Abstract
Background/objectives
Pentraxin 3 (PTX3) has been characterized as a soluble and multifunctional pattern recognition protein in the regulation of innate immune response. However, little is known about its role in adipose tissue inflammation and obesity. Herein, we investigated the role of PTX3 in the regulation of lipopolysaccharide (LPS)-induced inflammation in adipocytes and adipose tissue, as well as high-fat diet (HFD)-induced metabolic inflammation in obesity.
Methods
Ptx3 knockdown 3T3-L1 Cells were generated using shRNA for Ptx3 gene and treated with different inflammatory stimuli. For the in vivo studies, Ptx3 knockout mice were treated with 0.3 mg/kg of LPS for 6 h. Adipose tissues were collected for gene and protein expression by qPCR and western blotting, respectively. Ptx3 knockout mice were fed with HFD for 12 week since 6 week of age.
Results
We observed that the expression of PTX3 in adipose tissue and serum PTX3 were markedly increased in response to LPS administration. Knocking down Ptx3 in 3T3-L1 cells reduced adipogenesis and caused a more profound and sustained upregulation of proinflammatory gene expression and signaling pathway activation during LPS-stimulated inflammation in 3T3-L1 adipocytes. In vivo studies showed that PTX3 deficiency significantly exacerbated the LPS-induced upregulation of inflammatory genes and downregulation of adipogeneic genes in visceral and subcutaneous adipose tissue of mice. Accordingly, LPS stimulation elicited increased activation of nuclear factor-κB (NF-κB) and p44/42 MAPK (Erk1/2) signaling pathways in visceral and subcutaneous adipose tissue. The expression of PTX3 in adipose tissue was also induced by HFD, and PTX3 deficiency led to the upregulation of proinflammatory genes in visceral adipose tissue of HFD-induced obese mice.
Conclusions
Our results suggest a protective role of PTX3 in LPS- and HFD-induced sustained inflammation in adipose tissue.
Subject terms: Biochemistry, Genetics, Cell biology
Introduction
Low-grade chronic inflammation in adipose tissue is a causal link between obesity and insulin resistance [1]. Recent studies suggest that the activation of innate immune receptors, called pattern recognition receptors (PRR), triggers the inflammatory responses in adipose tissue [2]. The cellular PRR are either expressed on the cell surface such as toll-like receptors (TLRs, membrane bound PRR) or present in the cytoplasm such as nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs, cytoplasmic PRR). In addition, the humoral PRR including collectins and pentraxins are secreted and present in the body fluid [3–5]. Activation of cellular innate immune receptors TLRs and NLRs have been implicated in the recognition of metabolic stress and the initiation of inflammatory responses in various tissues during the development of metabolic diseases [6, 7]. The humoral recognition has also been found to mediate the innate immune response to microbial moieties via interacting with cellular PRR [8]. However, it remains largely unknown about the mechanisms of how the dysfunction of innate immune system contributes to adipose tissue inflammation during the onset of obesity and insulin resistance.
Pentraxin 3 (PTX3), also known as tumor necrosis factor (TNF)-inducible gene 14 protein (TSG-14), belongs to the pentraxin family of soluble and multifunctional pattern recognition proteins with a cyclic multimeric structure due to the presence of unique N-terminal domain. PTX3 acts as a predecessor of antibodies to facilitate pathogen recognition through interacting with several ligands, including growth factors, extracellular matrix components, and selected pathogens [9–11]. PTX3 also mediates C1q recognition via the site of sugar fucosylation and sialylation in N-terminal domain, playing a role in complement activation in response to proinflammatory signals and TLR stimulation [12, 13].
PTX3 can be produced and released by various cell types at the inflammatory site and is involved in innate immune responses [14–16]. On the one hand, Ptx3 gene promoters contain activator protein-1 (AP-1), nuclear factor-kappa B (NF-κB), and selective promoter 1 (SP1) [17]. Ptx3 gene expression is strongly upregulated by inflammatory inducers lipopolysaccharide (LPS), IL1β, and TNFα [18, 19], as well as high-density lipoprotein [20]. On the other hand, PTX3 regulate the immune response in dendritic cells, apoptotic cells [21, 22], and macrophages [23]. The above information suggests the potential role of PTX3 in the control of inflammation and metabolism.
Emerging evidence has shown that PTX3 is a useful new serological marker, modulating the immunoinflammatory response under diverse clinical conditions, including infection [24], female fertility [25], and vascular pathology [26]. However, little is known about the role of PTX3 in adipose tissue inflammation in obesity. In cell-based studies, PTX3 has been shown to be secreted from adipocytes in response to TNFα stimulation [27]. In obese mouse models, Ptx3 mRNA levels are increased in adipose tissue of genetically obese (ob/ob and db/db) mice[27]. In obese humans, while PTX3 mRNA levels in the visceral adipose tissue elevate, plasma PTX3 protein levels reduce [28, 29]. Although these preclinical findings strongly suggest that PTX3 is a potential link between chronic low-level inflammatory state and obesity, whether and how PTX3 plays its role in the intersection of immunology and metabolism during obesity remains poorly understood.
In this study, we investigated the role of PTX3 in the regulation of inflammation in adipocytes and adipose tissue by LPS stimulation in Ptx3-knockdown (kd) adipocytes and Ptx3-/- mice, as well as metabolic inflammation induced by high-fat diet (HFD) feeding in Ptx3-/- mice. Our results suggest that PTX3 plays a protective role against sustained inflammation in adipose tissue via modulating NF-κB and p44/42 MAPK (Erk1/2) signaling pathway activation. PTX3 also plays a protective role against chronic inflammation in adipose tissues in obesity.
Materials and methods
Animals
Ptx3-null (Ptx3-/-) mice were generated by Dr. Martin M Matzuk’s laboratory at Baylor College of Medicine, Houston, Texas. Male Ptx3-null mice were backcrossed with C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME) for ten generations before mice were used for the experiments. Since Ptx3-/- females are subfertile due to defects in the integrity of the cumulus cell–oocyte complex [30], heterozygous mating scheme was used to generate wild-type (WT) and Ptx3-/- mice for the experiments. Mice were housed in a specific pathogen-free facility and were given free access to water and food. All experimental procedures were approved by the University of Minnesota animal care and use committee. Animal handling was performed according to National Institutes of Health guidelines. For the experiments in high-fat diet (HFD)-induced obese mice, age-matched male and female WT and Ptx3 knockout (KO) mice were allocated into groups and fed an HFD (fat calories: 60%, Bio-Serv, F3282; New Brunswick, NJ) for 12 weeks starting at 6 weeks of age.
Serum analyses
Serum triglyceride and cholesterol were determined using enzymatic assay kits (Stanbio Laboratory, Boerne, TX). Serum levels of insulin and adiponectin were determined using a mouse insulin enzyme-linked immunosorbent assay (ELISA) kit (Therm Scientific, Frederick, MD) and a mouse adiponectin ELISA kit (R&D Systems, Minneapolis, MN), respectively.
3T3-L1 cell culture
3T3-L1 cells were grown in Dulbecco’s Modified Eagle Medium (DMEM) with 10% bovine calf serum (Sigma-Aldrich) and 100 IU/ml penicillin–streptomycin (Invitrogen) until confluence. The cells were then induced for differentiation with the differentiation cocktail containing 1 µg/ml insulin (Sigma), 0.25 µM dexamethasone (Sigma), 0.5 mM 1-methyl-3-isobutyl-xanthine (IBMX, Sigma-Aldrich) for 2 days. The cells were then cultured in DMEM with 100 IU/ml penicillin–streptomycin, 10% fetal bovine serum, and 1 μg/ml insulin. The culture media were replaced by fresh ones every 2 days for 6 days. On day 8, the differentiated adipocytes were used for in vitro experiments as described in details in the result section.
Generation of Ptx3 knockdown 3T3-L1 cells
The short hairpin RNA (shRNA) was generated with ViraPower Lentiviral Expression Systems (Invitrogen, Carlsbad, CA) by the Minnesota Obesity Center, University of Minnesota. The shRNA sequence variants for Ptx3 gene or nonspecific scrambled were synthesized and cloned into a lentiviral-based RNAi vector, pLKO.1. The selected oligomers targeting Ptx3 sense sequence were 5′- GCATCCTGTGAGACCAATGAA-3′. In brief, when 3T3-L1 fibroblasts reached to 70–80% confluence, they were transducted with different concentrations of virus supplemented with 6 μg/ml polybrene (Sigma-Aldrich, St. Louis, MO) for incubation overnight. The culture media containing the virus were then removed and the cells were gently washed by PBS and replaced by fresh culture media. After 6 h of incubation, 2 µg/ml of puromycin (Sigma-Aldrich) was added for the selection of stably transduced cells. Nontransducted cells died after 2–3 days, and the survived transducted cells were passaged for testing Ptx3 gene and protein expression. The successfully transducted cells were then induced to differentiation for experiments.
RNA isolation and relative quantitative RT-PCR
Total RNA was extracted from cells and frozen tissues with TRIZOL reagent (Invitrogen, Carlsbad, CA). First-strand cDNA was synthesized from DNase-treated total RNA using a Superscript II reverse transcriptase kit (Invitrogen). Quantitative amplification by PCR was performed using PowerUp SYBR Green qPCR Master Mix (SABiosciences, Frederick, MD) by a StepOne Real-Time PCR System (Applied Biosystem, Foster City, CA). The ΔΔCt method was used to calculate the results. For quantification, Tbp mRNA served as an endogenous control. The primer sequences for amplifying the target genes and the Genebank accession number are summarized in Table S1.
Western blot analysis
The cells and frozen tissues were homogenized in a lysis buffer RIPA (Sigma-Aldrich) with protease inhibitor cocktails (Diagnostic Roche, Branchburg, NJ) before subjecting to western blot analysis with specific antibodies. The primary antibodies included goat polyclonal antibody to PTX3 (R&D Systems, Cat#AF2166), mouse monoclonal antibodies to NF-κB (Santa Cruz Biotechnology, Dallas, Texas, Cat#SC-8008), and rabbit monoclonal antibodies to phospho-NF-κB p65 (Cat# 3033), phospho-p42/44 MAPK (pErk ½, Cat#4377), p42/44 MAPK (Erk ½, Cat#4695), phospho-p38 MAPK (Cat#9215), p38 MAPK (Cat#9212), phospho-Stat3 (Cat#9131), Stat3 (4904), and β-actin (Cat#4967), (Cell Signaling Technology, Danvers, MA). Densitometric quantification was determined using ImageJ software (National Institutes of Health; Bethesda, MD) and reported as a ratio to total protein or β-actin as indicated in the result sections.
Oil red O staining
3T3-L1 adipocytes were fixed in cold Baker’s Formalin for 30 min at 4 °C and stained in Oil Red O (Sigma-Aldrich) for 10 min. Cells were then rinsed in running distilled water for 5 min and mounted with glycerol gelatin (Sigma-Aldrich).
Statistical analysis
Results are expressed as mean ± SEM or mean ± SD for gene and protein expression, respectively. F-test was used to test the variance similarity between groups. A two-tailed Student’s test was used to test for differences between genotypes or treatment. p-values < 0.05 were considered significant
Results
Regulation of PTX3 expression in adipocytes by inflammatory stimulation
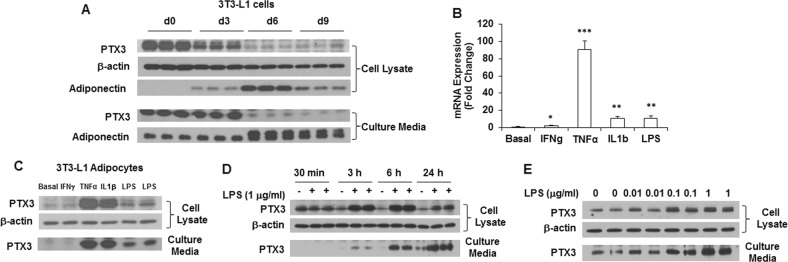
Previous studies have shown that PTX3 is produced locally at the inflammatory site by several cell types, including monocytes, dendritic cells, endothelial cells, smooth muscle cells, and mesangial cells when exposed to different inflammatory stimuli [31–35]. However, limited information is available regarding PTX3 expression in adipocytes. Since adipose tissue consists of adipose and stromal-vascular (SV) cells (a mixture of multiple non-adipose cell types), it is of importance to determine which types of cells specifically account for the upregulation of PTX3 expression in adipose tissue during inflammation. To that end, we examined the expression and secretion of PTX3 protein in 3T3-L1 preadipocytes and adipocytes during adipocyte differentiation. The results showed that PTX3 was highly expressed in 3T3-L1 preadipocytes; its expression levels were decreased with the differentiation of adipocytes (Fig. 1a). Although the basal levels of PTX3 expression were low in adipocytes, the expression of Ptx3 gene was markedly induced when challenged with TNFα, IL1β, and LPS (Fig. 1b). This was further demonstrated by PTX3 expression in response to these stimuli at the protein level (Fig. 1c). Compared to TNFα, IL1β, and LPS, interferon-γ (IFNγ) was less potent in terms of its induction of PTX3 expression in 3T3-L1 adipocytes (Fig. 1b, c).
Fig. 1.
PTX3 expression during adipocyte differentiation and by inflammatory stimulation in 3T3-L1 adipocytes. a The protein expression and secretion of PTX3 and adiponectin in 3T3-L1 cells during adipocyte differentiation. Induction of Ptx3 mRNA (b) and protein (c) expression by 24 h treatment of IFNγ (10 ng/ml), TNFα (1 nM), IL1β (1 nM), and LPS (1 μg/ml) in 3T3-L1 adipocytes. d Time course of PTX3 protein expression and secretion in 3T3-L1 adipocytes by 1 μg/ml of LPS stimulation. e PTX3 protein expression and secretion in 3T3-L1 adipocytes treated with various doses of LPS for 24 h. For cell culture studies, experiments were repeated 2–3 times yielding similar results. All values are mean ± SEM (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001 vs. basal levels
It has been well documented that toll-like receptors (TLRs) are the major innate immune receptors that recognize pathogens/metabolic insults such as LPS and mediate inflammatory responses [8]. We next assessed the regulation of PTX3 expression by LPS in adipocytes. We found that treatment of 3T3-L1 adipocytes with LPS resulted in a time- and dose-dependent increase in PTX3 expression (Fig. 1d, e). PTX3 protein expression was increased by LPS (1 μg/ml) stimulation with a peak response at 6 h and returned to the basal level after 24 h (Fig. 1d). Similarly, PTX3 secretion was increased by LPS and reached a peak level at 6 h. Twenty-four hours treatment further increased PTX3 secretion but this increase was not proportional to the increased treatment time (Fig. 1d), indicating that the extent of PTX3 secretion may be decreased from 6 h to 24 h. Moreover, 24 h treatment with various doses of LPS increased PTX3 expression and secretion in a dose-dependent manner (Fig. 1e).
PTX3 silencing leads to an increase in the basal and LPS-stimulated expression of inflammatory cytokines and extracellular matrix molecules in 3T3-L1 adipocytes
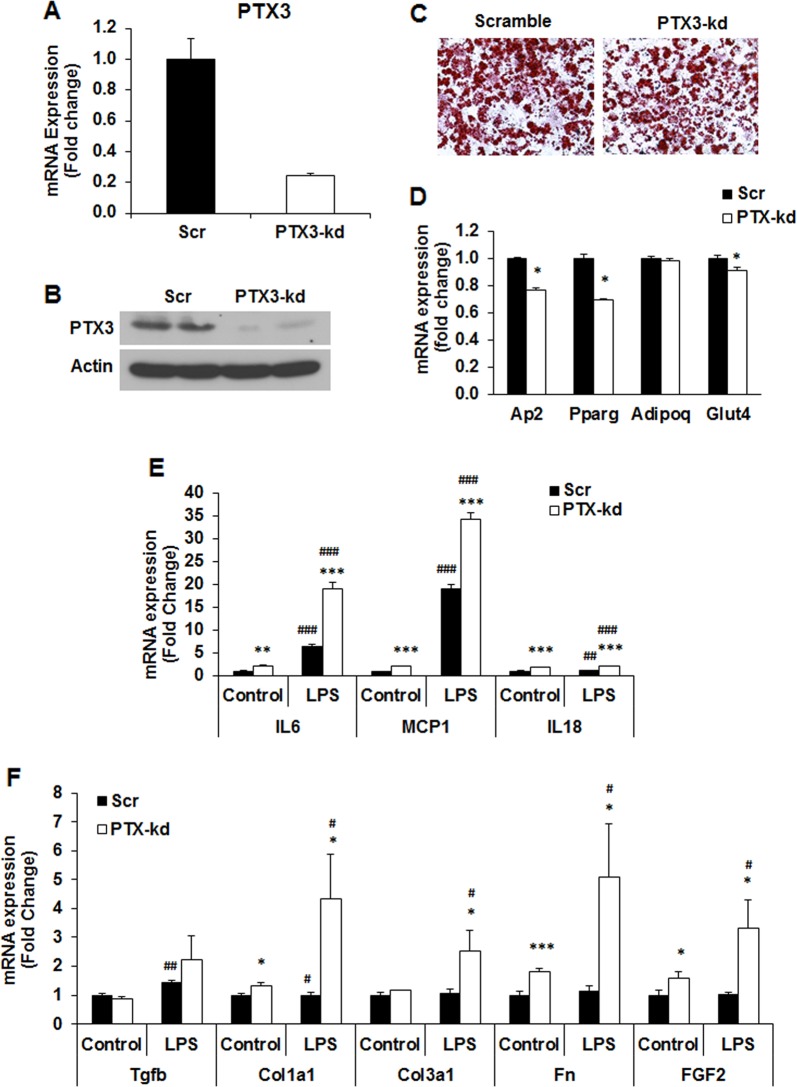
In order to determine the role of PTX3 in the regulation of inflammation in adipose tissue, we established Ptx3 knockdown (kd) and scrambled cell lines using lentivirus delivery of shRNA system. As shown in Fig. 2a, b, both PTX3 mRNA and protein expression was knocked down in 3T3-L1 adipocytes by 80% and 90%, respectively. Oil Red O staining of adipocytes on day 8 of differentiation indicates that Ptx3 knockdown (kd) induced a slight reduction in morphological differentiation of adipocytes compared with scrambled (Scr) controls (Fig. 2c). This is confirmed with a decrease in the mRNA expression of adipogenic genes, including peroxisome proliferator–activated receptor-γ (Pparg), adipocyte fatty acid–binding protein (aP2), and glucose transporter 4 (Glut4) in Ptx3-kd adipocytes compared with those in Scr controls (Fig. 2d). No difference was observed in adiponectin (Adipoq) mRNA expression between two genotypes (Fig. 2d).
Fig. 2.
Effect of PTX3 silencing on LPS-induced inflammation in 3T3–L1 adipocytes. a Ptx3 mRNA expression in 3T3-L1 adipocytes. b Protein expression of PTX3 in 3T3-L1 adipocytes. c Oil Red-O staining of 3T3-L1 adipocytes on day 8 of differentiation. d Adipogenic gene expression in 3T3-L1 adipocytes on day 8 of differentiation. Gene expression of inflammatory cytokines e and ECM gene expression f in 3T3-L1 adipocytes by LPS stimulation (1 μg/ml) for 24 h. The experiments were repeated three times. For cell culture studies, experiments were repeated 2–3 times yielding similar results. Data are expressed relative to the values for scrambled cells. The values are mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 vs. Scr; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. control. Scr: scramble; kd: knockdown
The promoter region of Ptx3 gene contains several inflammation-regulated transcription factor binding elements such as AP-1, NF-κB, and NF-IL-6 [17]. These inflammatory transcription factors together with Ptx3 gene can be significantly upregulated by LPS, suggesting the involvement of PTX3 in LPS-induced inflammation. We then assessed the effect of Ptx3 silencing on the inflammation in adipocytes in response to LPS stimulation. Interestingly, Ptx3 kd adipocytes had increased basal mRNA expression levels of Il6, Mcp1, and Il18 when compared to Scr cells (Fig. 2e). In response to LPS stimulation, increased mRNA expression of Il6, Mcp1, and Il18 is markedly exacerbated in Ptx3 kd adipocytes (Fig. 2e). Moreover, Ptx3-kd adipocytes expressed significantly higher levels of extracellular matrix molecules (ECMs), including transforming growth factor beta (Tgfb), collagen type I alpha 1 (Col1a1), collagen type III alpha 1 (Col3a1), fibronectin (Fn), and fibroblast growth factor 2 (Fgf2) in both basal and LPS-stimulated states (Fig. 2f).
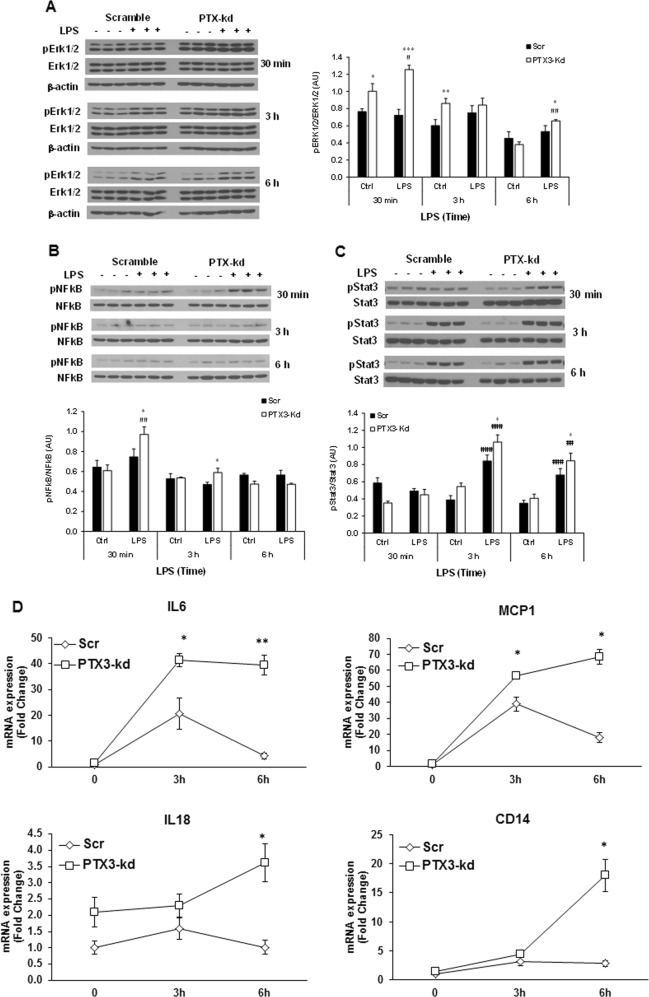
PTX3 silencing increases LPS-induced activation of inflammatory signaling pathways in 3T3-L1 adipocytes
It has been known that LPS induces the activation of multiple proinflammatory transcription factors and signaling pathways via TLR4, including NF-κB, the mitogen-activated protein kinase (MAPK), and c-jun-NH2-kinase (JNK), leading to the induction of inflammatory cytokines. Stat3 has been reported to directly interact with NF-κB, contributing to the sustained activation of NF-κB and inflammation [36]. We subsequently examined a time course of the activation of TLR4-mediated inflammatory signaling pathways, including NF-κB p65, Erk1/2, and Stat3 in Scr and Ptx3-kd adipocytes in response to LPS stimulation. LPS treatment for 30 min induced a higher level of the phosphorylation of Erk1/2 and NF-κB p65 in Ptx3-kd adipocytes compared to Scr controls (Fig. 3a, b). Different from a rapid increase of Erk1/2 and NF-κB p65 phosphorylation at 30 min after LPS treatment, the peak of Stat3 phosphorylation occurred at 3 h indicating that Stat3 is activated later than NF-κB signaling (Fig. 3c). Interestingly, levels of Stat3 and Erk1/2 phosphorylation remained higher in Ptx3-kd adipocytes compared to Scr cells at 6 h of LPS treatment. While LPS stimulates a peak level of PTX3 expression at 6 h (Fig. 1d), the peak activation of Erk1/2 and Stat3 occurs at 3 h. This suggests a role of PTX3 in the resolution rather than initiation of inflammation. To prove this hypothesis, we conducted a time course of LPS-induced expression of inflammatory cytokines in Scr and Ptx3-kd adipocytes. Consistent with the increased activation of inflammatory signaling pathways, LPS-stimulated expression levels of inflammatory cytokines Il6 and Mcp1 were significantly increased and reached a peak level at 3 h of LPS treatment. After 6 h of LPS treatment, the expression levels of these two cytokines reduced significantly compared to the levels at 3 h in Scr adipocytes (Fig. 3d). Intriguingly, Ptx3-kd adipocytes exhibited significantly increased expression of Il6 and Mcp1 at 3 h and maintained these higher expression levels at 6 h of LPS treatment. Similarly, the expression levels of Cd14 and Il18 were significantly higher in Ptx3-kd adipocytes compared to Scr cells at 6 h of LPS treatment (Fig. 3d). All the above data suggest that PTX3 may play a role in resolving inflammation and protecting against sustained inflammation.
Fig. 3.
Effect of PTX3 silencing on LPS-induced expression of cytokines and activation of inflammatory signaling pathways in 3T3-L1 adipocytes. Representative western blots for phosphorylated Erk1/2 (a), NF-κB p65 (b), and Stat3 (c) in 3T3-L1 adipocytes treated with LPS (1 μg/ml) for 30 min, 3 h, and 6 h, respectively. d Time course of gene expression of inflammatory cytokines in 3T3-L1 adipocytes treated with LPS (1 μg/ml). For cell culture studies, experiments were repeated 2–3 times yielding similar results. The values are mean ± SEM for gene expression and mean ± SD for the densitometric quantification of protein expression, respectively. *p < 0.05, **p < 0.01, ***p < 0.001 vs. Scr; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. control. Scr: scramble; kd: knockdown; Ctrl: control
PTX3 deficiency exacerbates LPS-induced inflammation in adipose tissues in mice
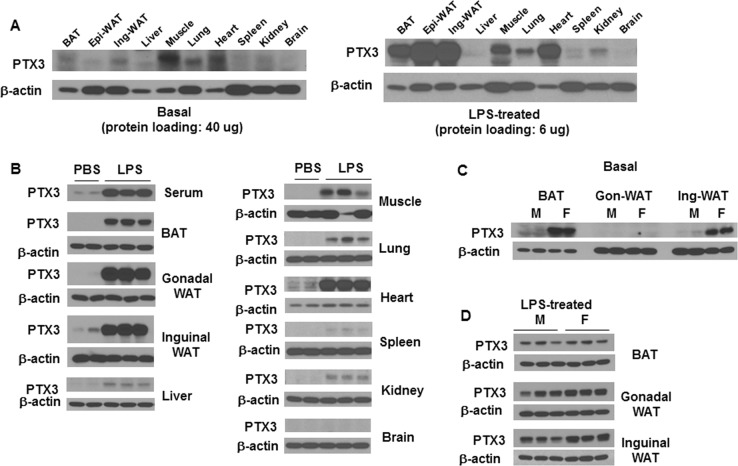
In diet-induced obesity, it has been suggested that increased plasma LPS is potentially an endogenous inflammatory insult that mediates high-fat diet (HFD)-induced inflammation in adipose tissue [37, 38]. Hence, investigating the role of PTX3 in LPS-stimulated adipose tissue inflammation in mice is of physiological relevance. First, we determined PTX3 expressing tissues in mice in the basal and LPS-stimulated inflammatory condition. Male C57/B6 mice were treated with LPS via intraperitoneal injection for 6 h. In the basal condition, little or no expression of PTX3 was observed in most of the tissues examined except for skeletal muscle, heart, and lung with 40 µg of proteins loaded for western blotting (Fig. 4a). Six hours after LPS administration, the protein expression of PTX3 was markedly increased in brown adipose tissue (BAT), epididymal white adipose tissue (Epi-WAT), inguinal white adipose tissue (Ing-WAT), and heart even with 6 µg of proteins loaded for western blotting, while much less increase or no increase was seen in muscle, lung, liver, kidneys, spleen, and brain (Fig. 4a, b). These results suggest that adipose tissues and heart are the main responders of PTX3 expression to LPS-stimulated inflammation. In addition, we observed a marked increase in serum PTX3 (Fig. 4b), indicating an increased secretion of PTX3 by LPS stimulation.
Fig. 4.
Depot- and sex-different expression of PTX3 by LPS stimulation. a Tissue distribution of PTX3 expression from mice without and with LPS treatment (0.3 mg/kg, intraperitoneal injection) for 6 h. b Induction of PTX3 in different tissues by LPS treatment for 6 h in mice (n = 6 per group). Comparison of PTX3 expression in different adipose depots in the basal (c) and 6 h LPS-treated (d) condition between male and female mice (n = 6 per group)
Since there are depot and sex differences in inflammatory response and adipogenesis in adipose tissue, it is of interest to investigate the depot and sex differences in PTX3 expression by inflammatory stimulation. Next, we examined the basal and LPS-stimulated expression of PTX3 in three adipose depots in normal male and female mice at the age of 16 weeks. The basal level of PTX3 protein expression in gonadal-WAT was undetectable in both male and female mice even though 50 µg of proteins were loaded (Fig. 4c). Female mice expressed much higher levels of PTX3 in BAT (40 µg of proteins loaded for western blotting) and Ing-WAT (10 µg of proteins loaded) than male mice (Fig. 4c). However, upon LPS treatment, PTX3 expression was significantly upregulated in all three adipose depots in both male and female mice with 6 µg of proteins loaded for Western Blotting. The LPS-stimulated levels of PTX3 expression were higher in gonadal and inguinal-WAT in females than that in males (Fig. 4d). These data suggest that female mice have higher basal levels of PTX3 expression in BAT and Ing-WAT than male mice, and gonadal and inguinal WATs are most responsive to LPS stimulation of PTX3 expression.
PTX3 deficiency potentiates LPS-induced adipose tissue inflammation
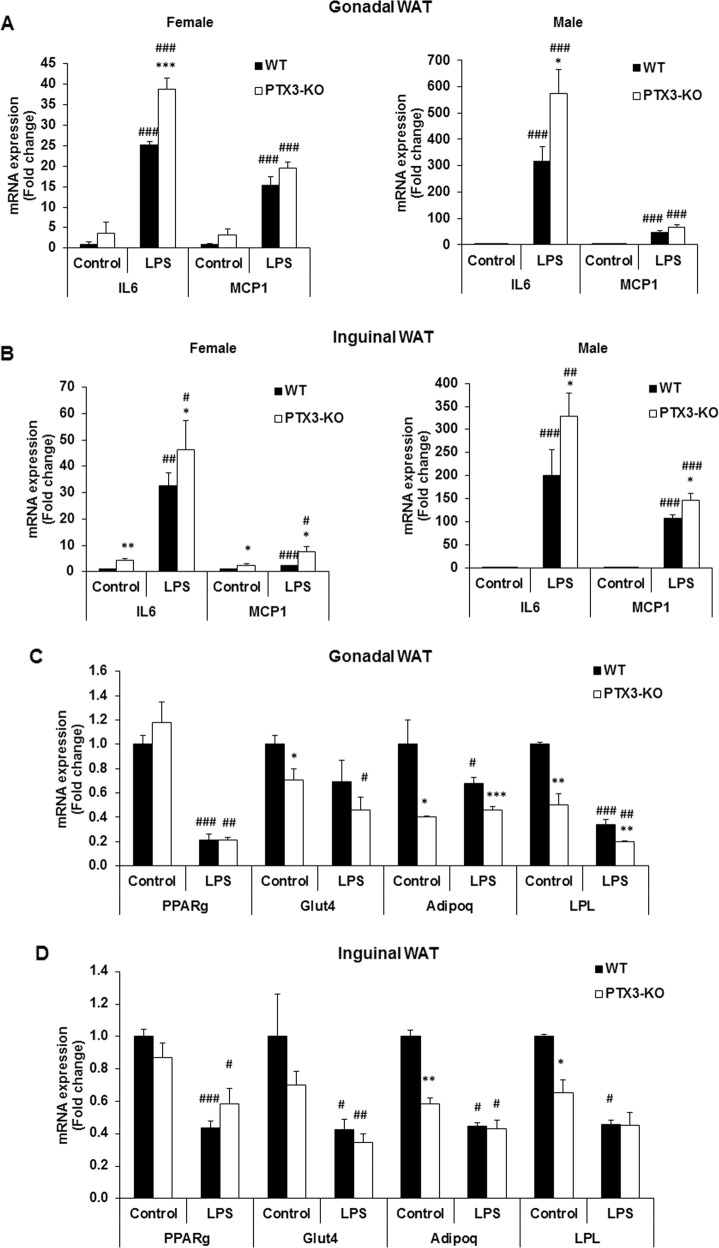
To evaluate the role of PTX3 in LPS-induced inflammation in adipose tissues, we assessed the expression of inflammatory genes and the activation of inflammatory signaling pathways in adipose tissues of WT and Ptx3-/- mice after LPS challenge. Although the expression of Il6 and Mcp1 genes was significantly upregulated in gonadal and inguinal-WAT of both WT and Ptx3-/- mice in males and females, Ptx3-/- mice had significantly higher expression levels of these two cytokines than WT mice in both males and females (Fig. 5a, b). No significant difference was observed in BAT from male and female mice between two genotypes (data not shown). Moreover, the expression of adipocyte-specific genes such as Glut4, adiponectin, and Lpl was significantly downregulated in gonadal-WAT of Ptx3-/- mice compared to WT mice in the basal and LPS-stimulated condition (Fig. 5c). While the expression of adiponectin and Lpl genes was significantly decreased in inguinal-WAT of Ptx3-/- mice, LPS-stimulated expression levels of these two genes were not different between WT and Ptx3-/- mice (Fig. 5d).
Fig. 5.
Effect of PTX3 deficiency on LPS-induced adipose tissue inflammation. The expression of inflammatory genes in epididymal (a) and inguinal adipose tissue (b) from male and female mice treated with LPS for 6 h. The expression of genes involved in adiogenesis in perigonadal (c) and inguinal (d) adipose tissue from female mice upon 6 h of LPS treatment. n = 6 per group. The values were mean ± SEM for gene expression. *p < 0.05, **p < 0.01, ***p < 0.001 vs. WT; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. control. WT: wild-type; KO: knockout
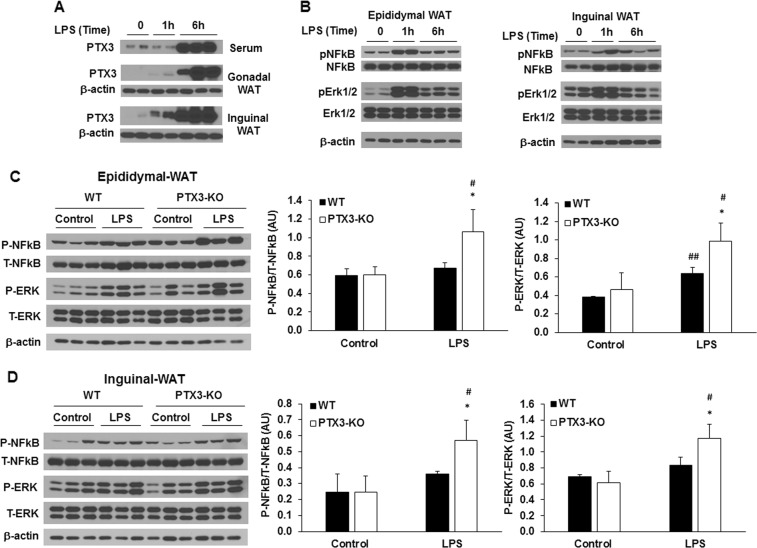
It is widely accepted that efficient resolution of inflammation prevents inflammation-caused tissue damage and maintains tissue in a healthy state [39]. Impaired resolution is an important contributor to inflammatory pathologies. Our above data from 3T3-L1 adipocytes have suggested the role of PTX3 in the resolution of inflammation. To provide further evidence supporting this conclusion, we performed the time course of PTX3 expression and inflammatory signaling pathway activation by LPS treatment in adipose tissues in mice. Similar to the results from in vitro studies, we showed the time-dependent expression and secretion of PTX3 in mice. In WT mice, 1 h treatment of LPS did not change PTX3 expression in gonadal-WAT and Inguinal-WAT and serum levels of PTX3 (Fig. 6a). However, a marked induction of PTX3 expression in gonadal-WAT and inguinal-WAT and increased serum PTX3 were observed at 6 h after LPS stimulation (Fig. 6a). Interestingly, inflammatory signaling pathways including Erk1/2 and NF-κB p65 phosphorylation were elevated in gonadal-WAT and inguinal-WAT at 1 h and then significantly decreased after 6 h of LPS treatment (Fig. 6b). Furthermore, we examined the changes in inflammatory signaling pathways in adipose tissue of Ptx3-/- mice after 6 h treatment of LPS. Similar to what we have observed in Ptx3 silencing 3T3-L1 adipocytes, Ptx3-/- mice exhibited elevated phosphorylation of NF-κB and Erk1/2 induced by 6 h treatment of LPS in gonadal and inguinal WATs compared with WT mice (Fig. 6c, d).
Fig. 6.
Effect of PTX3 deficiency on LPS-induced activation of inflammatory signaling pathways in adipose tissue. Time course of PTX3 protein expression (a) and activation of inflammatory signaling pathways (b) in adipose tissues treated with LPS in WT mice. Representative western blots for phosphorylated NF-κB p65 and phosphorylated Erk1/2 in epididymal (c) and inguinal (d) adipose tissue upon LPS treatment. n = 6 per group. The values are mean ± SD for the densitometric quantification of protein expression. *p < 0.05, **p < 0.01, ***p < 0.001 vs. WT; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. control. WT: wild-type; KO: knockout
PTX3 deficiency potentiates high-fat diet-induced adipose tissue inflammation
To evaluate the role of PTX3 in obesity-related inflammation in a more physiologically relevant setting, the Ptx3-KO mice were challenged with a high-fat diet (HFD) and the changes in inflammation in adipose tissues were investigated. We observed that HFD feeding could induce the expression of PTX3 in adipose tissues, especially in gonadal-WAT (Fig. 7a). Although the body weight was increased with time by HFD feeding in male and female WT and Ptx3-KO mice, no difference was observed between two genotypes (Fig. 7b). There was an increase in serum levels of insulin and a trend towards an increase in serum levels of total cholesterol in female Ptx3-KO mice (Table 1), suggesting a potential role of PTX3 in insulin resistance in female mice. We also found a trend towards a decrease in adiponectin in sera and a significant downregulation of adiponectin gene expression in gonadal adipose tissue (Fig. 7c) but not in inguinal adipose tissue (data not shown) of female HFD-fed Ptx3-KO mice. HFD-fed Ptx3-KO mice also displayed the upregulation of M1 markers, including IL6, TNFa, and IL18 and the downregulation of M2 marker arginase 1 (Arg1) in gonadal-WAT compared to WT mice (Fig. 7d).
Fig. 7.
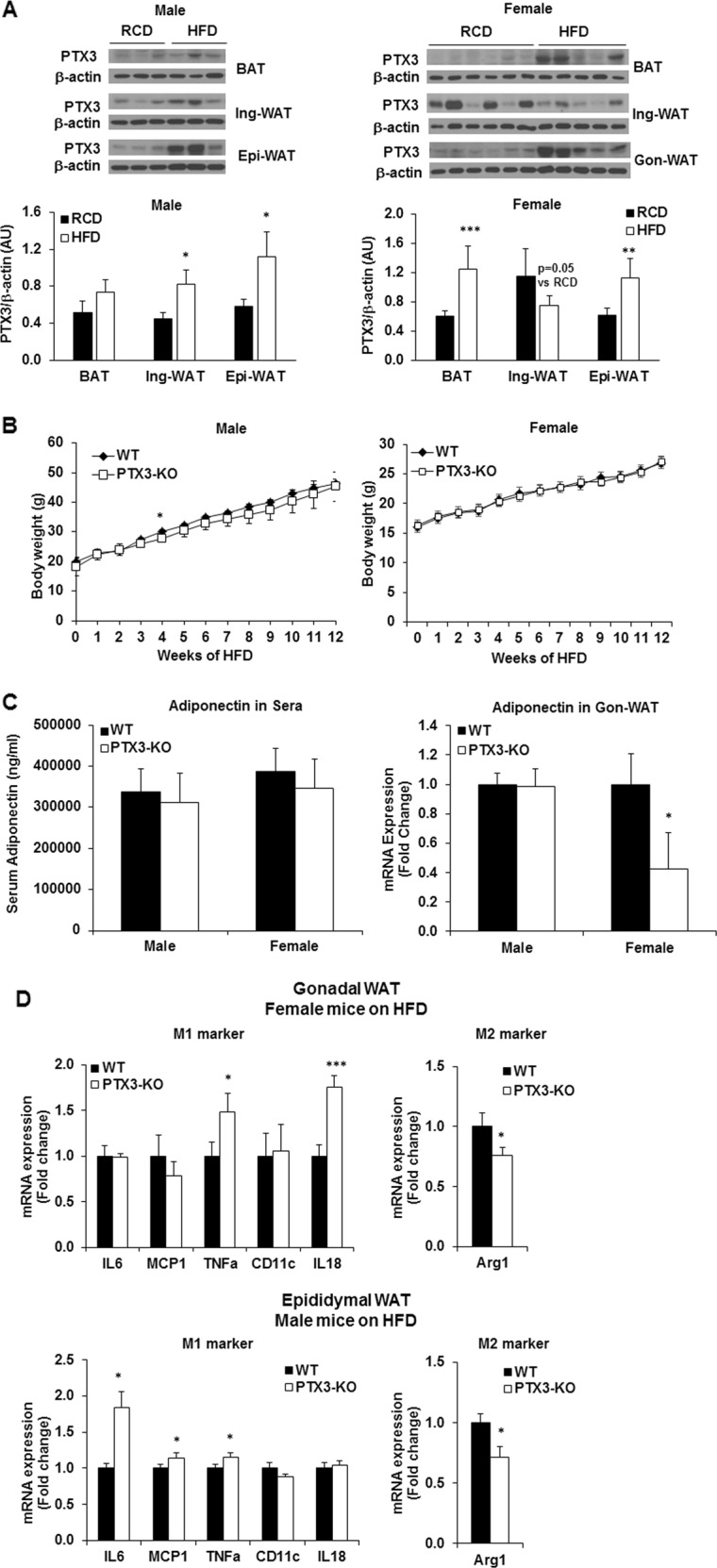
Effect of PTX3 deficiency on HFD-induced adipose inflammation a PTX3 protein expression and quantification in adipose tissues of WT mice by 12 weeks of high-fat diet (HFD) feeding. The age-matched mice fed regular chow diet (RCD) serve as controls. Male, RCD: n = 5, HFD: n = 6; Female, RCD: n = 7, HFD: n = 5. The values were mean ± SD for protein expression. b Body weight in WT and Ptx3-KO male and female mice during 12 weeks of HFD feeding. Male, WT n = 6, Ptx3-KO n = 8; Female, n = 8 per group. c Serum levels of adiponectin and mRNA expression in visceral adipose tissue of WT and Ptx3-KO male and female mice on HFD. Male, WT n = 6, Ptx3-KO n = 8; Female, n = 8 per group. d Proinflammatory (M1 marker) and anti-inflammatory (M2 marker) gene expression in visceral WAT of WT and Ptx3-KO male and female mice on HFD. The values were mean ± SEM for gene expression. *p < 0.05, **p < 0.01, ***p < 0.001 vs. WT; WT: wild-type; KO: knockout; WAT: white adipose tissue
Table 1.
Serum levels of glucose, insulin, and lipids in high-fat diet-induced obese mice of both genders
| Male | Female | |||
|---|---|---|---|---|
| WT (n = 6) |
Ptx3-KO (n = 8) |
WT (n = 8) |
Ptx3-KO (n = 8) |
|
| Glucose (mg/dl) | 113.3 ± 26.5 | 106.0 ± 20.7 | 119.0 ± 8.9 | 113.0 ± 9.7 |
| Insulin (pg/ml) | 591.9 ± 163.3 | 489.9 ± 116.2 | 85.9 ± 19.0 | 251.8 ± 70.5* |
| Triglycerides (mg/dl) | 57.0 ± 11.5 | 61.5 ± 15.4 | 60.8 ± 15.9 | 54.2 ± 20.2 |
| Total cholesterol (mg/dl) | 80.2 ± 2.7 | 80.3 ± 5.4 | 59.5 ± 2.4 | 70.1 ± 11.9# |
| Free fatty acid (mM) | 0.35 ± 0.04 | 0.35 ± 0.05 | 0.36 ± 0.08 | 0.37 ± 0.05 |
Data are mean ± standard deviation (SD)
WT wild-type, KO knockout
*p < 0.05, #p = 0.086 vs. WT (Student’s t-test)
Discussion
In the present study, we have shown that PTX3 deficiency potentiates LPS-induced inflammation in 3T3-L1 adipocytes in vitro and in white adipose tissues in vivo. We demonstrated that PTX3 could be markedly induced by LPS in 3T3-L1 adipocytes in vitro as well as in adipose tissues and heart in vivo. Ptx3-kd adipocytes are more sensitive to LPS-stimulated expression of proinflammatory cytokines and activation of inflammatory signaling pathways. Consistent with the findings in vitro, Ptx3-/- mice displayed upregulation of inflammatory genes and inflammatory signaling in white adipose tissues by LPS treatment in vivo. Moreover, Ptx3-kd adipocytes remain a higher level of inflammatory cytokine expression and inflammatory signaling pathway activation during the resolution phase of inflammation. Our results indicate that PTX3 may play a protective role in resolving inflammation in adipocytes and white adipose tissues through modulating inflammatory signaling pathways. We have also shown that PTX3 can be induced by high-fat diet feeding in adipose tissues, especially in gonadal-WAT. PTX3 deficiency potentiates high-fat diet-induced inflammation in visceral adipose tissues.
Since the first discovery and identification of PTX3 in human endothelial cells and fibroblasts as a TNFα or IL1β-inducible gene and protein, increasing reports have demonstrated the induction of PTX3 by various stimuli in other cell populations, including macrophages, smooth muscle cells, alveolar epithelial cells as well as adipocytes [18, 40]. Based on the molecular structure of PTX3 and the existence of enhancer-binding elements, including NF-κB, AP-1, and SP1 in the PTX3 promoter region, PTX3 is hypothesized to have a function in the regulation of inflammation. Consistent with this hypothesis, studies have shown that PTX3 expression is highly induced by proinflammatory cytokines such as TNFα and IL1β in lung epithelial cells and endothelial cells [20, 41]. In addition, various microbial moieties or intact microorganisms have been shown to induce the synthesis and release of PTX3 from macrophages, dendritic cells, and leukocytes [8, 14]. Moreover, PTX3 synthesis and release can be stimulated by TLR agonist such as LPS in dendritic cells and leukocytes [32, 42]. Consistent with the previous report that PTX3 expression can be induced by TNFα in 3T3-L1 adipocytes [27], we showed that PTX3 protein expression and secretion is stimulated by multiple inflammatory stimuli, including TNFα, LPS, and IL1β in 3T3-L1 adipocytes.
Chronic low-grade inflammation in adipose tissue plays a key role in the regulation of adipose tissue metabolism and function during obesity. Plasma levels of LPS are increased in obesity, and LPS may be associated with adipose tissue inflammation and impairment of adipocyte differentiation observed in obesity [37, 43]. Studies have shown that PTX3 expression is upregulated in gonadal-WAT in obese subjects [28, 29, 44]. This information leads us to hypothesize that PTX3, the humoral PRR, may inevitably be involved in the regulation of inflammation and metabolism in adipose tissues in obesity. Interestingly, although PTX3 expression can be induced by LPS in a variety of tissues in mice, we found that PTX3 is induced most pronouncedly in gonadal-WAT, inguinal-WAT, heart, and BAT in mice in response to LPS stimulation for 6 h. This suggests that PTX3 may play a role in adipose tissue inflammation.
To determine the role of PTX3 in the regulation of adipose tissue inflammation, we established a stable Ptx3-kd 3T3-L1 cell line and investigated the impact of Ptx3 knockdown on adipocyte inflammation. Our results demonstrated that adipocytes lacking Ptx3 are more sensitive to LPS-induced inflammation and express increased levels of inflammatory genes, including Il6, Mcp1, and Il18 when compared to WT controls. Moreover, we showed that Ptx3-kd adipocytes have higher levels of phosphorylated Erk1/2 and NF-κB at 30 min and 3 h after LPS stimulation. Upon the initiation of inflammation, protective mechanisms are also induced to ensure the resolution of the inflammatory response in a limited and specific time manner [45]. Interestingly, Stat3, which is known to be related to the sustained activation of inflammation, is increased in Ptx3-kd adipocytes at the late time (3 h and 6 h) of LPS stimulation. Altogether with a peak increase of PTX3 6 h after LPS stimulation, these results suggest a potential role of PTX3 in protecting against sustained inflammation. This conclusion is further supported by the results from a time course of LPS-induced gene expression of cytokines in 3T3-L1 adipocytes and Ptx3-/- mice. For instance, while the expression of Il6 and Mcp1 reaches a peak level at 3 h and is recovered at 6 h after LPS treatment in Scr adipocytes, higher levels of LPS-induced expression of these cytokines remain in Ptx3-kd adipocytes at 3 h particularly 6 h after LPS stimulation. In normal mice, we showed that inflammatory signaling pathways were highly induced by 1 h of LPS treatment but reduced after 6 h of LPS treatment. Interestingly, the peak increase of PTX3 protein expression in adipose tissues and serum PTX3 occurred 6 h after LPS treatment in WT mice. This suggests the potential role of PTX3 in the resolution of inflammation induced by LPS. In the absence of PTX3, the resolution of inflammation may be impaired leading to the sustained elevation of inflammatory signaling pathway activation during the resolution phase of inflammation. Indeed, we saw the upregulated inflammatory cytokines and impaired adipogenesis in white adipose tissues after LPS treatment for 6 h in Ptx3-/- mice. In line with the upregulation of proinflammatory cytokines by 6 h of LPS treatment, the activation of NF-κB and Erk1/2 in gonadal and inguinal adipose tissue is also increased in Ptx3-/- mice. Taken together, our findings suggest the potential anti-inflammatory role of PTX3 by resolving the inflammation in adipose tissue.
In addition to the role of PTX3 in acute inflammation in adipose tissues by LPS, we also documented the effect of PTX3 deficiency on HFD-induced chronic low-grade inflammation in adipose tissue, which is physiologically relevant to human obesity. We showed that Ptx3-KO mice challenged with 12 weeks of HFD feeding displayed increased expression of M1 macrophage markers and decreased expression of M2 marker in gonadal-WAT compared to WT controls, suggesting a potential role of PTX3 in the regulation in diet-induced metabolic inflammation in adipose tissue. It is well acknowledged that obesity-associated chronic low-grade inflammation is correlated with the development of insulin resistance [46]. HFD-induced obesity is also associated with hyperinsulinemia, hyperlipidemia, and low adiponectin levels [47]. Our results showed that female but not male Ptx3-KO mice have increased serum insulin, a trend increase in serum total cholesterol levels, and decreased adiponectin expression in gonadal-WAT after 12 weeks of HFD feeding. These findings suggest that PTX3 plays an important role in inflammatory and metabolic homeostasis during HFD-induced obesity, particularly in female mice. The gender-different effect of PTX3 may be attributed to the gender-different expression of PTX3 in fat depots and warrants further investigation.
In summary, our data revealed a protective role of PTX3 in LPS- and HFD-induced inflammation in adipose tissue. PTX3 is markedly induced following the peak induction of inflammatory gene expression and signaling pathway activation in adipose tissues by LPS. PTX3 is also highly induced in adipose tissues by HFD. The upregulation of PTX3 is likely a protective mechanism against inflammation and inflammation-associated metabolic deterioration. Specifically, the release of PTX3 from adipose tissue functions in resolving inflammation, thereby protecting against sustained and chronic inflammation.
Supplementary information
Acknowledgements
We thank Dr. Martin Matzuk from Department of Pathology and Immunology, Baylor College of Medicine, Houston, TX, USA for kindly providing PTX3 knockout mice for the project.
Funding
The project described was supported by grant (16GRNT31210025) from American Heart Association to XC and grant (1–18-IBS-287) from American Diabetes Association.
Author contributions
HG designed and performed the experiments, analyzed the data, and drafted the article; XQ, JD, and TL performed the experiments; XC researched, conceived and designed the experiments, analyzed the data, and revised/edited the article. XC is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version of this article (10.1038/s41366-019-0402-4) contains supplementary material, which is available to authorized users.
References
- 1.Kalupahana NS, Claycombe KJ, Moustaid-Moussa N. (n-3) fatty acids alleviate adipose tissue inflammation and insulin resistance: mechanistic insights. Adv Nutr. 2011;2(June):304–16. doi: 10.3945/an.111.000505.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin C, Henao-Mejia J, Flavell RA. Innate immune receptors: key regulators of metabolic disease progression. Cell Metabolism. 2013;17:873–82. doi: 10.1016/j.cmet.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Abdel-Latif MM, Duggan S, Reynolds JV, Kelleher D. Inflammation and esophageal carcinogenesis. Curr Opin Pharmacol. 2009;9:396–404. doi: 10.1016/j.coph.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Gordon S. Pattern recognition receptors: doubling up for the innate immune response. Cell. 2002;111:927–30. doi: 10.1016/S0092-8674(02)01201-1. [DOI] [PubMed] [Google Scholar]
- 5.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev. Immunol. 2001;1:135–45. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 6.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Ann Rev Immunol. 2011;29:415–45. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 7.Tanti JF, Ceppo F, Jager J, Berthou F. Implication of inflammatory signaling pathways in obesity-induced insulin resistance. Front Endocrinol. 2013;3(JAN):1–15. doi: 10.3389/fendo.2012.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeannin P, Bottazzi B, Sironi M, Doni A, Rusnati M, Presta M, et al. (2005). Complexity and complementarity of outer membrane protein A recognition by cellular and humoral innate immunity receptors. Immunity. 10.1016/j.immuni.2005.03.008 [DOI] [PubMed]
- 9.Camozzi M, Rusnati M, Bugatti A, Bottazzi B, Mantovani A, Bastone A, et al. Identification of an antiangiogenic FGF2-binding site in the N terminus of the soluble pattern recognition receptor PTX3. J Biol Chem. 2006;281:22605–13. doi: 10.1074/jbc.M601023200. [DOI] [PubMed] [Google Scholar]
- 10.Barbati E, Specchia C, Villella M, Rossi ML, Barlera S, Bottazzi B, et al. (2012). Influence of pentraxin 3 (PTX3) genetic variants on myocardial infarction risk and PTX3 plasma levels. PLoS ONE. 10.1371/journal.pone.0053030 [DOI] [PMC free article] [PubMed]
- 11.Bottazzi B, Garlanda C, Salvatori G, Jeannin P, Manfredi A, Mantovani A. Pentraxins as a key component of innate immunity. Curr Opin Immunol. 2006;18:10–15. doi: 10.1016/j.coi.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Inforzato A, Peri G, Doni A, Garlanda C, Mantovani A, Bastone A, et al. Structure and function of the long pentraxin PTX3 glycosidic moiety: fine-tuning of the interaction with C1q and complement activation. Biochemistry. 2006;45:11540–51. doi: 10.1021/bi0607453. [DOI] [PubMed] [Google Scholar]
- 13.Nauta AJ, Bottazzi B, Mantovani A, Salvatori G, Kishore U, Schwaeble WJ, et al. Biochemical and functional characterization of the interaction between pentraxin 3 and C1q. Eur J Immunol. 2003;33:465–73. doi: 10.1002/immu.200310022. [DOI] [PubMed] [Google Scholar]
- 14.Deban L, Russo RC, Sironi M, Moalli F, Scanziani M, Zambelli V, et al. Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat Immunology. 2010;11:328–34. doi: 10.1038/ni.1854. [DOI] [PubMed] [Google Scholar]
- 15.Garianda C, Hirsch E, Bozza S, Salustri A, De Acetis M, Nota R, et al. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature. 2002;420:182–6. doi: 10.1038/nature01195. [DOI] [PubMed] [Google Scholar]
- 16.Bottazzi B, Doni A, Garlanda C, Mantovani A. An integrated view of humoral innate immunity: pentraxins as a paradigm. Ann Rev Immunol. 2010;28:157–83. doi: 10.1146/annurev-immunol-030409-101305. [DOI] [PubMed] [Google Scholar]
- 17.Basile A, Sica A, D’Aniello E, Breviario F, Garrido G, Castellano M, et al. Characterization of the promoter for the human long pentraxin PTX3. Role of NF-kappaB in tumor necrosis factor-alpha and interleukin-1beta regulation. J Biol Chem. 1997;272:8172–8. doi: 10.1074/jbc.272.13.8172. [DOI] [PubMed] [Google Scholar]
- 18.Introna M, Alles VV, Castellano M, Picardi G, De Gioia L, Bottazzai B, et al. Cloning of mouse ptx3, a new member of the pentraxin gene family expressed at extrahepatic sites. Blood. 1996;87:1862–72. doi: 10.1016/1043-4666(94)90299-2. [DOI] [PubMed] [Google Scholar]
- 19.Vouret-Craviari V, Cenzuales S, Poli G, Mantovani A. Expression of monocyte chemotactic protein-3 in human monocytes exposed to the mycobacterial cell wall component lipoarabinomannan. Cytokine. 1997;9:992–8. doi: 10.1006/cyto.1997.0242. [DOI] [PubMed] [Google Scholar]
- 20.Norata GD, Marchesi P, Pirillo A, Uboldi P, Chiesa G, Maina V, et al. Long pentraxin 3, a key component of innate immunity, is modulated by high-density lipoproteins in endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:925–31. doi: 10.1161/ATVBAHA.107.160606. [DOI] [PubMed] [Google Scholar]
- 21.Mantovani A, Garlanda C, Bottazzi B. Pentraxin 3, a non-redundant soluble pattern recognition receptor involved in innate immunity. Vaccine. 2003;21(SUPPL. 2):43–7. doi: 10.1016/S0264-410X(03)00199-3. [DOI] [PubMed] [Google Scholar]
- 22.Baruah P, Propato A, Dumitriu IE, Rovere-Querini P, Russo V, Fontana R, et al. The pattern recognition receptor PTX3 is recruited at the synapse between dying and dendritic cells, and edits the cross-presentation of self, viral, and tumor antigens. Blood. 2006;107:151–8. doi: 10.1182/blood-2005-03-1112. [DOI] [PubMed] [Google Scholar]
- 23.Xu W, Roos A, Schlagwein N, Woltman AM, Daha MR, van Kooten CIL. IL-10-producing macrophages preferentially clear early apoptoticcells. Blood. 2006 doi: 10.1182/blood-2005-10-4144. [DOI] [PubMed] [Google Scholar]
- 24.Olesen R, Wejse C, Velez DR, Bisseye C, Sodemann M, Aaby P, et al. DC-SIGN (CD209), pentraxin 3 and vitamin D receptor gene variants associate with pulmonary tuberculosis risk in West Africans. Genes Immunity. 2007;8:456–67. doi: 10.1038/sj.gene.6364410. [DOI] [PubMed] [Google Scholar]
- 25.May L, Kuningas M, van Bodegom D, Meij HJ, Frolich M, Slagboom PE, et al. Genetic variation in pentraxin (PTX) 3 gene associates with PTX3 production and fertility in women. Biol Reproduc. 2010;82:299–304. doi: 10.1095/biolreprod.109.079111. [DOI] [PubMed] [Google Scholar]
- 26.Presta M, Camozzi M, Salvatori G, Rusnati M. Role of the soluble pattern recognition receptor PTX3 in vascular biology. J. Cell. Mol. Med. 2007;11:723–38. doi: 10.1111/j.1582-4934.2007.00061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abderrahim-Ferkoune A, Bezy O, Chiellini C, Maffei M, Grimaldi P, Bonino F, et al. Characterization of the long pentraxin PTX3 as a TNFalpha-induced secreted protein of adipose cells. J Lipid Res. 2003;44:994–1000. doi: 10.1194/jlr.M200382-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Ogawa T, Kawano Y, Imamura T, Kawakita K, Sagara M, Matsuo T, et al. (2010). Reciprocal contribution of pentraxin 3 and C-reactive protein to obesity and metabolic syndrome. Obesity. 10.1038/oby.2009.507 [DOI] [PubMed]
- 29.Osorio-Conles O, Guitart M, Chacón MR, Maymo-Masip E, Moreno-Navarrete JM, Montori-Grau M, et al. Plasma PTX3 protein levels inversely correlate with insulin secretion and obesity, whereas visceral adipose tissue PTX3 gene expression is increased in obesity. Am J Physiol Endocrinol Metab. 2011;301:E1254–61. doi: 10.1152/ajpendo.00163.2011. [DOI] [PubMed] [Google Scholar]
- 30.Varani S, Elvin JA, Yan C, DeMayo J, DeMayo FJ, Horton HF, et al. Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol Endocrinol. 2002;16:1154–67. doi: 10.1210/mend.16.6.0859. [DOI] [PubMed] [Google Scholar]
- 31.Desvergne B, Michalik L, Wahli W. Transcriptional regulation of metabolism. Physiol Rev. 2006;86:465–514. doi: 10.1152/physrev.00025.2005. [DOI] [PubMed] [Google Scholar]
- 32.Doni A, Peri G, Chieppa M, Allavena P, Pasqualini F, Vago L, et al. Production of the soluble pattern recognition receptor PTX3 by myeloid, but not plasmacytoid, dendritic cells. Eur J Immunol. 2003;33:2886–93. doi: 10.1002/eji.200324390. [DOI] [PubMed] [Google Scholar]
- 33.Klouche M, Peri G, Knabbe C, Eckstein HH, Schmid FX, Schmitz G, et al. Modified atherogenic lipoproteins induce expression of pentraxin-3 by human vascular smooth muscle cells. Atherosclerosis. 2004;175:221–8. doi: 10.1016/j.atherosclerosis.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 34.Rolph MS. Production of the long pentraxin PTX3 in advanced atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2002;22:10e–14. doi: 10.1161/01.ATV.0000015595.95497.2F. [DOI] [PubMed] [Google Scholar]
- 35.Bussolati B, Peri G, Salvidio G, Verzola D, Mantovani A, Camussi G. The long pentraxin PTX3 is synthesized in IgA glomerulonephritis and activates mesangial cells. J Immunol (Baltimore, Md.: 1950) 2003;170:1466–72. doi: 10.4049/jimmunol.170.3.1466. [DOI] [PubMed] [Google Scholar]
- 36.Lee H, Herrmann A, Deng JH, Kujawski M, Niu G, Li Z, et al. Persistently activated Stat3 maintains constitutive NF-κB activity in tumors. Cancer Cell. 2009;15:283–93. doi: 10.1016/j.ccr.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cani PD, Bibiloni R, Knauf C, Neyrinck AM, Delzenne NM. Changes in gut microbiota control metabolic diet–induced obesity and diabetes in mice. Diabetes. 2008;57:1470–81. doi: 10.2337/db07-1403.Additional. [DOI] [PubMed] [Google Scholar]
- 38.Dasu MR, Jialal I. Free fatty acids in the presence of high glucose amplify monocyte inflammation via Toll-like receptors. AJP: Endocrinol Metab. 2011;300:E145–54. doi: 10.1152/ajpendo.00490.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Dyke TE, Serhan CN. Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases. J Dental Res. 2003;82:82–90. doi: 10.1177/154405910308200202. [DOI] [PubMed] [Google Scholar]
- 40.Bottazzi B, Garlanda C, Cotena A, Moalli F, Jaillon S, Deban L, et al. The long pentraxin PTX3 as a prototypic humoral pattern recognition receptor: Interplay with cellular innate immunity. Immunol Rev. 2009;227:9–18. doi: 10.1111/j.1600-065X.2008.00719.x. [DOI] [PubMed] [Google Scholar]
- 41.Han B, Mura M, Andrade CF, Okutani D, Lodyga M, dos Santos CC, et al. TNF-induced long pentraxin PTX3 expression in human lung epithelial cells via JNK. J Immunol. 2005;175:8303–11. doi: 10.4049/jimmunol.175.12.8303. [DOI] [PubMed] [Google Scholar]
- 42.Maina V, Cotena A, Doni A, Nebuloni M, Pasqualini F, Milner CM, et al. Coregulation in human leukocytes of the long pentraxin PTX3 and TSG-6. J Leukoc Biol. 2009;86:123–32. doi: 10.1189/jlb.0608345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Original article. Diabetes. 2007;56(July):1761–72. doi: 10.2337/db06-1491.P.D.C. [DOI] [PubMed] [Google Scholar]
- 44.Goodman AR, Levy DE, Reis LF, Vilcek J. Differential regulation of TSG-14 expression in murine fibroblasts and peritoneal macrophages. J Leukoc Biol. 2000;67:387–95. doi: 10.1002/jlb.67.3.387. [DOI] [PubMed] [Google Scholar]
- 45.Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O’Neill LAJ, et al. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325–32. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saltiel AR, Olefsky JM, Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease Find the latest version: Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127:1–4. doi: 10.1172/JCI92035.systems. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tilg H, Moschen AR. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–83. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.