Abstract
Chemotherapy is an important cancer treatment method. Tumor chemotherapy resistance is one of the main factors leading to tumor progression. Like other malignancies, bladder cancer, especially muscle-invasive bladder cancer, is prone to chemotherapy resistance. Additionally, only approximately 50% of muscle-invasive bladder cancer responds to cisplatin-based chemotherapy. miRNAs are a class of small, endogenous, noncoding RNAs that regulate gene expression at the posttranscriptional level, which results in the inhibition of translation or the degradation of mRNA. In the study of miRNAs and cancer, including gastric cancer, prostate cancer, liver cancer, and colorectal cancer, it has been found that miRNAs can regulate the expression of genes related to tumor resistance, thereby promoting the progression of tumors. In bladder cancer, miRNAs are also closely related to chemotherapy resistance, suggesting that miRNAs can be a new therapeutic target for the chemotherapy resistance of bladder cancer. Therefore, understanding the mechanisms of miRNAs in the chemotherapy resistance of bladder cancer is an important foundation for restoring the chemotherapy sensitivity of bladder cancer and improving the efficacy of chemotherapy and patient survival. In this article, we review the role of miRNAs in the development of chemotherapy-resistant bladder cancer and the various resistance mechanisms that involve apoptosis, the cell cycle, epithelial-mesenchymal transition (EMT), and cancer stem cells (CSCs).
Keywords: miRNAs, chemoresistant, bladder cancer, biomarkers, targeted therapy
Background
Bladder cancer (BCa) is the ninth most common cancer in the world, and the incidence is higher in men than in women.1,2 Of the cases of initially diagnosed BCa, 70% are non-muscle-invasive bladder cancer (NMIBC), and approximately 30% are muscle-invasive bladder cancer (MIBC).3 Although the incidence of MIBC is lower than that of NMIBC, MIBC has a worse prognosis and has become a great challenge for urologists. The standard treatment for patients with MIBC is radical cystectomy. However, despite aggressive treatment, the five-year survival rate for patients with advanced BCa is only 20–40%.4 To improve unsatisfactory treatment efficacy, cisplatin-based combination chemotherapies, such as methotrexate, vinblastine, doxorubicin and cisplatin (MVAC) and gemcitabine and cisplatin (GC),5 have become important adjuvant therapies for MIBC and have been used since the late 1980s; however, the median progression time of the GC regimen is only 6 months, and the regimen has no effect on overall survival after radical cystectomy in high-risk patients.5 Furthermore, only 50% of patients with MIBC respond to cisplatin-based chemotherapy.6 In addition, chemotherapy has failed in a large proportion of patients due to the gradual occurrence of chemoresistance, which leads to the relapse and progression of tumors. Therefore, overcoming multidrug resistance and exploring a novel safe and effective treatment strategy is urgently needed for BCa, especially MIBC.
Recent research has indicated that posttranscriptional regulatory mechanisms play a crucial role in various tumor biological properties, including chemotherapy resistance, and the most important molecules involved include miRNAs and human antigen R.7,8 miRNAs are a class of endogenous small noncoding RNAs that are approximately 18–25 nt in length and were first discovered in 1993.9 Since their discovery, an increasing number of miRNAs have been identified. miRNAs recognize and bind to the 3ʹ-untranslated region (3ʹ-UTR) of target mRNAs with complete or incomplete complementary pairs and cause the degradation of the target mRNA or the inhibition of translation, thereby negatively regulating the expression of cancer-related molecules.10 Studies have revealed that nearly one-third of human genes, including those involved in tumor development, angiogenesis, invasion, metastasis and drug resistance, can be regulated by miRNAs. Therefore, miRNAs act as oncogenes or tumor suppressor genes, depending on their complex regulatory mechanisms.11 In recent years, tumor-related studies have shown that miRNAs are involved in the biological properties of BCa, including chemoresistance. Based on their important roles in BCa, miRNAs have been widely studied as therapeutic targets for BCa treatment. This paper will review the role of miRNAs in the chemoresistance of BCa and explore the related targeted therapeutic strategies.
Expression Patterns of Chemoresistance-Related miRNAs in BCa
Dysregulated expression of miRNAs in tumors involves pathophysiological processes.12,13 With the development of microarray analysis methods, real-time polymerase chain reaction (RT-PCR) and bioinformatic techniques, numerous cancer-related miRNAs have been discovered, most of which are closely associated with chemoresistance in many tumors, including BCa.14–17
The 5637 cell line is the most multichemosensitive cell line of the BCa cell lines (5637, T24, EJ, H-bc and Biu87), and H-bc is the most resistant cell line. An RNA-seq-based miRomic analysis of the 5637 and H-bc cell lines showed that 83 miRNAs were differentially expressed by at least twofold (37 were more highly expressed and 45 were less highly expressed in the 5637 cells than in the H-bc cells).18 Microarray analysis of gemcitabine-resistant and parental cells revealed 66 differentially expressed miRNAs, including 41 miRNAs and 25 unidentified human miRPlus sequences in the miRBase database.19 Among these differentially expressed miRNAs, miR-1290 and miR-138 showed increased expression levels in gemcitabine-resistant cells, while let-7b and let-7i exhibited decreased expression.18,19 Transfection of pre-miR-138 and pre-miR-1290 into parental cells attenuated gemcitabine-induced cell death, while transfection of pre-miR-let-7b and pre-miR-let-7i into the resistant cells augmented cell death.18,19 These results demonstrated the role of these miRNAs in the gemcitabine resistance of BCa and implied the potential role of targeted therapy. Several studies have shown that the expression of multiple chemoresistance-related miRNAs in BCa tissues and cell lines was either upregulated or downregulated. As found by Li et al20 the expression of miR-34a was frequently decreased in MIBC tissues and cell lines (5637, HT1376, J82, T24), while its upregulation promoted the sensitivity of BCa cells to cisplatin. Bu et al21 also found that the expression of miR-101 was downregulated in the BCa-resistant cell line T24/CDDP, while overexpression of miR-101 significantly enhanced cisplatin-induced apoptosis. Therefore, the differential expression of miRNAs in BCa confers the significance of miRNAs as oncogenes or tumor suppressor genes.
miRNAs and BCa Chemoresistance
Although chemotherapy plays an irreplaceable role in the treatment of advanced BCa, there are still numerous patients who are unable to tolerate it, and the emergence of drug resistance greatly limits the long-term curative effect of chemotherapy. Recent research has indicated that miRNA-mediated posttranscriptional regulation plays an important role in chemoresistance and can affect drug efficacy by regulating the expression of multiple drug-resistance-related proteins. We hypothesize that the effect of targeting miRNAs is stronger than the effect of targeting individual drug-associated proteins.
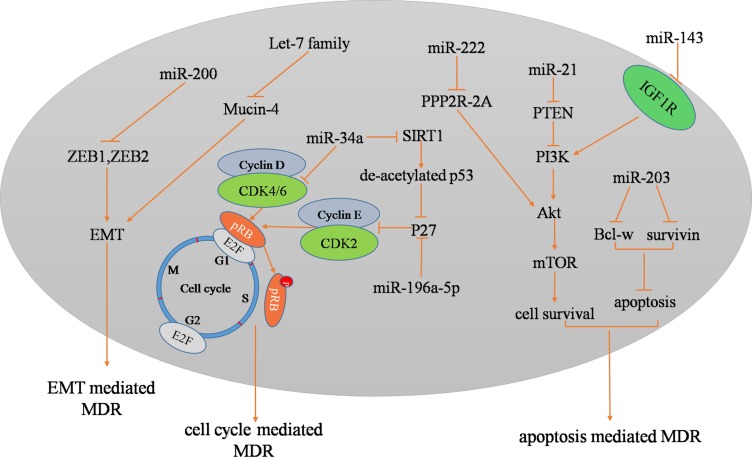
Regarding BCa, the first identified drug-resistance-related miRNAs belonged to the miR-200 family. In 2009, Adam et al22 showed that the stable expression of miR-200 in mesenchymal UMUC3 cells increased E-cadherin levels and sensitivity to EGFR blockers (cetuximab) and decreased the expression of ZEB1, ZEB2, and ERRFI-1 and cell migration. Since then, studies have focused on the role of miRNAs in the chemoresistance of BCa. For instance, miR-21 promoted cell proliferation and increased cell resistance to doxorubicin treatment in the BCa cell line T24.23 miR-203 was associated with cancer progression and poor prognosis of BCa patients who received cisplatin-based adjuvant chemotherapy.24 The restoration of miR-203 expression enhanced the sensitivity of BCa cells to cisplatin by promoting cell apoptosis by targeting Bcl-w and survivin. miR-218 increased the sensitivity of BCa to cisplatin by targeting Glut1 (glucose transporter isoform 1).25 miR-193a-3p has been found to be more highly expressed in resistant cell lines (H-bc and UM-UC-3) than in chemosensitive cell lines (5637), and miR-193a-3p was shown to not only mediate BCa chemoresistance at the cellular level but was promote paclitaxel resistance by inhibiting the expression of SRSF2 and LOXL4 in nude mouse BCa tumor xenografts.26 Similarly, it has been shown that other BCa chemoresistance-related miRNAs include miR-196a-5p, miR-203, miR-22-3p, miR-143, miR-193a-3b, miR-222, miR-27a, miR-145, miR-294, and miR-193b-3p (Table 1 and Figure 1).27–38 Based on these intracellular and preclinical studies, clinical research on miRNA-targeted therapeutic strategies will be the focus of cancer treatment. We believe that gene-targeted therapy will open a new chapter in cancer therapy.
Table 1.
Drug Resistance Related miRNAs in Bladder Cancer
| miRNA | Expression | Cell Lines | Corresponding Drugs | Targets | Effects | First Author and Year, Refs |
|---|---|---|---|---|---|---|
| miR-193a-3p | down | 5637,T24,EJ,H-bc,Biu87 | Pirarubicin, Paclitaxel,Adriamycin, and Epirubicin Hydrochloride | SRSF2,PLAU, HIC2 |
MiR-193a-3p promotes both growth and paclitaxel chemoresistance. | Lv 201418 |
| miR-1290,miR-138 | up | RT4, RT112, CUBIII,TCCSUP, UM-UC-3, J82 |
gemcitabine | mucin-4 | miRNAs 1290, 138, let-7i, and let-7b in imparting resistance to gemcitabine in UCB cell lines in part through the modulation of mucin-4. | Kozinn 201319 |
| let-7b,let-7i | down | |||||
| miR-34a | down | 5637,HT1376,J82,T24 and 5 MIBC tissues | cisplatin | CD44 | Increased miR-34a expression significantly sensitized MIBC cells to cisplatin. | Li 201420 |
| miR-101 | down | T24/CDDP | cisplatin | COX-2 | Enforced expression of miR-101 enhances cisplatin sensitivity in human bladder cancer cells. | Bu 201421 |
| miR-200 | up | epithelial cell lines | EGFR-blocking agents | ZEB1,ZEB2,ERRFI-1 | Expression of miR-200 is sufficient to restore EGFRdependency. | Adam 200922 |
| miR-21 | up | T24 | doxorubicin | PTEN | MiR-21 could modulate chemosensitivity of T24 cells to doxorubicin. | Tao 201123 |
| miR-203 | down | 5637,T24 and 108 patients tissue | cisplatin | Bcl-w,Survivin | MiR-203 overexpression can enhance cisplatin sensitization. | Zhang 201524 |
| miR-218 | up | T24,EJ | cisplatin | Glut1 | MiR-218 increases the sensitivity of bladder cancer to cisplatin by targeting Glut1. | Li 201725 |
| miR-193a-3p | down | 5637,T24, Biu87 | Pirarubicin, Paclitaxel, Adriamycin, Cisplatin, Epirubicin Hydrochloride | SRSF2,LOXL4 | MiR-193a-3p promotes both the growth and chemoresistance of the BCa cell derived tumor xenografts in nude mice. | Deng 201426 |
| miR-196a-5p | up | 5637 | cisplatin/gemcitabine | p27 | The reduced expression of miR-196a-5p enforced sensitivity to the growth inhibition effects of cisplatin/gemcitabine. | Pan 201627 |
| miR-22-3p | up | H-bc | pirarubicin;paclitaxel; Adriamycin; hydrochloride;hydroxycamptothecin;cisplatin | NET1 | MiR-22-3p promotes BCa chemoresistance by targeting NET1. | Xiao 201828 |
| miR-143 | down | T24,5637 and 20 pairs tissues | gemcitabine | IGF-1R | Overexpression of miR-143 inhibited cell proliferation and promoted chemosensitivity of bladder cancer 5637 cells to gemcitabine. | Wang 201729 |
| miR-193a-3p | down | 5637 | pirarubicin,epirubicin | HOXC9 | MiR-193a-3p promotes the multi-chemoresistance of bladder cancer by targeting the HOXC9 gene. | Lv 201530 |
| miR-193a-3p | down | 5637,T24,EJ,H-bc,Biu88 | Pirarubicin, Paclitaxel,Adriamycin, and Epirubicin Hydrochloride | PSEN1 | The miR-193a-3p regulated PSEN1 gene suppresses the multi-chemoresistance of bladder cancer. | Deng 201431 |
| miR-193a-3p | down | 5637,T24,EJ,H-bc,Biu89 | Pirarubicin, Paclitaxel,Adriamycin, and Epirubicin Hydrochloride | ING5 | The miR-193a-3p-regulated ING5 gene activates the DNA damage response pathway and inhibits multi-chemoresistance in bladder cancer. | Li 201432 |
| miR-222 | down | T24,5637 | cisplatin | PPP2R2A/Akt/mTOR axis | MiR-222 induces resistance of bladder cancer cells to cisplatin. | Zeng 201633 |
| miR-27a | down | cisplatin-resistant cell lines (EJ-R, D4-R,and G7-R) | cisplatin | SLC7A11 | Overexpression of miRNA-27a reduces levels of SLC7A11 and intracellular glutathione, and resensitizes resistant cells to cisplatin. | Drayton 201434 |
| miR-27a | down | 89 patients tissue,cell lines | cisplatin,Adriamycin,paclitaxel | RUNX-1 | MiR-27a modulates sensitivity of chemotherapy through directly inhibiting RUNX-1 expression. | Deng 201535 |
| miR-145 | up | T24,5673 | gemcitabine | NA | lncRNA-LET/NF90/miR-145 in UBC cells to increase CSC populations and promote chemoresistance. | Zhuang 201736 |
| miR-294 | up | J82, HT1376, T24, SW780 | cisplatin | NA | MiR 294 suppression could promote the sensitivity of T24 cells to cisplatin. | Li 201637 |
| miR-193b-3p | up | NTUB1 | cisplatin | ETS1,Cyclin D1 | MiR-193b-3p expression upon CDDP treatment | Lin 201638 |
| miR-34a | down | 27 patients tissue | cisplatin | Cdk6,SIRT-1 | Increased miR-34a expression levels correlated with increased chemosensitivity. | Vinall 201161 |
Abbreviations: COX 2, cyclooxygenase-2; PTEN, Phosphatase and tension homolog deleted on chromosome 10; Glut1:Glucose transporter isoform 1; NET1, neuroepithelial cell transforming 1; Cdk6, Cyclin dependent kinase 6; SIRT-1, sirtuin-1; IGF 1R, Insulin-like growth factor 1 receptor; HOXC9, homeobox C9; LOXL4, lysyl oxidase-like 4; PSEN1, presenilin 1; ING5, inhibitor of growth 5;RUNX1, Runt-related transcription factor1; PPP2R2A, protein phosphatase 2A subunit B.
Figure 1.
In BCa, these miRNAs are associated with chemoresistance.
miRNA-Mediated Pathways in the Chemoresistance of BCa
In terms of mechanisms, accumulating studies have revealed that miRNAs regulate the chemoresistance of BCa through a variety of signaling pathways. Current research indicates that the signaling pathways involving miRNAs are related to the following aspects:
Regulation of Cell Apoptosis
Apoptosis is one of the most important mechanisms by which multicellular organisms respond to environmental changes and maintain tissue homeostasis.39–41 Recent studies have shown that miRNAs play a key role in regulating apoptosis and its relationship with chemoresistance in BCa. miRNAs mediate chemoresistance in BCa by regulating the expression of members of the Bcl-2 family. Zhan et al24 showed that overexpression of miR-203 enhanced cisplatin sensitization by promoting apoptosis by directly targeting Bcl-w and survivin. Survivin is a key member of the inhibitor of apoptosis protein (IAP) family and exerts its antiapoptotic function by blocking caspase activity in a complex with the X-linked inhibitor of apoptosis protein (XIAP).42 In addition, studies have demonstrated that miR-133b regulates the proliferation and apoptosis of BCa cells (T24) by targeting Akt and Bcl-w.43 In BCa cells, Bcl-2 is regulated by miR-21, miR-192, miR-221, miR-9, miR-675, miR-29c, and Mcl-1 and is regulated by miR-192 and miR-29c.44–49 These miRNAs mediate the proliferation and apoptosis of BCa cells by regulating the expression of members of the Bcl-2 family. We speculate that the above miRNAs also play an important role in the chemotherapy resistance of BCa.
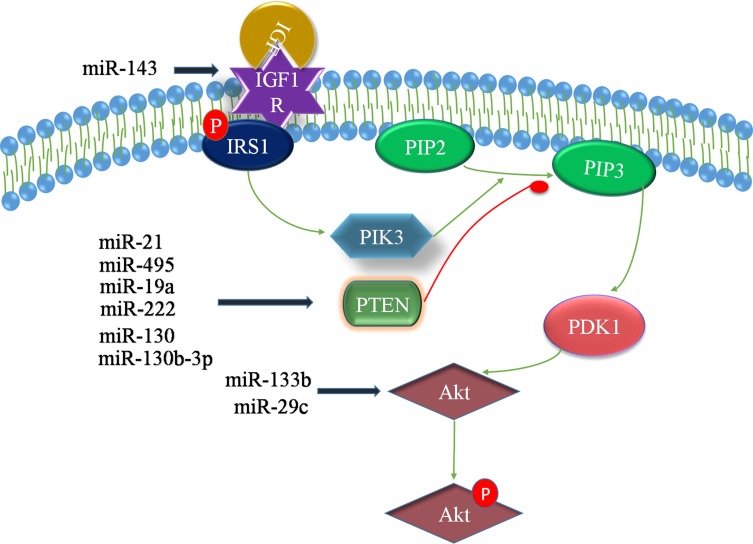
The PTEN/PI3K/Akt/mTOR signaling pathway plays a key role in the progression of BCa. The roles of Akt in cells are diverse, but all of the roles lead to antiapoptosis or cell proliferation. It is well known that the PI3K/Akt oncogenic signaling pathway is activated in response to various growth factors and extracellular matrix (ECM) proteins.50,51 PTEN has been reported to act as a dual-specific phosphatase, which, on the one hand, regulates cell growth, apoptosis, invasion and differentiation by negatively regulating the PI3K/Akt signaling pathway. On the other hand, PTEN downregulates the level of the lipid second messenger phosphoinositide-3,4,5-triphosphate (PIP3) via dephosphorylation and subsequently inhibits Akt phosphorylation.52 Studies have revealed that miRNAs mediate chemoresistance in BCa by regulating the PTEN/PI3K/Akt/mTOR signaling pathway. miR-21 can target PTEN and promote the proliferation and chemotherapy resistance (doxorubicin) of BCa cells (T24) through the PI3K-Akt pathway.53 miR-222 activates the Akt/mTOR pathway and directly inhibits cisplatin-induced autophagy in BCa cells by directly targeting the protein phosphatase 2A subunit B (PPP2R2A).54 In summary, a variety of miRNAs, including miR-21, miR-130b-3p, miR-495, miR-19a, miR-222, and the miR-130 family, have been found to target PTEN.53–58 Interestingly, miR-218 can indirectly regulate the expression of PTEN by targeting BMI-1, which plays a carcinogenic role and is related to tumor progression, inhibition of cell apoptosis and so on.59 The insulin-like growth factor-1 receptor (IGF-1R) and its ligand play an important role in regulating cell proliferation and apoptosis. The combination of IGF-1R and its ligand triggers the downstream PI3K/Akt signaling pathway (Figure 2). Wang et al showed that miR-143 can target IGF-1R and promote the chemosensitivity of BCa cells (5637) to gemcitabine.29
Figure 2.
PI3K/PIP3 signaling activates Akt signaling via Akt/PDK-1 activation, which results in the downregulation of apoptosis. However, the conversion of PIP2 to PIP3 is reversed by PTEN. In addition, insulin-like growth factor-1 receptor (IGF-1R) and its ligand play an essential role in regulating cellular proliferation and apoptosis. The binding of the ligand to IGF-1R triggers various downstream signaling pathways, including the PI3K/Akt pathway, which is essential for cell survival. In BCa, these miRNAs regulate the PTEN/PI3K/Akt/mTOR signaling pathway.
Regulation of the Cell Cycle
With advancements in our understanding of the basic mechanisms of tumor-related processes, cell cycle physiology, and apoptosis mechanisms, it is becoming increasingly apparent that the cell cycle plays a key role in chemosensitivity, especially sensitivity to combined chemotherapy.60 Recent studies have shown that miRNAs participate in the processes related to cell cycle-related chemoresistance in BCa.
Increased miR-34a expression levels have been found to promote the chemosensitivity of BCa cell lines to cisplatin treatment by targeting CDK6 and SIRT-1.61 However, the inhibition of CDK6 and SIRT-1 was not as effective as the inhibition of pre-miR-34a in mediating chemosensitization. We speculate that this difference lies in the fact that miRNAs can simultaneously regulate multiple target genes, indicating that targeting these miRNAs may be more effective than targeting a single chemoresistance-related gene. UCA1, a class of lncRNAs, activates the transcription factor CREB, which leads to the expression of miR-196a-5p by binding with its promoter. miR-196a-5p is involved in the inhibition of apoptosis by UCA1 that is induced by cisplatin/gemcitabine by targeting p27.27 These data suggest that the mechanisms of miRNA-mediated chemosensitivity involve a complex network system, that the novel lncRNA/miRNA/target gene axis plays an important role in tumor therapy and that any component of the axis can serve as a tumor treatment target. In addition, HOX gene family member-HOXC9 as an oncogene and a novel miR-193a-3p target has also been found to exert a promoting effect of miR-193a-3p in BCa chemoresistance. HOXC9 has been reported to bind to and activate the expression of a large number of genes involved in the DNA damage response, such as TP53 and E2F6.62 Therefore, the miR-193a-3p/HOXC9/DNA damage response axis plays a key role in the chemoresistance of BCa. We found that the role of miRNAs depends on complex network axes and that an increasing number of upstream regulatory genes as well as downstream targets are involved. We hypothesize that the therapeutic effect of targeting upstream genes is superior to that of targeting downstream genes; however, complex network regulation axes still require further investigation. Studies have demonstrated that miRNAs mediate cell cycle-regulated chemoresistance by targeting CDK and p27. We also summarized that these miRNAs, including miR-29c, miR-124, miR-449a, miR-320c, miR-106a, miR-20b, and miR-195, can target CDK.63–69 In addition, miR-221 and miR-192 can target p27.70 The roles of most miRNAs in the chemoresistance of BCa have not been studied, although these miRNAs can regulate the biological characteristics of BCa, which is one of the directions to be explored in the future.
Tumor Stem Cells
Stem cells are undifferentiated cells that have the ability to self-renew while producing differentiated tissues or organ-specific cells by asymmetric cell division. Knowledge of the importance of stem cells in normal tissue biology has led to the belief that cancer may also come from a pool of progenitor cells (the cancer stem cell (CSC) hypothesis). CSCs are a subpopulation of cancer cells responsible for tumor initiation, differentiation, recurrence, metastasis and drug resistance.71–73 CSCs can be isolated from a large number of tumor cells based on characteristic cell surface markers, such as CD44 and CD133. CD44 is also described as a marker for bladder CSCs that are resistant to therapeutic drugs.74,75 CD44 is targeted by miR-34a in MIBC cells after cisplatin treatment, and increased expression of CD44 could effectively reverse the effects of miR-34a on the proliferation, cloning potential and chemosensitivity of MIBC cells.76 The mechanism by which CSCs are involved in chemoresistance may be related to the fact that they produce drug-resistant daughter cells under the pressure of drug action. However, the exact mechanism remains unclear.
Epithelial-Mesenchymal Transition
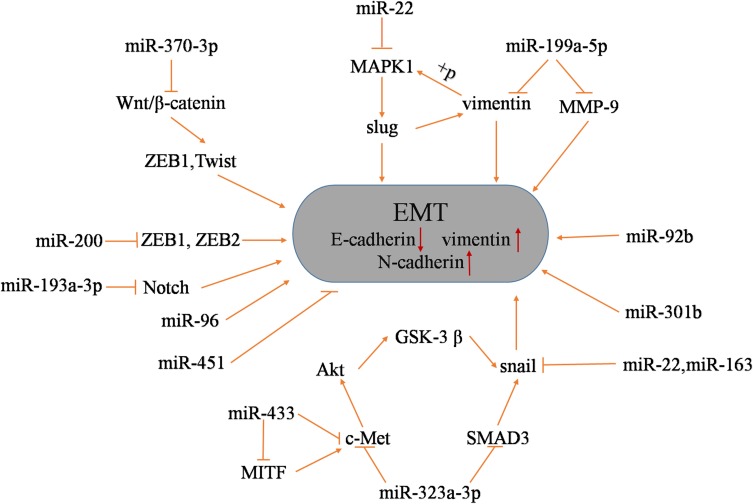
Epithelial-mesenchymal transition (EMT) is a process that plays major roles in development and wound healing and is characterized by the loss of homotypic adhesion and cell polarity and increased invasion and migration. At the molecular level, EMT is characterized by the loss of E-cadherin and the increased expression of several transcriptional repressors of E-cadherin expression (ZEB1, ZEB2, Twist, Snail, and Slug).77,78 Studies have shown that EMT is involved in the resistance of a variety of cancer drug treatments79–82 and is regulated by multiple signaling pathways.83,84 In BCa, the stable expression of miR-200 in mesenchymal UMUC3 cells increases E-cadherin levels; decreases the expression of ZEB1, ZEB2 and the tumor suppressor gene ERRFI-1 and cell migration; and increases EGFR blocker sensitivity.22 In addition, it has been shown that ERRFI-1 can be targeted by miR-200.22 The differential expression of the following miRNAs in BCa is associated with the EMT process: miR-433, miR-323a-3p, miR-22, miR-92, miR-96, miR-199a-5p, miR-301b, miR-613, miR-370-3p and miR-451.85–94 These miRNAs are associated with BCa cell proliferation, migration, invasion and EMT. For instance, miR-433 targets c-Met and CREB1 and inhibits EMT in BCa cells by modulating the c-Met/Akt/GSK-3β/Snail signaling pathway.85 miR-323a-3p regulates the EMT progression of BCa by targeting c-Met and SMAD3 and by negatively regulating their expression by modulating the c-Met/SMAD3/Snail pathway.86 In addition, the overexpression of miR-613 enhanced the expression of the epithelial biomarker E-cadherin and inhibited the expression of mesenchymal biomarkers (vimentin, Snail and N-cadherin).92 Moreover, sphingosine kinase 1 (Sphk1), as an oncogene, promotes tumor cell survival by converting ceramide to sphingosine and has been identified as a direct target gene of miR-613 in BCa cells.92 The recovery of Sphk1 partially reversed the inhibition of the proliferation, invasion and EMT of BCa cells induced by miR-613. Therefore, miR-613 exerts a tumor suppressive effect in BCa by targeting Sphk1. The Notch pathway has a negative regulatory effect on EMT, and DNA methylation in BCa regulates the high expression of mir-193a-3p, thus inhibiting the Notch pathway to promote EMT-induced multidrug resistance.18,95 Figure 3 shows the miRNAs associated with BCa EMT as well as their target genes and the EMT regulatory pathways.
Figure 3.
In BCa, these miRNAs regulate the EMT process.
Other Pathways
Generally, the chemoresistance of tumors involves complex networks. Mechanisms of multidrug resistance have been the subject of a great amount of research. In addition to the abovementioned signaling pathways, miRNAs can also regulate chemoresistance through other novel signaling pathways.
Low expression of miR-101 induced cell survival and cisplatin resistance by negatively regulating COX-2 expression.21 In addition, COX-2 was also shown to be a direct target of miR-101.21 COX-2 plays an important role in a variety of tumor drug resistances; therefore, strategies targeting the miR-101/COX-2 axis may be increasingly effective. Li et al found that miR-218 increased the sensitivity of BCa cells to cisplatin by targeting Glut1.25 Glut1 is a key rate-limiting enzyme that controls glycolysis flux in cells and plays a crucial role in tumorigenesis and progression.96,97 Overexpression of Glut1 enhances glycolysis activity, increases cancer cell proliferation, promotes tumor invasion and metastasis and is associated with poor prognosis of various malignancies, including BCa.98–100 In addition, miR-22-3p has been shown to promote chemotherapy resistance in BCa by targeting NET1. NET1 is a member of the transmembrane 4 superfamily (TM4SF)101 and is a novel tumor-associated gene associated with many malignancies through multiple regulatory mechanisms.102–105 Finally, overexpression of miRNA-27a has been reported to be associated with reduced levels of SLC7A11 and the intracellular levels of glutathione and to cause resistant cells to become resensitized to cisplatin.34
Notably, the tumor microenvironment, including the extracellular matrix and stromal cells, is closely related to the increase in drug resistance.106 Stromal cells, such as macrophages and fibroblasts, can produce growth factors to change the characteristics of tumor cells and increase drug resistance.106 Interactions between the tumor microenvironment and tumor, as well as the interior of the microenvironment, are mediated by important signal molecules. As an important signal molecule, miRNA levels are closely related to the tumor microenvironment. In BCa, it has been shown that tumor-related macrophages can promote EMT by increasing miR-30a levels,107 and EMT is one of the important mechanisms of drug resistance in BCa. Although there is limited evidence of the relationship between the tumor microenvironment and miRNA levels in BCa, this is an important direction and deserves further study.
Conclusions
Tumor-related research has evolved from studying a single oncogene or tumor suppressor gene to developing the current network regulation axis model. Changes in various biological properties of tumors, including the development of chemoresistance, are closely related to the dysregulation of miRNAs. lncRNAs, circRNAs and RNA-binding proteins are involved in the regulation of miRNA expression. The lncRNA/miRNA/target gene axis, the circRNA/miRNA/target gene axis, the RBP/miRNA/target gene axis, etc., constitute a complex tumor regulatory network. miRNAs may be the core factor, and the therapeutic effect of targeting miRNAs may be more effective than the effect of targeting a single oncogene. In tumors, miRNAs are involved in a variety of chemoresistance-related signaling pathways to regulate tumor resistance. Currently, miRNA-based therapeutic strategies include the use of agomir-miRNA, miR-101 mimic, and miRNA inhibitors. Extensive cell experiments have demonstrated the potential of these strategies to reverse chemoresistance in chemotherapy. miRNAs also have potential value in the treatment of chemotherapy resistance in BCa. In addition, the bladder is a smooth-muscle organ with an independent cavity that can be treated by perfusion administration, which greatly reduces targeting and safety problems. Therefore, miRNA-targeted therapy strategies to treat BCa chemotherapy resistance are a very promising treatment direction. Unfortunately, there are still many components of miRNA-targeted therapy strategies that need to be further elucidated. For example, how many miRNAs are associated with BCa resistance? Which miRNA is the most important? What duration of miRNA targeting is required to regulate drug resistance? Research on these issues will demonstrate the utility of miRNA-targeted therapeutic strategies and lay the foundation for further clinical trials.
Funding Statement
This work is supported by the grant from National Natural Science Foundation of China (Grant No. 81671448).
Data Sharing Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ploeg M, Aben KK, Kiemeney LA. The present and future burden of urinary bladder cancer in the world. World J Urol. 2009;27(3):289–293. doi: 10.1007/s00345-009-0383-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bo J, Yang G, Huo K, et al. microRNA-203 suppresses bladder cancer development by repressing bcl-w expression. FEBS J. 2011;278(5):786–792. doi: 10.1111/j.1742-4658.2010.07997.x [DOI] [PubMed] [Google Scholar]
- 3.Botteman MF, Pashos CL, Redaelli A, et al. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics. 2003;21(18):1315–1330. doi: 10.1007/BF03262330 [DOI] [PubMed] [Google Scholar]
- 4.Noguchi S, Mori T, Hoshino Y, et al. MicroRNA-143 functions as a tumor suppressor in human bladder cancer T24 cells. Cancer Lett. 2011;307(2):211–220. doi: 10.1016/j.canlet.2011.04.005 [DOI] [PubMed] [Google Scholar]
- 5.Witjes JA, Compérat E, Cowan NC, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol. 2014;65(4):778–792. doi: 10.1016/j.eururo.2013.11.046 [DOI] [PubMed] [Google Scholar]
- 6.Herr HW, Dotan Z, Donat SM, et al. Defining optimal therapy for muscle invasive bladder cancer. J Urol. 2007;177(2):437–443. doi: 10.1016/j.juro.2006.09.027 [DOI] [PubMed] [Google Scholar]
- 7.Srikantan S, Gorospe M. HuR function in disease. Front Biosci (Landmark Ed). 2012;17:189–205. doi: 10.2741/3921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Roretz C, Di Marco S, Mazroui R, et al. Turnover of AU-rich-containing mRNAs during stress: a matter of survival. Wiley Interdiscip Rev RNA. 2011;2(3):336–347. doi: 10.1002/wrna.55 [DOI] [PubMed] [Google Scholar]
- 9.Liang H, Gong F, Zhang S, et al. The origin, function, and diagnostic potential of extracellular microRNAs in human body fluids. Wiley Interdiscip Rev RNA. 2014;5:285–300. doi: 10.1002/wrna.1208 [DOI] [PubMed] [Google Scholar]
- 10.Lytle JR, Yario TA, Steitz JA. Target mRNAs are repressed as efficiently by microRNA-binding sites in the 5ʹ UTR as in the 3ʹ UTR. Proc Natl Acad Sci U S A. 2007;104(23):9667–9672. doi: 10.1073/pnas.0703820104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song JH, Meltzer SJ. MicroRNAs in pathogenesis, diagnosis, and treatment of gastroesophageal cancers. Gastroenterology. 2012;143(1):35–47.e2. doi: 10.1053/j.gastro.2012.05.003 [DOI] [PubMed] [Google Scholar]
- 12.Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–355. doi: 10.1038/nature02871 [DOI] [PubMed] [Google Scholar]
- 13.Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell. 2006;11(4):441–450. doi: 10.1016/j.devcel.2006.09.009 [DOI] [PubMed] [Google Scholar]
- 14.Fadejeva I, Olschewski H, Hrzenjak A, et al. MicroRNAs as regulators of cisplatin-resistance in non-small cell lung carcinomas. Oncotarget. 2017;8(70):115754–115773. doi: 10.18632/oncotarget.v8i70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang W, Ma J, Zhou W, et al. Molecular mechanisms and theranostic potential of miRNAs in drug resistance of gastric cancer. Expert Opin Ther Targets. 2017;21(11):1063–1075. doi: 10.1080/14728222.2017.1389900 [DOI] [PubMed] [Google Scholar]
- 16.Li F, Mahato RI. MicroRNAs and drug resistance in prostate cancers. Mol Pharm. 2014;11(8):2539–2552. doi: 10.1021/mp500099g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tölle A, Ratert N, Jung K, et al. miRNA panels as biomarkers for bladder cancer. Biomark Med. 2014;8(5):733–746. doi: 10.2217/bmm.14.26 [DOI] [PubMed] [Google Scholar]
- 18.Lv L, Deng H, Li Y, et al. The DNA methylation-regulated miR-193a-3p dictates the multi-chemoresistance of bladder cancer via repression of SRSF2/PLAU/HIC2 expression. Cell Death Dis. 2014;5:e1402. doi: 10.1038/cddis.2014.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kozinn SI, Harty NJ, Delong JM, et al. MicroRNA profile to predict gemcitabine resistance in bladder carcinoma cell lines. Genes Cancer. 2013;4(1–2):61–69. doi: 10.1177/1947601913484495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H, Yu G, Shi R, et al. Cisplatin-induced epigenetic activation of miR-34a sensitizes bladder cancer cells to chemotherapy. Mol Cancer. 2014;13:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bu Q, Fang Y, Cao Y, et al. Enforced expression of miR-101 enhances cisplatin sensitivity in human bladder cancer cells by modulating the cyclooxygenase-2 pathway. Mol Med Rep. 2014;10(4):2203–2209. doi: 10.3892/mmr.2014.2455 [DOI] [PubMed] [Google Scholar]
- 22.Adam L, Zhong M, Choi W, et al. miR-200 expression regulates epithelial-to-mesenchymal transition in bladder cancer cells and reverses resistance to epidermal growth factor receptor therapy. Clin Cancer Res. 2009;15(16):5060–5072. doi: 10.1158/1078-0432.CCR-08-2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tao J, Lu Q, Wu D, et al. microRNA-21 modulates cell proliferation and sensitivity to doxorubicin in bladder cancer cells. Oncol Rep. 2011;25(6):1721–1729. doi: 10.3892/or.2011.1245 [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Zhang Y, Liu X, et al. MicroRNA-203 is a prognostic indicator in bladder cancer and enhances chemosensitivity to cisplatin via apoptosis by targeting Bcl-w and survivin. PLoS ONE. 2015;10(11):e0143441. doi: 10.1371/journal.pone.0143441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li P, Yang X, Cheng Y, et al. MicroRNA-218 increases the sensitivity of bladder cancer to cisplatin by targeting Glut1. Cell Physiol Biochem. 2017;41(3):921–932. doi: 10.1159/000460505 [DOI] [PubMed] [Google Scholar]
- 26.Deng H, Lv L, Li Y, et al. miR-193a-3p regulates the multi-drug resistance of bladder cancer by targeting the LOXL4 gene and the oxidative stress pathway. Mol Cancer. 2014;13:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan J, Li X, Wu W, et al. Long non-coding RNA UCA1 promotes cisplatin/gemcitabine resistance through CREB modulating miR-196a-5p in bladder cancer cells. Cancer Lett. 2016;382(1):64–76. doi: 10.1016/j.canlet.2016.08.015 [DOI] [PubMed] [Google Scholar]
- 28.Xiao J, Niu S, Zhu J, et al. miR‑22‑3p enhances multi‑chemoresistance by targeting NET1 in bladder cancer cells. Oncol Rep. 2018;39(6):2731–2740. doi: 10.3892/or.2018.6355 [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Li Q, Niu X, et al. miR-143 inhibits bladder cancer cell proliferation and enhances their sensitivity to gemcitabine by repressing IGF-1R signaling. Oncol Lett. 2017;13(1):435–440. doi: 10.3892/ol.2016.5388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lv L, Li Y, Deng H, et al. MiR-193a-3p promotes the multi-chemoresistance of bladder cancer by targeting the HOXC9 gene. Cancer Lett. 2015;357(1):105–113. doi: 10.1016/j.canlet.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 31.Deng H, Lv L, Li Y, et al. The miR-193a-3p regulated PSEN1 gene suppresses the multi-chemoresistance of bladder cancer. Biochim Biophys Acta. 2015;1852(3):520–528. doi: 10.1016/j.bbadis.2014.12.014 [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Deng H, Lv L, et al. The miR-193a-3p-regulated ING5 gene activates the DNA damage response pathway and inhibits multi-chemoresistance in bladder cancer. Oncotarget. 2015;6(12):10195–10206. doi: 10.18632/oncotarget.3555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng LP, Hu ZM, Li K, et al. attenuates cisplatin-induced cell death by targeting the PPP2R2A/Akt/mTOR axis in bladder cancer cells. J Cell Mol Med. 2016;20(3):559–567. doi: 10.1111/jcmm.12760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drayton RM, Dudziec E, Peter S, et al. Reduced expression of miRNA-27a modulates cisplatin resistance in bladder cancer by targeting the cystine/glutamate exchanger SLC7A11. Clin Cancer Res. 2014;20(7):1990–2000. doi: 10.1158/1078-0432.CCR-13-2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deng Y, Bai H, Hu H. rs11671784 G/A variation in miR-27a decreases chemo-sensitivity of bladder cancer by decreasing miR-27a and increasing the target RUNX-1 expression. Biochem Biophys Res Commun. 2015;458(2):321–327. doi: 10.1016/j.bbrc.2015.01.109 [DOI] [PubMed] [Google Scholar]
- 36.Zhuang J, Shen L, Yang L, et al. TGFβ1 promotes gemcitabine resistance through regulating the LncRNA-LET/NF90/miR-145 signaling axis in bladder cancer. Theranostics. 2017;7(12):3053–3067. doi: 10.7150/thno.19542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Shan Z, Liu C, et al. MicroRNA-294 promotes cellular proliferation and motility through the PI3K/AKT and JAK/STAT pathways by upregulation of NRAS in bladder cancer. Biochemistry (Mosc). 2017;82(4):474–482. doi: 10.1134/S0006297917040095 [DOI] [PubMed] [Google Scholar]
- 38.Lin SR, Yeh HC, Wang WJ, et al. MiR-193b mediates CEBPD-induced cisplatin sensitization through targeting ETS1 and Cyclin D1 in human urothelial carcinoma cells. J Cell Biochem. 2017;118(6):1563–1573. doi: 10.1002/jcb.25818 [DOI] [PubMed] [Google Scholar]
- 39.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116(2):205–219. doi: 10.1016/S0092-8674(04)00046-7 [DOI] [PubMed] [Google Scholar]
- 40.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/S0092-8674(00)81683-9 [DOI] [PubMed] [Google Scholar]
- 41.Ashkenazi A. Directing cancer cells to self-destruct with pro-apoptotic receptor agonists. Nat Rev Drug Discov. 2008;7(12):1001–1012. doi: 10.1038/nrd2637 [DOI] [PubMed] [Google Scholar]
- 42.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3(8):917–921. doi: 10.1038/nm0897-917 [DOI] [PubMed] [Google Scholar]
- 43.Chen XN, Wang KF, Xu ZQ, et al. MiR-133b regulates bladder cancer cell proliferation and apoptosis by targeting Bcl-w and Akt1. Cancer Cell Int. 2014;14:70. doi: 10.1186/s12935-014-0070-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou C, Ding J, Wu Y. Resveratrol induces apoptosis of bladder cancer cells via miR‑21 regulation of the Akt/Bcl‑2 signaling pathway. Mol Med Rep. 2014;9(4):1467–1473. doi: 10.3892/mmr.2014.1950 [DOI] [PubMed] [Google Scholar]
- 45.Jin Y, Lu J, Wen J, et al. Regulation of growth of human bladder cancer by miR-192. Tumour Biol. 2015;36(5):3791–3797. doi: 10.1007/s13277-014-3020-8 [DOI] [PubMed] [Google Scholar]
- 46.Fu B, Wang Y, Zhang X, et al. MiR-221-induced PUMA silencing mediates immune evasion of bladder cancer cells. Int J Oncol. 2015;46(3):1169–1180. doi: 10.3892/ijo.2015.2837 [DOI] [PubMed] [Google Scholar]
- 47.Wang H, Zhang W, Zuo Y, et al. miR-9 promotes cell proliferation and inhibits apoptosis by targeting LASS2 in bladder cancer. Tumour Biol. 2015;36(12):9631–9640. doi: 10.1007/s13277-015-3713-7 [DOI] [PubMed] [Google Scholar]
- 48.Liu C, Chen Z, Fang J, et al. H19-derived miR-675 contributes to bladder cancer cell proliferation by regulating p53 activation. Tumour Biol. 2016;37(1):263–270. doi: 10.1007/s13277-015-3779-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu XD, Wu XH, Fan YR, et al. Exosome-derived microRNA-29c induces apoptosis of BIU-87 cells by down regulating BCL-2 and MCL-1. Asian Pac J Cancer Prev. 2014;15(8):3471–3476. doi: 10.7314/APJCP.2014.15.8.3471 [DOI] [PubMed] [Google Scholar]
- 50.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27(41):5497–5510. doi: 10.1038/onc.2008.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28(6):1075–1083. doi: 10.1200/JCO.2009.25.3641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Askham JM, Platt F, Chambers PA, et al. AKT1 mutations in bladder cancer: identification of a novel oncogenic mutation that can co-operate with E17K. Oncogene. 2010;29(1):150–155. doi: 10.1038/onc.2009.315 [DOI] [PubMed] [Google Scholar]
- 53.Lei M, Xie W, Sun E, et al. microRNA-21 regulates cell proliferation and migration and cross talk with PTEN and p53 in bladder cancer. DNA Cell Biol. 2015;34(10):626–632. doi: 10.1089/dna.2015.2868 [DOI] [PubMed] [Google Scholar]
- 54.Calderaro J, Rebouissou S, de Koning L, et al. PI3K/AKT pathway activation in bladder carcinogenesis. Int J Cancer. 2014;134(8):1776–1784. doi: 10.1002/ijc.28518 [DOI] [PubMed] [Google Scholar]
- 55.Tan M, Mu X, Liu Z, et al. microRNA-495 promotes bladder cancer cell growth and invasion by targeting phosphatase and tensin homolog. Biochem Biophys Res Commun. 2017;483(2):867–873. doi: 10.1016/j.bbrc.2017.01.019 [DOI] [PubMed] [Google Scholar]
- 56.Feng Y, Liu J, Kang Y, et al. miR-19a acts as an oncogenic microRNA and is up-regulated in bladder cancer. J Exp Clin Cancer Res. 2014;A,33::67. doi: 10.1186/s13046-014-0067-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lv M, Zhong Z, Chi H, et al. Genome-wide screen of miRNAs and targeting mRNAs reveals the negatively regulatory effect of miR-130b-3p on PTEN by PI3K and Integrin β1 signaling pathways in bladder carcinoma. Int J Mol Sci. 2016;18(1). doi: 10.3390/ijms18010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Egawa H, Jingushi K, Hirono T, et al. miR-130 family promotes cell migration and invasion in bladder cancer through FAK and Akt phosphorylation by regulating PTEN. Sci Rep. 2016;6:20574. doi: 10.1038/srep20574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheng Y, Yang X, Deng X, et al. MicroRNA-218 inhibits bladder cancer cell proliferation, migration, and invasion by targeting BMI-1. Tumour Biol. 2015;36(10):8015–8023. doi: 10.1007/s13277-015-3532-x [DOI] [PubMed] [Google Scholar]
- 60.Cheng JD, Werness BA, Babb JS, Meropol NJ. Paradoxical correlations of cyclin-dependent kinase inhibitors p21waf1/cip1 and p27kip1 in metastatic colorectal carcinoma. Clin Cancer Res. 1999;5(5):1057–1062. [PubMed] [Google Scholar]
- 61.Vinall RL, Ripoll AZ, Wang S. MiR-34a chemosensitizes bladder cancer cells to cisplatin treatment regardless of p53-Rb pathway status. Int J Cancer. 2012;130(11):2526–2538. doi: 10.1002/ijc.26256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang X, Choi JH, Ding J, et al. HOXC9 directly regulates distinct sets of genes to coordinate diverse cellular processes during neuronal differentiation. BMC Genomics. 2013;14:830. doi: 10.1186/1471-2164-14-830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao X, Li J, Huang S, et al. MiRNA-29c regulates cell growth and invasion by targeting CDK6 in bladder cancer. Am J Transl Res. 2015;7(8):1382–1389. [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang T, Wang J, Zhai X, et al. MiR-124 retards bladder cancer growth by directly targeting CDK4. Acta Biochim Biophys Sin (Shanghai). 2014;46(12):1072–1079. doi: 10.1093/abbs/gmu105 [DOI] [PubMed] [Google Scholar]
- 65.Chen H, Lin YW, Mao YQ, et al. MicroRNA-449a acts as a tumor suppressor in human bladder cancer through the regulation of pocket proteins. Cancer Lett. 2012;320(1):40–47. doi: 10.1016/j.canlet.2012.01.027 [DOI] [PubMed] [Google Scholar]
- 66.Wang X, Wu J, Lin Y, et al. MicroRNA-320c inhibits tumorous behaviors of bladder cancer by targeting Cyclin-dependent kinase 6. J Exp Clin Cancer Res. 2014;33:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shin SS, Park SS, Hwang B, et al. MicroRNA-106a suppresses proliferation, migration, and invasion of bladder cancer cells by modulating MAPK signaling, cell cycle regulators, and Ets-1-mediated MMP-2 expression. Oncol Rep. 2016;36(4):2421–2429. doi: 10.3892/or.2016.5015 [DOI] [PubMed] [Google Scholar]
- 68.Park SL, Cho TM, Won SY, et al. MicroRNA-20b inhibits the proliferation, migration and invasion of bladder cancer EJ cells via the targeting of cell cycle regulation and Sp-1-mediated MMP-2 expression. Oncol Rep. 2015;34(3):1605–1612. doi: 10.3892/or.2015.4119 [DOI] [PubMed] [Google Scholar]
- 69.Lin Y, Wu J, Chen H, et al. Cyclin-dependent kinase 4 is a novel target in micoRNA-195-mediated cell cycle arrest in bladder cancer cells. FEBS Lett. 2012;586(4):442–447. doi: 10.1016/j.febslet.2012.01.027 [DOI] [PubMed] [Google Scholar]
- 70.Lu Q, Lu C, Zhou GP, et al. MicroRNA-221 silencing predisposed human bladder cancer cells to undergo apoptosis induced by TRAIL. Urol Oncol. 2010;28(6):635–641. doi: 10.1016/j.urolonc.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 71.Galluzzi L, Vitale I, Michels J, et al. Systems biology of cisplatin resistance: past, present and future. Cell Death Dis. 2014;5:e1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Magee JA, Piskounova E, Morrison SJ. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell. 2012;21(3):283–296. doi: 10.1016/j.ccr.2012.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Valent P, Bonnet D, De Maria R, et al. Cancer stem cell definitions and terminology: the devil is in the details. Nat Rev Cancer. 2012;12(11):767–775. doi: 10.1038/nrc3368 [DOI] [PubMed] [Google Scholar]
- 74.Patrawala L, Calhoun T, Schneider-Broussard R, et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25(12):1696–1708. doi: 10.1038/sj.onc.1209327 [DOI] [PubMed] [Google Scholar]
- 75.Patrawala L, Calhoun-Davis T, Schneider-Broussard R, et al. Hierarchical organization of prostate cancer cells in xenograft tumors: the CD44+alpha2beta1+ cell population is enriched in tumor-initiating cells. Cancer Res. 2007;67(14):6796–6805. doi: 10.1158/0008-5472.CAN-07-0490 [DOI] [PubMed] [Google Scholar]
- 76.Yu G, Yao W, Xiao W, et al. MicroRNA-34a functions as an anti-metastatic microRNA and suppresses angiogenesis in bladder cancer by directly targeting CD44. J Exp Clin Cancer Res. 2014;33:779. doi: 10.1186/s13046-014-0115-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7(6):415–428. doi: 10.1038/nrc2131 [DOI] [PubMed] [Google Scholar]
- 78.Baumgart E, Cohen MS, Silva Neto B, et al. Identification and prognostic significance of an epithelial-mesenchymal transition expression profile in human bladder tumors. Clin Cancer Res. 2007;13(6):1685–1694. doi: 10.1158/1078-0432.CCR-06-2330 [DOI] [PubMed] [Google Scholar]
- 79.Wang Z, Li Y, Kong D, et al. Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res. 2009;69(6):2400–2407. doi: 10.1158/0008-5472.CAN-08-4312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim HP, Han SW, Song SH, et al. Testican-1-mediated epithelial-mesenchymal transition signaling confers acquired resistance to lapatinib in HER2-positive gastric cancer. Oncogene. 2014;33(25):3334–3341. doi: 10.1038/onc.2013.285 [DOI] [PubMed] [Google Scholar]
- 81.Du F, Wu X, Liu Y, et al. Acquisition of paclitaxel resistance via PI3K‑dependent epithelial‑mesenchymal transition in A2780 human ovarian cancer cells. Oncol Rep. 2013;30(3):1113–1118. doi: 10.3892/or.2013.2567 [DOI] [PubMed] [Google Scholar]
- 82.Kajiyama H, Shibata K, Terauchi M, et al. Chemoresistance to paclitaxel induces epithelial-mesenchymal transition and enhances metastatic potential for epithelial ovarian carcinoma cells. Int J Oncol. 2007;31(2):277–283. [PubMed] [Google Scholar]
- 83.Horiguchi K, Shirakihara T, Nakano A, et al. Role of Ras signaling in the induction of snail by transforming growth factor-beta. J Biol Chem. 2009;284(1):245–253. doi: 10.1074/jbc.M804777200 [DOI] [PubMed] [Google Scholar]
- 84.Levy L, Hill CS. Smad4 dependency defines two classes of transforming growth factor {beta} (TGF-{beta}) target genes and distinguishes TGF-{beta}-induced epithelial-mesenchymal transition from its antiproliferative and migratory responses. Mol Cell Biol. 2005;25(18):8108–8125. doi: 10.1128/MCB.25.18.8108-8125.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu X, Zhu Y, Liang Z, et al. and CREB1 are involved in miR-433-mediated inhibition of the epithelial-mesenchymal transition in bladder cancer by regulating Akt/GSK-3β/Snail signaling. Cell Death Dis. 2016;7:e2088. doi: 10.1038/cddis.2015.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li J, Xu X, Meng S, et al. MET/SMAD3/SNAIL circuit mediated by miR-323a-3p is involved in regulating epithelial-mesenchymal transition progression in bladder cancer. Cell Death Dis. 2017;8(8):e3010. doi: 10.1038/cddis.2017.331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu M, Li J, Wang X, et al. MiR-22 suppresses epithelial-mesenchymal transition in bladder cancer by inhibiting Snail and MAPK1/Slug/vimentin feedback loop. Cell Death Dis. 2018;9(2):209. doi: 10.1038/s41419-017-0206-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang J, Wang B, Hui K, et al. miR-92b targets DAB2IP to promote EMT in bladder cancer migration and invasion. Oncol Rep. 2016;36(3):1693–1701. doi: 10.3892/or.2016.4940 [DOI] [PubMed] [Google Scholar]
- 89.He C, Zhang Q, Gu R, et al. miR-96 regulates migration and invasion of bladder cancer through epithelial-mesenchymal transition in response to transforming growth factor-β1. J Cell Biochem. 2018. doi: 10.1002/jcb.27172 [DOI] [PubMed] [Google Scholar]
- 90.Zhou M, Wang S, Hu L, et al. miR-199a-5p suppresses human bladder cancer cell metastasis by targeting CCR7. BMC Urol. 2016;16(1):64. doi: 10.1186/s12894-016-0181-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yan L, Wang Y, Liang J. et al. MiR-301b promotes the proliferation, mobility, and epithelial-to-mesenchymal transition of bladder cancer cells by targeting EGR1. Biochem Cell Biol. 2017;95(5):571–577. doi: 10.1139/bcb-2016-0232 [DOI] [PubMed] [Google Scholar]
- 92.Yu H, Duan P, Zhu H, et al. miR-613 inhibits bladder cancer proliferation and migration through targeting SphK1. Am J Transl Res. 2017;9(3):1213–1221. [PMC free article] [PubMed] [Google Scholar]
- 93.Huang X, Zhu H, Gao Z, et al. Wnt7a activates canonical Wnt signaling, promotes bladder cancer cell invasion, and is suppressed by miR-370-3p. J Biol Chem. 2018;293(18):6693–6706. doi: 10.1074/jbc.RA118.001689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zeng T, Peng L, Chao C, et al. miR-451 inhibits invasion and proliferation of bladder cancer by regulating EMT. Int J Clin Exp Pathol. 2014;7(11):7653–7662. [PMC free article] [PubMed] [Google Scholar]
- 95.Fang D, Kitamura H. Cancer stem cells and epithelial-mesenchymal transition in urothelial carcinoma: possible pathways and potential therapeutic approaches. Int J Urol. 2018;25(1):7–17. doi: 10.1111/iju.2018.25.issue-1 [DOI] [PubMed] [Google Scholar]
- 96.Moreno-Sánchez R, Rodríguez-Enríquez S, Marín-Hernández A, et al. Energy metabolism in tumor cells. FEBS J. 2007;274(6):1393–1418. doi: 10.1111/j.1742-4658.2007.05686.x [DOI] [PubMed] [Google Scholar]
- 97.Koch A, Lang SA, Wild PJ, et al. Glucose transporter isoform 1 expression enhances metastasis of malignant melanoma cells. Oncotarget. 2015;6(32):32748–32760. doi: 10.18632/oncotarget.v6i32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Amann T, Maegdefrau U, Hartmann A, et al. GLUT1 expression is increased in hepatocellular carcinoma and promotes tumorigenesis. Am J Pathol. 2009;174(4):1544–1552. doi: 10.2353/ajpath.2009.080596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shen YM, Arbman G, Olsson B, et al. Overexpression of GLUT1 in colorectal cancer is independently associated with poor prognosis. Int J Biol Markers. 2011;26(3):166–172. doi: 10.5301/JBM.2011.8550 [DOI] [PubMed] [Google Scholar]
- 100.Zhang W, Liu Y, Chen X, et al. Novel inhibitors of basal glucose transport as potential anticancer agents. Bioorg Med Chem Lett. 2010;20(7):2191–2194. doi: 10.1016/j.bmcl.2010.02.027 [DOI] [PubMed] [Google Scholar]
- 101.Serru V, Dessen P, Boucheix C, et al. Sequence and expression of seven new tetraspans. Biochim Biophys Acta. 2000;1478(1):159–163. doi: 10.1016/S0167-4838(00)00022-4 [DOI] [PubMed] [Google Scholar]
- 102.Ye K, Chang S, Li J, et al. A functional and protein-protein interaction analysis of neuroepithelial cell transforming gene 1 in hepatocellular carcinoma. Tumour Biol. 2014;35(11):11219–11227. doi: 10.1007/s13277-014-2454-3 [DOI] [PubMed] [Google Scholar]
- 103.Zhang J, Wang J, Chen L, et al. Expression and function of NET-1 in human skin squamous cell carcinoma. Arch Dermatol Res. 2014;306(4):385–397. doi: 10.1007/s00403-013-1423-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ecimovic P, Murray D, Doran P, et al. Propofol and bupivacaine in breast cancer cell function in vitro - role of the NET1 gene. Anticancer Res. 2014;34(3):1321–1331. [PubMed] [Google Scholar]
- 105.Fang L, Zhu J, Ma Y, et al. Neuroepithelial transforming gene 1 functions as a potential prognostic marker for patients with non-small cell lung cancer. Mol Med Rep. 2015;12(5):7439–7446. doi: 10.3892/mmr.2015.4385 [DOI] [PubMed] [Google Scholar]
- 106.Assaraf YG, Brozovic A, Gonçalves AC, et al. The multi-factorial nature of clinical multidrug resistance in cancer. Drug Resist Updat. 2019;46:100645. doi: 10.1016/j.drup.2019.100645 [DOI] [PubMed] [Google Scholar]
- 107.Zhang Q, Mao Z, Sun J. NF-κB inhibitor, BAY11-7082, suppresses M2 tumor-associated macrophage induced EMT potential via miR-30a/NF-κB/Snail signaling in bladder cancer cells. Gene. 2019;710:91–97. doi: 10.1016/j.gene.2019.04.039 [DOI] [PubMed] [Google Scholar]