Abstract
Previous clinical studies highlighted nonalcoholic fatty liver disease (NAFLD) as a hepatic facet of metabolic syndrome, which progresses toward Type 2 diabetes along with an elevation of HbA1c in the blood. Longitudinal observations were performed in a cohort of 2811 participants with no liver disease at inception. The rate of the conversion into NAFLD was 15.7% (440/2811), with a steady increase in prevalence observed in sub-cohorts with increasing HbA1c levels. Moreover, regression analysis indicated that HbA1c levels serve as the risk factors for NAFLD after multiple adjustments (odds ratio: 1.58, P-value < 0.004). When HbA1c-related molecular networks were investigated using natural language programming algorithms, multiple genetic/small molecular (SM) pathways were highlighted as connectors between the HbA1c levels and the development of NAFLD, including ones for nitric oxide, hypoxia and receptor for advanced glycation end products (RAGE). Our results suggest that increased levels of HbA1c may contribute to the progression of NAFLD either directly, by stimulating RAGE or indirectly, through the promotion of hypoxia and suppression of the release of NO. Further studies are needed to test the impact of HbA1c on the development of the chronic liver disease.
Keywords: HbA1c, non-alcoholic fatty liver disease, pathway analysis, RAGE
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a common chronic disorder characterized by fatty degeneration of hepatocytes, which is not caused by excessive consumption of alcohol. The pathological spectrum of NAFLD starts as simple steatosis, followed by its progression to nonalcoholic steatohepatitis (NASH), which may lead to the development of cirrhosis and/or liver cancer [1]. Being the most common cause of elevated liver enzymes, NAFLD became a major epidemiological concern worldwide [2], with the prevalence in the last decade reaching 12–24% [3,4]. Individuals with NAFLD have a high frequency of metabolic comorbidities to the degree that NAFLD was dubbed a hepatic manifestation of metabolic syndrome [5]. Moreover, NAFLD is also the most common cause of elevated liver enzymes, and thereby became a major epidemiological concern worldwide [2], with the prevalence in the last decade reaching 12–24% [3]. The pathophysiology of NAFLD and its progression to NASH and to liver fibrosis is influenced by both genetics and environmental factors, which augment each other as a set of multiple parallel hits, with oxidative stress as a prominent promoter of the damage to hepatic parenchyma [6,7].
Glycosylated hemoglobin (HbA1c) is a product of the non-enzymatic chemical reaction between glucose and the N-terminus of the h chain (valine) amino groups on hemoglobin protein. From the molecular biology point of view, HbA1c belongs to a diverse group of molecules termed advanced glycation end-products (AGEs). In each individual, diabetic or non-diabetic, relative levels of HbA1c reflect the duration and the severity of hyperglycemic episodes as well as overall exposure to the extracellular glucose present in circulation. At varying cut-offs, the levels of HbA1c were developed as biomarkers both for the diagnosis of diabetes and for the premorbid chronic glycemic control [8,9].
While an association of diabetes with NAFLD and NASH is well documented [10–12], the relationship of the HbA1c levels to the development of NAFLD is less clear. All aspects of the pathophysiology of NAFLD [13], including individuals’ propensity to form advanced glycation end-products (AGE) including HbA1C [14], and the intensity of pro-inflammatory signal produced by binding of AGE to various isoforms of its receptor RAGE [15,16] are influenced by inherited gene variants.
To untangle the complex relationships between HbA1C as a functional molecule and NAFLD, we integrated large-scale literature-based relation data with the clinical and performed a comprehensive multiple level analysis. Obtained results may aid in the understanding of the pathophysiology of both NAFLD and Type 2 diabetes.
Materials and methods
We studied the association between NAFLD and HbA1c at three levels: first, we conducted a clinical study to dissect the relationship between NAFLD and HbA1c; second, we explored the relationship between NAFDLD and HbA1c as a molecule through a large scale literature-based pathway analysis; finally, we analyzed small molecules connecting elevation of HbA1c levels to the development of NAFLD.
The clinical study design
The participant’s selection
Study participants (N = 4905; age range: 45–89) were recruited from the South China area (Ningbo, Zhejiang province) in 2012. The study population included 2753 males and 2152 females. After 3 years of annual follow up (2013, 2014 and 2015) and relative data quality control, 2094 participants were excluded from the study, while 2811 subjects met the criteria of the study (male/female: 1664/1147; sex ratio 1.45: 1; average age: 58.2 ± 9.8 years). The study was reviewed and approved by the Ethics Committee of the Zhenhai Lianhua Hospital, Ningbo, China. Informed consent was obtained from all individual participants. All methods were performed in accordance with the relevant guidelines and regulations.
The exclusion criteria were as follows: (1) participant diagnosed with a specific liver disease, including fatty liver, viral hepatitis, Wilson’s disease, autoimmune liver disease or drug-induced liver disease; (2) participants have a long history of alcohol consumption or consumptions of ethanol were more than 140 g/week for men and more than 70 g/week for women; (3) participants have been recently treated with liver protecting drugs; (4) participants signed the consent but waived the physical examinations; (5) the data collection for a participant was not complete.
Diagnosis of NAFLD
The diagnosis of NAFLD was based on the criteria suggested by the Chinese Liver Disease Association (http://www.heporg.com), and the clinical diagnostic standards [17] (Fan et al. 2011) for the determination of NAFLD as described as our previous publication [18] (Chen 2017).
Detection of HbA1c
A 10 ml volume of fasting venous blood was drawn from the cubital vein and centrifuged to prepare the serum, which was subsequently used for biochemical analysis and HbA1c index test. The biochemical indices include ALT, AST, GGT, TG, TC, HDL-C, UA, FPG, etc. Each of these biochemical indicators was tested using the same Au640 fully automatic biochemical analyzer (Olympus, Kobe, Japan). HbA1c was tested using an HLC-723 G7 fully automatic HbA1c analyzer (Tosoh Chemical Company, Tokyo, Japan), and reagents and quality control products were provided by the same company. The analysis was conducted by experimental methodological tests.
Statistical analysis
Statistical analysis was performed by the SPSS 18.0 software package (IBM Corp., Armonk, NY, U.S.A.). The variables were expressed as the mean ± standard deviation or median ± quartile interval. Continuous variables were compared using the T-test (normal distribution) or Mann–Whitney test (not consistent with normal distribution). The chi-square test was used to examine categorical data rates. The Cox proportional hazards model was used to evaluate the risk factors for NAFLD, with the following factors included prior to running the analysis: age, gender, waist circumference, body mass index, systolic pressure, diastolic pressure, total cholesterol, triacylglycerol, low-density lipoprotein cholesterol, fasting blood glucose, high-density lipoprotein cholesterol and serum HbA1c. The difference was considered to be statistically significant when P < 0.05.
HbA1c–gene and NAFLD–gene relation data acquisition
We acquired the HbA1c–gene and NAFLD–gene relation data from Pathway Studio (www.pathwaystudio.com) and presented the related information in a searchable table file HbA1c_NAFLD, which is publicly available at (http://gousinfo.com/database/Data_Genetic/HbA1c_NAFLD.xlsx). The set of genes with shared association with both NAFLD and HbA1c was analyzed with Gene Set Enrichment Analysis (GSEA) against Gene Ontology (GO) and Pathway Studio Ontology (PSO).
The study of the compounds acting upon HbA1c and NAFLD
The relationship of HbA1c and NAFLD was explored by querying a set of compounds with evidence of influencing either levels of HbA1C or the NAFLD or both. The relation data were also acquired from Pathway Studio as described above, with all supporting data provided in the database HbA1c_NAFLD.
Shortest-path analysis
Based on the molecular relations identified, pathways that connecting HbA1c and NAFLD were build using the ‘Shortest-Path’ model within the Pathway Studio environment (www.pathwaystudio.com), with the purpose to identify genes and compounds/drugs, through which HbA1c and NAFLD may influence each other. The information of the references supporting the relationships in a pathway was provided in the Supplementary Material (HbA1c_NAFLD). Within Pathway Studio, there is a Confidence Score to evaluate the quality of a relationship. The higher the score, the higher the quality of a relation.
Result
Patients with ‘upper normal’ HbA1C levels are more likely to develop NAFLD than patients with ‘core normal’ levels
This prospective study aimed at detecting the accumulation of NAFLD diagnosis in a cohort of patients with healthy livers and normal levels of HbA1c (5.6% or less) levels of HbA1c at baseline. Notably, among the 2811 participants of the present study, we have identified 193 subjects with healthy liver and abnormal (pre-diabetic) levels of HbA1c (between 5.7% and 6.5%). On average, patients with abnormal levels of HbA1c at baseline were older (N = 193; mean age: 64.3 ± 10.4 years) than those with the levels of HbA1c falling within a normal range (N = 2618, mean age: 57.8 ± 10.2 years). At the end of 3 years follow-up, the cumulative incidence of NAFLD was 440/2811 cases (15.7%), including 285 males (17.1%) and 155 females (13.5%). As expected, the incidence of NAFLD in patients with normal levels of HbA1c was lower (391/2618 or 14.9%) than that in the pre-diabetic group (49/193 or 25.4%) (χ2 = 6.717, P < 0.010).
At the next stage of analysis, patients with normal levels of HbA1c were further subdivided into ‘upper normal’ (HbA1c between 5.4% and 5.6%, N = 773) and ‘core normal’ (HbA1c of 5.3% or less, N = 1411) groups. As could be seen from Table 1, the levels of HbA1c continued to have a significant relation with the incidence rate of NAFLD when all three groups were compared (df = 2, χ2 = 8.59, P < 0.014).
Table 1. The incidence of NAFLD with different levels of HbA1c.
| Percentage of glycosylated hemoglobin | ||||
|---|---|---|---|---|
| ≤5.3% | >5.3% - ≤5.6% | 5.7–6.5% | Total | |
| Did not develop NAFLD | 1213 | 651 | 507 | 2371 |
| Developed NAFLD | 198 | 122 | 120 | 440 |
| Incidence rate | 14.03% | 15.78% | 19.14% | 15.65% |
The relationship between the levels of HbA1c and the likelihood to develop NAFLD is linear
To eliminate confounding effects of other risk factors when considering the influence of the levels of HbA1c on NAFLD, the relationships between NAFLD and each clinical parameter were tested in the multivariate Cox proportional hazards regression model. As could be seen in Table 2, maximally adjusted model 4 suggests that NAFLD is more likely to occur in individuals with elevated HbA1c levels, with the odds ratio of 1.58, and P-value < 0.004. Other parameters contributing to the risk of NAFLD included gender, age, the levels of uric acid, AST, ALT and triglycerides as well as body mass index and waist circumference (all P-values < 0.05) (Table 2).
Table 2. Multivariate Cox proportional hazards regression analyses in entire cohort.
| β | SE | Wald x2 value | P value | OR | 95% CI | |
|---|---|---|---|---|---|---|
| Model 1 | 0.586 | 0.152 | 14.935 | <0.001 | 1.796 | 1.335–2.418 |
| Model 2 | 0.674 | 0.155 | 18.956 | <0.001 | 1.963 | 1.449–2.659 |
| Model 3 | 0.587 | 0.157 | 14.07 | <0.001 | 1.799 | 1.324–2.445 |
| Model 4 | 0.458 | 0.188 | 8.454 | <0.005 | 1.580 | 1.161–2.152 |
Note: Model 1 was unadjusted. Model 2 was adjusted for age and sex. Model 3 was adjusted for age, sex and body mass index. Model 4 was adjusted for all Model 3 variables associated plus these associated with metabolic syndrome, including waist circumference, systolic blood pressure, diastolic blood pressure, triglycerides, HDL cholesterol, and fasting blood sugar. β is partial regression coefficient; SE is standard error of partial regression coefficient; OR is odds ratio; CI is confidence interval. P-value specifies the possibility that a parameter is not a risk factor for NAFLD.
Other potential influential factors of NAFLD
During the 3-year follow-up period, 440 incident NAFLD cases (285 men and 155 women) were identified, and the cumulative incidence was 15.7% (440/2811). The baseline characteristics of participants were compared according to follow-up outcomes. Compared with remained NAFLD-free participants, incident NAFLD participants had higher baseline waist circumference, body mass index, systolic and diastolic blood pressure, triacylglycerol, total and low-density lipoprotein cholesterol, glutamyl-transpeptidase, fasting blood glucose, uric acid, hemoglobin and HbA1c levels, while lower baseline high-density lipoprotein cholesterol levels (Table 3).
Table 3. Comparison of baseline characteristics of participates according to follow-up outcomes.
| Variable | Did not develop NAFLD (n = 2371) | Developed NAFLD (n = 440) | t value | P value |
|---|---|---|---|---|
| Age (year) | 58.4±10.0 | 57.2±9.2 | −2.42 | 0.016 |
| Waist circumference (cm) | 80.6±8.2 | 85.8±7.6 | 12.2+ | <0.001 |
| Body mass index (kg/m2) | 22.5±2.5 | 24.5±2.4 | 15.21 | <0.001 |
| Systolic blood pressure (mmHg) | 125.6±16.2 | 128.9±16.3 | 3.87 | <0.001 |
| Diastolic blood pressure (mmHg) | 77.8±9.5 | 80.0±9.7 | 4.39 | <0.001 |
| Total cholesterol (mmol/l) | 5.1 (4.5,5.7) | 5.3 (4.6,5.9) | 3.05* | 0.002 |
| Triglycerides (mmol/l) | 1.0 (0.7,1.3) | 1.3 (1.0,1.8) | 12.34* | <0.001 |
| HDL cholesterol (mmol/l) | 1.7±0.4 | 1.5±0.3 | −9.73 | <0.001 |
| LDL cholesterol (mmol/l) | 2.9±0.7 | 3.1±0.8 | 3.87 | <0.001 |
| Glutamine transpeptidase (U/l) | 19 (14,26) | 25 (18,34) | 9.83* | <0.001 |
| Fasting blood sugar (mmol/l) | 5.2±0.9 | 5.4±1.1 | 2.56 | 0.010 |
| Uric acid (μmol/l) | 287.6±75.5 | 315.4±74.5 | 7.11 | <0.001 |
| Hemoglobin (g/l) | 133.6±13.7 | 137.4±12.9 | 5.41 | <0.001 |
| Glycosylated hemoglobin (%) | 5.4±0.5 | 5.5±0.7 | 2.59 | 0.010 |
Abbreviations: *, z value; HDL, high-density lipoprotein; LDL, low-density lipoprotein
Genes contributing to the levels of HbA1c and to NAFLD
Pathway Studio-guided analysis highlighted a total of 71 genes associated with the levels of HbA1c. This list of genes was supported by 141 peer-reviewed sources published from 1982 to 2018. For NAFLD, we identified 898 associated genes, supported by over 4000 references published from 1999 to 2018. A significant overlap of 54 genes (Fisher-Exact test P-value<1.82e-65) between the HbA1c- and NAFLD-associated gene sets was detected. This overlap covered approximately 76.1% of the HbA1c gene set (54/71) and 6.01% of the NAFLD gene set (54/898). For the list of these 54 genes and the supporting reference, please refer to HbA1c_NAFLD→ 54 Common Genes and HbA1c_NAFLD→ Ref for 54 Common Genes.
A Gene Set Enrichment Analysis (GSEA) was conducted to evaluate the functional profile of the 54 genes associated with both HbA1c and NAFLD. The top 10 significantly enriched pathways (FDR-corrected p-value7e-15) are presented in Table 4. The complete list of 29 pathways/gene sets with p-values for their enrichment below the cut-off of < 10e-10 are provided in HbA1c_NAFLD→ GSEA.
Table 4. Functional pathways enriched by 54 genes contributing to both NAFLD and HbA1c levels.
| Name | Overlap genes | Jaccard similarity | P-value after FDR | P-value before FDR |
|---|---|---|---|---|
| Response to nutrient levels | 24 | 0.040268 | 2.07E-20 | 1.55E-24 |
| Response to extracellular stimulus | 24 | 0.038339 | 5.3E-20 | 5.28E-24 |
| Response to hormone | 26 | 0.0282 | 9.73E-19 | 1.46E-22 |
| Insulin -> STAT Expression Targets | 20 | 0.103627 | 1.96E-17 | 6.84E-21 |
| Response to peptide hormone | 19 | 0.04034 | 3.68E-16 | 1.84E-19 |
| Insulin -> CEBPA/CTNNB/FOXA/FOXO Expression Targets | 19 | 0.093137 | 1.43E-15 | 8.19E-19 |
| Response to peptide | 19 | 0.036538 | 2.25E-15 | 1.35E-18 |
| Aging | 18 | 0.040359 | 2.78E-15 | 1.74E-18 |
| Insulin -> MEF/MYOD Expression Targets | 19 | 0.090047 | 2.78E-15 | 1.8E-18 |
| Insulin -> ELK/SRF/HIF1A/MYC/SREBF Expression Targets | 19 | 0.086364 | 6.77E-15 | 4.73E-18 |
Possible co-regulatory events shared for HbA1c levels and NAFLD
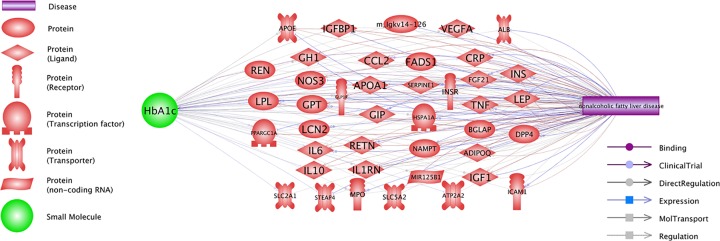
Pathway Studio guided Shortest-Path analysis identified multiple molecular networks, through which the levels of HbA1c and the development of NAFLD may be mutually influencing each other (Figure 1). These networks encompass 41 out of 54 shared contributors, with a total of 97 relations supported by 1001 references (Figure 1). For the detailed description of each of these HbA1c–gene–NAFLD relations, please refer to HbA1c_NAFLD→Co-regulation Network, where the supporting references and corresponding sentences are systematically described.
Figure 1. Positive co-regulation of the levels of HbA1c and the development of NAFLD.
Small molecules capable of influencing both the development of NAFLD and the levels of HbA1C
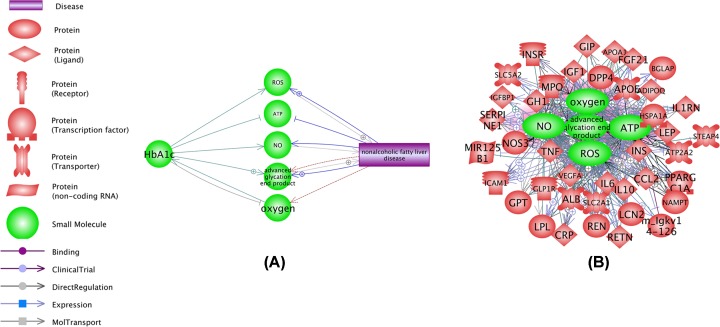
Figure 2A shows an output of ‘Shortest Path’ analysis, which identified 5 small molecules/ drugs bridging HbA1c and NAFLD. The relations presented in Figure 2A were supported by 237 references (see HbA1c_NAFLD→Ref for 5 SMs).
Figure 2. Small molecules contributing to, or responding to both the levels of HbA1c and the development of NAFLD.
(A) The levels of HbA1c and the development of NAFLD are connected by 5 molecules. (B) Relation network which integrated 5 small molecules highlighted by the ‘Shortest Path’ analysis, and 40 out of 41 genes that connect elevated levels of HbA1c and the development of NAFLD.
Notably, 40 out of 41 genes in Figure 1 also displayed a strong set of relations with all the 5 small molecular interactors (Figure 2B). These relations were supported by over 10,000 references. For the detailed description of the disease–SM–gene relations, please refer HbA1c_NAFLD→5 SMs_41 Genes.
Discussion
Blood levels of glycated hemoglobin (HbA1c) are commonly used as a screening tool for detecting insulin resistance in general, and the presence of Type 2 diabetes [19]. Insulin resistance is a known pathophysiological driver of NAFLD [20–22]. Recent studies suggest that the levels of HbA1c levels may contribute to the development of NAFLD directly [10,11]. In this work, and also some previous works, an association of HbA1c levels and the risk for NAFLD was demonstrated for nondiabetic individuals, independently of obesity and other metabolic components [11]. Notably, the same study also showed that the magnitude of association of HOMA-IR scores with HbA1c levels in subjects already diagnosed with NAFLD is greater than that in those with healthy livers [11].
We confirmed the association between the levels of HbA1c and NAFLD in metabolically intact patients (HbA1C levels of 5.6% or lower). The rate of the conversion into NAFLD was 15.7% (440/2811) for an entire cohort, with a steady increase in prevalence observed in sub-cohorts with increasing HbA1c levels. Moreover, regression analysis highlighted HbA1c served the risk factors for NAFLD after multiple adjustments (odds ratio: 1.58, P-value < 0.004).
We have also explored the relationship between the levels of HbA1c and the development of NAFLD by systematic mining of existing literature, and have identified multiple pathophysiologically relevant connections. In particular, disease–gene relation data analysis showed that a majority of the genes associated with the levels of HbA1c were also implicated with NAFLD, with a significant overlap between two gene sets (P-value < 1.82e-65). Genes that belong to this overlap were significantly enriched in pathways critical for the response to nutrient levels [23,24] and hormonal stimulation [25,26] (see Table 4 and HbA1c_NAFLD→GSEA).
Further analysis demonstrated that the levels of HbA1c and the development of NAFLD are mutually and positively influencing each other. For example, one of the pathways presented in Figure 2 is HbA1c→NOS3→NAFLD. It has been shown that increased concentrations of HbA1c are capable of significantly down-regulating the expression of NOS3 [27]; resultant deficiency of NOS3 is a known contributor to NAFLD [28]. Another example is the HbA1c→ IL10→NAFLD pathway. While IL10 plays a protective role in NAFLD pathogenesis [29], high concentrations of HbA1c influence the production of this molecule negatively and precipitate the development of the disease [30]. The discovery of these connecting pathways suggests an engagement of a positive feedback loop. For the information on other connecting pathways presented in Figure 2, please refer to HbA1c_NAFLD→ Co-regulation Network.
A set of 5 small molecules related to both the levels of HbA1c and NAFLD (Figure 2A) and related literature are summarized in HbA1c_NAFLD→Ref for 5 SMs. Elevated amounts of HbA1c lead to a decrease in the production of nitric oxide (NO) [31] and the development of the state of hypoxia [32], which both have important implications for NAFLD [33,34]. Further, a recent study showed that persistent elevation of HbA1c levels could lead to the production and accumulation of advanced glycation end products (AGE) [35], yet another NAFLD contributor [36]. As HbA1c is one of high volume AGE products, Hb-AGE, it is worthwhile to note that a close correlation of the amounts of HbA1c and Hb-AGE across diabetic subjects was previously observed, with the magnitude of this effect increases in the cohorts with least degree of glycemic control [37].
Additionally, we noted that 40 out of 41 genes included in the HbA1c→gene→NAFLD pathways (Figure 1) also had demonstrated strong associations with all the 5 drugs presented at Figure 2A, with a total of 317 relations supported by over 10,000 references (see Figure 2B and HbA1c_NAFLD→5 SMs_41 Genes). The remarkable density of gene–small molecule interaction networks that connects both HbA1a and NAFLD indicates close, possibly functional inter-relationship between these two aspects of metabolic syndrome. Biological mechanisms mediating HbA1c→gene/SM→NAFLD network are worthy of further study.
One limitation of the present study was that the clinical data were short of insulin resistance (IR) measurement, which could be a causal factor that leads to increased HbA1c levels and NAFLD incidence. Therefore, we speculate that the HbA1c level in the range of 5.4 to 5.6% may be reflective of early insulin resistance and thus may not be entirely healthy. Further study is needed to test the impact of IR on the HbA1c−NAFLD association observed here.
Conclusion
The results of the present study suggested that increased levels of HbA1c may contribute to the development of NAFLD. This form of altered hemoglobin may contribute to the progression of NAFLD either directly, by stimulating RAGE or indirectly, through the promotion of hypoxia and suppression of the release of NO. Multiple molecular pathways identified by applying natural language programming algorithms may aid in improving our understanding of the underlying pathophysiology of NAFLD.
Acknowledgements
We would like to thank Dr. Ancha Baranova from George Mason University for her contribution to the editing of the manuscript.
Abbreviations
- NAFLD
nonalcoholic fatty liver disease
- RAGE
receptor for advanced glycation end products
- SM
small molecular
Contributor Information
Juan Du, Email: j.du@gousinfo.com.
Hongbao Cao, Email: caohon2010@gmail.com.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This project is partially supported by the National Key R&D Program of China (NO. 2017YFC0908103); Ningbo Natural Science Foundation [grant number 2017A610276]; The Social Development Program of Ningbo, China [grant number 2013C50044]; The Social Development and Science and Techology Program of Zhenhai, Ningbo, China [grant numbers 2013S1002 and 2014020].; Ningbo Natural Science Foundation [grant number 2017A610276]; The Social Development Program of Ningbo, China [grant number 2013C50044]; The Social Development and Science and Technology Program of Zhenhai, Ningbo, China [grant numbers 2013S1002 and 2014020].
Author Contribution
C.C. and J.D. conceived and designed the study strategy. C.C., Z.Z., Y.M., Y.X., J.D. and X.T. recruited the participants and collected all the information and blood samples. C.C. and JD. with an aid of H.C. and X.T. collected and analyzed the data. C.C., J.D., H.C. and X.T. wrote and revised the manuscript. All authors read and approved the final manuscript.
Data Availability
Data are available by contacting the corresponding author.
References
- 1.Feijo S.G. et al. (2013) The spectrum of non alcoholic fatty liver disease in morbidly obese patients: prevalence and associate risk factors. Acta Cirurgica Brasileira 28, 788–793 10.1590/S0102-86502013001100008 [DOI] [PubMed] [Google Scholar]
- 2.Vernon G., Baranova A. and Younossi Z.M. (2011) Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 34, 274–285 10.1111/j.1365-2036.2011.04724.x [DOI] [PubMed] [Google Scholar]
- 3.Fan J.G. (2013) Epidemiology of alcoholic and nonalcoholic fatty liver disease in China. J. Gastroenterol. Hepatol. Suppl. 1, 11–17 10.1111/jgh.12036 [DOI] [PubMed] [Google Scholar]
- 4.Younossi Z. et al. (2018) Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 15, 11–20 10.1038/nrgastro.2017.109 [DOI] [PubMed] [Google Scholar]
- 5.Abenavoli L. et al. (2016) Metabolic aspects of adult patients with nonalcoholic fatty liver disease. World J. Gastroenterol. 22, 7006–7016 10.3748/wjg.v22.i31.7006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masarone M. et al. (2018) Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxid Med. Cell Longev 2018, 9547613 10.1155/2018/9547613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakaguchi S., Takahashi S., Sasaki T., Kumagai T. and Nagata K. (2011) Progression of alcoholic and non-alcoholic steatohepatitis: common metabolic aspects of innate immune system and oxidative stress. Drug Metab. Pharmacokinet. 26, 30–46 10.2133/dmpk.DMPK-10-RV-087 [DOI] [PubMed] [Google Scholar]
- 8.Chamberlain J.J., Rhinehart A.S., Shaefer C.F. and Neuman A. (2016) Diagnosis and Management of Diabetes: Synopsis of the 2016 American Diabetes Association Standards of Medical Care in Diabetes. Ann. Intern. Med. 164, 542–552 10.7326/M15-3016 [DOI] [PubMed] [Google Scholar]
- 9.Bennett C.M., Guo M. and Dharmage S.C. (2007) HbA(1c) as a screening tool for detection of Type 2 diabetes: a systematic review. Diabet. Med. 24, 333–343 10.1111/j.1464-5491.2007.02106.x [DOI] [PubMed] [Google Scholar]
- 10.Fan J.G. et al. (2011) Guidelines for the diagnosis and management of nonalcoholic fatty liver disease: update 2010. J. Dig. Dis. 12, 38–44 10.1111/j.1751-2980.2010.00476.x [DOI] [PubMed] [Google Scholar]
- 11.Bae J.C. et al. (2010) Impact of nonalcoholic fatty liver disease on insulin resistance in relation to GH levels in nondiabetic subjects. Am. J. Gastroenterol. 105, 2389–2395 10.1038/ajg.2010.275 [DOI] [PubMed] [Google Scholar]
- 12.Ma H. et al. (2013) Independent association of HbA1c and nonalcoholic fatty liver disease in an elderly Chinese population. BMC Gastroenterol. 13, 3 10.1186/1471-230X-13-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danford C.J., Yao Z.M. and Jiang Z.G. (2018) Non-alcoholic fatty liver disease: a narrative review of genetics. J. Biomed Res. 32, 389–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams J.N. et al. (2016) Genetic analysis of advanced glycation end products in the DHS MIND study. Gene 584, 173–179 10.1016/j.gene.2016.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reynaert N.L., Gopal P., Rutten E.P.A., Wouters E.F.M. and Schalkwijk C.G. (2016) Advanced glycation end products and their receptor in age-related, non-communicable chronic inflammatory diseases; Overview of clinical evidence and potential contributions to disease. Int. J. Biochem. Cell Biol. 81, 403–418 10.1016/j.biocel.2016.06.016 [DOI] [PubMed] [Google Scholar]
- 16.Mehta R. et al. (2018) Polymorphisms in the receptor for advanced glycation end-products (RAGE) gene and circulating RAGE levels as a susceptibility factor for non-alcoholic steatohepatitis (NASH). PLoS One 13, e0199294 10.1371/journal.pone.0199294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan J.G. et al. (2011) Chinese Association for the Study of Liver Disease. Guidelines for the diagnosis and management of nonalcoholic fatty liver disease: update 2010. J. Dig. Dis. 12, 38–44 10.1111/j.1751-2980.2010.00476.x [DOI] [PubMed] [Google Scholar]
- 18.Chen C.X. et al. (2017) Possible association between Helicobacter pylori infection and nonalcoholic fatty liver disease. Appl. Physiol. Nutr. Metab. 42, 295–301 10.1139/apnm-2016-0499 [DOI] [PubMed] [Google Scholar]
- 19.Onal Z.E. et al. (2014) Association of glycosylated hemoglobin (HbA1c) levels with Iinsulin resistance in obese children. Afr. Health Sci. 14, 533–538 10.4314/ahs.v14i3.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Utzschneider K.M. and Kahn S.E. (2006) Review: The role of insulin resistance in nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 91, 4753–4761 10.1210/jc.2006-0587 [DOI] [PubMed] [Google Scholar]
- 21.Kitade H., Chen G., Ni Y. and Ota T. (2017) Nonalcoholic Fatty Liver Disease and Insulin Resistance: New Insights and Potential New Treatments. Nutrients 9, 387–400 10.3390/nu9040387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stephen S., Baranova A. and Younossi Z.M. (2012) Nonalcoholic fatty liver disease and bariatric surgery. Expert Rev. Gastroenterol. Hepatol. 6, 163–171 10.1586/egh.11.97 [DOI] [PubMed] [Google Scholar]
- 23.Jiang J. et al. (2012) Dietary fiber intake is associated with HbA1c level among prevalent patients with type 2 diabetes in Pudong New Area of Shanghai, China. PLoS One 7, e46552 10.1371/journal.pone.0046552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antonucci L., Porcu C., Iannucci G., Balsano C. and Barbaro B. (2017) Non-Alcoholic Fatty Liver Disease and Nutritional Implications: Special Focus on Copper. Nutrients. 9, 1137–1149 10.3390/nu9101137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pampanelli S. et al. (1996) Long-term intensive insulin therapy in IDDM: effects on HbA1c, risk for severe and mild hypoglycaemia, status of counterregulation and awareness of hypoglycaemia. Diabetologia 39, 677–686 10.1007/BF00418539 [DOI] [PubMed] [Google Scholar]
- 26.Meli R., Mattace Raso G. and Calignano A. (2014) Role of innate immune response in non-alcoholic Fatty liver disease: metabolic complications and therapeutic tools. Front. Immunol. 5, 177 10.3389/fimmu.2014.00177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bo J. et al. (2015) Impairment of Endothelial Cell Function Induced by Hemoglobin A(1c) and the Potential Mechanisms. Exp. Clin. Endocrinol. Diabetes 123, 529–535 10.1055/s-0035-1554622 [DOI] [PubMed] [Google Scholar]
- 28.Nozaki Y. et al. (2015) Deficiency of eNOS exacerbates early-stage NAFLD pathogenesis by changing the fat distribution. BMC Gastroenterol. 15, 177 10.1186/s12876-015-0409-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMahan R.H. et al. (2013) Bile acid receptor activation modulates hepatic monocyte activity and improves nonalcoholic fatty liver disease. J. Biol. Chem. 288, 11761–11770 10.1074/jbc.M112.446575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zahran W.E., Salah El-Dien K.A., Kamel P.G. and El-Sawaby A.S. (2013) Efficacy of Tumor Necrosis Factor and Interleukin-10 Analysis in the Follow-up of Nonalcoholic Fatty Liver Disease Progression. Indian J. Clin. Biochem. 28, 141–146 10.1007/s12291-012-0236-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manju M., Mishra S., Toora B.D., kumar V. and Vinod R. (2014) Relationship between Glycosylated Hemoglobin, Serum Nitric Oxide and Mean Arterial Blood Pressure. Int. J. Biomed. Sci. 10, 252–257 [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider M.P. et al. (2013) Poor glycemic control is related to increased nitric oxide activity within the renal circulation of patients with type 2 diabetes. Diabetes Care. 36, 4071–4075 10.2337/dc13-0806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Oliveira C.P. et al. (2006) Hepatic gene expression profile associated with non-alcoholic steatohepatitis protection by S-nitroso-N-acetylcysteine in ob/ob mice. J. Hepatol. 45, 725–733 10.1016/j.jhep.2006.05.017 [DOI] [PubMed] [Google Scholar]
- 34.Sundaram S.S. et al. (2016) Nocturnal hypoxia-induced oxidative stress promotes progression of pediatric non-alcoholic fatty liver disease. J. Hepatol. 65, 560–569 10.1016/j.jhep.2016.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kadłubowska J., Malaguarnera L., Wąż P. and Zorena K. (2016) Neurodegeneration and Neuroinflammation in Diabetic Retinopathy: Potential Approaches to Delay Neuronal Loss. Curr. Neuropharmacol. 14, 831–839 10.2174/1570159X14666160614095559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdelmegeed M.A. et al. (2012) Critical role of cytochrome P450 2E1 (CYP2E1) in the development of high fat-induced non-alcoholic steatohepatitis. J. Hepatol. 57, 860–866 10.1016/j.jhep.2012.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turk Z., Mesić R. and Benko B. (1998) Comparison of advanced glycation endproducts on haemoglobin (Hb-AGE) and haemoglobin A1c for the assessment of diabetic control. Clin. Chim. Acta 277, 159–170 10.1016/S0009-8981(98)00128-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available by contacting the corresponding author.