Abstract
Anastomotic leakage is a complication of colorectal surgery. C-reactive protein (CRP) is an acute-phase marker that can indicate surgical complications. We determined whether serum CRP levels in patients who had undergone colorectal surgery can be used to exclude the presence of anastomotic leakage and allow safe early discharge. We included 90 patients who underwent colorectal surgery with primary anastomosis. Serum CRP levels were measured retrospectively on postoperative days (PODs) 1 – 7. Patients with anastomotic leakage (n = 11) were compared to those without leakage (n = 79). We statistically analysed data and plotted receiver operating characteristic curves. The incidence of anastomotic leakage was 12.2%. Diagnoses were made on PODs 3 – 24. The overall mortality rate was 3.3% (18.2% in the leakage group, 1.3% in the non-leakage group; P < 0.045). CRP levels were most accurate on POD 4, with a cutoff level of 180 mg/L, showing an area under the curve of 0.821 and a negative predictive value of 97.2%. Lower CRP levels after POD 2 and levels <180 mg/L on POD 4 may indicate the absence of anastomotic leakage and may allow safe discharge of patients who had undergone colorectal surgery with primary anastomosis.
Subject terms: Colonic diseases, Colorectal cancer
Introduction
Despite advances in surgical techniques and stapling devices, anastomotic leakage remains one of the most devastating complications of colorectal surgery1,2 with mortality rates reaching 30%3. The incidence of anastomotic leakage varies from 1% to 30%4, and it is most common in extraperitoneal anastomosis2,5,6. This variation in the incidence rate is attributed to the variety of definitions of anastomotic leakage found in the medical literature3.
Anastomotic leakage can be defined as a defect leading to the communication or extravasation of intra- and extraluminal contents4,7. The presence of a pelvic abscess or an abscess near the site of anastomosis can also be considered as leakage5. The most frequently reported risk factors for anastomotic leakage include male gender, smoking, obesity, preexisting disease (e.g., diabetes mellitus or chronic renal failure), nutritional status, use of neoadjuvant therapy, and emergency surgery2,8–11. Measures such as individualized hydration management, mechanical preparation of the colon, and use of epidural anaesthesia do not appear to influence the risk of leakage11,12.
Early diagnosis is essential to reduce mortality, length of hospital stay, postoperative complications, tumour recurrence, and costs1,5. Because of the high mortality rate4, scores were developed to help identify patients at high risk for this complication13. Clinical status may vary widely, however, from benign symptoms to signs of peritonitis and septic shock, and surgical intervention is frequently necessary4,7,14,15. Computed tomography (CT), endoscopic examination, biomarkers and abdominal drain secretion analysis are the most commonly used tools in clinical practice to diagnose anastomotic leakage2,14,16,17.
C-reactive protein (CRP) is the most widely studied biomarker2,4,18,19, first described in 1930 by Willian S. Tillet and Thomas Francis. It is considered an acute-phase protein20–23. Secretion begins at 4 to 10 hours following inflammatory stimulation, peaks in the plasma at 48 hours, and returns to baseline after the inflammatory stimulus ceases20,21,24,25. Because of its short half-life (19 hours), CRP is a reliable marker following surgical procedures25–27.
In addition to being used for the diagnosis of anastomotic leakage, CRP is also used as a marker of severity in gastrointestinal pathologies and infectious complications of open and laparoscopic surgeries26,28–33. The objective of this study was to determine whether serum CRP levels of patients who had undergone emergency or elective colorectal surgery with primary anastomosis can be used to exclude the presence of anastomotic leakage and allow for safe and early discharge.
Methods
Ninety colorectal surgeries with primary anastomoses (ileocolic, colocolic, or colorectal) were performed in the General Surgery Department of Carapicuíba General Hospital between June 2014 and July 2018. The Research Ethics Committee of our institution approved this retrospective study and waived the need for informed consent. This study included patients of both gender who underwent elective or emergency colorectal surgery with primary anastomosis. The diseases that determined surgical treatment are presented in Table 1. Indications for emergency surgery included acute appendicitis, diverticulitis, and obstructive or perforated neoplasia. Patients who did not present at least 3 serum CRP levels within the first 7 postoperative days (PODs) were excluded. The patients were divided into 2 groups: leakage (n = 11) and non-leakage (n = 79). Clinical and demographic characteristics of the groups are shown in Table 1.
Table 1.
Clinical and demographic characteristics.
| Characteristic | Total (n = 90) | Non–leakage (n = 79) | Leakage (n = 11) | P value |
|---|---|---|---|---|
| Age, median (range), y | 56.0 (36.2–68.0) | 55.0 (37.5–67.0) | 68.0 (36.0–77.5) | 0.211 |
| Gender, n (%) | 1.000 | |||
| Female | 40 (44.4) | 35 (44.3) | 5 (45.5) | |
| Male | 50 (55.6) | 44 (55.7) | 6 (54.5) | |
| Hospital stay, median (range), d | 7.0 (7.0–9.0) | 7.0 (7.0–7.5) | 15.0 (10.5–22.0) | <0.001 |
| Surgical indication, n (%) | 0.182 | |||
| Acute abdomen | 23 (25.6) | 23 (29.1) | 0 | |
| Colon adenocarcinoma | 28 (28.9) | 23 (29.1) | 3 (27.3) | |
| Rectal adenocarcinoma | 3 (3.3) | 3 (3.8) | 0 | |
| Intestinal endometriosis | 1 (1.1) | 1 (1.3) | 0 | |
| Ostomy closure | 33 (36.7) | 26 (32.9) | 7 (63.6) | |
| Colovesical fistula | 1 (1.1) | 1 (1.3) | 0 | |
| Megacolon | 1 (1.1) | 1 (1.3) | 0 | |
| Surgical procedure, n (%) | 0.648 | |||
| Right colectomy | 25 (27.8) | 23 (29.1) | 2 (18.2) | |
| Left colectomy | 3 (3.2) | 3 (3.3) | 0 | |
| Segmental colectomy | 4 (4.4) | 3 (3.8) | 1 (9.1) | |
| Transit reconstruction | 33 (36.7) | 27 (34.2) | 6 (54.5) | |
| Rectosigmoidectomy | 25 (27.8) | 23 (29.1) | 2 (18.2) | |
| Anastomosis, n (%) | 0.292 | |||
| Ileocolic | 39 (43.3) | 35 (44.3) | 4 (36.4) | |
| Colorectal | 42 (46.7) | 39 (49.4) | 3 (27.3) | |
| Colocolic | 9 (10.0) | 5 (6.3) | 4 (36.4) | |
| Anastomosis technique, n (%) | 0.059 | |||
| Handsewn | 20 (22.2) | 15 (19.0) | 5 (45.5) | |
| Stapled | 70 (77.8) | 64 (81.0) | 6 (54.5) | |
| Surgical planning, n (%) | 0.508 | |||
| Emergency | 26 (28.9) | 24 (30.4) | 2 (18.2) | |
| Elective | 64 (71.1) | 55 (69.6) | 9 (81.8) | |
| Abdominal drain, n (%) | 0.694 | |||
| No | 19 (21.1) | 16 (20.3) | 3 (27.3) | |
| Yes | 71 (78.9) | 63 (79.7) | 8 (72.7) | |
| Death, n (%) | 0.045 | |||
| No | 87 (96.7) | 78 (98.7) | 9 (81.8) | |
| Yes | 3 (3.3) | 1 (1.3) | 2 (18.2) |
P-values obtained using the Mann–Whitney U test.
Serum CRP levels were evaluated on PODs 1 through 7 by immunoassays using the turbidimetric method with an Architect Plus C4000 analyser (Abbot, Lake Bluff, IL, USA). CRP levels >5 mg/L were considered altered. Patients were evaluated daily for the presence of abdominal pain, fever, volume, return of bowel habits, and/or appearance of abdominal drainage. Patients with altered parameters underwent laboratory and imaging examinations (CT or radiography). All patients received antibiotic prophylaxis, and mechanical preparation of the colon was conducted only for elective surgeries (71.1%).
Anastomotic leakage was defined using the following clinical and radiologic criteria: 1) presence of air or abscess near the site of anastomosis identified on CT, 2) purulent discharge or enteric secretion through the drain, and 3) clinical signs of peritonitis and/or presence of faecal or purulent discharge during surgical re-approach. Antibiotics were restarted in patients with leakage.
Categorical variables were presented as frequency and percentage, and quantitative variables, as median and interquartile range. The Shapiro–Wilk test was used to define normality, whereas the Mann–Whitney U test was used for bivariate comparisons. Receiver operating characteristic (ROC) curves were plotted using the values generated by logistic regression analysis. Sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV), accuracy, and area under the curve (AUC) were calculated. R language software (RStudio, Inc, Boston, MA, USA; www.rstudio.com) was used for statistical analysis with the level of significance set at 5% (P < 0.05).
Ethical approval
The Research Ethics Committee of São Camilo University Centre approved this retrospective study by CAAE number: 66510317.6.0000.0062.
Informed consent
The Research Ethics Committee of our institution approved this retrospective study and waived the need for informed consent.
Results
During the study period, 90 patients underwent colorectal surgery with primary anastomosis. The median age was 56 years and 55.6% of patients were male (Table 1). Ostomy closure (36.7%) and colon adenocarcinoma resection (28.9%) were the most common surgical indications. The use of abdominal drainage did not affect the onset of leakage (P = 0.694). Colic anastomoses were created using a mechanical stapler in 70 patients (77.8%) and sutures in 20 patients (22.2%).
The postoperative mortality rate was significantly higher in patients with anastomotic leakage than in those without leakage (18.2% vs 1.3%; P < 0.045). The rate of postoperative complications was 26.6% (n = 24), including 11 patients (12.2%) in the leakage group: 10 (11.1%) with surgical site infection, two (2.2%) with evisceration, and one (1.1%) with pneumonia. Leakage was diagnosed between PODs 3 and 24 (median, 7.7 days) (Online Resource 1). The median hospital stay was significantly longer in the leakage group than in the non-leakage group (15 vs 7 days; P < 0.001). CT was used to diagnose anastomotic leakage in 72.7% of patients. Ten patients (91.9%) with leakage underwent surgical treatment, including four (36.4%) Hartmann’s colectomies, three (27.3%) colectomies with terminal ileostomy, three (27.3%) reanastomoses, and one (9.1%) abdominal drainage.
There were no statistically significant differences in serum CRP levels in the first 3 PODs. After POD 4, however, there was a significant increase in serum CRP levels in patients with anastomotic leakage (median, 246.4 mg/L) compared with those without leakage (median, 113.5 mg/L; P = 0.002) (Online Resource 2). Serum CRP levels increased from POD 2 in patients with leakage and decreased in those without leakage (Fig. 1). Peak levels were seen on POD 5 in patients with leakage and on POD 2 in those without leakage.
Figure 1.
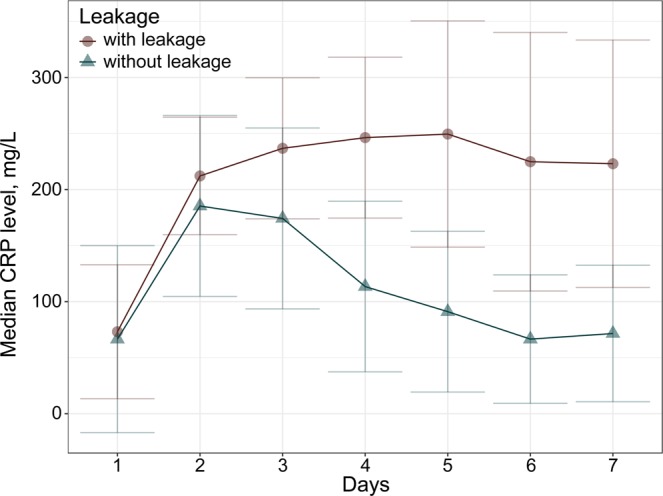
Serum C-reactive protein (CRP) levels in patients with and without primary colic anastomotic leakage.
Analyses of the ROC curves from PODs 3 through 5 are presented in Tables 2–4. Sensitivity, specificity, NPV, PPV, accuracy, and AUC are presented in Figs. 2–4. A cutoff value of 220 mg/L was established on POD 3 with an AUC of 0.643, NPV of 89.3%, PPV of 20%, sensitivity of 71%, and specificity of 45%. On POD 4, with a cutoff value of 180 mg/L, the AUC was 0.821, NPV was 97.2%, sensitivity was 72.3%, and specificity was 88.9%. Patients with CRP levels <180 mg/L on POD 4 had a 12.2% probability of developing anastomotic leakage.
Table 3.
Results of ROC curve analysis on POD 4 (cutoff value, 180 mg/L).
| Accuracy | PPV | NPV | AUC | Sensitivity | Specificity |
|---|---|---|---|---|---|
| 0.743 | 0.307 | 0.972 | 0.821 | 0.723 | 0.889 |
AUC, area under the curve; NPV, negative predictive value; POD, postoperative day; PPV, positive predictive value; ROC, receiver operating characteristic.
Figure 3.
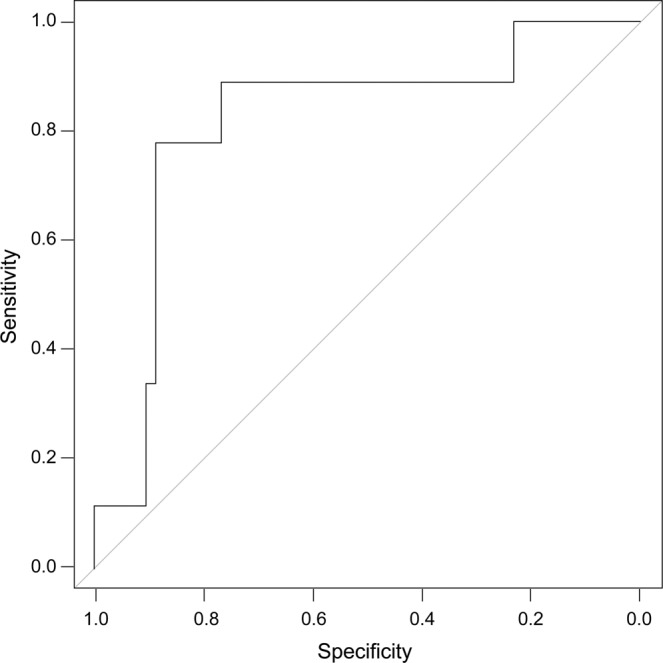
Receiver operating characteristic curve analysis of C-reactive protein level on postoperative day 4.
Table 2.
Results of ROC curve analysis on POD 3 (cutoff value, 220 mg/L).
| Accuracy | PPV | NPV | AUC | Sensitivity | Specificity |
|---|---|---|---|---|---|
| 0.679 | 0.200 | 0.893 | 0.643 | 0.714 | 0.455 |
AUC, area under the curve; NPV, negative predictive value; POD, postoperative day; PPV, positive predictive value; ROC, receiver operating characteristic.
Table 4.
Results of ROC curve analysis on POD 5 (cutoff value, 160 mg/L).
| Accuracy | PPV | NPV | AUC | Sensitivity | Specificity |
|---|---|---|---|---|---|
| 0.789 | 0.333 | 0.931 | 0.796 | 0.818 | 0.6 |
AUC, area under the curve; NPV, negative predictive value; POD, postoperative day; PPV, positive predictive value; ROC, receiver operating characteristic.
Figure 2.
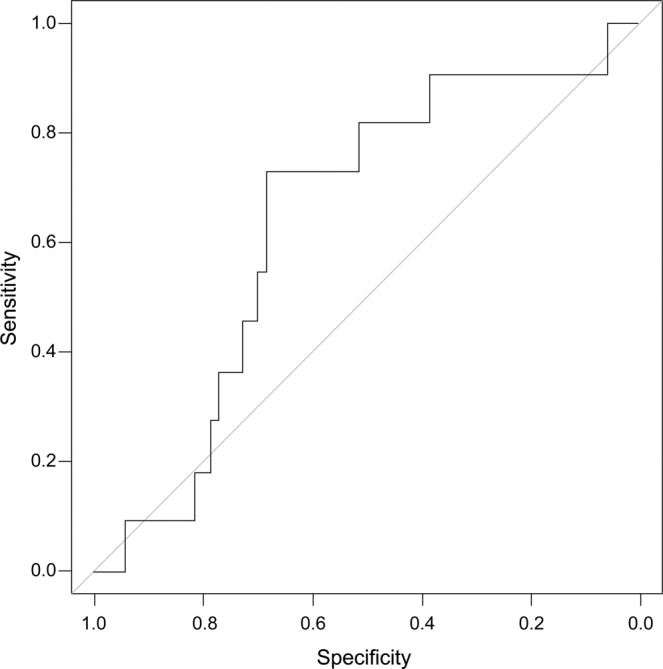
Receiver operating characteristic curve analysis of C-reactive protein level on postoperative day 3.
Figure 4.
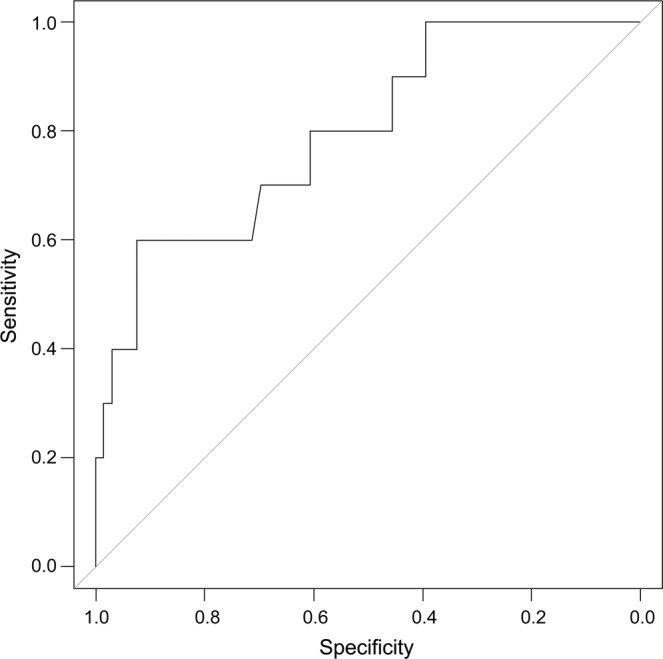
Receiver operating characteristic curve analysis of C-reactive protein level on postoperative day 5.
Discussion
Anastomotic leakage is an undesirable complication of colorectal surgery1, resulting in increased length of hospitalization, increased treatment costs, delayed return of intestinal homeostasis, and decreased survival34,35. Because vital signs and leukocyte numbers are slow in responding, it is important to identify tools to detect early leakage36,37. Early diagnosis is essential to reduce morbidity and mortality33,38–40 because delayed diagnosis can increase mortality by 18%37.
CRP is an acute-phase protein produced by hepatocytes after inflammatory stimulation18,25. It is a useful marker to monitor and identify postoperative complications because it has a short half-life38,40. This protein has been shown to be as effective and sensitive as a predictor of anastomotic leakage34,35,39–41 and postoperative infection15,19,38. In fact, increased CRP levels are more sensitive to diagnose surgical complications than are increased erythrocyte sedimentation rate, leukocytes, body temperature, and heart rate25. Nevertheless, because of the individual regulation of inflammatory responses, disagreement among serum CRP levels is not uncommon18,25. Su’a et al. analysed 11 studies on anastomotic leakage and identified a wide variation in CRP cutoff values, ranging from 94 to 190 mg/L, on the same postoperative day22. Medications such as corticosteroids and statins may also alter this response, which could decrease serum CRP levels and alter the interpretation of cutoff levels22.
The surgical approach also influences serum CRP levels. Waterland et al. found higher CRP levels in patients who underwent open surgery than in those who underwent laparoscopic surgery40. They reported that a level of 123.5 mg/L on POD 4 after conventional surgery was the most predictive of anastomotic leakage. However, their study included only elective colorectal surgeries. Almeida et al. evaluated several types of colorectal resections and found that a CRP cutoff level of 140 mg/L on POD 3 had a significant association with the presence of anastomotic leakage29. Lagoutte et al. reported a cutoff value of 125 mg/L on POD 435, whereas Granero-Garcia et al. reported that a cutoff level of 135 mg/L on POD 5 was a good predictor of leakage39. In another study, Muñoz et al. evaluated only patients who underwent elective laparoscopic colorectal cancer resection using the enhanced recovery after surgery (ERAS) protocol. In their study, CRP had a high NPV on POD 3 with a cutoff level of 163 mg/L33.
Singh et al. conducted a systematic review of 6 studies including >2400 patients41 and found that CRP levels were comparable in terms of accuracy on PODs 3, 4, and 5. On the other hand, Warschkow et al. conducted a meta-analysis and reported that CRP levels were more accurate on POD 4, demonstrating a high NPV for postoperative complications, with a cutoff value of 135 mg/L (38). Our study showed a high NPV, sensitivity, and specificity with a cutoff value of 180 mg/L on POD 4. This high cutoff value may be related to the inclusion of patients who underwent emergency colorectal surgery. This study also identified decreased CRP levels on POD 2 in patients without leakage, similar to the findings reported by Woeste et al.34. However, it is difficult to compare studies because of the non-standardization of anastomotic leakage definitions, day and time of CRP testing, patient selection, and surgical approach22,34,41.
Nonetheless, most studies support the notion that patients with anastomotic leakage present higher and sustained elevation of serum CRP levels in the postoperative period compared with patients without leakage33,34,36,39,42. According to several studies, increased serum CRP levels precede radiologic and clinical diagnosis of anastomotic leakage. They reported that the detection of sustained serum CRP elevation may decrease the time for indicating reoperation, which could lead to lower mortality rates and hospital costs34,39.
Sawyer et al. analysed the differences between short and extended use of antibiotics in >500 patients who underwent complicated intra-abdominal infection treatment and colorectal surgery. They found no significant differences in terms of surgical site infection, recurrent intra-abdominal infection, or death43. These findings suggest that with short-term use of effective and safe antibiotic therapy, patients undergoing emergency colorectal surgery could also benefit from the analysis of serial CRP levels, which could provide the possibility of early and safe hospital discharge43.
Patients tend to be discharged early, between PODs 4 and 5, with the advent of multimodal accelerated postoperative recovery protocols such as ERAS33,35,41. Because most surgical complications occur after patients are discharged, between PODs 5 and 8, a marker such as CRP, which has a high NPV on POD 4, could be used to exclude anastomotic leakage and other postoperative complications38. In addition to the use of scores to identify patients at high risk of anastomotic leakage, postoperative investigation protocols for patients with sustained elevation of CRP levels after POD 2 or with levels above the cutoff on POD 4 should be generated13,38. Because of the high NPV, serum CRP levels on POD 4 seem to play an important role in the exclusion of anastomotic leakage33,35,39,40.
In conclusion, serum CRP levels can be routinely analysed in patients who undergo elective or emergency colorectal surgery. Decreased CRP levels after POD 2 can exclude anastomotic leakage because they are not influenced by factors such as individual inflammatory response, type of approach, or surgical indication. A cutoff level of 180 mg/L on POD 4 can indicate high reliability for hospital discharge due to a low probability of anastomotic leakage.
Postoperative serum CRP levels in patients who undergo colorectal surgery with primary anastomosis could become a useful marker for the exclusion of anastomotic leakage. This was a single-centre study with a small sample size; therefore, prospective multicentre studies with a greater number of patients are necessary to confirm our findings and extend them to clinical practice.
Supplementary information
Author contributions
B.A.M., E.R.M., R.V.B., K.C.T. and S.S.S. performed data analysis. B.A.M. and J.W. designed the study. B.A.M. and E.R.M. contributed to acquisition of data. B.A.M., E.R.M., R.V.B. and K.C.T. did literature review. B.A.M. and J.W. drafted the manuscript. S.S.S. performed interpretation of the data. R.V.B. and K.C.T. did medical and technical analysis, respectively. J.W. revised the manuscript. All authors reviewed the manuscript.
Data availability
All data generated or analysed during this study are included in this published article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-58780-3.
References
- 1.Cikot M, et al. The marker C-reactive protein is helpful in monitoring the integrity of anastomosis: plasma calprotectin. Am. J. Surg. 2016;212:53–61. doi: 10.1016/j.amjsurg.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 2.Vallance A, et al. A collaborative review of the current concepts and challenges of anastomotic leaks in colorectal surgery. Colorectal Dis. 2017;19:O1–O12. doi: 10.1111/codi.13534. [DOI] [PubMed] [Google Scholar]
- 3.Moghadamyeghaneh Z, et al. Contemporary management of anastomotic leak after colon surgery: assessing the need for reoperation. Am. J. Surg. 2016;211:1005–1013. doi: 10.1016/j.amjsurg.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 4.Kingham TP, Pachter HL. Colonic anastomotic leak: risk factors, diagnosis, and treatment. J. Am. Coll. Surg. 2009;208:269–278. doi: 10.1016/j.jamcollsurg.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 5.Park JS, et al. Risk factors of anastomotic leakage and long-term survival after colorectal surgery. Medicine. 2016;95:e2890. doi: 10.1097/MD.0000000000002890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neutzling CB, Lustosa AS, Proenca IM, da Silva EM, Matos D. Stapled versus handsewn methods for colorectal anastomosis surgery. Cochrane Database Syst. Rev. 2012;2:CD003144. doi: 10.1002/14651858.CD003144.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Rahbari NN, et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the international Study Group of Rectal Cancer. Surgery. 2010;147:339–351. doi: 10.1016/j.surg.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Parthasarathy M, Greensmith M, Bowers D, Groot-Wassink T. Risk factors for anastomotic leakage after colorectal resection: a retrospective analysis of 17 518 patients. Colorectal Dis. 2017;19:288–298. doi: 10.1111/codi.13476. [DOI] [PubMed] [Google Scholar]
- 9.Rojas-Machado SA, et al. Prediction of anastomotic leak in colorectal cancer surgery based on a new prognostic index PROCOLE (prognostic colorectal leakage) developed from the meta-analysis of observational studies of risk factors. Int. J. Colorectal Dis. 2016;31:197–210. doi: 10.1007/s00384-015-2422-4. [DOI] [PubMed] [Google Scholar]
- 10.Pettersson D, et al. Interim analysis of the Stockholm III trial of preoperative radiotherapy regimens for rectal cancer. Br. J. Surg. 2010;97:580–587. doi: 10.1002/bjs.6914. [DOI] [PubMed] [Google Scholar]
- 11.McDermott FD, et al. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br. J. Surg. 2015;102:462–479. doi: 10.1002/bjs.9697. [DOI] [PubMed] [Google Scholar]
- 12.Halabi WJ, et al. Epidural analgesia in laparoscopic colorectal surgery: a nationwide analysis of use and outcomes. JAMA Surg. 2014;149:130–136. doi: 10.1001/jamasurg.2013.3186. [DOI] [PubMed] [Google Scholar]
- 13.Daams F, Wu Z, Lahaye MJ, Jeekel J, Lange JF. Prediction and diagnosis of colorectal anastomotic leakage: a systematic review of literature. World J. Gastrointest. Surg. 2014;6:14–26. doi: 10.4240/wjgs.v6.i2.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gessler B, Eriksson O, Angenete E. Diagnosis, treatment, and consequences of anastomotic leakage in colorectal surgery. Int. J. Colorectal Dis. 2017;32:549–556. doi: 10.1007/s00384-016-2744-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McSorley ST, Khor BY, MacKay GJ, Horgan PG, McMillan DC. Examination of a CRP first approach for the detection of postoperative complications in patients undergoing surgery for colorectal cancer: a pragmatic study. Medicine. 2017;96:e6133. doi: 10.1097/MD.0000000000006133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kornmann VN, et al. Systematic review on the value of CT scanning in the diagnosis of anastomotic leakage after colorectal surgery. Int. J. Colorectal Dis. 2013;28:437–445. doi: 10.1007/s00384-012-1623-3. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda T, et al. Endoscopic evaluation of clinical colorectal anastomotic leakage. J. Surg. Res. 2015;193:126–134. doi: 10.1016/j.jss.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 19.Nason GJ, et al. Early rise in C-reactive protein is a marker for infective complications in laparoscopic colorectal surgery. Surg. Laparoscop. Endosc. Percutan. Tech. 2014;24:57–61. doi: 10.1097/SLE.0b013e31828fa03e. [DOI] [PubMed] [Google Scholar]
- 20.Cousin F, et al. Diagnostic accuracy of procalcitonin and C-reactive protein for the early diagnosis of intra-abdominal infection after elective colorectal surgery: a meta-analysis. Ann. Surg. 2016;264:252–256. doi: 10.1097/SLA.0000000000001545. [DOI] [PubMed] [Google Scholar]
- 21.Smith SR, et al. Biomarkers and anastomotic leakage in colorectal surgery: C-reactive protein trajectory is the gold standard. ANZ. J. Surg. 2018;88:440–444. doi: 10.1111/ans.13937. [DOI] [PubMed] [Google Scholar]
- 22.Su’a BU, Mikaere HL, Rahiri JL, Bissett IB, Hill AG. Systematic review of the role of biomarkers in diagnosing anastomotic leakage following colorectal surgery. Br. J. Surg. 2017;104:503–512. doi: 10.1002/bjs.10487. [DOI] [PubMed] [Google Scholar]
- 23.Wu Y, Potempa LA, El Kebir D, Filep JG. C-reactive protein and inflammation: conformational changes affect function. Biol. Chem. 2015;396:1181–1197. doi: 10.1515/hsz-2015-0149. [DOI] [PubMed] [Google Scholar]
- 24.Rickert A, Willeke F, Kinle P, Post S. Management and outcome of anastomotic leakage after colonic surgery. Colorectal Dis. 2010;12:e216–e223. doi: 10.1111/j.1463-1318.2009.02152.x. [DOI] [PubMed] [Google Scholar]
- 25.Aguiar FJ, et al. C-reactive protein: clinical applications and proposals for a rational use. Rev. Assoc. Med. Bras. 2013;59:85–92. doi: 10.1590/S0104-42302013000100016. [DOI] [PubMed] [Google Scholar]
- 26.Mik M, Berut M, Dziki L, Dziki A. Does C-reactive protein monitoring after colorectal resection with anastomosis give any practical benefit for patients with intra-abdominal septic complications? Colorectal Dis. 2016;18:O252–O259. doi: 10.1111/codi.13386. [DOI] [PubMed] [Google Scholar]
- 27.Kostić Z, et al. Diagnostic value of serial measurement of C-reactive protein in serum and matrix metalloproteinase-9 in drainage fluid in the detection of infectious complications and anastomotic leakage in patients with colorectal resection. Vojnosanit. Pregl. 2015;72:889–898. doi: 10.2298/VSP140723011K. [DOI] [PubMed] [Google Scholar]
- 28.Du Clos TW. Function of C-reactive protein. Ann. Med. 2000;32:274–278. doi: 10.3109/07853890009011772. [DOI] [PubMed] [Google Scholar]
- 29.Almeida AB, et al. Elevated serum C-reactive protein as a predictive factor for anastomotic leakage in colorectal surgery. Int. J. Surg. 2012;10:87–91. doi: 10.1016/j.ijsu.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Vermeire S, Van Assche G, Rutgeerts P. The role of C-reactive protein as an inflammatory marker in gastrointestinal diseases. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005;2:580–586. doi: 10.1038/ncpgasthep0359. [DOI] [PubMed] [Google Scholar]
- 31.Ji L, Wang T, Tian L, Gao M. The early diagnostic value of C-reactive protein for anastomotic leakage post radical gastrectomy for esophagogastric junction carcinoma: a retrospective study of 97 patients. Int. J. Surg. 2016;27:182–186. doi: 10.1016/j.ijsu.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 32.Scepanovic MS, et al. C-reactive protein as an early predictor for anastomotic leakage in elective abdominal surgery. Tech. Coloproctol. 2013;17:541–547. doi: 10.1007/s10151-013-1013-z. [DOI] [PubMed] [Google Scholar]
- 33.Muñoz JL, et al. Procalcitonin and C-reactive protein as early markers of anastomotic leak after laparoscopic colorectal surgery within an enhanced recovery after surgery (ERAS) program. Surg. Endosc. 2018;32:4003–4010. doi: 10.1007/s00464-018-6144-x. [DOI] [PubMed] [Google Scholar]
- 34.Woeste G, Müller C, Bechstein WO, Wullstein C. Increased serum levels of C-reactive protein precede anastomotic leakage in colorectal surgery. World J. Surg. 2010;34:140–146. doi: 10.1007/s00268-009-0304-z. [DOI] [PubMed] [Google Scholar]
- 35.Lagoutte N, et al. C-reactive protein and procalcitonin for the early detection of anastomotic leakage after elective colorectal surgery: pilot study in 100 patients. J. Visc. Surg. 2012;149:e345–e349. doi: 10.1016/j.jviscsurg.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Reynolds IS, et al. C-reactive protein as a predictor of anastomotic leak in the first week after resection for rectal cancer. Colorectal Dis. 2017;19:812–818. doi: 10.1111/codi.13649. [DOI] [PubMed] [Google Scholar]
- 37.Alves A, Panis Y, Pocard M, Regimbeau JM, Valleur P. Management of anastomotic leakage after nondiverted large bowel resection. J. Am. Coll. Surg. 1999;189:554–559. doi: 10.1016/S1072-7515(99)00207-0. [DOI] [PubMed] [Google Scholar]
- 38.Warschkow R, et al. Safe and early discharge after colorectal surgery due to C-reactive protein: a diagnostic meta-analysis of 1832 patients. Ann. Surg. 2012;256:245–250. doi: 10.1097/SLA.0b013e31825b60f0. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Granero A, et al. Procalcitonin and C-reactive protein as early predictors of anastomotic leak in colorectal surgery: a prospective observational study. Dis. Colon Rectum. 2013;56:475–483. doi: 10.1097/DCR.0b013e31826ce825. [DOI] [PubMed] [Google Scholar]
- 40.Waterland P, et al. Using CRP to predict anastomotic leakage after open and laparoscopic colorectal surgery: is there a difference? Int. J. Colorectal Dis. 2016;31:861–868. doi: 10.1007/s00384-016-2547-0. [DOI] [PubMed] [Google Scholar]
- 41.Singh PP, et al. Systematic review and meta-analysis of use of serum C-reactive protein levels to predict anastomotic leak after colorectal surgery. Br. J. Surg. 2014;101:339–346. doi: 10.1002/bjs.9354. [DOI] [PubMed] [Google Scholar]
- 42.Matthiessen P, et al. Increase of serum C-reactive protein is an early indicator of subsequent symptomatic anastomotic leakage after anterior resection. Colorectal Dis. 2008;10:75–80. doi: 10.1111/j.1463-1318.2007.01452.x. [DOI] [PubMed] [Google Scholar]
- 43.Sawyer RG, et al. Trial of short-course antimicrobial therapy for intraabdominal infection. N. Engl. J. Med. 2015;372:1996–2005. doi: 10.1056/NEJMoa1411162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article.


