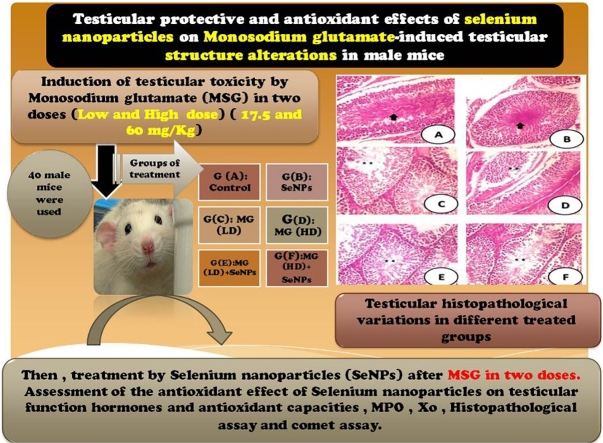
Graphical abstract
Keywords: Monosodium glutamate, Selenium nanoparticles, Testis, Testosterone, Oxidative stress, Antioxidant, Histological changes, DNA damage
Abstract
Background
Selenium has a protective antioxidant effect on several tissues. Monosodium glutamate (MSG), MSG has been known as flavor enhancer that influences reversely on male reproductive systems and having a number of side effects, including reproductive toxicity.
Objectives
The current study aims to evaluate the possible ameliorative functions of selenium nanoparticles (SeNPs) on MSG-induced reproductive toxicity.
Materials and methods
In total, 42 male mice included in this study were divided into six groups: control, MSG (LD), MSG (HD), SeNPs, MSG (LD) plus SeNPs and finally MSG (HD) plus SeNPs. Testosterone hormone, tumor necrosis factor-alpha (TNF-α), as well as the antioxidant biomarkers: superoxide dismutase [SOD], glutathione peroxidase [GPx], catalase [CAT] and marker of lipid peroxidation [MDA], were examined. Histological and comet assay variations in the testicular tissues as markers of testicular damage after the MSG administration in two doses (MSG-LD and MSG-HD) either alone or combined with SeNPs.MSG in two doses (LD and HD) genotoxic effects were also evaluated and the ameliorative role of SeNPs on the testicular tissues were recorded.
Results
Results proved that the administration of SeNPs diminished the effect of MSG (LD and HD)-that induced decrease in testosterone hormone levels and elevated oxidative stress markers markedly. SeNPs had a potent antioxidant effect and elevated the antioxidant enzymes significantly and decreased lipid peroxidation markers as compared with MSG either (LD and HD) groups.
Conclusion
It is clear from the data that SeNPs inhibit testicular injury and improve the antioxidant state in male mice.
1. Introduction
Monosodium glutamate (MSG), is a crystalline powder which is white in colour [1]. MSG is a food additive and flavor enhancing [2]. The safe and toxic concentrations of MSG in foods of human is still a varied issue.
Gao et al. [3] found that the higher dosage of MSG caused injury in neonatal animals. Also, MSG is involved in male infertility by causing testicular degeneration and modification of the sperm cell populace and morphology [4]. In details, MSG actuates a critical oligozoospermia and increments of anomalous sperm structure in male rats [5].
MSG has been involved in testicular hemorrhage and sperm cell morphology alterations [6]. Testicular morphological changes, testosterone level and sperm concentration were sensitive to high doses of MSG because it may cause to somewhat infertility in males. So the consumption of high dose MSG must be avoided [7]. Thus, The MSG using should be by the supervision of health authorities and should be limited to be used in very small amounts [8].
Previous studies have revealed that both oxidative stress and changes in the antioxidant enzyme system are the two most important factors leading to reproductive dysfunction. In this context, Hamza and Al-Baqami [9] found that oxidative stress resulting in lipid and protein peroxidation due to MSG administration.
Selenium (Se) is an essential trace element. It is incorporated into selenoproteins and representing the most important part of the active center of the enzymatic activities [10]. The importance of selenium (Se) for successful reproduction has been recognized. It is important in male reproduction due to its role in testosterone biosynthesis and subsequently in the formation and typical development of spermatozoa. Thus, Se is a key factor in spermatogenesis and male fertility [11].
Nanomedicine has emerged as a boon to the present day pharmaceutical applications. Among different NPs, inorganic NPs of metals like selenium which possess a significant place owing to their unique bioactivities in nanoforms [10]. SeNPs serve as an attractive platform for drug and gene delivery that has been rapid in the last five years [12].
Recently, elemental SeNPs have become a point of interest in nanotechnology treatment due to their unique biological activities and low toxicity. Based on the improved properties of SeNPs over selenium, they have been explored in various disease conditions. SeNPs offer improved bioavailability with the added advantage of decreased toxicity [10].
Thus, the goal of the study was to clarify the antioxidant role of administration of SeNPs in mitigating the reproductive toxicity induced by MSG in two doses either low or high and to study SeNPs enhancing effect on reproductive capacities and antioxidant capacities in male mice in respect to the testicular oxidative damage induced by MSG.
2. Materials and methods
2.1. Chemicals
MSG (Monosodium glutamate (C5H9NO·Na) with high purity (99 %) was sold from (Universal Chemicals, India).MSG was used in two doses (High and low dose) MSG–low dose (17.5 mg/Kg); MSG–high dose (60 mg/Kg) [13]. SeNPs were obtained from Sigma Co. Dose of SeNPs are (1.7 ppm) [14].
2.2. Experimental animals
Forty "30" to "35" g adult male albino mice were maintained in metal cages with good aeration conditions, by following the European guides on animal care no. (86/609/EEC). The mice were chosen and were handled by following the rules for the security of animal welfare, this work was approved by the animal ethical committee of Zagazig University, the Painful procedures were carried out under light anesthesia to minimize the stress that could inflict on the mice.
2.3. Experimental design
Male mice were divided into 6 groups: 1st control group was given 1 ml of DMSO (1%) as a vehicle; 2nd group treated with SeNPs dose (1.7 ppm), 3rd and 4th groups were treated with low and high doses of MSG (MSG-LD) (17.5 mg/Kg) and (MSG-HD) (60 mg/Kg), 5th and 6th groups were treated with MSG -LD + SeNPs and MSG-HD + SeNPs, treatment were i.p for consecutive 30 days for all groups.
2.4. Preparation of testis homogenates
Male mice were fasted for 8 h and then were light anesthetized by ketamine at the end of treatment to minimize the stress on the mice, then, the male mice were sacrificed by cervical dislocation. Testis tissues (∼0.23 g) were used to determine the oxidative stress, testis tissues were homogenized in ice saline sodium phosphate buffer (100 mM, pH 7.4), the homogenates were centrifuged at 10,000g at 4°C for 20 min. Then, the resulted supernatant was transferred into Eppendorfs and was kept till use.
2.5. Determination of the enzymatic antioxidant biomarkers in testis
Catalase activity (CAT) was determined by Aebi [15]. Superoxide dismutase (SOD) was estimated by Marklund and Marklund [16]. Malondialdehyde (MDA) levels were estimated by Ohkawa et al. [17]. Glutathione reductase (GRx) was assessed by Bio-Diagnostic kit.
Glutathione peroxidase (GPx) activity was determined by Hafeman et al. [18]. Myeloperoxidase (MPO) activity was determined by Suzuki et al. [19]. Xanthine oxidase (XO) activity was determined by Litwack et al. [20].
2.6. Determination of the testosterone hormone and Inhibin-B level
Testosterone hormone level was measured by (Elecsys, Roche diagnostics) Elisa kit by Wheeler [21]. Inhibin-B was determined by using (Biomatik) ELISA kit, the detection range was (12.35–1000 pg/ml).
2.7. Determination of plasma 8-hydroxydeoxyguanosine (8-OHDG)
Level of (8-OHDG) was determined by ELISA kit using manual guide.
2.8. Histological evaluation
A part of testis tissues were fixed in 10 % neutral buffered formalin and by applying other processes as described by Gabe [22].
2.9. The Single cell gel electrophoresis (SCGE) (Comet assay)
Testis tissues were put into a petri dish with addition of ice-solution of (Ca2+- and Mg2+-free HBSS), cells viability was indirectly determined by the comet images [23]. The assay was carried out in alkaline conditions. Collins and Dunsinka [24]. Then, the photos were analyzed [25].
2.10. Statistical analysis
Data are expressed as mean ± S.E. Statistical analysis was performed by using one-way ANOVA by using Duncan’s post hoc test. The Duncan’s Test [26] was used.
3. Results
3.1. The oxidative stress biomarkers
There were variations in the antioxidant enzymes of the testicular tissues in groups treated with MSG in two doses either low or high dose. Current results showed an increment in MDA levels in the testicular tissues of the MSG-treated group especially in MSG-HD as elucidated by the increment of MDA levels. The co-administration of SeNPs with MSG either (MSG-LD and/or MSG-HD) moderated the level of lipid peroxides and reduced markedly the MDA levels (Table 1).
Table 1.
Effect of selenium nanoparticles (SeNPs) and Monosodium glutamate (MSG) in two doses (MSG-HD) and (MSG-LD) on MDA, SOD, CAT, GPx and GRx levels in testis tissues of male mice.
| Parameters | Control | SeNPs | MSG (LD) | MSG (HD) | MSG (LD) and SeNPs | MSG (HD) and SeNPs |
|---|---|---|---|---|---|---|
| MDA (U/g) | 9.59 ± 1.24e | 9.10 ± 2.25e | 55.96 ± 2.74b | 87.36 ± 2.16 a | 19.25 ± 1.25d | 21.3 ± 1.69 cd |
| SOD (U/g) | 17.39 ± 2.15b | 17.61 ± 1.38b | 12.41 ± 1.49 c | 9.36 ± 1.97 a | 15.02 ± 1.39a | 14.25 ± 1.14a |
| CAT (U/g) | 20.58 ± 1.15a | 21.03 ± 1.12a | 13.04 ± 2.45c | 10.35 ± 1.15d | 17.25 ± 1.12b | 16.25 ± 1.79b |
| GRx (U/g) | 5.33 ± 0.78a | 5.36 ± 0.47a | 2.88 ± 0.12c | 1.67 ± 0.45d | 4.69 ± 0.78b | 4.31 ± 0.74b |
| GPx (U/g) | 7.74 ± 1.37ab | 8.78 ± 1.29 a | 4.65 ± 0.98 e | 2.69 ± 0.65f | 5.25 ± 1.17 d | 6.01 ± 1.65 cd |
Means within the same column in each category (mean ± SE) carrying different letters are significant at P ≤ 0.05, where the highest mean value has the symbol (a) and decreasing in value were assigned alphabetically. SeNPs: selenium nanoparticles; MSG: Monosodium glutamate; MDA: Malondialdehyde; SOD: Superoxide dismutase; CAT: Catalase; GRx: Glutathione reductase; GPx: Glutathione peroxidase; LD: Low dose; HD: High dose.
In MSG-groups either (HD or LD), SOD, GRx, GPx and CAT activities in testicular tissues were reduced significantly as compared to control group, particularly in MSG-HD group. Administration of SeNPs with MSG either MSG-LD and/or MSG-HD ameliorated the antioxidant enzyme activities as compared to MSG-LD and MSG-HD treated groups (Table 1).
The significant increase in the levels of MPO and XO were recorded particularly in MSG-HD treated group. These biomarkers were decreased and reversed to normal levels after the treatment with the combination of SeNPs with MSG-HD (Table 2).
Table 2.
Effect of selenium nanoparticles (SeNPs) and Monosodium glutamate (MSG) in two doses (MSG-HD) and (MSG-LD) on MPO and XO in testicular tissues of male mice.
| Parameters | Control | SeNPs | MSG (LD) | MSG (HD) | MSG (LD) and SeNPs | MSG (HD) and SeNPs |
|---|---|---|---|---|---|---|
| MPO (nM/min/mg) | 7.11 ± 1.11d | 7.21 ± 0.51d | 24.18 ± 1.14b | 41.15 ± 1.14a | 14.78 ± 1.65c | 12.58 ± 1.69c |
| XO (U/g) | 8.10 ± 1.15d | 8.22 ± 0.78d | 31.31 ± 1.25b | 44.21 ± 1.27a | 10.14 ± 1.24c | 11.41 ± 1.23c |
Means within the same column in each category (mean ± SE) carrying different letters are significant at P ≤ 0.05, where the highest mean value has the symbol (a) and decreasing in value were assigned alphabetically. SeNPs: selenium nanoparticles; MSG (LD): Monosodium glutamate low dose; MSG (HD): Monosodium glutamate high dose; Myeloperoxidase (MPO) and Xanthine oxidase (XO); SH: Thiol, PC: Protein carbonyl; LD: Low dose ; HD: High dose.
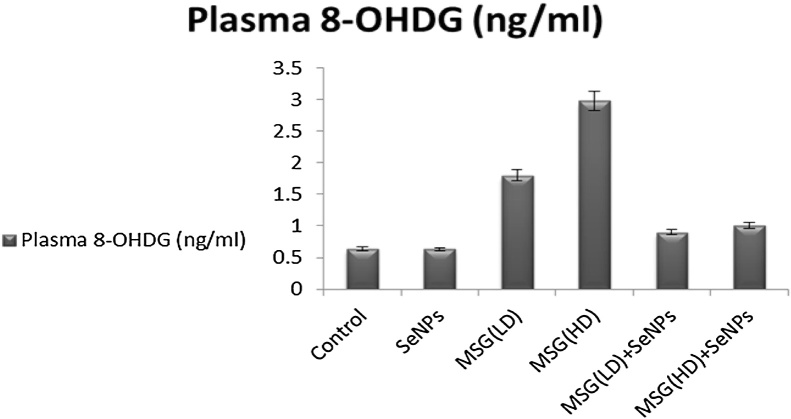
3.2. Plasma (8-OHDG) level
8-OHDG activity of MSG treated group particularly MSG (High dose) was significantly increased as compared with control group (Table 1). Administration of SeNPs after MSG (LD or HD) administration decreased 8-OHDG activity as compared with MSG-LD and/or MSG-HD treated groups (Fig. 1).
Fig. 1.
Effect of selenium nanoparticles (SeNPs) and Monosodium glutamate (MSG) on Plasma 8- hydroxydeoxyguanosine.
SeNPS: Selenium nanoparticles; MSG: Monosodium glutamate; plasma 8−OHDG: plasma 8- hydroxydeoxyguanosine; LD: Low dose; HD: High dose.
3.3. Testosterone hormone and inhibin B levels
Testosterone hormone and Inhibin-B levels were reduced significantly in MSG-groups in two doses (MSG-LD and/or MSG-HD). SeNPs treated group elevated significantly both testosterone hormone and inhibin-B levels as compared to control group. While, treatment of male mice with SeNPs combined with MSG-LD and/or MSG-HD increased both testosterone hormone and Inhibin-B levels when compared to MSG-LD or MSG-HD groups (Table 3).
Table 3.
Effect of selenium nanoparticles (SeNPs) and Monosodium glutamate (MSG) in two doses (MSG-HD) and (MSG-LD) on testosterone hormone and inhibin B levels of male mice.
| Parameters | Control | SeNPs | MSG (LD) | MSG (HD) | MSG (LD) and SeNPs | MSG (HD) and SeNPs |
|---|---|---|---|---|---|---|
| Testosterone (μIU/mL) | 1.23 ± 0.11b | 1.49 ± 0.14a | 0.78 ± 0.21d | 0.66 ± 0.10d | 1.18 ± 0.24c | 1.16 ± 0.13c |
| Inhibin B (ng/ml) | 0.35 ± 0.11b | 0.49 ± 0.12a | 0.10 ± 0.01c | 0.08 ± 0.03c | 0.32 ± 0.01b | 0.30 ± 0.02b |
Means within the same column in each category (mean ± SE) carrying different letters are significant at P ≤ 0.05, where the highest mean value has the symbol (a) and decreasing in value were assigned alphabetically. SeNPs: Selenium nanoparticles; MSG: Monosodium glutamate; LD: Low dose; HD: High dose.
3.4. Histological changes
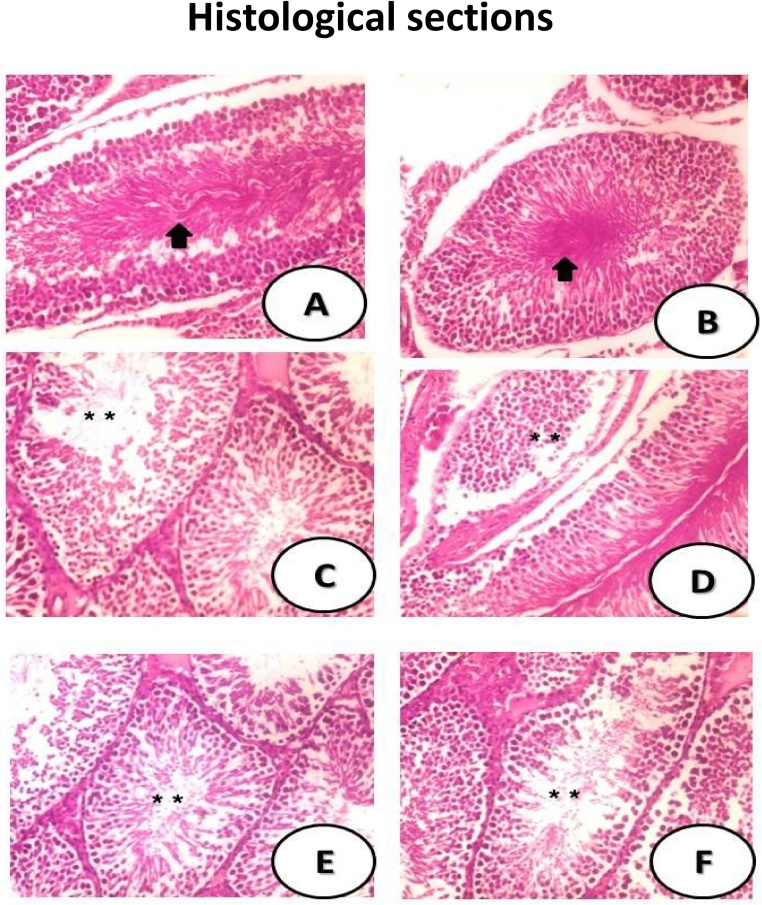
The control group's testicular tissues were normal in structure with plenty of sperms (Black arrow) (Fig. 2A) showed cells with normal spermatogonia stacked in several layers on seminiferous tubule. SeNPs–group demonstrated normal seminiferous tubules and normal spermatogenesis with plenty of sperm (Black arrow) (Fig. 2B). MSG-LD induced testicular structural variations by appearance of few number of spermatids (**) (Fig. 2C). MSG-HD showing Leydig cells with hyperplasia and showing disorganized germinal epithelium with no sperm aggregations and some degenerated parts (**) (Fig. 2D). The clear improvement was noticed in MSG-LD and SeNPs-group where there was some restoration of the germinal cells and some spermatozoa (**) with plenty layers of spermatogenic cells (Fig. 2E). The seminiferous tubules with normal structure and mild sperm (**) with Leydig cells were found iMSG-HD and SeNPs treated group (Fig. 2F).
Fig. 2.
Histological sections of the testes of mice of different treated group. The testicular tissues from control group was normal in appearance with plenty of sperms (Black arrow) (Fig. 2A) showed cells of normal spermatogonia stacked in several layers on seminiferous tubule. SeNPs–group showed normal seminiferous tubules and normal spermatogenesis with plenty of sperm (Black arrow) (Fig. 2B). MSG-LD induced histopathological changes in the testicular tissues as distended seminiferous tubules by some stages of spermatogenic cells, with few number of spermatids (**) (Fig. 2C). MSG-HD showing Leydig cells hyperplasia and disorganized germinal epithelium and no aggregations of sperms and some degenerated parts (**) (Fig. 2D). MSG-LD and SeNPs treated group where there was some restoration of germinal cells and some spermatozoa (**) as well as seminiferous tubules that were lined by numerous spermatogenic cells (Fig. 2E). The normal structure of seminiferous tubules with mild sperm (**) with Leydig cells were found in the group that received MSG-HD and SeNPs (Fig. 2F).
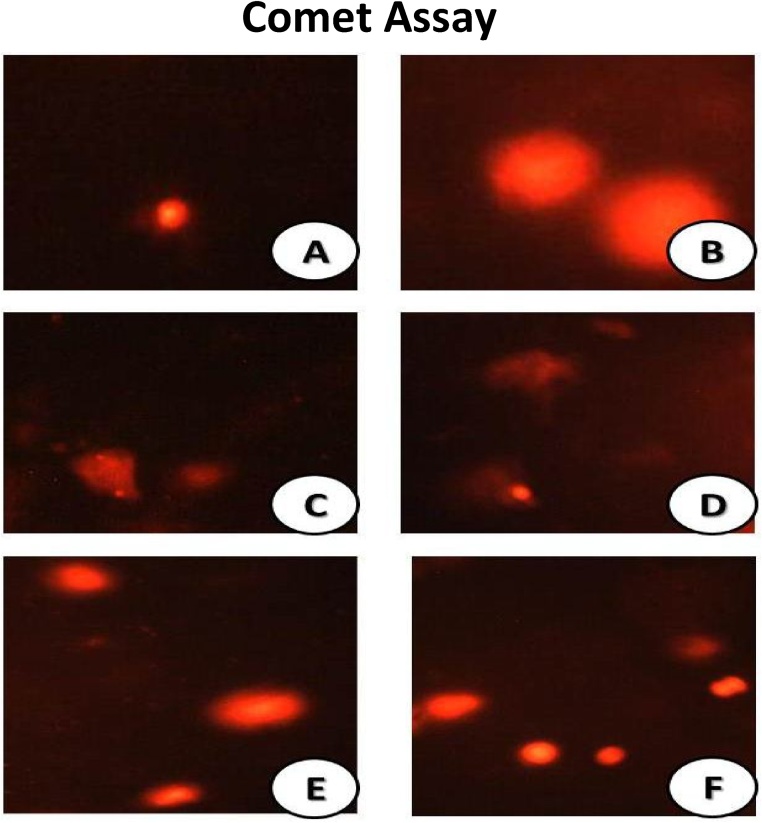
3.5. Comet assay
Comet images of control group showed intact nuclei of testicular tissues, rounded cell with no appearance of tail (Fig. 3A). The SeNPs- group showed an intact nuclei with undamaged DNA (Fig. 3B). MSG-LD group showed DNA strand and damaged breaks, the cell contains a head like a comet, and with tail appear as hollow area (Fig. 3C). MSG-HD showed the appearance of highly damage degree with a lot of apoptotic cells (Fig. 3D). MSG-LD + SeNPs revealed high amelioration of the cells as with a few record in the percent of damaged DNA (Fig. 3E). MSG-HD + SeNPs showed more % of intact DNA molecules and less comet cell counts (Fig. 3F).
Fig. 3.
Comet images of cells derived from the testicular tissues of different treated groups. control group cells derived from testis showed intact nuclei and rounded cell with no tail (Fig. 3A). The SeNPs- treated group showed nuclei in intact form with undamaged DNA (Fig. 3B). MSG-LD group showed damaged DNA strand breaks and the cell contains a head like a comet, and with tail appear as hollow area (Fig. 3C). MSG-HD clarified the appearance of highly degree of damage with a lot of apoptotic cells (Fig. 3D). MSG-LD + SeNPs revealed amelioration of the cells as with a few record in the percent of damaged DNA (Fig. 3E). MSG-HD + SeNPs showed more percent of intact form of DNA and less count of comet cells (Fig. 3F).
4. Discussion
MSG pleasant taste has led to its inclusion as a flavoring agent in foods. Its excessive inclusion in foods has required its safety evaluation, which has raised the public concerns about its transfer into the blood and thus increasing the brain glutamate levels, thus causing functional disruptions and affected other organs of the body because MSG is a neurotransmitter [27], this concern originally raised greatly and has led to an extensive series of scientific studies to examine this issue, conducted primarily in rodents.
The key findings have previously reported that MSG ingestion in the diet does not produce appreciable increases in glutamate concentrations in the blood as the blood-brain barrier effectively restricts the passage of glutamate from the blood into the brain. The brain glutamate levels rise only when the blood glutamate concentrations are raised experimentally via injection intraperitoneally [27], this explain the cause of using the intraperitoneal injection of MSG in the current study instead of oral administration of MSG.
MSG is a flavor enhancer that is excessively used in many foods. Recently, nanoparticle molecules exerted protective roles against tissues injuries, therefore the main point of the present investigation was to assess the health implications after exposure to SeNPs in the testis of mice that induced toxicity using MSG in two doses either low or high dose, by evaluating the biochemical, histopathological and the genotoxicity testicular changes induced by MSG and the possible testicular protective effects of SeNPs.
According to Fernstrom [28], The author concluded that MSG administration to various animal generation, the expected pathophysiologic signs resulting from MSG injection are not found in animals that consuming enormous amounts of MSG orally through their life cycles, also reported that injection of MSG help in crossing blood-brain barrier and thus confirming incidence of MSG toxicity. So, we want to give a real assessment of MSG reproductive toxicity and also to ensure that MSG crosses the blood-brain barrier and thus evaluate its side effects on reproductive tissues and thus the current administration rout of MSG was intraperitoneal.
In the current study, the LPO levels and testicular antioxidant enzymes activities which include SOD, CAT, GPx, MPO, and XO evaluated as markers of testicular damage in rats by MSG-induction, the results also showed that MSG in two doses either (MSG-LD) or (MSG-HD) caused a notable rise in testicular LPO and a concurrent diminish in the antioxidant state of the rats.
The increase in LPO suggested that MSG produced ROS which may result in oxidative destruction of the testicular tissue. Suppression of SOD and CAT in MSG-LD or MSG-HD treated groups could be due to the intensive increase in superoxide radical and H2O2 and thus lead to the damage of the testis.
The elevation of LPO in MSG-treated mice is in parallel to the findings of Hamza et al. [5]. They found that MSG increased LPO in the testis of rats.
Whereas, GPx preserved against oxidative injury by minimizing H2O2 to water [29]. Thus, the observed diminish in testicular GRx and GPx activity indicated the anti-fertility effects of MSG and strongly suggested that MSG may antagonistically influence the GSH metabolic pathway which promotes oxidative injury and reduces testosterone levels.
The current results were approved previously by Hamza and Al-Harbi [13] who demonstrated that the testicular tissue damage main reason is the highly lipid peroxidation level which is clarified by high level of MDA and concluded that MSG caused promotion of oxidative stress by elevating the oxidative stress markers.
Additionally, Diab and Hamza [30] proved that MSG induced severe oxidative damage in sperms by affecting on the plasma membrane of the sperms and recording very low percent of antioxidant enzymes which clarify the alterations occurred in the sperm functions, motility impairment and may have deleterious effect on the spermatozoa development.
The defense antioxidant system consists of some antioxidant enzymes as SOD, GPx and CAT [31]. The current study reported that MSG caused significant decrease in SOD, CAT and GPx activities and these findings are in accordance with Hamza et al. [5] who demonstrated a significant amelioration in both SOD and GPx after administration of MSG in combination with antioxidant compound. These enzymes also have a noticeable effect in keeping the antioxidant pathway balance and the testicular tissues that have higher antioxidant enzyme levels [32].
The testis is the critical organ that is exposed heavily to oxidative stress due to its membrane polyunsaturated lipids high contents [33].
MSG may also effect on the male reproductive function [34]. In this study MSG caused several histological and ultrastructural changes like spermatogenic arrest, edema, and hypospermia and azospermia, it may be related to oxidative effects of MSG on testis cell membrane and also testis tissues damages.
Additionally, severe oxidative injury resulted from MSG exposure in two doses that could lead to DNA damage, the comet assay was used to evaluate DNA damage and strand breaks [24], this is greatly in accordance with the present results as the two groups treated with MSG-LD and MSG-HD induced DNA damage as appeared clearly.
DNA damage which resulted in an oxidative injury is one of the important bio-monitoring parameters on the effect of dietary antioxidants on the human's health [35], as comet assay is excessively used for measuring the oxidative DNA damage [36] and this was observed also in increasing 8-OHDG levels in MSG groups while damaged DNA breaks are revealed.
The results are consistent with Hamid et al. [37] who demonstrated that trend of research is now being directed towards synthesis and application of Se NPs. due to its higher biological activity and lower toxicity compared to selenium ions.
Selenium has ameliorated all the biochemical parameters and antioxidant enzyme capacities and this come in agreement with Surhio et al. [38] who demonstrated that selenium is an essential mineral for human’s good health and is required in many biological functions.
Selenium is an essential part of the strong antioxidant defense system in human body. Its nutritional deficiency causes failure of organ system, immune dysfunctions, cancer, cardiac diseases [39].
These results were similar to those of Abu El-Zahab et al. [39] who demonstrated that Se improved the testicular parameters to be identical to control levels after treatment by cadmium. Se ameliorated the reduction in the reproductive organs weights, sperm characteristics.
Additionally, Se has a positive effect on ejaculate volume and semen quality in goats [40],glutathione and glutathione-related enzymes are involved in the metabolism and detoxification of cytotoxic and carcinogenic compounds as well as ROS [41].
After administration of Se, serum testosterone level was significantly increased parallel to the reduction in LPO concentration, this could be attributed to a relationship between steroidogenesis and ROS [42], the improvement in the histological structure of testes, epididymis and accessory sex glands was correlated to the potential role of Se in scavenging ROS generated by MSG.
The histopathological studies revealed the improvement in the testis structure in comparison with MSG-groups in two doses either low or high and thus, the current finding is in agreement with Hamza and Al-Harbi [13] who investigated that MSG-treated group in high dose showing few sperm number, additionally, the current results suggest that group treated with MSG in either (LD or HD) afforded a significant protection for the sperm formation qualities and this reinforced the antioxidant abilities of SeNPs in mitigating the reproductive toxicity induced by MSG either (HD or LD) dose.
The current histological findings of the testicular tissues were greatly reinforced by Alalwani et al. [34] who revealed the disorganization of the spermatogenic cells in the seminiferous tubules with irregular basement membrane and some leydg’s cells necrosis and edema that is linked with the cellular infiltration in MSG -treated group which is approved by the current finding.
Apoptosis is known as the programmed cell death and it is characterized by cellular changes and biochemical variations that make the cell to be as a suicide bag [43], an altered apoptotic process has been noticed in MSG groups' testis tissues.
In the current study, SeNPs clarified a significant protective effect against MSG. This may be due to MSG- impaired absorption or due to the high potent antioxidant effect of SeNPs [44]. SeNPs may prevent oxidative injury to the membrane lipids by protecting the cellular membranes from oxidative stress [45].
The current finding are in complete accordance with Zanfirescu et al. [46] who reported in their review some morphological changes in testes, as well as sperm abnormalities in adult male rats following the intraperitoneal MSG administration and this may be due to the excessive production of free radicals and incidence of oxidative injury, taking into consideration the high doses used which reflects the importance of the high dose used in this study to clarify the effect of excessive use of MSG on reproductive capacities.
In accordance with the genotoxicity test that clarified deleterious genotoxic effect of MSG in two doses either (LD or HD) that was a consequence of generating of more free radicals as previously reported by Hassan et al. [47] who clarified that the free radical generation leads to DNA damage, protein degradation, LPO and finally culminating into damage to various vital tissues like liver, kidney and brain.
5. Conclusion
MSG in two doses either (LD or HD) increased the oxidative injury markedly in testicular tissues, that leading to the disruption and impairment of the testicular histological characteristics. MSG down regulated the antioxidant enzymes, and up regulated the lipid peroxide biomarkers. The present study confirmed that SeNPs have protective role on the reproductive capacity against MSG that afforded a reproductive toxicity, It's enhancement role of the structural and biochemical parameters were parallel with the improvement role of the cellular structure. Therefore, we need to increase the public awareness about the toxic effects of MSG on reproductive capacities and the possible ameliorative role of SeNPs as potent antioxidant.
Declaration of Competing Interest
The author declares that there are not any conflicts of interest.
References
- 1.Furst P., Stehle P. What are the essential elements needed for the determination of amino acid requirements in humans? J. Nutr. 2004;34:1558–1565. doi: 10.1093/jn/134.6.1558S. [DOI] [PubMed] [Google Scholar]
- 2.Farombi E.O., Onyema O.O. Monosodium glutamate-induced oxida-tive damage and genotoxicity in the rat: modulatory role of vitamin C, vitamin E and guercetin. Hum. Exp. Toxicol. 2006;125:251–259. doi: 10.1191/0960327106ht621oa. [DOI] [PubMed] [Google Scholar]
- 3.Gao J., Wu J., Zhao X.N., Zhang Z.X., Zhang Y.Y., Zhang Z.X. Transplacental neurotoxic effects of monosodium glutamate on structures and functions of specific brain areas of filial mice. Acta Physiol. Sin. 1994;46(1):44–51. [PubMed] [Google Scholar]
- 4.Igwebuike U.M. The effects of oral administration of monosodium glutamate (MSG) on the testicular morphology and cauda epididymal sperm reserves of young and adult male rats. Vet. Arh. 2011;8194:525–534. [Google Scholar]
- 5.Hamza R.Z., Al-Salmi F.A., Laban H., El-Shenawy N.S. Ameliorative role of green tea and zinc oxide nanoparticles complex against monosodium glutamate-induced testicular toxicity in male rats. Curr. Pharm. Biotechnol. 2019;(Dec 2) doi: 10.2174/1389201020666191203095036. [DOI] [PubMed] [Google Scholar]
- 6.Nayanatara A., Vinodini N., Damadar G., Ahemed B., Ramaswamy C., Shabarinath M., Bhat M. Role of ascorbic acid in monosodium glutamate mediated effect on testicular weight sperm morphology and sperm count in rat testis. J. Chin. Clin. Med. 2008;3:1–5. [Google Scholar]
- 7.Iamsaard S., Sukhorum W., Samrid R., Yimdee J., Kanla P., Chaisiwamongkol K., Hipkaeo W., Fongmoon D., Kondo H. The sensitivity of male rat reproductive organs to monosodium glutamate. Acta Med. Acad. 2014;43(1):3–9. doi: 10.5644/ama2006-124.94. [DOI] [PubMed] [Google Scholar]
- 8.Wahdan A., Shareef M. Study of the protective effect of vitamin C on monosodium glutamate induced cardiotoxicity in adult male albino rats. Ain Shams J. Forensic Med. Clin. Toxicol. 2016;27:49–56. [Google Scholar]
- 9.Hamza R.Z., Al-Baqami N.M. Testicular protective effects of ellagic acid on monosodium glutamate-induced testicular structural alterations in male rats. Ultrastruct. Pathol. 2019;43(4–5):1–14. doi: 10.1080/01913123.2019.1671569. [DOI] [PubMed] [Google Scholar]
- 10.Khurana A., Sravani T., Mohd A.S., Pooladanda V., Chandraiah G. Therapeutic applications of selenium nanoparticles. Biomed. Pharmacother. 2019;111:802–812. doi: 10.1016/j.biopha.2018.12.146. [DOI] [PubMed] [Google Scholar]
- 11.Das R.S., Ghosh S.K. Long term effects of monosodium glutamate on spermatogenesis following neonatal exposure in albino mice—a histological study. Nepal Med. Coll. J. 2010;12(3):149–153. [PubMed] [Google Scholar]
- 12.Staroverov S.A., Volkov A.A., Larionov S.V., Mezhennyy P.V., Kozlov S.V., Fomin A.S., Dykman L.A. Study of transmissible gastroenteritis virus antigenconjugated immunogenic properties of selenium nanoparticles and gold. Life Sci. J. 2014;11(11):456–460. [Google Scholar]
- 13.Hamza R.Z., AL-Harbi M.S. Monosodium glutamate induced testicular toxicity and the possible ameliorative role of vitamin E or selenium in male rats. Toxicol. Rep. 2014;1:1037–1045. doi: 10.1016/j.toxrep.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jang D.Y., Kim S.J., Jeong J.H., Nam S.Y., Kim J.S., Yun Y.W., Lee B.J. Protective effects of sodium selenite and selenium nanoparticles against experimental colon carcinogenesis in mice. J. Prev. Vet. Med. 2016;40:101–108. [Google Scholar]
- 15.Aebi H. Catalase in vitro. Meth. Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 16.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Clin. Chem. Clin. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 17.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1970;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 18.Hafeman D.G., Sunde R.A., Hoekstra W.G. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J. Nutr. 1974;104:580–587. doi: 10.1093/jn/104.5.580. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki K., Ota H., Sasagawa S., Sakatani T., Fujikura T. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal. Biochem. 1983;132:345–352. doi: 10.1016/0003-2697(83)90019-2. [DOI] [PubMed] [Google Scholar]
- 20.Litwack G., Bothwell J.W., Williams J.N., Elvehjem C.A. A colorimetric assay for xanthine oxide in rat liver homogenates. J. Biol. Chem. 1953;200:303–310. [PubMed] [Google Scholar]
- 21.Wheeler M.J. The determination of bio-available testosterone. Ann. Clin. Biochem. 1995;32:345–357. doi: 10.1177/000456329503200401. [DOI] [PubMed] [Google Scholar]
- 22.Gabe M. Masson Publisher; Paris: 1968. Techniques Histologiques [Histological Techniques] [Google Scholar]
- 23.Endoh D., Okui T., Ozawa S., Yamato O., Kon Y., Arikawa J., Hayashi M. Protective effect of a lignan-containing flaxseed extract against CCl (4)-induced hepatic injury. J. Vet. Med. Sci. 2002;64(9):761–765. doi: 10.1292/jvms.64.761. [DOI] [PubMed] [Google Scholar]
- 24.Collins A.R. The comet assay for DNA damage and repair: principles, applications, and limitations. Mol. Biotechnol. 2004;26(3):249–261. doi: 10.1385/MB:26:3:249. [DOI] [PubMed] [Google Scholar]
- 25.Liu X., Yao J., Pisha E., Yang Y., Hua Y., Breemen R.B., Bolton J.L. Oxidative DNA damage induced in equine estrogen metabolites: the role of estrogen receptor alpha. Chem. Res. Toxicol. 2002;15:512–519. doi: 10.1021/tx0101649. [DOI] [PubMed] [Google Scholar]
- 26.Duncan D.B. Multiple range and multiple F-test. Biometrics. 1955;11:1–42. [Google Scholar]
- 27.Gong S.L., Xia F.Q., Wei J., Li X.Y., Sun T.H., Lu Z., Liu S.Z. Harmful effects of MSG on function of the hypothalamus-pituitary-target gland system. Biomed. Environ. Sci. 1995;8(4):310–317. [PubMed] [Google Scholar]
- 28.Fernstrom J.D. Monosodium glutamate in the diet does not raise brain glutamate concentrations or disrupt brain functions. Ann. Nutr. Metab. 2018;73(suppl 5):43–52. doi: 10.1159/000494782. [DOI] [PubMed] [Google Scholar]
- 29.Dalto D., Matte J.J. Pyridoxine (vitamin B6) and the glutathione peroxidase system; a link between one-carbon metabolism and antioxidation. Nutrition. 2017;9(3):189. doi: 10.3390/nu9030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diab A.A., Hamza R.Z. Monosodium glutamate induced hepatotoxicity and the possible mitigating effect of vitamin C and propolis. J. Adv. Med. Pharm. Sci. 2016;7(4):1–10. [Google Scholar]
- 31.Demir F., Uzun F.G., Durak D., Kalender Y. Subacute chlorpyrifos-induced oxidative stress in rat erythrocytes and the protective effectsof catechin and quercetin. Pestic. Biochem. Phys. 2011;99:77–81. [Google Scholar]
- 32.Jihen E.H., Imed M., Fatima H., Abdelhamid K. Protective effects ofselenium (Se) and zinc (Zn) on cadmium (Cd) toxicity in the liver of the rat: effects on the oxidative stress. Ecotoxicol. Environ. Saf. 2009;72:1559–1564. doi: 10.1016/j.ecoenv.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Debnath D., Mandal T.K. Study of quinalphos (an environmental oestrogenic insecticide) formulation (Ekalux 25 E.C.)-induced damage of the testicular tissues and antioxidant defence systems in Sprague-Dawley albino rats. J. Appl. Toxicol. 2000;20:197–204. doi: 10.1002/(sici)1099-1263(200005/06)20:3<197::aid-jat634>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 34.Alalwani D.A. Monosodium glutamate induced testicular lesions in rats (histological study) Middle East Fertil. Soc. J. 2014;19(4):274–280. [Google Scholar]
- 35.Nandhakumar S., Parasuraman S., Shanmugam M.M., Rao K.R., Chand P., Bhat B.V. Evaluation of DNA damage using single-cell gel electrophoresis (Comet Assay) J. Pharmacol. Pharmacother. 2011;2(2):107–111. doi: 10.4103/0976-500X.81903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simon L., Brunborg G., Stevenson M., Lutton D., McManus J., Lewis S.E. Clinical significance of sperm DNA damage in assisted reproduction outcome. Hum. Reprod. 2010;25(7):1594–1608. doi: 10.1093/humrep/deq103. [DOI] [PubMed] [Google Scholar]
- 37.Hamid F., Mahboubeh A., Maryam N., Mitra M., Bagher A., Ahmad R.S., Mojtaba S. Antioxidant and cytotoxic effect of biologically synthesized selenium nanoparticles in comparison to selenium dioxide. J. Trace Elem. Med. Biol. 2014;28:75–79. doi: 10.1016/j.jtemb.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Surhioa M.M., Yufen W., Ping X., Shah F., Jinglei L., Ming Y. Antihyperlipidemic and hepatoprotective properties of selenium modified polysaccharide from Lachnum sp. Int. J. Biol. Macromol. 2017;99:88–95. doi: 10.1016/j.ijbiomac.2017.01.148. [DOI] [PubMed] [Google Scholar]
- 39.Abu-El-Zahab H.S., Hamza R.Z., Montaser M.M., Al-Mahdi M.M., Al-Harthi W.A. Antioxidant, antiapoptotic, antigenotoxic, and hepatic ameliorative effects of L-carnitine and selenium on cadmium-induced hepatotoxicity and alterations in liver cell structure in male mice. Ecotoxicol. Environ. Saf. 2019;173:419–428. doi: 10.1016/j.ecoenv.2019.02.041. [DOI] [PubMed] [Google Scholar]
- 40.Shi L., Zhang C., Yue W., Shi L., Zhu X., Lei F. Short-term effect of dietary selenium enriched yeast on semen parameters, antioxidant status and Se concentration in goat seminal plasma. Anim. Feed Sci. Technol. 2010;157:104–108. [Google Scholar]
- 41.Knapen M.F., Zusterzeel P.L., Peters W.H., Steegers E.A. Glutathione and glutathionerelated enzymes in reproduction. A review. Eur. J. Obstet. Gynecol. Reprod. Biol. 1999;82:171–184. doi: 10.1016/s0301-2115(98)00242-5. [DOI] [PubMed] [Google Scholar]
- 42.Aybek H., Aybek Z., Rota S., Sxen N., Akbulut M. The effects of diabetes mellitus, age, and vitamin E on testicular oxidative stress. Fertil. Steril. 2008;90:755–760. doi: 10.1016/j.fertnstert.2007.01.101. [DOI] [PubMed] [Google Scholar]
- 43.Vaux D.L., Flavell R.A. Apoptosis genes and autoimmunity. Curr. Opin. Immunol. 2000;12:719–724. doi: 10.1016/s0952-7915(00)00168-0. [DOI] [PubMed] [Google Scholar]
- 44.Giadinis N., Koptopoulus G., Roubies N., Siarkou V., Papasteriades A. Selenium and vitamin E effect on antibody production of sheep vaccinated against enzootic abortion (Chlamydia psittaci) Comp. Immunol. Microbiol. Infect. Dis. 2000;23:129–137. doi: 10.1016/s0147-9571(99)00066-1. [DOI] [PubMed] [Google Scholar]
- 45.Gupta S., Gupta H.K., Soni J. Effect of vitamin E and selenium supplementation on concentrations of plasma cortisol and erythrocyte lipid peroxides and the incidence of retinal fetal membranes in crossbred dairy cattle. Theriogenology. 2005;64:1273–1286. doi: 10.1016/j.theriogenology.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Zanfirescu A., Ungurianu A., Tsatsakis A.M., Nițulescu G.M., Kouretas D., Veskoukis A., Tsoukalas D., Engin A.B., Aschner M., Margina D. A review of the alleged health hazards of monosodium glutamate. Compr. Rev. Food Sci. Food Saf. 2019;18:1111–1134. doi: 10.1111/1541-4337.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hassan A.S., Abo El-Ela F.I., Abdel-Aziz A.M. Investigating the potential protective effects of natural product quercetin against imidacloprid-induced biochemical toxicity and DNA damage in adults rats. Toxicol. Rep. 2019;6:727–735. doi: 10.1016/j.toxrep.2019.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]