and age-specific standards, children's age and sex were excluded in the adjustment.
Model 2: Model 1 + maternal lifestyle, socioeconomic and other related factors: smoking status (no, past, current), marital status, education (secondary school, senior high school, bachelor, master), family monthly income (<5000, 5000–8000, ≥8000 yuan), occupation of mother (farmer/worker, office worker, service professional worker, unemployed person, and other), hypertensive disorders of pregnancy, number of childbirth, treatment of GDM (none, insulin, lifestyle control).
Model 3: Model 2 + children's variables: feeding patterns (exclusive breast feeding, mixed breast and formula feeding, and exclusive formula feeding), lactation duration, outdoor physical activity time, sleeping time, vegetable intake frequency, fruit intake frequency, history of disease in recent 3 mo (no, yes).
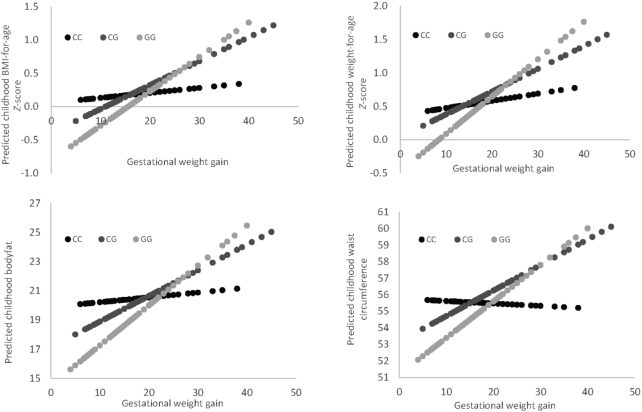
Figure 2 shows the predicted indicators of offspring's childhood obesity with maternal gestational weight gain (per 1 kg) by the MTNR1B genotype. Maternal gestational weight gain showed positive associations with all indicators of offspring's childhood obesity; and the associations were stronger among women carrying the G allele than those without the allele. Maternal gestational weight gain was associated with greater childhood BMI-for-age z-scores in women with the MTNR1B rs10830962 GG genotype (β = 0.061; P < 0.0001) than in women with the CC or CG genotype (β = 0.009, 0.026, respectively; P = 0.601, 0.011, respectively). Maternal gestational weight gain was also associated with greater childhood weight-for-age z-score, body fat, and waist circumference in women carrying the GG genotype.
FIGURE 2.
Predicted indicators of offspring's childhood obesity, according to gestational weight gain (per 1 kg) by the MTNR1B rs10830962 genotype. The slope represents the β coefficient. The β coefficients were: 0.009 (SE 0.018), 0.026 (SE 0.010), and 0.061 (SE 0.010) for childhood BMI-for-age z-score; 0.004 (SE 0.016), 0.021 (SE 0.009), and 0.063 (SE 0.009) for weight-for-age z-score; 0.017 (0.077), 0.106 (0.044), and 0.263 (0.047) for waist circumference (cm); and 0.064 (SE 0.117), 0.134 (SE 0.060), and 0.373 (SE 0.059) for body fat percentage, respectively (P-interaction = 0.012, 0.001, 0.003, and 0.012, respectively). Maternal gestational weight gain was significantly associated with greater childhood BMI-for-age z-scores,weight-for-age z-score, body fat, and waist circumference in women carrying the GG genotype (all P < 0.0001).C, cytosine; G, guanine; SE, standard error.
Stratified analysis by maternal GDM status
In the stratified analysis by maternal GDM status, we observed that the interactions between maternal gestational weight gain and the maternal MTNR1B genotype on indicators of offspring's obesity were consistently significant (all P-interaction < 0.05), and the interaction patterns appeared to be similar to those observed in the whole population (Table 3).
TABLE 3.
Association of offspring's childhood obesity-related quantitative traits with gestational weight gain by MTNR1B rs10830962 genotype–stratified maternal GDM status1
| CC (n = 203) | CG (n = 543) | GG (n = 368) | |||||
|---|---|---|---|---|---|---|---|
| β (SE) | P | β (SE) | P | β (SE) | P | P for interaction2 | |
| Non-GDM mothers (n = 554) | |||||||
| Weight-for-age z-score | 0.008 (0.023) | 0.712 | 0.027 (0.013) | 0.032 | 0.061 (0.012) | <0.0001 | 0.022 |
| BMI-for-age z-score | −0.004 (0.026) | 0.885 | 0.032 (0.014) | 0.023 | 0.057 (0.013) | <0.0001 | 0.044 |
| Waist circumference, cm | −0.038 (0.100) | 0.708 | 0.082 (0.060) | 0.176 | 0.221 (0.055) | <0.0001 | 0.034 |
| Body fat percentage | 0.070 (0.179) | 0.697 | 0.165 (0.081) | 0.043 | 0.303 (0.075) | <0.0001 | 0.015 |
| GDM mothers (n = 560) | |||||||
| Weight-for-age z-score | −0.003 (0.026) | 0.908 | 0.004 (0.015) | 0.782 | 0.066 (0.018) | 0.0003 | 0.003 |
| BMI-for-age z-score | 0.012 (0.027) | 0.669 | 0.009 (0.016) | 0.554 | 0.067 (0.019) | 0.0005 | 0.026 |
| Waist circumference, cm | 0.049 (0.120) | 0.682 | 0.073 (0.068) | 0.279 | 0.351 (0.095) | 0.0003 | 0.004 |
| Body fat percentage | 0.142 (0.172) | 0.412 | 0.078 (0.094) | 0.408 | 0.491 (0.109) | <0.0001 | 0.045 |
1The β coefficient represents children's traits in obesity trait per 1-kg increment of gestational weight gain. C, cytosine; G, guanine; GDM, gestational diabetes mellitus; MTNR1B, melatonin receptor 1B.
Adjusting for children's age, sex, birth weight, maternal age at pregnancy, history of GDM, gestational age at delivery, maternal prepregnancy BMI, smoking status (no, past, current), marital status, education (secondary school, senior high school, bachelor, master), family monthly income (<5000, 5000–8000, ≥8000 yuan), occupation of mother (farmer/worker, office worker, service professional worker, unemployed person, and other), hypertensive disorders of pregnancy, number of childbirth, treatment of GDM (none insulin, lifestyle control), feeding patterns (exclusive breast feeding, mixed breast and formula feeding, and exclusive formula feeding), lactation duration, outdoor physical activity time, sleeping time, vegetable intake frequency, fruit intake frequency, history of disease in recent 3 mo (no, yes). For weight-for-age z-score and BMI-for-age z-score, which were calculated based on sex- and age-specific standards, children's age and sex were excluded in the adjustment. The interactions between the maternal MTNR1B genotype, GDM status, and gestational weight gain on childhood weight-for-age z-score, BMI-for-age z-score, waist circumference, and body fat percentage were 0.572, 0.912, 0.595, and 0.817, respectively.
Discussion
In this study of 1114 mother-child pairs, we found significant interactions between the maternal MTNR1B genotype and gestational weight gain on offspring's childhood obesity. We found more pronounced, positive relations between maternal gestational weight gain and indicators of obesity among children whose mothers were carrying the more GDM-predisposing G allele at the MTNR1B locus. The associations remained consistently significant in women with and without GDM. We also noted that the major childhood obesity measures such as BMI-for-age z-score associated with gestational weight gain showed up to a ∼7-fold difference across the maternal MTNR1B genotype (7-fold difference is derived by comparing 0.009 and 0.061).
Compelling evidence supports the vicious intergenerational cycle of metabolic disorders between mothers and their offspring (17, 18). Numerous studies indicate that maternal gestational weight gain can affect the offspring's obesity especially during childhood (19–21). Previous studies have consistently shown that women with GDM are affected by excess maternal gestational weight gain (6, 7). In our earlier analysis, we found that greater maternal weight gain during pregnancy was related to the offspring's enhanced indicators of childhood obesity including BMI, waist circumference, and body fat, consistent with the findings from other studies (20, 22, 23).
The variant in the MTNR1B gene, rs10830962, was found to be associated with the risk of GDM in a recent genome-wide association study (8). Several studies reported that the MTNR1B genotype was also related to body weight regulation among women (24), and associated with BMI and obesity (25, 26). In the present study, we found that the associations between indicators of offspring's childhood obesity and maternal gestational weight gain were significantly modified by the maternal MTNR1B genotype. The mechanisms underlying such modification effects remain unclear. Mothers carrying the GDM-increasing MTNR1B G allele were found to have elevated fasting glucose concentrations (8, 27), which might lead to greater gestational weight gain. Therefore, we assumed that the maternal MTNR1B variant might act by modifying metabolic status, such as glucose concentrations, in ways that might modulate the effects of gestational weight gain on the intrauterine environment, and subsequently affect the offspring's metabolic traits such as body weight (28). Women with the MTNR1B G allele have a higher risk of hyperglycemia, and increased glucose transfer to the placenta could lead to higher fetal insulin secretion and offspring obesity (29–31). In addition, it was found that the MTNR1B glucose-raising allele was associated with higher offspring birth weight (28), which is also associated with an increased risk of obesity during childhood (32, 33). The MTNR1B gene encodes the melatonin MT2 receptor, and it is well known that maternal melatonin crosses the placenta and affects fetal growth and maturation, so it is likely that the modified maternal melatonin secretion pattern could affect the fetus by prolonging the immediate effects or altering the chronobiotic effects of melatonin (34).
We also found that maternal MTNR1B genotype modified the relation of gestational weight gain with offspring's waist circumference and body fat, suggesting such interactions might also affect fat distribution. Limited studies have investigated the effects of gestational weight gain on offspring's fat distribution, such as waist circumference and body fat, and found that greater gestational weight gain was related to greater waist circumference and body fat (35, 36). In our previous study, we found that children born to mothers with a history of GDM had greater waist circumferences and body fat (14), and the MTNR1B genotype might influence total body fat composition (37). Waist circumference has been found to be a better predictor of cardiometabolic disease in children than BMI (38).
To the best of our knowledge, this is the first study to assess the interactions between the maternal MTNR1B genotype and gestational weight gain on childhood obesity. Our study used a large cohort of GDM mother–child pairs worldwide, which is the same as the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study (39). We directly measured indicators of childhood obesity including body weight, waist circumference, and body fat using standardized methods. Moreover, a variety of potential confounding factors were measured and controlled in the present study. However, there were several potential limitations. First, parity might be a potential confounder in the analyses (40, 41). Because of the 1-child policy in China from 1979 to 2015 (42), only 2.44% of the women included in our study had more than one parity history (43); therefore, parity bias less likely affected our results (44). Second, our study participants were Chinese mother–child pairs, which might limit the generalizability of our findings to other populations. Third, we used self-reported data—maternal prepregnancy weight and gestational weight gain, which might cause recall bias. However, validation studies have found that mothers’ self-reported information during pregnancy is highly consistent with clinical records (45). Fourth, we did not analyze children's genotype, which might partly explain the observed associations due to the potential correlations with mothers’ genotypes. Future studies would consider children's genotypes in the analyses.
In conclusion, we found that the maternal GDM-predisposing variant at the MTNR1B locus significantly interacted with gestational weight gain on offspring's childhood obesity, and children whose mothers carry the MTNR1B G genotype had a higher risk of childhood obesity than those with other genotypes. Our data indicate that maternal genetic variations can modify the relations between prenatal risk factors and offspring's risk of obesity; these findings highlight the importance of gestational weight management particularly in mothers with high genetic risk of GDM.
Supplementary Material
ACKNOWLEDGEMENTS
We thank all the participants in the study for their dedication and contribution to the research.
The authors’ contributions were as follows—ZL: contributed to the study concept and design; data acquisition, analysis, and interpretation; statistical analysis; drafting and revising the manuscript; YC, TZ, YH: contributed to analysis, interpretation, and critical revision of the manuscript; HL, LW, WL, JL, JW, RG, GH: contributed to data acquisition and the critical revision of the manuscript for important intellectual content; GH, LQ: were involved in the collection and assembly of data and obtained funding for the study; LQ: contributed to the study concept and design, acquisition of data, analysis, and interpretation of data, drafting and revising the manuscript, statistical analysis, and funding and study supervision; and all authors: read and approved the final manuscript.
Author disclosures: The authors report no conflicts of interest.
Notes
This study was supported by grants from the European Foundation for the Study of Diabetes (EFSD)/Chinese Diabetes Society (CDS)/Lilly programme for Collaborative Research between China and Europe, and the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK100790). This project also used core facilities supported by the NORC Center Grant P30 DK072476, and the COBRE Center Grant P30 GM118430. LQ was supported by grants from the National Heart, Lung, and Blood Institute (HL071981, HL034594, HL126024), the National Institute of Diabetes and Digestive and Kidney Diseases (DK115679, DK091718, DK100383, DK078616), and the Fogarty International Center (TW010790). GH was partly supported by a grant from the National Institute of General Medical Sciences (U54GM104940). ZL was supported by a grant from the National Natural Science Foundation of China (81974234, 81701465).
Data described in the manuscript, code book, and analytic code will not be made available because the project is in cooperation with other institutions and we do not have the authority to make data public. LQ is the guarantor and has full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Supplemental Figure 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: GDM, gestational diabetes mellitus; MTNR1B, melatonin receptor 1B; OGTT, oral-glucose-tolerance test; SNP, single nucleotide polymorphism.
References
- 1. Weihrauch-Blüher S, Schwarz P, Klusmann JH. Childhood obesity: increased risk for cardiometabolic disease and cancer in adulthood. Metabolism. 2019;92:147–52. [DOI] [PubMed] [Google Scholar]
- 2. Haidar YM, Cosman BC. Obesity epidemiology. Clin Colon Rectal Surg. 2011;24(4):205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Twig G, Yaniv G, Levine H, Leiba A, Goldberger N, Derazne E, Ben-Ami Shor D, Tzur D, Afek A, Shamiss A et al.. Body-mass index in 2.3 million adolescents and cardiovascular death in adulthood. N Engl J Med. 2016;374(25):2430–40. [DOI] [PubMed] [Google Scholar]
- 4. Dabelea D, Pettitt DJ.. Intrauterine diabetic environment confers risks for type 2 diabetes mellitus and obesity in the offspring, in addition to genetic susceptibility. J Pediatr Endocrinol Metab. 2001;14(8):1085–91. [DOI] [PubMed] [Google Scholar]
- 5. Viswanathan M, Siega-Riz AM, Moos MK, Deierlein A, Mumford S, Knaack J, Thieda P, Lux LJ, Lohr KN. Outcomes of maternal weight gain. Evid Rep Technol Assess (Full Rep). 2008;(168):1–223. [PMC free article] [PubMed] [Google Scholar]
- 6. Egan AM, Dennedy MC, Al-Ramli W, Heerey A, Avalos G, Dunne F. ATLANTIC-DIP: excessive gestational weight gain and pregnancy outcomes in women with gestational or pregestational diabetes mellitus. J Clin Endocrinol Metab. 2014;99(1):212–9. [DOI] [PubMed] [Google Scholar]
- 7. Black MH, Sacks DA, Xiang AH, Lawrence JM. The relative contribution of prepregnancy overweight and obesity, gestational weight gain, and IADPSG-defined gestational diabetes mellitus to fetal overgrowth. Diabetes Care. 2013;36(1):56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kwak SH, Kim SH, Cho YM, Go MJ, Cho YS, Choi SH, Moon MK, Jung HS, Shin HD, Kang HM et al.. A genome-wide association study of gestational diabetes mellitus in Korean women. Diabetes. 2012;61(2):531–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nisa H, Qi KHT, Leng J, Zhou T, Liu H, Li W, Wang L, Li N, Hu G, Qi L. The circadian rhythm-related MTNR1B genotype, gestational weight gain, and postpartum glycemic changes. J Clin Endocrinol Metab. 2018;103(6):2284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang S, Liu H, Zhang C, Wang L, Li N, Leng J, Li Y, Liu G, Fan X, Yu Z et al.. Maternal glucose during pregnancy and after delivery in women with gestational diabetes mellitus on overweight status of their children. Biomed Res Int. 2015;2015:543038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hu G, Tian H, Zhang F, Liu H, Zhang C, Zhang S, Wang L, Liu G, Yu Z, Yang X et al.. Tianjin Gestational Diabetes Mellitus Prevention Program. Study design, methods, and 1-year interim report on the feasibility of lifestyle intervention program. Diabetes Res Clin Pract. 2012;98(3):508–17. [DOI] [PubMed] [Google Scholar]
- 12. World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Geneva: WHO; 1999. [Google Scholar]
- 13. Li W, Zhang S, Liu H, Wang L, Zhang C, Leng J, Yu Z, Yang X, Tian H, Hu G. Different associations of diabetes with β-cell dysfunction and insulin resistance among obese and nonobese Chinese women with prior gestational diabetes mellitus. Diabetes Care. 2014;37(9):2533–9. [DOI] [PubMed] [Google Scholar]
- 14. Wang J, Wang L, Liu H, Zhang S, Leng J, Li W, Zhang T, Li N, Li W, Baccarelli A et al.. Maternal gestational diabetes and different indicators of childhood obesity: a large study. Endocr Connect. 2018;7(12):1464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines; Rasmussen KM, Yaktine AL, editors. Weight gain during pregnancy: reexamining the guidelines. Washington (DC): National Academies Press (US); 2009. [PubMed] [Google Scholar]
- 16. World Health Organization. The WHO child growth standards [Internet]. WHO; 2006. Available from: http://www.who.int/childgrowth/standards/en/. [Google Scholar]
- 17. Tam WH, Ma RCW, Ozaki R, Li AM, Chan MHM, Yuen LY, Lao TTH, Yang X, Ho CS, Tutino GE et al.. In utero exposure to maternal hyperglycemia increases childhood cardiometabolic risk in offspring. Diabetes Care. 2017;40(5):679–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dabelea D. The predisposition to obesity and diabetes in offspring of diabetic mothers. Diabetes Care. 2007;30(Suppl 2):S169–74. [DOI] [PubMed] [Google Scholar]
- 19. Guo L, Liu J, Ye R, Liu J, Zhuang Z, Ren A. Gestational weight gain and overweight in children aged 3–6 years. J Epidemiol. 2015;25(8):536–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lau EY, Liu J, Archer E, McDonald SM, Liu J. Maternal weight gain in pregnancy and risk of obesity among offspring: a systematic review. J Obes. 2014;2014:524939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Von Kries R, Ensenauer R, Beyerlein A, Amann-Gassner U, Hauner H, Rosario AS. Gestational weight gain and overweight in children: results from the cross-sectional German KiGGS study. Int J Pediatr Obes. 2011;6(1):45–52. [DOI] [PubMed] [Google Scholar]
- 22. Mamun AA, Mannan M, Doi SA. Gestational weight gain in relation to offspring obesity over the life course: a systematic review and bias-adjusted meta-analysis. Obes Rev. 2014;15(4):338–47. [DOI] [PubMed] [Google Scholar]
- 23. Nehring I, Lehmann S, Von Kries R. Gestational weight gain in accordance to the IOM/NRC criteria and the risk for childhood overweight: a meta-analysis. Pediatr Obes. 2013;8(3):218–24. [DOI] [PubMed] [Google Scholar]
- 24. Goni L, Cuervo M, Milagro FI, Martínez JA. Gene-gene interplay and gene-diet interactions involving the MTNR1B rs10830963 variant with body weight loss. J Nutrigenet Nutrigenomics. 2014;7(4-6):232–42. [DOI] [PubMed] [Google Scholar]
- 25. Stancáková A, Kuulasmaa T, Paananen J, Jackson AU, Bonnycastle LL, Collins FS, Boehnke M, Kuusisto J, Laakso M. Association of 18 confirmed susceptibility loci for type 2 diabetes with indices of insulin release, proinsulin conversion, and insulin sensitivity in 5,327 nondiabetic Finnish men. Diabetes. 2009;58(9):2129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Andersson EA, Holst B, Sparsø T, Grarup N, Banasik K, Holmkvist J, Jørgensen T, Borch-Johnsen K, Egerod KL, Lauritzen T et al.. MTNR1B G24E variant associates with BMI and fasting plasma glucose in the general population in studies of 22,142 Europeans. Diabetes. 2010;59(6):1539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spégel P, Bugliani M, Saxena R, Fex M, Pulizzi N et al.. A common variant in the melatonin receptor gene (MTNR1B) is associated with increased risk of future type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41(1):82–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beaumont RN, Warrington NM, Cavadino A, Tyrrell J, Nodzenski M, Horikoshi M, Geller F, Myhre R, Richmond RC, Paternoster L et al.. Genome-wide association study of offspring birth weight in 86577 women identifies five novel loci and highlights maternal genetic effects that are independent of fetal genetics. Hum Mol Genet. 2018;27(4):742–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Philipps LH, Santhakumaran S, Gale C, Prior E, Logan KM, Hyde MJ, Modi N.. The diabetic pregnancy and offspring BMI in childhood: a systematic review and meta-analysis. Diabetologia. 2011;54(8):1957–66. [DOI] [PubMed] [Google Scholar]
- 30. Fraser A, Lawlor DA.. Long-term health outcomes in offspring born to women with diabetes in pregnancy. Curr Diab Rep. 2014;14(5):489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pedersen J. Diabetes and pregnancy; blood sugar of newborn infants during fasting and glucose administration. Nord Med. 1952;47(30):1049. [PubMed] [Google Scholar]
- 32. Ren J, Wu J, Ji M, Rong F, Li Y, Gao E, Ji H. The effect of high birth weight on overweight and obesity in childhood and adolescence. A cohort study in China. Saudi Med J. 2013;34(6):623–31. [PubMed] [Google Scholar]
- 33. Qiao Y, Ma J, Wang Y, Katzmarzyk PT, Chaput JP, Fogelholm M, Johnson WD, Kuriyan R, Kurpad A, Lambert EV. Birth weight and childhood obesity: a 12-country study. Int J Obes Suppl. 2015;5(Suppl 2):S74–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Karamitri A, Jockers R. Melatonin in type 2 diabetes mellitus and obesity. Nat Rev Endocrinol. 2019;15(2):105–25. [DOI] [PubMed] [Google Scholar]
- 35. Fraser A, Tilling K, Macdonald-Wallis C, Sattar N, Brion MJ, Benfield L, Ness A, Deanfield J, Hingorani A, Nelson SM et al.. Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation. 2010;121(23):2557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reynolds RM, Osmond C, Phillips DI, Godfrey KM. Maternal BMI, parity, and pregnancy weight gain: influences on offspring adiposity in young adulthood. J Clin Endocrinol Metab. 2010;95(12):5365–9. [DOI] [PubMed] [Google Scholar]
- 37. Goni L, Sun D, Heianza Y, Wang T, Huang T, Martínez JA, Shang X, Bray GA, Smith SR, Sacks FM et al.. A circadian rhythm-related MTNR1B genetic variant modulates the effect of weight-loss diets on changes in adiposity and body composition: the POUNDS Lost trial. Eur J Nutr. 2019;58(4):1381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Savva SC, Tornaritis M, Savva ME, Kourides Y, Panagi A, Silikiotou N, Georgiou C, Kafatos A. Waist circumference and waist-to-height ratio are better predictors of cardiovascular disease risk factors in children than body mass index. Int J Obes Relat Metab Disord. 2000;24(11):1453–8. [DOI] [PubMed] [Google Scholar]
- 39. Lowe WL Jr, Scholtens DM, Lowe LP, Kuang A, Nodzenski M, Talbot O, Catalano PM, Linder B, Brickman WJ, Clayton P et al.. Association of gestational diabetes with maternal disorders of glucose metabolism and childhood adiposity. JAMA. 2018;320(10):1005–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stamnes Køpp UM, Dahl-Jørgensen K, Stigum H, Frost Andersen L, Næss Ø, Nystad W. The associations between maternal pre-pregnancy body mass index or gestational weight change during pregnancy and body mass index of the child at 3 years of age. Int J Obes (Lond). 2012;36(10):1325–31. [DOI] [PubMed] [Google Scholar]
- 41. Ehrenthal DB, Maiden K, Rao A, West DW, Gidding SS, Bartoshesky L, Carterette B, Ross J, Strobino D. Independent relation of maternal prenatal factors to early childhood obesity in the offspring. Obstet Gynecol. 2013;121(1):115–21. [DOI] [PubMed] [Google Scholar]
- 42. Zeng Y, Hesketh T. The effects of China's universal two-child policy. Lancet. 2016;388(10054):1930–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang F, Dong L, Zhang CP, Li B, Wen J, Gao W, Sun S, Lv F, Tian H, Tuomilehto J et al.. Increasing prevalence of gestational diabetes mellitus in Chinese women from 1999 to 2008. Diabet Med. 2011;28(6):652–7. [DOI] [PubMed] [Google Scholar]
- 44. Shen Y, Wang P, Wang L, Zhang S, Liu H, Li W, Li N, Li W, Leng J, Wang J et al.. Gestational diabetes with diabetes and prediabetes risks: a large observational study. Eur J Endocrinol. 2018;179(1):51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dietz P, Bombard J, Mulready-Ward C, Gauthier J, Sackoff J, Brozicevic P, Gambatese M, Nyland-Funke M, England L, Harrison L et al.. Validation of self-reported maternal and infant health indicators in the Pregnancy Risk Assessment Monitoring System. Matern Child Health J. 2014;18(10):2489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.