Abstract
Fibroblast growth factor 21 (FGF21) is an important regulator of energy metabolism. FGF21 is inactivated by fibroblast activation protein (FAP). We investigated whether FGF21 and/or FAP are secreted from human white adipose tissue of individuals with obesity by measuring total FGF21, active FGF21, and FAP concentrations in arterialized blood and venous blood draining the subcutaneous abdominal adipose tissue (scAT). Measurements were performed under fasting conditions and after a high fat meal before and after diet-induced weight loss in 16 adults with BMI 27–35 kg/m2. FGF21 was not released from scAT, neither before nor after weight loss in agreement with an undetectable gene expression of FGF21 in this tissue. Although scAT showed significant gene expression of FAP, no release of FAP from the tissue could be detected. The high fat meal increased postprandial circulating FGF21 but not FAP. Circulating levels of FAP but not FGF21 were significantly reduced after weight loss. On the other hand, FAP expression in scAT was increased. In conclusion, release from scAT does not appear to contribute to circulating concentrations of FGF21 and FAP and their responses to ingestion of a high fat meal or weight loss, respectively, in individuals with obesity.
Subject terms: Endocrinology, Obesity
Introduction
Fibroblast growth factor 21 (FGF21), a regulator of energy metabolism, is mainly expressed and secreted by the liver, but other tissues, such as white and brown adipose tissue, skeletal muscle and cardiac muscle, also express and secrete FGF21 under certain circumstances in humans [1–3]. Whether FGF21 from these tissues contributes to circulating FGF21 concentrations and under what conditions is less clear [1]. Plasma concentrations of FGF21 are increased in human obesity. Several studies have investigated the effects of diet-induced weight loss on circulating FGF21 concentrations after weight loss with inconsistent outcomes [4–8]. Whether changes in adipose tissue secretion of FGF21 contribute to changes in circulating FGF21 concentrations with weight loss is unknown. Human FGF21 is inactivated by fibroblast activation protein (FAP) [9]. FAP exists both on the cell surface and in a soluble, circulating form in the blood. The cellular origin of soluble FAP is unknown [10].
In this study we measured total FGF21, active FGF21, and FAP concentrations in arterialized blood and venous blood draining subcutaneous abdominal adipose tissue (scAT) in obese volunteers under fasting conditions, after a high-fat meal and before and after diet-induced weight loss.
Subjects and methods
Adult volunteers (BMI between 28 and 35 kg/m2) were randomized to either a low calorie diet (LCD; 1250 kcal/d) for 12 weeks or a very low calorie diet (VLCD; 500 kcal/d) for 5 weeks. Both groups lost similar amounts of weight during this period. Subsequently, all participants returned to a weight maintenance diet and kept this for 4 weeks (weight stable period). Measurements were performed at T1 (baseline), T2 (end of the energy-restricted diet period), and T3 (end of weight stable period). Arterio-venous difference measurements were performed in 16 of the volunteers at T1 and T3, whereas microarray gene expression analysis in adipose tissue biopsies was performed at T1, T2, and T3. The study design and methods used for the measurements of arterio-venous differences across adipose tissue and the microarray analysis have been described in detail elsewhere [11–13].
Total FGF21 was measured via ELISA using a combination of three monoclonal antibodies: a mid-domain (amino acids 136–143), a C-terminal domain and a polyclonal anti-FGF21 detection antibody based on [14]. Active FGF21 was captured with the C-terminal specific antibody (custom mouse anti-FGF21 antibody developed by Morphosys/AbD Serotec) in combination with the same polyclonal anti-FGF21 detection antibody. FAP was measured with the FAP Human ELISA Kit (abcam (ab193701).
Data are presented as mean ± standard deviation (SD) for normally distributed variables or as median ± interquartile range (IQR) for nonnormally distributed variables (FGF21 concentrations) and appropriate parametric or nonparametric statistical tests were used.
Results
Fasting total FGF21 (totFGF21) and active FGF21 (actFGF21) were highly variable among individuals. TotFGF21 ranged from undetectable (<5 pg/ml; n = 1) to 490 pg/ml. ActFGF21 ranged from undetectable (<5 pg/ml; n = 5) to 170 pg/ml. For the data analysis the undetectable concentrations were set to zero. On average 15% of totFGF21 circulated in its active form.
There were no statistically significant differences in fasting concentrations of totFGF21, actFGF21, or FAP between arterialized and scAT venous plasma, neither before nor after weight loss (Table 1). The fluxes of totFGF21, actFGF21, and FAP across scAT were also not significantly different from 0 before or after weight loss (all P > 0.20).
Table 1.
Subcutaneous adipose tissue plasma flow, concentrations of total FGF21, active FGF21, and FAP in plasma from arterialized and subcutaneous adipose tissue venous blood, their arterio-venous differences and fluxes under fasting conditions before and after weight lossa
| Before weight loss (median ± IQR) | After weight loss (median ± IQR) | P value for difference before vs after | |
|---|---|---|---|
| ATPF ((ml/min)/100 ml) (*) | 0.69 ± 0.36 | 0.68 ± 0.38 | 0.940 |
| Arterialized totFGF21 (pg/ml) | 121.3 ± 190.6 | 111.2 ± 73.0 | 0.191 |
| scAT venous totFGF21 (pg/ml) | 120.5 ± 188.9 | 111.6 ± 73.4 | 0.211 |
| a-v difference totFGF21 (pg/ml) | −1.7 ± 18.7 | 0.1 ± 5.4 | 0.609 |
| totFGF21 flux ((pg/min).100 ml) | −1.3 ± 10.5 | 0.2 ± 3.0 | 0.865 |
| Arterialized actFGF21 (pg/ml) | 8.5 ± 51.0 | 10.4 ± 25.1 | 0.136 |
| scAT venous actFGF21 (pg/ml) | 19.8 ± 50.5 | 17.0 ± 22.8 | 0.064 |
| a-v difference actFGF21 (pg/ml) | 0.0 ± 13.6 | 0.0 ± 6.0 | 0.701 |
| actFGF21 flux ((pg/min).100 ml) | 0.0 ± 7.3 | 0.0 ± 3.6 | 0.382 |
| Arterialized FAP (ng/ml) (*) | 116.9 ± 28.4 | 100.5 ± 23.4 | 0.017 |
| scAT venous FAP (ng/ml) (*) | 116.2 ± 27.4 | 104.0 ± 21.9 | 0.100 |
| a-v difference FAP (ng/ml) (*) | 0.8 ± 12.1 | −3.5 ± 11.0 | 0.279 |
| FAP flux ((ng/min).100 ml) (*) | 1.6 ± 9.0 | −1.9 ± 6.4 | 0.248 |
| Arterialized actFGF21 (%totFGF21) (*) | 12.7 ± 14.2 | 11.5 ± 11.3 | 0.676 |
| scAT venous actFGF21 (%totFGF21) (*) | 16.8 ± 14.7 | 11.5 ± 9.1 | 0.055 |
ATPF adipose tissue plasma flow, totFGF21 total FGF21, actFGF21 active FGF21, scAT subcutaneous adipose tissue, a-v arterio-venous, SD standard deviation, IQR interquartile range
aAverage of the values at t = −30 and 0 min; (*) mean ± SD
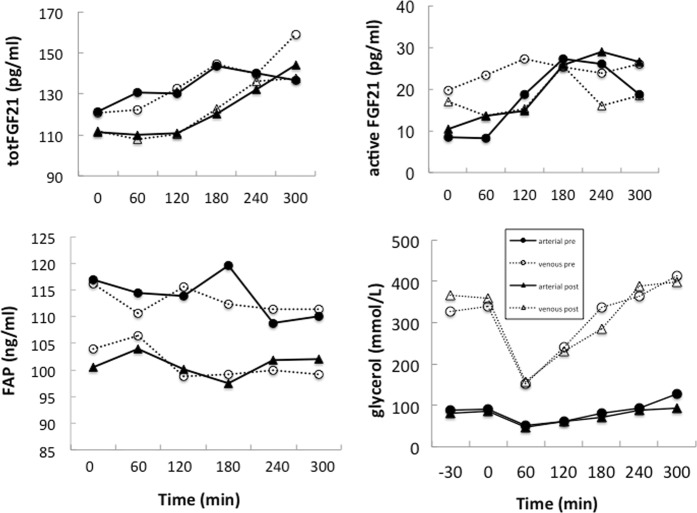
Arterial and scAT venous concentrations of totFGF21 (P = 0.002 and P = 0.001, respectively) and actFGF21 (P = 0.003 and P = 0.086, respectively) but not of FAP, increased after consumption of the high-fat meal at baseline and similarly after weight loss (Fig. 1). There was no evidence for FGF21 or FAP secretion from scAT after consumption of the high-fat meal. Figure 1 also shows the concentrations of glycerol in arterialized and venous plasma supporting the adequacy of the arterio-venous balance technique.
Fig. 1.
Arterial and subcutaneous adipose tissue venous concentrations of total FGF21 (totFGF21) (median), active FGF21 (actFGF21) (median), FAP (mean), and glycerol (mean) at baseline (t = −30 and 0 min) and after consumption (t = 60 to 300 min) of a high fat mixed meal, before and after weight loss. Continuous lines and closed symbols arterialized concentrations, dotted lines and open symbols subcutaneous adipose tissue venous concentrations; circles before weight loss, triangles after weight loss
After weight loss arterial and scAT venous plasma concentrations of totFGF21, actFGF21, or FAP were lower under fasting conditions (Table 1), but only the change in arterialized FAP concentrations was statistically significant (P = 0.017). Furthermore, mean concentrations of totFGF21 and actFGF21 over the 5-h test did not differ significantly from before weight loss. The mean arterialized and venous concentrations of FAP were significantly higher before than after weight loss (P = 0.033 and P = 0.041, respectively).
Gene expressions of FGF21 and its receptor FGFR1 were very low and did not pass the noise filtering. Gene expression of FAP was significantly increased after weight loss (fold change (FC) 1.18, q = 0.021), gene expression of KLB (ß-klotho) was not significantly changed (FC − 1.09, q = 0.149).
Discussion
The results demonstrate that FGF21 and FAP are not released from abdominal subcutaneous adipose tissue in individuals with obesity, neither before nor after weight loss nor after a high-fat meal. This is in agreement with undetectable gene expression of FGF21 in this tissue under fasting conditions. Although scAT showed significant gene expression of FAP, no release of FAP from the tissue could be detected. Therefore, release from scAT does not appear to contribute to circulating concentrations of FGF21 and FAP and their responses to ingestion of a high-fat meal or weight loss, respectively. Furthermore, the high-fat meal increased postprandial circulating FGF21 but not FAP and the 10% weight loss did not affect fasting or postprandial FGF21 concentrations, whereas postprandial FAP was significantly reduced.
Release of FGF21 from human adipocytes in vitro has been reported [3], but studies in mice suggest that although FGF21 is expressed in white adipose tissue and secreted from adipocytes, FGF21 acts locally in an autocrine or paracrine fashion instead of entering the circulation [1, 15]. In agreement with these data, our results indicate that, even if FGF21 is produced in human adipose tissue, it is not secreted into the circulation under the conditions studied.
The literature on the effects of meal ingestion on plasma FGF21 concentrations in humans is inconsistent [16–19]. Our data suggest a postprandial increase in plasma FGF21. Since none of these studies included a fasting time control arm, it cannot be excluded that circadian changes in FGF21 have interfered with these results [20]. Moreover, meal compositions were different in the different studies.
Weight loss did not affect fasting or postprandial circulating FGF21 levels in our study. Equivocal results have been reported: increases [4, 7], decreases [5, 7, 8], and unchanged levels [6] have been found. Differences in baseline concentrations of FGF21, reflecting differences in baseline characteristics, amount of weight loss and concomitant level and type of energy restriction, and the duration of weight loss may contribute to this variation.
Gene expression of FGF21 was undetectable in scAT of our obese participants in agreement with other studies in humans, e.g. [16]. In contrast, other groups did report FGF21 gene expression in human scAT, although without clear quantification [3, 4]. Although we cannot exclude that FGF21 gene expression in scAT can be stimulated, expression appears to be extremely low after an overnight fast. In contrast, there was a clear expression of FAP, KLB, and FGFR1 in human scAT indicating that FGF21 is likely to act in scAT and its activity can be regulated locally.
FAP gene expression in scAT increased after weight loss, but no release of FAP from scAT before or after weight loss was found. Plasma concentrations of FAP were reduced after weight loss, suggesting reduced FAP secretion from other tissues than scAT or increased FAP clearance after weight loss.
In conclusion, human subcutaneous adipose tissue is not a source of circulating FGF21 or FAP in overweight and moderately obese individuals, neither before nor after weight loss or in response to a high fat mixed meal. Circulating levels of FAP, the enzyme that inactivates FGF21, were reduced after weight loss. On the other hand, FAP expression in scAT was increased, which may explain the tendency for lower scAT venous levels of active FGF21 after weight loss.
Author contributions
MAvB and ECMM conceived the study and RGV and NJTR performed it. ACA and CCC were responsible for the analysis of FGF21 and FAP. MAvB wrote the manuscript. All authors commented on the content and approved the final version.
Compliance with ethical standards
Conflict of interest
MAvB, ECM, RGV, and NJTR declare that they have no conflict of interest. CCC and ACA are employed by Eli Lilly and Company. This study was supported by the Netherlands Organisation for Scientific Research TOP, Grant Number: 200500001.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.BonDurant LD, Potthoff MJ. Fibroblast growth factor 21: a versatile regulator of metabolic homeostasis. Annu Rev Nutr. 2018;38:173–96. doi: 10.1146/annurev-nutr-071816-064800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Indrakusuma I, Sell H, Eckel J. Novel mediators of adipose tissue and muscle crosstalk. Curr Obes Rep. 2015;4:411–7. doi: 10.1007/s13679-015-0174-7. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X, Yeung DC, Karpisek M, Stejskal D, Zhou ZG, Liu F, et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57:1246–53. doi: 10.2337/db07-1476. [DOI] [PubMed] [Google Scholar]
- 4.Mraz M, Bartlova M, Lacinova Z, Michalsky D, Kasalicky M, Haluzikova D, et al. Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clin Endocrinol. 2009;71:369–75. doi: 10.1111/j.1365-2265.2008.03502.x. [DOI] [PubMed] [Google Scholar]
- 5.Christodoulides C, Dyson P, Sprecher D, Tsintzas K, Karpe F. Circulating fibroblast growth factor 21 is induced by peroxisome proliferator-activated receptor agonists but not ketosis in man. J Clin Endocrinol Metab. 2009;94:3594–601. doi: 10.1210/jc.2009-0111. [DOI] [PubMed] [Google Scholar]
- 6.Mai K, Schwarz F, Bobbert T, Andres J, Assmann A, Pfeiffer AF, et al. Relation between fibroblast growth factor-21, adiposity, metabolism, and weight reduction. Metabolism. 2011;60:306–11. doi: 10.1016/j.metabol.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Lips MA, de Groot GH, Berends FJ, Wiezer R, van Wagensveld BA, Swank DJ, et al. Calorie restriction and Roux-en-Y gastric bypass have opposing effects on circulating FGF21 in morbidly obese subjects. Clin Endocrinol. 2014;81:862–70. doi: 10.1111/cen.12496. [DOI] [PubMed] [Google Scholar]
- 8.Haluzikova D, Lacinova Z, Kavalkova P, Drapalova J, Krizova J, Bartlova M, et al. Laparoscopic sleeve gastrectomy differentially affects serum concentrations of FGF-19 and FGF-21 in morbidly obese subjects. Obesity. 2013;21:1335–42. doi: 10.1002/oby.20208. [DOI] [PubMed] [Google Scholar]
- 9.Coppage AL, Heard KR, DiMare MT, Liu Y, Wu W, Lai JH, et al. Human FGF-21 is a substrate of fibroblast activation protein. PLoS ONE. 2016;11:e0151269. doi: 10.1371/journal.pone.0151269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keane FM, Yao TW, Seelk S, Gall MG, Chowdhury S, Poplawski SE, et al. Quantitation of fibroblast activation protein (FAP)-specific protease activity in mouse, baboon and human fluids and organs. FEBS Open Bio. 2013;4:43–54. doi: 10.1016/j.fob.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vink RG, Roumans NJ, Arkenbosch LA, Mariman EC, van Baak MA. The effect of rate of weight loss on long-term weight regain in adults with overweight andobesity. Obesity. 2016;24:321–7. doi: 10.1002/oby.21346. [DOI] [PubMed] [Google Scholar]
- 12.Vink RG, Roumans NJ, van der Kolk BW, Fazelzadeh P, Boekschoten MV, Mariman EC, et al. Adipose tissue meal-derived fatty acid uptake before and after diet-induced weight loss in adults with overweight and obesity. Obesity. 2017;25:1391–9. doi: 10.1002/oby.21903. [DOI] [PubMed] [Google Scholar]
- 13.Roumans NJT, Vink RG, Bouwman FG, Fazelzadeh P, van Baak MA, Mariman ECM. Weight loss-induced cellular stress in subcutaneous adipose tissue and the risk for weight regain in overweight and obese adults. Int J Obes. 2017;41:894–901. doi: 10.1038/ijo.2016.221. [DOI] [PubMed] [Google Scholar]
- 14.Umberger TS, Sloan JH, Chen J, Cheng C, Siegel RW, Qian Y, et al. Novel sandwich immunoassays for the measurement of total and active FGF21. Bioanalysis. 2014;6:3283–93. doi: 10.4155/bio.14.241. [DOI] [PubMed] [Google Scholar]
- 15.Dutchak PA, Katafuchi T, Bookout AL, Choi JH, Yu RT, Mangelsdorf DJ, et al. Fibroblast growth factor-21 regulates PPARgamma activity and the antidiabetic actions of thiazolidinediones. Cell. 2012;148:556–67. doi: 10.1016/j.cell.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dushay J, Chui PC, Gopalakrishnan GS, Varela-Rey M, Crawley M, Fisher FM, et al. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology. 2010;139:456–63. doi: 10.1053/j.gastro.2010.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matikainen N, Taskinen MR, Stennabb S, Lundbom N, Hakkarainen A, Vaaralahti K, et al. Decrease in circulating fibroblast growth factor 21 after an oral fat load is related to postprandial triglyceride-rich lipoproteins and liver fat. Eur J Endocrinol. 2012;166:487–92. doi: 10.1530/EJE-11-0783. [DOI] [PubMed] [Google Scholar]
- 18.Schmid A, Leszczak S, Ober I, Karrasch T, Schaffler A. Short-term and divergent regulation of FGF-19 and FGF-21 during oral lipid tolerance test but not oral glucose tolerance test. Exp Clin Endocrinol Diabetes. 2015;123:88–94. doi: 10.1055/s-0034-1395635. [DOI] [PubMed] [Google Scholar]
- 19.Samms RJ, Lewis JE, Norton L, Stephens FB, Gaffney CJ, Butterfield T, et al. FGF21 is an insulin-dependent postprandial hormone in adult humans. J Clin Endocrinol Metab. 2017;102:3806–13. doi: 10.1210/jc.2017-01257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu H, Xia F, Lam KS, Wang Y, Bao Y, Zhang J, et al. Circadian rhythm of circulating fibroblast growth factor 21 is related to diurnal changes in fatty acids in humans. Clin Chem. 2011;57:691–700. doi: 10.1373/clinchem.2010.155184. [DOI] [PubMed] [Google Scholar]