Abstract
The growing incidence of cancer raises an urgent need to develop effective diagnostic and therapeutic strategies. With the rapid development of nanomedicine, nanoscale metal-organic frameworks (NMOFs) presented promising potential in various biomedical applications in the last 2 decades, especially in cancer theranostics. Due to the unique features of NMOFs, including structural diversities, enormous porosity, multifunctionality and biocompatibility, they have been widely used to deliver imaging contrast agents and therapeutic drugs. Moreover, multiple types of contrast agents, anti-cancer drugs and targeting ligands could be co-delivered through one single NMOF to enable combination therapy. Co-delivering system using NMOFs helped to avoid multidrug resistance, to reduce adverse effects, to achieve imaging-guided precise therapy and to enhance anti-cancer efficacy. This review summarized the recent research advances on the application of NMOFs in biomedical imaging and cancer treatments in the last few years. The current challenges that impeding their translation to clinical practices and the perspectives for their future applications were also highlighted and discussed.
Keywords: nanomedicine, theranostic platform, biomedical imaging, combination therapy, clinical translation
Introduction
The incidence of cancer is increasing rapidly and it is still one of the most devastating diseases threatening people’s health.1 Therapeutic strategies, including surgical intervention, radiotherapy (RT), and chemotherapy, are routine methods of cancer treatment in the clinic. However, owing to poor effects, intolerably systemic toxicity and high risk of recurrence, traditional treatments often fail to meet the clinical needs. Thus, there is an urgent need for safer and more effective treatment strategies to improve the survival rate of cancer patients.
By integrating functions of diagnostics and therapeutics into a single system, cancer theranostics has attracted great attention in cancer research.2,3 With the rapid development of nanotechnology in the last 2 decades, nanoparticles have been used in cancer theranostics for more efficient early diagnoses and early therapies.4 Metal-organic frameworks (MOFs) are a series of crystalline hybrid materials composed of metal ions or clusters and organic ligands.5,6 Recently, MOFs were scaled down to the nanometer sizes, namely nanoscale MOFs (NMOFs). NMOFs combine many unique properties into a single loading and delivery system, including high loading capacities, compositional and structural tenability, controllable and sustained drug release, excellent biocompatibility and intrinsic biodegradable.7,8 In addition, postsynthetic modification offers NMOFs more desired functionalities.9,10 Nanoscopic size of NMOFs enables them to accumulate at the tumor sites due to the enhanced permeability and retention (EPR) effect of tumors.11 These unique features promote NMOFs’ widely application in delivering imaging contrast agents and anti-cancer drugs as attractive theranostic platforms.12
Based on the different microenvironment between normal and tumor tissues, tumor-targeting NMOFs can be designed to reduce the damage to normal tissues. According to the EPR effect, NMOFs can be accumulated at tumor sites, which is passive targeting.13 On the other hand, active targeting can be achieved by adding targeting ligands on the surface of NMOFs. Recent researches demonstrated the successful fabrication of stimuli-responsive NMOF systems based on acidic pH conditions in tumor microenvironment14–16 and the overexpressed biomarkers in cancer cells, such as vascular endothelial growth factor (VEGF)17 and folate reporter (FR).18,19
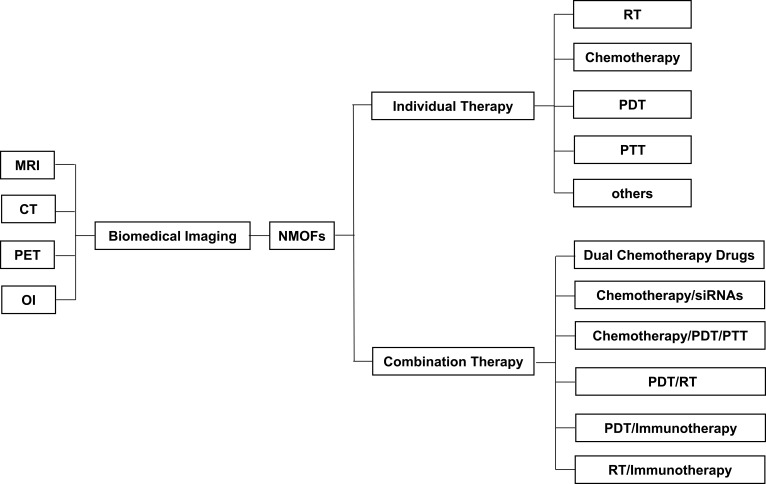
As shown in Figure 1, this review summarized the studies on the diagnostic and therapeutic applications of NMOFs in the last 5 years. The implication of NMOFs in combination cancer therapies was especially emphasized. The remaining challenges and future directions of NMOFs translational studies were highlighted.
Figure 1.
The applications of NMOFs in diagnostic and therapeutic strategies for cancers.
Abbreviations: MRI, magnetic resonance imaging; CT, computed tomography; PET, positron emission tomography; OI, optical imaging; NMOFs, nanoscale metal-organic frameworks; RT, radiotherapy; PDT, photodynamic therapy; PTT, photothermal therapy.
Implication of NMOFs in Biomedical Imaging
The rapid development of biomedical imaging has greatly facilitated the early detection of cancers. The metal ions or clusters on NMOFs make them promising candidates as contrast agents for biomedical imaging, such as magnetic resonance imaging (MRI), computed tomography (CT), positron emission tomography (PET), and optical imaging (OI). Compared with traditional small-molecule contrast agents that are restricted by nonspecific distribution and rapid pharmacokinetics, NMOFs-based contrast agents are easily functionalized. In addition, NMOFs can co-deliver multiple biomolecules at the same time, so they can be used for multimodal imaging and image-guided therapy. The implication of NMOFs as imaging agents is illustrated in Table 1.
Table 1.
The Applications of NMOFs in Biomedical Imaging
| NMOFs | Imaging Methods | References |
|---|---|---|
| Eu,Gd-NMOF@SiO2 | MRI | [20] |
| Mn-IR825@PDA-PEG | MRI | [21] |
| Fe3O4@IRMOF-3/FA | MRI | [22] |
| Mn2(Cmdcp)2(H2O)2]·H2O}n [Gd(Cmdcp)(H2O)3](NO3)·3H2O}n |
MRI | [23] |
| MOF@HA@ICG NPs | MRI | [24] |
| NMOF-SNO | MRI | [25] |
| Fe3O4-ZIF-8 | MRI | [26] |
| TPZ/Hf/TCPP/PEG | CT | [27] |
| Au@MIL‐88(Fe) | MRI/CT | [28] |
| DOX@Gd-MOFs-Glu | MRI/CT | [29] |
| 89Zr-UiO-66/Py-PGA-PEG-F3 | PET | [30] |
| Fe3O4@OCMC@IRMOF-3/FA | OI | [31] |
| DOX@NPMOFs | OI | [32] |
| [Rs⊂nMOF-801]-GS/[R6G⊂nUiO-67]-GS | OI | [33] |
| UiO‐66@DOPA‐LB | OI | [34] |
| UCNP@Fe‐MIL‐101_NH2 | MRI/OI | [35] |
Abbreviations: MRI, magnetic resonance imaging; CT, computed tomography; PET, positron emission tomography; OI, optical imaging; PDA, polydopamine; PEG, polyethylene glycol; FA, folate acid; HA, hyaluronic acid; ICG, indocyanine green; NPs, nanoparticles; SNO, S-nitrosothiol; TPZ, tirapazamine; TCPP, tetra(4-carboxyphenyl)porphine; Py-PGA-PEG, pyrene-derived polyethylene glycol; Glu, glucose; DOX, doxorubicin; OCMC, O-carboxymethyl chitosan; DOPA-LB, 1,2‐dioleoyl‐sn‐glycero‐3‐phosphate lipid bilayer.
MRI
NMOFs have recently been used as potential MRI agents owing to their large metal payload, which greatly improved MRI quality and diagnostic sensitivity.
Lin’s group36 reported the successful application of Gd3+-based NMOFs as MRI contrast agents for the first time in 2006. Long relaxation time of Gd3+ ensured the capability of MRI. In 2016, Wang et al20 synthesized a Gd and Eu co-doped NMOF. By integrating T1 and T2 contrast agents into a nanoparticle, the Eu,Gd-NMOFs@SiO2 simultaneously modulated T1- and T2-weighted contrasts, suggesting their great potential as T1-T2 dual-modal imaging probes.
In recent years, researchers tried to synthesize stimuli-responsive MRI contrast agents to improve the sensitivity of early detection and to increase the efficacy of imaging-guided precision therapy. Ray and Sahu22 demonstrated that magnetic Fe3O4@IRMOF-3/FA can function as strong T2-weighted MRI contrast agents and delivery anti-cancer drug delivery agents at the same time. Fe3O4 were used to offer strong contrast in T2-weighted MRI. FA conjugated to the NMOF surface served as the targeted reagent. Cell viability assays indicated that Fe3O4@IRMOF-3/FA were nontoxic towards HeLa and NIH3T3 cells. Lin et al26 successfully synthesized Fe3O4-ZIF-8 as pH- and glutathione (GSH)-responsive MRI contrast agents due to acidic conditions and overexpressed GSH in the tumor microenvironment. The disassembled Fe3O4-ZIF-8 released the Fe3O4 nanoparticles in tumor tissues, leading to transformation from T2 to T1 contrast enhancement, and providing a large inverse contrast compared with the T2 contrast enhancement for normal tissues.
CT
Due to high spatial resolution, deep tissue penetration, and 3-dimensional (3D) visibility, CT has been found increasing use in the diagnosis and treatment of cancers in recent years.37 NMOFs served as contrast agents for CT imaging due to the incorporation of high Z element.38 Owing to the large X‐ray absorption coefficient, gold nanoparticles were widely used as contrast agents for CT imaging.39,40 Shang et al28 synthesized small‐scale core–shell nanoparticles, named as Au@MIL-88(Fe). The modified nanocomposites possessed both CT enhancement ability and the T2‐weighted MRI property. Therefore, Au@MIL-88(Fe) served as multimodality imaging agents to integrate various image‐enhancing behaviors into a single system for multimodality imaging. In addition, MTT assay showed that this nanoparticle had no significant cytotoxicity towards U87MG cells even at high concentrations. Liu et al27 also suggested that TPZ/Hf/TCPP/PEG acted as an efficient CT contrast agent due to the strong X-ray attenuation of Hf. In vivo CT imaging ability was validated in 4T1 tumor-bearing mice.
PET
Compared with other imaging methods, PET imaging displays superior sensitivity, deeper tissue penetration capability, and better quantitative capacity, which made it widely used as diagnostic tools from preclinical to clinical research.41 The conventional PET imaging agent Fluorodeoxyglucose (FDG) hardly targets cancer cells specifically. Fortunately, this can be overcome by incorporated PET imaging agents into NMOFs. Chen et al30 designed and synthesized a radioactive MOF nanomaterial, 89Zr-UiO-66/Py–PGA-PEG-F3. F3 peptide functioned as tumor-targeting ligand because it exhibited potent binding to tumor cells. In addition, the NMOFs showed a high loading capacity of DOX. This study suggested the potential of NMOFs for PET-guided tumor-targeted drugs delivery. In addition, in vivo safety evaluation confirmed that there was no observable acute, medium, or chronic toxicity.
OI
OI is increasingly applied to medical and biological research with its high resolution and high sensitivity. NMOFs have been widely used in OI due to their attractive water solubility. In 2016, Chowdhuri et al31 successfully designed a magnetic NMOF, Fe3O4@OCMC@IRMOF-3/FA. Highly fluorescent carbon dots were conjugated on the surface of NMOFs for optical imaging and drug tracking. In 2017, Liu et al32 validated that zirconium-porphyrin MOFs (NPMOFs) were an ideal OI-guided therapy system. Porphyrin provided strong fluorescence, but it was hydro-phobic with a tendency to aggregate. NPMOFs helped to overcome these disadvantages and retained the photostability of the porphyrin. Ryu et al33 encapsulated dye molecules within pores of NMOFs and then found that Dye⊂NMOFs could be successfully utilized for fluorescence imaging of human cells. More recently, Zhang et al34 reported that UiO-66@DOPA-LB exhibited improved biostability and prolonged circulation time, which endowed it great potential to serve as a nanocarrier for imaging agents. When labeling with NIR dye, IR‐800, UiO-66@DOPA-LB-IR-800 exhibited superior capability for the detection of small tumor lesions at early stages.
Implication of NMOFs in Individual Cancer Therapy
A major reason for the failure of conventional cancer treatment is the inability of therapeutic drugs to be efficiently directed to tumor sites without damage to healthy tissues and organs. NOMFs not only improve the effects of traditional treatments such as RT and chemotherapy, but also benefit the newly development methods of phototherapy due to their excellent characteristics (Table 2).
Table 2.
The Examples of NMOFs in Individual Cancer Therapy
| Therapy Strategies | NMOFs | Cancer Cell Types | Animal Models | References |
|---|---|---|---|---|
| RT-RDT | Hf-DBB-Ru | MC38/CT26 cells | Mice bearing MC38/CT26 tumors | [42] |
| W18@Hf12-DBB-Ir | MC38/CT26 cells | Mice bearing MC38/CT26 tumors | [43] | |
| Chemotherapy | VEGF-responsive DOX-loaded NMOFs | MDA-MB-231cells | None | [17] |
| Fe-MIL-53-NH2-FA-5-FAM/5-FU | MGC-803 cells | None | [19] | |
| Fe3O4@IRMOF-3/FA | HeLa cells | None | [22] | |
| DOX@Gd-MOFs-Glu | HeLa cells | Mice bearing Hela tumors | [29] | |
| 89Zr-UiO-66/Py-PGA-PEG-F3 | MDA-MB-231cells | Mice bearing MDA-MB-231 tumors | [30] | |
| Fe3O4@OCMC@IRMOF-3/FA | HeLa cells | None | [31] | |
| Fe3O4@ZIF-8 | MCF-7 cells | None | [44] | |
| UiO-66/UiO-67 | U-87 MG/HSC-3 cells | None | [45] | |
| DOX@UiO-68-FA | HepG2 cells | Mice bearing HepG2 tumors | [46] | |
| UCNP@ZIF-8/FA | HeLa cells | None | [47] | |
| P@ZIF‐8 | MDA‐MB‐231 cells | None | [48] | |
| Cisplatin@NMOF-1/DOX@NMOF-1 | HeLa cells | None | [49] | |
| NH2-MIL-53(Al) | HepG2 cells | None | [50] | |
| PDT | FA-PCN-224 | HeLa/A549 cells | None | [18] |
| DBP-UiO | SQ20B cells | Mice bearing SQ20B tumors | [51] | |
| DBC-UiO | CT26/HT29 cells | Mice bearing CT26/HT29 tumors | [52] | |
| PS@MOF-FA | HeLa cells | None | [53] | |
| NP-1 | HepG2/HCT116 cells | Mice bearing HCT116 tumors | [54] | |
| (nUiO-67)-Ru(bpy)32+ NPs | A549 cells | None | [55] | |
| UiO-66-TPP-SH | HeLa cells | None | [56] | |
| ZnDTPP-I2⊂UiO-66 | HepG2 cells | None | [57] | |
| PCN-224-Pt | HeLa/4T1 cells | Mice bearing H22 tumors | [58] | |
| MB@THA-NMOF-76@cRGD | A549 cells | None | [59] | |
| Ti-TBP | CT26 cells | Mice bearing CT26 tumors | [60] | |
| Au@ZIF-8 | EMT-6 cells | Mice bearing EMT-6 tumors | [61] | |
| MOF QDs | HeLa cells | Mice bearing HeLa tumors | [62] | |
| PTT | Mn-IR825@PDA-PEG | 4T1 cells | Mice bearing 4T1 tumors | [21] |
| MOF@HA@ICG NPs | MCF-7 cells | Mice bearing MCF-7 tumors | [24] | |
| UiO‐66@PAN | CT26/HCT116 cells | Mice bearing CT26 tumors | [63] | |
| THA@Eu-NMOF@Fe/TA | None | None | [64] |
Abbreviations: RT-RDT, radiotherapy-radiodynamic therapy; PDT, photodynamic therapy; PTT, photothermal therapy; DOX, doxorubicin; FA, folate acid; OCMC, O-carboxymethyl chitosan; 5-FAM, 5-carboxyfluorescein; 5-FU, fluorouracil; Py-PGA-PEG, pyrene-derived polyethylene glycol; Glu, glucose; PS, photosensitizer; NPs, nanoparticles; PCN, porous coordination network; TPP, tris(4-chlorophenyl)porphyrin; MB, methylene blue; HTHA, 4,4,4-trifluoro-1-(9-hexylcarbazol-3-yl)-1,3-butanedione; cRGD, cyclic Arg-Gly-Asp peptide; TBP, 5,10,15,20-tetra(p-benzoato)porphyrin; PDA, polydopamine; PNA, polyaniline; HA, hyaluronic acid; ICG, indocyanine green.
Implication of NMOFs in RT
RT is a potent strategy to eliminate tumors. About half of cancer patients need at least one course of RT at different stages of treatment.65 However, traditional radio-therapeutic approaches lacked specificities to cancer cells and may trigger severe side effects. Therefore, it is of significant importance to explore effective methods to enhance the efficacy of RT in tumor tissues and reduce its adverse effects on normal tissues. NMOFs containing high-Z elements showed promising potential to enhance RT-induced tumor fading due to their strong X-ray attenuation capabilities.66 Ni et al42 reported that Hf-DBB-Ru served as a mitochondria-targeted NMOF for radiotherapy-radiodynamic therapy (RT-RDT). Hf-DBB-Ru exhibited strong mitochondria-targeting properties due to the presence of Ru(bpy)32+. Upon irradiation with low doses of highly penetrating X-rays, Hf-DBB-Ru depolarized the mitochondrial membrane to initiate cancer cell apoptosis. In vivo study demonstrated that NMOF-enabled mitochondria-targeted RT-RDT led to obvious regression of MC38/CT26 xenograft tumors. More recently, Lan et al43 synthesized a multifarious radioenhancer by integrating three high-Z components (Hf12, DBB-Ir and W18) into one NMOF. Upon X-ray irradiation, W18@Hf12-DBB-Ir showed superb anticancer efficacy on two murine colorectal adenocarcinoma models.
Implication of NMOFs in Chemotherapy
Low bioavailability, poor tumor specificity and intolerable systemic toxicity are main obstacles for the clinical practice of chemotherapy.67 To overcome these disadvantages, NMOFs were used as drug delivery platforms to specifically target tumor tissues and controllably release anti-cancer drugs.17,68,69 Several chemotherapeutic drugs successfully delivered by NMOFs in the last 2 decades, such as cisplatin,68 5-fluorouracil (5-FU),47,69 Doxorubicin (DOX),31,48 paclitaxel.14,22
In 2016, Chowdhuri et al31 synthesized a new series of hybrid magnetic NMOFs that combined cancer-targeted drug delivery/imaging, high DOX loading capacity, and pH-sensitive DOX release into one single system. FA encapsulation on the surface of IRMOF-3 specifically targeted folate-overexpressed cancer cells. Chitosan was used for controlled and pH-responsive drug release. Carbon dots were used for optical bioimaging. The as-synthesized Fe3O4@OCMC@IRMOF-3/FA showed high DOX loading efficiency. In vitro cytotoxicity study showed that Fe3O4@OCMC@IRMOF-3/FA without DOX were nontoxic towards both HeLa cancer cells and L929 normal cells. While DOX encapsulated Fe3O4@OCMC@IRMOF-3/FA caused toxicity toward both normal and cancer cells, toxicity was more prominent in cancer cells than in normal cells. In 2018, Chen et al17 developed VEGF-induced DOX-loaded NMOFs to achieve specific killness of cancer cells. Overexpressed VEGF in cancer cells functioned as a gate to achieve controlled release of DOX. Apoptosis experiments revealed selective cytotoxicity of the DOX-loaded NMOFs towards the MDA-MB-231 breast cancer cells.
Samui et al50 developed NMOFs as targeted drug carriers and biomedical imaging by a single step synthetic procedure. Lactobionic acid (LA) is the ligand of asialoglycoprotein receptors overexpressing in hepatocellular cells.70 The LA-conjugated NMOFs performed as an effective targeting ligand towards HepG2 cells for drug delivery. The MTT assay revealed that the DOX-loaded LA-modified NMOFs showed greater cytotoxicity towards HepG2 cells compared to normal HEK cells. Zhang et al29 developed a novel strategy for the design of smart NMOF-based nanoplatforms for MRI-guided tumor-targeted precise chemotherapy. The glucose not only acted as a targeting ligand to glucose-transported proteins in cancer cells, but also endowed the NMOFs with a pH-responded release of DOX. The presence of Gd3+ ions ensured the MRI capability. Therefore, targeted MRI-guided pH-responded chemotherapy was achieved by NMOFs-Glu nanocomposites.
In conclusion, smart theranostic platforms that combine tumor targeting, stimuli-responded release of anti-cancer drugs can be achieved by NMOFs, thus to realize imaging-guided precise chemotherapy.
Implication of NMOFs in Phototherapy
Phototherapy is an effective anticancer intervention that relies on light activation of photoactive materials localized in tumors, and is commonly implemented in the forms of photodynamic therapy (PDT) and photothermal therapy (PTT).71 Photosensitizers (PSs) are required to enhance the efficacy and selectivity of phototherapy via more singlet oxygen (1O2) generation in PDT and photothermal responses in PTT.72 In both cases, delivery of PSs to the tumor tissues is of significant importance.
Implication of NMOFs in PDT
PDT combines 3 components, namely PS, light source and oxygen, and has emerged as an attractive new method of tumor treatment. Upon irradiation, the excited PS transfers energy to the surrounding oxygen to generate reactive oxygen species (ROS), particularly 1O2, resulting in cell death and tumor-specific immunity.71,73 PDT provides an effective local cancer treatment by eradicating malignant tumors without damages to the surrounding normal tissues. However, owing to the poor water solubility and light penetration depth of traditional PSs, the clinical application of PDT is potentially limited. Recently, photosensitive agent-based NMOFs were designed to enhance PDT.51,52,61,74 Proper size and the micropore of NMOFs allowed high PS loadings without self-quenching, whereas the porous structures of NMOFs facilitated the diffusion of ROS, thus leading to improved efficacy in PDT.74
Lin’s group reported the first successful synthesis of a porphyrin-based NMOF, DBP-UiO, as an efficient PS in PDT for resistant head and neck cancer.51 They further optimized the design of NMOF via reducing the porphyrin ligands to its chlorin counterpart and synthesized the first chlorin‐based NMOF, DBC-UiO. The authors demonstrated the superior PDT efficacy of DBC‐UiO over DBP-UiO both in vitro and in vivo in 2 colon cancer models.52
In addition, Park et al18 synthesized the size-controllable NMOF, PCN-224 to enhance PDT efficacy. This nanoparticle demonstrated active targeting property due to postsynthetic FA modification. Jia et al59 successfully synthesized the MB@THA-NMOF-76@cRGD, which achieved NIR-triggered targeting PDT. Modifying cRGD on the surface of MB@THA-NMOF-76 had better biocompatibility and targeted properties into cancer cells. Recently, Ma et al61 described the synthesis and characteristics of an oxygen self-supplied nanodelivery system with embedded AuNPs on the NMOF surface as a catalase-like nanozyme and encapsulating Chlorin e6(Ce6) inside as a PS. This powerful nanoplatform not only made full use of NMOFs to fully utilize the advantages of PSs, but also produced O2 in a more effective way to relieve tumor hypoxia.
Implication of NMOFs in PTT
PTT causes thermal ablation and death of tumor cells with photoabsorbing agents to generate heat from light activation, preferably near-infrared (NIR) light.75 It was recognized as a safe method to reduce the normal tissue damage and system adverse effects. The widely used PTT agents, such as inorganic gold nanomaterials and nanorods, are nonbiodegradable and have the potential for long-term toxicity. NMOFs-based PTT agents are biodegradable, exhibiting high tumor-homing ability and strong NIR absorption coefficients.
Wang et al63 synthesized polymer MOF hybrids, UiO-66@PAN, which not only indicated significant photothermal therapeutic effect in vitro, but also effectively inhibited the growth of colon cancers in vivo. Cai et al24 synthesized a multifunctional nanoplatform for imaging-guided, anti-cancer PTT. Hyaluronic acid (HA) was conjugated to the MOF nanoparticles to target tumor cells. Indocyanine green (ICG), was incorporated function as NIR organic dye. And the Fe(III) ions in the MOF nanoparticles were utilized in T2-weighted MRI. In vitro study showed that the MOF@HA@ICG nanoparticles exhibited strong NIR absorbance and low cytotoxicity. In vivo study indicated that the MOF@HA@ICG nanoparticles could accumulate at tumor sites due to their targeting capability and effectively inhibited the growth of MCF-7 xenograft tumors.
Yan et al64 designed and assembled a smart all-in-one nanoparticle THA@Eu-NMOF@Fe/TA by postsynthetical functionalization of an Eu(III)-based NMOF, which achieved precise temperature sensing and optical heating simultaneously. Fe/TA had PTT effects induced by 808 nm laser irradiation. The long emission lifetime and ultrasensitive transition of the Eu3+ endowed THA@Eu-NMOFs with the ability of real-time temperature sensing.
All these studies suggested PTT agents based on NMOFs significantly enhanced the lethal effects of PTT and reduce systemic side effects.
Implication of NMOFs in Other Cancer Therapies
Nucleic Acid-Based Cancer Detection and Therapeutics
NMOFs were used to deliver nucleic acids such as DNA,76 small interfering RNA (siRNA)68,77 and microRNA.78,79
Morris et al76 demonstrated that UiO-66-N3 NMOFs had the ability to deliver DNA to HeLa cells to achieve intracellular gene regulation. In addition, multiple research demonstrated that siRNA could be loaded by NMOFs to overcome multidrug resistance (MDR).68,77 Wu et al79 developed NMOF-based strategies for multiplexed microRNA (miRNA) detection in living cancer cells for early diagnosis of cancer. Previous researches demonstrated that miRNA functioned as specific biomarkers on different kinds of cancer cells.80 For example, miRNA‐21 is overexpressed in MCF-7 breast cancer cells, and miRNA-221 is overexpressed in OVCAR-3 ovarian cancer cells.81,82 Chen et al78 designed miRNA‐21- and miRNA‐221‐responded DOX-loaded NMOFs for the treatment of breast cancer and ovarian cancer.
Nitric Oxide (NO) Delivery
Recently, free-radical gas NO has drawn widespread attention in cancer treatment.83,84 NMOFs have been reported for use in NO delivery due to suitable characteristics.85 Zhang et al25 developed a Mn-porphyrin NMOF platform as a novel theranostic system for MRI-guided controllable NO release and PTT synergetic therapy under single NIR irradiation. This platform showed effective tumor accumulation in vivo.
Implication of NMOFs in Combination Cancer Therapy
Generally, individual cancer therapy approach cannot eliminate tumor effectively. The combination of two or more therapeutic treatments has been considered as a promising cancer treatment strategy due to fewer side effects and synergistic anti-cancer efficacy.86 The realization of combination therapy based on NMOFs relied heavily on their multimodal loading capability. Recently, various types of therapeutic combinations, such as dual chemotherapy drugs,69 chemotherapy/siRNAs,68,77 chemotherapy/PDT,27,32 PDT/immunotherapy87–90 or RT/immunotherapy91,92 based on NMOFs have been examined for tumor therapies both in vitro and in vivo (Table 3).
Table 3.
The Examples of NMOFs in Combination Cancer Therapy
| Therapy Strategies | NMOFs | Cancer Cell Types | Animal Models | References | |
|---|---|---|---|---|---|
| Dual Chemo-drugs | 5-Fu/DOX | 5-FU@ZIF-90-DOX | None | None | [69] |
| Chemo/siRNAs | Cisplatin/siRNAs | siRNA/UiO-Cis | SKOV-3 cells | None | [68] |
| Paclitaxel/siRNAs | Se/Ru@MIL-101-(P+V)siRNA | MCF-7/T cells | Mice bearing MCF-7/T tumors | [77] | |
| Chemo/PDT | DOX/PDT | DOX@NPMOFs | HepG2 cells | Mice bearing HepG2 tumors | [32] |
| DOX/PDT | DOX/UCMOFs | 4T1 cells | Mice bearing 4T1 tumors | [93] | |
| DOX/PDT | NH2-MIL-125/PEG | MCF-7 cells | None | [94] | |
| Tirapazamine/PDT | TPZ/Hf/TCPP/PEG | 4T1 cells | Mice bearing 4T1 tumors | [27] | |
| Banoxantrone/PDT | A@UiO‐66‐H‐P | U87MG cells | Mice bearing U87MG tumors | [95] | |
| Chemo/PTT | DOX/PTT | Au@Cu3(BTC)2NPs | A549 cells | Mice bearing A549 tumors | [96] |
| Chemo/PTT/PDT | CPT/PTT/PDT | AuNR@MOFs@CPT | 4T1 cells | Mice bearing 4T1 tumors | [97] |
| PTT/NO | NMOF–SNO | MCF-7 cells | Mice bearing MCF-7/T tumors | [25] | |
| PDT/RT | Hf-TCPP NMOF-PEG | 4T1 cells | Mice bearing 4T1 tumors | [66] | |
| PDT/Immunotherapy | PDT/IDOi | IDOi@TBC-Hf | CT26/MC38 cells | Mice bearing CT26/MC38 tumors | [88] |
| PDT/αPD-1 | TBP-nMOF | 4T1 cells | Mice bearing 4T1 tumors | [89] | |
| PDT/αPD-L1 | Fe-TBP | CT26 cells | Mice bearing CT26 tumors | [87] | |
| RT/Immunotherapy | RT/αPD-L1 | Hf6-DBA and Hf12-DBA | CT26 cells | Mice bearing CT26 tumors | [91] |
Abbreviations: RT-RDT, radiotherapy-radiodynamic therapy; PDT, photodynamic therapy; PTT, photothermal therapy; IDOi, Indoleamine 2.3-dioxygenase inhibitor; αPD-1, anti-programmed death-1; α-PD-L1, anti-programmed death-ligand 1; 5-FU, 5-fluorouracil; DOX, doxorubicin; NP,nanoparticles; PEG, polyethylene glycol; TPZ, tirapazamine; TCPP, tetra(4-carboxyphenyl)porphine; BTC, 1,3,5-benzenetricarboxylate; CPT, camptothecin; SNO, S-Nitrosothiol; TCPP, tetra(4-carboxyphenyl)porphine; TBC, tetra(p-benzoato)chlorin; TBP, 5,10,15,20-tetra(p-benzoato)porphyrin; DBA, 2.5-di(p-benzoato)aniline.
Dual Chemotherapy Drugs
Single chemotherapy drug always needs a high dose, which brings severe side effects. Zhang et al69 firstly established a co-delivery platform of 2 different chemical drugs based on nanoscale ZIF-90, which not only reduced the drug toxicity, but also achieved effective therapeutic synergy. Owing to high surface area and excellent biocompatibility, ZIF-90 was chosen as the framework of the NMOFs. This co-delivery system was generated by attaching DOX on the surface of nanoscale ZIF-90, while encapsulating 5-FU into the pores of the framework at the same time. Moreover, ZIF-90 exhibited more stability at high pH, while at lower pH, the framework of ZIF-90 was instable. Due to the acidic environment at tumor sites, 5-FU@ZIF-90-DOX could achieve cancer-targeted co-delivery and controlled release of DOX and 5-Fu.
Chemotherapy/siRNAs
RNA interference (RNAi) is a sequence-specific and post-transcriptional gene silencing technique, which can be used to regulate protein expression and is currently used as a tool to modulate disease-related genes, such as MDR genes.98 Several studies have shown that silencing MDR genes using RNAi reversed cisplatin resistance in ovarian cancer cells.99–101 Several NMOFs have been developed to deliver chemotherapeutic drugs and siRNAs targeting MDR to chemo-resistant cancer cells to overcome MDR.68,77
He et al68 reported the first use of UiO NMOFs for the co-delivery of cisplatin and pooled siRNAs to reverse MDR in ovarian cancer cells. Cisplatin prodrug was encapsulated into the pores of UiO, whereas pooled siRNAs binded to metal ions on the NMOFs surfaces. UiO protected siRNAs against nuclease degradation, and facilitated the siRNAs internalization to enhance the silencing efficiency of MDR genes. Chen et al77 used a similar strategy to utilize Se/Ru@MIL‐101‐(P+V)siRNA nanoparticles for pooled siRNAs delivery to overcome MDR in Taxol-resistant breast cancer cells. MIL-101 had a large surface area. Selenium (Se)-based complexes exhibited excellent anti-tumor effects and low systemic toxicity,102 and ruthenium (Ru) anticancer drugs showed a remarkable anti-metastatic activity.103 In vivo study indicated that Se@MIL-101-(P+V)siRNA significantly suppressed cancer growth in MCF-7/T cells xenografts model.
Chemotherapy/PDT or Chemotherapy/PTT
Several studies demonstrated that combining chemotherapy based on PDT potentially enhanced anticancer efficacy.27,95 The main obstacle of PDT is the hypoxia of tumor environment, which significantly limits its application. Therefore, relieving tissue hypoxia by NMOFs can be used to enhance the efficacy of PDT. Liu et al27 synthesized TPZ/Hf/TCPP/PEG with high loading capacity of the hypoxia-activated prodrug tirapazamine. This study demonstrated the potential of NMOF-based platforms to combine PDT and chemotherapy. More recently, He et al95 synthesized multifunctional A@UiO‐66‐H‐P NMOFs, which also achieved a combination of NMOFs-based PDT with hypoxia‐activated chemotherapy. In addition, due to the large surface area of A@UiO‐66‐H‐P NMOFs and concentration gradient of phosphate between plasma and cells, efficient prodrug loading and controlled prodrug release can be realized.
The combination of chemotherapy with PTT also showed a synergistic anti-cancer effect. He et al96 manufactured multifunctional core–shell Au@Cu3(BTC)2 nanoparticles, which showed both high DOX loading efficiency and excellent photothermal effect.
With the unique features of good biocompatibility and high sensitivity, Au nanoparticles were recognized as ideal materials of this NMOF. Due to good stability, low toxicity and strong NIR absorbance, Cu3(BTC)2 was selected to enhance the photothermal effect. In vitro cytotoxicity studies revealed that even at a high concentration, Au@Cu3(BTC)2 nanoparticles exhibited very low cytotoxicity on A549, beas-2b, MCF-7 and HeLa cells. In vivo study indicated that there were no obvious body weight losses of A549 xenograft model, suggesting its safety. This work inspired further researches on the invention of more multifunctional theranostic platforms based on NMOFs.
PDT/RT
Severe toxicity greatly limits the potential of RT. Combining RT with other therapeutic approaches has drawn great attention in the past few years, which significantly enhanced anti-cancer efficacy and decreased systemic toxicity.104,105 In 2016, Liu et al66 reported the successful synthesis of novel NMOFs that were used for the combination of PDT and RT in a murine breast cancer model. In this NMOFs platform, TCPP served as a PS of PDT, and Hf4+ with strong X-ray attenuation ability functioned as a radiosensitizer to enhance RT-induced tumor killing. In addition, these NMOFs showed tumor targeting due to the EPR effect. In vivo study indicated that the mice bearing 4T1 tumors showed greatly tumor shrinking in the combined therapy group (i.v. injection with NMOF-PEG + X-ray + Laser) compared with respective mono-therapies.
PDT/Immunotherapy
PDT can efficiently eliminate local tumors but is unable to control distant metastases. Immunotherapy, especially the application of immune checkpoint inhibitors, has achieved promising anti-cancer efficacy, but only worked for a few patients. It is also ineffective against poorly immunogenic tumors. Lu et al52 demonstrated that NMOF-mediated PDT induced immunogenic cell death, which endowed them with potential applications to enhance cancer immunotherapy. In recent years, there have been several studies about combining PDT and immunotherapy that elicited superb anticancer efficacy both in local and distant tumor in animal models.87–90
Indoleamine 2,3-dioxygenase (IDO) is an immune checkpoint. In 2016, Lu et al88 synthesized a chlorin-based nMOF, TBC-Hf, to load an IDO inhibitor (IDOi), which achieved an synergistic anti-cancer effect by combining NMOF-enabled PDT and immunotherapy. The combination therapy not only eliminated local tumors, but also achieved abscopal effects in breast and colorectal cancer mouse models. The mechanism study revealed an increased T cell infiltration in the tumor microenvironment after PDT treatment with IDOi@TBC-Hf. This study indicated the potential of NMOFs to enhance cancer immunotherapy. Similar strategies were also used to reduce primary and metastatic lung tumors.90
To overcome the hypoxia of tumor microenvironment, Lan et al87 synthesized NMOF to improve the PDT-induced immune response even under hypoxic conditions. Fe-TBP-based PDT significantly improved the efficacy of anti-programmed death-ligand 1 (α-PD-L1) treatment and elicited abscopal effects in a mouse model of colorectal cancer by recruiting both CD4+ and CD8+ cytotoxic T cells. Meanwhile, Zeng et al89 synthesized TBP-NMOF, which can also function as a superb PS of PDT under hypoxic conditions. When combined with anti-programmed death-1 (αPD-1) antibody, abscopal effects were elicited.
RT/Immunotherapy
RT has the function of local immunomodulatory, which changes the microenvironment of irradiated tumors and synergizes with immune checkpoint blockade. Ni et al91 reported the design of Hf-based NMOFs, Hf6-DBA and Hf12-DBA, as highly effective radioenhancers, which combined NMOF-mediated RT and an α-PD-L1 antibody, achieving local and distant rejection of colorectal tumors in mouse models. In addition, Lu et al92 described that combined NMOF-enabled RD-RDT therapy with IDOi both local and systemic tumors in mouse models of breast and colorectal cancer.
Challenges of the Clinical Translation of NMOFs
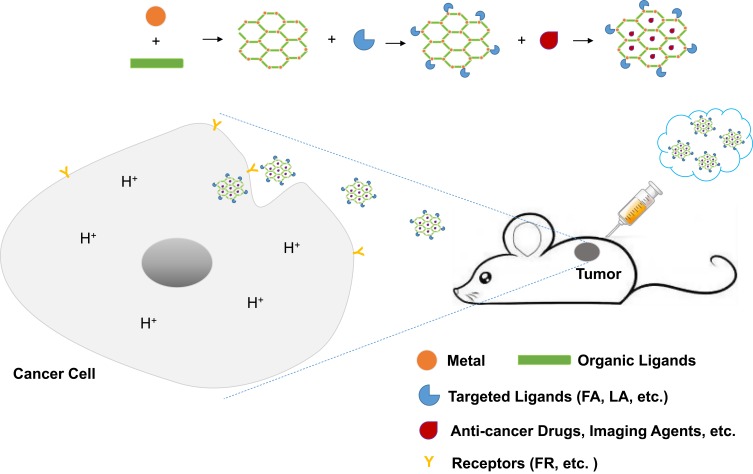
By combining both biomedicine imaging agents and anti-cancer drugs in a single NMOF platform, we can achieve both diagnosis and therapy of cancers at the same time. Due to passive targeting and active targeting of NMOFs, imaging agents and therapeutic drugs can accumulate at the tumor sites. Controlled and sustained drug delivery can be realized due to the existence of stimuli-responsive NMOFs. In addition, many surface modification approaches, such as PEG modification, have been employed, which improved the stability and long-circulating property of NMOFs efficiently. Moreover, combined therapeutic strategies based on NMOFs substantially enhanced the anti-tumor effect (Table 3), and researchers highly valued the importance of in vivo study. Figure 2 presents the schematic of the synthesis and the implication of NMOFs in cancer theranostics briefly.
Figure 2.
Schematic presentation of the synthesis and the implication of NMOFs in cancer theranostics.
Abbreviations: FA, folate acid; LA, lactobionic acid.
Although significant progress has been made in the development of NMOFs for biomedical imaging and anti-cancer drug delivery, there were still some challenges which limit their translation to clinical settings. Firstly, the long‐term toxicity and biosafety of NMOFs still need to be further evaluated. Although most NMOFs systems have no significant acute or subacute cytotoxicity in mice, but the long‐term safety is to be examined. Secondly, there are significant differences between animal models and humans. Although many NMOFs platforms displayed efficient anti-cancer effects in animal models, its functions in the human body are still to be investigated. In addition, although targeted ligands can be incorporated into the NMOFs to realize tumor-targeted delivery of imaging agents and anti-cancer drugs, it is still quite difficult to distinguish cancerous and normal cells. Moreover, the construction of NMOFs systems often involves complex chemical synthesis and post-modifications Further studies are required to fully validate the implication of NMOFs at the clinical translation stage.
Given recent technical advancements over the last decades, smart and targeted NMOFs as cancer theranostic platforms will be further improved to enhance anti-cancer effects and better life quality for cancer patients.
Acknowledgments
This study was supported by National Natural Science Foundation of China (grant numbers 81372498, 81572967, 81773236, and 81800429); National Project for Improving the Ability of Diagnosis and Treatment of Difficult Diseases, National Key Clinical Speciality Construction Program of China (grant number [2013]544); the Fundamental Research Funds for the Central Universities (grant numbers 2042018kf0065 and 2042018kf1037); Health Commission of Hubei Province Scientific Research Project (grant numbers WJ2019H002 and WJ2019Q047); Wuhan City Huanghe Talents Plan, and Zhongnan Hospital of Wuhan University Science, Technology and Innovation Seed Fund (grant numbers znpy2016050, znpy2017001, znpy2017049, and znpy2018028).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2.Zhao X, Yang CX, Chen LG, Yan XP. Dual-stimuli responsive and reversibly activatable theranostic nanoprobe for precision tumor-targeting and fluorescence-guided photothermal therapy. Nat Commun. 2017;8:14998. doi: 10.1038/ncomms14998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai J, Jia X, Zhen W, Cheng W, Jiang X. A facile ion-doping strategy to regulate tumor microenvironments for enhanced multimodal tumor theranostics. J Am Chem Soc. 2018;140(1):106–109. doi: 10.1021/jacs.7b11114 [DOI] [PubMed] [Google Scholar]
- 4.Ng KK, Lovell JF, Zheng G. Lipoprotein-inspired nanoparticles for cancer theranostics. Acc Chem Res. 2011;44(10):1105–1113. doi: 10.1021/ar200017e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui Y, Li B, He H, Zhou W, Chen B, Qian G. Metal-organic frameworks as platforms for functional materials. Acc Chem Res. 2016;49(3):483–493. doi: 10.1021/acs.accounts.5b00530 [DOI] [PubMed] [Google Scholar]
- 6.Della Rocca J, Liu D, Lin W. Nanoscale metal-organic frameworks for biomedical imaging and drug delivery. Acc Chem Res. 2011;44(10):957–968. doi: 10.1021/ar200028a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He C, Liu D, Lin W. Nanomedicine applications of hybrid nanomaterials built from metal-ligand coordination bonds: nanoscale metal-organic frameworks and nanoscale coordination polymers. Chem Rev. 2015;115(19):11079–11108. doi: 10.1021/acs.chemrev.5b00125 [DOI] [PubMed] [Google Scholar]
- 8.Li S, Huo F. Metal-organic framework composites: from fundamentals to applications. Nanoscale. 2015;7(17):7482–7501. doi: 10.1039/C5NR00518C [DOI] [PubMed] [Google Scholar]
- 9.Wang Z, Cohen SM. Postsynthetic modification of metal-organic frameworks. Chem Soc Rev. 2009;38(5):1315–1329. doi: 10.1039/b802258p [DOI] [PubMed] [Google Scholar]
- 10.Islamoglu T, Goswami S, Li Z, Howarth AJ, Farha OK, Hupp JT. Postsynthetic tuning of metal-organic frameworks for targeted applications. Acc Chem Res. 2017;50(4):805–813. doi: 10.1021/acs.accounts.6b00577 [DOI] [PubMed] [Google Scholar]
- 11.Sun T, Zhang YS, Pang B, Hyun DC, Yang M, Xia Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew Chem Int Ed Engl. 2014;53(46):12320–12364. doi: 10.1002/anie.201403036 [DOI] [PubMed] [Google Scholar]
- 12.Lu K, Aung T, Guo N, Weichselbaum R, Lin W. nanoscale metal-organic frameworks for therapeutic, imaging, and sensing applications. Adv Mater. 2018;30(37):e1707634. doi: 10.1002/adma.v30.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen F, Hong H, Zhang Y, et al. In vivo tumor targeting and image-guided drug delivery with antibody-conjugated, radiolabeled mesoporous silica nanoparticles. ACS Nano. 2013;7(10):9027–9039. doi: 10.1021/nn403617j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen W, Yu X, Cecconello A, Sohn YS, Nechushtai R, Willner I. Stimuli-responsive nucleic acid-functionalized metal-organic framework nanoparticles using pH- and metal-ion-dependent DNAzymes as locks. Chem Sci. 2017;8(8):5769–5780. doi: 10.1039/C7SC01765K [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahn JS, Freage L, Enkin N, Garcia MA, Willner I. Stimuli-responsive DNA-functionalized metal-organic frameworks (MOFs). Adv Mater. 2017;29(6). doi: 10.1002/adma.201602782 [DOI] [PubMed] [Google Scholar]
- 16.Tan LL, Li H, Qiu YC, et al. Stimuli-responsive metal-organic frameworks gated by pillar[5]arene supramolecular switches. Chem Sci. 2015;6(3):1640–1644. doi: 10.1039/C4SC03749A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen WH, Yang Sung S, Fadeev M, Cecconello A, Nechushtai R, Willner I. Targeted VEGF-triggered release of an anti-cancer drug from aptamer-functionalized metal-organic framework nanoparticles. Nanoscale. 2018;10(10):4650–4657. doi: 10.1039/C8NR00193F [DOI] [PubMed] [Google Scholar]
- 18.Park J, Jiang Q, Feng D, Mao L, Zhou HC. Size-controlled synthesis of porphyrinic metal-organic framework and functionalization for targeted photodynamic therapy. J Am Chem Soc. 2016;138(10):3518–3525. doi: 10.1021/jacs.6b00007 [DOI] [PubMed] [Google Scholar]
- 19.Gao X, Zhai M, Guan W, Liu J, Liu Z, Damirin A. Controllable synthesis of a smart multifunctional nanoscale metal-organic framework for magnetic resonance/optical imaging and targeted drug delivery. ACS Appl Mater Interfaces. 2017;9(4):3455–3462. doi: 10.1021/acsami.6b14795 [DOI] [PubMed] [Google Scholar]
- 20.Wang GD, Chen H, Tang W, Lee D, Xie J. Gd and Eu Co-doped nanoscale metal-organic framework as a T1-T2 dual-modal contrast agent for magnetic resonance imaging. Tomography. 2016;2(3):179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y, Liu J, Liang C, et al. Nanoscale metal-organic particles with rapid clearance for magnetic resonance imaging-guided photothermal therapy. ACS Nano. 2016;10(2):2774–2781. doi: 10.1021/acsnano.5b07882 [DOI] [PubMed] [Google Scholar]
- 22.Ray BD, Sahu SK. Magnetic nanoscale metal organic frameworks for potential targeted anticancer drug delivery, imaging and as an MRI contrast agent. Dalton Trans. 2016;45(7):2963–2973. doi: 10.1039/C5DT03736K [DOI] [PubMed] [Google Scholar]
- 23.Qin L, Sun ZY, Cheng K, et al. Zwitterionic manganese and gadolinium metal-organic frameworks as efficient contrast agents for in vivo magnetic resonance imaging. ACS Appl Mater Interfaces. 2017;9(47):41378–41386. doi: 10.1021/acsami.7b09608 [DOI] [PubMed] [Google Scholar]
- 24.Cai W, Gao H, Chu C, et al. Engineering phototheranostic nanoscale metal-organic frameworks for multimodal imaging-guided cancer therapy. ACS Appl Mater Interfaces. 2017;9(3):2040–2051. doi: 10.1021/acsami.6b11579 [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Tian XT, Shang Y, Li YH, Yin XB. Theranostic Mn-porphyrin metal-organic frameworks for magnetic resonance imaging-guided nitric oxide and photothermal synergistic therapy. ACS Appl Mater Interfaces. 2018;10(34):28390–28398. doi: 10.1021/acsami.8b09680 [DOI] [PubMed] [Google Scholar]
- 26.Lin J, Xin P, An L, et al. Fe3O4-ZIF-8 assemblies as pH and glutathione responsive T2-T1 switching magnetic resonance imaging contrast agent for sensitive tumor imaging in vivo. Chem Commun (Camb). 2019;55(4):478–481. doi: 10.1039/C8CC08943D [DOI] [PubMed] [Google Scholar]
- 27.Liu M, Wang L, Zheng X, Liu S, Xie Z. Hypoxia-triggered nanoscale metal-organic frameworks for enhanced anticancer activity. ACS Appl Mater Interfaces. 2018;10(29):24638–24647. doi: 10.1021/acsami.8b07570 [DOI] [PubMed] [Google Scholar]
- 28.Shang W, Zeng C, Du Y, et al. Core-Shell Gold Nanorod@Metal-organic framework nanoprobes for multimodality diagnosis of glioma. Adv Mater. 2017;29(3):1604381. doi: 10.1002/adma.v29.3 [DOI] [PubMed] [Google Scholar]
- 29.Zhang H, Shang Y, Li YH, Sun SK, Yin XB. Smart metal-organic framework-based nanoplatforms for imaging-guided precise chemotherapy. ACS Appl Mater Interfaces. 2019;11(2):1886–1895. doi: 10.1021/acsami.8b19048 [DOI] [PubMed] [Google Scholar]
- 30.Chen D, Yang D, Dougherty CA, et al. In vivo targeting and positron emission tomography imaging of tumor with intrinsically radioactive metal-organic frameworks nanomaterials. ACS Nano. 2017;11(4):4315–4327. doi: 10.1021/acsnano.7b01530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chowdhuri AR, Singh T, Ghosh SK, Sahu SK. Carbon dots embedded magnetic nanoparticles @Chitosan @metal organic framework as a nanoprobe for pH sensitive targeted anticancer drug delivery. ACS Appl Mater Interfaces. 2016;8(26):16573–16583. doi: 10.1021/acsami.6b03988 [DOI] [PubMed] [Google Scholar]
- 32.Liu W, Wang YM, Li YH, et al. Fluorescent imaging-guided chemotherapy-and-photodynamic dual therapy with nanoscale porphyrin metal-organic framework. Small. 2017;13(17). [DOI] [PubMed] [Google Scholar]
- 33.Ryu U, Yoo J, Kwon W, Choi KM. Tailoring nanocrystalline metal-organic frameworks as fluorescent dye carriers for bioimaging. Inorg Chem. 2017;56(21):12859–12865. doi: 10.1021/acs.inorgchem.7b01684 [DOI] [PubMed] [Google Scholar]
- 34.Zhang R, Qiao C, Jia Q, et al. Highly stable and long-circulating metal-organic frameworks nanoprobes for sensitive tumor detection in vivo. Adv Healthc Mater. 2019;8(19):e1900761. doi: 10.1002/adhm.v8.19 [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Tang J, He L, et al. Core-shell upconversion nanoparticle@metal-organic framework nanoprobes for luminescent/magnetic dual-mode targeted imaging. Adv Mater. 2015;27(27):4075–4080. doi: 10.1002/adma.201501779 [DOI] [PubMed] [Google Scholar]
- 36.Rieter WJ, Taylor KM, An H, Lin W, Lin W. Nanoscale metal-organic frameworks as potential multimodal contrast enhancing agents. J Am Chem Soc. 2006;128(28):9024–9025. doi: 10.1021/ja0627444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ju Y, Zhang H, Yu J, et al. Monodisperse Au-Fe2C Janus nanoparticles: an attractive multifunctional material for triple-modal imaging-guided tumor photothermal therapy. ACS Nano. 2017;11(9):9239–9248. doi: 10.1021/acsnano.7b04461 [DOI] [PubMed] [Google Scholar]
- 38.deKrafft KE, Xie Z, Cao G, et al. Iodinated nanoscale coordination polymers as potential contrast agents for computed tomography. Angew Chem Int Ed Engl. 2009;48(52):9901–9904. doi: 10.1002/anie.v48:52 [DOI] [PubMed] [Google Scholar]
- 39.Kattumuri V, Katti K, Bhaskaran S, et al. Gum arabic as a phytochemical construct for the stabilization of gold nanoparticles: in vivo pharmacokinetics and X-ray-contrast-imaging studies. Small. 2007;3(2):333–341. doi: 10.1002/(ISSN)1613-6829 [DOI] [PubMed] [Google Scholar]
- 40.Kim D, Park S, Lee JH, Jeong YY, Jon S. Antibiofouling polymer-coated gold nanoparticles as a contrast agent for in vivo X-ray computed tomography imaging. J Am Chem Soc. 2007;129(24):7661–7665. doi: 10.1021/ja071471p [DOI] [PubMed] [Google Scholar]
- 41.Hong H, Chen F, Zhang Y, Cai W. New radiotracers for imaging of vascular targets in angiogenesis-related diseases. Adv Drug Deliv Rev. 2014;76:2–20. doi: 10.1016/j.addr.2014.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ni K, Lan G, Veroneau SS, Duan X, Song Y, Lin W. Nanoscale metal-organic frameworks for mitochondria-targeted radiotherapy-radiodynamic therapy. Nat Commun. 2018a;9(1):4321. doi: 10.1038/s41467-018-06655-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lan G, Ni K, Veroneau SS, Luo T, You E, Lin W. Nanoscale metal-organic framework hierarchically combines high-Z components for multifarious radio-enhancement. J Am Chem Soc. 2019;141(17):6859–6863. doi: 10.1021/jacs.9b03029 [DOI] [PubMed] [Google Scholar]
- 44.Zhuang J, Kuo CH, Chou LY, Liu DY, Weerapana E, Tsung CK. Optimized metal-organic-framework nanospheres for drug delivery: evaluation of small-molecule encapsulation. ACS Nano. 2014;8(3):2812–2819. doi: 10.1021/nn406590q [DOI] [PubMed] [Google Scholar]
- 45.Filippousi M, Turner S, Leus K, et al. Biocompatible Zr-based nanoscale MOFs coated with modified poly(epsilon-caprolactone) as anticancer drug carriers. Int J Pharm. 2016;509(1–2):208–218. doi: 10.1016/j.ijpharm.2016.05.048 [DOI] [PubMed] [Google Scholar]
- 46.Li Y, Zhao XD, Yin HP, Chen GJ, Yang S, Dong YB. A drug-loaded nanoscale metal-organic framework with a tumor targeting agent for highly effective hepatoma therapy. Chem Commun (Camb). 2016;52(98):14113–14116. doi: 10.1039/C6CC07321B [DOI] [PubMed] [Google Scholar]
- 47.Chowdhuri AR, Laha D, Pal S, Karmakar P, Sahu SK. One-pot synthesis of folic acid encapsulated upconversion nanoscale metal organic frameworks for targeting, imaging and pH responsive drug release. Dalton Trans. 2016;45(45):18120–18132. doi: 10.1039/C6DT03237K [DOI] [PubMed] [Google Scholar]
- 48.Zhou W, Wang L, Li F, et al. Selenium-containing polymer@metal-organic frameworks nanocomposites as an efficient multiresponsive drug delivery system. Adv Funct Mater. 2017;27(6):1605465. doi: 10.1002/adfm.201605465 [DOI] [Google Scholar]
- 49.Samanta D, Roy S, Sasmal R, et al. Solvent adaptive dynamic metal-organic soft hybrid for imaging and biological delivery. Angew Chem Int Ed Engl. 2019;58(15):5008–5012. doi: 10.1002/anie.201900692 [DOI] [PubMed] [Google Scholar]
- 50.Samui A, Pal K, Karmakar P, Sahu SK. In situ synthesized lactobionic acid conjugated NMOFs, a smart material for imaging and targeted drug delivery in hepatocellular carcinoma. Mater Sci Eng C Mater Biol Appl. 2019;98:772–781. doi: 10.1016/j.msec.2019.01.032 [DOI] [PubMed] [Google Scholar]
- 51.Lu K, He C, Lin W. Nanoscale metal-organic framework for highly effective photodynamic therapy of resistant head and neck cancer. J Am Chem Soc. 2014;136(48):16712–16715. doi: 10.1021/ja508679h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu K, He C, Lin W. A chlorin-based nanoscale metal-organic framework for photodynamic therapy of colon cancers. J Am Chem Soc. 2015;137(24):7600–7603. doi: 10.1021/jacs.5b04069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang L, Lei J, Ma F, Ling P, Liu J, Ju H. A porphyrin photosensitized metal-organic framework for cancer cell apoptosis and caspase responsive theranostics. Chem Commun (Camb). 2015;51(54):10831–10834. doi: 10.1039/C5CC03028E [DOI] [PubMed] [Google Scholar]
- 54.Ma Y, Li X, Li A, Yang P, Zhang C, Tang B. H2 S-activable MOF nanoparticle photosensitizer for effective photodynamic therapy against cancer with controllable singlet-oxygen release. Angew Chem Int Ed Engl. 2017;56(44):13752–13756. doi: 10.1002/anie.201708005 [DOI] [PubMed] [Google Scholar]
- 55.Chen R, Zhang J, Chelora J, et al. Ruthenium(II) complex incorporated UiO-67 metal-organic framework nanoparticles for enhanced two-photon fluorescence imaging and photodynamic cancer therapy. ACS Appl Mater Interfaces. 2017;9(7):5699–5708. doi: 10.1021/acsami.6b12469 [DOI] [PubMed] [Google Scholar]
- 56.Kan JL, Jiang Y, Xue A, et al. Surface decorated porphyrinic nanoscale metal-organic framework for photodynamic therapy. Inorg Chem. 2018;57(9):5420–5428. doi: 10.1021/acs.inorgchem.8b00384 [DOI] [PubMed] [Google Scholar]
- 57.Zhou LL, Guan Q, Li YA, Zhou Y, Xin YB, Dong YB. One-pot synthetic approach toward porphyrinatozinc and heavy-atom involved Zr-NMOF and its application in photodynamic therapy. Inorg Chem. 2018;57(6):3169–3176. doi: 10.1021/acs.inorgchem.7b03204 [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y, Wang F, Liu C, et al. Nanozyme decorated metal-organic frameworks for enhanced photodynamic therapy. ACS Nano. 2018;12(1):651–661. doi: 10.1021/acsnano.7b07746 [DOI] [PubMed] [Google Scholar]
- 59.Jia J, Zhang Y, Zheng M, et al. Functionalized Eu(III)-based nanoscale metal-organic framework to achieve near-IR-triggered and -targeted two-photon absorption photodynamic therapy. Inorg Chem. 2018;57(1):300–310. doi: 10.1021/acs.inorgchem.7b02475 [DOI] [PubMed] [Google Scholar]
- 60.Lan G, Ni K, Veroneau SS, et al. Titanium-based nanoscale metal-organic framework for type I photodynamic therapy. J Am Chem Soc. 2019;141(10):4204–4208. doi: 10.1021/jacs.8b13804 [DOI] [PubMed] [Google Scholar]
- 61.Ma YC, Zhu YH, Tang XF, et al. Au nanoparticles with enzyme-mimicking activity-ornamented ZIF-8 for highly efficient photodynamic therapy. Biomater Sci. 2019;7(7):2740–2748. doi: 10.1039/C9BM00333A [DOI] [PubMed] [Google Scholar]
- 62.Wang H, Yu D, Fang J, et al. Renal-clearable porphyrinic metal-organic framework nanodots for enhanced photodynamic therapy. ACS Nano. 2019;13(8):9206–9217. doi: 10.1021/acsnano.9b03531 [DOI] [PubMed] [Google Scholar]
- 63.Wang W, Wang L, Li Y, Liu S, Xie Z, Jing X. Nanoscale polymer metal-organic framework hybrids for effective photothermal therapy of colon cancers. Adv Mater. 2016;28(42):9320–9325. doi: 10.1002/adma.201602997 [DOI] [PubMed] [Google Scholar]
- 64.Yan H, Ni H, Jia J, et al. Smart all-in-one thermometer-heater nanoprobe based on postsynthetical functionalization of a Eu(III)-metal-organic framework. Anal Chem. 2019;91(8):5225–5234. doi: 10.1021/acs.analchem.8b05960 [DOI] [PubMed] [Google Scholar]
- 65.Skliarenko J, Warde P. Practical and clinical applications of radiation therapy. Medicine. 2016;44(1):15–19. doi: 10.1016/j.mpmed.2015.10.016 [DOI] [Google Scholar]
- 66.Liu J, Yang Y, Zhu W, et al. Nanoscale metal-organic frameworks for combined photodynamic & radiation therapy in cancer treatment. Biomaterials. 2016;97:1–9. doi: 10.1016/j.biomaterials.2016.04.034 [DOI] [PubMed] [Google Scholar]
- 67.Illes B, Wuttke S, Engelke H. Liposome-coated iron fumarate metal-organic framework nanoparticles for combination therapy. Nanomaterials. 2017;7(11):351. doi: 10.3390/nano7110351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.He C, Lu K, Liu D, Lin W. Nanoscale metal-organic frameworks for the co-delivery of cisplatin and pooled siRNAs to enhance therapeutic efficacy in drug-resistant ovarian cancer cells. J Am Chem Soc. 2014;136(14):5181–5184. doi: 10.1021/ja4098862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang FM, Dong H, Zhang X, et al. Postsynthetic modification of ZIF-90 for potential targeted codelivery of two anticancer drugs. ACS Appl Mater Interfaces. 2017;9(32):27332–27337. doi: 10.1021/acsami.7b08451 [DOI] [PubMed] [Google Scholar]
- 70.Zeng Y, Zhang D, Wu M, et al. Lipid-AuNPs@PDA nanohybrid for MRI/CT imaging and photothermal therapy of hepatocellular carcinoma. ACS Appl Mater Interfaces. 2014;6(16):14266–14277. doi: 10.1021/am503583s [DOI] [PubMed] [Google Scholar]
- 71.Dong Z, Feng L, Hao Y, et al. Synthesis of hollow biomineralized CaCO3-polydopamine nanoparticles for multimodal imaging-guided cancer photodynamic therapy with reduced skin photosensitivity. J Am Chem Soc. 2018;140(6):2165–2178. doi: 10.1021/jacs.7b11036 [DOI] [PubMed] [Google Scholar]
- 72.Qiu WX, Liu LH, Li SY, Lei Q, Luo GF, Zhang XZ. ACPI conjugated gold nanorods as nanoplatform for dual image guided activatable photodynamic and photothermal combined therapy in vivo. Small. 2017;13(18). doi: 10.1002/smll.201603956 [DOI] [PubMed] [Google Scholar]
- 73.Guo T, Wu Y, Lin Y, et al. Black phosphorus quantum dots with renal clearance property for efficient photodynamic therapy. Small. 2018;14(4). [DOI] [PubMed] [Google Scholar]
- 74.Horcajada P, Gref R, Baati T, et al. Metal-organic frameworks in biomedicine. Chem Rev. 2012;112(2):1232–1268. doi: 10.1021/cr200256v [DOI] [PubMed] [Google Scholar]
- 75.Cheng L, Wang C, Feng L, Yang K, Liu Z. Functional nanomaterials for phototherapies of cancer. Chem Rev. 2014;114(21):10869–10939. doi: 10.1021/cr400532z [DOI] [PubMed] [Google Scholar]
- 76.Morris W, Briley WE, Auyeung E, Cabezas MD, Mirkin CA. Nucleic acid-metal organic framework (MOF) nanoparticle conjugates. J Am Chem Soc. 2014;136(20):7261–7264. doi: 10.1021/ja503215w [DOI] [PubMed] [Google Scholar]
- 77.Chen Q, Xu M, Zheng W, Xu T, Deng H, Liu J. Se/Ru-decorated porous metal-organic framework nanoparticles for the delivery of pooled sirnas to reversing multidrug resistance in taxol-resistant breast cancer cells. ACS Appl Mater Interfaces. 2017;9(8):6712–6724. doi: 10.1021/acsami.6b12792 [DOI] [PubMed] [Google Scholar]
- 78.Chen W, Luo GF, Sohn YS, Nechushtai R, Willner I. miRNA-specific unlocking of drug-loaded metal-organic framework nanoparticles: targeted cytotoxicity toward cancer cells. Small. 2019;15(17):e1900935. [DOI] [PubMed] [Google Scholar]
- 79.Wu Y, Han J, Xue P, Xu R, Kang Y. Nano metal-organic framework (NMOF)-based strategies for multiplexed microRNA detection in solution and living cancer cells. Nanoscale. 2015;7(5):1753–1759. doi: 10.1039/C4NR05447D [DOI] [PubMed] [Google Scholar]
- 80.Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol. 2014;9:287–314. doi: 10.1146/annurev-pathol-012513-104715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heneghan HM, Miller N, Lowery AJ, Sweeney KJ, Kerin MJ. MicroRNAs as novel biomarkers for breast cancer. J Oncol. 2009;2009:950201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoheisel J, Dahiya N, Sherman-Baust CA, et al. MicroRNA expression and identification of putative miRNA targets in ovarian cancer. PLoS One. 2008;3(6):e2436. doi: 10.1371/journal.pone.0002436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fan W, Yung BC, Chen X. Stimuli-responsive NO release for on-demand gas-sensitized synergistic cancer therapy. Angew Chem Int Ed Engl. 2018;57(28):8383–8394. doi: 10.1002/anie.v57.28 [DOI] [PubMed] [Google Scholar]
- 84.Mocellin S, Bronte V, Nitti D. Nitric oxide, a double edged sword in cancer biology: searching for therapeutic opportunities. Med Res Rev. 2007;27(3):317–352. doi: 10.1002/(ISSN)1098-1128 [DOI] [PubMed] [Google Scholar]
- 85.McKinlay AC, Xiao B, Wragg DS, Wheatley PS, Megson IL, Morris RE. Exceptional behavior over the whole adsorption-storage-delivery cycle for NO in porous metal organic frameworks. J Am Chem Soc. 2008;130(31):10440–10444. doi: 10.1021/ja801997r [DOI] [PubMed] [Google Scholar]
- 86.Bayat Mokhtari R, Homayouni TS, Baluch N, et al. Combination therapy in combating cancer. Oncotarget. 2017;8(23):38022–38043. doi: 10.18632/oncotarget.16723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lan G, Ni K, Xu Z, Veroneau SS, Song Y, Lin W. Nanoscale metal-organic framework overcomes hypoxia for photodynamic therapy primed cancer immunotherapy. J Am Chem Soc. 2018;140(17):5670–5673. doi: 10.1021/jacs.8b01072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu K, He C, Guo N, et al. Chlorin-based nanoscale metal-organic framework systemically rejects colorectal cancers via synergistic photodynamic therapy and checkpoint blockade immunotherapy. J Am Chem Soc. 2016;138(38):12502–12510. doi: 10.1021/jacs.6b06663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zeng JY, Zou MZ, Zhang M, et al. pi-extended benzoporphyrin-based metal-organic framework for inhibition of tumor metastasis. ACS Nano. 2018;12(5):4630–4640. doi: 10.1021/acsnano.8b01186 [DOI] [PubMed] [Google Scholar]
- 90.Song W, Kuang J, Li CX, et al. Enhanced immunotherapy based on photodynamic therapy for both primary and lung metastasis tumor eradication. ACS Nano. 2018;12(2):1978–1989. doi: 10.1021/acsnano.7b09112 [DOI] [PubMed] [Google Scholar]
- 91.Ni K, Lan G, Chan C, et al. Nanoscale metal-organic frameworks enhance radiotherapy to potentiate checkpoint blockade immunotherapy. Nat Commun. 2018;9(1):2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lu K, He C, Guo N, et al. Low-dose X-ray radiotherapy-radiodynamic therapy via nanoscale metal-organic frameworks enhances checkpoint blockade immunotherapy. Nat Biomed Eng. 2018;2(8):600–610. doi: 10.1038/s41551-018-0203-4 [DOI] [PubMed] [Google Scholar]
- 93.Li Y, Di Z, Gao J, et al. Heterodimers made of upconversion nanoparticles and metal-organic frameworks. J Am Chem Soc. 2017;139(39):13804–13810. doi: 10.1021/jacs.7b07302 [DOI] [PubMed] [Google Scholar]
- 94.Rengaraj A, Puthiaraj P, Heo NS, et al. Porous NH2-MIL-125 as an efficient nano-platform for drug delivery, imaging, and ROS therapy utilized low-intensity visible light exposure system. Colloids Surf B Biointerfaces. 2017;160:1–10. doi: 10.1016/j.colsurfb.2017.09.011 [DOI] [PubMed] [Google Scholar]
- 95.He Z, Dai Y, Li X, et al. Hybrid nanomedicine fabricated from photosensitizer-terminated metal-organic framework nanoparticles for photodynamic therapy and hypoxia-activated cascade chemotherapy. Small. 2019;15(4):e1804131. doi: 10.1002/smll.201804131 [DOI] [PubMed] [Google Scholar]
- 96.He J, Dong J, Hu Y, Li G, Hu Y. Design of Raman tag-bridged core-shell Au@Cu3(BTC)2 nanoparticles for Raman imaging and synergistic chemo-photothermal therapy. Nanoscale. 2019;11(13):6089–6100. doi: 10.1039/C9NR00041K [DOI] [PubMed] [Google Scholar]
- 97.Zeng JY, Zhang MK, Peng MY, Gong D, Zhang XZ. Porphyrinic metal–organic frameworks coated gold nanorods as a versatile nanoplatform for combined photodynamic: photothermal: chemotherapy of tumor. Adv Funct Mater. 2017;201705451:1–13. [Google Scholar]
- 98.Xu J, Wang XF, Chen P, et al. RNA interference in moths: mechanisms, applications, and progress. Genes (Basel). 2016;7(10):88. doi: 10.3390/genes7100088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xiong XB, Lavasanifar A. Traceable multifunctional micellar nanocarriers for cancer-targeted co-delivery of MDR-1 siRNA and doxorubicin. ACS Nano. 2011;5(6):5202–5213. doi: 10.1021/nn2013707 [DOI] [PubMed] [Google Scholar]
- 100.Shahzad MM, Lopez-Berestein G, Sood AK. Novel strategies for reversing platinum resistance. Drug Resist Updat. 2009;12(6):148–152. doi: 10.1016/j.drup.2009.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yellepeddi VK, Vangara KK, Kumar A, Palakurthi S. Comparative evaluation of small-molecule chemosensitizers in reversal of cisplatin resistance in ovarian cancer cells. Anticancer Res. 2012;32(9):3651–3658. [PubMed] [Google Scholar]
- 102.Wang H, Zhang J, Yu H. Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: comparison with selenomethionine in mice. Free Radic Biol Med. 2007;42(10):1524–1533. doi: 10.1016/j.freeradbiomed.2007.02.013 [DOI] [PubMed] [Google Scholar]
- 103.Levina A, Mitra A, Lay PA. Recent developments in ruthenium anticancer drugs. Metallomics. 2009;1(6):458–470. doi: 10.1039/b904071d [DOI] [PubMed] [Google Scholar]
- 104.Hsu HW, Wall NR, Hsueh CT, et al. Combination antiangiogenic therapy and radiation in head and neck cancers. Oral Oncol. 2014;50(1):19–26. doi: 10.1016/j.oraloncology.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 105.Iwamoto Y, Ishii K, Kanda H, et al. Combination treatment with naftopidil increases the efficacy of radiotherapy in PC-3 human prostate cancer cells. J Cancer Res Clin Oncol. 2017;143(6):933–939. doi: 10.1007/s00432-017-2367-9 [DOI] [PubMed] [Google Scholar]