ABSTRACT
Background
Results from observational studies regarding associations between fish (including shellfish) intake and cardiovascular disease risk factors, including blood pressure (BP) and BMI, are inconsistent.
Objective
To investigate associations of fish consumption and associated urinary metabolites with BP and BMI in free-living populations.
Methods
We used cross-sectional data from the International Study of Macro-/Micronutrients and Blood Pressure (INTERMAP), including 4680 men and women (40–59 y) from Japan, China, the United Kingdom, and United States. Dietary intakes were assessed by four 24-h dietary recalls and BP from 8 measurements. Urinary metabolites (2 timed 24-h urinary samples) associated with fish intake acquired from NMR spectroscopy were identified. Linear models were used to estimate BP and BMI differences across categories of intake and per 2 SD higher intake of fish and its biomarkers.
Results
No significant associations were observed between fish intake and BP. There was a direct association with fish intake and BMI in the Japanese population sample (P trend = 0.03; fully adjusted model). In Japan, trimethylamine-N-oxide (TMAO) and taurine, respectively, demonstrated area under the receiver operating characteristic curve (AUC) values of 0.81 and 0.78 in discriminating high against low fish intake, whereas homarine (a metabolite found in shellfish muscle) demonstrated an AUC of 0.80 for high/nonshellfish intake. Direct associations were observed between urinary TMAO and BMI for all regions except Japan (P < 0.0001) and in Western populations between TMAO and BP (diastolic blood pressure: mean difference 1.28; 95% CI: 0.55, 2.02 mmHg; P = 0.0006, systolic blood pressure: mean difference 1.67; 95% CI: 0.60, 2.73 mmHg; P = 0.002).
Conclusions
Urinary TMAO showed a stronger association with fish intake in the Japanese compared with the Western population sample. Urinary TMAO was directly associated with BP in the Western but not the Japanese population sample. Associations between fish intake and its biomarkers and downstream associations with BP/BMI appear to be context specific. INTERMAP is registered at www.clinicaltrials.gov as NCT00005271.
Keywords: biomarkers, blood pressure, body mass index, fish, homarine, hypertension, INTERMAP metabolomics, metabonomics, seafood, shellfish
Introduction
Fish (including shellfish), represents an important source of protein for many populations; it is also a source of iodine, selenium, vitamin D, and long-chain omega-3 PUFAs (n–3 PUFAs) (1). Fish contributes to established cardioprotective dietary patterns such as the Mediterranean and Nordic diets (2, 3) and is encouraged by American Heart Association and other dietary guidelines (4, 5). The Lugalawa study was one of the first to report a beneficial association between fish intake and blood pressure (BP); reporting that the consumption of 300–600 g of freshwater fish per day resulted in a lower prevalence of hypertension compared with a vegetarian diet (6). However, previous findings from prospective studies are inconclusive regarding the benefit of fish intake in the management of BP. Meta-analyses of prospective studies found a direct effect of n–3 PUFAs on BP but no association with overall fish intake (7), whereas another reported that dietary fish intake, rather than n–3 PUFAs, had a consistent inverse association with cerebrovascular disease – an end point of hypertension (8). It has also been suggested that acute intake of lean fish may promote lipid catabolism via increased mitochondrial efficiency (9), but studies showing a benefit of fish intake on body weight are lacking (10, 11). Possible contributors to these inconsistencies include the variability of fish intake between and within populations in terms of regularity, amount, and the species consumed, thereby complicating the interpretation of results. Recent research has suggested that there may be differences in the associations between fish intake and cardiovascular disease (CVD) mortality across geographic regions, specifically Asian and Western regions (12). The lack of objective biomarkers of fish intake applied in observational studies remains a further limitation.
Metabolic profiling has proven a useful tool in advancing the identification of objective biomarkers of dietary intakes (13, 14). Identifying metabolic markers of fish intake has potential to ensure more robust measurements of intake and to elucidate mechanisms underpinning the relation between fish intake and cardiovascular health outcomes. Several studies have previously identified PUFAs, polychlorinated biphenyls, and methyl mercury as metabolic markers of fish consumption in serum (15, 16). Trimethylamine-N-oxide (TMAO), taurine, and 1-methylhistidine have been proposed as urinary biomarkers of fish intake (17–20). A few studies have reported an inverse relation between urinary taurine excretion and BP (21–23). Controversy remains regarding the association of high plasma TMAO with CVD; recent studies propose a detrimental impact on kidney function (24) but conversely, reduced type 2 diabetes risk (25). The overall aims of this investigation are to: 1) measure the associations of fish intake with CVD risk factors across Asian and Western population samples, and 2) explore associations between candidate metabolites as urinary biomarkers of fish intake in free-living individuals and their associations with BP and BMI.
Methods
Study population
The International Study of Macro-/Micronutrients and Blood Pressure (INTERMAP) from 1996 to 1999 surveyed 4680 men and women aged 40 to 59 y from Japan, the People's Republic of China (PRC), the UK, and USA (26). Participants were selected randomly from community or workplace population lists, arrayed into 4 age/sex strata. Each participant attended 4 clinic visits, 2 on consecutive days and 2 further visits on consecutive days on average 3 wk later (Supplemental Figure 1). Of the 4895 recruited into the study, participants were excluded if they failed to attend all 4 clinic visits (n = 110) or had incomplete/missing data or 24-h urine sample (n = 61), provided unreliable dietary data (n = 7), or had extreme total energy intake (>5000 kcal/d for women and >8000 kcal/d for men) from any 24-h recall (n = 37). As there are no agreed parameters to determine extremes of energy intake reporting, the upper limits of 5000 and 8000 kcal/d were selected through consensus agreement between country nutritionists. The final sample size was 4680 participants for inclusion in the study (Supplemental Figure 2). Institutional ethics committee approval was obtained for each site; all participants provided written informed consent. INTERMAP is registered at www.clinicaltrials.gov as NCT00005271.
Assessment of fish and dietary intakes
Dietary intakes were assessed via 4 24-h dietary recalls administered by staff trained and certified according to a standardized protocol following the triphasic method (27). In Japan, PRC, and the UK, standardized 24-h paper records were collected and coded using country-specific food tables. The US recall data were entered electronically via the Nutrition Data System, Nutrition coordinating Centre (Version 2.91, University of Minnesota) to generate nutrient intake data. These data were then standardized across all countries (26, 27). Alcohol intake during the previous week was estimated from 7-d diaries. To account for individual differences in reporting and total energy intake, energy adjusted dietary variables were calculated using the nutrient density method (quantity per 1000 kcal) (28).
We defined fish as all “marine animals that live in the sea and in freshwater lakes and rivers” (e.g., salmon, tuna, trout, and tilapia) including shellfish (e.g., shrimp, crab, and oysters) (4). Fish intake was estimated per person and included fish that formed part of a composite dish (e.g., fish pie). Fish intakes were then classified into the following groups (where data permitted): shellfish, oily/fatty, lean, fried, raw, and salted (Supplemental Table 1). Oily/fatty fish was categorized based on the US definition of providing 588 mg or more of combined EPA and DHA per 100 g of fish (4) where data were available. The reliability of total fish intake for individuals was estimated using the following formula: 1/[1 + (ratio/2)] × 100. The ratio is intraindividual variance divided by interindividual variance, estimated separately by country. The averages of the first 2 and second 2 visits were used to account for higher correlation between dietary intakes on consecutive days. The reliability estimate gives an indication of the effect of random error (day-to-day variability) on the associations with BP and BMI (29, 30).
Assessment of BP and BMI
BP was measured according to a standardized protocol by trained staff at each visit following ≥5 min rest with a random-zero sphygmomanometer (26). The mean of the 8 BP measurements was calculated for use in the analyses. At clinical visits, participants' height and weight were measured twice and BMI calculated as weight (kg)/height2 (m2).
Urine collection
Each participant provided 2 borate-preserved timed 24-h urine collections ∼3 wk apart; aliquots of urine were frozen on site (−20°C) and air-freighted frozen to the Central Laboratory (Leuven, Belgium) for biochemical analyses (26). Urinary potassium and sodium were measured using emission flame photometry and excretion values were calculated per participant as the product of concentrations in urine and urinary volumes corrected to 24 h. The mean of the 2 samples was calculated for use in the current study.
Proton NMR spectroscopic analysis of urine
Urine samples were thawed completely before mixing 500 µL of urine with 250 µL of phosphate buffer (0.2 M) for the stabilization of urinary pH at 7.4 (± 0.5), and 75 µL of sodium 3-trimethylsilyl-(2,2,3,3-d)-1-propionate (TSP) in a deuterium oxide (D2O; final concentration 0.1 mg/mL) solution for chemical shift referencing of TSP (δ 0.0). Proton NMR (1H NMR) spectra of the urine specimens were obtained at 300 K using a Bruker Avance 600 spectrometer (Bruker Biospin) (31). A standard one-dimensional pulse sequence (recycle delay - 90° - t1 - 90° - tm - 90° acquisition was used, with a relaxation delay of 2 s and mixing time of 100 ms. A total of 64 free induction decays were collected into 32 K data points using a spectral width of 20 ppm (32). Baseline correction, phasing, referencing to TSP were performed in MATLAB and spectra were reduced to 7100 variables by integrating the intensity in adjacent spectral bins using a segment width of δ 0.001, corresponding to the regions δ = 0.5–9.5. The region δ 4.5–6.4, containing the residual water and urea resonances were excluded from analyses (31). The urine samples were randomized for the analysis and 8% of samples were split at source and analyzed blindly as a quality check; analytical reproducibility was high (>98%) and published previously (33). The mean coefficients of variation for the paired split samples, excluding those falling below the signal-to-noise threshold (S/N ratio = 10), were estimated to be 6%, 11%, and 13%, respectively, for TMAO (N pair = 598), taurine (N pair = 520), and homarine (N pair = 225).
Identification of urinary metabolic biomarkers of fish intake
Analyses were performed over the whole 1H NMR spectrum, utilizing the combined samples across the 4 populations to uncover metabolites associated with fish consumption in our study. Out of the study population of N = 4680, due to missing samples (N = 50) and 575 samples considered spectral outliners based on the principal component analysis 95% Hotelling T2 statistic, 4055 urine samples were used for metabolite discovery analyses (31).
Univariate partial correlation analyses were performed separately on the 2 24-h urinary samples for each of the 7100 spectral variables against dietary fish and separately for shellfish intake recall data (in grams), adjusting for age, sex, and study center. Significantly correlated peak variables after Bonferroni correction (P threshold = 7×10−6) in both the first and second samples were annotated, where possible, with reference to in-house databases and open source databases, such as the Human Metabolome Database, and to the literature, two-dimensional heteronuclear single-quantum correlation, total correlation spectroscopy, and by spiking chemical standards in urine samples (Supplemental Table 2 and Supplemental Figures 3–7).
Assessment of covariates
Education, occupation, physical activity, smoking, medical history, family history of high BP, and current medication data were collected using interviewer-guided questionnaires. Participants were also asked if they were currently following a special diet (e.g., weight loss, health care professional advised diet).
Statistical analyses
All statistical analyses were undertaken using SAS version 9.4 (SAS Institute Inc.) and R programming software packages version 3.5. Baseline characteristics were reported by country (mean and SD). Associations across categorical variables were analyzed using the chi-squared (χ²) test. Associations between total fish intake and BP were initially analyzed stratified by country. Fish intake in the PRC, UK, and USA was highly skewed with a high frequency of nonconsumers. Therefore, individuals were grouped based on mean intake and classified as: nonconsumers (no intake of fish recorded at any recall), low consumers (below the median intake for consumers by country: PRC: 14.6 g/1000 kcal; UK: 12.0 g/1000 kcal; USA: 11.8 g/1000 kcal), and high consumers (at or above the median intake by country). Due to comparable median intakes for the USA and UK, these population samples were pooled and the combined median of 11.9 g/1000 kcal was used as the cut point to categorize low and high total fish consumers. For the PRC, the population sample was categorized as nonconsumers, consumers below 14.6 g/1000 kcal (low consumers), and equal to or above 14.6 g/1000 kcal (high consumers). As fish intake in Japan followed an approximate normal distribution, participants were classified into tertiles of fish intake (tertile 1: 0–35.11 g/1000 kcal; tertile 2: >35.11–53.60 g/1000 kcal; tertile 3: >53.60–174.07 g/1000 kcal). Characteristics across country-specific intake groups were compared using general linear models adjusted for gender, age, and study center. Post hoc Bonferroni correction was applied to correct for multiple comparisons to determine significant differences between the 3 groups (P < 0.017). We then used multivariable linear regression to examine associations between fish consumption with systolic BP (SBP), diastolic BP (DBP), and BMI by country (PRC, Japan) and region (Western: USA and UK combined; coefficients were pooled, weighted by inverse of their variance), and total fish and shellfish intakes were analyzed with respect to differences corresponding to 2 SD of intake.
To test for associations between urinary metabolites identified as putative biomarkers of fish intake, the data were split into 2 groups by study visit (Supplementary Figure 1) to align with each 24-h timed urine collection (i.e., the first urine collection, aligned to mean dietary intakes from clinic visits 1 and 2 and the second urine collection, aligned to mean dietary intakes from visits 3 and 4). Using this methodological approach we have previously shown reproducible patterns of metabolite excretion (34).
Partial Pearson correlation coefficients were calculated (adjusted for age, sex, and study center) by country. Receiver operating characteristic (ROC curves) analyses were performed using the “pROC” package in R (35). Associations between metabolites and SBP, DBP, and BMI were tested by linear regression analyses using the mean metabolite concentration from the 2 visits. Logarithmic transformations were applied to urinary metabolites to approximate for normality across analyses.
Three sequential models were constructed to test associations between fish intake and metabolites with BP and BMI: Model 1 adjusted for: age (years, continuous), sex, study center, education (years), physical activity (h/d), alcohol (g/d), smoking (yes/no), vitamin supplement usage (yes/no), antihypertensive medication (yes/no), family history of hypertension (yes/no), diagnosed CVD (yes/no); Model 2: as Model 1 with additional adjustment for the following continuous dietary variables: energy intake (kcal), calcium (mg/1000 kcal), magnesium (mg/1000 kcal), SFAs (% energy intake), plant protein (% energy intake), fiber (g/1000 kcal), sodium (urinary), and potassium (urinary). As obesity may lie on the causal pathway between diet and BP, BMI (continuous variable) was added to Model 2 separately (Model 3).
Sensitivity analyses were also performed, including analyses of fish intake of Japanese participants only to explore potential differential associations between categories of fish (shellfish, oily, lean, raw, and salted fish) intake with BP and BMI; stratified analyses by sex and category of BMI (<25, >25) and by country. Analyses were additionally adjusted for antihypertensive medication usage by adding 15 mmHg and 10 mmHg to SBP and DBP, respectively (36).
Results
Descriptive statistics
Across the 4 countries the prevalence of fish consumers was highest in Japan with 99% of participants reporting fish or shellfish consumption at 1 or more of the study visits. The PRC had the lowest frequency of fish consumption with 26% reporting fish or shellfish consumption at any study visit (Table 1). Concordantly, Japan had the highest intake of fish (mean 40.3, SD 22.3 g/1000 kcal) and the PRC recorded the lowest (mean 5.5, SD 14.2 g/1000 kcal) . For all countries, the majority of fish consumption came from fish; Japan had the highest shellfish intake (mean 10.6, SD 11.2 g/1000 kcal). Fish contributed 24.4% of the protein intake in Japan, 5.1% in the USA, 4.0% in the UK, and 2.9% in the PRC. Fish contributed 12.7% of the vitamin D intake in the USA and 16.7% of the selenium intake in Japan. High fish consumers in all countries had lower adjusted mean intakes of energy from saturated fat compared with non/low consumers. (Supplemental Table 3). In Japan and the UK, higher fish consumers were older compared with non/low consumers (Japan mean age 50.4 compared with 48.2 y; UK mean age 50.2 compared with 48.7 y). The prevalence of fish intake varied by geographic region in the PRC, USA, and Japan; in the PRC 93.6% of those in the Southern Guangxi province sample were classified as high consumers whereas the Shanxi province sample in the North had the highest prevalence of nonconsumers (42.9%); in the USA, Honolulu had the greatest proportion of high consumers (56.9%) and Corpus Christi the highest proportion of nonconsumers (59.4%); (Supplemental Tables 4and5). Reliability estimates of total fish intake in Japan were 83%, and the UK 84%, USA 88%, and PRC 92%.
TABLE 1.
Baseline nutritional intake characteristics of INTERMAP study participants by seafood (fish and shellfish) consumption by country (consumers and nonconsumers)
| Japan | PRC | UK | USA | |
|---|---|---|---|---|
| Variable | (n = 1145) | (n = 839) | (n = 501) | (n = 2195) |
| Consumers of fish, n (%) | 1142 (99) | 217 (26) | 277 (55) | 1156 (53) |
| Total fish, g/1000 kcal | 40.28 ± 22.26 | 5.46 ± 14.20 | 8.52 ± 12.42 | 8.98 ± 15.00 |
| Fish (exc. shellfish), g/1000 kcal | 36.81 ± 18.86 | 5.25 ± 13.94 | 7.90 ± 12.16 | 6.79 ± 13.17 |
| Shellfish, g/1000 kcal | 10.56 ± 11.21 | 0.20 ± 1.96 | 0.63 ± 2.35 | 2.19 ± 6.38 |
| “Oily” fish, g/1000 kcal1 | 19.82 ± 13.73 | — | 2.58 ± 8.84 | 2.10 ± 7.83 |
| Fried fish, g/1000 kcal | 1.08 ± 3.01 | 0.27 ± 1.61 | 1.91 ± 5.90 | — |
| Salted/preserved fish, g/100 kcal | 8.04 ± 8.56 | 0.00 ± 0.00 | 0.05 ± 0.75 | 0.05 ± 0.57 |
| Selenium intake, mcg/1000 kcal | 80.08 ± 31.08 | 16.56 ± 4.26 | 44.13 ± 13.90 | 59.47 ± 25.92 |
| Vitamin D intake mcg/1000 kcal | — | — | — | 2.30 ± 1.52 |
| % Energy from PUFA | 6.24 ± 1.45 | 5.88 ± 2.22 | 6.25 ± 1.91 | 7.16 ± 2.28 |
| Cholesterol, mg/1000 kcal | 191.43 ± 65.06 | 89.28 ± 84.74 | 120.47 ± 47.87 | 131.85 ± 57.53 |
| % Protein from fish | 24.41 ± 10.76 | 2.89 ± 6.93 | 4.03 ± 5.94 | 5.05 ± 7.85 |
| % Total fat from fish | 10.52 ± 7.42 | 0.99 ± 2.90 | 1.45 ± 3.21 | 0.79 ± 2.09 |
| % PUFA from fish | 9.38 ± 6.54 | 0.92 ± 3.08 | 0.89 ± 2.20 | 7.16 ± 2.28 |
| % Selenium from fish | 37.63 ± 16.66 | 4.36 ± 10.88 | 6.47 ± 9.14 | 7.33 ± 10.90 |
| % Vitamin D from fish | — | — | — | 12.66 ± 20.23 |
| % Cholesterol from fish | 29.71 ± 16.61 | 7.10 ± 16.57 | 4.02 ± 6.78 | 5.61 ± 9.77 |
Values are means ± SD unless otherwise indicated; n = 4680. "—" data not available. PRC, People's Republic of China.
Defined as containing 588 mg DPA + EPA per 100 g of fish.
Associations of fish intake with BP and BMI
In the combined UK and US population sample, no associations were observed between fish intake and BP or BMI in either stratified analyses by intake category (Table 2) or linear regression analyses (Supplemental Table 6). For Japanese participants, no associations were observed between fish intake and BP, however, there was a direct association between fish intake and BMI; the highest compared with lowest tertiles was 24.33, SE: 0.23 compared with 23.82, SE: 0.23, P = 0.03; P for trend = 0.0249. There was no association between fish intake and BP or BMI by category of intake among PRC participants. Linear regression analyses showed a direct association with fish intake and BMI in the Japanese population sample (mean difference 0.52; 95% CI: 0.21, 0.82; P = 0.001; fully adjusted model).
TABLE 2.
Estimated differences in blood pressure and BMI across total fish (fish and shellfish) intake groups for Japan and the People's Republic of China INTERMAP participants1
| Categories of fish intake2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Japan (n = 1145) | People's Republic of China (n = 839) | USA and UK (n = 2696) | ||||||||||
| Low consumers | Med consumers | High consumers | P | Nonconsumers | Low consumers | High consumers | P | Nonconsumers | Low consumers | High consumers | P | |
| Participants, n (%) | 381 (33) | 382 (33) | 382 (33) | 622 (74) | 108 (13) | 109 (13) | 1263 (47) | 716 (26) | 171 (27) | |||
| Systolic blood pressure, mmHg | ||||||||||||
| Model 1 | 122.88 (0.97) | 122.77 (0.97) | 121.57 (0.97) | 0.28 | 129.52 (1.59) | 128.18 (2.05) | 129.73 (2.13) | 0.66 | 120.55 (0.44) | 120.96 (0.53) | 120.37 (0.55) | 0.66 |
| Model 2 | 122.84 (1.01) | 122.74 (0.98) | 121.80 (1.01) | 0.54 | 129.74 (1.58) | 128.58 (2.05) | 130.30 (2.17) | 0.66 | 122.80 (1.15) | 124.81 (1.29) | 125.06 (1.29) | 0.17 |
| Model 3 | 122.23 (0.97) | 121.69 (0.94) | 120.53 (0.98) | 0.40 | 128.81 (1.56) | 127.44 (2.03) | 129.06 (2.15) | 0.62 | 122.62 (1.12) | 124.16 (1.26) | 124.58 (1.25) | 0.28 |
| Diastolic blood pressure, mmHg | ||||||||||||
| Model 1 | 77.01 (0.74) | 76.53 (0.73) | 75.78 (0.74) | 0.20 | 76.48 (0.97) | 75.74 (1.26) | 76.80 (1.31) | 0.65 | 74.93 (0.31) | 75.12 (0.37) | 74.86 (0.38) | 0.84 |
| Model 2 | 77.07 (0.76) | 76.54 (0.74) | 75.90 (0.76) | 0.34 | 76.50 (0.98) | 75.79 (1.27) | 77.10 (1.34) | 0.58 | 79.04 (0.80) | 79.51 (0.90) | 79.43 (0.90) | 0.86 |
| Model 3 | 76.60 (0.73) | 75.73 (0.71) | 74.92 (0.74) | 0.09 | 74.73 (0.95) | 74.85 (1.24) | 76.07 (1.31) | 0.55 | 78.96 (0.80) | 79.23 (0.90) | 79.22 (0.89) | 0.95 |
| BMI, kg/m2 | ||||||||||||
| Model 1 | 23.82 (0.23) | 24.29 (0.23) | 24.33 (0.23) | 0.03a | 24.14 (0.33) | 24.48 (0.43) | 24.55 (0.44) | 0.40 | 29.53 (0.19) | 29.41 (0.23) | 29.28 (0.23) | 0.63 |
| Model 2 | 23.85 (0.22) | 24.26 (0.21) | 24.48 (0.22) | 0.03b | 24.21 (0.33) | 24.46 (0.42) | 24.58 (0.45) | 0.57 | 27.74 (0.40) | 28.43 (0.45) | 28.18 (0.45) | 0.33 |
Values are adjusted means and SEM; n= 4680.
Categories of intake per population sample: Japan: low consumers (>0.0 g/1000 kcal –28.4 g/1000 kcal); medium consumers (28.4 g/1000 kcal –45.3 g/1000 kcal); high consumers (45.3 g/1000 kcal –165.5 g/1000 kcal). The PRC: medium consumers (0.3 g/1000 kcal –14.3 g/1000 kcal); high consumers (14.6 g/1000 kcal –155.5 g/1000 kcal). The UK and USA combined: medium consumers (0.1 g/1000 kcal –11.8 g/kcal); high consumers (11.9 g/1000 kcal –155.3 g/1000 kcal) . Model 1: age (years, continuous), sex, study center, education (years), physical activity (hours per day), alcohol (average g/day), smoking, vitamin supplement usage (yes/no), antihypertensive medication (yes/no), family history of hypertension (yes/no), diagnosed cardiovascular disease (yes/no). Model 2: as Model 1 additional adjustment for the following continuous dietary variables: energy intake (kcal) calcium (mg/1000 kcal), magnesium (mg/1000 kcal), %energy intake SFA, %energy intake vegetable protein, fiber (g/1000 kcal), sodium (urinary, mmol/24 h), and potassium (urinary, mmol/24 h). Model 3: as Model 2 + BMI. asignificant difference between groups 1 and 3, P for trend = 0.0249, bsignificant difference between groups 1 and 3, P for trend = 0.0097.
Associations of type of fish intake with BP and BMI
Subgroup analyses of Japanese populations showed a borderline inverse association between oily fish intake and DBP in Model 1 (mean difference −1.10; 95% CI: −2.22, 0.02 mmHg; P = 0.05), this association was significant in the fully adjusted model including BMI (mean difference −1.38; 95% CI: −2.54, −0.24; P = 0.02). Following adjustment for dietary factors, all categories of fish (total, oily, and lean) except for raw fish showed a direct association with BMI (Supplemental Table 7).
Urinary metabolic biomarkers of fish intake
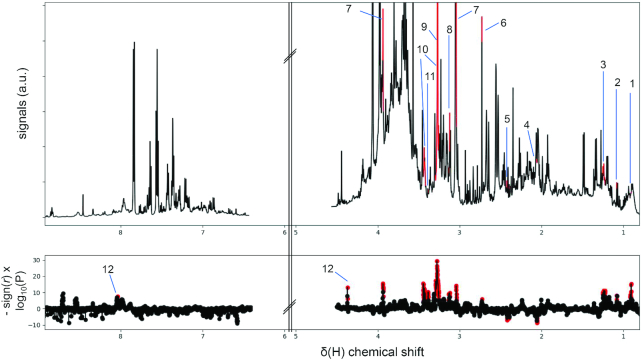
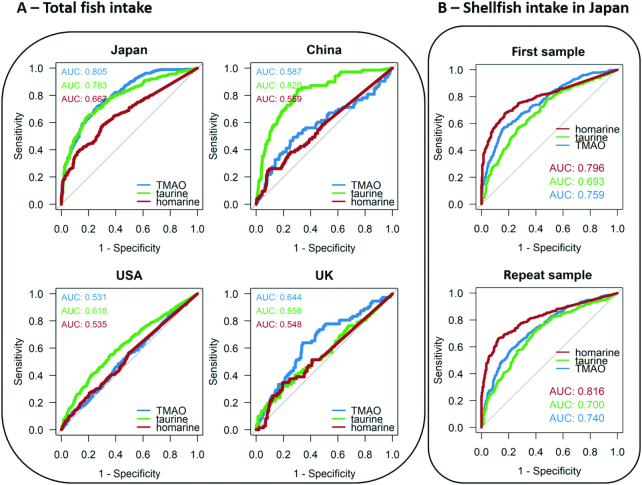
TMAO (P < 1×10−23), taurine (P < 1×10−15), creatine (P < 1×10−15), homarine (P < 1×10−13), ethyl glucuronide (P < 1×10−11), trimethyllysine (P < 1×10−9), and dimethylamine (P = 1×10−6) were found to be significantly correlated with fish consumption (Figure 1), and homarine in particular, was found to be strongly associated with shellfish consumption (P < 1×10−20; Supplemental Figures 3 and 4). Partial correlation analyses performed using the concentrations of these selected metabolites from the first urine samples against fish and shellfish intakes (Supplemental Figure 8) showed that in Japanese participants, TMAO, homarine, and taurine were significantly correlated with fish intake (P < 0.0001); homarine showed a stronger correlation with shellfish (r = 0.46) than fish (r = 0.30). Among Chinese participants, taurine was correlated with fish intake (r = 0.31, P < 0.0001). ROC curve analyses were also performed (Figure 2) which show that in Japanese participants, TMAO and taurine, respectively, demonstrated an area under the receiver operating characteristic curve (AUC) values of 0.81 and 0.78 for discriminating high against low total fish intake (including shellfish). In the Japanese population sample, homarine demonstrated an AUC of 0.80 for discriminating high shellfish intake against participants with no recorded shellfish intake (Figure 2B). Taurine also demonstrated an AUC of 0.82 for discriminating high against low total fish intake. Validation using the second 24-h urinary data showed similar results (Supplemental Figures 9and10).
FIGURE 1.
NMR spectral variables correlating to fish intake. Partial correlation analyses of total fish intake were performed. Fish intake was correlated against each NMR intensity variable using the combined sample population from 4 countries and analyses were adjusted for age, sex, and center. Analyses were performed on first and repeat visit samples independently. Bonferroni threshold was used for multiple testing corrections (P threshold = 7×10−6) with repeat visit samples used as validation set. Upper panel: median spectrum of first visit samples with significantly correlated NMR variable annotated/highlighted in red. Lower panel: Manhattan plot showing signed -log10(P) indicates the level of significance of the correlating NMR variables. Significantly correlated NMR variables were annotated/highlighted in red. r: partial correlation coefficient. 1: 3-methyladipic acid; 2: 3-hydroxyisobutyrate; 3: ethyl glucuronide; 4: N-acetyl neuraminate; 5: pyroglutamate; 6: dimethylamine; 7: creatine; 8: trimethyllysine; 9: trimethylamine N-oxide; 10: taurine; 11: theophylline; 12: homarine.
FIGURE 2.
Receiver operating characteristic (ROC) curves were used to assess the predictive ability of urinary metabolites of interest in discriminating total fish intake (including shellfish) and separately for shellfish only. A) Panels illustrate by country the ability of urinary metabolites of interest in discriminating high fish intake from nonfish consumers (China, USA, UK) and low fish intake from nonfish consumers (Japan) using samples from the first visit. N = 753 (Japan), N = 757 (China), N = 1777 (USA), N = 409 (UK). Japan: consumers of high fish intake N = 380, consumers of low fish intake N = 373; China: consumers of high fish intake N = 73, nonconsumers N = 684; USA: consumers of high fish intake N = 384, nonconsumers N = 1393; UK: consumers of high fish intake N = 72, nonconsumers N = 337. B) Panel illustrates the ability of urinary metabolites of interest in discriminating high shellfish intake from nonconsumers of shellfish (Japan only). Analysis was independently performed for first and repeat visit urine samples. TMAO, trimethylamine-N-oxide.
Associations of urinary metabolites of fish intake with BP and BMI
Analyses by country (Japan, the PRC) and region (Western: the UK and USA) found no associations between urinary homarine and BP (Table 3); direct associations were observed between homarine and BMI in Japanese and Western population samples (P < 0.05), which were attenuated following adjustment for energy intake and other dietary factors. In Western population samples, there was a direct association between TMAO and DBP (mean difference per 2 SD 1.28; 95% CI: 0.55, 2.02 mmHg; P = 0.0006; fully adjusted for lifestyle and dietary intake), this remained significant after further adjustment for BMI (mean difference 0.92; 95% CI: 0.19, 1.64 mmHg; P = 0.01). No significant associations were found between TMAO and BP for Japanese or PRC samples. There was a direct association between TMAO and BMI in all population samples; and following adjustment for other dietary variables this association remained significant in PRC and Western population samples (P < 0.0001). In Western samples, there was an inverse association between taurine and DBP (mean difference −0.81; 95% CI: −1.48, −0.15 mmHg; P = 0.02; fully adjusted for lifestyle and dietary intake); following adjustment for BMI this was attenuated. There was an inverse association between taurine and BMI in the Chinese population sample (mean difference −1.05; 95% CI: −1.60, −0.50; P = 0.0002; fully adjusted for lifestyle and dietary intake), but not in the other population samples.
TABLE 3.
Estimated differences in blood pressure with 2 SD higher urinary excretion of trimethylamine N-oxide, homarine, and taurine in Western, Asian, and combined INTERMAP participants
| Systolic blood pressure (mmHg) | Diastolic blood pressure (mmHg) | BMI | ||||
|---|---|---|---|---|---|---|
| Difference (95% CI) | P | Difference (95% CI) | P | Difference (95% CI) | P | |
| TMAO (N = 4609*,2SD = 1.66) | ||||||
| Japan | ||||||
| Model 1 | −0.14 (−1.61, 1.33) | 0.85 | −0.20 (−1.30, 0.91) | 0.73 | 0.61 (0.28, 0.95) | 0.0004 |
| Model 2 | −0.08 (−1.69, 1.53) | 0.92 | −0.19 (−1.40, 1.02) | 0.76 | 0.31 (−0.04, 0.66) | 0.08 |
| PRC | ||||||
| Model 1 | −0.23 (−2.53, 2.07) | 0.84 | 0.26 (−1.13, 1.65) | 0.71 | 1.35 (0.89, 1.80) | <0.0001 |
| Model 2 | 0.21 (−2.19, 2.61) | 0.86 | 0.26 (−1.20, 1.72) | 0.73 | 1.16 (0.69, 1.63) | <0.0001 |
| Western (UK and USA) | ||||||
| Model 1 | 1.82 (0.80, 2.84) | 0.0005 | 1.29 (0.59, 1.99) | 0.0003 | 1.79 (1.38, 2.20) | <0.0001 |
| Model 2 | 1.67 (0.60, 2.73) | 0.002 | 1.28 (0.55, 2.02) | 0.0006 | 1.10 (0.68, 1.51) | <0.0001 |
| Homarine (N = 3677*,2SD = 2.46) | ||||||
| Japan | ||||||
| Model 1 | −0.06 (−1.64, 1.53) | 0.94 | −0.76 (−1.95, 0.44) | 0.21 | 0.46 (0.10, 0.82) | 0.01 |
| Model 2 | 0.21 (−1.46, 1.88) | 0.80 | −0.66 (−1.91, 0.60) | 0.31 | 0.30 (−0.06, 0.66) | 0.10 |
| PRC | ||||||
| Model 1 | 0.38 (−2.21, 2.97) | 0.77 | 0.15 (−1.39, 1.69) | 0.85 | −0.03 (−0.55, 0.49) | 0.91 |
| Model 2 | 0.52 (−2.08, 3.12) | 0.70 | 0.12 (−1.43, 1.67) | 0.88 | −0.18 (−0.70, 0.33) | 0.49 |
| Western (UK and US) | ||||||
| Model 1 | 0.43 (−0.71, 1.56) | 0.46 | −0.02 (−0.80, 0.76) | 0.95 | 0.55 (0.09, 1.02) | 0.02 |
| Model 2 | 0.46 (−0.69, 1.61) | 0.43 | 0.00 (−0.79, 0.79) | 0.99 | 0.27 (−0.18, 0.72) | 0.24 |
| Taurine (N = 4347*,2SD = 1.86) | ||||||
| Japan | ||||||
| Model 1 | −1.70 (−4.04, 0.63) | 0.15 | −1.52 (−3.28, 0.23) | 0.09 | 0.45 (−0.09, 0.98) | 0.10 |
| Model 2 | −2.10 (−4.58, 0.39) | 0.10 | −1.89 (−3.75, −0.03) | 0.05 | −0.10 (−0.65, 0.44) | 0.71 |
| PRC | ||||||
| Model 1 | −0.38 (−3.11, 2.36) | 0.79 | 0.25 (−1.39, 1.89) | 0.76 | −0.62 (−1.16, −0.09) | 0.02 |
| Model 2 | −0.56 (−3.37, 2.25) | 0.70 | 0.03 (−1.67, 1.73) | 0.97 | −1.05 (−1.60, −0.50) | 0.0002 |
| Western | ||||||
| Model 1 | −0.34 (−1.29, 0.62) | 0.49 | −0.53 (−1.19, 0.13) | 0.11 | 0.20 (−0.19, 0.59) | 0.31 |
| Model 2 | −0.76 (−1.73, 0.20) | 0.12 | −0.81 (−1.48, −0.15) | 0.02 | −0.29 (−0.67, 0.08) | 0.12 |
Metabolites log transformed. Pooled data weighted by center. *Samples available for analysis <4680 due to missing samples.
Model 1 adjusted for age, sex, education (years), physical activity (hours per day), alcohol (average g/d), smoking, vitamin supplement usage (yes/no), antihypertensive medication (yes/no), family history of hypertension (yes/no), diagnosed cardiovascular disease (yes/no).
Model 2 additional adjustment for the following continuous dietary variables: energy intake (kcal), calcium (mg/1000 kcal), magnesium (mg/1000 kcal), %EI SFA, %EI vegetable protein, fiber (g/1000 kcal), sodium (urinary, mmol/24 h), and potassium (urinary, mmol/24 h).
TMAO: trimethylamine N-oxide.
Sensitivity analyses
No significant associations between fish intake (g/1000 kcal) and BP were observed in analyses stratified by sex and BMI category (not shown). Analyses adjusted for antihypertensive medication did not indicate any change in association between fish intake for any country (not shown).
Discussion
To our knowledge, this is the first study to report results from rigorous and standardized dietary assessment and BP measurement across Asian and Western population samples investigating associations between fish intake and urinary fish metabolites with BP and BMI. Our null finding between fish intake and BP supports results of a meta-analysis of controlled trials investigating fish intake and vascular risk factors (37), with only 1 study reporting a significant change in SBP (−4 mmHg) following a 4-wk protocol of 125 g salmon per day (38). Although these amounts are higher than mean daily intakes reported by INTERMAP Japanese participants (∼45 g/d for oily/fatty fish), we did find an association between oily fish consumption and DBP. Oily fish are high in EPA (20:5n–3) and DHA (22:6n–3), which are precursors to prostaglandins and are thought to exert beneficial effects on BP via vasodilatory activity (39). One potential explanation for the null finding in the Japanese population sample for oily fish with SBP is the combined high oily fish intake with high dietary cholesterol. We have previously reported from INTERMAP a low-order direct association between dietary cholesterol and BP (40). A study in hamsters showed that dietary cholesterol intake modified n–3 PUFA metabolism and subsequently increased circulating LDL cholesterol concentrations (41). The interaction between dietary cholesterol and n–3 PUFA intake warrants further investigation in humans and in relation to BP.
Our observed direct association between energy-adjusted fish intake and BMI in the Japanese population sample is contrary to previous findings from a large-scale European longitudinal study reporting no difference in body weight or waist circumference in relation to fish consumption (10, 11). Differences in ethnicity and measurement methods may account for differences in observations; also, in the current study we were unable to test the association between fish intake and distribution of body fat (e.g., waist circumference). In European populations, high fish intake is associated with a healthy dietary pattern (6). In contrast, a study based on data from the national dietary survey in Japan observed no association between fish intake and adherence to Japanese healthy eating guidelines (42), possibly reflecting that the guidelines for animal product intake do not differentiate between fish, shellfish, egg, and meat intake. Public health nutrition guidance for the PRC, UK, and USA include eating fish as part of a healthy diet: PRC: aquatic product 40–75 g/d (43), UK: at least 2 portions per week, one of which should be oily (portion: 140 g/4.9 oz) (44), USA: 2 servings of fish per week (serving: 4 oz) (4). We found fish intake to be an important contributor to selenium, n–3 PUFAs, and vitamin D (where vitamin D data were available) intake. Thus, despite the lack of association between fish intake and BP in our current study, fish and shellfish consumption have other potential nutritional health benefits. Our findings regarding fish intake are in contrast with the direct associations we previously reported between meat intake and BP in INTERMAP (45) and thus, highlight potential differences in nutrient profile and health outcomes between sources of animal protein. This difference may be due to fatty acid profiles, with high saturated fat in red meat compared with n–3 PUFAs in fish or to unidentified bioactive compounds within the food matrix.
We identified 3 predominant metabolites associated with fish intake – TMAO, homarine, and taurine. To our knowledge this is the first time homarine, a metabolite found in the muscle of shellfish (crustacean and mollusks) (46, 47), has been identified as a candidate urinary marker for shellfish intake in humans. Although we observed homarine to have a strong correlation and specificity with shellfish compared with nonshellfish, it was not possible in the current study to establish if homarine can differentiate shellfish consumers from nonshellfish consumers in the Japanese population sample as the majority of participants consumed both shellfish and nonshellfish.
Fish, along with meat, are sources of taurine, which is an amino sulfonic acid (48). A recent meta-analysis of human supplement trials indicated taurine supplementation (0.5–6 g/d) reduced both DBP and SBP by ∼3 mmHg (49). However, these trials used isolated taurine rather than increasing intake via food and supplementation was ≤150 times higher than estimated background intake in adults of 40–400 mg/d (48). We observed taurine to have an inverse association with BMI, concordant with the limited human studies that have reported weight loss with long-term taurine supplementation, and suggested a potential mechanistic pathway via the inhibition of white adipocyte differentiation (50).
TMAO is found in variable concentrations in fish (51) and in agreement with previous work (18, 19, 51), we identified TMAO as a urinary biomarker of fish intake. However, TMAO can also be an indirect urinary biomarker of red meat and egg consumption. Trimethylamine (TMA) is synthesized via gut bacteria from dietary choline and carnitine (52) (found in eggs and red meat) and oxidized to TMAO in the liver prior to renal excretion. Moreover, gut microbiota profiles characterized by an enriched ratio of Firmicutes to Bacteroidetes and low diversity have been associated with higher TMA production in humans (53); such a gut microbiota profile is associated with obesity (54) and consuming a diet of poorer nutritional quality (55). Previously, in the INTERMAP UK and US participants, we reported a direct association between urinary TMA and BMI (56). In the present study we observed that the association between TMAO and BMI was independent of energy intake and key nutrients in all population samples except the Japanese sample. We also reported an association between urinary TMAO and DBP independent of BMI in the INTERMAP Western population samples. A nested case-control study in a Chinese cohort found that urinary TMAO concentrations were directly associated with central adiposity, but in contrast to our findings, reported an inverse association with hypertension (57). The authors also reported stronger associations with deep-fried meat and fish consumption compared with meat and fish prepared by other cooking methods and deep-fried plant-based foods (57). The wider profile of a population's diet should be considered when interpreting the clinical relevance of urinary TMAO to cardiometabolic health outcomes. We found no evidence that urinary TMAO is associated with BP or BMI in a population sample with a relatively high level of fish intake where TMAO is strongly associated with fish intake. However, TMAO in the Western population samples with relatively low fish intake (where TAMO is a low predictive marker of fish intake), we observed direct associations between TMAO with BP and BMI – potentially reflecting the intake of other dietary components. Due to the complexity of associating urinary TMAO directly to the consumption of specific food groups, further research is needed to elucidate these differential associations observed between populations. The 4 population samples included in this study showed large differences in fish intake. Therefore, this is likely to contribute to the variation in the correlations and predictive values we observed between metabolites and intake, with the strongest reported in the Japanese population sample which is likely to reflect their high fish intake.
The main strengths of our study are 1) the detailed dietary assessment aligned to 2 timed 24-h urine collections facilitating the identification of potential novel urinary biomarkers of fish intake and 2) the standardized methods of measurement and data collection applied across population samples drawn from 4 countries allowing, for the first time, standardized investigation of fish intake, BP and BMI across Asian and Western population samples. There are also several limitations. First, fish is a food group with sporadic patterns of intake in the PRC, UK, and USA; therefore, 4 24-h recalls may not reflect true intake; however, the days of recall were selected at random with ≥1 rest day to represent different days of the week and reliability estimates were high. Consumption in Japan was much higher than in the other 3 countries, which limited the ability to combine data across countries. We observed significant differences in patterns of intake across geographic regions in the PRC, therefore the limits of generalizability should also be acknowledged in applying our findings outside of these regions. As part of this study we did not determine intake of meat and eggs, which may affect TMAO excretion: this may also have contributed to the differences in the results observed between Asian and Western samples and warrants further investigation. TMAO varies between species depending on depth and salinity of fish habitat (58), a potential determinant of urinary TMAO, however, this level of detail was not available. Since the data collected are cross-sectional, our observed association between fish intake and BMI in Asian samples requires further longitudinal assessment. Our analyses adjusted for intake of energy and other nutrients related to diet quality (fiber, saturated fat, vegetables, and selected minerals) but residual confounding from sample-specific dietary variables cannot be ruled out. The method of processing and cooking fish can impact upon the nutritional profile of the fish consumed. The Coronary Artery Risk Development in Young Adults (CARDIA) study at 20 y of follow-up observed inverse associations of nonfried fish consumption on the incidence of hypertension and BP (59). Although, we controlled our analyses for fat intake, due to insufficient data and/or low intake of fried fish products, we were unable to test if fried fish, independent of fat intake, was associated with BP.
In conclusion, the INTERMAP results overall showed no significant association between fish intake and BP in population samples with a low and high intake of fish. However, we did observe population sample-specific observations. Urinary TMAO was directly associated with BMI independent of other dietary factors in population samples with lower fish intake, and with BP in Western but not Asian population samples. These differences in association with health outcomes between countries require further investigation. Future work should consider how specific foods are consumed as part of geographic region-specific dietary patterns and gut microbiota characterization. Additionally, exploration of the potential interaction between n–3 PUFAs and dietary cholesterol on vascular function would advance understanding of the potential mechanisms of nutrient–nutrient interactions.
We also identified homarine as a novel urinary biomarker of shellfish intake, which requires further validation in other observational studies and controlled feeding studies. If our observation can be replicated and validated in future studies, this finding will contribute to the incremental advancement towards the discovery of objective dietary biomarkers.
Supplementary Material
ACKNOWLEDGEMENTS
We thank all INTERMAP staff at local, national, and international centers for their invaluable efforts. A partial listing of these colleagues is given in Reference 24 of this paper (26). We thank every participant in the study for their invaluable contributions. We thank Dr Lea Maitre for her helpful suggestion on metabolite identification of homarine for this manuscript.
The authors’ contributions were as follows—KM, AD, HU, LZ, PE, and JS: designed INTERMAP, conducted the fieldwork, and collected data; RG, CEL, and QC: performed the analysis, interpreted the data, and prepared the manuscript; RLL, EC, and EH: contributed to the biochemical analysis for biomarkers and revised the work critically for important intellectual content; and TMDE and AD: contributed substantially to the statistical analysis and revised the work critically for important intellectual content; KM, HU, LZ, PE, MLD, JS, and LVH: revised the work critically for important intellectual content; EH and QC: were responsible for the final content; and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
Notes
INTERMAPwas supported by grants R01-HL50490, R01-HL65461, R01-HL84228, and R01-HL135486 from the National Heart, Lung, and Blood Institute, NIH (Bethesda, MD, USA) and by the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Grant-in-Aid for Scientific Research [A], No. 090357003 and No. 17H01553), and the United Kingdom (a project grant from the West Midlands National Health Service Research and Development, and grant R2019EPH from the Chest, Heart and Stroke Association).
Supplemental Figures 1–10 and Supplemental Tables 1–7 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
RG and C-HEL are joint first authors.
Abbreviations used: BP, blood pressure; CVD, cardiovascular disease; DBP, diastolic blood pressure;1H NMR, proton NMR; INTERMAP, International Study of Macro-/Micronutrients and Blood Pressure; n–3 PUFA, long-chain omega-3 PUFA; PRC, People's Republic of China; ROC, receiver operating characteristic; SBP, systolic blood pressure; TMA, trimethylamine; TMAO, trimethylamine-N-oxide; TSP, trimethylsilyl-(2,2,3,3-d)-1-propionate.
References
- 1. Weichselbaum E, Coe S, Buttriss J, Stanner S. Fish in the diet: a review. Nutr Bull. 2013;38:128–77. [Google Scholar]
- 2. Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348(26):2599–608. [DOI] [PubMed] [Google Scholar]
- 3. Calton EK, James AP, Pannu PK, Soares MJ. Certain dietary patterns are beneficial for the metabolic syndrome: reviewing the evidence. Nutr Res. 2014;34(7):559–68. [DOI] [PubMed] [Google Scholar]
- 4. US Department of Health and Human Services and US Department of Agriculture. 2015–2020 Dietary Guidelines for Americans [Internet]. 8th ed December 2015. Available from: http://health.gov/dietaryguidelines/2015/guidelines/. [Google Scholar]
- 5. Van Horn L, Carson JA, Appel LJ, Burke LE, Economos C, Karmally W, Lancaster K, Lichtenstein AH, Johnson RK, Thomas RJ et al.. Recommended dietary pattern to achieve adherence to the American Heart Association/American College of Cardiology (AHA/ACC) guidelines: a scientific statement from the American Heart Association. Circulation. 2016;134(22):e505–e29. [DOI] [PubMed] [Google Scholar]
- 6. Pauletto P, Puato M, Caroli MG, Casiglia E, Munhambo AE, Cazzolato G, Bittolo Bon G, Angeli MT, Galli C, Pessina AC. Blood pressure and atherogenic lipoprotein profiles of fish-diet and vegetarian villagers in Tanzania: the Lugalawa study. Lancet. 1996;348(9030):784–8. [DOI] [PubMed] [Google Scholar]
- 7. Yang B, Shi MQ, Li ZH, Yang JJ, Li D. Fish, long-chain n-3 PUFA and incidence of elevated blood pressure: a meta-analysis of prospective cohort studies. Nutrients. 2016;8(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chowdhury R, Stevens S, Gorman D, Pan A, Warnakula S, Chowdhury S, Ward H, Johnson L, Crowe F, Hu FB et al.. Association between fish consumption, long chain omega 3 fatty acids, and risk of cerebrovascular disease: systematic review and meta-analysis. BMJ. 2012;345:e6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schmedes M, Aadland EK, Sundekilde UK, Jacques H, Lavigne C, Graff IE, Eng O, Holthe A, Mellgren G, Young JF et al.. Lean-seafood intake decreases urinary markers of mitochondrial lipid and energy metabolism in healthy subjects: metabolomics results from a randomized crossover intervention study. Mol Nutr Food Res. 2016;60(7):1661–72. [DOI] [PubMed] [Google Scholar]
- 10. Jakobsen MU, Dethlefsen C, Due KM, May AM, Romaguera D, Vergnaud AC, Norat T, Sorensen TI, Halkjaer J, Tjonneland A et al.. Fish consumption and subsequent change in body weight in European women and men. Br J Nutr. 2013;109(2):353–62. [DOI] [PubMed] [Google Scholar]
- 11. Jakobsen MU, Due KM, Dethlefsen C, Halkjaer J, Holst C, Forouhi NG, Tjonneland A, Boeing H, Buijsse B, Palli D et al.. Fish consumption does not prevent increase in waist circumference in European women and men. Br J Nutr. 2012;108(5):924–31. [DOI] [PubMed] [Google Scholar]
- 12. Jayedi A, Shab-Bidar S, Eimeri S, Djafarian K. Fish consumption and risk of all-cause and cardiovascular mortality: a dose-response meta-analysis of prospective observational studies. Public Health Nutr. 2018;21(7):1297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mancano G, Mora-Ortiz M, Claus SP. Recent developments in nutrimetabolomics: from food characterisation to disease prevention. Curr Opin Food Sci. 2018;22:145–52. [Google Scholar]
- 14. Brennan L. Metabolomics: a tool to aid dietary assessment in nutrition. Curr Opin Food Sci. 2017;16:96–9. [Google Scholar]
- 15. Guertin KA, Moore SC, Sampson JN, Huang WY, Xiao Q, Stolzenberg-Solomon RZ, Sinha R, Cross AJ. Metabolomics in nutritional epidemiology: identifying metabolites associated with diet and quantifying their potential to uncover diet-disease relations in populations. Am J Clin Nutr. 2014;100(1):208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Turunen AW, Mannisto S, Kiviranta H, Marniemi J, Jula A, Tiittanen P, Suominen-Taipale L, Vartiainen T, Verkasalo PK. Dioxins, polychlorinated biphenyls, methyl mercury and omega-3 polyunsaturated fatty acids as biomarkers of fish consumption. Eur J Clin Nutr. 2010;64(3):313–23. [DOI] [PubMed] [Google Scholar]
- 17. Mori M, Mori H, Hamada A, Yamori Y. Taurine in morning spot urine for the useful assessment of dietary seafood intake in Japanese children and adolescents. J Biomed Sci. 2010;17(Suppl 1):S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kruger R, Merz B, Rist MJ, Ferrario PG, Bub A, Kulling SE, Watzl B. Associations of current diet with plasma and urine TMAO in the KarMeN study: direct and indirect contributions. Mol Nutr Food Res. 2017;61(11). doi:10.1002/mnfr.201700363. [DOI] [PubMed] [Google Scholar]
- 19. Lloyd AJ, Fave G, Beckmann M, Lin W, Tailliart K, Xie L, Mathers JC, Draper J. Use of mass spectrometry fingerprinting to identify urinary metabolites after consumption of specific foods. Am J Clin Nutr. 2011;94(4):981–91. [DOI] [PubMed] [Google Scholar]
- 20. Cheung W, Keski-Rahkonen P, Assi N, Ferrari P, Freisling H, Rinaldi S, Slimani N, Zamora-Ros R, Rundle M, Frost G et al.. A metabolomic study of biomarkers of meat and fish intake. Am J Clin Nutr. 2017;105(3):600–8. [DOI] [PubMed] [Google Scholar]
- 21. Kohashi N, Katori R.. Decrease of urinary taurine in essential-hypertension. Jpn Heart J. 1983;24(1):91–102. [DOI] [PubMed] [Google Scholar]
- 22. Mizushima S, Moriguchi EH, Ishikawa P, Hekman P, Nara Y, Mimura G, Moriguchi Y, Yamori Y. Fish intake and cardiovascular risk among middle-aged Japanese in Japan and Brazil. J Cardiovasc Risk. 1997;4(3):191–9. [PubMed] [Google Scholar]
- 23. Sagara M, Murakami S, Mizushima S, Liu LJ, Mori M, Ikeda K, Nara Y, Yamori Y. Taurine in 24-h urine samples is inversely related to cardiovascular risks of middle aged subjects in 50 populations of the world. Adv Exp Med Biol. 2015;803:623–36. [DOI] [PubMed] [Google Scholar]
- 24. Manor O, Zubair N, Conomos MP, Xu X, Rohwer JE, Krafft CE, Lovejoy JC, Magis AT. A multi-omic association study of trimethylamine N-oxide. Cell Rep. 2018;24(4):935–46. [DOI] [PubMed] [Google Scholar]
- 25. Papandreou C, Bullo M, Zheng Y, Ruiz-Canela M, Yu E, Guasch-Ferre M, Toledo E, Clish C, Corella D, Estruch R et al.. Plasma trimethylamine-N-oxide and related metabolites are associated with type 2 diabetes risk in the Prevencion con Dieta Mediterranea (PREDIMED) trial. Am J Clin Nutr. 2018;108(1):163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stamler J, Elliott P, Dennis B, Dyer AR, Kesteloot H, Liu K, Ueshima H, Zhou BF. INTERMAP: background, aims, design, methods, and descriptive statistics (nondietary). J Hum Hypertens. 2003;17(9):591–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dennis B, Stamler J, Buzzard M, Conway R, Elliott P, Moag-Stahlberg A, Okayama A, Okuda N, Robertson C, Robinson F et al.. INTERMAP: the dietary data – process and quality control. J Hum Hypertens. 2003;17(9):609–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4 Suppl):1220S–8S. [DOI] [PubMed] [Google Scholar]
- 29. Dyer AR, Elliott P, Shipley M. Urinary electrolyte excretion in 24 hours and blood pressure in the INTERSALT Study: II. Estimates of electrolyte-blood pressure associations corrected for regression dilution bias. Am J Epidemiol. 1994;139(9):940–51. [DOI] [PubMed] [Google Scholar]
- 30. Grandits GA, Bartsch GE, Stamler J. Method issues in dietary data analyses in the Multiple Risk Factor Intervention Trial. Am J Clin Nutr. 1997;65(1):211S–27S. [DOI] [PubMed] [Google Scholar]
- 31. Holmes E, Loo RL, Stamler J, Bictash M, Yap IKS, Chan Q, Ebbels T, De Iorio M, Brown IJ, Veselkov KA et al.. Human metabolic phenotype diversity and its association with diet and blood pressure. Nature. 2008;453(7193):396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Loo RL, Coen M, Ebbels T, Cloarec O, Maibaum E, Bictash M, Yap I, Elliott P, Stamler J, Nicholson JK et al.. Metabolic profiling and population screening of analgesic usage in nuclear magnetic resonance spectroscopy-based large-scale epidemiologic studies. Anal Chem. 2009;81(13):5119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dumas M, Maibaum EC, Teague C, Ueshima H, Zhou B, Lindon JC, Nicholson JK, Stamler J, Elliott P, Chan Q et al.. Assessment of analytical reproducibility of 1H NMR spectroscopy based metabonomics for large-scale epidemiological research: the INTERMAP Study. Anal Chem. 2006;78(7):2199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Elliott P, Posma JM, Chan Q, Garcia-Perez I, Wijeyesekera A, Bictash M, Ebbels TMD, Ueshima H, Zhao L, van Horn L et al.. Urinary metabolic signatures of human adiposity. Sci Transl Med. 2015;7(285):285ra62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, Müller M. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12(77). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med. 2005;24(19):2911–35. [DOI] [PubMed] [Google Scholar]
- 37. Alhassan A, Young J, Lean MEJ, Lara J. Consumption of fish and vascular risk factors: a systematic review and meta-analysis of intervention studies. Atherosclerosis. 2017;266:87–94. [DOI] [PubMed] [Google Scholar]
- 38. Lara JJ, Economou M, Wallace AM, Rumley A, Lowe G, Slater C, Caslake M, Sattar N, Lean ME. Benefits of salmon eating on traditional and novel vascular risk factors in young, non-obese healthy subjects. Atherosclerosis. 2007;193(1):213–21. [DOI] [PubMed] [Google Scholar]
- 39. Cottin SC, Sanders TA, Hall WL. The differential effects of EPA and DHA on cardiovascular risk factors. Proc Nutr Soc. 2011;70(2):215–31. [DOI] [PubMed] [Google Scholar]
- 40. Sakurai M, Stamler J, Miura K, Brown IJ, Nakagawa H, Elliott P, Ueshima H, Chan Q, Tzoulaki I, Dyer AR et al.. Relationship of dietary cholesterol to blood pressure: the INTERMAP study. J Hypertens. 2011;29(2):222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lin MH, Lu SC, Huang PC, Liu YC, Liu SY. The amount of dietary cholesterol changes the mode of effects of (n-3) polyunsaturated fatty acid on lipoprotein cholesterol in hamsters. Ann Nutr Metab. 2004;48(5):321–8. [DOI] [PubMed] [Google Scholar]
- 42. Nishimura T, Murakami K, Livingstone MB, Sasaki S, Uenishi K. Japan Dietetic Students' Study for N, Biomarkers G. Adherence to the food-based Japanese dietary guidelines in relation to metabolic risk factors in young Japanese women. Br J Nutr. 2015;114(4):645–53. [DOI] [PubMed] [Google Scholar]
- 43. Wang SS, Lay S, Yu HN, Shen SR. Dietary guidelines for Chinese residents (2016): comments and comparisons. J Zhejiang Univ Sci B. 2016;17(9):649–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Public Health England. The Eatwell Guide. Version 28 January2019; [Internet]. Available from: https://www.nhs.uk/live-well/eat-well/the-eatwell-guide/ (accessed 1 May 2019). [Google Scholar]
- 45. Oude Griep LM, Seferidi P, Stamler J, Van Horn L, Chan Q, Tzoulaki I, Steffen LM, Miura K, Ueshima H, Okuda N et al.. Relation of unprocessed, processed red meat and poultry consumption to blood pressure in East Asian and Western adults. J Hypertens. 2016;34(9):1721–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Netherton JC 3rd, Gurin S. Biosynthesis and physiological role of homarine in marine shrimp. J Biol Chem. 1982;257(20):11971–5. [PubMed] [Google Scholar]
- 47. Polychronopoulos P, Magiatis P, Skaltsounis A-L, Tillequin F, Vardala-Theodorou E, Tsarbopoulos A. Hormarine, a common metabolite in edible Mediterranean molluscs: occurrence, spectral data and revision of related structure. Natural Product Letters. 2001;15(6):411–8. [DOI] [PubMed] [Google Scholar]
- 48. Wojcik OP, Koenig KL, Zeleniuch-Jacquotte A, Costa M, Chen Y. The potential protective effects of taurine on coronary heart disease. Atherosclerosis. 2010;208(1):19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Waldron M, Patterson SD, Tallent J, Jeffries O. The effects of oral taurine on resting blood pressure in humans: a meta-analysis. Curr Hypertens Rep. 2018;20(9):81. [DOI] [PubMed] [Google Scholar]
- 50. Kim KS, Jang MJ, Fang S, Yoon SG, Kim IY, Seong JK, Yang HI, Hahm DH. Anti-obesity effect of taurine through inhibition of adipogenesis in white fat tissue but not in brown fat tissue in a high-fat diet-induced obese mouse model. Amino Acids. 2019;51(2):245–54. [DOI] [PubMed] [Google Scholar]
- 51. Svensson BG, Akesson B, Nilsson A, Paulsson K. Urinary excretion of methylamines in men with varying intake of fish from the Baltic Sea. J Toxicol Environ Health. 1994;41(4):411–20. [DOI] [PubMed] [Google Scholar]
- 52. Rath S, Heidrich B, Pieper DH, Vital M. Uncovering the trimethylamine-producing bacteria of the human gut microbiota. Microbiome. 2017;5(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cho CE, Taesuwan S, Malysheva OV, Bender E, Tulchinsky NF, Yan J, Sutter JL, Caudill MA. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: a randomized controlled trial. Mol Nutr Food Res. 2017;61(1). doi: 10.1002/mnfr.201600324. [DOI] [PubMed] [Google Scholar]
- 54. Koliada A, Syzenko G, Moseiko V, Budovska L, Puchkov K, Perederiy V, Gavalko Y, Dorofeyev A, Romanenko M, Tkach S et al.. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017;17(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Conlon MA, Bird AR.. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2014;7(1):17–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Elliott P, Posma JM, Chan Q, Garcia-Perez I, Wijeyesekera A, Bictash M, Ebbels TM, Ueshima H, Zhao L, van Horn L et al.. Urinary metabolic signatures of human adiposity. Sci Transl Med. 2015;7(285):285ra62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yu D, Shu XO, Rivera ES, Zhang X, Cai Q, Calcutt MW, Xiang YB, Li H, Gao YT, Wang TJ et al.. Urinary levels of trimethylamine-N-oxide and incident coronary heart disease: a prospective investigation among urban Chinese adults. J Am Heart Assoc. 2019;8(1):e010606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kelly RH, Yancey PH. High contents of trimethylamine oxide correlating with depth in deep-sea teleost fishes, skates, and decapod crustaceans. Biol Bull. 1999;196(1):18–25. [DOI] [PubMed] [Google Scholar]
- 59. Kim YS, Xun P, Iribarren C, Van Horn L, Steffen L, Daviglus ML et al.. Intake of fish and long-chain omega-3 polyunsaturated fatty acids and incidence of metabolic syndrome among American young adults: a 25-year follow-up study. Eur J Nutr. 2016;55(4):1707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.