Abstract
Community structure of many systems changes across space in many different ways (e.g., gradual, random or clumpiness). Accessing patterns of species spatial variation in ecosystems characterized by strong environmental gradients, such as estuaries, is essential to provide information on how species respond to them and for identification of potential underlying mechanisms. We investigated how environmental filters (i.e., strong environmental gradients that can include or exclude species in local communities), spatial predictors (i.e., geographical distance between communities) and temporal variations (e.g., different sampling periods) influence benthic macroinfaunal metacommunity structure along salinity gradients in tropical estuaries. We expected environmental filters to explain the highest proportion of total variation due to strong salinity and sediment gradients, and the main structure indicating species displaying individualistic response that yield a continuum of gradually changing composition (i.e., Gleasonian structure). First we identified benthic community structures in three estuaries at Todos os Santos Bay in Bahia, Brazil. Then we used variation partitioning to quantify the influences of environmental, spatial and temporal predictors on the structures identified. More frequently, the benthic metacommunity fitted a quasi-nested pattern with total variation explained by the shared influence of environmental and spatial predictors, probably because of ecological gradients (i.e., salinity decreases from sea to river). Estuarine benthic assemblages were quasi-nested likely for two reasons: first, nested subsets are common in communities subjected to disturbances such as one of our estuarine systems; second, because most of the estuarine species were of marine origin, and consequently sites closer to the sea would be richer while those more distant from the sea would be poorer subsets.
Subject terms: Community ecology, Community ecology
Introduction
Understanding how community structure of many systems changes across space and how mechanisms, driven mostly by dispersal and environmental filters, determine species distribution patterns in local communities is a central question in community ecology1–3. Testing how community assembly mechanisms determine species distribution has also become important in metacommunity ecology, an offshoot of community ecology, which has emerged to describe processes occurring at local and regional scales1,4. A metacommunity can be defined as a set of local communities potentially, but not necessarily, linked by the dispersal of multiple, likely interacting, species5,6. Assessing processes that affect metacommunity composition particularly in ecosystems characterized by strong environmental gradients is important to provide useful information on species responses to environmental changes across ecological gradients.
To understand patterns of spatial variation in species composition, two different and complementary metacommunity approaches have been proposed7: one focusing on patterns7,8 and another focusing on mechanisms1,9. The pattern-based approach evaluates the characteristics of species distributions along environmental gradients (i.e., random, checkerboard, nested subsets, evenly-spaced, Gleasonian, or Clementsian patterns)7,8 (Table 1). The mechanistic approach considers the roles of niche (i.e., environmental filters and biotic interactions) and dispersal-related processes in determining such metacommunity structures. Both approaches have provided insights into the different processes that structure communities across different ecosystems10–13, but have not been applied along well-defined ecological gradients.
Table 1.
Six idealized structures to identify species distribution among sites. Patterns represent idealized characteristics hypothesized as a result of ecological processes. References indicates early description of these patterns, Description explains each pattern, and Processes is a potentially important ecological or biogeographical cause.
| Pattern | Reference | Description | Processes |
|---|---|---|---|
| Checkerboard | Diamond 1975 | Combinations of mutually exclusive species that occur independently of other pairs along the gradient. | Biotic process may prevent coexistence of particular sets of species that interact antagonistically. |
| Nested subsets | Patterson and Atmar 1986 | Species-poor communities are subsets of species-richer communities. | Species-specific characteristics, such as dispersal ability and tolerance to abiotic conditions. |
| Clementsian | Clements 1916 | Groups of species show similar responses to environmental gradients, which replace each other as a group, and can be classified into distinctive community types. | Biotic process may prevent coexistence of particular sets of species that interact antagonistically. |
| Gleasonian | Gleason 1926 | Communities are structured along some gradient, but species display individualistic responses that yield a continuum of gradually changing composition. | Idiosyncratic responses to abiotic factors, with coexistence resulting from change similarities in requirements or tolerance. |
| Evenly spaced gradients | Tilman 1982 | Species are distributed more uniformly than expected by chance. | Strong interspecific competition. |
| Random | Simberloff 1983 | There are no gradients or other patterns in species distribution among sites. | Indicator of stochastic processes. |
The framework devised by Leibold and Mikkelson8 to identify patterns of metacommunity structure (later expanded7) is based on evaluating three metrics – coherence, turnover and boundary clumping (known as Elements of Metacommunity Structure, herein called EMS) – calculated from a presence–absence matrix8,14. Coherence, the first element of the hierarchical EMS framework, is related to the level at which species respond to the same environmental gradient, while turnover relates to the way species composition changes across communities, and boundary clumping measures the level of distinctiveness of blocks of species. Following coherence, turnover and boundary clumping, it is possible to identify species’ distribution patterns among sites (Fig. 1). Accordingly, EMS analyzes multiple models simultaneously, comparing them against each other to assess which one best fits a particular metacommunity pattern along a single major ordination axis (i.e., a latent environmental gradient) in the data7,8,10,15. Among the idealized structures for identifying species distributions among sites, community structure can change across space in many different ways (e.g., gradual, random, clumpiness) (Table 1). However, EMS indicates but does not directly inform the processes underlying patterns, such as the role of environmental filtering or dispersal effects in metacommunity structuring2,4. Therefore, combining the EMS approach with variation partitioning techniques (environmental and spatial variation) is strongly recommended to assess the main drivers of observed metacommunity structure3. For instance, different functional groups of freshwater benthic invertebrate communities12 may display Clementsian or random patterns (identified by the pattern-based approach) likely due to different causes (identified by the mechanistic approach), respectively environmental heterogeneity and dispersal mode.
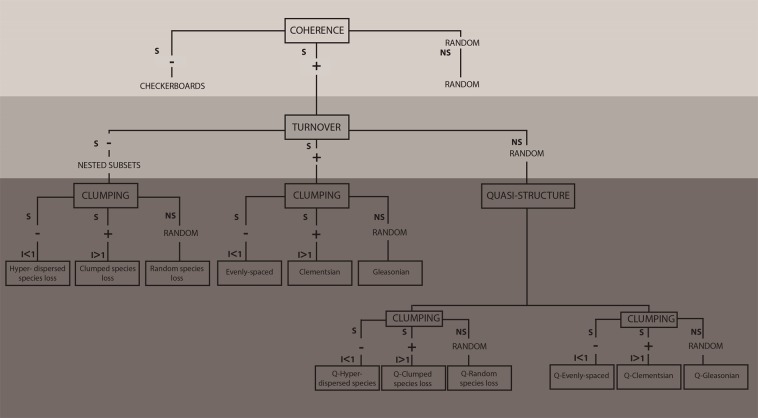
Figure 1.
Schematic representation used to examine how the EMS (i.e., coherence, turnover and boundary clumping) results in six main metacommunity structures (i.e., checkerboards, random, nested, evenly spaced, Gleasonian and Clementsian) and quasi-structures. S = significant; NS = non-significant; “ + ” = positive; “−” = negative; “I” = Morisita’s index value. Modified from Presley et al.7 and Brasil et al.65.
Strong environmental gradients, like salinity gradients in estuaries, may be important metacommunity structure drivers, generating non-random and ecologically meaningful patterns3,10,16. Such gradients can be understood as environmental filters that can include or exclude species, at different sites along the gradient, through species interactions with the physical and chemical characteristics of the environment3,17. Environmental filters may be more pronounced than dispersal limitation or interactions between species in environments with strong environmental gradients. Furthermore, spatial variation plays an important role on the arrangement of environmental gradients and consequently on communities’ final distribution, but there is still a lack of formal tests on strong gradients that include spatial information when analyzing metacommunity structure patterns3,10,12,18. Temporal variation, such as sampling periods, should also be taken into account in metacommunity studies, since communities are not static, but dynamic16,19,20, notably in estuaries21,22. In addition, the relative roles of different local and regional processes in determining community structure and metacommunity pattern remain unclear in estuaries.
Estuarine systems are characterized by their transitional position between marine and freshwater ecosystems, which can generate strong and well-defined gradients (i.e., salinity and sediment grain size)23,24. It is well known that benthic communities play important roles in estuaries and, considering that component species have sedentary or relatively low mobility habits, changes in environmental gradients are frequently detected in benthic organisms resulting in benthic community changes23. Hence, the environmental estuarine gradient along with the benthic community life mode represents an opportunity to explore metacommunity patterns. Also, knowing the type of structure and the drivers shaping metacommunities in estuaries is important for providing information on how species respond to salinity gradients and, consequently, on the underlying mechanisms responsible for the general functioning of these systems21,26,27. Thus, estuaries are highly productive systems and provide several goods and services as they are often used as feeding areas and nursery grounds by various species, and serve as natural pollution filters and storm buffers21,24. However, despite their high ecological and economic importance, estuaries experience a wide array of human impacts, such as increased urbanization and industrialization and altered connection to marine and freshwater systems due to shoreline development25. These influences can compromise their ecological integrity and consequently change the structure of benthic assemblages. Environmental condition changes in systems characterized by strong gradients such as estuaries may reflect alterations in species replacement9,22, and impacts over time might modify the organisms’ ranges along the salinity gradient23, highlighting the importance of knowing how such communities are structured and the main drivers of structure.
We used an empirical framework linking the EMS approach and variation partitioning (environmental, spatial and temporal variation)3 to understand the emergence of benthic macroinfaunal metacommunity structure along salinity gradients in tropical estuaries. Even though benthic species diversity patterns along tropical estuaries21,22 (decreasing from marine to freshwater zones) had challenged previous well-accepted paradigms (i.e., Remane, 193428), metacommunity patterns of estuarine species are still unclear. This study offers insight into the debates on diversity patterns along estuaries through a metacommunity approach. We first expected a Gleasonian pattern (i.e., species displaying individualistic responses producing a continuum of gradually changing composition), since estuarine benthic fauna had been previously associated with ecocline23 and species replacement22 ideas. Thus, we predicted that the benthic metacommunity would be organized according to salinity preferences21–23 following a non-clumped association (i.e., Gleasonian distribution). Due to the strong salinity gradient, we expected environmental filters to be more important in explaining benthic community variation than were spatial and temporal predictors.
Materials and Methods
Study area
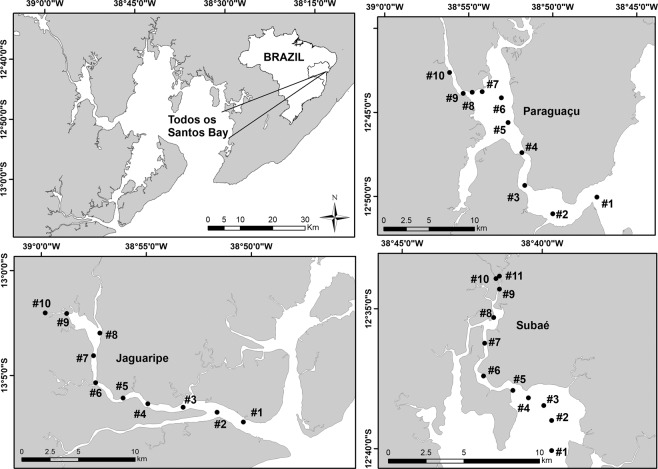
We conducted our study in the estuarine portion of the three main tributaries of Todos os Santos Bay located in Bahia state in Brazil: Paraguaçu (56,300 km2), Subaé (600 km2) and Jaguaripe (2,200 km2) Rivers29 (Fig. 2). Several anthropogenic activities, such as industrial effluents, untreated sewage, urbanization, agriculture, ports and mining activities, have decreased the environmental quality in some specific regions of our study area30. Since we were interested in analyzing the influence of environmental filters on the structure of metacommunities, each estuary studied encompassed the effect of a salinity gradient ranging from approximately 0.5 to 40 along 10 or 11 randomly chosen stations (Fig. 3). Each one of the 10 (Jaguaripe and Paraguaçu) or 11 stations (Subaé) along the salinity gradient had two randomly chosen sites. A total of 270 sites were sampled for all estuarine systems over time. In each estuary, we sampled in a gradient from the most seaward and generally deepest station (i.e., lower-numbered stations in Fig. 2) to the furthest inland and shallowest station (i.e., higher-numbered stations in Fig. 2).
Figure 2.
Map of the study area showing sampled stations (black dots) for each estuary (Subaé, Jaguaripe, and Paraguaçu) at Todos os Santos Bay, in Bahia, Brazil.
Figure 3.
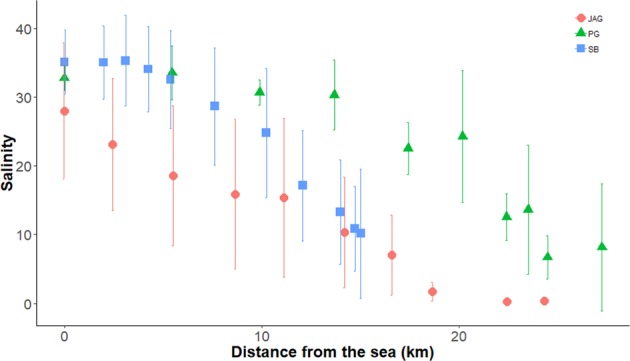
Salinity gradient from sea to freshwater in sampled sites for Jaguaripe (circles), Paraguaçu (triangles) and Subaé (squares) estuaries at Baía de Todos os Santos.
The survey took place over time in different estuaries. The Subaé estuary was sampled in five periods: Jun-2004, Mar-2006, Dec-2009, Apr-2011, and Mar-2013. The Jaguaripe estuary was sampled in four periods: May-2006, Aug-2007, Jul-2010, and Aug-2014. Finally, the Paraguaçu estuary was sampled in four periods: May and Dec-2005, Jun-2011, and Aug-2014.
Sample collection and processing
In the Paraguaçu estuary, the six replicates were collected at each station using a van Veen grab (0.05 m2, 3.2 L). In Subaé and Jaguaripe, at each station eight replicates were collected manually by divers, using corers (10 cm diameter, 0.008 m2, 1.2 L). Core sampling was not suitable in the Paraguaçu River due to the depth at some stations ( > 35 m), very strong water current and zero visibility. For both types of gear, sample infauna was collected from the water–sediment interface to a depth of 15 cm31. All macroinfaunal samples were sieved through a 0.5 mm mesh in the field, preserved in 70% alcohol and taken to the laboratory for further processing and identification. Most invertebrates were identified to family level; family has been shown to be a taxonomically sufficient descriptor of estuarine benthic invertebrates in habitats with strong gradients32 but still provide information about community identities and their temporal drift33. Also, family level was a good choice due to the scarcity of taxonomical studies of the local benthic invertebrates (with several undescribed species) and allowed comparison of the taxon distribution patterns observed in other regions21.
The environmental variables measured included salinity and sediment type (i.e., grain size). Salinity of the superficial water was measured at spring low ebb tides and recorded using a Hydrolab Data Sonde. One sediment sample was collected at each station for grain size analysis, using a 0.05 m2 van Veen grab for Paraguaçu River and a 0.008 m2 corer for Subaé and Jaguaripe. Sediment particle size was determined by standard techniques34. Salinity and each fraction of sediment grain size (i.e., pebble, gravel, very coarse sand, coarse sand, medium sand, fine sand, very fine sand and silt sand clay) were treated as environmental predictors.
Sample adequacy
In order to evaluate whether the benthic macroinfaunal family of each estuary was representatively sampled and to avoid artifactual patterns because the probability of detection of species varied, we calculated the relationship between sampling effort and family richness for each estuary for the total sampling time. The specaccum function and the species accumulation method random were used in the vegan package35 in the R environment36. Sample-based rarefaction is preferable than individual-based rarefaction to account for natural levels of sample heterogeneity in the data37.
A total of 11,328 individuals of benthic invertebrates were sampled, mainly belonging to 144 taxa of Polychaeta, Mollusca and Crustacea. Polychaeta was the most abundant phylum, followed by Mollusca and Crustacea. In spite of differences in the sampling methods (i.e., sampling gear, total area sampled, number of sites and replicates) among estuaries, most of the systems showed a near stabilization of the relationship between number of stations and richness, allowing further analyses (Supplementary Fig. 1).
Elements of metacommunity structure
We used incidence matrices (i.e., presence–absence) to estimate Elements of Metacommunity Structure (EMS). We followed the ‘range perspective’ in our analysis, which is defined by species range turnover and range boundary clumping8 as recommended38. These incidence matrices were subsequently subjected to a reciprocal averaging (also known as Correspondence Analysis, CA), an unconstrained ordination method, which positions sites having similar species composition close to each other and locating species having similar occurrence among the sites close to each other along the ordination axis39. Because we focused only on the first ordination axis, other ordination methods, such as detrended correspondence analysis, will give the same results8. When a single or at least a predominant gradient structures a community data set, the reciprocal averaging arranged matrix will display this gradient effectively.
Coherence, the first EMS metric, is based on calculating the number of embedded absences (i.e., interruptions in species distribution or in the composition of the sites) in the ordinated matrix and then comparing the empirical observed value of embedded absences (EmbAbs) to a null distribution created from simulated matrices with 1,000 iterations7,8. A large number of embedded absences (i.e., EmbAbs significantly larger than expected by chance) suggests negative coherence and leads to a checkerboard distribution of species; non-significant coherence refers to a random metacommunity type; and a small number of embedded absences (i.e., EmbAbs significantly lower than expected by chance) suggests positive coherence related to nestedness, evenly spaced, Gleasonian or Clementsian gradients8 (Fig. 1).
Turnover is evaluated if coherence is significant and positive (Fig. 1). It is measured by the number of times one species replaces another between two sites (i.e., number of replacements) in an ordinated matrix. To do this, the number of empirical replacements (turnover) was compared to the distribution of randomly generated values based on a null model distribution that randomly shifts entire ranges of species8. Significant negative turnover (i.e., replacement significantly lower than expected by chance) refers to nested subsets, while significant positive turnover (i.e., replacement significantly larger than expected by chance) refers to evenly spaced gradients (Gleasonian or Clementsian structures), requiring further analysis of boundary clumping to distinguish among them8. Furthermore, cases where coherence is significant and positive and turnover is non-significant can be regarded as quasi-structures, indicating that the effects of structuring mechanisms are weaker than in idealized structures7 (Fig. 1).
Boundary clumping is analysed using the Morisita’s Index40 and a chi-square test comparing observed and expected distributions of range boundary locations. Non-significant clumping, and values of Morisita’s index that are not different from 1, indicate randomly distributed species loss in nested subsets when turnover is negative or Gleasonian distribution when turnover is positive. Values significantly larger than 1 indicate clumped species loss in nested subsets when turnover is negative or Clementsian distribution when turnover is positive. Values significantly less than 1 indicate hyperdispersed species loss in nested subsets when turnover is negative and an evenly spaced metacommunity type when turnover is positive (Fig. 1).
The significance of coherence and turnover was tested separately using the fixed-proportional null model, where the species richness of each site was maintained (i.e., row sums were fixed), but species frequencies of occurrence (i.e., columns) were filled based on their marginal probabilities. Random matrices were produced by the r1 method using the R package vegan35 for the fixed-proportional null model, which has a more desirable combination of Type I and Type II error properties7 and has been applied successfully15,16,38,41,42. All EMS analyses were done using the metacom package43 in the R environment (version 1.5.0)36.
Nestedness
We performed ‘nestedness metric based on overlap and decreasing fill’ (NODF)44 to accurately identify nestedness along salinity gradients in estuarine benthic communities. There is some criticism about the EMS framework used to investigate idealized metacommunity patterns and especially whether the turnover test is adequate for detecting a nested pattern, as turnover and nestedness are not necessarily exclusive or opposite38,45,46. Schmera et al.46 showed that even though high turnover is frequently related to low nestedness, low turnover does not predict high nestedness. We performed NODF using the oecosimu function from the vegan package35 in the R environment (version 2.0-10)36.
Spatial predictors
We used Principal Components of Neighbour Matrices (PCNM) to generate spatial variables from geographical coordinates (e.g., latitude and longitude) represented as a Euclidean distance matrix47,48. The PCNM technique represents the spatial configuration of sample points using principal coordinates of a truncated geographic distance matrix between sampling sites. We used the resulting PCNM eigenvectors associated with the positive eigenvalues as spatial components in a global test and in a forward selection prior to variation partitioning47,49,50. PCNM analyses were done using the function pcnm in the vegan package35 in the R environment (version 2.0-10)36.
Environmental predictors
We converted environmental data to standardized Z-scores by subtracting each environmental variable from their mean and dividing by their standard deviation. The new standardized variables are thus dimensionless, with a mean of 0 and a standard deviation of 151. In addition, we tested multicollinearity using a variance inflation factor (VIF)52. When the VIF values indicated a high level of collinearity, we removed the predictor with the highest VIF value. We then recalculated VIF and repeated this process until all VIFs were below a pre-selected threshold (VIF < 3)52,53. Standardized Z-score and VIF analyses were done using the functions scale and vif.cca, respectively, in the vegan package35 in the R environment (version 2.0-10)36.
Variation partitioning
We used partial Redundancy Analysis (pRDA)51 to quantify the pure and shared contributions of environmental filters (i.e., salinity and each fraction of sediment grain size), spatial variables (i.e., variables created using PCNM) and time (i.e., sampling occasions) structuring the benthic metacommunity in the estuaries. RDA can be best understood as an extension of multiple regression that has multiple response variables (i.e., species) and a common matrix of predictors (i.e., environmental and spatial predictors)54. pRDA or variation partitioning51 may indicate the relative strength of association between each component and the metacommunity pattern of benthic macroinvertebrates. We expected environmental variables to be the main influencer of benthic metacommunity structure. In situations where environmental gradients determine most of the variation in the living community, the amount of variation in species data explained by environmental variables is fairly high55. We also included sampling period as a temporal predictor in variation partitioning because time may influence community structure, but we did not have specific expectations regarding temporal variation in benthic structures.
Prior to the pRDA, we Hellinger-transformed abundance matrices and report values based on adjusted R2 to provide unbiased estimates of explained variation and valid comparisons between sets of factors for explaining community structure54. Hellinger transformation consists of transforming the site-by-species data into relative values per site by dividing each value by the site sum, and then taking the square root of the resulting values42. It is suitable for community composition data in comparative analysis because it reduces the importance of high species abundance.
We first did a global RDA test to prevent the inflation of Type I error, and only if it was significant proceeded with forward selection using the double-stopping criterion: the usual alpha significance level (p < 0.05) and the adjusted coefficient of multiple determination (R2)49. Each eigenvector was counted as a single predictor since this approach is the most conservative in its penalization of degrees of freedom and adjusted R2 statistics50. We used forward selection to determine the environmental and spatial filters to be used in variation partitioning. Forward selection analyses for spatial and environmental predictors were done using the ordiR2step function in the vegan package35 in the R environment (version 2.0-10)36.
We carried out pRDA using the function varpart in the vegan package35 in the R environment (version 2.0-10)36. We report adjusted R2 and test the significance of the pure environmental, pure spatial and pure temporal components (P < 0.05). The total percentage of variation explained by the model (R2) is partitioned into unique and common contributions of sets of predictors54. It offers a way of dealing with the importance of spatial correlation when observations are not independent, the number of degrees of freedom in the sample is smaller than expected based on the number of observations used in the analysis, and Type I errors increase, leading to incorrect conclusions about the effect of the environment on community structure56. Statistical significance of RDA in global models was based on 999 permutations and assessed at a significance level of 0.05.
Results
Elements of metacommunity structure
The EMS analysis indicated five metacommunity patterns among the six idealized patterns57–62 and the quasi-structures7 (Table 2). We found that the Q-nested (n = 6) metacommunity type structure was the most common followed by nested (n = 3), Q-Clementsian (n = 2), Clementsian (n = 1), and Q-Gleasonian (n = 1) (Supplementary Fig. 2). As expected, for all estuaries the first step of EMS analysis (Fig. 1), indicated that metacommunity structure was positively coherent (P < 0.001). That is, EmbAbs was significantly lower than expected by chance (Table 2) likely due to the salinity gradient (Supplementary Fig. 2). The second EMS step (Fig. 1) revealed that turnover was not significant (P > 0.05) in most cases (9 out of 13), displaying quasi-structures7 and predominantly negative turnover. That is, replacement was lower than expected by chance) (Table 2) (Supplementary Fig. 2). Even though spatial turnover among sites was more often linked to environmental gradients49, these results indicated that benthic macroinfaunal metacommunities did not always follow a species replacement structure. Finally, the boundary clumping third step (Fig. 1), showed that Morisita’s index was higher (P < 0.005) than 1 for most (11) of the cases, indicating positive clumping structures or clumped species loss for the Q-nested and nested structures (Supplementary Fig. 2).
Table 2.
The Elements of Metacommunity Structure results for each estuary (Subaé, Jaguaripe and Paraguaçu). These results were based on the fixed-proportional (r1) null model. Interpretations followed Leibold & Mikkelson8 and Presley et al.7. Abbreviations: embABS = embedded absences; Coh Z = Z-value of coherence; Tur Z = Z-value of turnover; Q = Quasi. Significant p-values are indicated by bold font.
| Estuary Month Year | Coherence | Turnover | Boundary clumping | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| embAbs | Coh Z | P | Sim mean | Sim sd | Turnover | Tur Z | P | Sim mean | Sim sd | Index | P | df | Interpretation | |
| Subaé 03 2013 | 95 | 6.52 | < 0.001 | 179 | 13 | 921 | −0.37 | 0.716 | 875 | 126 | 1.04 | 0.267 | 8 | Q-Gleasonian |
| Subaé 04 2011 | 94 | 3.14 | < 0.001 | 131 | 12 | 537 | 1.10 | 0.271 | 658 | 111 | 1.41 | 0.008 | 8 | Q-nested* |
| Subaé 12 2009 | 101 | 7.58 | < 0.001 | 280 | 24 | 886 | 6.01 | < 0.001 | 2228 | 2229 | 4.29 | 0.001 | 8 | Nested* |
| Subaé 03 2006 | 57 | 7.26 | < 0.001 | 196 | 19 | 962 | 2.94 | < 0.001 | 1296 | 117 | 1.20 | 0.107 | 8 | Nested# |
| Subaé 06 2004 | 36 | 8.54 | < 0.001 | 130 | 11 | 911 | 1.55 | 0.123 | 1095 | 119 | 4.00 | 0.001 | 7 | Q-nested* |
| Jaguaripe 08 2014 | 85 | 1.06 | < 0.001 | 210 | 12 | 2103 | −0.27 | 0.787 | 2024 | 290 | 2.08 | 0.001 | 7 | Q-Clementsian |
| Jaguaripe 07 2010 | 104 | 6.07 | < 0.001 | 193 | 15 | 1150 | 1.25 | 0.204 | 1374 | 179 | 1.73 | 0.009 | 7 | Q-nested* |
| Jaguaripe 08 2007 | 98 | 6.79 | < 0.001 | 184 | 13 | 1058 | 0.36 | 0.721 | 1113 | 156 | 1.74 | 0.008 | 7 | Q-nested* |
| Jaguaripe 05 2006 | 35 | 5.71 | < 0.001 | 749 | 7 | 339 | −1.10 | 0.270 | 287 | 47 | 1.74 | 0.002 | 6 | Q-Clementsian |
| Paraguaçu 08 2014 | 263 | 1.01 | < 0.001 | 392 | 13 | 3895 | 0.99 | 0.320 | 4270 | 378 | 1.36 | 0.001 | 6 | Q-nested* |
| Paraguaçu 06 2011 | 130 | 1.18 | < 0.001 | 288 | 14 | 3481 | 0.37 | 0.715 | 3651 | 469 | 1.95 | 0.003 | 7 | Q-nested* |
| Paraguaçu 12 2005 | 91 | 1.13 | < 0.001 | 253 | 15 | 2195 | 258 | < 0.001 | 2852 | 255 | 1.99 | 0.008 | 7 | Nested# |
| Paraguaçu 05 2005 | 89 | 1.01 | < 0.001 | 229 | 14 | 1886 | −2.02 | < 0.05 | 1527 | 177 | 1.35 | 0.001 | 7 | Clementsian |
Nestedness
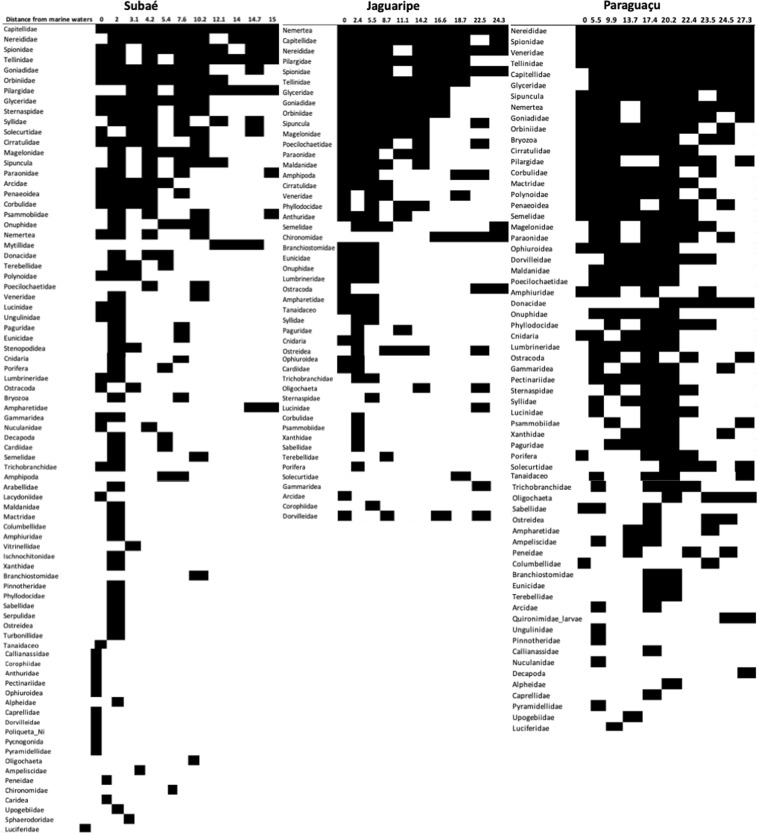
NODF results suggested that in Subaé and Paraguaçu systems, benthic macroinfaunal metacommunity followed an intermediate nested distribution for while the Jaguaripe system had a highly nested structure (Supplementary Table 1). Taxa vs. sites occurrence resulting from the overall NODF analysis based on incidence matrices along salinity gradients for Todos os Santos Bay estuarine systems showed that most of the taxa found follow Q-nested and nested species composition (Fig. 4).
Figure 4.
Taxa vs. sites, incidence matrices along the salinity gradient at the sampled stations for Jaguaripe, Paraguaçu and Subaé estuaries after ordination according to occurrence resulting from the overall NODF analysis. Black squares indicates the presence of a taxon, while white squares indicates the absence of a taxon along the salinity gradient indicated as distance to marine waters (km).
Variation partitioning
The RDA used in the spatial global model was significant for all estuaries (Subaé: adjusted R2 = 0.28, P < 0.001; Jaguaripe: adjusted R2 = 0.48, P < 0.001; Paraguaçu: adjusted R2 = 0.26, P < 0.001), so forward selection was carried out to select spatial variables among PCNM eigenvectors associated with the positive eigenvalues before variation partitioning (Table 3).
Table 3.
Environmental and spatial factors selected through forward selection, after checking multicollinearity using variance inflation factor (VIF) for each variable, during the sampled periods in the three main tributaries (Subaé, Jaguaripe and Paraguaçu) at Todos os Santos Bay.
| Estuarine system | Spatial selected variables | Environmental selected variables | VIF |
|---|---|---|---|
| Subaé | PCNM 1, 3, 2, 5 | Salinity | 1.72 |
| Medium sand | 1.81 | ||
| Fine sand | 1.29 | ||
| Pebble | 1.39 | ||
| Jaguaripe | PCNM 1, 2 | Coarse sand | 1.92 |
| Salinity | 1.70 | ||
| Very fine sand | 1.47 | ||
| Medium sand | 1.50 | ||
| Paraguaçu | PCNM 1, 3, 2 | Fine sand | 1.78 |
| Salinity | 2.21 |
The multicollinearity test for environmental predictors for all systems did not show a high VIF value for salinity even before dropping the predictor with the highest VIF value, indicating that there was no problem of multicollinearity among salinity and other predictors. However, sediment predictors showed a high VIF value and the predictor with highest value (VIF < 3) was removed for each system (Subaé: coarse sand, granular gravel, and silt/clay; Jaguaripe: fine sand, very coarse sand, and silt/clay; Paraguaçu: coarse sand, granular gravel and silt/clay). The remaining variables had a VIF value smaller than the threshold (Supplementary Table 2). The environmental global model was significant (Subaé: adjusted R2 = 0.24, P = 0.001; Jaguaripe: adjusted R2 = 0.38, P = 0.001; Paraguaçu: adjusted R2 = 0.25, P = 0.001), so forward selection of environmental variables was also carried out to select environmental filters after the removal of collinear explanatory variables (Table 3).
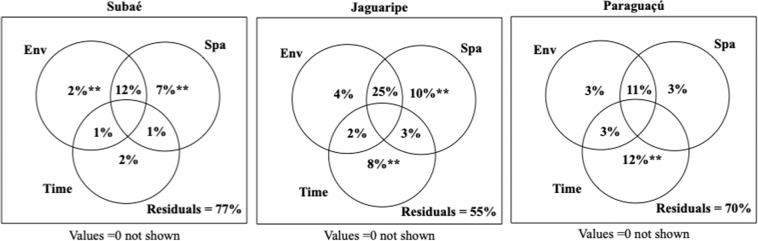
The shared influence of environmental and spatial predictors explained a high proportion of benthic metacommunity structure (Fig. 5). For example, in the Subaé estuary, the shared influence of environmental and spatial predictors explained 12% of the variance, and spatial factors alone explained 7% (Fig. 5). Similarly, shared environmental and spatial predictors explained 25% and spatial predictors explained 10% in the Jaguaripe estuary. (Fig. 5). Finally, in the Paraguaçu estuary, temporal components explained 12% of metacommunity structure followed by 11% explained by shared environmental and spatial components (Fig. 5). Purely environmental, purely spatial and purely temporal predictors were significant (P < 0.01) influences in all three estuaries (Supplementary Table 3).
Figure 5.
Explained proportion of variance partitioning for each estuarine system (Subaé, Jaguaripe and Paraguaçu): the effects related to environmental filters (Env), those related to spatial patterns (Spa), those resulting from temporal patterns (Time), shared influence of environmental filters and spatial descriptors (Env + Spa), environmental filters and temporal patterns (Env + Time), and spatial descriptors and temporal patterns (Spa + Time). Numbers indicate the explained proportion of variation partitioning for each estuarine system; values = 0 are not shown. **P < 0.01 (Supplementary Table 3).
Discussion
We used EMS combined with a variation partitioning techniques to identify the relationships between environmental, spatial and temporal predictors structuring benthic metacommunities in estuarine systems. However, our prediction that benthic macroinfaunal metacommunities would follow a non-clumped associations characterized by a continual change in species composition along environmental gradients without the formation of discrete assemblages (i.e., Gleasonian distribution) was not supported. Instead, we found Q-nested and nested species composition with clumped species loss as the most frequent patterns. We also did not corroborate our prediction of the higher importance of pure environmental filters, but found a higher importance of the shared fraction between environment and space influencing benthic metacommunity. Therefore, in this system benthic communities along estuaries were generally subsets of a large pool of species (i.e. nested or Q-nested) and with space, salinity and sediment size were most strongly associated with this pattern.
Even though we observed six metacommunity types7, the overall metacommunity fitted Q-nested (Q-clumped species loss) or nested subsets (clumped species loss). Nestedness may arise when sites with lower species richness are subsets of richer sites as a result of environmental conditions of the habitats or species-specific characteristics, such as dispersal ability or tolerance of abiotic conditions62. Nested structures are not rare, and they have already been reported for aquatic metacommunities10,15,20,41,63. Most species found in estuarine systems have marine origin and diversification23, so the sites closer to the sea are richer while the sites closer to freshwater have poorer subsets, as fewer estuarine species can arrive and/or survive in such conditions (e.g., lower salinity and depth).
Some taxa, like polychaetes from the families Nereididae and Capitellidae, and also Tellinidae mollusks, showed a wide distribution along all estuarine systems (Fig. 4). However, Chironomidae (Insecta) and Oligochaeta, for example, are adapted to freshwater systems and occurred only at the sites farthest from the sea (Fig. 4). This partially explains nestedness being not so strong (Q-nested) and the distinct patterns found on different sampling occasions. Another important consideration is that, at timescales of months and years, the same taxa might migrate up or down the estuarine gradient in order to physiologically couple with environmental variability.
The emergence of a Q-Gleasonian gradient occurred only for one sampling period for the Subaé estuary. The upper zone (more freshwater) of this estuary is well known for its inorganic pollution in the sediments64.Given the high importance of the strong salinity gradient in estuarine systems, we expected the dominance of Gleasonian patterns at metacommunity level for all estuarine systems, as a result of species having differential responses to the environmental gradients. Nevertheless, it seems that a nestedness situation, where most of the taxa can live in more salty regions (richer sites) and some of them will tolerate different levels of freshwater, is predominant. The emergence of a Clementsian structure for one sampling period for the Paraguaçu estuary and a Q-Clementsian structure for two sampling periods for the Jaguaripe estuary was also unexpected. The Clementsian gradient may be related to historical biogeographic features such as the process of communities’ isolation and/or environmental variation65 showing that sets of species respond similarly to environmental variation, but the occurrence of environmental stochastic stress zones lead to clumped boundaries. Moreover, our study clearly shows that whenever metacommunity patterns are under investigation it is imperative to have replicates in time and space at landscape level (e.g., different estuaries, lakes, rivers etc sampled at different times) because such patterns are dynamic.
We observed a high amount of benthic metacommunity variation explained by the shared influence of environmental filters and spatial predictors for all estuaries. However, since the variation was not exclusively caused by spatial variables, we can still argue that environmental filters (i.e. salinity and sediment) are important in shaping benthic metacommunity structure3. Spatial predictors, measured as geographical distance, were considered to play a significant role in the similarity of species compositions between sites1. Accordingly, environmental variables in estuaries, especially salinity, were spatially structured, which may explain why the highest proportion of total variance was explained by the shared influence of environmental and spatial predictors (Fig. 5). Salinity decreases according to distance from sea to river (Fig. 3), and consequently may affect benthic metacommunity structure from high species and feeding-guild diversities to dominance by a single species or a feeding group27 and decrease in diversity at family level21,22.
Communities change dynamically in richness and composition over time, and consequently the underlying mechanisms are not static over time either4,19,20,41. Unlike the other systems, the Paraguaçu estuary had two sample campaigns in the same year representing two different seasons and it was the system in which variation partitioning showed temporal predictors as the most important component for explaining total variation. There is also a possibility that freshwater inflows into the Paraguaçu estuary may have greater variability than would naturally occur because of construction of the Pedra do Cavalo Dam during the 1980s and the implementation of the Pedra do Cavalo Hydroeletric Power Plant for energy generation in 200566. Our results suggest that potential differences between those metacommunity structures (e.g. Paraguaçu Dec-2005 nested vs. Paraguaçu May-2005 Clementsian) and temporal components are worth additional research. We strongly suggest that future studies should include hypotheses explicitly related to temporal variation (i.e., seasons, drought/flood) with specific changes in metacommunity patterns.
For the same estuaries sampled in this study, Barros et al.21 found a decrease in diversity of benthic macroinfaunal assemblages, at family level, from marine to freshwater zones. Likewise, Barros et al.22 showed that α-diversity decreased along marine to freshwater conditions, while β-diversity was driven by replacement or nestedness depending on the level and distribution of disturbances in estuaries subjected to anthropogenic stressors. Both studies contradict the most popular estuarine model, the Remane model28, which suggests a diversity minimum zone called the arteminimum.
Contrastingly, environmental disturbances may result in environmental homogenization due to high dispersal, which can result in homogenization of metacommunities, increasing nestedness and decreasing species replacement65,67. The Subaé estuary is well-known to be impacted by human activity, with high levels of inorganic contaminants in the upper estuary30 and showed a nested pattern for four sampling periods out of five (Table 2). Jaguaripe and Paraguaçu estuaries also had a decrease in concentrations of contaminants seawards, but they are considered relatively well conserved. The less disturbed Jaguaripe estuary, displayed less nestedness (in two out of four sampling periods) than Paraguaçu (three out of four) (Table 2). Nestedness was more often found in the Subaé estuary, where the upper region (contaminated) is poorer in species richness compared to the lower region, likely accentuating the nestedness pattern from sea to freshwater.
There is some criticism about the EMS framework used to investigate idealized metacommunity patterns and especially whether the turnover test is adequate for detecting nested patterns, as turnover and nestedness are not necessarily mutually exclusive or opposite38,45,46. Nestedness can be measured using various metrics26, such as NODF, that may not be directly comparable to the one used in the context of the EMS framework. Consequently, results based on EMS to evaluate nestedness may be inconsistent if compared to these indices, which ordinate matrices based on richness of sites and species incidence. However, the reciprocal averaging method used in the EMS analysis for nested subsets discerns inter-site variation in response to a latent environmental gradient enhancing the association of mechanisms with nested structures and the form of species loss7. Also, if EMS are studied for a wide range of taxa and locations as in this study, general associations may emerge between particular idealized patterns of distribution and specific taxa38.
Since nestedness is among the non-random distribution patterns related to species-specific characteristics such as dispersal ability, habitat specialization and tolerance to abiotic conditions, future studies should integrate temporal dynamics, spatial predictors and environmental filters with dispersal traits and disturbances in the EMS approach. Dispersal mode is a regional process considered a strong driver for benthic metacommunity distribution pattern4,11,12,19,67 as more dispersive species are more controlled by the environment than less dispersive species60. Considering that many natural systems are subjected to human impacts, such as the upper region of the Subaé estuary, integrating the knowledge of how local (i.e., environmental filters, geographical distance) and regional (i.e., dispersal mode) processes structure natural systems may improve management.
Estuaries are strongly impacted by human activities, in addition to natural stressors, which can affect assemblage structure affecting the functioning of these important systems26,68. Knowing the type of structure and the drivers that shape metacommunities along different estuarine gradients and how that structure changes over time is important for future studies aiming at conservation to help to establish effective conservation policies3,26. Our study indicated that benthic metacommunities follow Q-nested and nested structures and are highly influenced by the shared influence of environmental and spatial predictors. We identified many common taxa occurring across all salinity gradients (e.g., Capitelllidae and Nereididae) as well as the most habitat-specialist taxa, with occurrence only in more freshwater sites (e.g., Chironomidae and Oligochaeta). More importantly, by studying a strong environmental gradient at different times we showed that metacommunity patterns will differ since environmental conditions will vary. We believe our study advances the knowledge on how estuarine benthic communities are structured. We showed that salinity and proximity (space) are major drivers and also highlighted the importance of spatial and temporal replication whenever investigating stronger ecological gradients. Future studies should incorporate functionality and explicit time-related hypotheses.
Supplementary information
Supplementary Dataset - Environmental Data.
Supplementary Dataset - Spatial Coordinates.
Acknowledgements
The authors thank the Programa de Pós-Graduação em Ecologia: Teoria, Aplicação e Valores of the Universidade Federal da Bahia and Instituto Kirimurê Baía de Todos os Santos for their support and facilities. ATA is thankful to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), DKP is grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the post-graduate scholarships, FB was supported by CNPq (PQ 306332/2014-0; 304907/2017-0). We gratefully acknowledge Eduardo Mariano Neto for helping with suggestions on an early version of this manuscript. We thank Pavel Dodonov and Juliana Deo Dias for insightful comments. We thank Daniel Evangelista Santos for helping with the schematic in Fig. 1 and Antônio Carlos Dórea Pereira Filho for helping with the map of the study area. We also thank the Laboratório de Ecologia Bentônica team involved in the fieldwork and laboratory analyses of the data used in this paper.
Author contributions
A.T.A. and F.B. conceived the ideas; F.B. carried out field and lab work and supervision of all stages; A.T.A. and D.K.P. performed statistical analyses; A.T.A. analysed the results; A.T.A. wrote the first draft of the manuscript, and coordinated revisions. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-58631-1.
References
- 1.Leibold MA, et al. The metacommunity concept: a framework for multi-scale community ecology. Ecol. Lett. 2004;7:601–613. doi: 10.1111/j.1461-0248.2004.00608.x. [DOI] [Google Scholar]
- 2.Logue JB, et al. Empirical approaches to metacommunities: a review and comparison with theory. Trends Ecol. Evol. 2011;26:482–491. doi: 10.1016/j.tree.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Gascón S, et al. Environmental filtering determines metacommunity structure in wetland microcrustaceans. Oecologia. 2016;181:193–205. doi: 10.1007/s00442-015-3540-y. [DOI] [PubMed] [Google Scholar]
- 4.Erős T, Takács P, Specziár A, Schmera D, Sály P. Effect of landscape context on fish metacommunity structuring in stream networks. Freshw. Biol. 2017;62:215–228. doi: 10.1111/fwb.12857. [DOI] [Google Scholar]
- 5.Gilpin ME, Hanski IA. Metapopulation Dynamics: Empirical and Theoretical Investigations. London: Academic Press; 1991. [Google Scholar]
- 6.Wilson DS. Complex interactions in metacommunities, with implications for biodiversity and higher levels of selection. Ecol. 1992;73:1984–2000. doi: 10.2307/1941449. [DOI] [Google Scholar]
- 7.Presley SJ, Higgins CL, Willig MR. A comprehensive framework for the evaluation of metacommunity structure. Oikos. 2010;119:908–917. doi: 10.1111/j.1600-0706.2010.18544.x. [DOI] [Google Scholar]
- 8.Leibold MA, Mikkelson GM. Coherence, species turnover, and boundary clumping: elements of meta-community structure. Oikos. 2002;97:237–250. doi: 10.1034/j.1600-0706.2002.970210.x. [DOI] [Google Scholar]
- 9.Cottenie K. Integrating environmental and spatial processes in ecological community dynamics. Ecol. Lett. 2005;8:1175–1182. doi: 10.1111/j.1461-0248.2005.00820.x. [DOI] [PubMed] [Google Scholar]
- 10.Henriques-Silva R, Lindo Z, Peres-Neto PR. A community of metacommunities: exploring patterns in species distributions across large geographical areas. Ecol. 2013;94:627–639. doi: 10.1890/12-0683.1. [DOI] [PubMed] [Google Scholar]
- 11.Petsch DK, Pinha GD, Dias JD, Takeda AM. Temporal nestedness in Chironomidae and the importance of environmental and spatial factors in species rarity. Hydrobiologia. 2015;745:181–93. doi: 10.1007/s10750-014-2105-0. [DOI] [Google Scholar]
- 12.Petsch DK, Pinha GD, Takeda AM. Dispersal mode and flooding regime as drivers of benthic metacommunity structure in a Neotropical floodplain. Hydrobiologia. 2017;788:131–141. doi: 10.1007/s10750-016-2993-2. [DOI] [Google Scholar]
- 13.da Silva FR, Rossa-Feres DC. Fragmentation gradients differentially affect the species range distributions of four taxonomic groups in semi-deciduous Atlantic forest. Biotropica. 2017;43:283–292. doi: 10.1111/btp.12362. [DOI] [Google Scholar]
- 14.Dallas T. Metacom: an R package for the analysis of metacommunity structure. Ecography. 2014;37:402–405. doi: 10.1111/j.1600-0587.2013.00695.x. [DOI] [Google Scholar]
- 15.Heino J, et al. Elements of metacommunity structure and community- environment relationships in stream organisms. Freshw. Biol. 2015;60:973–988. doi: 10.1111/fwb.12556. [DOI] [Google Scholar]
- 16.Erős T, et al. Quantifying temporal variability in the metacommunity structure of stream fishes: the influence of non-native species and environmental drivers. Hydrobiologia. 2014;722:31–43. doi: 10.1007/s10750-013-1673-8. [DOI] [Google Scholar]
- 17.Otegui MBP, Brauko KM, Pagliosa PR. Matching ecological functioning with polychaete morphology: Consistency patterns along sedimentary habitats. J. Sea Res. 2016;114:13–21. doi: 10.1016/j.seares.2016.05.001. [DOI] [Google Scholar]
- 18.Landeiro VL, Magnusson WE, Melo AS, Espirito-Santo HMV, Bini LM. Spatial eigenfunction analyses in stream networks: do watercourse and overland distances produce different results? Freshw. Biol. 2011;56:1184–1192. doi: 10.1111/j.1365-2427.2010.02563.x. [DOI] [Google Scholar]
- 19.Fernandes IM, Henriques-Silva R, Penha J, Zuanon J, Peres-Neto PR. Spatiotemporal dynamics in a seasonal metacommunity structure is predictable: the case of floodplain-fish communities. Ecography. 2013;37:464–475. doi: 10.1111/j.1600-0587.2013.00527.x. [DOI] [Google Scholar]
- 20.Valanko S, Heino J, Westerbom M, Viitasalo M, Norkko A. Complex metacommunity structure for benthic invertebrates in a low diversity coastal system. Ecol. Evol. 2015;5:5203–5215. doi: 10.1002/ece3.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barros F, de Carvalho GC, Costa Y, Hatje V. Subtidal benthic macroinfaunal assemblages in tropical estuaries: Generality amongst highly variable gradients. Mar. Env. Res. 2012;81:43–52. doi: 10.1016/j.marenvres.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Barros F, Blanchet H, Hammerstrom K, Sauriau PG, Oliver J. A framework for investigating general patterns of benthic β-diversity along estuaries. Estuar. Coast. Shelf Sci. 2014;149:223–231. doi: 10.1016/j.ecss.2014.08.025. [DOI] [Google Scholar]
- 23.Attrill MJ, Rundle SD. Ecotone or ecocline: Ecological boundaries in estuaries. Estuar. Coast. Shelf Sci. 2002;55:929–936. doi: 10.1006/ecss.2002.1036. [DOI] [Google Scholar]
- 24.Mclusky, D. S. & Elliot, M. The Estuarine Ecosystem - Ecology, Threats, Management, Oxford University Pres (2004).
- 25.Kennish MJ. Environmental threats and environmental future of estuaries. Env. Conserv. 2002;29:78–107. doi: 10.1017/S0376892902000061. [DOI] [Google Scholar]
- 26.Baselga A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010;19:134–143. doi: 10.1111/j.1466-8238.2009.00490.x. [DOI] [Google Scholar]
- 27.Magalhães WF, Barros F. Structural and functional approaches to describe polychaete assemblages: Ecological implications for estuarine ecosystems. Mar. Freshw. Res. 2011;62:918–926. doi: 10.1071/MF10277. [DOI] [Google Scholar]
- 28.Remane A. Die Brackwasserfauna. Zoologische Anz. 1934;7:34–74. [Google Scholar]
- 29.Cirano M, Lessa GC. Oceanographic Characteristics of Baía de Todos os Santos, Brazil. Rev. Bras. Geof. 2007;25:363–387. doi: 10.1590/S0102-261X2007000400002. [DOI] [Google Scholar]
- 30.Hatje V, Barros F. Overview of the 20th century impact of trace metal contamination in the estuaries of Todos os Santos Bay: past, present and future scenarios. Mar. Pollut. Bull. 2012;64:2603–2614. doi: 10.1016/j.marpolbul.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 31.de Souza, G. B. G. & Barros, F. Cost/benefit and the effect of sample preservation procedures on quantitative patterns in benthic ecology. Helgol Mar Res 71–21, 10.1186/s10152-017-0501-3 (2017).
- 32.Souza GBG, Barros F. Analysis of sampling methods of estuarine benthic macrofaunal assemblages: sampling gear, mesh size, and taxonomic resolution. Hydrobiologia. 2015;743:157–174. doi: 10.1007/s10750-014-2033-z. [DOI] [Google Scholar]
- 33.De Biasi AM, Bianchi CN, Morri C. Analysis of macrobenthic communities at different taxonomic levels: an example from an estuarine environment in the Ligurian Sea (NW Mediterranean) Estuar. Coast. Shelf Sci. 2003;58:99–106. doi: 10.1016/S0272-7714(03)00063-5. [DOI] [Google Scholar]
- 34.Folk RL, Ward WC. Brazos river bar: a study in the significance of grain size parameters. J. Sediment. Pet. 1957;27:3–26. doi: 10.1306/74D70646-2B21-11D7-8648000102C1865D. [DOI] [Google Scholar]
- 35.Oksanen, J. et al. vegan: Community Ecology Package. R package version 2.0-10, https://cran.r-project.org/web/packages/vegan/index.html (2013).
- 36.R Development Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, https://www.r-project.org/ (2013).
- 37.Gotelli NJ, Colwell RK. Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 2001;4:379–391. doi: 10.1046/j.1461-0248.2001.0230.x. [DOI] [Google Scholar]
- 38.Presley SJ, Higgins CL, Lopez-Gonzalez C, Stevens RD. Elements of metacommunity structure of Paraguayan bats: multiple gradients require analysis of multiple ordination axes. Oecologia. 2009;160:781–793. doi: 10.1007/s00442-009-1341-x. [DOI] [PubMed] [Google Scholar]
- 39.Gauch HG. Multivariate Analysis in Community Ecology. Cambridge: Cambridge University Press; 1982. [Google Scholar]
- 40.Morisita M. Composition of the I-index. Res. Popul. Ecol. 1971;13:1–27. doi: 10.1007/BF02530774. [DOI] [Google Scholar]
- 41.Heino J, Soininen J, Alahuhta J, Lappalainen J, Virtanen R. A comparative analysis of metacommunity types in the freshwater realm. Ecol. Evol. 2015;5:1525–1537. doi: 10.1002/ece3.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heino J, Soininen J, Alahuhta J, Lappalainen J, Virtanen R. Metacommunity ecology meets biogeography: effects of geographical region, spatial dynamics and environmental filtering on community structure in aquatic organisms. Oecologia. 2017;183:121–137. doi: 10.1007/s00442-016-3750-y. [DOI] [PubMed] [Google Scholar]
- 43.Dallas, T. Metacom: analysis of the “elements of metacommunity structure”. R package version 1.5.0, https://cran.r-project.org/web/packages/metacom/index.html (2013).
- 44.Almeida-Neto M, Guimarães P, Guimarães PR, Loyola RD, Ulrich W. A consistent metric for nestedness analysis in ecological systems: reconciling concept and measurement. Oikos. 2008;117:1227–1239. doi: 10.1111/j.2008.0030-1299.16644.x. [DOI] [Google Scholar]
- 45.Ulrich W, Gotelli NJ. Pattern detection in null model analysis. Oikos. 2013;122:2–18. doi: 10.1111/j.1600-0706.2012.20325.x. [DOI] [Google Scholar]
- 46.Schmera D, Podani J, Botta-Dukát Z, Erős T. On the reliability of the Elements of Metacommunity Structure framework for separating idealized metacommunity patterns. Ecol. Indic. 2018;85:853–860. doi: 10.1016/j.ecolind.2017.11.022. [DOI] [Google Scholar]
- 47.Dray S, Legendre P, Peres-Neto PR. Spatial modelling: a comprehensive framework for principal coordinate analysis of neighbor matrices (PCNM) Ecol. Model. 2006;196:483–493. doi: 10.1016/j.ecolmodel.2006.02.015. [DOI] [Google Scholar]
- 48.Borcard D, Legendre P. All-scale spatial analysis of ecological data by means of principal coordinates of neighbour matrices. Ecol. Model. 2002;153:51–68. doi: 10.1016/S0304-3800(01)00501-4. [DOI] [Google Scholar]
- 49.Blanchet FG, Legendre P, Borcard D. Forward selection of explanatory variables. Ecol. 2008;89:2623–2632. doi: 10.1890/07-0986.1. [DOI] [PubMed] [Google Scholar]
- 50.Gilbert B, Bennett JR. Partitioning variation in ecological communities: do the numbers add up? J. Appl. Ecol. 2010;47:1071–1082. doi: 10.1111/j.1365-2664.2010.01861.x. [DOI] [Google Scholar]
- 51.Legendre P, Legendre L. Numerical Ecology. 2nd edn. Amsterdam: Elsevier; 1998. [Google Scholar]
- 52.Zuur AF, Ieno EN, Elphick CS. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2009;1:3–14. doi: 10.1111/j.2041-210X.2009.00001.x. [DOI] [Google Scholar]
- 53.Mansfield ER, Helms BP. Detecting multicollinearity. Am. Stat. 1982;36(3a):158–160. doi: 10.2307/2683167. [DOI] [Google Scholar]
- 54.Peres-Neto PR, Legendre P, Dray S, Borcard D. Variation partitioning of species data matrices: estimation and comparison of fractions. Ecol. 2006;87:2614–2625. doi: 10.1890/0012-9658(2006)87[2614:VPOSDM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 55.Borcard D, Legendre P, Drapeau P. Partialling out the spatial component of ecological variation. Ecol. 1992;73:1045–1055. doi: 10.2307/1940179. [DOI] [Google Scholar]
- 56.Peres-Neto PR, Legendre P. Estimating and controlling for spatial structure in ecological communities. Glob. Ecol. Biogeogr. 2010;19:174–184. doi: 10.1111/j.1466-8238.2009.00506.x. [DOI] [Google Scholar]
- 57.Clements FE. Plant succession: an analysis of the development of vegetation. Washington: Carnegie Institution of Washington; 1916. [Google Scholar]
- 58.Gleason HA. The individualistic concept of the plant association. Bull. Torrey Bot. Club. 1926;53:7–26. doi: 10.2307/2479933. [DOI] [Google Scholar]
- 59.Simberloff D. Competition theory, hypothesis testing, and other community ecological buzzwords. Am. Nat. 1983;122:626–635. doi: 10.1086/284163. [DOI] [Google Scholar]
- 60.Diamond, J. M. Assembly of species communities. In Cody, M. L., Diamond, J. D. (eds), Ecology and Evolution of Communities. Harvard University Press, Harvard (1975).
- 61.Tilman D. Resource Competition and Community Structure. Princeton: Princeton University; 1982. [PubMed] [Google Scholar]
- 62.Atmar W, Patterson BD. The measure of order and disorder in the distribution of species in fragmented habitat. Oecologia. 1993;96:373–382. doi: 10.1007/bf00317508. [DOI] [PubMed] [Google Scholar]
- 63.Josefson AB. Species sorting of benthic invertebrates in a salinity gradient Importance of dispersal limitation. PLoS ONE. 2016;11(12):1–21. doi: 10.1371/journal.pone.0168908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hatje V, Barros F, Figueiredo DG, Santos VLCS, Peso-Aguiar MC. Trace metal contamination and benthic assemblages in Subaé estuarine system, Brazil. Mar. Pollut. Bull. 2006;52:969–987. doi: 10.1016/j.marpolbul.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 65.Brasil LS, Vieira TB, Oliveira-Junior JMB, Dias-Silva K, Juen L. Elements of metacommunity structure in Amazonian Zygoptera among streams under different spatial scales and environmental conditions. Ecol. Evol. 2017;7:3190–3200. doi: 10.1002/ece3.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Genz F, Lessa GC, Cirano M. Vazão mínima para estuários: um estudo de caso do Rio Paraguaçu/BA. Rev. Bras. Rec. Hid. 2008;13:73–82. [Google Scholar]
- 67.Gianuca AT, Declerck SA, Lemmens P, De Meester L. Effects of dispersal and environmental heterogeneity on the replacement and nestedness components of β-diversity. Ecol. 2017;98:525–533. doi: 10.1002/ecy.1666/suppinfo. [DOI] [PubMed] [Google Scholar]
- 68.Lotze HK, et al. Depletion, degradation, and recovery potential of estuaries and coastal seas. Sci. 2006;312:1806–1809. doi: 10.1126/science.1128035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Dataset - Environmental Data.
Supplementary Dataset - Spatial Coordinates.