Abstract
Diaphyseal fractures represent a complex biological entity that could often end into impaired bone-healing, with delayed union and non-union occurring up to 10% of cases. The role of the modern orthopaedic surgeon is to optimize the fracture healing environment, recognize and eliminate possible interfering factors, and choose the best suited surgical fixation technique. The impaired reparative process after surgical intervention can be modulated with different surgical techniques, such as dynamization or exchange nailing after failed intramedullary nailing. Moreover, the mechanical stability of a nail can be improved through augmentation plating, bone grafting or external fixation techniques with satisfactory results. According to the “diamond concept”, local therapies, such as osteoconductive scaffolds, bone growth factors, and osteogenic cells can be successfully applied in “polytherapy” for the enhancement of delayed union and non-union of long bones diaphyseal fractures. Moreover, systemic anti-osteoporosis anabolic drugs, such as teriparatide, have been proposed as off-label treatment for bone healing enhancement both in fresh complex shaft fractures and impaired unions, especially for fragility fractures. The article aims to review the biological and mechanical principles of failed reparative osteogenesis of diaphyseal fractures after surgical treatment. Moreover, the evidence about the modern non-surgical and pharmacological options for bone healing enhancement will discussed.
Keywords: Diaphyseal fractures, Bone healing, Nonunions, Bone enhancement, Bone substitutes, Cell therapy, Diamond concept
Highlights
-
•
Bone healing of shaft fractures depends on mechanical and biological factors
-
•
Surgical treatments aim to restore the mechanical stability at fracture site
-
•
Biological treatments comprehend scaffolds, growth factors and cell therapies
-
•
No evidence supports biophysical stimulation (PEMF, LIPUS) for acute shaft fractures
-
•
Poor evidence exists supporting anabolic agents therapies for acute shaft fractures
-
•
The use of multiple combined treatments (polytherapy) provides higher union rates
1. Introduction
Long bones diaphyseal fractures have typical morphology patterns, with displacement and comminution which are held responsible for low bone contact and vascular supply disruption that adversely affects the healing process. Other factors, such as severe bone defects, soft tissue damages, open fractures and patients' related risk factors could lead to delayed unions and non-unions, which are estimated to occur in 1.9% and 10% of all diaphyseal fractures (Mills et al., 2017; Nandra et al., 2016).
Modern research efforts in orthopaedics are oriented into bone callus enhancement by surgical, pharmacological, cellular, and biophysical strategies in order to improve fracture healing process (Djouad et al., 2009; Mark Fisher et al., 2013; Hannemann et al., 2014).
The current article aims to review the bio-mechanical basis of failed reparative osteogenesis in diaphyseal fractures. Moreover, the surgical, biological, physical and pharmacological treatment options for bone healing enhancement of acute fractures and delayed unions will be highlighted.
2. Biology and bio-mechanical basis of surgical treatment in diaphyseal fractures
Diaphyseal fractures of long bones can heal by either direct fracture healing or indirect fracture healing. The healing pathway of a diaphyseal fracture depends on the complexity of fracture pattern (simple or multi-fragmentary fracture), the blood supply and the bio-mechanical environment at the fracture site consequent to the operative or non-operative treatment (Table 1).
Table 1.
Different bone healing processes promoted according to osteosynthesis system in long bone shaft fractures (Claes and Heigele, 1999; Perren, 2003).
| Osteosynthesis type | Fracture type | Interfragmentary strain | Healing pathway | Callus type |
|---|---|---|---|---|
| Intramedullary nail | Simple Multifragmentary |
+ ++ |
Direct Indirect |
Cortical Periosteal Endosteal |
| Dynamic compression plate | Simple | − | Direct | Cortical |
| Locking compression plate | Multifragmentary | ++ | Indirect | Periosteal Endosteal |
| External fixator |
Open Simple Multifragmentary |
+ +++ |
Direct Indirect |
Cortical Periosteal Endosteal |
Historically Wolff's law (Wolff, 1986), in 1982 first described the physiological response of normal bone to its mechanical environment during bone healing and remodelling. Then, Frost's concept of the “mechanostat” defined bone homeostasis as an independent unit responding to variations in the mechanical environment (Frost, 1987). One of the most accepted theories is Perren's “strain theory” (Perren, 1989), in which is stated that bony bridging between the distal and proximal callus can only occur when local strain (i.e., deformation) is less than the forming woven bone can tolerate (Perren, 1989). Therefore, when the interfragmentary strain (IFS), expressed as the function of movement and gap fracture width, is <2% bone repair occurs by direct healing, while for intermediate amount of IFS (5–10%) the fracture heals by indirect healing. If the stresses exceed the deformation tolerance of the fracture gap, producing a high-strain environment (IFS > 10%), the fracture will eventually culminate in pseudarthrosis (Perren, 2003). The same deforming force produces more strain at the site of a simple fracture than at that of a multi-fragmentary fractures, which tolerate more motion between the two main fragments because the overall movement is shared by several fracture planes.
Claes et al. demonstrated that in diaphyseal simple fractures, direct healing can only occur when the cortical continuity of the fractured fragments is restored and rigid fixation is provided, resulting in low inter-fragmentary movements (Claes and Heigele, 1999; Shapiro, 1988; Claes et al., 1997). Particularly, a gap between fragments lower than 0.01 mm an inter-fragmentary strain lower than 2%, is needed in order to have the fracture unite by intramembranous ossification (Shapiro, 1988). Therefore, surgical techniques with absolute stability, such as plate internal fixation, are mandatory. In complex, multi-fragmentary, diaphyseal fractures the process of indirect healing is enhanced by micro-movements and weight-bearing, therefore anatomic reduction and absolute stability are not required. Relative stability osteosynthesis techniques, such as external fixation, intramedullary nailing (IMN) and bridging plating, are more suitable since they further tolerate deforming forces. If the interfragmentary deformation is excessive or the fracture gap is too wide, bony bridging by hard callus is not obtained in spite of good callus formation, and could result in malunion, delayed union or hypertrophic non-union. On the other hand, if the fixation device is too stiff and the gap too wide will be produced a low-strain environment which will result in delayed healing and non-union (Claes et al., 1997). Therefore, the choice of the type of treatment affect the stability and lead to a specific pathway of bone repair. The ideal fixation system should provide a temporary support that protect callus formation and lead the fracture to union in the shorter time possible, allowing anatomy restoration and early mobilization.
More recent evolution of these theories, highlighted the role of the “biological fixation” (Perren, 2003). ‘Biological’ internal fixation avoids anatomic reduction, especially of the intermediate fragments, and prefers indirect reduction. Indirect reduction aims only to align the fragments and limits exposure of the bone thus reducing the surgical trauma (Baumgaertel et al., 2002). This principle applies equally to locked intramedullary nailing (IMN), bridge plating such as minimally invasive percutaneous osteosynthesis (MIPO) and less invasive stabilization system (LISS) systems, and internal fixator-like devices (Bong et al., 2007; Saini et al., 2013; Zhang et al., 2017). Callus formation is, therefore, achieved through flexible fixation using wide bridging of the fractured gap, and avoiding an extensive implant-to-bone contact. This should provide the optimal biological conditions for healing rather than absolute stability. Results of this approach showed excellent results becoming the preferred fixation especially for unstable, complex, multifragmentary diaphyseal fractures (Piétu and Ehlinger, 2017; Celebi et al., 2006; Kesemenli et al., 2002; El-Desouky et al., 2016). On the other hand, for simple shaft fractures, rigid fixation with absolute stability still represent the ideal treatment (Wenger et al., 2017; van de Wall et al., 2019). Although correct surgical indications generally lead to good clinical outcomes, delayed union and non-unions rates are still a consistent threat (Table 2).
Table 2.
Non-union rates of non-unions after surgical and conservative treatment of diaphyseal fractures (Nandra et al., 2016; Rupp et al., 2018; Zura et al., 2016).
| Fracture | Note | Treatment | Non-union rate |
|---|---|---|---|
| Humeral shaft | Debate over conservative vs operative management with plate fixation | Conservative Plate fixation Anterograde IMN Retrograde IMN |
0–13% 0–4% 1.6–33%. 4.5% |
| Femoral shaft | Requires stable fixation, early mobilization. MIPO and LISS are best plating choices. IMN fixation is the preferred system (dynamization option) | Plate fixation Antegrade IMN Unreamed Retrograde IMN |
2–7% 2.5% 13.8% 5.8% |
| Tibial shaft | High energy trauma, associated soft tissue injury. In plating risk for wound complications. IMN fixation is the preferred system (dynamization option) |
Conservative + cast Plate fixation Antegrade IMN Unreamed |
1–17% 1–7% 0–5.5% 11% |
In 2016, Elliot et al. presented their bone healing and nonunion theory (BHN) with the aim to produce an unified theory that links established facts about the physiology of bone and homeostasis with those involved in the healing of fractures and the development of nonunion. The key point is that, according to Wolff's and Frost's theories, a prolonged increase in strain will result in increased bone formation, while prolonged reduction in strain results in bone loss.
The homeostasis state is represented by a balance in osteoblast and osteoclast function and consequent with a slow bone turnover. In case of fracture, the so-called bone-healing unit act as a specific functional entity which produces a physiological response to the biological and mechanical environment leading to the normal healing of bone. The bone-healing unit evolves trough the different stages of reparative osteogenesis producing different tissues (hematoma, granulation tissue, cartilage and bone), that can tolerate various levels of strain.
The theory recognize three different types of bone healing mechanisms. A normal response to fracture in which initially, the strain is high, granulation tissue forms and the healing process gradually stiffens the area until the strain reduces and bone can form and finally remodel through normal homeostasis. This type correspond to the type of bone healing seen after nonoperative treatment of fractures and operative fixation with relative stability. The healing by callus type occurs when higher strains are within tolerable levels maximizing the formation of bone with large volumes of callus. It is typically associated with relative stability surgical techniques such as intramedullary nailing. Then primary bone healing type occurs when a fracture is treated with anatomic reduction and absolute stability. In this low-strain environment, bone healing is the result of normal homeostatic remodelling of the local bone, therefore, healing is slow and there is no callus formation. According to the BHN theory nonunion occurs primarily due to mechanical or biological origin, representing the two main path that can lead to bone healing impairment. Mechanical instability recovers the dominant role in clinical practice, and in most nonunions there is an intact bone-healing unit maintaining its biological potential of healing. When very high strain persists, the movement at the fracture site breaks down the bone-healing unit. Therefore, the main strategy for bone healing enhancement is represented by the restoration of mechanical stability and reduction of strain. In addition biological factor can positively or negatively modulate the response of the bone-healing unit to strains and the mechanical environment.
3. Biological factors influencing bone healing
The process of fracture healing can suffer from many biological factors that may interfere with its development. Biological factors are classified in patient – related factors (i.e. living habits and comorbidity) and fracture – related factors (i.e. topography, soft tissue injuries) (Zura et al., 2016; Santolini et al., 2015) (Table 3).
Table 3.
Risk factors contributing to fracture delayed union and non-union (Zura et al., 2016; Santolini et al., 2015).
| Patient-related factors | Fracture-related factors |
|---|---|
| Age | High-energy trauma |
| Smoking | Soft tissue injury |
| Alcohol consumption | Open fracture with High Gustilo-Anderson Grade |
| Poorly controlled diabetes | Large inter-fragmentary gaps |
| Malnutrition, protein deficiency | Complex or comminuted fractures |
| Reduced muscle mass, sarcopenia | Biomechanical instability |
| Osteoporosis | Large fracture haematoma |
| Vitamin D, calcium | Infection |
| Post-menopausal females | Prolonged immobilisation |
| Genetic polymorphisms | Perioperative or prolonged non-steroidal anti-inflammatory drugs (NSAID) use |
Age is the patient – related factors that plays the most important role. The periosteum of children and young adults is rich in osteoblasts and has a strong blood flow. In the elderly, instead, the periosteum is partially fibrous and originates, therefore, a slower callus formation (Cheung et al., 2016). In osteoporotic patients, both type I (postmenopausal estrogenic deficiency) and type II (aging), a delayed expression of estrogenic receptor was shown during the healing process that correlated to impairment in callus formation capacity. Other factors including progenitor cell recruitment, differentiation, and proliferation during the early phase of fracture healing are reduced due to low production of growth factors (BMP) and both qualitative and quantitative deficiency of mesenchymal cells (Cheung et al., 2016). Nikolaou et al. reported that the average time of consolidation of diaphyseal femoral fractures treated with intramedullary nailing in patients with osteoporosis was 3 weeks longer compared to a control group of healthy patients (Nikolaou et al., 2009). Moreover, surgical procedures of fractures fixation in osteoporotic patients have generally poorer outcome and higher rates of complications such as loss of reduction, implant failure and delayed union or non-union, instead (Marongiu et al., 2013). Osteoprogenitor cells activity is influenced by genetic factors. Recently Ma and O'Connor, in an experimental study on rats, identified several genes (C57BL/6, DBA/2, C3H9) that affected the process of fracture consolidation (Manigrasso and O'Connor, 2008). In mice with C57BL/6 genes, fracture consolidation was achieved in a shorter time. Hofmann et al. have shown altered cell viability and down regulated gene expression of signalling molecules (Wnt-, IGF-, TGF-β-, and FGF) in osteoblasts of patients suffering from hypertrophic non-union of long bones (Hofmann et al., 2008). Other specific molecules polymorphisms and genetic profiles were reported to affect both the cellular differentiation and the process of enchondral callus generation (Jepsen et al., 2008).
Diabetes can significantly compromise the healing process of a fracture. Shaft fractures in patients with uncontrolled diabetes often suffer impaired vascularization and therefore fracture healing is prolonged with a significantly higher risk of complications such as impaired wound healing, delayed union, and non-union (Jiao et al., 2015). On the other hand, patients under proper insulin therapy have lower risk of delayed and non-union (Simpson et al., 2011).
Also some living habits of the patients represent a well-known risk factors. Smoking increases the risk of delayed union and non-union; nicotine inhibits cellular proliferation during repair processes by altering the activity of macrophages, fibroblast and osteoblasts. In addition, it acts as a vasoconstrictor, causing impaired perfusion resulting in hypoxia and ischemia. Patients who smoke >10 cigarettes a day have shown a deficit of formation of hematoma at fracture site. The reduced vascular supply lead to atrophic non-union (Adams et al., 2001; Ziran et al., 2005).
In chronic alcoholics, high doses of Ethanol (>1000 cc/day) inhibits the ossification of newly-formed bone which is poorly mineralized and has reduced mechanical stability (Chakkalakal et al., 2005). Also the use of NSAIDs for a period > 4 weeks immediately after surgery reduces osteoblastic activity and inhibition prostaglandin synthesis with delay in callus formation (Dodwell et al., 2010).
The patient's nutritional status, especially protein/amino acid malnutrition, can negatively affect fracture healing pathways, considering that all the stages of bone healing are strictly regulated by protidic mediators such as collagens and BMPs (Meesters et al., 2018). A systematic review examining the role that vitamin D plays in fracture healing found variable results investigating the effect of vitamin D on the specific stages of bone healing. Vitamin D supplementation has been shown to stimulate osteogenesis, increase the production of osteocalcin and stimulate osteoclast-mediated bone resorption (Gorter et al., 2014). Moreover, Vitamin D deficiencies seem to promote non-union development (OR 1.14; 1.05–1.22) (Zura et al., 2016), therefore advice on diet is important particularly for older fragile patients. Also adequate calcium intake is required for callus mineralization; for patients older than 50 years of age, the recommended daily intake of calcium is 1200 mg and 800 UI of vitamin D (Fischer et al., 2018).
Fracture-related factors characterize the so-called “fracture personality”. They include the injury mechanism (i.e. a high or low energy trauma) and the vascular and soft tissue damage. Moreover, the number and the comminution of fragments, the extension of inter-fragmentary gap, the displacement and the type of surgery influence the healing pattern (Lepri et al., 2018a).
In the last decades, several clinical trials and cohort studies attempted to quantify the influence of all these factors on the healing potential of diaphyseal fractures. In a recent multicentre observational study, the FRACTING study, these general and local factors were combined in a score, calculated immediately after the treatment, and used in the attempt to predict the healing time of tibial diaphyseal fractures (Massari et al., 2013). The authors concluded that patients with higher scores more likely underwent non-union and had longer healing time (Massari et al., 2018). The resulting score could be used for selecting patients who might be receiving therapeutic interventions to enhance fracture healing (i.e. surgical techniques, cell therapy, growth factors, drugs, or physical stimuli).
4. Surgical strategies for bone healing enhancement
As mentioned before, the normal process of bone regeneration could result impaired or simply insufficient due to both mechanical and biological underlying factors. Giannoudis et al. in their “Diamond concept” theory, emphasized the role of mechanical environment as a crucial factor for bone healing enhancement, in addition to other cell and tissue-based engineering strategies (Giannoudis et al., 2007). Surgical approaches aim to restore mechanical stability of the fracture site and to stimulate or augment bone gap, through fracture fixation and bone grafting techniques, either alone or in combination. Each of the proposed treatment have a different effectiveness according to the type of bone healing impairment (delayed union, hypertrophic non-union, hypotrophic and atrophic non-union) in which is applied (Fig. 1, Fig. 2, Fig. 3).
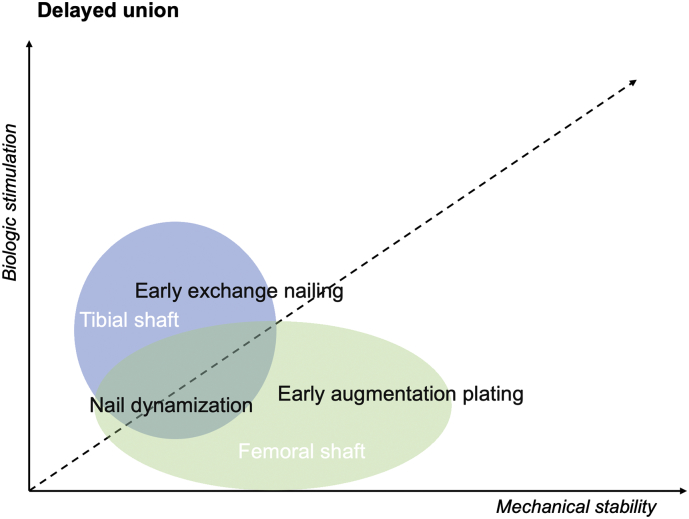
Fig. 1.
Biomechanical rationale and efficacy of surgical interventions for delayed unions of long bones shaft fractures (Litrenta et al., 2015; Vicenti et al., 2019; Ateschrang et al., 2013).
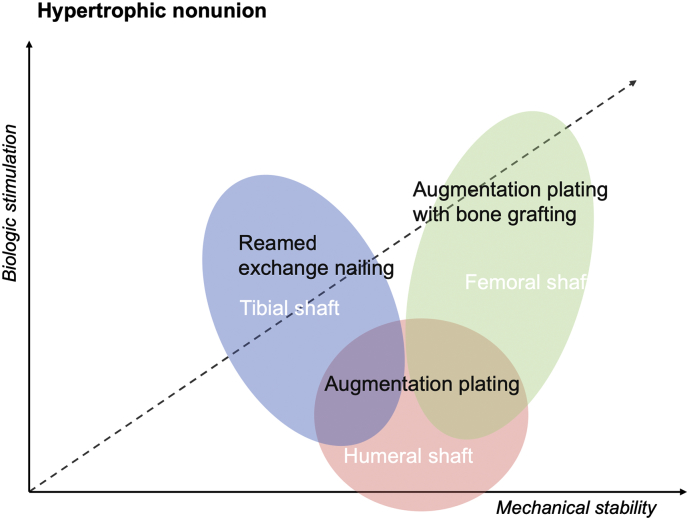
Fig. 2.
Biomechanical rationale and efficacy of surgical interventions for hypertrophic nonunions of long bones shaft fractures (Gogus et al., 2007; Brinker and O'Connor, 2007; Kashayi-Chowdojirao et al., 2017).
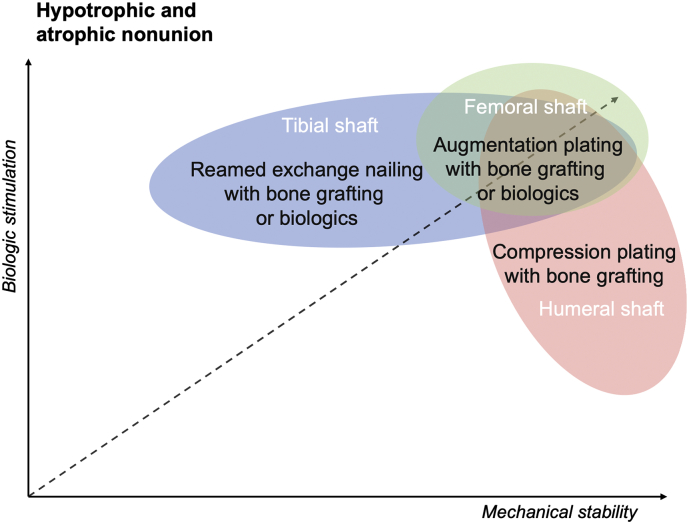
Fig. 3.
Biomechanical rationale and efficacy of surgical interventions for hypotrophic and atrophic non-union of long bones shaft fractures (Gogus et al., 2007; Dimitriou et al., 2005; Gessmann et al., 2016; Chiang et al., 2016).
The graph shows that delayed union of tibial shaft fractures, without bone defects, are more likely treated with nail dynamization and early exchange nailing which provide additional biologic stimulation at fracture site. Exchange nailing for femoral delayed union showed lower success rates and is considered a second line therapy. No evidence of surgical treatment for humeral delayed union is reported.
Reamed exchange nailing improves mechanical stability and provide biological stimulation trough auto-grafting. Augmentation plating represents an effective treatment for both femoral and humeral hypertrophic nonunions which need primarily the enhancement of the mechanical stability. Bone grafting augmentation improves the biologic stimulation after plating.
Hypotrophic and atrophic non-unions are the result of a poor biologic response at the fracture site. For humeral, tibial and femoral hypotrophic and atrophic non-unions surgical interventions has to be combined with bone grafting and/or other biologic treatments. For femoral shaft fractures exchange nailing is regarded as second line therapy, compared to augmentation plating. Humeral shaft fracture benefit both from compression and augmentation plating with bone grafting (or biologics).
4.1. Nail dynamization
Nail dynamization provides fracture compression trough the removal of interlocking screws of the IM nail, either proximal or distal to the fracture site. The bio-mechanical pathway promoted by dynamization is the enhancement of micro-movement at the fracture site resulting in stimulation of osteogenesis (Glatt et al., 2017). However, excess of movement could lead to loss of reduction and leg-length or rotation discrepancies, particularly in highly comminuted fractures and wide fracture gaps. Therefore, a fracture is considered suitable for dynamization only when sufficient stability at the fracture site is obtained. Other risk factors for unsuccessful dynamization are open fractures and unstable atrophic non-unions, whereas in unstable hypertrophic non-unions dynamization is considered an appropriate treatment option (Papakostidis et al., 2011).
In the past nail dynamization was recommended as the standard procedure after locked intramedullary nailing of long bone shaft fractures, 2 to 4 months after the surgery, while nowadays it is indicated mainly for delayed union or non-union (Egger et al., 1993). There is not consensus about the best timing for the procedure but available results suggest that dynamization of delayed union is more promising than dynamization of femoral and tibial diaphyseal non-unions (Vaughn et al., 2016). Vicenti et al. in their retrospective analysis of femoral shaft fractures, suggested to perform dynamization between three and six months after trauma. The overall healing rate was 94.1% for both femoral delayed union and non-union (Vicenti et al., 2019). Litrenta et al. in a large series of IMN dynamization for tibial shaft fracture, reported a mean 5.2 months from the trauma to the procedure. They found out union in 83% of cases and reported worst results in patients with a wide fracture gap (Litrenta et al., 2015).This technique cannot be easily applied after IM nailing of a humeral shaft fracture while humerus is not a weight bearing bone (Congia et al., 2019).
4.2. Exchange nailing
Exchange nailing involves removal of the IM nail, reaming, and replacement with a larger nail at least one millimetre thicker in its diameter. The main advantage of this technique is the additional mechanical stability provided from a larger diameter nail (Court-Brown et al., 2018). Mechanical stiffness is often improved using a multiple locking screws construct. Moreover, reaming procedure with subsequent transport of mesenchymal stem cells into the non-union site, act like an “internal autologous bone grafting” enhancing the healing process (Ghiasi et al., 2017). Several studies reported successful results after exchange nailing, with healing rate ranging from 72% to 96% (Brinker and O'Connor, 2007). Recently, results of a large series of tibial delayed union, reported superior success rate after exchange nailing compared to nail dynamization (90% vs 83%; p = 0.02) (Litrenta et al., 2015). Some studies have reported lower non-union healing rates after exchange nailing in femoral non-unions (Weresh et al., 2000) and therefore was then recommended as a second line therapy.
4.3. Augmentation plating
Augmentation plating over a retained intramedullary nail is reported as a viable option for managing long bones fracture non-unions. Plating over a retained nail improve the stiffness of the construct, reducing micromotions. Moreover, compression at non-union site can be achieved through a LC – DCP. Results in the literature are substantially satisfactory, even if not large series have been reported. Chiang et al. obtained a 96.6% of healing in 30 femur fractures with augmentation plating of after failed IMN as the index procedure (Chiang et al., 2016). Excellent union rates were reported for the treatment of both tibial (94.6%) (Ateschrang et al., 2013) and humeral shaft (97%) failed IMN nailing (Gessmann et al., 2016).
4.4. External fixation techniques
Several external fixation techniques, with both mono-axial and ring fixators, have been proposed for delayed unions and non-unions of long bones diaphyseal fractures. Ilizarov (circular) fixators allow compression at the fracture site (axial dynamization and active compression), which restores the cortical contact, confers stability to the fracture and neutralizes inter-fragmentary strain, promoting bone healing trough enchondral remodelling (Ilizarov, 1989). Aro has described this process as “healing for second contact” (Aro et al., 2006). In case of recalcitrant non-union, the fibrous fracture site can be resected leaving a bone gap which can be filled trough cortical compression.
The daily gradual (1 mm/day) distraction with fixators, enhance bone transport trough “distraction osteogenesis”, which involves intramembranous ossification pathways (direct healing) (Ilizarov, 2006). High union rates up to 97% were reported in patients suffering from femoral or tibial non-unions treated with a monolateral or circular external fixator. External fixation showed its superiority compared to the other techniques especially in the treatment of humeral non-unions (98% union rate) and in the treatment of infected non-unions (Yin et al., 2015).
5. Biological local approaches to enhance bone healing
In addition to the stability of mechanical environment, bone healing can be modulated through the use of cell- and tissue-based engineering therapies with osteoconductive, osteoinductive and osteogenic function.
Currently, we can classify the biotechnological systems for the enhancement of primary healing or management of delayed union and non-unions of long-bone fractures in 3 main categories: osteoconductive scaffolds, growth factors and osteogenic cells (Table 4).
Table 4.
Mechanism of action and efficacy of scaffold, growth factors and cell therapies in bone healing stimulation of diaphyseal fractures (Calcei and Rodeo, 2019; Schottel and Warner, 2017; Malhotra et al., 2015; Boyce et al., 1999; Calori et al., 2015; Calori et al., 2008; Sen and Miclau, 2007).
| Osteogenicity | Osteoconductivity | Osteoinductivity | Growth factors | |
|---|---|---|---|---|
| Autograft | ++ | +++ | ++ | ++ |
| Allograft | +++ | + | ||
| Demineralized bone matrix (DBM) | ++ | + | ||
| Calcium phosphate Hydroxyapatite |
+ | |||
| Bioactive glass | ++ | |||
| BMPs | +++ | +++ | ||
| Platelet rich plasma (PRP) | + | ++ | ++ | |
| Bone marrow aspirate concentrate (BMAC) | +++ | ++ | ++ |
5.1. Bone grafting
Bone grafting techniques are applied in several orthopaedic and trauma procedures to provide bone augmentation and regeneration is needed. Cortical structural grafts, used as biological plates or intramedullary support in combination with other internal fixation devices, improve mechanical stability (Gogus et al., 2007; Kashayi-Chowdojirao et al., 2017; Marongiu, 2016).
Autologous bone graft (ABG) combines all properties required in a biological graft: osteogenic, osteoconductive and osteoinductive properties. Limitations of ABG extensive use are the limited availability, graft dimension and donor site morbidity (i.e. iliac crest or fibula) (Sen and Miclau, 2007).
Allogenic bone grafts (Allograft) are available in different shapes and dimension as cortical, cancellous, osteochondral and whole-bone segments. The main disadvantage of allograft is the loss of osteogenic potential due to the removal of bone cells during the graft preparation. ABG, both vascularized and non-vascularized, have been widely applied in diaphyseal long bones fractures, with severe comminution and bone loss, in support to osteo-synthesis techniques. Zhen reported excellent results after plating and vascularized ABG for tibial shaft complex fractures. All the patients achieved union, but 10 out 38 experimented donor-site morbidities (Zhen et al., 2011). In a 45 patients' series, structural allograft associated to ORIF for complex humeral and femoral shaft fractures provided 93% and 88% union rates, respectively (Gogus et al., 2007). Moreover, bone grafting represents an additional option in all the osteoporosis related and so-called fragility fractures (Marongiu et al., 2013). Several studies have shown that cortical strut allografts represent a reliably solution in the treatment of periprosthetic femoral fractures (Capone, 2017; Tomás Hernández and Holck, 2015; Marongiu et al., 2019). Particularly, grafts have been applied successfully in type B2 or B3 in young patients, in type C and in re-osteosynthesis of a previously failed fixation, with union rates ranging from 89 to 99% (Tomás Hernández and Holck, 2015). Due to increased infection and time to union, allograft struts should be used cautiously for the treatment of Vancouver B1 factures (Moore et al., 2014). The application cortical and cancellous ABG and allograft have been widely studied in non-unions of long bones shaft fractures with consistent union rates ranging from 80 to 94% (Kashayi-Chowdojirao et al., 2017; Miska et al., 2016).
5.2. Bone substitutes
The demineralized bone matrix (DBM) is a highly processed allograft without nearly 60% of the mineral content while collagens, non-collagenous proteins and growth factors are maintained (Urist et al., 1968). DBM allograft materials satisfies both principles of osteoconductive and osteoinductive healing. The osteoconductivity of the DBM is conferred by providing a suitable matrix for cells to infiltrate, populate and for generate new bone. DBM can also aid the healing response through osteoinductive pathways, in which mesenchymal cells are stimulated by native bioactive molecules to differentiate into bone-forming cells, and can trigger the endochondral ossification cascade at the site of implantation (Boyce et al., 1999). DMB, due to its inferior structural mechanical integrity than ABG and strut-allograft is mainly applied for filling bone defects. Particularly, results in long bones fracture surgery have shown good results. Lindsey et al. reported 90% bone healing after DBM augmentation compared to 75% after iliac crest autograft in 20 consecutive long bone fractures (Lindsey et al., 2006). Another report showed shorter healing time when atypical sub-trochanteric femoral fractures were treated with DBM in addition to IMN fixation (Kulachote et al., 2016). However, the clinical level of evidence that supports the use of DBM in trauma and orthopaedic surgery is limited and consists mainly of poor quality and retrospective case-series and the resulting Grades of Recommendation are very low (van der Stok et al., 2017).
Calcium sulphate, calcium phosphate (CaP) ceramics and cements, bioactive glass or their combinations are the currently more used synthetic bone substitutes. They all share similar mechanical characteristics and the aim to mimic the osteoconductive properties of bone graft and are mainly used as void fillers in large segmental defects (Calcei and Rodeo, 2019). The use of β-tricalcium phosphate combined with demineralized bone matrix as a primary hybrid grafting were studied in femoral and tibial comminuted fractures treated by plate fixation. Antibiotics powder was added to the graft in all open fractures. All the fractures healed with solid union without any implant failure (Ayoub and El-Rosasy, 2014). The application of bioactive glass showed promising results (Civinini et al., 2017). Sun et al., in a RCT, compared the use of reamed IMN alone versus reamed IMN combined with Bioglass 45S5 grafting at the fracture sites for treating 78 high-energy tibial shaft fractures. All the patient in the experimental group healed within 6 months while 4/38 patients (10.5%) in the control group underwent to delayed union (Sun et al., 2009). Moreover, these substitutes may be combined with biologically active osteoinductive and osteogenic substrates such as bone marrow aspirate, platelet rich plasma, or BMPs (Calcei and Rodeo, 2019).
5.3. Growth factors
Synthetic growth factors, including Bone morphogenetic proteins (BMP), fibroblast growth factors (FGF), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and insulin-like growth factor (IGF), have been proposed with regard to their stimulation activity of bone healing.
The family of Bone Morphogenetic Proteins (BMP), mainly BMP-2, BMP-4 and BMP-7, has found wide application in the orthopaedic field (Dimitriou et al., 2005; Calori et al., 2015; Cho et al., 2002). BMPs regulate the activity of osteoprogenitor cells and their differentiation into osteoblasts and stimulate chondrocyte proliferation during endochondral bone formation. In experimental models of fracture healing, BMP-2 and BMP-7 increased the amount of cartilage and bone formation within the callus and reduced the time needed to reach callus mineralization and bone remodelling (Yu et al., 2010). Several clinical trials showed that BMP-2 and BMP-7 reduced the risk of fracture non-union and decreased the time to union following surgical treatment of tibial shaft fractures (Ristiniemi et al., 2007). Govender et al. in a RCT of 450 open tibia shaft fractures, showed that the application of 1.50 mg/mL recombinant (rh) BMP-2 was useful in order to reduce the healing time and the risk of non-union (Govender et al., 2002). Other studies have shown controversial results. Aro et al. showed that the healing of open tibial fractures treated with reamed intramedullary nail fixation was not significantly accelerated by the addition of an absorbable collagen sponge containing rhBMP-2 (Aro et al., 2011). Lyon et al., in patients with closed tibial fractures treated with reamed intramedullary nailing, found that the time to fracture union and pain-free full weight-bearing were not significantly reduced by 2.0-mg/mL of rhBMP-2/CPM compared with standard of care alone (Lyon et al., 2013).
The application of BMPs to treat fracture non-unions, according to several authors appeared to be a favourable alternative to autologous bone grafting. Particularly, BMP-2 or BMP-7 resulted in similar union rates (75–89%) as those achieved with autologous bone grafting (Calori et al., 2008; Kanakaris et al., 2008). As reported by Klenke and Siebenrock, interestingly, RCTs investigating the application of BMPs and autograft have not been published so far (Klenke and Siebenrock, 2016). Although the initial enthusiasm, several concerns have been raised about the use of BMPs. The induction of heterotopic ossifications has been reported from 9 to 18% of tibia fractures treated with BMPs, even if conversely other studies did not show a direct correlation (Aro et al., 2011; Lyon et al., 2013). Moreover, other morbidities are associated with its use, particularly in off-label and high dosage use, including the potential for carcinogenesis, renal and hepatic failure, and compartment syndrome (Barcak and Beebe, 2017; Guo et al., 1999). In conclusion, the actual evidence seems to limit the use of rhBMP-2 for treating open tibia fractures, specifically, Gustilo-Anderson type 3 injuries, and the use of rhBMP-7 for treating tibia shaft non-unions (Barcak and Beebe, 2017).
Fibroblast growth factors are secreted by monocytes, mesenchymal stem cells, osteoblasts and chondrocytes, from the early stages of fracture healing throughout the whole healing process. Kawaguchi et al. reported the results of rhFGF for treating tibial shaft fracture in a clinical trial including 70 patients (Kawaguchi et al., 2010). Reamed intramedullary nailing represented the standard care, then patients were randomly injected with either placebo or 0.8 mg rhFGF-2 or 2.4 mg rhFGF-2 at the fracture site. The authors found at x-rays analysis shorter healing time and higher facture union rates in both rhFGF treated groups compared to the hydrogel-only treated group.
Platelet-derived growth factor is a signalling molecule that is released by degranulating platelets in the early phases of fracture healing through its important role in chemotaxis. Although, the application of rhPDGF in bone healing have been approved by the FDA for hindfoot ankle fusion (DiGiovanni et al., 2013), currently there are no PDGF agents approved specifically for use in fracture healing (Calcei and Rodeo, 2019). Similarly, vascular endothelial growth factor has also been demonstrated to have osteoinductive function (Keramaris et al., 2008). However, all the available studies have been performed in different animal models (Kentleach et al., 2006; Eckardt et al., 2003; Buettmann et al., 2019), there is not available for clinical use.
5.4. Cells therapies
Autologous bone marrow aspirate concentrate (BMAC) has demonstrated positive outcomes in the treatment of musculoskeletal injuries. Bone marrow, specifically red marrow, contains 2 types of adult stem cells: hematopoietic stem cells and mesenchymal stem cells (MSCs). The MSCs are considered progenitor cells with demonstrated ability to repair bone defects and also have a potential variable for the development of mesenchymal tissues. However the concentration of MSC in bone marrow is <1 on 100,000 cells or <600 progenitor cells/cm3 (Hernigou et al., 2006), therefore, the ultimate goal of BMAC is to concentrate the number of progenitor cells delivered in the aspirate. Hernigou et al. reported that bone marrow from Iliac crest aspiration, after concentration, contained >2500 progenitor cells/cm3 (Hernigou et al., 2006). Although the indications for the use of BMAC in the treatment of acute fractures are not as well established, some authors described the primary fracture fixation with BMAC-enriched allografts for complex shaft fractures and bone defect, as alternative to autografts (Schottel and Warner, 2017; Jäger et al., 2011). The role of BMAC in delayed unions and non-unions of long bones is well-known. BMAC can be applied both alone or in combination with enriched scaffolds (autografts, allografts and DBM), PRPs and BMPs. Clinical case series have reported that bone marrow aspirate injection has a success of 75% to 90% in treating atrophic tibial and humeral non-unions (Rupp et al., 2018; Hernigou et al., 2006; Desai et al., 2015; Goel et al., 2005; Garnavos et al., 2010). Hernigou et al. proposed the use of BMAC alone for tibial non-union reporting brilliant results. BMAC was injected 8 months after the trauma in 60 patients, and union was obtained in 88% of cases at 4.5 months (Hernigou et al., 2006). Unfortunately, no comparative studies of bone marrow aspirate injection with other non-union treatment techniques, such as intramedullary nail dynamization, exchange nailing, or compressive plating with autologous bone grafting has been performed.
Platelet rich plasma (PRP) is an autologous blood concentrate suspension of platelets obtained via centrifugation techniques. The biologic rationale for its use in bone healing involves the local delivery of cytokines that are released from a pool of degranulating platelets. Local injection of PRP aim to mimic and augment the biological function of the hematoma at the fracture site. Specifically, the granules in platelets contain and release PDGF, transforming growth factor beta 1 (TGF-b1), VEGF, epidermal growth factor, fibroblast growth factor, and insulin-like growth factor which have been addressed to enhance the healing process of injured tissues (Oh et al., 2015). Although a number of preclinical studies reported favourable results to the use of PRP for fresh fracture enhancement (Wang and Rodeo, 2017), its clinical use in long bone fractures is mainly limited to the treatment of delayed union and non-union. In a prospective study, PRP for the treatment of 94 shaft fracture non-unions (35 tibia, 30 femur, 11 humerus, 4 radius, 12 ulna, 2 with both radius and ulna) resulted in 87% rate of union at 4 months (Malhotra et al., 2015). In randomized controlled trial, PRP injections were compared with exchange nailing for the treatment of 29 diaphyseal oligotrophic non-unions of tibia and femur, showing superior healing rate in the PRP group compared with exchange nailing group (93% vs 80%) (Duramaz et al., 2018). Despite early promising results, at the moment, the grades of recommendation for PRP use in bone healing indicates conflicting or poor-quality evidence and therefore further investigation is needed (Wang and Rodeo, 2017).
6. Biophysical stimulation of fracture healing
Adjuvant biophysical therapies have been employed in trauma surgery to optimize fracture-healing since 1950's, and their use had particular diffusion in the area of delayed unions and nonunions (Hannemann et al., 2014; Clin. Orthop. Relat. Res., 1977; Heckman et al., 1994; Scott and King, 1994; Capone et al., 2011).
6.1. Pulsed electromagnetic fields
The primary clinical application of pulsed electromagnetic fields (PEMF) was, in fact, nonunited fractures of long bones, with bone union reported up to 80% (Scott and King, 1994). In 2011 a Cochrane metanalysis showed inconclusive and insufficient results to inform current practice (Heckman et al., 1994). Other studies demonstrated successful results in the early treatment of delayed union, but the effects of the PEMF on fresh fractures were unclear (Heckman et al., 1994; Scott and King, 1994). In 2011, Adie et al. conducted a multicentre, double-blind, randomized trial including 259 patients with acute tibial shaft fractures. They concluded that adjuvant PEMF did not prevent secondary surgical interventions for delayed union or nonunion and did not improve radiographic union or patient-reported functional outcomes (Adie et al., 2011).
In 2014, a metanalysis of RCTs by Hannemann et al. investigated the effects of PEMF bone growth stimulation in acute fractures. They found significant results that suggest that the use of PEMF in acute diaphyseal fractures may accelerate the time to radiological and clinical union (Hannemann et al., 2014). The main advantages were found in patients who received conservative treatment rather than operative.
6.2. Low-intensity pulsed ultrasonography
Low-intensity pulsed ultrasonography (LIPUS) has also been applied in clinical settings for the treatment of long-bone fractures of the upper and lower extremities. First reports, provide good quality evidence on this topic but with discordant results. Heckman et al. in a prospective, randomized, double-blind trial with 77 closed tibia shaft fractures, nonoperatively treated, reported lower time of radiological and clinical union in the active-treatment group (96 ± 4.9 days) compared with the control group (154 ± 13.7 days) (p = 0.0001) (Heckman et al., 1994). On the contrary, Emami et al. in a prospective, randomized, double-blind trial with 32 closed tibia shaft fractures, treated with locked IMN, concluded that LIPUS treatment did not shorten healing time in fresh tibial fractures treated with a reamed locked IMN (Emami et al., 1999). In a Cochrane metanalysis of 12 trials, Griffin et al. assessed the effects of low-intensity ultrasound (LIPUS), high-intensity focused ultrasound (HIFUS) and extracorporeal shockwave therapies (ECSW) as part of the treatment of acute fractures in adults (Griffin et al., 2012; Griffin et al., 2014). The authors stated that a potential benefit of ultrasound for the treatment of acute fractures of the lower limb in adults cannot be excluded. However, the currently available evidence is insufficient to support the routine use of this intervention in clinical practice, due to the clinical heterogeneity of the trial included.
7. Systemic pharmacological therapy
The process of consolidation and bone remodelling of a fracture is strictly dependent by bone turnover and the calcium and phosphate metabolism. Therefore, pharmacological interventions which could modulate positively the healing process, have been recently merged to the treatment algorithm, which evolved from a diamond to a pentagon concept where the systemic therapy has the potential to represent the fifth interaction factor (Capone et al., 2014). Current research is being carried out into off-label use of two classes of anti-osteoporotic drugs, antiresorptive and anabolic agents, for complex conditions requiring enhancement of bone repair such as complicated fractures and non-union (Cheng and Shoback, 2019) (Table 5).
Table 5.
Summary of clinical evidence of pharmacological agents' effect on bone repair of long bones shaft fractures (Capone et al., 2014; Brandi, 2012).a
| Agent | Class | Clinical evidence | Quality of evidence |
|---|---|---|---|
| Calcium/vitamin D | Supplemental | May increase bone mineral density at fracture site | Weak |
| Bisphosphonates | Antiresorptive | No definitive evidence to suggest inhibition of healing. Unclear if there are benefits in augmenting healing. May increase BMD at fracture site when given 2 weeks after fracture. Improve fixation at bone implant interface |
Weak |
| Denosumab | Antiresorptive | Does not delay healing Unclear if benefits in enhancing healing |
Weak |
| Strontium ranelate | Anabolic | Case reports suggest benefit on fracture healing. Increased callus resistance and volume. May facilitate fusion after delayed union or nonunion. |
Weak |
| Teriparatide | Anabolic | Accelerated healing of long bone fractures and improved implant stabilization. Some evidence for improved healing of delayed unions, non-unions, atypical femoral fractures and periprosthetic femoral fractures |
Moderate |
Quality of clinical evidence was classified as weak, moderate, or strong based on the level of evidence of existing literature. A “weak” rating consisted of mainly level 3–4 studies. “Moderate” indicated multiple level 2 studies or conflicting level 1 data. “Strong” evidence consisted of multiple level 1 studies with generally consistent findings.
7.1. Bisphosphonates
Controversies exist on the usefulness of bisphosphonates (BP) on fracture healing. As antiresorptive drugs, inhibit bone reabsorption by blocking the action of osteoclasts, and this may cause a delay in bone remodelling. Animal studies showed larger callus and stronger mechanical strength of femoral shaft fractures after bisphosphonates administration in ovariectomized rats (Fu et al., 2013). Several studies have evaluated the effect of bisphosphonate therapy on osteoporotic women with distal radius fractures, showing that post-fracture bisphosphonate treatment led to increased bone density at the fracture site compared to placebo (Colón-Emeric et al., 2011). Moreover, the use of bisphosphonate in osteoporotic showed improved fixation stability at the bone – implant interface (Moroni et al., 2007). However weak evidence exists on the effects of bisphosphonates either to accelerate fracture healing or rescue impaired healing, and at the moment no clinical study reported results of its human use in long bones diaphyseal fractures (Hegde et al., 2016).
7.2. Denosumab
Denosumab (DMAB) is an anti-resorptive agent and monoclonal antibody directed against the receptor for nuclear factor-kappa B ligand (RANKL) and has a strong antiresorptive activity. The biggest evidence on the effect of DMAB for fracture healing originated from a subgroup analysis of the FREEDOM study, a double-blind, placebo controlled trial in post-menopausal women investigating fracture risk reduction by denosumab vs placebo (Adami et al., 2012). Results around DMAB and bone healing enhancement in non-vertebral fractures were substantially neutral: DMAB did not seem to delay fracture-healing or contribute to other complications, even when it is administered at or near the time of the fracture. Even for DMAB, clinical studies reporting results of its human use diaphyseal fractures are lacking.
7.3. Strontium ranelate
Strontium ranelate is an anti-osteoporotic drug and anabolic agent which has a dual mode activity on bone promoting both bone formation and its resorption, increasing the action of osteoblasts and reducing that of osteoclasts (Marie et al., 2001). In experimental study in ovariectomized rats with tibiae fracture, administration of strontium ranelate promoted fracture healing, increasing callus, bone mineral density and biomechanical strength at early period of fracture healing (4 weeks post fracture). Regarding the use of strontium ranelate on fracture repair in humans there are only reported clinical cases that indicate a potential benefit on long bones fractures (Alegre et al., 2012; Negri and Spivacow, 2012).
7.4. Parathormone analogues
Currently two parathormone (PTH) analogues, PTH 1–34 (or teriparatide) and PTH 1–84, are available for clinical treatment of osteoporosis (Díez-Pérez et al., 2019; Marongiu et al., 2018). The application of these osteoanabolic agents for bone healing purposes in shaft fractures of animal models have shown findings that indicates increased callus volume, mineralization, and mechanical strength (Nakajima et al., 2002; Nakazawa et al., 2005). However a wide range of different dosages and duration of the treatment have been proposed for both animal and human models (Ota et al., 2019). Although early successful clinical results were reported in two RCTs which analysed the effect of PTH (1–34) vs placebo in the treatment of distal radius and pelvis fractures (Aspenberg et al., 2010; Peichl et al., 2011), evidence that PTH analogues clinically improve fracture healing of long bones is limited to case reports or small case series.
The main reported application of teriparatide (TPTD) in bone healing is the enhancement of impaired diaphyseal fractures, such as femoral, tibial and humeral non-unions with mostly stated favourable reports (Nozaka et al., 2014; Takeuchi et al., 2019; Giannotti et al., 2013). In a prospective non-randomized non-controlled study, 32 patients with non-unions of femur, tibia, humerus and metatarsal bones were treated with 20 μg per day of teriparatide. After average 4.1 ± 1.5 months after PTH treatment, 30 of the 32 patients (95% of the cases) obtained a stable osseous consolidation of the non-union and regained full and pain-free weight-bearing (Kastirr et al., 2019).
Recently interest has focused on the use of teriparatide for the treatment of atypical femoral fractures (AFFs). AFFs are sub-trochanteric and diaphyseal fractures of the femur (between the lesser trochanter and proximal to the supracondylar flare) that occur after minimal or absence of trauma and are associated with long-term bisphosphonate or denosumab use (Lepri et al., 2018b; Shane et al., 2014). Several studies strongly support the use of PTH 1–34 in promoting bone union in AFFs (Miyakoshi et al., 2015; Watts et al., 2017; Chiang et al., 2013). Miyakoshi et al. (Miyakoshi et al., 2015) retrospectively reviewed 45 consecutive AFFs in 34 Japanese patients who received long-term oral BPs. For all AFFs treated surgically, mean time to fracture healing was significantly better in the TPTD group (5.4 ± 1.5 months) than in the non-TPTD group (8.6 ± 4.7 months, p = 0.012), and the frequency of delayed healing or non-union was significantly lower in the TPTD group than in the non-TPTD group (p = 0.014). Both Watts et al. (Watts et al., 2017) and Chiang et al. (Chiang et al., 2013) prospectively evaluated patients with AFFs and a history of previous BPs therapy. In their series, administration of 20 μg of daily TPTD for 6 to 24 months was associated with an increase in bone turnover markers, but, on the other hand, there was no consistent effect on fracture healing.
Teriparatide has been proposed for its possible benefits in the treatment of another type of shaft fractures: periprosthetic femoral fractures (PFFs). Even if encouraging results of case series are reported (Papaiordanidou et al., 2014; Kim et al., 2016), unfortunately there's a lack of high level clinical studies.
8. Conclusions
The main complication after the treatment of diaphyseal fractures is still represented by delayed union and nonunion. In order to avoid these threatening events, surgical and non-surgical approach has to be tailored differently for each fracture and each patient according to biological and biomechanical basis of bone healing.
In the last decade, the “diamond concept” for fracture healing, has given equal importance to the mechanical stability and the biologic environment, and offered a new paradigm for complex fractures and impaired union management. In this poly-therapeutic approach, modern bio-engineering technologies play a crucial role improving the traditional fixation techniques with other surgical and non-surgical approaches (Calcei and Rodeo, 2019). Additional local biological enhancement by addition of a scaffold, growth factors, and cell therapies whilst preserving the local vascular supply. The evidence available nowadays showed convincing results supporting the use of ‘polytherapy’ with the diamond concept over ‘monotherapy’, although some of the studies exploited all the aspects of the diamond concept only for high-risk patients, which may have biased the results (Andrzejowski and Giannoudis, 2019). Systemic drug therapies for long bones bone healing enhancement remains limited to anti-osteoporotic anti-resorptive and anabolic agents. The use of teriparatide in selected shaft fractures types, such as atypical femoral fractures, periprosthetic femoral fractures and recalcitrant non-unions, have yielded positive results but limited to case reports and case series. Several ongoing clinical trials, examining the effects of local enhancement approaches and the use of systemic anabolic agents would eventually provide the needed evidence on this topic. In the future results derived from computational simulation, finite element analysis and new imaging technologies such as those based on 3D modelling software, could add information and allow to monitor the bone healing process (Marongiu et al., 2020a; Lal and Patralekh, 2018; Wang et al., 2017; Marongiu et al., 2020b), in order to choose the best timing for each of the proposed treatment.
Principal institution
The work was performed at Orthopaedic and Trauma Clinic, Department of Surgical Sciences, University of Cagliari, Cagliari, Italy.
Author appointments
GM, AD, AC and MV equally contributed to the conception and design of the work and the acquisition, analysis and interpretation of data.
GM, AD, AC and MV equally contributed to manuscript's drafting for important intellectual content, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding disclosures
Each author certifies that he has no personal commercial associations that could inappropriately influence their work. This work was supported by the Open Access Publishing Fund of the University of Cagliari, with the funding of the Regione Autonoma della Sardegna – L.R. n. 7/2007.
Transparency document
Transparency document.
Footnotes
The Transparency document associated with this article can be found, in online version.
References
- Ilizarov G.A. The tension-stress effect on the genesis and growth of tissues: part I. The influence of stability. Clin. Orthop. Relat. Res. 1989;238:249–281. [PubMed] [Google Scholar]
- Adami S., Libanati C., Boonen S., Cummings S.R., Ho P.-R., Wang A., Siris E., Lane J. Denosumab treatment in postmenopausal women with osteoporosis does not interfere with fracture-healing. J. Bone Jt. Surgery-American. 2012;94:2113–2119. doi: 10.2106/JBJS.K.00774. [DOI] [PubMed] [Google Scholar]
- Adams C.I., Keating J.F., Court-Brown C.M. Cigarette smoking and open tibial fractures. Injury. 2001 doi: 10.1016/s0020-1383(00)00121-2. [DOI] [PubMed] [Google Scholar]
- Adie S., Harris I.A., Naylor J.M., Rae H., Dao A., Yong S., Ying V. Pulsed electromagnetic field stimulation for acute tibial shaft fractures. J. Bone Jt. Surgery-American. 2011;93:1569–1576. doi: 10.2106/JBJS.J.00869. [DOI] [PubMed] [Google Scholar]
- Alegre D.N., Ribeiro C., Sousa C., Correia J., Silva L., de Almeida L. Possible benefits of strontium ranelate in complicated long bone fractures. Rheumatol. Int. 2012;32:439–443. doi: 10.1007/s00296-010-1687-8. [DOI] [PubMed] [Google Scholar]
- Andrzejowski P., Giannoudis P.V. The ‘diamond concept’ for long bone non-union management. J. Orthop. Traumatol. 2019;20:21. doi: 10.1186/s10195-019-0528-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aro H.T., Kelly P.J., Lewallen D.G., Chao E.Y.S. The effects of physiologic dynamic compression on bone healing under external fixation. Clin. Orthop. Relat. Res. 2006 [PubMed] [Google Scholar]
- Aro H.T., Govender S., Patel A.D., Hernigou P., Perera de Gregorio A., Popescu G.I., Golden J.D., Christensen J., Valentin A. Recombinant human bone morphogenetic protein-2: a randomized trial in open tibial fractures treated with reamed nail fixation. J. Bone Jt. Surgery-American. 2011;93:801–808. doi: 10.2106/JBJS.I.01763. [DOI] [PubMed] [Google Scholar]
- Aspenberg P., Genant H.K., Johansson T., Nino A.J., See K., Krohn K., García-Hernández P.A., Recknor C.P., Einhorn T.A., Dalsky G.P., Mitlak B.H., Fierlinger A., Lakshmanan M.C. Teriparatide for acceleration of fracture repair in humans: a prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J. Bone Miner. Res. 2010;25:404–414. doi: 10.1359/jbmr.090731. [DOI] [PubMed] [Google Scholar]
- Ateschrang A., Karavalakis G., Gonser C., Liener U., Freude T., Stöckle U., Walcher M., Zieker D. Exchange reamed nailing compared to augmentation compression plating leaving the inserted nail in situ in the treatment of aseptic tibial non-union: a two-centre study. Wien. Klin. Wochenschr. 2013 doi: 10.1007/s00508-013-0355-x. [DOI] [PubMed] [Google Scholar]
- Ayoub M.A., El-Rosasy M.A. Hybrid grafting of post-traumatic bone defects using β-tricalcium phosphate and demineralized bone matrix. Eur. J. Orthop. Surg. Traumatol. 2014;24:663–670. doi: 10.1007/s00590-013-1253-7. [DOI] [PubMed] [Google Scholar]
- Barcak E.A., Beebe M.J. Bone morphogenetic protein. Orthop. Clin. North Am. 2017;48:301–309. doi: 10.1016/j.ocl.2017.03.004. [DOI] [PubMed] [Google Scholar]
- Baumgaertel F., Buhl M., Rahn B.A. Fracture healing in biological plate osteosynthesis. Injury. 2002 doi: 10.1016/s0020-1383(98)95002-1. [DOI] [PubMed] [Google Scholar]
- Bong M.R., Kummer F.J., Koval K.J., Egol K.A. Intramedullary nailing of the lower extremity: biomechanics and biology. J. Am. Acad. Orthop. Surg. 2007 doi: 10.5435/00124635-200702000-00004. [DOI] [PubMed] [Google Scholar]
- Boyce T., Edwards J., Scarborough N. Allograft bone. Orthop. Clin. North Am. 1999;30:571–581. doi: 10.1016/s0030-5898(05)70110-3. [DOI] [PubMed] [Google Scholar]
- Brandi M.L. Drugs for bone healing. Expert Opin. Investig. Drugs. 2012;21:1169–1176. doi: 10.1517/13543784.2012.696610. [DOI] [PubMed] [Google Scholar]
- Brinker M.R., O'Connor D.P. Exchange nailing of ununited fractures. J. Bone Jt. Surg. - Ser. A. 2007 doi: 10.2106/JBJS.F.00742. [DOI] [PubMed] [Google Scholar]
- Buettmann E.G., McKenzie J.A., Migotsky N., Sykes D.A.W., Hu P., Yoneda S., Silva M.J. VEGFA from early osteoblast lineage cells (Osterix+) is required in mice for fracture healing. J. Bone Miner. Res. 2019;34:1690–1706. doi: 10.1002/jbmr.3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcei J.G., Rodeo S.A. Orthobiologics for bone healing. Clin. Sports Med. 2019;38:79–95. doi: 10.1016/j.csm.2018.08.005. [DOI] [PubMed] [Google Scholar]
- Calori G.M., Tagliabue L., Gala L., D'Imporzano M., Peretti G., Albisetti W. Application of rhBMP-7 and platelet-rich plasma in the treatment of long bone non-unions. Injury. 2008;39:1391–1402. doi: 10.1016/j.injury.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Calori G.M., Colombo M., Bucci M., Mazza E.L., Fadigati P., Mazzola S. Clinical effectiveness of Osigraft in long-bones non-unions. Injury. 2015;46:S55–S64. doi: 10.1016/S0020-1383(15)30056-5. [DOI] [PubMed] [Google Scholar]
- Capone A. Periprosthetic fractures: epidemiology and current treatment. Clin. Cases Miner. Bone Metab. 2017;14:189. doi: 10.11138/ccmbm/2017.14.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone A., Podda D., Ennas F., Iesu C., Casciu L., Civinini R. Hyperbaric oxygen therapy for transient bone marrow oedema syndrome of the hip. HIP Int. 2011;21:211–216. doi: 10.5301/HIP.2011.6492. [DOI] [PubMed] [Google Scholar]
- Capone A., Orgiano F., Pianu F., Planta M. Orthopaedic surgeons' strategies in pharmacological treatment of fragility fractures. Clin. Cases Miner. Bone Metab. 2014;11:105–109. [PMC free article] [PubMed] [Google Scholar]
- Celebi L., Can M., Muratli H.H., Yagmurlu M.F., Yuksel H.Y., Bicimoǧlu A. Indirect reduction and biological internal fixation of comminuted subtrochanteric fractures of the femur. Injury. 2006 doi: 10.1016/j.injury.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Chakkalakal D.A., Novak J.R., Fritz E.D., Mollner T.J., McVicker D.L., Garvin K.L., McGuire M.H., Donohue T.M. Inhibition of bone repair in a rat model for chronic and excessive alcohol consumption. Alcohol. 2005 doi: 10.1016/j.alcohol.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Cheng C., Shoback D. Mechanisms underlying normal fracture healing and risk factors for delayed healing. Curr. Osteoporos. Rep. 2019;17:36–47. doi: 10.1007/s11914-019-00501-5. [DOI] [PubMed] [Google Scholar]
- Cheung W.H., Miclau T., Chow S.K.H., Yang F.F., Alt V. Fracture healing in osteoporotic bone. Injury. 2016 doi: 10.1016/S0020-1383(16)47004-X. [DOI] [PubMed] [Google Scholar]
- Chiang C.Y., Zebaze R.M.D., Ghasem-Zadeh A., Iuliano-Burns S., Hardidge A., Seeman E. Teriparatide improves bone quality and healing of atypical femoral fractures associated with bisphosphonate therapy. Bone. 2013;52:360–365. doi: 10.1016/j.bone.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Chiang J.C., Johnson J.E., Tarkin I.S., Siska P.A., Farrell D.J., Mormino M.A. Plate augmentation for femoral nonunion: more than just a salvage tool? Arch. Orthop. Trauma Surg. 2016 doi: 10.1007/s00402-015-2365-9. [DOI] [PubMed] [Google Scholar]
- Cho T.-J., Gerstenfeld L.C., Einhorn T.A. Differential temporal expression of members of the transforming growth factor β superfamily during murine fracture healing. J. Bone Miner. Res. 2002;17:513–520. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
- Civinini R., Capone A., Carulli C., Matassi F., Nistri L., Innocenti M. The kinetics of remodeling of a calcium sulfate/calcium phosphate bioceramic. J. Mater. Sci. Mater. Med. 2017;28:137. doi: 10.1007/s10856-017-5940-5. [DOI] [PubMed] [Google Scholar]
- Claes L.E., Heigele C.A. Magnitudes of local stress and strain along bony surfaces predict the course and type of fracture healing. J. Biomech. 1999 doi: 10.1016/s0021-9290(98)00153-5. [DOI] [PubMed] [Google Scholar]
- Claes L., Augat P., Suger G., Wilke H.J. Influence of size and stability of the osteotomy gap on the success of fracture healing. J. Orthop. Res. 1997 doi: 10.1002/jor.1100150414. [DOI] [PubMed] [Google Scholar]
- The classic: fundamental aspects of fracture treatment by Iwao Yasuda, reprinted from J. Kyoto Med. SocClin. Orthop. Relat. Res. 1977;4:395–406. (1953) [PubMed] [Google Scholar]
- Colón-Emeric C., Nordsletten L., Olson S., Major N., Boonen S., Haentjens P., Mesenbrink P., Magaziner J., Adachi J., Lyles K.W., Hyldstrup L., Bucci-Rechtweg C., Recknor C. Association between timing of zoledronic acid infusion and hip fracture healing. Osteoporos. Int. 2011;22:2329–2336. doi: 10.1007/s00198-010-1473-1. [DOI] [PubMed] [Google Scholar]
- Congia S., Palmas A., Marongiu G., Capone A. Is antegrade nailing a proper option in 2- and 3-part proximal humeral fractures? Musculoskelet. Surg. 2019 doi: 10.1007/s12306-019-00610-5. [DOI] [PubMed] [Google Scholar]
- Court-Brown C., Keating J., Christie J., McQueen M. Exchange intramedullary nailing. Its use in aseptic tibial nonunion. J. Bone Joint Surg. Br. 2018 [PubMed] [Google Scholar]
- Desai P., Hasan S.M., Zambrana L., Hegde V., Saleh A., Cohn M.R., Lane J.M. Bone mesenchymal stem cells with growth factors successfully treat nonunions and delayed unions. HSS J. 2015;11:104–111. doi: 10.1007/s11420-015-9432-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díez-Pérez A., Marin F., Eriksen E.F., Kendler D.L., Krege J.H., Delgado-Rodríguez M. Effects of teriparatide on hip and upper limb fractures in patients with osteoporosis: a systematic review and meta-analysis. Bone. 2019;120:1–8. doi: 10.1016/j.bone.2018.09.020. [DOI] [PubMed] [Google Scholar]
- DiGiovanni C.W., Lin S.S., Baumhauer J.F., Daniels T., Younger A., Glazebrook M., Anderson J., Anderson R., Evangelista P., Lynch S.E. Recombinant human platelet-derived growth factor-BB and beta-tricalcium phosphate (rhPDGF-BB/β-TCP): an alternative to autogenous bone graft. J. Bone Jt. Surgery-American. 2013;95:1184–1192. doi: 10.2106/JBJS.K.01422. [DOI] [PubMed] [Google Scholar]
- Dimitriou R., Dahabreh Z., Katsoulis E., Matthews S.J., Branfoot T., Giannoudis P.V. Application of recombinant BMP-7 on persistent upper and lower limb non-unions. Injury. 2005;36:S51–S59. doi: 10.1016/j.injury.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Djouad F., Bouffi C., Ghannam S., Noël D., Jorgensen C. Mesenchymal stem cells: innovative therapeutic tools for rheumatic diseases. Nat. Rev. Rheumatol. 2009 doi: 10.1038/nrrheum.2009.104. [DOI] [PubMed] [Google Scholar]
- Dodwell E.R., Latorre J.G., Parisini E., Zwettler E., Chandra D., Mulpuri K., Snyder B. NSAID exposure and risk of nonunion: a meta-analysis of case-control and cohort studies. Calcif. Tissue Int. 2010 doi: 10.1007/s00223-010-9379-7. [DOI] [PubMed] [Google Scholar]
- Duramaz A., Ursavaş H.T., Bilgili M.G., Bayrak A., Bayram B., Avkan M.C. Platelet-rich plasma versus exchange intramedullary nailing in treatment of long bone oligotrophic nonunions. Eur. J. Orthop. Surg. Traumatol. 2018;28:131–137. doi: 10.1007/s00590-017-2024-7. [DOI] [PubMed] [Google Scholar]
- Eckardt H., Bundgaard K.G., Christensen K.S., Lind M., Hansen E.S., Hvid I. Effects of locally applied vascular endothelial growth factor (VEGF) and VEGF-inhibitor to the rabbit tibia during distraction osteogenesis. J. Orthop. Res. 2003;21:335–340. doi: 10.1016/S0736-0266(02)00159-6. [DOI] [PubMed] [Google Scholar]
- Egger E.L., Gottsauner-Wolf F., Palmer J., Aro H.T., Chao E.Y.S. Effects of axial dynamization on bone healing. J. Trauma - Inj. Infect. Crit. Care. 1993 doi: 10.1097/00005373-199302000-00001. [DOI] [PubMed] [Google Scholar]
- El-Desouky I.I., Mohamed M.M., Kandil A.E. Clinical outcome of conventional versus biological fixation of subtrochanteric fractures by proximal femoral locked plate. Injury. 2016 doi: 10.1016/j.injury.2016.03.016. [DOI] [PubMed] [Google Scholar]
- Emami A., Petrén-Mallmin M., Larsson S. No effect of low-intensity ultrasound on healing time of intramedullary fixed tibial fractures. J. Orthop. Trauma. 1999 doi: 10.1097/00005131-199905000-00005. [DOI] [PubMed] [Google Scholar]
- Fischer V., Haffner-Luntzer M., Amling M., Ignatius A. Calcium and vitamin D in bone fracture healing and post-traumatic bone turnover. Eur. Cells Mater. 2018 doi: 10.22203/eCM.v035a25. [DOI] [PubMed] [Google Scholar]
- Frost H.M. Bone “mass” and the “mechanostat”: a proposal. Anat. Rec. 1987;219:1–9. doi: 10.1002/ar.1092190104. [DOI] [PubMed] [Google Scholar]
- Fu L., Tang T., Hao Y., Dai K. Long-term effects of alendronate on fracture healing and bone remodeling of femoral shaft in ovariectomized rats. Acta Pharmacol. Sin. 2013;34:387–392. doi: 10.1038/aps.2012.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnavos C., Mouzopoulos G., Morakis E. Fixed intramedullary nailing and percutaneous autologous concentrated bone-marrow grafting can promote bone healing in humeral-shaft fractures with delayed union. Injury. 2010 doi: 10.1016/j.injury.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Gessmann J., Königshausen M., Coulibaly M.O., Schildhauer T.A., Seybold D. Anterior augmentation plating of aseptic humeral shaft nonunions after intramedullary nailing. Arch. Orthop. Trauma Surg. 2016 doi: 10.1007/s00402-016-2418-8. [DOI] [PubMed] [Google Scholar]
- Ghiasi M.S., Chen J., Vaziri A., Rodriguez E.K., Nazarian A. Bone fracture healing in mechanobiological modeling: a review of principles and methods. Bone Reports. 2017 doi: 10.1016/j.bonr.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannotti S., Bottai V., Dell'Osso G., de Paola G., Pini E., Guido G. Atrophic femoral nonunion successfully treated with teriparatide. Eur. J. Orthop. Surg. Traumatol. 2013;23:291–294. doi: 10.1007/s00590-012-1143-4. [DOI] [PubMed] [Google Scholar]
- Giannoudis P.V., Einhorn T.A., Marsh D. Fracture healing: the diamond concept. Injury. 2007 doi: 10.1016/s0020-1383(08)70003-2. [DOI] [PubMed] [Google Scholar]
- Glatt V., Evans C.H., Tetsworth K. A concert between biology and biomechanics: the influence of the mechanical environment on bone healing. Front. Physiol. 2017 doi: 10.3389/fphys.2016.00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A., Sangwan S.S., Siwach R.C., Ali A.M. Percutaneous bone marrow grafting for the treatment of tibial non-union. Injury. 2005;36:203–206. doi: 10.1016/j.injury.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Gogus A., Ozturk C., Tezer M., Çamurdan K., Hamzaoglu A. “Sandwich technique” in the surgical treatment of primary complex fractures of the femur and humerus. Int. Orthop. 2007;31:87–92. doi: 10.1007/s00264-006-0134-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorter E.A., Hamdy N.A.T., Appelman-Dijkstra N.M., Schipper I.B. The role of vitamin D in human fracture healing: a systematic review of the literature. Bone. 2014 doi: 10.1016/j.bone.2014.04.026. [DOI] [PubMed] [Google Scholar]
- Govender S., Csimma C., Genant H.K., Valentin-Opran A. Recombinant human bone morphogenetic protein-2 for treatment of open Tibial fractures. J. Bone Jt. Surgery-American. 2002;84:2123–2134. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]
- Griffin X.L., Smith N., Parsons N., Costa M.L. In: Ultrasound and Shockwave Therapy for Acute Fractures in Adults. Griffin X.L., editor. John Wiley & Sons, Ltd; Chichester, UK: 2012. (Cochrane Database Syst. Rev). [DOI] [PubMed] [Google Scholar]
- Griffin X.L., Parsons N., Costa M.L., Metcalfe D. Ultrasound and shockwave therapy for acute fractures in adults. Cochrane Database Syst. Rev. 2014:CD008579. doi: 10.1002/14651858.CD008579.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Gorlick R., Ladanyi M., Meyers P.A., Huvos A.G., Bertino J.R., Healey J.H. Expression of bone morphogenetic proteins and receptors in sarcomas. Clin. Orthop. Relat. Res. 1999;365:175–183. doi: 10.1097/00003086-199908000-00023. [DOI] [PubMed] [Google Scholar]
- Hannemann P.F.W., Mommers E.H.H., Schots J.P.M., Brink P.R.G., Poeze M. The effects of low-intensity pulsed ultrasound and pulsed electromagnetic fields bone growth stimulation in acute fractures: a systematic review and meta-analysis of randomized controlled trials. Arch. Orthop. Trauma Surg. 2014 doi: 10.1007/s00402-014-2014-8. [DOI] [PubMed] [Google Scholar]
- Heckman J.D., Ryaby J.P., McCabe J., Frey J.J., Kilcoyne R.F. Acceleration of tibial fracture-healing by non-invasive, low-intensity pulsed ultrasound. J. Bone Jt. Surg. - Ser. A. 1994 doi: 10.2106/00004623-199401000-00004. [DOI] [PubMed] [Google Scholar]
- Hegde V., Jo J.E., Andreopoulou P., Lane J.M. Effect of osteoporosis medications on fracture healing. Osteoporos. Int. 2016;27:861–871. doi: 10.1007/s00198-015-3331-7. [DOI] [PubMed] [Google Scholar]
- Hernigou P., Mathieu G., Poignard A., Manicom O., Beaujean F., Rouard H. Percutaneous autologous bone-marrow grafting for nonunions: surgical technique. JBJS Essent. Surg. Tech. 2006;os-88:322–327. doi: 10.2106/JBJS.F.00203. [DOI] [PubMed] [Google Scholar]
- Hofmann A., Ritz U., Hessmann M.H., Schmid C., Tresch A., Rompe J.D., Meurer A., Rommens P.M. Cell viability, osteoblast differentiation, and gene expression are altered in human osteoblasts from hypertrophic fracture non-unions. Bone. 2008 doi: 10.1016/j.bone.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Ilizarov G.A. The tension-stress effect on the genesis and growth of tissues. Clin. Orthop. Relat. Res. 2006 [PubMed] [Google Scholar]
- Jäger M., Herten M., Fochtmann U., Fischer J., Hernigou P., Zilkens C., Hendrich C., Krauspe R. Bridging the gap: bone marrow aspiration concentrate reduces autologous bone grafting in osseous defects. J. Orthop. Res. 2011;29:173–180. doi: 10.1002/jor.21230. [DOI] [PubMed] [Google Scholar]
- Jepsen K.J., Price C., Silkman L.J., Nicholls F.H., Nasser P., Hu B., Hadi N., Alapatt M., Stapleton S.N., Kakar S., Einhorn T.A., Gerstenfeld L.C. Genetic variation in the patterns of skeletal progenitor cell differentiation and progression during endochondral bone formation affects the rate of fracture healing. J. Bone Miner. Res. 2008 doi: 10.1359/JBMR.080317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao H., Xiao E., Graves D.T. Diabetes and its effect on bone and fracture healing. Curr. Osteoporos. Rep. 2015 doi: 10.1007/s11914-015-0286-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanakaris N.K., Calori G.M., Verdonk R., Burssens P., De Biase P., Capanna R., Vangosa L.B., Cherubino P., Baldo F., Ristiniemi J., Kontakis G., Giannoudis P.V. Application of BMP-7 to tibial non-unions: a 3-year multicenter experience. Injury. 2008;39(Suppl. 2):S83–S90. doi: 10.1016/S0020-1383(08)70019-6. [DOI] [PubMed] [Google Scholar]
- Kashayi-Chowdojirao S., Vallurupalli A., Chilakamarri V.K., Patnala C., Chodavarapu L.M., Kancherla N.R., Khazi Syed A.H. Role of autologous non-vascularised intramedullary fibular strut graft in humeral shaft nonunions following failed plating. J. Clin. Orthop. Trauma. 2017;8:S21–S30. doi: 10.1016/j.jcot.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastirr I., Reichardt M., Andresen R., Radmer S., Schröder G., Westphal T., Mittlmeier T., Schober H.C. Therapy of aseptic nonunions with parathyroid hormone. Eur. J. Orthop. Surg. Traumatol. 2019;29:169–173. doi: 10.1007/s00590-018-2269-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi H., Oka H., Jingushi S., Izumi T., Fukunaga M., Sato K., Matsushita T., Nakamura K. A local application of recombinant human fibroblast growth factor 2 for tibial shaft fractures: a randomized, placebo-controlled trial. J. Bone Miner. Res. 2010;25:2735–2743. doi: 10.1002/jbmr.146. [DOI] [PubMed] [Google Scholar]
- Kentleach J., Kaigler D., Wang Z., Krebsbach P., Mooney D. Coating of VEGF-releasing scaffolds with bioactive glass for angiogenesis and bone regeneration. Biomaterials. 2006;27:3249–3255. doi: 10.1016/j.biomaterials.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Keramaris N.C., Calori G.M., Nikolaou V.S., Schemitsch E.H., Giannoudis P.V. Fracture vascularity and bone healing: a systematic review of the role of VEGF. Injury. 2008;39:S45–S57. doi: 10.1016/S0020-1383(08)70015-9. [DOI] [PubMed] [Google Scholar]
- Kesemenli C., Subasi M., Necmioglu S., Kapukaya A. Treatment of multifragmentary fractures of the femur by indirect reduction (biological) and plate fixation. Injury. 2002 doi: 10.1016/s0020-1383(02)00166-3. [DOI] [PubMed] [Google Scholar]
- Kim J.T., Jeong H.J., Lee S.J., Kim H.J., Yoo J.J. Adjuvant teriparatide therapy for surgical treatment of femoral fractures; does it work? Hip Pelvis. 2016 doi: 10.5371/hp.2016.28.3.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenke F.M., Siebenrock K.A. Ref. Modul. Biomed. Sci. Elsevier; 2016. Osteology in orthopedics – bone repair, bone grafts and bone graft substitutes; pp. 1–15. [Google Scholar]
- Kulachote N., Sa-ngasoongsong P., Sirisreetreerux N., Chanplakorn P., Fuangfa P., Suphachatwong C., Wajanavisit W. Demineralized bone matrix add-on for acceleration of bone healing in atypical subtrochanteric femoral fracture: a consecutive case-control study. Biomed. Res. Int. 2016;2016:4061539. doi: 10.1155/2016/4061539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal H., Patralekh M.K. 3D printing and its applications in orthopaedic trauma: a technological marvel. J. Clin. Orthop. Trauma. 2018;9:260–268. doi: 10.1016/j.jcot.2018.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepri A.C., Capone A., Del Prete A., Soderi S., Muncibi F., Innocenti M. Surgical strategies for high-energy fractures in patients with osteoporosis. Clin. Cases Miner. Bone Metab. 2018 [Google Scholar]
- Lepri A.C., Capone A., Del Prete A., Soderi S., Muncibi F., Civinini R. Atypical femur fractures. Clin. Cases Miner. Bone Metab. 2018;15(1):43–59. [Google Scholar]
- Lindsey R.W., Sadasivian K.K., Wood G.W., Stubbs H.A., Block J.E. Grafting long bone fractures with demineralized bone matrix putty enriched with bone marrow: pilot findings. Orthopedics. 2006;29:939–941. doi: 10.3928/01477447-20061001-07. [DOI] [PubMed] [Google Scholar]
- Litrenta J., Tornetta P., Vallier H., Firoozabadi R., Leighton R., Egol K., Kruppa C., Jones C.B., Collinge C., Bhandari M., Schemitsch E., Sanders D., Mullis B. Dynamizations and exchanges: success rates and indications. J. Orthop. Trauma. 2015 doi: 10.1097/BOT.0000000000000311. [DOI] [PubMed] [Google Scholar]
- Lyon T., Scheele W., Bhandari M., Koval K.J. Efficacy and safety of recombinant human bone matrix for closed tibial diaphyseal fracture. J Bone Jt. Surg Am. 2013;95:2088–2097. doi: 10.2106/JBJS.L.01545. [DOI] [PubMed] [Google Scholar]
- Malhotra R., Kumar V., Garg B., Singh R., Jain V., Coshic P., Chatterjee K. Role of autologous platelet-rich plasma in treatment of long-bone nonunions: a prospective study. Musculoskelet. Surg. 2015;99:243–248. doi: 10.1007/s12306-015-0378-8. [DOI] [PubMed] [Google Scholar]
- Manigrasso M.B., O'Connor J.P. Comparison of fracture healing among different inbred mouse strains. Calcif. Tissue Int. 2008 doi: 10.1007/s00223-008-9144-3. [DOI] [PubMed] [Google Scholar]
- Marie P.J., Ammann P., Boivin G., Rey C. Mechanisms of action and therapeutic potential of strontium in bone. Calcif. Tissue Int. 2001;69:121–129. doi: 10.1007/s002230010055. [DOI] [PubMed] [Google Scholar]
- Mark Fisher D., Min-Leong Wong J., Crowley C., S. Khan W. Preclinical and clinical studies on the use of growth factors for bone repair: a systematic review. Curr. Stem Cell Res. Ther. 2013 doi: 10.2174/1574888x11308030011. [DOI] [PubMed] [Google Scholar]
- Marongiu G. Atypical periprosthetic acetabular fracture in long-term alendronate therapy. Clin. Cases Miner. Bone Metab. 2016;13:209–213. doi: 10.11138/ccmbm/2016.13.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marongiu G., Mastio M., Capone A. Current options to surgical treatment in osteoporotic fractures. Aging Clin. Exp. Res. 2013;25:15–17. doi: 10.1007/s40520-013-0081-2. [DOI] [PubMed] [Google Scholar]
- Marongiu G., Congia S., Verona M., Lombardo M., Podda D., Capone A. The impact of magnetic resonance imaging in the diagnostic and classification process of osteoporotic vertebral fractures. Injury. 2018;49(Suppl. 3):S26–S31. doi: 10.1016/j.injury.2018.10.006. [DOI] [PubMed] [Google Scholar]
- Marongiu G., Podda D., Mastio M., Capone A. Long-term results of isolated acetabular revisions with reinforcement rings: a 10- to 15-year follow-up. HIP Int. 2019 doi: 10.1177/1120700018802750. [DOI] [PubMed] [Google Scholar]
- Marongiu G., Prost R., Capone A. A new diagnostic approach for periprosthetic acetabular fractures based on 3D modeling: a study protocol. Diagnostics. 2020;10:15. doi: 10.3390/diagnostics10010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marongiu G., Prost R., Capone A. Use of 3D modelling and 3D printing for the diagnostic process, decision making and preoperative planning of periprosthetic acetabular fractures. BMJ Case Rep. 2020;13:e233117. doi: 10.1136/bcr-2019-233117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massari L., Falez F., Lorusso V., Zanon G., Ciolli L., La Cava F., Cadossi M., Chiarello E., De Terlizzi F., Setti S., Benazzo F.M. Can a combination of different risk factors be correlated with leg fracture healing time? J. Orthop. Traumatol. 2013 doi: 10.1007/s10195-012-0218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massari L., Benazzo F., Falez F., Cadossi R., Perugia D., Pietrogrande L., Aloj D.C., Capone A., D'Arienzo M., Cadossi M., Lorusso V., Caruso G., Ghiara M., Ciolli L., La Cava F., Guidi M., Castoldi F., Marongiu G., La Gattuta A., Dell'Omo D., Scaglione M., Giannini S., Fortina M., Riva A., De Palma P.L., Gigante A.P., Moretti B., Solarino G., Lijoi F., Giordano G., Londini P.G., Castellano D., Sessa G., Costarella L., Barile A., Borrelli M., Rota A., Fontana R., Momoli A., Micaglio A., Bassi G., Cornacchia R.S., Castelli C., Giudici M., Monesi M., Branca Vergano L., Maniscalco P., Bulabula M., Zottola V., Caraffa A., Antinolfi P., Catani F., Severino C., Castaman E., Scialabba C., Tovaglia V., Corsi P., Friemel P., Ranellucci M., Caiaffa V., Maraglino G., Rossi R., Pastrone A., Caldora P., Cusumano C., Squarzina P.B., Baschieri U., Demattè E., Gherardi S., De Roberto C., Belluati A., Giannini A., Villani C., Persiani P., Demitri S., Di Maggio B., Abate G., De Terlizzi F., Setti S. Can clinical and surgical parameters be combined to predict how long it will take a tibia fracture to heal? A prospective multicentre observational study: the FRACTING study. Biomed. Res. Int. 2018;2018:1–7. doi: 10.1155/2018/1809091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meesters D.M., Wijnands K.A.P., Brink P.R.G., Poeze M. Malnutrition and fracture healing: are specific deficiencies in amino acids important in nonunion development? Nutrients. 2018 doi: 10.3390/nu10111597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills L.A., Aitken S.A., Simpson A.H.R.W. The risk of non-union per fracture: current myths and revised figures from a population of over 4 million adults. Acta Orthop. 2017;88:434–439. doi: 10.1080/17453674.2017.1321351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miska M., Findeisen S., Tanner M., Biglari B., Studier-Fischer S., Grützner P.A., Schmidmaier G., Moghaddam A. Treatment of nonunions in fractures of the humeral shaft according to the diamond concept. Bone Joint J. 2016;98-B:81–87. doi: 10.1302/0301-620X.98B1.35682. [DOI] [PubMed] [Google Scholar]
- Miyakoshi N., Aizawa T., Sasaki S., Ando S., Maekawa S., Aonuma H., Tsuchie H., Sasaki H., Kasukawa Y., Shimada Y. Healing of bisphosphonate-associated atypical femoral fractures in patients with osteoporosis: a comparison between treatment with and without teriparatide. J. Bone Miner. Metab. 2015;33:553–559. doi: 10.1007/s00774-014-0617-3. [DOI] [PubMed] [Google Scholar]
- Moore R.E., Baldwin K., Austin M.S., Mehta S. A systematic review of open reduction and internal fixation of periprosthetic femur fractures with or without allograft strut, cerclage, and locked plates. J. Arthroplast. 2014;29:872–876. doi: 10.1016/j.arth.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Moroni A., Faldini C., Hoang-Kim A., Pegreffi F., Giannini S. Alendronate improves screw fixation in osteoporotic bone. J. Bone Jt. Surg. 2007;89:96–101. doi: 10.2106/JBJS.F.00484. [DOI] [PubMed] [Google Scholar]
- Nakajima A., Shimoji N., Shiomi K., Shimizu S., Moriya H., Einhorn T.A., Yamazaki M. Mechanisms for the enhancement of fracture healing in rats treated with intermittent low-dose human parathyroid hormone (1-34) J. Bone Miner. Res. 2002;17:2038–2047. doi: 10.1359/jbmr.2002.17.11.2038. [DOI] [PubMed] [Google Scholar]
- Nakazawa T., Nakajima A., Shiomi K., Moriya H., Einhorn T.A., Yamazaki M. Effects of low-dose, intermittent treatment with recombinant human parathyroid hormone (1–34) on chondrogenesis in a model of experimental fracture healing. Bone. 2005;37:711–719. doi: 10.1016/j.bone.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Nandra R., Grover L., Porter K. Fracture non-union epidemiology and treatment. Trauma. 2016;18:3–11. [Google Scholar]
- Negri A.L., Spivacow F.R. Healing of subtrochanteric atypical fractures after strontium ranelate treatment. Clin. Cases Miner. Bone Metab. 2012;9:166–169. http://www.ncbi.nlm.nih.gov/pubmed/23289032 [PMC free article] [PubMed] [Google Scholar]
- Nikolaou V.S., Efstathopoulos N., Kontakis G., Kanakaris N.K., Giannoudis P.V. The influence of osteoporosis in femoral fracture healing time. Injury. 2009 doi: 10.1016/j.injury.2008.10.035. [DOI] [PubMed] [Google Scholar]
- Nozaka K., Shimada Y., Miyakoshi N., Yamada S., Hongo M., Kasukawa Y., Saito H., Kijima H. Combined effect of teriparatide and low-intensity pulsed ultrasound for nonunion: a case report. BMC Res. Notes. 2014;7:317. doi: 10.1186/1756-0500-7-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J.H., Kim W., Park K.U., Roh Y.H. Comparison of the cellular composition and cytokine-release kinetics of various platelet-rich plasma preparations. Am. J. Sports Med. 2015;43:3062–3070. doi: 10.1177/0363546515608481. [DOI] [PubMed] [Google Scholar]
- Ota M., Takahata M., Shimizu T., Momma D., Hamano H., Hiratsuka S., Amizuka N., Hasegawa T., Iwasaki N. Optimal administration frequency and dose of teriparatide for acceleration of biomechanical healing of long-bone fracture in a mouse model. J. Bone Miner. Metab. 2019;37:256–263. doi: 10.1007/s00774-018-0930-3. [DOI] [PubMed] [Google Scholar]
- Papaiordanidou M., Billot M., Varray A., Martin A. Neuromuscular fatigue is not different between constant and variable frequency stimulation. PLoS One. 2014;9:e84740. doi: 10.1371/journal.pone.0084740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papakostidis C., Psyllakis I., Vardakas D., Grestas A., Giannoudis P.V. Femoral-shaft fractures and nonunions treated with intramedullary nails: the role of dynamisation. Injury. 2011 doi: 10.1016/j.injury.2011.06.024. [DOI] [PubMed] [Google Scholar]
- Peichl P., Holzer L.A., Maier R., Holzer G. Parathyroid hormone 1-84 accelerates fracture-healing in pubic bones of elderly osteoporotic women. J. Bone Jt. Surgery-American. 2011;93:1583–1587. doi: 10.2106/JBJS.J.01379. [DOI] [PubMed] [Google Scholar]
- Perren S.M. The biomechanics and biology of internal fixation using plates and nails. Orthopedics. 1989;12:21–34. doi: 10.3928/0147-7447-19890101-06. [DOI] [PubMed] [Google Scholar]
- Perren S.M. Evolution of the internal fixation of long bone fractures: the scientific basis of biological internal fixation: choosing a new balance between stability and biology. J. Bone Jt. Surg. 2003 doi: 10.1302/0301-620x.84b8.13752. [DOI] [PubMed] [Google Scholar]
- Piétu G., Ehlinger M. Minimally invasive internal fixation of distal femur fractures. Orthop. Traumatol. Surg. Res. 2017 doi: 10.1016/j.otsr.2016.06.025. [DOI] [PubMed] [Google Scholar]
- Ristiniemi J., Flinkkilä T., Hyvönen P., Lakovaara M., Pakarinen H., Jalovaara P. RhBMP-7 accelerates the healing in distal tibial fractures treated by external fixation. J. Bone Joint Surg. Br. 2007;89-B:265–272. doi: 10.1302/0301-620X.89B2.18230. [DOI] [PubMed] [Google Scholar]
- Rupp M., Biehl C., Budak M., Thormann U., Heiss C., Alt V. Diaphyseal long bone nonunions — types, aetiology, economics, and treatment recommendations. Int. Orthop. 2018 doi: 10.1007/s00264-017-3734-5. [DOI] [PubMed] [Google Scholar]
- Saini P., Kumar R., Shekhawat V., Joshi N., Bansal M., Kumar S. Biological fixation of comminuted subtrochanteric fractures with proximal femur locking compression plate. Injury. 2013;44:226–231. doi: 10.1016/j.injury.2012.10.037. [DOI] [PubMed] [Google Scholar]
- Santolini E., West R., Giannoudis P.V. Risk factors for long bone fracture non-union: a stratification approach based on the level of the existing scientific evidence. Injury. 2015 doi: 10.1016/S0020-1383(15)30049-8. [DOI] [PubMed] [Google Scholar]
- Schottel P.C., Warner S.J. Role of bone marrow aspirate in orthopedic trauma. Orthop. Clin. North Am. 2017;48:311–321. doi: 10.1016/j.ocl.2017.03.005. [DOI] [PubMed] [Google Scholar]
- Scott G., King J.B. A prospective, double-blind trial of electrical capacitive coupling in the treatment of non-union of long bones. J. Bone Jt. Surg. - Ser. A. 1994 doi: 10.2106/00004623-199406000-00005. [DOI] [PubMed] [Google Scholar]
- Sen M.K., Miclau T. Autologous iliac crest bone graft: should it still be the gold standard for treating nonunions? Injury. 2007;38:S75–S80. doi: 10.1016/j.injury.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Shane E., Burr D., Abrahamsen B., Adler R.A., Brown T.D., Cheung A.M., Cosman F., Curtis J.R., Dell R., Dempster D.W., Ebeling P.R., Einhorn T.A., Genant H.K., Geusens P., Klaushofer K., Lane J.M., McKiernan F., McKinney R., Ng A., Nieves J., O'Keefe R., Papapoulos S., Sen Howe T., van der Meulen M.C., Weinstein R.S., Whyte M.P. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2014;29:1–23. doi: 10.1002/jbmr.1998. [DOI] [PubMed] [Google Scholar]
- Shapiro F. Cortical bone repair. The relationship of the lacunar-canalicular system and intercellular gap junctions to the repair process. J. Bone Jt. Surg. - Ser. A. 1988 [PubMed] [Google Scholar]
- Simpson C.M., Calori G.M., Giannoudis P.V. Diabetes and fracture healing: the skeletal effects of diabetic drugs. Expert Opin. Drug Saf. 2011 doi: 10.1517/14740338.2012.639359. [DOI] [PubMed] [Google Scholar]
- Sun J., Hao S., Sun R., Yang Y. Treatment of high-energy tibial shaft fractures with internal fixation and early prophylactic NovaBone grafting. Orthop. Surg. 2009;1:17–21. doi: 10.1111/j.1757-7861.2008.00004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Matsubara H., Yamamoto N., Hayashi K., Miwa S., Igarashi K., Inatani H., Tsuchiya H. Successful treatment of pathologic femoral shaft fracture associated with large arteriovenous malformations using a 3-dimensional external fixator and teriparatide: a case report. BMC Surg. 2019;19:35. doi: 10.1186/s12893-019-0498-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomás Hernández J., Holck K. Periprosthetic femoral fractures: when I use strut grafts and why? Injury. 2015;46:S43–S46. doi: 10.1016/j.injury.2015.08.012. [DOI] [PubMed] [Google Scholar]
- Urist M.R., Dowell T.A., Hay P.H., Strates B.S. Inductive substrates for bone formation. Clin. Orthop. Relat. Res. 1968;59:59–96. [PubMed] [Google Scholar]
- van de Wall B.J.M., Theus C., Link B.C., van Veelen N., van de Leeuwen R.J.H., Ganzert C., Babst R., Beeres F.J.P. Absolute or relative stability in plate fixation for simple humeral shaft fractures. Injury. 2019 doi: 10.1016/j.injury.2019.08.004. [DOI] [PubMed] [Google Scholar]
- van der Stok J., Hartholt K.A., Schoenmakers D.A.L., Arts J.J.C. The available evidence on demineralised bone matrix in trauma and orthopaedic surgery. Bone Joint Res. 2017;6:423–432. doi: 10.1302/2046-3758.67.BJR-2017-0027.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn J., Gotha H., Cohen E., Fantry A.J., Feller R.J., Van Meter J., Hayda R., Born C.T. Nail dynamization for delayed union and nonunion in femur and tibia fractures. Orthopedics. 2016 doi: 10.3928/01477447-20160819-01. [DOI] [PubMed] [Google Scholar]
- Vicenti G., Bizzoca D., Carrozzo M., Nappi V., Rifino F., Solarino G., Moretti B. The ideal timing for nail dynamization in femoral shaft delayed union and non-union. Int. Orthop. 2019;43:217–222. doi: 10.1007/s00264-018-4129-y. [DOI] [PubMed] [Google Scholar]
- Wang D., Rodeo S.A. Platelet-rich plasma in orthopaedic surgery. JBJS Rev. 2017;5:e7. doi: 10.2106/JBJS.RVW.17.00024. [DOI] [PubMed] [Google Scholar]
- Wang M., Yang N., Wang X. A review of computational models of bone fracture healing. Med. Biol. Eng. Comput. 2017 doi: 10.1007/s11517-017-1701-3. [DOI] [PubMed] [Google Scholar]
- Watts N.B., Aggers D., McCarthy E.F., Savage T., Martinez S., Patterson R., Carrithers E., Miller P.D. Responses to treatment with teriparatide in patients with atypical femur fractures previously treated with bisphosphonates. J. Bone Miner. Res. 2017;32:1027–1033. doi: 10.1002/jbmr.3081. [DOI] [PubMed] [Google Scholar]
- Wenger R., Oehme F., Winkler J., Perren S.M., Babst R., Beeres F.J.P. Absolute or relative stability in minimal invasive plate osteosynthesis of simple distal meta or diaphyseal tibia fractures? Injury. 2017 doi: 10.1016/j.injury.2017.03.005. [DOI] [PubMed] [Google Scholar]
- Weresh M.J., Hakanson R., Stover M.D., Sims S.H., Kellam J.F., Bosse M.J. Failure of exchange reamed intramedullary nails for ununited femoral shaft fractures. J. Orthop. Trauma. 2000 doi: 10.1097/00005131-200006000-00005. [DOI] [PubMed] [Google Scholar]
- Wolff J. Springer Berlin Heidelberg; Berlin, Heidelberg: 1986. The Law of Bone Remodelling. [Google Scholar]
- Yin P., Ji Q., Li T., Li J., Li Z., Liu J., Wang G., Wang S., Zhang L., Mao Z., Tang P. A systematic review and meta-analysis of Ilizarov methods in the treatment of infected nonunion of tibia and femur. PLoS One. 2015 doi: 10.1371/journal.pone.0141973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y.Y., Lieu S., Lu C., Colnot C. Bone morphogenetic protein 2 stimulates endochondral ossification by regulating periosteal cell fate during bone repair. Bone. 2010;47:65–73. doi: 10.1016/j.bone.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.B., Zhang Y.B., Wang S.H., Zhang H., Liu P., Zhang W., Ma J.L., Wang J. Clinical efficacy and safety of limited internal fixation combined with external fixation for Pilon fracture: a systematic review and meta-analysis. Chinese J. Traumatol. - English Ed. 2017 doi: 10.1016/j.cjtee.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen P., Liu X.-Y., Lu H., Li X.-S. Fixation and reconstruction of severe tibial shaft fractures with vascularized fibular grafting. Arch. Orthop. Trauma Surg. 2011;131:93–99. doi: 10.1007/s00402-010-1121-4. [DOI] [PubMed] [Google Scholar]
- Ziran B., Cheung S., Smith W., Westerheide K. Comparative efficacy of 2 different demineralized bone matrix allografts in treating long-bone nonunions in heavy tobacco smokers. Am. J. Orthop. (Belle Mead. NJ) 2005;34(7):329–332. [PubMed] [Google Scholar]
- Zura R., Xiong Z., Einhorn T., Watson J.T., Ostrum R.F., Prayson M.J., Della Rocca G.J., Mehta S., McKinley T., Wang Z., Steen R.G. Epidemiology of fracture nonunion in 18 human bones. JAMA Surg. 2016 doi: 10.1001/jamasurg.2016.2775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.