Abstract
The key players of calcium (Ca2+) homeostasis and Ca2+ signal generation, which are Ca2+ channels, Ca2+/H+ antiporters, and Ca2+-ATPases, are present in all fungi. Their coordinated action maintains a low Ca2+ baseline, allows a fast increase in free Ca2+ concentration upon a stimulus, and terminates this Ca2+ elevation by an exponential decrease – hence forming a Ca2+ signal. In this respect, the Ca2+ signaling machinery is conserved in different fungi. However, does the similarity of the genetic inventory that shapes the Ca2+ peak imply that if “you’ve seen one, you’ve seen them all” in terms of physiological relevance? Individual studies have focused mostly on a single species, and mechanisms elucidated in few model organisms are usually extrapolated to other species. This mini-review focuses on the physiological relevance of the machinery that maintains Ca2+ homeostasis for growth, virulence, and stress responses. It reveals common and divergent functions of homologous proteins in different fungal species. In conclusion, for the physiological role of these Ca2+ transport proteins, “seen one,” in many cases, does not mean: “seen them all.”
Keywords: calcium signal, calcium signaling, calcium channel, calcium pump, calcium proton antiporter, filamentous fungi, yeast
Introduction
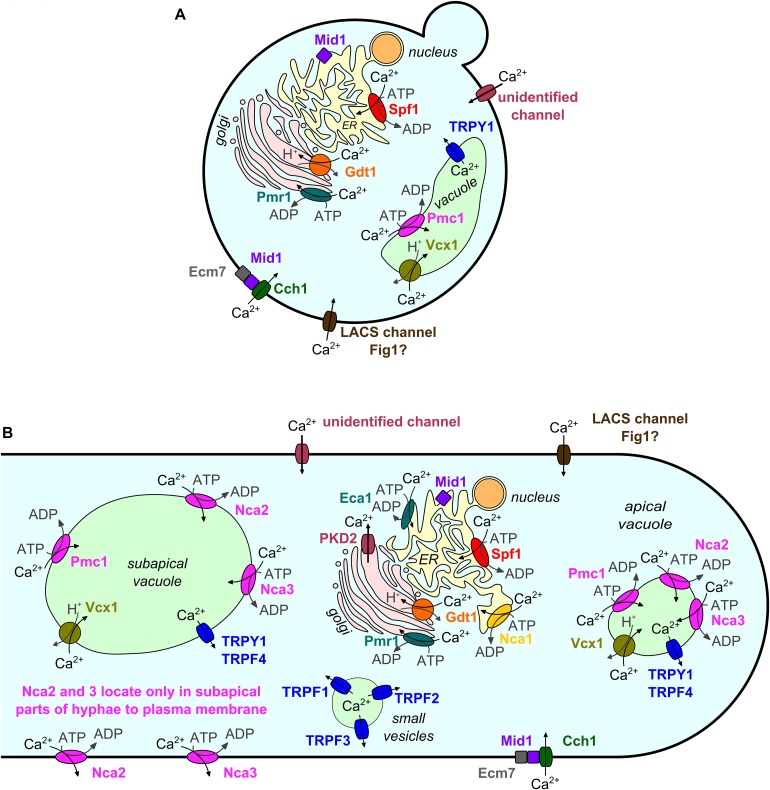
In fungi, as in other higher organisms, many stimuli and developmental cues excite calcium (Ca2+) signals, which again initiate appropriate downstream responses by changing the conformation of Ca2+-binding proteins. Ca2+ signals are usually characterized by a sharp rise in cytosolic free Ca2+ ([Ca2+]cyt) followed by an exponential decrease (Cui et al., 2009; Carbó et al., 2017). The basal [Ca2+]cyt level is low (∼100 nM in Neurospora crassa; Tamuli et al., 2013). This level is maintained by Ca2+ pumps and antiporters that export Ca2+ or sequester it into organelles (in N. crassa mainly into the vacuole; Tamuli et al., 2013). In response to a stimulus, Ca2+ channels open and allow Ca2+ to passively enter the cytosol along the concentration gradient from extracellular space or intracellular stores. Ca2+-sensitive Ca2+ channels may further amplify the signal by Ca2+-induced Ca2+ release (CICR) (Goncalves et al., 2014). Ca2+/H+ antiporters utilize the proton motive force, and Ca2+ pumps use ATP to transport Ca2+ against a concentration gradient out of the cytosol. Thereby, Ca2+/H+ antiporters and Ca2+ pumps decrease the [Ca2+]cyt again to the basal level. This set of Ca2+ transport proteins identified in the model yeast Saccharomyces cerevisiae is displayed in Figure 1A, and equivalent mechanisms found in other fungi are shown in Figure 1B. For details on mechanisms of Ca2+ homeostasis, the reader is referred to excellent general reviews, for example Cunningham (2011) for yeast or Tamuli et al. (2013) for N. crassa. A simulation of Ca2+ homeostasis in yeast is presented by Cui et al. (2009). Supplementary File 1 contains a collection of recent studies on the regulation of Ca2+ transport and homeostasis, which is not the focus of this mini-review.
FIGURE 1.
Subcellular localization of Ca2+ channels, Ca2+/H+ exchangers, and Ca2+ ATPases in the model yeast S. cerevisiae (A) and other fungi (B). Homologs are depicted in identical colors. Subcellular localizations are shown as described or assumed in the literature. Data from fungi other than S. cerevisiae are depicted in a simplified model of a fungal hypha as most of these data were gained from filamentous fungi. Note the more complex localization patterns and larger number of protein family members in non-yeast fungi.
As in other organisms, in fungi Ca2+ signals are decoded and modulated by Ca2+-sensitive proteins, such as calmodulin (CaM) and calcineurin (CN). CaM binds to CaM-dependent proteins and modulates their activity by Ca2+-induced conformational changes. The protein phosphatase CN is activated by Ca2+ itself and by CaM. CN activates the transcription factor Crz1 by dephosphorylation, thus triggering its translocation into the nucleus (Stathopoulos-Gerontides et al., 1999). Crz1 is also a central downstream target of Ca2+ signals in filamentous fungi (Schumacher et al., 2008; Choi et al., 2009).
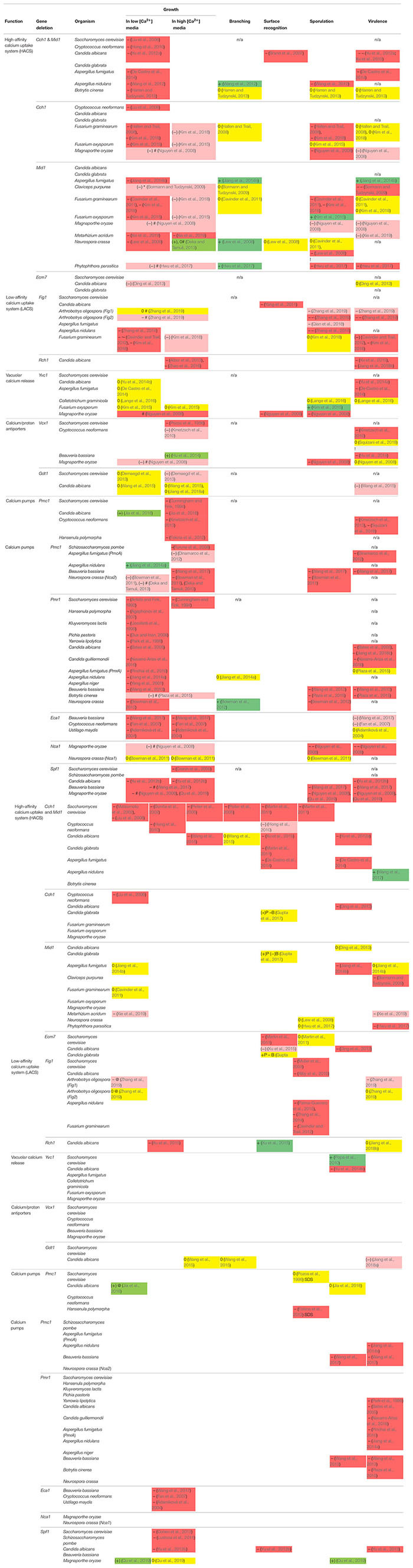
A considerable number of studies have elucidated the processes that contribute to the generation of Ca2+ signals and the physiological roles of Ca2+ transport proteins in fungi. Thereby, individual studies focus mostly on a single species, and mechanisms elucidated in few model species are usually extrapolated to other species. In this mini-review, we query the validity of this generalization by comparing findings on the impact of the Ca2+ signaling machinery in diverse fungal species. The phylogenetic diversity of mutant phenotypes with respect to growth, branching, surface recognition, sporulation, and virulence, as well as resistance to diverse stresses is condensed in Table 1. Throughout this review, the phenotype descriptions refer to this table.
TABLE 1.
Compilation of observed phenotypes for Ca2+-signaling defect mutants in different fungi. For explanation of colors and symbols, see legend below table.
 |
Ca2+ Channels – Generating a Ca2+ Signal Upon a Stimulus
The High-Affinity Ca2+ Uptake System in the Plasma Membrane
In S. cerevisiae, a high-affinity Ca2+ uptake system (HACS) is formed by Cch1, Mid1, and Ecm7. Cch1 is a homolog of the α subunit of mammalian L-type voltage-gated Ca2+ channels (Fischer et al., 1997). The transmembrane protein Mid1 interacts with Cch1 (Locke et al., 2000). Consistently, most phenotypes of deletions in either one or both genes are identical. However, MID1 has also been claimed to function independently as stretch-activated Ca2+-permeable channel localized largely in the ER (Kanzaki et al., 1999; Yoshimura et al., 2004). Ecm7 is involved in HACS-mediated Ca2+ influx, but deletion phenotypes are less drastic than those of Δcch1 or Δmid1 mutants (Martin et al., 2011; Kato et al., 2017).
In good agreement with the high affinity of HACS for Ca2+, the system is important for growth of diverse fungal species when external Ca2+ is limited. Claviceps purpurea is a notable exception in that a Δmid1 strain grows less vigorously than the wild type, but addition of Ca2+ inhibits growth even further. However, in N. crassa high-Ca2+ media lead to enhanced growth (Deka and Tamuli, 2013). In Fusarium graminearum, growth was more strongly affected in mid1 than in cch1 mutants. Only mid1 of F. graminearum produced more conidia, again pointing to independent functions of this HACS subunit (Kim et al., 2015). In some fungal species, reduced growth upon HACS deletion is associated with hyperbranching or a defect in surface recognition, while for others this is not the case. Sporulation and the tolerance to a wide variety of stresses depend on HACS in many fungi, but this requirement varies to some extent between species. The importance of HACS for virulence strongly depends on the fungal species, and ranges from essential to detrimental. In summary, the role of HACS for growth in low [Ca2+] environments is widely conserved, but other functions of the HACS vary more or less between different fungi.
The Low-Affinity Ca2+ Uptake System in the Plasma Membrane
The molecular identity of the low-affinity Ca2+ uptake system (LACS) is still unclear. Fig1, a plasma membrane protein, has been proposed to be either the LACS Ca2+ channel itself or an important regulator of it. It is needed for Ca2+ influx and normal mating in all fungi analyzed so far. In S. cerevisiae and Candida albicans, Fig1 was shown to be important for Ca2+ influx during mating and cell fusion (Muller et al., 2003; Yang et al., 2011). Deletion of Fig1 also causes retardation in vegetative growth, which can be rescued by the addition of Ca2+ in N. crassa but not in F. graminearum. In the latter species, Fig1 is more important than HACS in the generation of disease symptoms (Kim et al., 2018). Arthrobotrys oligospora has two Fig genes. Here, Fig1 is more important for stress tolerance, while Fig2 is crucial for growth, sporulation, and virulence (Zhang et al., 2019).
The plasma membrane protein Rch1 is a negative regulator of Ca2+ uptake, but the underlying mechanism is not clear (Alber et al., 2013). Rch1 is expressed under high-Ca2+ stress and important for growth in these conditions (Zhao et al., 2016). In C. albicans, Rch1 is essential for full virulence, whereby it genetically interacts with CaPMR1 (Jiang et al., 2018b).
Ca2+ Release Channels in the Vacuolar Membrane
TRPY1 (synonym Yvc1) is a Ca2+ channel of the Transient Receptor Potential family in the vacuolar membrane of S. cerevisiae (Palmer et al., 2001; Denis and Cyert, 2002; Hamamoto et al., 2018; Amini et al., 2019). It is activated by stretch (Zhou et al., 2003) and amplifies hyperosmotic shock-triggered Ca2+ signals by CICR (Su et al., 2009). In these aspects, TRPY1s of Kluyveromyces lactis (Zhou et al., 2005), C. albicans (Zhou et al., 2005), and the filamentous fungus F. graminearum (Ihara et al., 2013) resemble ScTRPY1. However, the channel of F. graminearum, but not that of S. cerevisiae, is negatively regulated by inositol phosphates (Ihara et al., 2013). The physiological relevance of TRPY1 is highly diverse between different fungi. In S. cerevisiae, a deletion of TRPY1 leads to increased resistance to oxidative stress (Popa et al., 2010); no other phenotypical differences were reported (Chang et al., 2010). In contrast, C. albicans needs TRPY1 to survive oxidative stress (Yu et al., 2014b). In C. albicans (Yu et al., 2014a) and Aspergillus fumigatus (De Castro et al., 2014), TRPY1 is important for biofilm formation and virulence, but not for growth on agar. Colletotrichum graminicola has four TRPY1 homologs (Lange et al., 2016). In this fungus, deletion of any of those genes did not lead to any differences in Ca2+ signal generation, in growth with or without stress, or in virulence. In contrast to all other fungal species analyzed so far, Magnaporthe oryzae requires TRPY1 for axenic growth on agar and for virulence (Nguyen et al., 2008). In summary, the physiological roles of TPRY1 homologs are highly diverse in the fungi studied so far.
Ca2+/H+ Antiporters – High-Capacity Low-Affinity Ca2+ Sequestration
Ca2+/H+ Exchange Over the Vacuolar Membrane
Vcx1 is a low-affinity, high-capacity Ca2+/H+ antiporter in the vacuolar membrane of S. cerevisiae. It can also sequester Mn2+. Therefore, Vcx1 allows cells to grow at very high extracellular concentrations of these ions (Cunningham and Fink, 1996; Pozos et al., 1996). Vcx1 serves to recover basal [Ca2+]cyt after a Ca2+ peak in S. cerevisiae, whereas the vacuolar Ca2+ ATPase Pmc1 (see the section “Ca2+-ATPases Sequestering Ca2+ Into the Vacuole and Exporting Ca2+ out of the Cell”) maintains the basal [Ca2+]cyt prior to a signal (Denis and Cyert, 2002). This recovery is slowed down by a repression of Vcx1 by Ca2+-activated CN (Rusnak and Mertz, 2000).
Vcx1 of Cryptococcus neoformans also localizes to the vacuolar membrane and is needed to grow on high Ca2+ but not on standard media (Kmetzsch et al., 2010). Here, both the antiporter and the ATPase maintain the [Ca2+]cyt baseline and sequestrate cytosolic Ca2+ after a peak (Kmetzsch et al., 2013). The effect of vcx1Δ for virulence of this fungus is disputed (Kmetzsch et al., 2010; Squizani et al., 2018). A knockdown of each of the four M. oryzae Vcx1 genes results in no to slight reduction of growth speed on standard media. It causes a clear reduction in sporulation and appressorium formation, but there is no effect on pathogenicity (Nguyen et al., 2008). In contrast, deletion of individual Vcx1 genes in the insect-pathogenic fungus Beauveria bassiana, which has five Vcx1 homologs, results in a moderate reduction in pathogenicity. In this fungus growth is not impaired by Vcx1 deletion on standard media, while there is a slight effect on high-Ca2+ media (Hu et al., 2014).
In summary, sequestering high Ca2+ concentrations seems to be the common job of Vcx1, while effects on growth and virulence are highly species-specific.
Ca2+/H+ Exchange Over Golgi Membranes
Gdt1 is a putative Ca2+/H+ and Mn2+/H+ antiporter of S. cerevisiae which is localized to membranes of the cis- and medial-Golgi. It is believed to be important for supplying the Golgi with Ca2+ and Mn2+, and for sequestration of high [Ca2+]cyt (Demaegd et al., 2013; Colinet et al., 2016). Gdt1 deletion causes late-Golgi glycosylation defects in particular in high-Ca2+ media, pointing to a primary role in Mn2+ transport for glycosylation (Dulary et al., 2018). Consensus motives in the transmembrane helices 1 and 4 are important for Gdt1 function (Colinet et al., 2017).
A Gdt1 homolog of C. albicans complements the respective deletion in S. cerevisiae (Wang et al., 2015). Gdt1 has been suggested to remove Ca2+ from the cytosol also in this fungus (Jiang et al., 2018a), and the mutant shows a reduced virulence (Wang et al., 2015). There is clearly more research required on the function and the physiological roles of the Gdt1 family in different fungi.
Ca2+-ATPases – Keeping the Cytosolic Free Ca2+ Concentration at a Low Basal Level and Supplying Organelles With Ca2+
Ca2+-ATPases Sequestering Ca2+ Into the Vacuole and Exporting Ca2+ Out of the Cell
The Ca2+-ATPase Pmc1 localizes to the vacuolar membrane and mediates Ca2+ sequestration, which is essential for growth of S. cerevisiae and C. albicans in high-Ca2+ media. Pmc1 activity is partially inhibited through physical interaction with Nyv1 at basal [Ca2+]cyt (Takita et al., 2001). Under conditions of high [Ca2+]cyt, expression of Nyv1 stays constant, while Pmc1 expression is induced via CaM-CN-Crz1 signaling to keep [Ca2+]cyt stable.
Aspergillus fumigatus harbors three Pmc1 homologs (PmcA, PmcB, and PmcC). A deletion of PmcC is lethal. Deletion of PmcA results in impairment of spore germination, growth at high [Ca2+] in rich (but not in minimal) media, and virulence (Dinamarco et al., 2012). B. bassiana also has three Pmc genes. Here, ΔpmcB is massively impaired in growth, while ΔpmcC is vital and only slightly impaired, and ΔpmcA grows like the wild type. PmcA-C of B. bassiana are important for full germination speed, conidiation, resistance to oxidative and cell wall stress, as well as for virulence (Wang et al., 2017). Pmc1 of C. neoformans is needed for growth on Ca2+-supplemented rich media, replication in its host, and virulence (Kmetzsch et al., 2013; Squizani et al., 2018). The non-pathogenic filamentous fungus N. crassa has two Pmc-type Ca2+-ATPases, Nca2 and Nca3 (for Nca1 see the section “Ca2+-ATPases Sequestering Ca2+ and Mn2+ Into the Golgi and ER”). Both locate to the vacuolar membrane network (VMN) and subapically also to the plasma membrane, as their mammalian homolog. Nca2 is needed to supply the VMN and export Ca2+ out of the cell. The protein is beneficial for growth in minimal media with low [Ca2+] and essential when [Ca2+]ext is high. Female spores of nca2Δ strains are infertile, and both genders produce less spores. Nca3 seems to be dispensable for growth, sporulation, and stress tolerance (Bowman et al., 2011).
Pmc1 sequesters Ca2+ into the vacuole in all fungi analyzed so far. In yeasts, Pmc1 is dispensable for normal growth but important under stressful conditions, whereas in other fungi this protein is important during the normal life cycle. In A. fumigatus and C. neoformans mutants for Pmc1 homologs, the impact of high [Ca2+]ext stress is greatly increased in richer media. Moreover, organisms with several Pmc genes show a considerable diversity in their relative importance.
Ca2+-ATPases Sequestering Ca2+ and Mn2+ Into the Golgi and ER
The phylogeny of another group of Ca2+-ATPases is separated in two clades: The Golgi-localized Pmr1 branch and the ER-borne SERCA-type Eca1/Nca1 branch (Antebi and Fink, 1992; Adamíková et al., 2004). Some fungi have only genes belonging to one of these types in their genome, but this is not conserved (Fan et al., 2007; Wang et al., 2017).
Pmr1 is required for growth in many yeast fungi, especially in low-Ca2+ or low-Mn2+ media. In S. cerevisiae, it also supports vitality of stationary phase cells irrespective of medium [Ca2+] (Rudolph et al., 1989). It is essential for protein mannosylation in different yeast species (Antebi and Fink, 1992; Bates et al., 2005; Agaphonov et al., 2007; Navarro-Arias et al., 2016). Pmr1Δ strains of C. albicans and Candida guilliermondii show a massively reduced virulence next to the phenotypes mentioned above (Bates et al., 2005; Navarro-Arias et al., 2016). In contrast to S. cerevisiae, reduced vitality is recovered by high external [Ca2+] in C. albicans (Bates et al., 2005).
Pmr1 is also important for growth of filamentous fungi, and even more so when Ca2+ availability is limited. These growth defects can be rescued in B. bassiana and N. crassa by addition of Mn2+ or Ca2+ (Bowman et al., 2012; Wang et al., 2013). Interestingly, growth defects in A. fumigatus and Aspergillus nidulans can be recovered by osmotic stabilization, but not by Ca2+ or Mn2+ (Pinchai et al., 2010; Jiang et al., 2014a). As in yeasts, Pmr1 is needed in filamentous fungi to resist cell wall stress. The relevance of Pmr1 for virulence of pathogenic fungi ranges from minor to highly important.
The Eca1 branch of this Ca2+-ATPase family was first revealed in Ustilago maydis. Eca1 resides, similar to mammalian SERCAs, in the ER (Adamíková et al., 2004). It was also found in C. neoformans (Fan et al., 2007). In B. bassiana both Pmr1 and Eca1 are present (Wang et al., 2017). In these fungi, Eca1 is responsible for removing excessive Ca2+ from the cytosol. Furthermore, it supplies the ER lumen with essential Ca2+ (Adamíková et al., 2004; Fan et al., 2007; Wang et al., 2017). Eca1 is important for growth, tolerance of ER stress, and Ca2+ signaling (Adamíková et al., 2004; Fan et al., 2007). The impact on virulence ranges from absent in U. maydis, via host- and temperature-dependent impairment in C. neoformans, to attenuated in B. bassiana.
Nca1 is closely related to Eca1. In N. crassa, Nca1 locates to the ER. In this fungus, deletion of Nca1 causes no phenotype (Bowman et al., 2011). However, in M. oryzae, knockdown of Nca1 results in a complete blockage of sporulation – rendering the fungus apathogenic – and in a slight reduction of colony growth (Nguyen et al., 2008).
In summary, there are only subtle functional differences within the Pmr1 branch between yeast and filamentous fungi. In spite of the bipartite phylogenetic relationship of the Pmr1 and SERCA-type ATPase family members, their molecular functions are quite similar. However, their relevance for virulence differs greatly between fungal species.
An Emerging Family of ER-Localized Ca2+-ATPases
Another Ca2+/Mn2+-ATPase, Spf1, is also localized to the ER membrane of S. cerevisiae. It supplies the ER lumen with Ca2+/Mn2+ and removes excessive cytosolic amounts of these ions (Suzuki and Shimma, 1999; Cronin et al., 2000; Cohen et al., 2013). Deletion of Spf1 results in reduced growth on high-Ca2+ media (Cronin et al., 2000) and defective sterol homeostasis (Sørensen et al., 2019). Furtheron, ΔSpf1 leads to Ca2+/Mn2+ deficiency in the ER lumen, which provokes protein misfolding and reduced resistance to ER stress (Cronin et al., 2000; Cohen et al., 2013). The Spf1 homolog of Schizosaccharomyces pombe, Cta4, has similar molecular functions as ScSpf1 (Lustoza et al., 2011). In C. albicans, Spf1 deletion results in similar defects and represses the formation of the more pathogenic hyphal state (Yu et al., 2012b, 2013). Spf1 of M. oryzae and B. bassiana is important for colony growth, sporulation, spore germination, and virulence (Nguyen et al., 2008; Wang et al., 2017; Qu et al., 2019). In most fungi, Spf1 is also important for stress tolerance.
Albeit examined in only few species yet, the function of Spf1 is, as known so far, very similar in different fungi as well as compared to the Pmr1/Eca1 family. This is contrasted by the clearly more diverse functions of Pmc1 in different species (see the section “Ca2+-ATPases Sequestering Ca2+ Into the Vacuole and Exporting Ca2+ Out of the Cell”). Therefore, phylogenetic similarity does not necessarily correlate with biological function.
Conclusion and Outlook
A wealth of data on transport proteins that maintain Ca2+ homeostasis and shape Ca2+ signals in fungi has been acquired in the past. However, there is also evidence that we are still missing some fundamental parts of the fungal Ca2+ signalosome. First, pharmacological Ca2+ signaling modulators have pronounced effects on Ca2+ signals in fungi (Lange and Peiter, 2016), although the canonical targets of these chemicals are often not present in the respective species. Also, mathematical modeling (Cui et al., 2009) and experimental evidence (Goncalves et al., 2014) indicate that at least two additional Ca2+ channels (next to Cch1-Mid1) must exist in the fungal plasma membrane. The LACS component Fig1 may be one interesting candidate in this respect.
Regarding the known players, the physiological role of some of the proteins involved in shaping Ca2+ signals (Pmr1, Eca1, and Spf1) appears to be quite conserved during fungal evolution, whereas in others (Mid1, TRPY1/Yvc1), there appear to be striking differences between species. Therefore, the “seen one, seen them all” principle should be applied very cautiously, in particular in translational studies aiming to develop antifungal drugs. An ensuing question remains to be answered however: What are the causes for this evolutionary divergence? Therefore, future work needs move from the description of phenotypes to the deciphering of mechanisms in phylogenetically diverse fungal species.
Author Contributions
ML and EP conceived, drafted, and finalized the manuscript, approved the final version of the article, and agreed to be accountable for all aspects of the work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG PE1500/2-1 within the Research Unit FOR 666). We acknowledge the financial support within the funding programme Open Access Publishing by the German Research Foundation (DFG).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.03100/full#supplementary-material
References
- Adamíková L., Straube A., Schulz I., Steinberg G. (2004). Calcium signaling is involved in dynein-dependent microtubule organization. Mol. Biol. Cell 15 1969–1980. 10.1091/mbc.E03-09-0675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agaphonov M. O., Plotnikova T. A., Fokina A. V., Romanova N. V., Packeiser A. N., Kang H. A., et al. (2007). Inactivation of the Hansenula polymorpha PMR1 gene affects cell viability and functioning of the secretory pathway. FEMS Yeast Res. 7 1145–1152. 10.1111/j.1567-1364.2007.00247.x [DOI] [PubMed] [Google Scholar]
- Alber J., Jiang L., Geyer J. (2013). CaRch1p does not functionally interact with the high-affinity Ca2+ influx system (HACS) of Candida albicans. Yeast 30 449–457. 10.1002/yea.2981 [DOI] [PubMed] [Google Scholar]
- Alby K., Schaefer D., Sherwood R. K., Jones S. K., Bennett R. J. (2010). Identification of a cell death pathway in Candida albicans during the response to pheromone. Eukaryot. Cell 9 1690–1701. 10.1128/Ec.00155-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini M., Wang H., Belkacemi A., Jung M., Bertl A., Schlenstedt G., et al. (2019). Identification of inhibitory Ca2+ binding sites in the upper vestibule of the yeast vacuolar TRP channel. iScience 11 1–12. 10.1016/j.isci.2018.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antebi A., Fink G. W. (1992). The yeast Ca2+-ATPase homologue. PMR1, is required for normal Golgi function and localizes in a novel Golgi-like distribution. Mol. Biol. Cell 3 633–654. 10.1091/mbc.3.6.633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates S., Maccallum D. M., Bertram G., Munro C. A., Hughes H. B., Buurman E. T., et al. (2005). Candida albicans Pmr1p, a secretory pathway P-type Ca2+/Mn2+-ATPase, is required for glycosylation and virulence. J. Biol. Chem. 280 23408–23415. 10.1074/jbc.M502162200 [DOI] [PubMed] [Google Scholar]
- Bonilla M., Nastase K. K., Cunningham K. W. (2002). Essential role of calcineurin in response to endoplasmic reticulum stress. EMBO J. 21 2343–2353. 10.1093/emboj/21.10.2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J., Tudzynski P. (2009). Deletion of Mid1, a putative stretch-activated calcium channel in Claviceps purpurea, affects vegetative growth, cell wall synthesis and virulence. Microbiology 155 3922–3933. 10.1099/mic.0.030825-0 [DOI] [PubMed] [Google Scholar]
- Bowman B. J., Abreu S., Johl J. K., Bowman E. J. (2012). The pmr gene, encoding a Ca2+-ATPase, is required for calcium and manganese homeostasis and normal development of hyphae and conidia in Neurospora crassa. Eukaryot. Cell 11 1362–1370. 10.1128/EC.00105-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman B. J., Abreu S., Margolles-Clark E., Draskovic M., Bowman E. J. (2011). Role of four calcium transport proteins, encoded by nca-1, nca-2, nca-3, and cax, in maintaining intracellular calcium levels in Neurospora crassa. Eukaryot. Cell 10 654–661. 10.1128/EC.00239-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A., Shanks S., Duncan V. M. S., Yang M., Mackenzie K., Glow N. A. R. (2007). Hyphal orientation of Candida albicans is regulated by a calcium-dependent mechanism. Curr. Biol. 17 347–352. 10.1016/j.cub.2006.12.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbó N., Tarkowski N., Ipina E. P., Dawson S. P., Aguilar P. S. (2017). Sexual pheromone modulates the frequency of cytosolic Ca2+ bursts in Saccharomyces cerevisiae. Mol. Biol. Cell 28 501–510. 10.1091/mbc.E16-07-0481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavinder B., Hamam A., Lew R. R., Trail F. (2011). Mid1, a mechanosensitive calcium ion channel, affects growth, development, and ascospore discharge in the filamentous fungus Gibberella zeae. Eukaryot. Cell 10 832–841. 10.1128/EC.00235-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavinder B., Trail F. (2012). Role of Fig1, a component of the low-affinity calcium uptake system, in growth and sexual development of filamentous fungi. Eukaryot. Cell 11 978–988. 10.1128/EC.00007-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y., Schlenstedt G., Flockerzi V., Beck A. (2010). Properties of the intracellular transient receptor potential (TRP) channel in yeast. Yvc1. FEBS Lett. 584 2028–2032. 10.1016/j.febslet.2009.12.035 [DOI] [PubMed] [Google Scholar]
- Choi J., Kim Y., Kim S., Park J., Lee Y.-H. (2009). MoCRZ1, a gene encoding a calcineurin-responsive transcription factor, regulates fungal growth and pathogenicity of Magnaporthe oryzae. Fungal Genet. Biol. 46 243–254. 10.1016/j.fgb.2008.11.010 [DOI] [PubMed] [Google Scholar]
- Cohen Y., Megyeri M., Chen O. C. W., Condomitti G., Riezman I., Loizides-Mangold U., et al. (2013). The yeast P5 type ATPase, Spf1, regulates manganese transport into the endoplasmic reticulum. PLoS One 8:e85519. 10.1371/journal.pone.0085519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colinet A.-S., Sengottaiyan P., Deschamps A., Colsoul M.-L., Thines L., Demaegd D., et al. (2016). Yeast Gdt1 is a Golgi-localized calcium transporter required for stress-induced calcium signaling and protein glycosylation. Sci. Rep. 6:24282. 10.1038/srep24282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colinet A.-S., Thines L., Deschamps A., Flémal G., Demaegd D., Morsomme P. (2017). Acidic and uncharged polar residues in the consensus motifs of the yeast Ca2+ transporter Gdt1p are required for calcium transport. Cell. Microbiol. 19:e12729. 10.1111/cmi.12729 [DOI] [PubMed] [Google Scholar]
- Cronin S. R., Khoury A., Ferry D. K., Hampton R. Y. (2000). Regulation of HMG-CoA reductase degradation requires the P-type ATPase Cod1p/Spf1p. J. Cell Biol. 148 915–924. 10.1083/jcb.148.5.915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J., Kaandorp J. A., Ositelu O. O., Beaudry V., Knight A., Nanfack Y. F., et al. (2009). Simulating calcium influx and free calcium concentrations in yeast. Cell Calcium 45 123–132. 10.1016/j.ceca.2008.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham K. W. (2011). Acidic calcium stores of Saccharomyces cerevisiae. Cell Calcium 50 129–138. 10.1016/j.ceca.2011.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham K. W., Fink G. R. (1994). Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J. Cell Biol. 124 351–363. 10.1128/MCB.16.5.2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham K. W., Fink G. R. (1996). Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol. Cell. Biol. 16 2226–2237. 10.1128/MCB.16.5.2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Castro P. A., Chiaratto J., Winkelströter L. K., Bom V. L. P., Ramalho L. N. Z., Goldman M. H. S., et al. (2014). The involvement of the Mid1/Cch1/Yvc1 calcium channels in Aspergillus fumigatus virulence. PLoS One 9:e103957. 10.1371/journal.pone.0103957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deka R., Tamuli R. (2013). Neurospora crassa ncs-1, mid-1 and nca-2 double-mutant phenotypes suggest diverse interaction among three Ca2+-regulating gene products. J. Genet. 92 559–563. 10.1007/s12041-013-0270-y [DOI] [PubMed] [Google Scholar]
- Demaegd D., Foulquier F., Colinet A. S., Gremillon L., Legrand D., Mariot P., et al. (2013). Newly characterized Golgi-localized family of proteins is involved in calcium and pH homeostasis in yeast and human cells. Proc. Natl. Acad. Sci. U.S.A. 110 6859–6864. 10.1073/pnas.1219871110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis V., Cyert M. S. (2002). Internal Ca2+ release in yeast is triggered by hypertonic shock and mediated by a TRP channel homologue. J. Cell Biol. 156 29–34. 10.1083/jcb.200111004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinamarco T. M., Freitas F. Z., Almeida R. S., Brown N. A., Dos Reis T. F., Ramalho L. N. Z., et al. (2012). Functional characterization of an Aspergillus fumigatus calcium transporter (PmcA) that is essential for fungal infection. PLoS One 7:e37951. 10.1371/journal.pone.0037591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X., Yu Q., Xu N., Wang Y., Cheng X., Qian K., et al. (2013). Ecm7, a regulator of HACS, functions in calcium homeostasis maintenance, oxidative stress response and hyphal development in Candida albicans. Fungal Genet. Biol. 57 23–32. 10.1016/j.fgb.2013.05.010 [DOI] [PubMed] [Google Scholar]
- Dulary E., Yu S.-Y., Houdo M., De Bettignies G., Decool V., Potelle S., et al. (2018). Investigating the function of Gdt1p in yeast Golgi glycosylation. Biochim. Biophys. Acta Gen. Subj. 1862 394–402. 10.1016/j.bbagen.2017.11.006 [DOI] [PubMed] [Google Scholar]
- Dux M. P., Inan M. (2006). Identification and characterization of calcium and manganese transporting ATPase (PMR1) gene of Pichia pastoris. Yeast 23 613–621. 10.1002/yea.1379 [DOI] [PubMed] [Google Scholar]
- Fan W., Idnurm A., Breger J., Mylonakis E., Heitman J. (2007). Eca1, a sarcoplasmic/endoplasmic reticulum Ca2+-ATPase, is involved in stress tolerance and virulence in Cryptococcus neoformans. Infect. Immun. 75 3394–3405. 10.1128/IAI.01977-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M., Schnell N., Chattaway J., Davies P., Dixon G., Sanders D. (1997). The Saccharomyces cerevisiae CCH1 gene is involved in calcium influx and mating. FEBS Lett. 419 259–262. 10.1016/S0014-5793(97)01466-X [DOI] [PubMed] [Google Scholar]
- Fokina A. V., Sokolov S. S., Kang H. A., Kalebina T. S., Ter-Avanesyan M. D., Agaphonov M. O. (2012). Inactivation of Pmc1 vacuolar Ca2+ ATPase causes G2 cell cycle delay in Hansenula polymorpha. Cell Cycle 11 778–784. 10.4161/cc.11.4.19220 [DOI] [PubMed] [Google Scholar]
- Furune T., Hashimoto K., Ishiguro J. (2008). Characterization of a fission yeast P5-type ATPase homologue that is essential for Ca2+/Mn2+ homeostasis in the absence of P2-type ATPases. Genes Genet. Syst. 83 373–381. 10.1266/ggs.83.373 [DOI] [PubMed] [Google Scholar]
- Goncalves A. P., Cordeiro J. M., Monteiro J., Munoz A., Correia-De-Sá P., Read N. D., et al. (2014). Activation of a TRP-like channel and intracellular Ca2+ dynamics during phospholipase-C-mediated cell death. J. Cell Sci. 127 3817–3829. 10.1242/jcs.152058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P., Meena R. C., Kumar N. (2017). Functional analysis of selected deletion mutants in Candida glabrata under hypoxia. 3 Biotech 7:193. 10.1007/s13205-017-0821-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallen H. E., Trail F. (2008). The L-type calcium ion channel Cch1 affects ascospore discharge and mycelial growth in the filamentous fungus Gibberella zeae (anamorph Fusarium graminearum). Eukaryot. Cell 7 415–424. 10.1128/EC.00248-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamamoto S., Mori Y., Yabe I., Uozumi N. (2018). In vitro and in vivo characterization of modulation of the vacuolar cation channel TRPY1 from Saccharomyces cerevisiae. FEBS J. 285 1146–1161. 10.1111/febs.14399 [DOI] [PubMed] [Google Scholar]
- Harren K., Tudzynski B. (2013). Cch1 and Mid1 are functionally required for vegetative growth under low-calcium conditions in the phytopathogenic ascomycete Botrytis cinerea. Eukaryot. Cell 12 712–724. 10.1128/EC.00338-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M.-P., Vu K., Bautos J., Gelli A. (2010). Cch1 restores intracellular Ca2+ in fungal cells during endoplasmic reticulum stress. J. Biol. Chem. 285 10951–10958. 10.1074/jbc.M109.056218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Wang J., Ying S.-H., Feng M.-G. (2014). Five vacuolar Ca2+ exchangers play different roles in calcineurin-dependent Ca2+/Mn2+ tolerance, multistress responses and virulence of a filamentous entomopathogen. Fungal Genet. Biol. 73 12–19. 10.1016/j.fgb.2014.09.005 [DOI] [PubMed] [Google Scholar]
- Hwu F.-Y., Lai M.-W., Liou R.-F. (2017). PpMID1 plays a role in the asexual development and virulence of Phytophthora parasitica. Front. Microbiol. 8:610. 10.3389/fmicb.2017.00610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara M., Hamamoto S., Miyanoiri Y., Takeda M., Kainosho M., Yabe I., et al. (2013). Molecular bases of multimodal regulation of a fungal Transient Receptor Potential (TRP) channel. J. Biol. Chem. 288 15303–15317. 10.1074/jbc.M112.434795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia C., Zhang K., Zhang D., Yu Q., Xiao C., Dong Y., et al. (2018). Effects of disruption of PMC1 in the tfp1Δ/Δ mutant on calcium homeostasis, oxidative and osmotic stress resistance in Candida albicans. Mycopathologia 183 315–327. 10.1007/s11046-017-0216-7 [DOI] [PubMed] [Google Scholar]
- Jiang H., Liu F., Zhang S., Lu L. (2014a). Putative PmrA and PmcA are important for normal growth, morphogenesis and cell wall integrity, but not for viability in Aspergillus nidulans. Microbiology 160 2387–2395. 10.1099/mic.0.080119-0 [DOI] [PubMed] [Google Scholar]
- Jiang H., Shen Y., Liu W., Lu L. (2014b). Deletion of the putative stretch-activated ion channel Mid1 is hypervirulent in Aspergillus fumigatus. Fungal Genet. Biol. 62 62–70. 10.1016/j.fgb.2013.11.003 [DOI] [PubMed] [Google Scholar]
- Jiang L., Wang J., Asghar F., Snyder N., Cunningham K. W. (2018a). CaGdt1 plays a compensatory role for the calcium pump CaPmr1 in the regulation of calcium signaling and cell wall integrity signaling in Candida albicans. Cell Commun. Signal. 16:33. 10.1186/s12964-018-0246-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Xu D., Hameed A., Fang T., Bakr Ahmad, Fazili A., et al. (2018b). The plasma membrane protein Rch1 and the Golgi/ER calcium pump Pmr1 have an additive effect on filamentation in Candida albicans. Fungal Genet. Biol. 115 1–8. 10.1016/j.fgb.2018.04.001 [DOI] [PubMed] [Google Scholar]
- Kanzaki M., Nagasawa M., Kojima I., Sato C., Naruse K., Sokabe M., et al. (1999). Molecular identification of a eucaryotic, strech-activated nonselective cation channel. Science 285 882–886. 10.1126/science.285.5429.882 [DOI] [PubMed] [Google Scholar]
- Kato T., Kubo A., Nagayama T., Kume S., Tanaka C., Nakayama Y., et al. (2017). Genetic analysis of the regulation of the voltage-gated calcium channel homolog Cch1 by the γ subunit homolog Ecm7 and cortical ER protein Scs2 in yeast. PLoS One 12:e0181436 10.1371/journal.pone.0181436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.-S., Kim J.-E., Frailey D., Nohe A., Duncan R., Czymmek K. J., et al. (2015). Roles of three Fusarium oxysporum calcium ion (Ca2+) channels in generating Ca2+ signatures and controlling growth. Fungal Genet. Biol. 82 145–157. 10.1016/j.fgb.2015.07.003 [DOI] [PubMed] [Google Scholar]
- Kim H.-S., Kim J.-E., Son H., Frailey D., Cirino R., Lee Y.-W., et al. (2018). Roles of three Fusarium graminearum membrane Ca2+ channels in the formation of Ca2+ signatures, growth, development, pathogenicity and mycotoxin production. Fungal Genet. Biol. 111 30–46. 10.1016/j.fgb.2017.11.005 [DOI] [PubMed] [Google Scholar]
- Kmetzsch L., Staats C. C., Cupertino J. B., Fonseca F. L., Rodrigues M. L., Schrank A., et al. (2013). The calcium transporter Pmc1 provides Ca2+ tolerance and influences the progression of murine cryptococcal infection. FEBS J. 280 4853–4864. 10.1111/febs.12458 [DOI] [PubMed] [Google Scholar]
- Kmetzsch L., Staats C. C., Simon E., Fonseca F. L., De Oliveira D. L., Sobrino L., et al. (2010). The vacuolar Ca2+ exchanger Vcx1 is involved in calcineurin-dependent Ca2+ tolerance and virulence in Cryptococcus neoformans. Eukaryot. Cell 9 1798–1805. 10.1111/febs.12458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange M., Peiter E. (2016). Cytosolic free calcium dynamics as related to hyphal and colony growth in the filamentous fungal pathogen Colletotrichum graminicola. Fungal Genet. Biol. 91 55–65. 10.1016/j.fgb.2016.04.001 [DOI] [PubMed] [Google Scholar]
- Lange M., Weihmann F., Schliebner I., Horbach R., Deising H. B., Wirsel S. G. R., et al. (2016). The Transient Receptor Potential (TRP) channel family in Colletotrichum graminicola: a molecular and physiological analysis. PLoS One 11:e0158561. 10.1371/journal.pone.0158561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew R. R., Abbas Z., Anderca M. I., Free S. J. (2008). Phenotype of a mechanosensitive channel mutant, mid-1, in a filamentous fungus, Neurospora crassa. Eukaryot. Cell 7 647–655. 10.1128/EC.00411-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Du P., Heinrich G., Cox G. M., Gelli A. (2006). Cch1 mediates calcium entry in Cryptococcus neoformans and is essential in low-calcium environments. Eukaryot. Cell 5 1788–1796. 10.1128/EC.00158-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke E. G., Bonilla M., Liang L., Takita Y., Cunningham K. W. (2000). A homolog of voltage-gated Ca2+ channels stimulated by depletion of secretory Ca2+ in yeast. Mol. Cell. Biol. 20 6686–6694. 10.1128/MCB.20.18.6686-6694.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustoza A. C. D. M., Palma L. M., Facanha A. R., Okorokov L. A., Okorokova-Facanha A. L. (2011). P5A-type ATPase Cta4p is essential for Ca2+ transport in the endoplasmic reticulum of Schizosaccharomyces pombe. PLoS One 6:e27843. 10.1371/journal.pone.0027843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. C., Kim H., Mackin N. A., Maldonado-Báez L., Evangelista C. C., Beaudry V. G., et al. (2011). New regulators of a high affinity Ca2+ influx system revealed through a genome-wide screen in yeast. J. Biol. Chem. 286 10744–10754. 10.1074/jbc.M110.177451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T. K., Ellsmore A. J., Cessna S. G., Low P. S., Pardo J. M., Bressnan R. A., et al. (2002). An osmotically induced cytosolic Ca2+ transient activates calcineurin signaling to mediate ion homeostasis and salt tolerance of Saccharomyces cerevisiae. J. Biol. Chem. 277 33075–33080. 10.1074/jbc.M205037200 [DOI] [PubMed] [Google Scholar]
- Muller E. M., Mackin N. A., Erdman S. E., Cunningham K. W. (2003). Fig1p facilitates Ca2+ influx and cell fusion during mating of Saccharomyces cerevisiae. J. Biol. Chem. 278 38461–38469. 10.1074/jbc.M304089200 [DOI] [PubMed] [Google Scholar]
- Navarro-Arias M. J., Defosse T. A., Dementhon K., Csonka K., Mellado-Mojica E., Valério A. D., et al. (2016). Disruption of protein mannosylation affects Candida guilliermondii cell wall, immune sensing, and virulence. Front. Microbiol. 7:1951. 10.3389/fmicb.2016.01951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Q. B., Kadotani N., Kasahara S., Tosa Y., Mayama S., Nakayahiki H. (2008). Systematic functional analysis of calcium-signalling proteins in the genome of the rice-blast fungus, Magnaporthe oryzae, using a high-throughput RNA-silencing system. Mol. Microbiol. 68 1348–1365. 10.1111/j.1365-2958.2008.06242.x [DOI] [PubMed] [Google Scholar]
- Palma-Guerrero J., Zhao J. H., Gonalves A. P., Starr T. L., Glass N. L. (2015). Identification and characterization of LFD-2, a predicted fringe protein required for membrane integrity during cell fusion in Neurospora crassa. Eukaryot. Cell 14 265–277. 10.1128/Ec.00233-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer C. P., Zhou X.-L., Lin J., Loukin S. H., Kung C., Saimi Y. (2001). A TRP homolog in Saccharomyces cerevisiae forms an intracellular Ca2+-permeable channel in the yeast vacuolar membrane. Proc. Natl. Acad. Sci. U.S.A. 98 7801–7805. 10.1073/pnas.141036198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C. S., Kim J.-Y., Crispino C., Chang C. C., Ryu D. D. Y. (1998). Molecular cloning of YlPMR1, a S. cerevisiae PMR1 homologue encoding a novel P-type secretory pathway Ca2+-ATPase, in the yeast Yarrowia lipolytica. Gene 206 107–116. 10.1016/S0378-1119(97)00573-8 [DOI] [PubMed] [Google Scholar]
- Peiter E., Fischer M., Sidaway K., Roberts S. K., Sanders D. (2005). The Saccharomyces cerevisiae Ca2+ channel Cch1pMid1p is essential for tolerance to cold stress and iron toxicity. FEBS Lett. 579 5697–5703. 10.1016/j.febslet.2005.09.058 [DOI] [PubMed] [Google Scholar]
- Pinchai N., Juvvadi P. R., Fortwendel J. R., Perfect B. Z., Rogg L. E., Asfaw Y. G., et al. (2010). The Aspergillus fumigatus P-type Golgi apparatus Ca2+/Mn2+ ATPase PmrA is involved in cation homeostasis and cell wall integrity but is not essential for pathogenesis. Eukaryot. Cell 9 472–476. 10.1128/EC.00378-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza V., Lagües Y., Carvajal M., Pérez-García L. A., Mora-Montes H. M., Canessa P., et al. (2015). bcpmr1 encodes a P-type Ca2+/Mn2+-ATPase mediating cell-wall integrity and virulence in the phytopathogen Botrytis cinerea. Fungal Genet. Biol. 76 36–46. 10.1016/j.fgb.2015.01.012 [DOI] [PubMed] [Google Scholar]
- Popa C.-V., Dumitru I., Ruta L. L., Danet A. F., Farcasanu I. C. (2010). Exogenous oxidative stress induces Ca2+ release in the yeast Saccharomyces cerevisiae. FEBS J. 277 4027–4038. 10.1111/j.1742-4658.2010.07794.x [DOI] [PubMed] [Google Scholar]
- Pozos T. C., Sekler I., Cyert M. S. (1996). The product of HUM1, a novel yeast gene, is required for vacuolar Ca2+/H+ exchange and is related to mammalian Na+/Ca2+ exchangers. Mol. Cell. Biol. 16 3730–3741. 10.1128/MCB.16.7.3730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H., Chen Q., Zhang S., Lu L. (2018). The claudin family protein FigA mediates Ca2+ homeostasis in response to extracellular stimuli in Aspergillus nidulans and Aspergillus fumigatus. Front. Microbiol. 9:977. 10.3389/fmicb.2018.00977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y., Wang J., Zhu X., Dong B., Liu X., Lu J., et al. (2019). The P5-type ATPase Spf1 is required for development and virulence of the rice blast fungus Pyricularia oryzae. Current Genetics 65:467–471. 10.1007/s00294-019-01030-5 [DOI] [PubMed] [Google Scholar]
- Rudolph H. K., Antebi A., Fink G. R., Buckley C. M., Dorman T. E., Levitre J., et al. (1989). The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+ ATPase family. Cell 58 133–145. 10.1016/0092-8674(89)90410-8 [DOI] [PubMed] [Google Scholar]
- Rusnak F., Mertz P. (2000). Calcineurin: form and function. Physiol. Rev. 80 1483–1521. 10.1152/physrev.2000.80.4.1483 [DOI] [PubMed] [Google Scholar]
- Schumacher J., De Larrinoa I. F., Tudzynski B. (2008). Calcineurin-responsive zinc finger transcription factor CRZ1 of Botrytis cinerea is required for growth, development, and full virulence on bean plants. Eukaryot. Cell 7 584–601. 10.1128/EC.00426-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen D. M., Holen H. W., Pedersen J. T., Martens H. J., Daniele Silvestro D., Stanchev L. D., et al. (2019). The P5A ATPase Spf1p is stimulated by phosphatidylinositol 4-phosphate and influences cellular sterol homeostasis. Mol. Biol. Cell 30 1069–1084. 10.1091/mbc.E18-06-0365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squizani E. D., Oliveira N. K., Reuwsaat J. C. V., Marques B. M., Lopes W., Gerber A. L., et al. (2018). Cryptococcal dissemination to the central nervous system requires the vacuolar calcium transporter Pmc1. Cell. Microbiol. 20:e12803 10.1111/cmi.12803 [DOI] [PubMed] [Google Scholar]
- Stathopoulos-Gerontides A., Guo J. J., Cyert M. S. (1999). Yeast calcineurin regulates nuclear localization of the Crz1p transcription factor through dephosphorylation. Genes Dev. 13 798–803. 10.1101/gad.13.7.798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z., Zhou X., Loukin S. H., Saimi Y., Kung C. (2009). Mechanical force and cytoplasmic Ca2+ activate yeast TRPY1 in parallel. J. Membr. Biol. 227 141–150. 10.1007/s00232-009-9153-9 [DOI] [PubMed] [Google Scholar]
- Suzuki C., Shimma Y. (1999). P-type ATPase spf1 mutants show a novel resistance mechanism for the killer toxin SMKT. Mol. Microbiol. 32 813–823. 10.1046/j.1365-2958.1999.01400.x [DOI] [PubMed] [Google Scholar]
- Takita Y., Engstrom L., Ungermann C., Cunningham K. W. (2001). Inhibition of the Ca2+-ATPase Pmc1p by the v-SNARE protein Nyv1p. J. Biol. Chem. 276 6200–6206. 10.1074/jbc.M009191200 [DOI] [PubMed] [Google Scholar]
- Tamuli R., Kumar R., Srivastava D. A., Deka R. (2013). “Calcium signaling,” in Neurospora: Genomics and Molecular Biology, ed. Kasbekar D. P., McCluskey K. (Poole: Caister Academic Press; ), 209–226. [Google Scholar]
- Uccelletti D., Farina F., Palleschi C. (1999). The KlPMR1 gene of Kluyveromyces lactis encodes for a P-type Ca2+-ATPase. Yeast 15 593–599. [DOI] [PubMed] [Google Scholar]
- Wang J., Zhou G., Ying S.-H., Feng M.-G. (2013). P-type calcium ATPase functions as a core regulator of Beauveria bassiana growth, conidiation and responses to multiple stressful stimuli through cross-talk with signalling networks. Environ. Microbiol. 15 967–979. 10.1111/1462-2920.12044 [DOI] [PubMed] [Google Scholar]
- Wang J., Zhu X.-G., Ying S.-H., Feng M.-G. (2017). Differential roles for six P-type calcium ATPases in sustaining intracellular Ca2+ homeostasis, asexual cycle and environmental fitness of Beauveria bassiana. Sci. Rep. 7:1420. 10.1038/s41598-017-01570-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Cao J., Liu X., Hu H., Shi J., Zhang S., et al. (2012). Putative calcium channels CchA and MidA play the important roles in conidiation, hyphal polarity and cell wall components in Aspergillus nidulans. PLoS One 7:e46564. 10.1371/journal.pone.0046564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang J., Cheng J., Xu D., Jiang L. (2015). Genetic interactions between the Golgi Ca2+/H+ exchanger Gdt1 and the plasma membrane calcium channel Cch1/Mid1 in the regulation of calcium homeostasis, stress response and virulence in Candida albicans. FEMS Yeast Res. 15:fov069. 10.1093/femsyr/fov069 [DOI] [PubMed] [Google Scholar]
- Xie M., Zhou X., Xia Y., Cao Y. (2019). Mid1 affects ion transport, cell wall integrity, and host penetration of the entomopathogenic fungus Metarhizium acridum. Appl. Microbiol. Biotechnol. 103 1801–1810. 10.1007/s00253-018-09589-8 [DOI] [PubMed] [Google Scholar]
- Xu D., Cheng J., Cao C., Wang L., Jiang L. (2015). Genetic interactions between Rch1 and the high-affinity calcium influx system Cch1/Mid1/Ecm7 in the regulation of calcium homeostasis, drug tolerance, hyphal development and virulence in Candida albicans. FEMS Yeast Res. 15:fov079. 10.1093/femsyr/fov079 [DOI] [PubMed] [Google Scholar]
- Yang J., Kang H. A., Ko S.-M., Chae S.-K., Ryu D. D. Y., Kim J.-Y. (2001). Cloning of the Aspergillus niger pmrA gene, a homologue of yeast PMR1, and characterization of a pmrA null mutant. FEMS Microbiol. Lett. 199 97–102. 10.1111/j.1574-6968.2001.tb10657.x [DOI] [PubMed] [Google Scholar]
- Yang M., Brand A., Srikantha T., Daniels K. J., Soll D. R., Gow N. A. R. (2011). Fig1 facilitates calcium influx and localizes to membranes destined to undergo fusion during mating in Candida albicans. Eukaryot. Cell 10 435–444. 10.1128/EC.00145-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura H., Tada T., Iida H. (2004). Subcellular localization and oligomeric structure of the yeast putative strech-activated Ca2+ channel component Mid1. Exp. Cell Res. 293 185–195. 10.1016/j.yexcr.2003.09.020 [DOI] [PubMed] [Google Scholar]
- Yu Q., Ding X., Zhang B., Xu N., Cheng X., Qian K., et al. (2013). The P-type ATPase Spf1 is required for endoplasmic reticulum functions and cell wall integrity in Candida albicans. Int. J. Med. Microbiol. 303 257–266. 10.1016/j.ijmm.2013.11.022 [DOI] [PubMed] [Google Scholar]
- Yu Q., Wang F., Zhao Q., Chen J., Zhang B., Ding X., et al. (2014a). A novel role of the vacuolar calcium channel Yvc1 in stress response, morphogenesis and pathogenicity of Candida albicans. Int. J. Med. Microbiol. 304 339–350. 10.1016/j.ijmm.2013.11.022 [DOI] [PubMed] [Google Scholar]
- Yu Q., Wang H., Cheng X., Xu N., Ding X., Xing L., et al. (2012a). Roles of Cch1 and Mid1 in morphogenesis, oxidative stress response and virulence in Candida albicans. Mycopathologia 174 359–369. 10.1007/s11046-012-9569-0 [DOI] [PubMed] [Google Scholar]
- Yu Q., Wang H., Xu N., Cheng X., Wang Y., Zhang B., et al. (2012b). Spf1 strongly influences calcium homeostasis, hyphal development, biofilm formation and virulence in Candida albicans. Microbiology 158 2272–2282. 10.1099/mic.0.057232-0 [DOI] [PubMed] [Google Scholar]
- Yu Q., Zhang B., Yang B., Chen J., Wang H., Jia C., et al. (2014b). Interaction among the vacuole, the mitochondria, and the oxidative stress response is governed by the transient receptor potential channel in Candida albicans. Free Radic. Biol. Med. 77 152–167. 10.1016/j.freeradbiomed.2014.09.011 [DOI] [PubMed] [Google Scholar]
- Zhang S., Zheng H., Long N., Carbó N., Chen P., Aguilar P. S., et al. (2014). FigA, a putative homolog of low-affinity calcium system member Fig1 in Saccharomyces cerevisiae, is involved in growth and asexual and sexual development in Aspergillus nidulans. Eukaryot. Cell 13 295–303. 10.1128/Ec.00257-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Hu C., Hussain M., Chen J., Xiang M., Liu X. (2019). Role of low-affinity calcium system member Fig1 homologous proteins in conidiation and trap-formation of nematode-trapping fungus Arthrobotrys oligospora. Sci. Rep. 9:4440. 10.1038/s41598-019-40493-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Yan H., Happeck R., Peiter-Volk T., Xu H., Zhang Y., et al. (2016). The plasma membrane protein Rch1 is a negative regulator of cytosolic calcium homeostasis and positively regulated by the calcium/calcineurin signaling pathway in budding yeast. Eur. J. Cell Biol. 95 164–174. 10.1016/j.ejcb.2016.01.001 [DOI] [PubMed] [Google Scholar]
- Zhou X.-L., Batiza A. F., Loukin S. H., Palmer C. P., Kung C., Saimi Y. (2003). The transient receptor potential channel on the yeast vacuole is mechanosensitive. Proc. Natl. Acad. Sci. U.S.A. 100 7105–7110. 10.1073/pnas.1230540100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X.-L., Loukin S. H., Coria R., Kung C., Saimi Y. (2005). Heterologously expressed fungal transient receptor potential channels retain mechanosensitivity in vitro and osmotic response in vivo. Eur. Biophys. J. 34 413–422. 10.1007/s00249-005-0465-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.