Abstract
Abiotic emergence of ordered information stored in the form of RNA is an important unresolved problem concerning the origin of life. A polymer longer than 40–100 nucleotides is necessary to expect a self-replicating activity, but the formation of such a long polymer having a correct nucleotide sequence by random reactions seems statistically unlikely. However, our universe, created by a single inflation event, likely includes more than 10100 Sun-like stars. If life can emerge at least once in such a large volume, it is not in contradiction with our observations of life on Earth, even if the expected number of abiogenesis events is negligibly small within the observable universe that contains only 1022 stars. Here, a quantitative relation is derived between the minimum RNA length lmin required to be the first biological polymer, and the universe size necessary to expect the formation of such a long and active RNA by randomly adding monomers. It is then shown that an active RNA can indeed be produced somewhere in an inflationary universe, giving a solution to the abiotic polymerization problem. On the other hand, lmin must be shorter than ~20 nucleotides for the abiogenesis probability close to unity on a terrestrial planet, but a self-replicating activity is not expected for such a short RNA. Therefore, if extraterrestrial organisms of a different origin from those on Earth are discovered in the future, it would imply an unknown mechanism at work to polymerize nucleotides much faster than random statistical processes.
Subject terms: RNA, Evolutionary theory, Astronomy and astrophysics, Cosmology, Exoplanets
Introduction
In spite of recent rapid development of biology, chemistry, Earth science and astronomy, the origin of life (abiogenesis) is still a great mystery in science1–5. A prominent feature of life is the ordered information stored in DNA/RNA, and how such information appeared from abiotic processes is a crucial issue. The RNA world hypothesis6–8 postulates an early era when RNA played both the genetic and catalytic roles before the DNA-protein world came into being. This is widely accepted due to strong supporting evidence including catalytic activities of RNA, especially its central role in a ribosome. However, a more fundamental and unsolved problem is how an RNA polymer long enough to have a self-replicating RNA polymerase activity (i.e., RNA replicase ribozyme) emerged from prebiotic conditions and then triggered Darwinian evolution.
A key quantity is the minimum RNA length required to show a self-replicating ability. RNA molecules shorter than 25 nucleotides (nt) do not show a specified function, but there is a reasonable hope to find a functioning replicase ribozyme longer than 40–60 nt8,9. RNA polymerase ribozymes produced by in vitro experiments so far have a length longer than 100 nt10–12. Furthermore, formation of just a single long strand may not be sufficient to initiate an abiogenesis event. Instead a pair of identical strands may be necessary if one serves as a replicase ribozyme and the other as a template.
Polymerization of RNA in water is a thermodynamically uphill process, and hence reacting monomers need to be activated. Non-enzymatic reactions of adding activated monomers (e.g., imidazole-activated ribonucleotides) to an RNA oligomer have been experimentally studied1,4,13. Reactions at inorganic catalytic sites (e.g. surface of minerals such as montmorillonite clay) may be particularly efficient14,15. Some experiments yielded production of up to 40-mers of RNA16,17, which may be long enough to have some biological activities. However, these results have not been reproducible, and only short oligomers of up to 10-mers were produced conclusively in recent experiments, with the abundance rapidly decreasing with the oligomer length13,18–20. This trend is also consistent with the theoretical expectation for random adding of monomers (see below). An experimental difficulty is that aggregates may easily be mistaken for polymers, depending on detection methods13,21.
It is theoretically speculated that terminal ligation of oligomers may hierarchically produce further longer polymers22, but there is no experimental or quantitative demonstration of this starting from realistic prebiotic conditions. A report23 of experimental production of long polymers (>120 nt) by ligation has been subject to reproducibility and the aggregate/polymer discrimination problem13,21,24. A high concentration of oligomers is necessary for ligation to work efficiently, but this may be difficult because oligomer abundance rapidly decreases with oligomer length in polymerization by monomers, even if such a ligase activity exists.
If we consider only the conservative abiotic polymerization, i.e., statistically adding monomers, the probability of abiogenesis may be extremely low on a terrestrial planet. This case is not in contradiction with our existence on Earth, because we would find ourselves on a planet where abiogenesis happened. The life on Earth is believed to have descended from the single last universal common ancestor (LUCA) with no evidence for multiple abiogenesis events, and we do not know any life of a different origin in the universe. The emergence of life early in the history of Earth is often used to argue for a high abiogenesis rate, but an arbitrarily low rate cannot be robustly excluded25–27 because the chance probability tab∕t⊕ ~ 0.1 is not negligible assuming a constant abiogenesis rate, where tab is the time of abiogenesis elapsed from the birth of Earth28, and t⊕ the present age of Earth. It may well be possible that early Earth was a more favorable environment for abiogenesis than present29,30. There may also be an anthropic selection effect favoring earlier abiogenesis on Earth, because an intelligent life must emerge before the increasing solar luminosity causes an end to Earth’s habitable state (estimated to be ~1 Gyr in future)31.
For the case of a low abiogenesis rate, the number of abiogenesis events is often considered in the Milky Way Galaxy containing about 1011 Sun-like stars32 or in the whole observable universe containing 1022 stars33 inside a spherical volume with a comoving radius of 46.3 Gly (or 13.8 Gly as a light travel time distance)26,34. However, the size of the observable universe is not related at all to its whole physical size. According to the widely accepted view of the inflationary cosmology35–40, the physical size of the universe created by an inflation event should be much larger, likely including more than 10100 stars (see below). In that case, even if the expected number of abiogenesis events is much less than unity in a volume size of the observable universe, it may still be consistent with our observations provided that abiogenesis is expected to occur somewhere in an inflationary universe.
The aim of this work is to examine this possibility quantitatively, assuming that the first biological RNA polymer was produced by randomly adding monomers. Koonin41 considered implications of the eternal inflation theory for the origin of life. In this scenario, most part of the universe inflates forever, self-reproducing many subregions that undergo a conventional inflation followed by a hot big-bang universe. Then an infinite number of stars and galaxies would be formed, and we expect emergence of life even if the abiogenesis probability is infinitely small. Though eternal inflation is a theoretically likely scenario42, it is difficult to confirm observationally, and a quantitative discussion is impossible. It is then interesting to ask if life can emerge within the homogeneous region in which we exist, assuming its minimal size necessary to explain observations. This work tries to give a quantitative answer to this question.
The Size of the Universe Created by an Inflation Event
The observable universe is highly homogeneous and spatially flat on scales many orders of magnitude larger than the causally connected scale (horizon) in the early universe, which are called the horizon and flatness problems, and cannot be explained by the standard big bang cosmology. Cosmic inflation is currently the only widely accepted solution to these problems, and furthermore, it naturally generates scale-invariant quantum density fluctuations that serve as the seed of galaxy formation and the large scale structure in the present universe. Its prediction is in quantitative agreement with the observations of the cosmic microwave background radiation anisotropy, already constraining some theoretical models43.
There are many models and scenarios about how inflation occurred in the early universe42, but all of them consider an epoch of exponential expansion as a ∝ exp(Hi ti), where a is the scale factor of the universe, Hi the Hubble parameter at the time of inflation, and ti the duration of inflation. If the inflation occurred at the energy scale of the grand unified theory of particle physics (1016 GeV), Hi would be about 1037 s−1. To solve the horizon and flatness problems, the e-folding number of inflation (Ni ≡ Hi ti) must be larger than44,45Ni,min ~ 60. If Ni = Ni,min, a causal patch region expanded by the inflation has now the same size as the observable universe. It would be a fine tuning if the inflation duration is extremely close to the minimal value to solve the problems (i.e., Ni − Ni,min ≪ Ni,min). Rather, we naturally expect Ni − Ni,min ≳ Ni,min. If the inflation duration is twice (three times) as much as that required to solve the problems, the homogeneous universe should extend e60 (e120) times as much as the currently observable universe, which is 1078 (10156) times as large in volume, thus including about 10100 (10178) stars.
Poissonian RNA Polymerization
Here we consider a cycle of RNA polymerization by randomly adding activated monomers to an oligomer as a Poisson process, taking an experiment on clay surfaces18 as a model case. Non-RNA nucleic acid analogues may have carried genetic information before the RNA world emerged1, but the formulations below can also be applied to such cases. Let xl be the abundance of l-nt long oligomers. After the injection of activated monomers at the time of initialization (t = 0), evolution of xl is described by the following differential equations:
| 1 |
where the dot denotes a time derivative. Here we assume that the coefficient κ (probability of a reaction with a monomer per unit time) does not depend on the oligomer length, which is approximately consistent with the trend found in the experiment18. We consider initial conditions of xl = 0 for l ≥ 2, and x1 can be approximated to be constant in the early phase. The second term on the right hand side can be neglected when xl+1 ≪ xl. Solving the equations iteratively under these conditions, the abundance xl at a time t is obtained as
| 2 |
where pr ≡ κ t is the reaction probability with a monomer up to the time t. A similar result is obtained by considering the Poisson distribution with an expectation value of pr; the only difference is a factor of exp(−pr) that is not important at pr ≲ 1. We should consider only the regime of pr ≲ 1, because by the time t ~ κ−1, a significant fraction of activated monomers are lost by the reactions, and hence the approximation of constant x1 is no longer valid and efficient polymerization is not expected beyond this point. If activated monomers are lost earlier by some other processes (e.g. hydrolysis), pr would be smaller than unity.
In RNA oligomerization on clay surfaces, the coefficient κ should be proportional to the concentration of activated monomers adsorbed on the clay surface. This clay-phase monomer concentration increases with that in aqueous phase, but according to the Langmuir adsorption isotherm, it saturates when the adsorbed monomer abundance reaches that of the exchangeable cations on clay surface. In the experiment18, montmorillonite has 0.8 mmol exchangeable cations per gram, and it starts to saturate at an aqueous monomer concentration of ~ 0.01 M (=mol/L). At the saturated clay-phase monomer concentration, the reaction rate is κ ~ 1 h−1, and thus pr ~ 1 is reached within a few hours, which is much shorter than the hydrolysis time scale of activated monomers. Aqueous monomer concentration needs to be higher than a certain level to keep κ large enough for pr ~ 1, and this may be achieved at some points during a cycle, for example, by variable amount of water expected in dry-wet cycles around warm little ponds29.
Probability of an Active RNA Formation in the Universe
Let lmin be the minimum length of an RNA that needs to be abiotically formed for an emergence of life, and suppose that a lmin-nt long, randomly polymerized RNA molecule acquires the necessary activity with a probability Pac by a correct informational sequence of nucleotides. Once such an active polymer is produced, it proceeds to the stage of Darwinian evolution with a probability Pev, thus completing an abiogenesis process. Then we can calculate the number of abiogenesis events in a region of the universe containing N* stars as
| 3 |
where fpl is the number of habitable planets per star, td the time during which abiotic RNA polymerization cycles continue, and rp the production rate of lmin-nt long RNA polymers on a planet. The production rate by the Poissonian process can be expressed using Eq. 2 in the previous section as
| 4 |
where Nm is the number of activated monomers participating in a cycle of polymerization on a planet, tc the repeating time interval of polymerization cycles, and an approximation of lmin ~ lmin − 1 is used for simplicity. The baseline value of pr is set to unity in the following analysis.
The probability Pac can be expressed as
| 5 |
where Nnb is the number of nucleobase types participating in polymerization, and Nac is the number of active sequences among all the possible sequences of a lmin-nt long RNA polymer. We adopt Nnb = 4 as the baseline from RNA/DNA of life as we know it, but probably this is an underestimate for abiotic polymerization, because regioselectivity, homochiral selectivity, or any other reacting molecules that stop further polymerization would effectively increase Nnb. The parameter Nac is highly uncertain. Here we convert this parameter into Δl (or leff ≡ lmin − Δl) defined by the relation , so that
| 6 |
Considering an example with lmin = 40, there are 440 ~ 1024 possible sequences of 40-mers, and perhaps Nac = 104 sequences out of them may have a replicase activity8, in this case Δl = 6.6. Here we take Δl = 0 as the baseline value, which is valid when Δl ≪ lmin.
Requiring Nlife = 1 and taking a logarithm of Eq. 3, we find the number of stars necessary to expect at least one abiogenesis event in their planetary systems, as
| 7 |
where
| 8 |
and lg and ln are the common and natural logarithms, respectively. We need to determine the five parameters included in C. Obviously there are huge uncertainties, probably more than 10 orders of magnitude in total. However, these parameters appear only logarithmically, and we will find that these uncertainties hardly affect the main conclusions derived in this work.
We use fpl = 0.1 as the baseline for the planet parameter46, which is the least uncertain among these, owing to the rapid development of exoplanet studies in recent years. The baseline for td is set to 0.5 Gyr as a plausible time scale from the birth of Earth to the abiogenesis28, and that for tc is set to 1 yr supposing a seasonal cycle (e.g. ref. 29), though 1 day may also be reasonable for a day-night cycle. The parameter Pev is highly uncertain, but Pev = 1 is set as the baseline, which is optimistic but may not be unreasonable because a long RNA polymer assembled by the Poisson process would be rare and there would be no competitor or predator around it. Any other essential factors involved in the origin of life, e.g., encapsulation by membrane vesicle formation, may significantly reduce this parameter. It has been known that both RNA polymerization and vesicle assembly are accelerated on clay surfaces1,4,14.
The amount of monomers, Nm, is probably the most uncertain parameter among the five in C. An upper limit may be estimated by the number of nucleotides in the present life on Earth, Nm = 7 × 1037 (3.7 × 1016 g in mass), which is estimated by the total biomass of 550 Gt-C (3.7 × 1012 wet ⋅ t)47 assuming that nucleic acids constitute 1% of the wet biomass. A rough amount of nucleobases delivered from space by meteorites can be estimated as follows (see ref. 29 for a more detailed modeling). The mass delivery rate of meteoroids from 4.5 to 4.0 Ga is 1020−25 kg/Gyr, and 0.1% of this mass belongs to meteoroids of a diameter 40–80 m, which efficiently deliver nucleobases avoiding melting or vaporization. Carbonaceous meteorites contain nucleobases with a mass fraction of 10−7, and they are deposited into warm little ponds which cover a fraction of 10−6 on the Earth surface. These nucleobases survive for 1 yr, i.e., a seasonal cycle before they are destroyed by UV radiation or seepage. Then we expect 1020−25 nucleobases (0.01–103 g in mass) in the ponds. Instead, nucleobases may also be produced on Earth, and it would not be unreasonable to assume a similar nucleobase/carbon mass ratio to that found in carbonaceous meteorites (10−5). Assuming a carbon abundance similar to the present seawater, we expect 1027 nucleobases in the ponds assuming their depth to be 1 m. We then use Nm = 1025 as the baseline, though it could be wrong by many orders of magnitude, depending on various scenarios of nucleotide formation and their activation under prebiotic conditions. Using the baseline parameter values thus determined, we find lnC = 75.3.
The Minimum RNA Length Versus the Universe Size
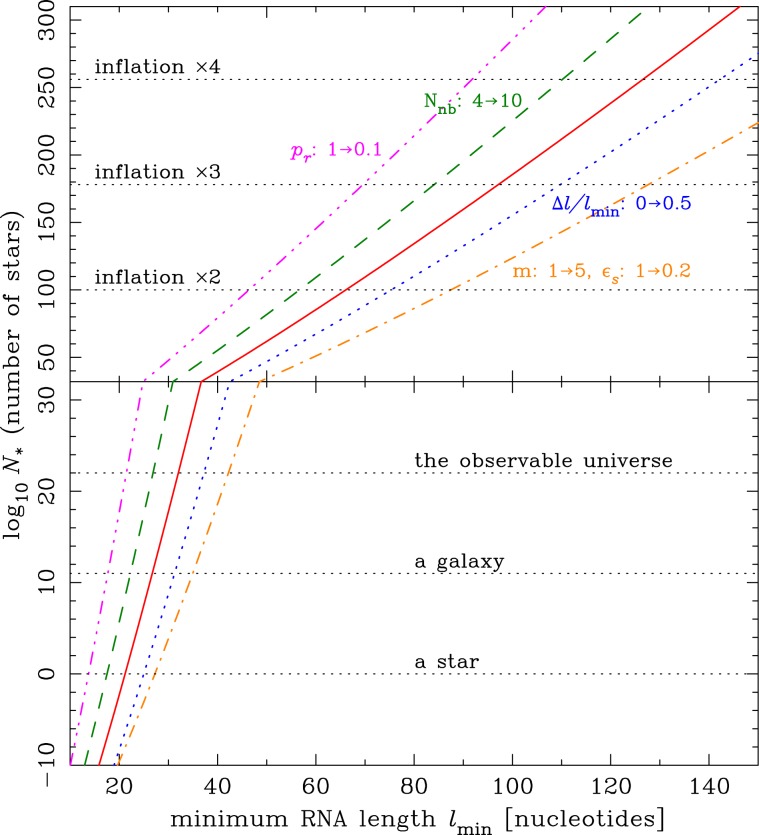
Figure 1 shows lgN* versus lmin for Nlife = 1 calculated by Eq. 7. When the baseline parameter values are used, the minimum RNA length must be lmin = 21, 27 and 32 to expect one abiogenesis event for a survey of a single star (lgN* = 0), a galaxy (lgN* = 11), and the observable universe (lgN* = 22), respectively. These lmin values are not sufficiently large compared with that (~40–100) required to expect an RNA replicase activity from a biological viewpoint, implying that abiogenesis is not easy even if we consider the entire volume of the observable universe. For lmin = 40 we find lgN* = 39. If we try to reduce this to lgN* = 22 or 0 for the same lmin by the uncertainty in C, this parameter needs to be increased by a factor of 1017 or 1039, respectively.
Figure 1.
Logarithm of the number of stars necessary to expect at least one abiogenesis event (lgN*) versus the minimum RNA length required to show a biological activity leading to abiogenesis (lmin). The difference between the top and bottom panels is just the scale of the vertical axis. Some important values of lgN* are indicated by horizontal dotted lines; "inflation ×2” means the universe size when the inflation lasted twice as long as that required to make the observable universe homogeneous. The red solid curve is the relation using the baseline model parameter values, and other curves are when some of the model parameters are changed from the baseline values, as indicated in the figure.
However, if we request one abiogenesis event somewhere in the whole physical volume created by an inflation, the chance of abiogenesis greatly increases. In a volume created by a twice, three, and four times as long inflation as that required to create the observable universe (lgN* = 100, 178, and 256), lmin becomes 66, 97, and 127, respectively. These lmin lengths now allow us to expect a self-replicating activity of an RNA molecule. If an identical pair of RNA strands is required for abiogenesis, lmin should be effectively twice as large as each of the identical strands. Then an inflationary universe can produce a pair with a length of ~33–64 nt for each, and we can still expect a replicase activity. It is also possible that the inflation duration is even longer than the examples considered here.
Using the Stirling’s approximation, Eq. 7 can be written as
| 9 |
when pr = 1 and Δl = 0. In the large limit of lmin, lnNnb − 1 can be neglected, and a useful approximated formula is:
| 10 |
It should be noted that lgN* changes only by 10 when a factor included in C is changed by 10 orders of magnitude; lgN* changes from 167 to 177 for lmin = 100 for example, which hardly affects the main conclusion of this work.
Figure 1 also shows the lgN*-lmin relation when some model parameters are changed. The main results described above are not seriously changed when we change Nnb = 4 → 10 or Δl∕lmin = 0 → 0.5. If we reduce pr from 1 to 0.1, lmin is reduced from 66 to 46 for lgN* = 100 (a twice as long inflation). A sufficient number of abiogenesis events may not be expected when pr ≪ 1, even in the total volume of an inflationary universe.
A possibly important process is polymerization over multiple cycles. In polymerization on clay surfaces, inactive monomers and oligomers left from the previous cycle must be released from a clay surface for the next cycle to work, but a fraction of long oligomers may remain on the surface. Adding newly activated monomers to such oligomers over many cycles may be an efficient way to assemble a long polymer. Such a polymerization process may be limited by a time scale of RNA oligomer destruction, e.g., by hydrolysis or UV radiation during the dry phase. As a toy model to consider this, suppose that a fraction ϵs of oligomers survive to the next cycle. If polymerization of an oligomer continues over m cycles, the most efficient path to form a lmin-nt polymer would be to repeat m times the process of adding lmin∕m monomers. Then the polymer production rate rp of Eq. 4 should be replaced by
| 11 |
The result for m = 5 and ϵs = 0.2 is shown in Fig. 1 as an example, using the Gamma function for the factorial when lmin/m is not an integer. In this case lmin becomes 42 for lgN* = 22, implying a possibility that abiogenesis has occurred more than once inside the observable universe. Though m and ϵs are highly uncertain, this possibility should not be overlooked.
Conclusions
It has been shown that the first RNA polymer with a replicase activity can be abiotically assembled by the most conservative polymerization process, i.e., random Poissonian adding of monomers, if we require that it occurs more than once somewhere in the physical volume of a universe created by an inflation, rather than inside the observable universe for us. This gives a simple solution to the abiotic polymerization problem to initiate the RNA world. Equation 7 relates two quantities on vastly difference scales: lgN* on an astronomical scale and lmin on a biologically microscopic scale, and uncertainties of other parameters are not important because most of them appear logarithmically. This reminds us of an ouroboros.
The result of this work may also give an explanation for the homochirality of life. Even if activated monomers supplied to the polymerization cycle are a racemic mixture, life emerging from them would be homochiral, if homochirality is a necessary requirement for an RNA polymer to show biological activities. Simply it needs more time or volume for a homochiral polymer to be assembled by random polymerization, with Nnb twice as large as when ignoring chirality. As shown in Fig. 1, change of Nnb by a factor of two does not seriously affect the expected number of abiogenesis events in an inflationary universe.
On the other hand, the expected number of abiogenesis events is much smaller than unity when we observe a star, a galaxy, or even the whole observable universe. This gives an explanation to the Fermi’s paradox. The observable universe is just a tiny part, whose volume is likely smaller than 1∕1078 of the whole universe created by an inflation, and there is no strong reason to expect more than one abiogenesis event in such a small region. Even if Earth is the only planet that harbors life inside the observable universe, it does not contradict the Copernican principle, because life would have emerged on countless planets in the whole inflationary universe in which we exist.
In the picture presented here, the probability of finding biosignatures from planets or satellites in the Solar System or from exoplanets is negligibly small, unless we consider interplanetary or interstellar traveling of microorganisms48,49. It should be noted, however, that the case of a high abiogenesis rate (Nlife ≳ 1 for N* = 1) cannot be excluded by this work, because we assumed that abiotic RNA polymerization occurs only by the random Poisson process of adding monomers. Potential roles of much more efficient processes on the origin of life, such as non-linear auto- or cross-catalytic reactions, have been studied theoretically50, though it is highly uncertain whether such processes really worked in realistic prebiotic conditions. If organisms having a different origin from those on Earth are found in future, it would suggest that such a mechanism is working at abiogenesis to reduce lmin. Although this possibility should not be excluded, what is shown by this work is that such a hypothetical process is not necessary if we request abiogenesis events to occur somewhere in an inflationary universe.
It is also worth pointing out that, in the lgN*-lmin relation for Nlife = 1, lgN* rapidly increases from 0 (a star) to 22 (the observable universe) in a short range of lmin = 21–32. Even if a non-linear process is working at some stages, the initial polymerization is likely statistical and random as considered here. Then it would be an extreme fine tuning if a biological parameter lmin is just close to the value corresponding to Nlife ~ 1 for a star (N* = 1). Rather, Nlife ≫ 1 or Nlife ≪ 1 is much more likely when we observe just one planetary system. As we have argued, the case of Nlife ≪ 1 is not in contradiction with observations, but the opposite case may be in tension with the lack of evidence for multiple abiogenesis events in the history of Earth or in laboratories.
A fundamental assumption in this work is that an abiotically assembled RNA polymer acquires a self-replicating ability if it is sufficiently long and has a correct nucleotide sequence. This may be rather trivial under the physical laws ruling this universe, because we know that ribozymes are actually working in life and can also be produced by in vitro experiments. This work considered only a single homogeneous region in the universe created by an inflation event, obeying the same physical laws that we observe. However, the multiverse hypothesis51 implies existence of other universes created by different inflation events, in which physical laws may be different from ours. A theoretically intriguing question is whether a chemical RNA-like long polymer is easily formed to contain information and show biological activities eventually leading to higher organisms, when physical laws are arbitrarily made, e.g., by random choices of fundamental physical constants. Perhaps this may be the ultimate mystery regarding the origin of life, which is, of course, far beyond the scope of this work.
Acknowledgements
The author was supported by the JSPS/MEXT KAKENHI Grant Numbers 18K03692 and 17H06362.
Author contributions
T.T. performed all work for this paper.
Competing interests
The author declares no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ruiz-Mirazo K, Briones C, de la Escosura A. Prebiotic systems chemistry: new perspectives for the origins of life. Chem. Rev. 2014;114:285–366. doi: 10.1021/cr2004844. [DOI] [PubMed] [Google Scholar]
- 2.Lanier KA, Williams LD. The Origin of Life: Models and Data. J. Mol. Evol. 2017;84:85–92. doi: 10.1007/s00239-017-9783-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szostak JW. The Narrow Road to the Deep Past: In Search of the Chemistry of the Origin of Life. Angew. Chem. Int. Ed. Engl. 2017;56:11037–11043. doi: 10.1002/anie.201704048. [DOI] [PubMed] [Google Scholar]
- 4.Kitadai Norio, Maruyama Shigenori. Origins of building blocks of life: A review. Geoscience Frontiers. 2018;9(4):1117–1153. doi: 10.1016/j.gsf.2017.07.007. [DOI] [Google Scholar]
- 5.Yamagishi, A., Kakegawa, T. & Usui, T. (eds.) Astrobiology: From the Origins of Life to the Search for Extraterrestrial Intelligence (Springer, 2019).
- 6.Gilbert W. Origin of life: The RNA world. Nature. 1986;319:618–618. doi: 10.1038/319618a0. [DOI] [Google Scholar]
- 7.Orgel LE. Prebiotic chemistry and the origin of the RNA world. Crit. Rev. Biochem. Mol. Biol. 2004;39:99–123. doi: 10.1080/10409230490460765. [DOI] [PubMed] [Google Scholar]
- 8.Robertson, M. P. & Joyce, G. F. The origins of the RNA world. Cold Spring Harb Perspect Biol4:a003608 (2012). [DOI] [PMC free article] [PubMed]
- 9.Szostak, J. W. & Ellington, A. D. In vitro selection of functional RNA sequences. In Gesteland, R. F. and Atkins, J. F. (eds.) The RNA world : the nature of modern RNA suggests a prebiotic RNA world, chap. 20, 511–533 (Cold Spring Harbor, NY, USA, 1993).
- 10.Johnston WK, Unrau PJ, Lawrence MS, Glasner ME, Bartel DP. RNA-catalyzed RNA polymerization: accurate and general RNA-templated primer extension. Science. 2001;292:1319–1325. doi: 10.1126/science.1060786. [DOI] [PubMed] [Google Scholar]
- 11.Horning DP, Joyce GF. Amplification of RNA by an RNA polymerase ribozyme. Proc. Natl. Acad. Sci. USA. 2016;113:9786–9791. doi: 10.1073/pnas.1610103113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wachowius F, Holliger P. Non-enzymatic assembly of a minimized RNA polymerase ribozyme. ChemSystemsChem. 2019;1:12–15. doi: 10.1002/syst.201900004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cafferty BJ, Hud NV. Abiotic synthesis of RNA in water: a common goal of prebiotic chemistry and bottom-up synthetic biology. Curr. Opin. Chem. Biol. 2014;22:146–157. doi: 10.1016/j.cbpa.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Ferris JP. Montmorillonite-catalysed formation of RNA oligomers: the possible role of catalysis in the origins of life. Philos. Transactions Royal Soc. B: Biol. Sci. 2006;361:1777–1786. doi: 10.1098/rstb.2006.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cleaves H, II., et al. Mineral-organic interfacial processes: potential roles in the origins of life. Chem. Soc. Rev. 2012;41:5502. doi: 10.1039/c2cs35112a. [DOI] [PubMed] [Google Scholar]
- 16.Huang, W. & Ferris, J. P. Synthesis of 35–40 mers of RNA oligomers from unblocked monomers. a simple approach to the RNA world Chem. Commun. 1458–1459 10.1039/b303134a (2003) [DOI] [PubMed]
- 17.Huang W, Ferris JP. One-step, regioselective synthesis of up to 50-mers of RNA oligomers by montmorillonite catalysis. J. Am. Chem. Soc. 2006;128:8914–8919. doi: 10.1021/ja061782k. [DOI] [PubMed] [Google Scholar]
- 18.Kawamura Kunio, Ferris James P. Kinetic and Mechanistic Analysis of Dinucleotide and Oligonucleotide Formation from the 5'-Phosphorimidazolide of Adenosine on Na+-Montmorillonite. Journal of the American Chemical Society. 1994;116(17):7564–7572. doi: 10.1021/ja00096a013. [DOI] [PubMed] [Google Scholar]
- 19.Joshi PC, Aldersley MF, Delano JW, Ferris JP. Mechanism of montmorillonite catalysis in the formation of RNA oligomers. J. Am. Chem. Soc. 2009;131:13369–13374. doi: 10.1021/ja9036516. [DOI] [PubMed] [Google Scholar]
- 20.Aldersley MF, Joshi PC, Huang Y. The comparison of hydrochloric acid and phosphoric acid treatments in the preparation of montmorillonite catalysts for RNA synthesis. Orig. Life Evol. Biospheres. 2017;47:297–304. doi: 10.1007/s11084-017-9533-6. [DOI] [PubMed] [Google Scholar]
- 21.Burcar BT, et al. Potential pitfalls in MALDI-TOF MS analysis of abiotically synthesized RNA oligonucleotides. Orig. Life Evol. Biospheres. 2013;43:247–261. doi: 10.1007/s11084-013-9334-5. [DOI] [PubMed] [Google Scholar]
- 22.Briones C, Stich M, Manrubia SC. The dawn of the RNA world: Toward functional complexity through ligation of random RNA oligomers. RNA. 2009;15:743–749. doi: 10.1261/rna.1488609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costanzo G, Pino S, Ciciriello F, Mauro ED. Generation of long RNA chains in water. J. Biol. Chem. 2009;284:33206–33216. doi: 10.1074/jbc.m109.041905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morasch M, Mast CB, Langer JK, Schilcher P, Braun D. Dry polymerization of 3′, 5′ -cyclic GMP to long strands of RNA. Chem. Bio. Chem. 2014;15:879–883. doi: 10.1002/cbic.201300773. [DOI] [PubMed] [Google Scholar]
- 25.Lineweaver CH, Davis TM. Does the rapid appearance of life on earth suggest that life is common in the universe? Astrobiology. 2002;2:293–304. doi: 10.1089/153110702762027871. [DOI] [PubMed] [Google Scholar]
- 26.Spiegel DS, Turner EL. Bayesian analysis of the astrobiological implications of life's early emergence on earth. Proc. Natl. Acad. Sci. 2012;109:395–400. doi: 10.1073/pnas.1111694108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J, Kipping D. On the rate of abiogenesis from a bayesian informatics perspective. Astrobiology. 2018;18:1574–1584. doi: 10.1089/ast.2018.1836. [DOI] [PubMed] [Google Scholar]
- 28.Pearce BK, Tupper AS, Pudritz RE, Higgs PG. Constraining the time interval for the origin of life on earth. Astrobiology. 2018;18:343–364. doi: 10.1089/ast.2017.1674. [DOI] [PubMed] [Google Scholar]
- 29.Pearce BKD, Pudritz RE, Semenov DA, Henning TK. Origin of the RNA world: The fate of nucleobases in warm little ponds. Proc. Natl. Acad. Sci. 2017;114:11327–11332. doi: 10.1073/pnas.1710339114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benner, S. A. et al. When did Life Likely Emerge on Earth in an RNA-First Process? ChemSystemsChem1, e1900035 (2019).
- 31.Lingam Manasvi, Loeb Abraham. Role of stellar physics in regulating the critical steps for life. International Journal of Astrobiology. 2019;18(6):527–546. doi: 10.1017/S1473550419000016. [DOI] [Google Scholar]
- 32.Licquia TC, Newman JA. Improved Estimates of the Milky Way’s Stellar Mass and Star Formation Rate from Hierarchical Bayesian Meta-Analysis. The Astrophys. J. 2015;806:96. doi: 10.1088/0004-637x/806/1/96. [DOI] [Google Scholar]
- 33.Rudnick G, et al. The rest-frame optical luminosity density, color, and stellar mass density of the universe from z=0 to z=3. The Astrophys. J. 2003;599:847–864. doi: 10.1086/379628. [DOI] [Google Scholar]
- 34.Sandberg, A.Drexler, E. & Ord, T. Dissolving the Fermi Paradox. arXiv e-prints arXiv:1806.02404 (2018).
- 35.Starobinsky A. A new type of isotropic cosmological models without singularity. Phys. Lett. B. 1980;91:99–102. doi: 10.1016/0370-2693(80)90670-x. [DOI] [Google Scholar]
- 36.Kazanas D. Dynamics of the universe and spontaneous symmetry breaking. The Astrophys. J. 1980;241:L59. doi: 10.1086/183361. [DOI] [Google Scholar]
- 37.Guth AH. Inflationary universe: A possible solution to the horizon and flatness problems. Phys. Rev. D. 1981;23:347–356. doi: 10.1103/physrevd.23.347. [DOI] [Google Scholar]
- 38.Sato K. First-order phase transition of a vacuum and the expansion of the universe. Mon. Notices Royal Astron. Soc. 1981;195:467–479. doi: 10.1093/mnras/195.3.467. [DOI] [Google Scholar]
- 39.Linde A. A new inflationary universe scenario: A possible solution of the horizon, flatness, homogeneity, isotropy and primordial monopole problems. Phys. Lett. B. 1982;108:389–393. doi: 10.1016/0370-2693(82)91219-9. [DOI] [Google Scholar]
- 40.Albrecht A, Steinhardt PJ. Cosmology for grand unified theories with radiatively induced symmetry breaking. Phys. Rev. Lett. 1982;48:1220–1223. doi: 10.1103/physrevlett.48.1220. [DOI] [Google Scholar]
- 41.Koonin EV. The cosmological model of eternal inflation and the transition from chance to biological evolution in the history of life. Biol. Direct. 2007;2:15. doi: 10.1186/1745-6150-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liddle, A. R. & Lyth, D. H.Cosmological Inflation and Large-Scale Structure (Cambridge University Press, 2000).
- 43.Ade PAR, et al. Planck 2015 results XX. Constraints on inflation. Astron. & Astrophys. 2016;594:A20. doi: 10.1051/0004-6361/201525898. [DOI] [Google Scholar]
- 44.Dodelson, S. & Hui, L. Horizon ratio bound for inflationary fluctuations. Phys. Rev. Lett. 91, 131301, 10.1103/physrevlett.91.131301 (2003). [DOI] [PubMed]
- 45.Liddle, A. R. & Leach, S. M., How long before the end of inflation were observable perturbations produced? Phys. Rev. D68, 103503, 10.1103/physrevd.68.103503 (2003).
- 46.Lissauer JJ, Dawson RI, Tremaine S. Advances in exoplanet science from Kepler. Nature. 2014;513:336–344. doi: 10.1038/nature13781. [DOI] [PubMed] [Google Scholar]
- 47.Bar-On YM, Phillips R, Milo R. The biomass distribution on earth. Proc. Natl. Acad. Sci. 2018;115:6506–6511. doi: 10.1073/pnas.1711842115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nicholson WL. Ancient micronauts: interplanetary transport of microbes by cosmic impacts. Trends Microbiol. 2009;17:243–250. doi: 10.1016/j.tim.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 49.Wesson PS. Panspermia, past and present: Astrophysical and biophysical conditions for the dissemination of life in space. Space Sci. Rev. 2010;156:239–252. doi: 10.1007/s11214-010-9671-x. [DOI] [Google Scholar]
- 50.Coveney PV, Swadling JB, Wattis JAD, Greenwell HC. Theory, modelling and simulation in origins of life studies. Chem. Soc. Rev. 2012;41:5430. doi: 10.1039/c2cs35018a. [DOI] [PubMed] [Google Scholar]
- 51.Carr, B. (ed.) Universe or Multiverse? (Cambridge University Press, 2009).