Graphical abstract
Keywords: Copper, Oxidative stress, Curcumin, D. melanogaster, Neurotoxicity
Highlights
-
•
Curcumin ameliorates Cu2+- induced oxidative stress, and modulated nitrite level in D. melanogaster.
-
•
Curcumin inhibited Cu2+-induced increase in Acetylcholinesterase activity in D. melanogaster.
-
•
Curcumin restored the Cu2+-induced alteration in dopamine levels in D. melanogaster.
-
•
Thus, it might be used for the treatment of oxidative stress-induced neurodegenerative diseases.
Abstract
Curcumin is a hydrophobic polyphenol derived from the rhizome of the Herb Curcuma longa belonging to the family Zingiberaceae. Curcumin possesses antioxidative, anti-inflammatory and anti-depressant-like properties. In this study, we evaluated the rescue role of Curcumin in Copper2+-induced toxicity in D. melanogaster. Adult, wild type flies were exposed to Cu2+ (1 mM) and/or Curcumin (0.2 and 0.5 mg/kg diet) in the diet for 7 days. The results indicated that Cu2+- fed flies had reduced survival compared to the control group. Copper toxicity was also associated with a marked decrease in total thiol (T-SH), as well as catalase and glutathione S-transferase activities, contemporaneous with increased acetylcholinesterase (AChE) activity, nitric oxide (nitrate and nitrite) and dopamine levels. Co-exposure of flies to Cu2+ and Curcumin prevented mortality, inhibited AChE activity and restored dopamine to normal levels (p < 0.05). Moreover, Curcumin restored eclosion rates, and the cellular antioxidant status, as well as alleviated the accumulation of nitric oxide level in the flies. Curcumin ameliorated oxidative damage in the flies as evidenced by the survival rates, longevity assay as well as the restoration of antioxidant status. Our findings thus suggest that Curcumin ameliorated Cu2+-induced neurotoxicity in D. melanogaster and as such could be considered an effective therapeutic agent in the prevention and treatment of disorders, where oxidative stress is implicated.
1. Introduction
Copper (Cu2+) is an essential trace element (ETE) for all biological organisms, ranging from bacterial cells to humans and is implicated in a variety of biological processes and vital functions such as cell respiration, maturation of erythrocytes, antioxidant defense [1,2]. Copper is also a cofactor required for structural and catalytic properties of enzymatic machineries involved in oxidative phosphorylation, protein synthesis, neurotransmission modulation including superoxide dismutase (Cu-Zn-SOD), dopamine–β-hydroxylase, ceruloplasmin and cytochrome oxidase [[3], [4], [5]]. However, when in excess, copper is cytotoxic and is capable of generating highly damaging free radicals such as hydroxyl radicals by Fenton or Haber-Weiss reaction, thereby contributing to oxidative stress [[6], [7], [8], [9]]. In addition, organelle dysfunction, lipid peroxidation, and formation of toxic alkenals such as 4-hydroxy nonenal, an inhibitor of pyruvate dehydrogenase and alpha-keto-glutarate dehydrogenase are damaging products of disrupted copper homeostasis [[10], [11], [12]].
Cu2+ from geological materials and particulate matter is released into the air [13], and a higher concentration of Cu2+ is found in surface water, groundwater, seawater and drinking-water in addition to 40 % of dietary copper that originates from yeast breads, white potatoes, tomatoes, cereals, beef and dried beans [14]. Collectively, these sources of Cu2+have been implicated in neurotoxicity and neurodegeneration [15]. Particularly, Cu2+ release can be via by-products of industrial wastes including pesticide and fungicide applications. Thus, this increased levels of Cu2+ in the soil and water surface in several countries has attracted attention due to its potential toxic effects [[16], [17], [18]].
The excessive ingestion of inorganic Cu2+ affects cognition and has been implicated in the pathogenesis of numerous neurological and neurodegenerative diseases, such as Parkinson’s disease (PD), Alzheimer’s disease (AD) and amyotrophic lateral sclerosis [[19], [20], [21]]. In Wilson’s disease for instance, copper overload induces liver failure and causes oxidative damage to the central nervous system, through free radical generation, lipid peroxidation and mitochondrial dysfunction [[22], [23], [24]].
Recently, plant phenolics and bioactive compounds are being explored as chemoprotective and chemopreventive agents in epidemiological and experimental studies to mitigate, suppress and regulate the progression of oxidative stress-related diseases [25,26].
Curcumin, chemically known as diferuloyl-methane, is the basis for the yellow pigment of turmeric [27]. It is a hydrophobic polyphenol derived from the rhizome of the Herb Curcuma longa belonging to family Zingiberaceae. It is widely known for its biological activities, such as antioxidant, anti-inflammatory and antidepressant properties [27]. Several reports have associated Curcumin with antioxidant, anti-inflammatory and anticancer activities in humans, animal and Drosophila models [[27], [28], [29]]. Recently, Curcumin, when administered singly and in combination with Quercetin attenuates oxidative stress, by reducing lipid peroxidation and elevating antioxidant enzymes activity [30]. Importantly, Curcumin can inhibit acetylcholinesterase activity, ameliorate cognitive deficits and reduce amyloid accumulation [27,31], which characterize AD. Further, Curcumin can inhibit the formations of amyloid oligomers, fibrils, bind plaques and reduce beta amyloid in vivo [32].
Moreover, the beneficial effects of Curcumin have been studied in neurodegenerative diseases such as AD and PD in rodent models [27,31,33]. However, the effect of Curcumin against Copper-induced neurotoxicity in vivo has not been elucidated in Drosophila melanogaster - an important model organism in genetic and toxicological studies. In addition, in previous studies, it was difficult to carry out the effects of Curcumin on longevity in rodents, which was easy with flies as reported here. In this study, D. melanogaster was used as an alternative and complementary model to study Cu2+-induced toxicity and the rescuing role of Curcumin. Thus, the aim of this study was to assess the rescue and protective role of Curcuminin in Cu2+-induced toxicity and associated oxidative damage in Drosophila melanogaster.
2. Materials and methods
2.1. Chemicals
The 1-chloro-2, 4-dinitrobenzene CDNB, 5,5-dithiobis 2-nitrobenzoic acid DTNB, acetylthiocholine iodide, were purchased from Sigma USA. Copper sulfate was procured from AK Scientific, 30,023 Ahern Ave, Union City, CA 94587, USA. Other reagents were commercial products of high analytical grade.
2.2. Drosophila melanogaster stock and culture
D. melanogaster wild-type (Harwich strain) flies (from National Species Stock Center (Bowling Green, OH, USA)) were obtained from Prof. JBT Rocha, Department of Biochemistry and Molecular Biology, Federal University of Santa Maria, Brazil. The flies were maintained and reared in Drosophila Laboratory, Biochemistry Department, University of Ibadan, Nigeria on cornmeal medium containing 1 % w/v brewer's yeast, 2 % w/v sucrose, 1 % w/v powdered milk, 1 % w/v agar, and 0.08 % v/w nipagin at constant temperature and humidity (22–24 °C; 60–70 % relative humidity) under 12 h dark/light cycle conditions.
2.3. Cu2+exposure and curcumin treatment
Human exposure to Cu2+ in drinking water depends on the source; a safe level of Cu2+ is about 1.3 mg/ml [34]. Thus, Copper sulfate (CuSO4) and Curcumin were added into the medium at a final dose of Cu2+(1 mM) and/or Curcumin (0.2 and 0.5 mg/kg diet)respectively. Wild-type D. melanogaster of both genders(1–3 days old) were divided into five groups of 50 flies in each: (1) Control; (2) Curcumin (0.2 mg/kg diet); (3) Curcumin (0.5 mg/kg diet); (4) Cu2+ (1 mM); (5) Cu2+(1 mM)+ Curcumin (0.2 mg/kg diet) (6) Cu2+(1 mM) + Curcumin (0.5 mg/kg diet). The 1 mM dose of Cu2+used in the ameliorative study was based on a survival curve and biochemical assays reported later in this study. Studies by Halmenschelager et al, [35] also informed our choice of 1 mM Cu2+ dose. The choice of Curcumin doses of 0.2 and 0.5 mg/kg diet was based on our previous study [36], and the observations that Curcumin in the range of 0.2-0.5 mg/g diet did not cause overt signs of toxicity in flies, evidenced by longevity curve and biochemical assays carried out in this study.
2.4. In vivo assays
2.4.1. Longevity, survival and emergence rate of flies
The survival rate and longevity studies were determined by recording daily mortality of flies and data were analyzed [37]. We evaluated emergence rate of D. melanogaster offspring after exposure of the parent flies to Copper and Curcumin as previously reported [37].
2.5. Ex vivo assays
2.5.1. Preparation of samples for biochemical assays
For the biochemical assays, 50 control and treated flies per group were anaesthetized on ice, weighed, homogenized in 0.1 M potassium phosphate buffer, pH 7.4 (1:10 (flies/volume (μL)), and centrifuged at 10,000 g for 10 min at 4 °C. The supernatants were separated from the pellets into Eppendorf tubes, kept at −20 °C freezer and used for the evaluation of biochemical assays: acetylcholinesterase (AChE), catalase and glutathione S-transferase (GST) activities, as well as total thiol (T-SH) and hydrogen peroxide (H2O2) levels. Notably, all the assays were carried out in duplicates for each of the 5 replicates of control and treated flies.
2.6. Biochemical assays
2.6.1. Determination of protein concentration
The concentration of protein was determined by the method of Lowry et al. [38], with minor modifications.
2.6.2. Determination of total thiol level
Total thiol content was determined according to the method described by Ellman [39]. The reaction mixture contained 510 μL of 0.1 M phosphate buffer (pH 7.4), 20 μL of sample, 35 μL of 1 mM DTNB and 35 μL of distilled water. Then, incubation was carried out at room temperature for 30 min. The absorbance was measured at 412 nm.
2.6.3. Determination of glutathione S-transferase activity
The activity of GST was evaluated using the method of Habig and Jakoby [40] where 1-chloro-2,4-dinitrobenzene (CDNB) was used as substrate. The reaction mixture contained 270 μL of a solution made up of (20 μL of 0.25 M potassium phosphate buffer, pH 7.0, with 2.5 mM EDTA, 10.5 μL of distilled water and 500 μL of 0.1 M GSH at 25 °C),10 μL of 25 mM CDNB and 20 μL of sample (1:5 dilution). The mixture was monitored for 5 min. (10 s intervals) at 340 nm using a spectrophotometer.
2.6.4. Determination of catalase activity
Catalase activity was determined by the method of Aebi [41]. The reaction mixture consisted of 50 mM potassium phosphate buffer (pH 7.0), 300 mM H2O2 and sample (1:50 dilution). The loss in absorbance of H2O2 was monitored for 2 min at 240 nm and thereafter used to calculate catalase activity expressed as μmol of H2O2 consumed per minutes per milligram of protein.
2.6.5. Determination of acetylcholinesterase activity
The activity of acetylcholinesterase was determined with the method of Ellman et al. [42]. The reaction was carried out in 0.1 M potassium phosphate buffer of pH 7.4, 1 mM DTNB and 0.8 mM acetylthiocholine, the initiator. The reaction was monitored for 2 min (30 s interval) at 412 nm. The enzyme activity was estimated as μmol of acetylthiocholine hydrolyzed/minute/mg protein.
2.6.6. Determination of hydrogen peroxide level
The level of hydrogen peroxide level was carried out using the method of Wolff [43]. The reaction mixture consisted of FOX 1 (10 ml of 100 mM xylenol orange, 50 ml of 250 mM ammonium ferrous sulfate, 10 ml of 100 mM sorbitol, 5 ml of 25 mM H2SO4 and 30 ml of distilled water) mixed with the sample. After 30 min incubation at room temperature, the absorbance was measured at 560 nm. The values were extrapolated from the standard curve and expressed in micromole per milligram protein.
2.6.7. Determination of nitric oxide (nitrate/nitrite) level
Nitric oxide (nitrate and nitrite) level was quantified by following the method of Griess reaction [44], by incubating 250 μL of sample with 250 μL of Griess reagent[0.1 % N-(1-naphthyl) ethylenediamine dihydrochloride; 1 % sulfanilamide in 5 % phosphoric acid; 1:1 ratio] at room temperature for 20 min. The absorbance at 550 nm (OD 550) was measured spectrophotometrically and nitrite concentration was calculated by comparison with the OD 550 of a standard solution of known sodium nitrite concentrations.
2.7. Analyses of dopamine concentrations
After the 7-day treatment, 50 flies per vial, n = 5, were frozen at −20 °C and their heads were separated from the bodies using forceps and sharp blade/cutter. Then, fly heads were homogenized in 0.1 M Perchloric acid and centrifuged using an Eppendorf Legend Micro 17R Centrifuge (fixed angle rotor) at 12000×g for 30 min at 4 °C. The supernatants were carefully separated from the pellets and stored at −20 °C until used within 24 h. Dopamine ELISA kit (Abnova, Taiwan) was thereafter used to determine the level of dopamine in the supernatants by following the manufacturer’s procedure. Spectramax Plus 384 Microplate Reader (Molecular Devices) was used to measure the absorbance at wavelength of 450 nm.
2.8. Statistical analyses
Analyses of data for survival and longevity were carried out using the Kaplan-Meier method, and comparisons were made with the log-rank tests. The biochemical data are presented as the Mean ± SEM. One-way Analysis of variance (ANOVA) was used to assess the significant differences among multiple groups under various treatments, followed by Dunett’s posthoc test. In all the groups, differences were considered statistically significant among groups with p < 0.05, using the GraphPad Prism5.0 software.
3. Results
3.1. Longevity and selected biochemical markers of flies after exposure to Curcumin
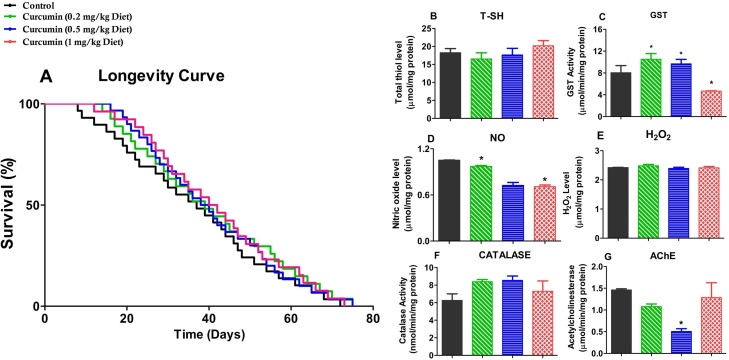
The effects of Curcumin on the longevity and selected biochemical markers in D. melanogaster are shown in Fig. 1.There was no significant difference in the longevity of flies treated with Curcumin compared with the control. Lifespan was extended by 4.2 % (0.2 and 0.5 mg/kg diet) and by 1.4 % for 1 mg/kg diet concentrations (Fig. 1A). Importantly, the levels of total thiol and H2O2, as well as catalase activity were not affected by Curcumin administration (Fig. 1B, E and F). In addition, Curcumin, 0.2 and 0.5 mg/kg diet caused a 20 % reduction in nitric oxide (nitrate and nitrite) level and a 67 % inhibition of AChE activity respectively after 7 days of treatment (Fig. 1D and G; p < 0.05). Curcumin (0.2 and 0.5 mg/kg diet) increased GST activity (about 1.25 folds compared to control p < 0.05) but offered a 42 % reduction at 1 mg/kg diet dose (Fig. 1C) compared with control. Hence, the 1 mg/kg dose was excluded from the ameliorative study.
Fig. 1.
Effects of Curcumin on longevity and selected biochemical parameters in D. melanogaster. Longevity (A), Total thiols (B), Glutathione S-transferase activity (C), Nitric oxide level (D), Hydrogen peroxide level (E), Catalase activity (F), and Acetylcholinesterase activity (G). in D. melanogaster after exposure to Curcumin (0.2, 0.5, and 1 mg/kg diet) for 7 days. Data are presented as Mean ± SEM of 30 flies/vial with 5 replicates per treatment group. Significant differences from the control group are indicated by *(p < 0.05). In (A), the p values (log – rank tests) for each group include: Ctrl versus 0.2 mg/kg diet (p = 0.4706), 0.5 mg/kg diet (p = 0.5567), 1 mg/kg diet (p = 0.3767). The maximum lifespan in each group represents the percentage of surviving flies.
3.2. Survival rate and selected biochemical markers after exposure to copper sulfate
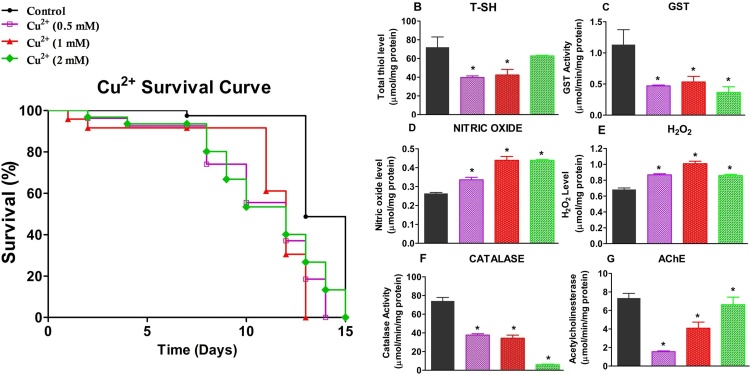
Fig. 2 shows the effects of Cu2+ on the survival and selected biochemical markers in D. melanogaster. Cu2+ exerted a toxic effect at all the doses used, thus, decreasing the survival of flies (Fig. 2A). We selected 1 mM Cu2+ dose and 7 days as treatment period in the ameliorative study, since no significant mortality was recorded at this dose and duration. In addition, after 7 days of treatment, Cu2+(1 mM) significantly depleted T-SH level (42 % reduction compared to the control group, p < 0.05; Fig. 2B), increased NO (nitrate and nitrite, Fig. 2D) and H2O2 (Fig. 2E) levels by 69 % and 47 % respectively compared to the control, p < 0.05). Further, Cu2+ at 1 mM caused more than half-fold reductions in GST activity (57 % reduction compared to control, Fig. 2C; p < 0.05) and catalase activity (53 % reduction compared to control, Fig. 2F; p < 0.05).
Fig. 2.
Effects of Cu2+on survival rate and biochemical parameters in D. melanogaster. Survival rate (A), Total thiol level (B), Glutathione S-transferase activity (C), Nitric oxide level D), Hydrogen peroxide level (E) and Catalase activity (F)of D. melanogaster exposed to Cu2+ (1 mM). Data are presented as Mean ± SEM of 30flies/vial with 5 replicates per treatment group. Significant differences from the control group are indicated by *(p < 0.05). In (A), the p values (log – rank tests) for each group include: Ctrl versus 0.5 mM (p = 0.0955), 1 mM (p = 0.0715), 2 mM (p = 0.1118). The maximum lifespan in each group represents the percentage of surviving flies.
3.3. Ameliorative role of Curcumin in Cu2+-induced toxicity in D. melanogaster
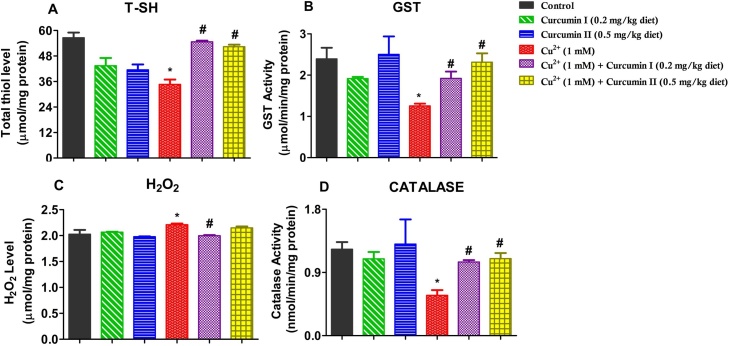
3.3.1. Curcumin ameliorates Cu2+-induced accumulations of H2O2level, depletion of T-SH, and inhibition of catalase and GST activities in D. melanogaster
Fig. 3 shows the effects of Cu2+ and Curcumin on selected oxidative stress and antioxidant parameters in D. melanogaster. After a 7-day exposure of D. melanogaster to Cu2+ and Curcumin, we observed that Curcumin restored Cu2+-induced elevation of H2O2 level (25 % reduction compared to Cu2+ treated group, Fig. 3C, p < 0.05), depletion of total thiol level (37 % protection compared to Cu2+ treated group, Fig. 3A, p < 0.05), and ameliorated Cu2+-induced inhibition of catalase (1.73 folds protection, Fig. 3D)and GST (1.85 folds protection, Fig. 3B) activities compared to the Cu2+ in D. melanogaster (p < 0.05).
Fig. 3.
Effects of Curcumin and Cu2+ on biochemical parameters in D. melanogaster. Total thiols level (A), Glutathione S-transferase activity (B), Hydrogen peroxide level (C) and Catalase activity (D) of D. melanogaster exposed to Cu2+(1 mM) and Curcumin (0.2 and 0.5 mg/kg diet) for 7 days. Values are expressed as Mean ± Standard Error of Mean (n = 5). Significant differences from the control are indicated by *(p < 0.05). Significant differences from the Cu2+ - treated group indicated by #(p < 0.05).
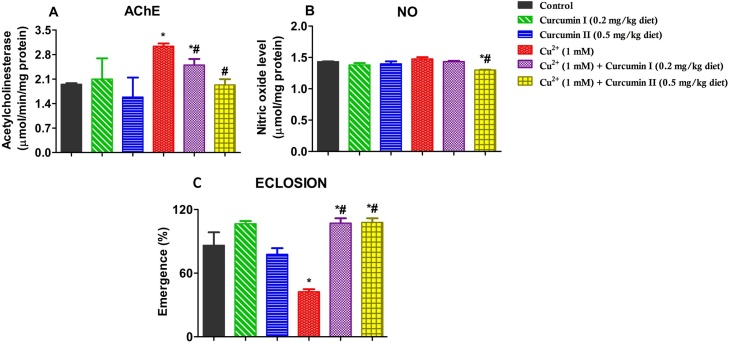
3.3.2. Curcumin improves Cu2+-induced, reduction of emergence of offspring and alteration of AChE activity in Drosophila melanogaster
Fig. 4 shows the effects of Cu2+ and Curcumin on markers of neurotoxicity and emergence. Our results show that 1 mM Cu2+dose effectuated a significant (56 %) increase in AChE activity. However, co-treatment with Curcumin (0.2 and 0.5 mg/kg diet) significantly restored AChE activity up to 1.57 folds compared to the Cu2+-fed group (Fig. 4A; p < 0.05). Further, emergence rate of offspring significantly reduced by 51 % in flies treated with 1 mM Cu2+. This reduction was ameliorated in flies co-exposed to Cu2+ and Curcumin with about 24 % improvement in eclosion rate (Fig. 4C, p < 0.05)
Fig. 4.
Effects of Curcumin and Cu2+ on behavioural, nitric oxide and eclosion rate inD. melanogaster. Acetylcholinesterase activity (A), Nitric oxide level, (B) and Flies emergence (C) in wild-type D. melanogaster. Values are expressed as Mean ± Standard Error of Mean (n = 5). Significant differences from the control are indicated by *(p < 0.05). Significant differences from the Cu2+ - treated group indicated by #(p < 0.05).
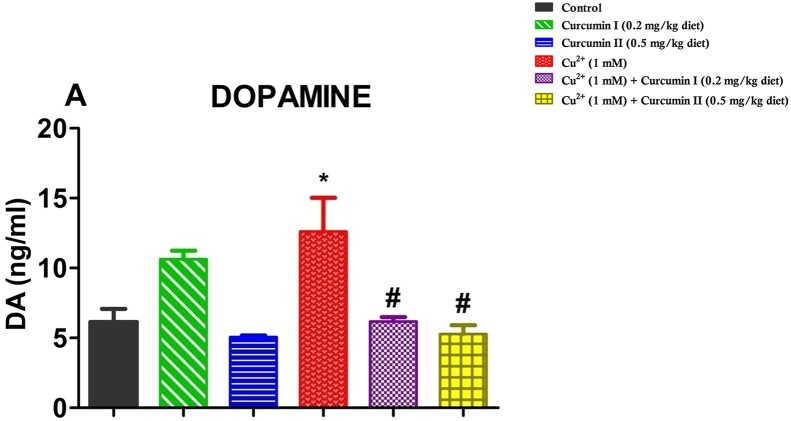
3.4. Effect of Curcumin on Cu2+-induced elevation of dopamine levels
Dopamine level in the brains of D. melanogaster treated with Cu2+ and Curcumin is shown in Fig. 5. We observed that flies from the Cu2+-treated group showed elevation (about 50 %) in the levels of dopamine compared to the control flies. Treatment with Curcumin restored Cu2+-induced elevation of dopamine level, offering about 60 % protection when compared withCu2+-treated flies (Fig. 5; p < 0.05)
Fig. 5.
Effect of Curcumin on Cu2+-induced neurotoxicity in treated flies. Dopamine levels in flies after treatment for 7 days. Values are expressed as Mean ± Standard Error of Mean (70 fly heads/replicate, n = 3). Significant differences from the control are indicated by *(p < 0.05). Significant differences from the Cu2+ - treated group indicated by #(p < 0.05).
4. Discussion
Drosophila melanogaster has been established as a model system in neurotoxicology and genetics due to ease of genetic manipulations, simple nervous system, and a relatively short life-span and generation time [45]. D. melanogaster has been previously used as an alternative and complementary model for studying disorders potentially associated with copper dyshomeostasis [9]. The objective of this study was to evaluate the attenuative role of Curcumin in Cu2+-induced toxicity in Wild-type D. melanogaster.
Despite the reported low bioavailability of Curcumin, it has been shown to possess antioxidative, anti-inflammatory and acetylcholinesterase inhibiting properties [46]. The bioavailability of Curcumin is dependent on the route of administration [47,48]. For instance, following oral administration to rats and humans, only 40 % of the administered dose was excreted unchanged in the feces [49,50]. Moreover, following intraperitoneal route of administration in mice, the plasma concentration of Curcumin decreased by 44 % and reduced rapidly within an hour [51,52]. Additionally, intravenous administration of Curcumin indicated only about 1 % bioavailability [53]. Moreover, a near limit of detection but effective plasma concentration of Curcumin was found when mice with intestinal tumour were fed with diets fortified with Curcumin [54]. The reported bioavailability of Curcumin in humans and animals [55], may not necessarily be concordant in D. melanogaster [56]. Considering the evidences regarding the potency and bioavailability of Curcumin, we investigated its rescue role in Cu2+-induced toxicity in D. melanogaster.
In this study, flies were exposed to varying doses of Cu2+ to establish a dose-response relationship. The flies exposed to Cu2+ showed decreased survival during the 10-day exposure period. This suggests that Cu2+ caused toxicity that is detrimental to the life-span of the flies, which may be due to increased oxidative insults. Furthermore, accumulating evidence reveals that consumption of diets fortified with antioxidant-rich supplements extended organismal life-span [[57], [58], [59]]. Our study demonstrated statistically indistinguishable life-span maintenance and extension capacity of Curcumin (0.2 and 0.5 mg/kg).
Glutathione S-transferases (GSTs) are phase II family of multifunctional enzymes with cysteine-rich domains [60,61]. They play important roles in the detoxification of xenobiotics by conjugating GSH with electrophilic molecules [61,62]. Redox active Cu2+has been implicated in the oxidation of thiol-containing proteins. The observation that Curcumin restored Cu2+-induced depletion of total thiol and inhibition of GST activity in D. melanogaster showed that Cu2+ oxidized thiol groups on GST leading to the modification of its native structure thereby altering its activity. Notably, Cu2+can also oxidize GSH, and indirectly interfere with GST activity by depleting its substrate [63]. Curcumin’s protective action may be through scavenging free radicals and an indirect effect via the activation of ARE (antioxidant responsive element), which includes Nrf-2 [64]. Thiols are compounds with carbon-bound sulfhydryl group [65]. Total thiols comprise of GSH and several other thiol-containing compounds in tissues. Total thiols mirror the redox state of –SH groups, which are fundamental for the activity of thousands of proteins (including several oxidoreductases involved in the maintenance of cell redox state) and for the maintenance of the GSH levels, which is a low molecular mass thiol very important to protect cells against pro-oxidant species. Our results showed that Curcumin restored Cu2+ - induced thiol oxidation and depletion of GSH.
In our study, Cu2+ exposure had no significant effect on nitric oxide (nitrite and nitrate) level in D. melanogaster. Indeed, nitric oxide (NO), a highly diffusible and short-lived free radical, rapidly recombines to form the stable metabolites nitrate and nitrite [66,67]. NO has been considered as a pro-inflammatory mediator because of its potential to react with superoxide anion and form more deleterious nitrite anion causing toxicity [68]. Similarly, Curcumin did not have significant effect on NO (nitrite and nitrate) level in the flies.
Cu2+can promote the Fenton reaction, i.e., the generation of hydroxyl radicals and/or other ROS as by product of the reaction of Cu2+ with hydrogen peroxide [69,70]. Here, Cu2+ caused accumulation of H2O2 and inhibited catalase activity in D. melanogaster. Antioxidants play protective roles by scavenging ROS and reducing the insults of oxidative damage [71]. Catalase, a heme-containing enzyme [39], catalyses the dismutation of H2O2 to molecular oxygen and water in order to minimize oxidative stress-mediated toxicity [72]. Therefore, the ability of Curcumin to restore Cu2+-induced inhibition of catalase activity and H2O2 accumulation suggests that it possesses both antioxidative and free radical scavenging properties.
Moreover, the observation that the treatment of flies with Cu2+ reduced eclosion rates in the flies is worthy of note. It was reported that copper has a toxic effect on the reproductive system due to decrease percentage of motile spermatozoa and decrease number of malformed sperm cells [73]. This might just be due to induced infertility in the flies. However, flies co-treated with Curcumin and Cu2+ showed increased emergence rate of flies comparable with control probably due to presumed increased fertility in the flies.
Curcumin ameliorated Cu2+- induced elevation of acetylcholinesterase activity in the flies. This enzyme hydrolyses acetylcholine, a neurotransmitter that plays a vital role in the regulation of motor function and locomotion [74]. Acetylcholinesterase is a vital neurotransmitter of the cholinergic system that modulates learning, memory and locomotor activities. In contrast, AChE, hydrolyzes ACh to choline and acetate thereby hampering cholinergic neurotransmission [75]. Sufficient information has elucidated the role of cholinergic system in the pathogenesis of neurodegenerative diseases especially in the aged [74,75]. While this holds in vertebrate models, the precise outcome in flies has not been clearly elucidated. Nevertheless, both an increase and a decrease in AChE may have toxic consequences in the flies [35]. In this study, our results revealed that Curcumin significantly decreased AChE activity when compared with control and ameliorated Cu2+-induced elevation of AChE activity. This finding agrees with observations in rodent [57], and our previous study where Curcumin inhibited the activity and mRNA expression of AChE [36]. Thus, the inhibitory action of Curcumin on AChE activity may increase acetylcholine level in the synaptic clefts leading to an efficient functioning of the cholinergic system in the flies.
Cu2+ exposure also resulted in an unanticipated significant increase in dopamine (DA) level. DA is an endogenous organic catecholamine that functions in the substantia nigra part of the central nervous system (mid-brain) and plays a unique role in the coordination of learning, cognition, emotion and locomotion. [76]. In Drosophila, signaling of DA plays diverse strategic roles in learning and memory [77]. Nonetheless, increased or reduced DA levels may impact memory retention, and cognition negatively [78]. Several pathogenetic mechanisms that might elicit DA-ergic dysfunction in disease states have been reported, revealing that Aβ oligomers possesses toxic and deleterious effects on neuronal arrangement and neurotransmission, leading to death of the neocortex and hippocampi neurons [79]. Also, there is evidence linking the ability of copper to form a complex with dopamine, allowing this metal to be transported by cells that have the ability to take up dopamine [80]. Our results showed significant increase in dopamine level observed in fly brainsin thegroups treated with Cu2+ only. This effect was attenuated to levels statistically indistinguishable from control in the Cu2+-curcumin treated flies. Due to present constraints, we could not carry out the protein levels of important copper importer (Ctr1A), efflux transporters (ATP7A and ATP7B) and chaperone (ATOX1) to understand the cause of increased level of DA in Cu2+-treated flies. Nevertheless, this outcome might be due to increased expression and levels of Cu2+ importers and decreased expression Cu2+ efflux transporters. Furthermore, Cu2+-mediated chaperone (ATOX1) that delivers copper to the ATPases, might be similarly affected. The ability of Curcumin to ameliorate these effects may present it as a suitable neuroprotective agent.
5. Conclusion
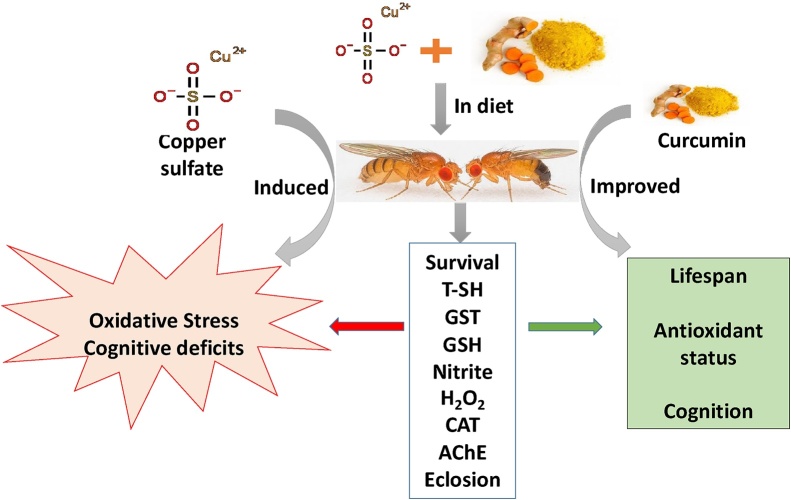
Taken together, we demonstrated that Curcumin possesses protective activity against Cu2+ toxicity in vivo. We posit that the rescue ability of Curcumin is due to its antioxidative effect evidenced by reduced mortality, modulation of oxidative stress-antioxidant homeostasis, as well as amelioration of altered dopamine level in D. melanogaster (Scheme 1). Thus, Curcumin may be considered an effective agent in the treatment and prevention of neurological disorders, where oxidative stress is implicated, such as in AD. Further, the results of the present study confirm the utility of D. melanogaster as a model to investigate therapeutic strategies that may be promising in the treatment of neurodegenerative diseases.
Scheme 1.
Protective mechanism of Curcumin in Cu2+-induced toxicity in Drosophila melanogaster.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgements
The study was partly supported by the International Centre for Genetic Engineering and Biotechnology (ICGEB), Italy via the grant used to purchase Spectramax Microplate Reader (Molecular Devices), −20 °C Freezer and analytical weighing balance that were used in this study. Prof. Michael Aschner was supported by National Institute of Health (NIH)R01 ES10563, R01 ES07331 and R01 ES020852 grants.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2020.01.015.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Letelier M.E., Lepe A.M., Faúndez M., Salazar J., Marín R., Aracena P. Possible mechanisms underlying copper-induced damage in biological membranes leading to cellular toxicity. Chem. Biol. Interact. 2005;151:71–82. doi: 10.1016/j.cbi.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Tumer Z., Moller L.B. Menkes disease. Eur. J. Hum. Genet. 2010;8:511–518. doi: 10.1038/ejhg.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Popescu B.F., Robinson C.A., Rajput A., Rajput A.H., Harder S.L., Nichol H. Iron, copper, and zinc distribution of the cerebellum. Cerebel. 2009;8:74–79. doi: 10.1007/s12311-008-0091-3. [DOI] [PubMed] [Google Scholar]
- 4.Lutsenko S., Gupta A., Burkhead J.L., Zuzel V. Cellular multitasking: the dual role of human Cu-ATPases in cofactor delivery and intracellular copper balance. Arch. Biochem. Biophys. 2008;476:22–32. doi: 10.1016/j.abb.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaggelli E., Kozlowski H., Valensin D., Valensin G. Copper homeostasis and neurodegenerative disorders (Alzheimer’s disease, prion, and Parkinson’s diseases and amyotrophic lateral sclerosis) Chem. Rev. 2006;106:1995–2044. doi: 10.1021/cr040410w. [DOI] [PubMed] [Google Scholar]
- 6.Dusek P., Litwin T., Czlonkowska A. Wilson disease and other neurodegeneration with metal accumulations. NeurolClin. 2015;33:175–204. doi: 10.1016/j.ncl.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Brewer G.J. The risks of free copper in the body and the development of useful anticopper drugs. Curr. Opin. Clin. Nutr. Metab. Care. 2008;11:727–732. doi: 10.1097/MCO.0b013e328314b678. [DOI] [PubMed] [Google Scholar]
- 8.Tumer Z., Moller L.B. Menkes disease. Eur. J. Hum. Genet. 2010;8:511–518. doi: 10.1038/ejhg.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Southon A., Burke R., Camakaris J. What can flies tell us about copper homeostasis? Metallomics. 2013;5(10):1346–1356. doi: 10.1039/c3mt00105a. [DOI] [PubMed] [Google Scholar]
- 10.Zischka H., Borchard S. Clinical and Translational Perspectives on Wilson disease. 2019. Mitochondrial copper toxicity with a focus on wilson disease; pp. 65–75. [Google Scholar]
- 11.Sheline C.T., Choi D.W. Cu2+ toxicity inhibition of mitochondrial dehydrogenases in vitro and in vivo. Ann. Neurol. 2004;55:645–653. doi: 10.1002/ana.20047. [DOI] [PubMed] [Google Scholar]
- 12.White A.R., Multhaup G., Maher F., Bellingham S., Camakaris J., Zheng H., Bush A.I., Beyreuther K., Masters C.L., Cappai R. The Alzheimer’s disease amyloid precursor protein modulates copper-induced toxicity and oxidative stress in primary neuronal cultures. J. Neurosci. 1999;19:9170–9179. doi: 10.1523/JNEUROSCI.19-21-09170.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klimaczewski C.V., Ecker A., Piccoli B., Aschner M., Barbosa N.V., Rocha J.B.T. Peumusboldusattenuates copper-induced toxicity in Drosophila melanogaster. Biomed. Pharmacother. 2018;97:1–8. doi: 10.1016/j.biopha.2017.09.130. [DOI] [PubMed] [Google Scholar]
- 14.Subar A.F., Krebs-Smith S.M., Cook A., Kahle L.L. Dietary sources of nutrients among US adults. J. Americ. Diet. Assoc. 1998;98:537–547. doi: 10.1016/S0002-8223(98)00122-9. [DOI] [PubMed] [Google Scholar]
- 15.Sparks D.L., Schreurs B.G. Trace amounts of copper in water induce beta-amyloid plaques and learning deficits in a rabbit model of alzheimer’s disease. Proc. Natl. Acad. Sci. U. S. A. 2003;100(19):11065–11069. doi: 10.1073/pnas.1832769100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwarzenbach R.P., Egli T., Hofstetter T.B., Von Gunten U., Wehrli B. Global water pollution and human health. Annu. Rev. Environ. Resour. 2010;35:109–136. [Google Scholar]
- 17.Cicatelli A., Castiglione S. A step forward in tree physiological research on soil copper contamination. Tree Physiol. 2016;36(4):403–406. doi: 10.1093/treephys/tpw014. [DOI] [PubMed] [Google Scholar]
- 18.Christen Y. Oxidative stress and Alzheimer disease. Am. J. Clin. Nutr. 2000;71(2):621–629. doi: 10.1093/ajcn/71.2.621s. [DOI] [PubMed] [Google Scholar]
- 19.Ferrante R.J., Browne S.E., Shinobu L.A., Bowling A.C., Baik M.J., Mac Garvey U., Kowall N.W., Brown R.H., Beal M.F. Evidence of increased oxidative damage in both sporadic and familial amyotrophic lateral sclerosis. J. Neurochem. 1997;69(5):2064–2074. doi: 10.1046/j.1471-4159.1997.69052064.x. [DOI] [PubMed] [Google Scholar]
- 20.Eskici G., Axelsen P.H. Copper and oxidative stress in the pathogenesis of Alzheimer’s disease. Biochem. 2012;14:6289–6311. doi: 10.1021/bi3006169. [DOI] [PubMed] [Google Scholar]
- 21.Brewer G.J. Copper toxicity in Alzheimer’s disease: cognitive loss from ingestion of inorganic copper. J. Trace Elem. Med. Biol. 2012;26(2–3):89–92. doi: 10.1016/j.jtemb.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 22.Schilsky M.L. Wilson disease: current status and the future. Biochimie. 2009;91:1278–1281. doi: 10.1016/j.biochi.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Shi L.-M., Jiang H., Wang J., Ma Z.-G., Xie J.-X. Mitochondria dysfunction was involved in copper-induced toxicity in MES23.5 cells. Neurosci. Bull. 2008;24(2):79–83. doi: 10.1007/s12264-008-0079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu W.R., Jiang H., Wang J., Xie J.-X. Copper (Cu2+) induces degeneration of dopaminergic neurons in the nigrostriatal system of rats. Neurosci. Bull. 2008;24(2):73–78. doi: 10.1007/s12264-008-0073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruby A.J., Kuttan G., Babu K.D., Rajasekharan K.N., Kuttan R. Anti-tumor and antioxidant activity of natural curcuminoids. Cancer Lett. 1995;94(1):79–83. doi: 10.1016/0304-3835(95)03827-j. [DOI] [PubMed] [Google Scholar]
- 26.Sharma A., Kulkarni S.K. Evaluation of learning and memory mechanisms employing plus maze in rats and mice. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1992;16:117–125. doi: 10.1016/0278-5846(92)90014-6. [DOI] [PubMed] [Google Scholar]
- 27.Mythri R.B., Bharath M.M. Curcumin: a potential neuroprotective agent in Parkinson’s disease. Curr. Pharm. Des. 2012;18:91–99. doi: 10.2174/138161212798918995. [DOI] [PubMed] [Google Scholar]
- 28.Maruta H. Herbal therapeutics that block the oncogenic kinase PAK1: a practical approach towards PAK1- dependent diseases and longevity. Phytother. Res. 2014;28:656–672. doi: 10.1002/ptr.5054. [DOI] [PubMed] [Google Scholar]
- 29.Lee S., Bang S.M., Lee J.W., Cho K.S. Evaluation of traditional medicines for neurodegenerative diseases using Drosophila models. Evid. Complement. Alternat. Med. 2014:1–14. doi: 10.1155/2014/967462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdel-Diam M.M., Samak D.H., El-Sayed Y.S., Aleya L., Alarifi S., Alkahtani S. Curcumin and quercetin synergistically attenuate subacute diazinon-induced inflammation and oxidative neurohepatic damage, and acetylcholinesterase inhibition in albino rats. Environ. Sci. Pollut. R. Int. 2019;26(4):3659–3665. doi: 10.1007/s11356-018-3907-9. [DOI] [PubMed] [Google Scholar]
- 31.Yang F., Lim G.P., Begum A.N., Ubeda O.J., Simmons M.R., Ambegaokar S.S. Curcumin inhibits formation of amyloidoligomers and fibrils, binds plaques and reduces amyloid in vivo. J. Biol. Chem. 2005;280(7):5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 32.Adewale O.O., Samuel E.S., Manubolu M., Pathakoti K. Curcumin protects Sodium nitrite-induced hepatotoxicity in wistar rats. Toxicol. Reports. 2019;6:1006–1011. doi: 10.1016/j.toxrep.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hung L.W., Villemagne V.L., Cheng L., Sherratt N.A. The hypoxia imaging agent CuII(atsm) is neuroprotective and improves motor and cognitive functions in multiple animal models of Parkinson’s disease. J. Exp. Med. 2012;209:837–854. doi: 10.1084/jem.20112285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brewer G.J. Copper toxicity in the general population. Clin. Neurophysiol. 2010;121:459–460. doi: 10.1016/j.clinph.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 35.Halmenschelager P.T., Rocha J.B.T. Biochemical CuSO4 Toxicity in Drosophila melanogaster depends on sex and developmental stage of exposure. Biol. Trace Elem. Res. 2018 doi: 10.1007/s12011-018-1475-y. [DOI] [PubMed] [Google Scholar]
- 36.Akinyemi A.J., Oboh G., Ogunsuyi O., Abolaji A.O., Udofia A. Curcumin-supplemented diets improve antioxidant enzymes and alter acetylcholinesterase genes expression level in D. melanogaster model. Metab. Brain Dis. 2018;33(2):369–375. doi: 10.1007/s11011-017-0100-7. [DOI] [PubMed] [Google Scholar]
- 37.Farombi E.O., Abolaji A.O., Farombi T.H., Oropo A.S., Owoje O.A., Awunah M.T. Garciniakolaseedsbiflavonoid fraction (Kolaviron), increases longevity and attenuates rotenone induced toxicity in Drosophila melanogaster. Pestic. Biochem. Physiol. 2018;145:39–45. doi: 10.1016/j.pestbp.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 39.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 40.Habig W.H., Jakoby W.B. Assays for differentiation of glutathione-S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- 41.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 42.Ellman G.L., Courtney K.D., Andres V., Feathers-Stone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 43.Wolff S.P. Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods Enzymol. 1994;233:182–189. [Google Scholar]
- 44.Green L.C., Wagner D.A., Glogowski J., Skipper P.L., Wishnok J.S., Tannenbaum S.R. Analysis of nitrate, nitrite and (15N) nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 45.Kim S.I., Jung J.W., Ahn Y.J., Restifo L.L., Kwon H.W. Drosophila as a model system for studying lifespan and neuroprotective activities of plant-derived compounds. J. Asia–Pacific Entomol. 2011;14:509–517. [Google Scholar]
- 46.Hongsibsong S., Stuetz W., Sus N., Prapamontol T., Grune T., Frank J. Dietary exposure to continous small doses of alpha-cypermethrin in the presence or absence of dietary curcumin does not induced oxidative stress in male wisar rats. Toxicol. Rep. 2014;1:1106–1114. doi: 10.1016/j.toxrep.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Asai A., Miyazawa T. Occurrence of orally administered curcuminoid as glucuronide and glucuronide/sulfate conjugates in rat plasma. Life Sci. 2000;67:2785–2793. doi: 10.1016/s0024-3205(00)00868-7. [DOI] [PubMed] [Google Scholar]
- 48.Her C., Venier-Julienne M.C., Roger E. Improvement of Curcumin bioavailability for medical applications. Med. Aromat. Plants (Los Angel) 2018;7:326. [Google Scholar]
- 49.Ravindranath V., Chandrasekhara N. Absorption and tissue distribution of curcumin in rats. Toxicology. 1980;16:259–265. doi: 10.1016/0300-483x(80)90122-5. [DOI] [PubMed] [Google Scholar]
- 50.Sharma R.A., Mclelland H.R., Hill K.A., Sharma R.A., Mclelland H.R. Pharmacodynamic and Pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin. Cancer Res. 2001;7:1894–1900. [PubMed] [Google Scholar]
- 51.Pan M.H., Huang T.M., Lin J.K. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab. Dispos. 1999;27:486–494. [PubMed] [Google Scholar]
- 52.Irving G.R.B., Karmokar A., Berry D.P., Brown K., Steward W.P. Curcumin: the potential for efficacy in gastrointestinal diseases. Best Pract. Res. Clin. Gastroenterol. 2011;25:519–534. doi: 10.1016/j.bpg.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 53.Yang K.Y., Lin L.C., Tseng T.Y., Wang S.C., Tsai T.H. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LCMS/MS. J. Chromatogr. B. Anal. Technol. Biomed. Life Sci. 2007;853:183–189. doi: 10.1016/j.jchromb.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 54.Perkins S., Verschoyle R.D., Hill K., Perkins S., Verschoyle R.D. Chemopreventive Efficacy and pharmacokinetics of Curcumin in the Min /+ Mouse, a model of familial adenomatous polyposis. Cancer Epidemiol. Biomarkers Prev. 2002;11:535–540. [PubMed] [Google Scholar]
- 55.Liu W., Zhai Y., Heng X., Che F.Y., Chen W., Sun D., Zhai G. Oral bioavailability of curcumin: problems and advancements. J .Drug Target. 2016;24(8):694–702. doi: 10.3109/1061186X.2016.1157883. [DOI] [PubMed] [Google Scholar]
- 56.Phom L., Debasmita B.A., Muralidhara P.A., Yenisett S.C. Curcumin’s neuroprotective efficacy in Drosophila model of idiopathic Parkinson’s Disease is phase specific: implication of its therapeutic effectiveness. Rejuvenation Res. 2014;17:6. doi: 10.1089/rej.2014.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shen L.R., Xiao F., Yuan P., Chen Y., Gao Q.K., Parnell L.D., Meydani M., Ordovas J.M., Li D., Lai C.Q. Curcumin-supplemented diets increase superoxide dismutase activity and mean lifespan in Drosophila melanogaster. Age. 2013;35:1133–1142. doi: 10.1007/s11357-012-9438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang H.L., Sun Z.O., Rehman R.U., Wang H., Wang Y.F., Wang H. Rosemary extract-mediated lifespan extension and attenuated oxidative damage in Drosophila melanogaster fed on high-fat diet. J. Food Sci. 2017;82(4):1006–1011. doi: 10.1111/1750-3841.13656. [DOI] [PubMed] [Google Scholar]
- 59.Kumar A., Christian P.K., Panchal K., Guruprasad B.R., Tiwari A.K. Supplementation of Spirulina (Arthrospira platensis) improves lifespan and locomotor activity in paraquat-sensitive DJ-1βΔ93 flies, a Parkinson’s disease model in Drosophila melanogaster. J. Diet. Suppl. 2017;14(5):573–588. doi: 10.1080/19390211.2016.1275917. [DOI] [PubMed] [Google Scholar]
- 60.Desai V., Kaler S.G. Role of copper in human neurological disorders. Am. J. Clin. Nutr. 2008;88(3):855–858. doi: 10.1093/ajcn/88.3.855S. [DOI] [PubMed] [Google Scholar]
- 61.Hatem E., Veronique B., Michele D., Gilles L., Peggy B., Meng-Er H., Stephane Jean C. Glutathione is essential to preserve nuclear function and cell survival under oxidative stress. Free Radic. Biol. Med. 2014;67:103–114. doi: 10.1016/j.freeradbiomed.2013.10.807. [DOI] [PubMed] [Google Scholar]
- 62.Abolaji A.O., Kamdem J.P., Lugokenski T.H., Nascimento T.K., Waczuk E.P., Farombi E.O., Loreto E.L., Rocha J.B.T. Involvement of oxidative stress in 4-vinylcyclohexene-induced toxicity in Drosophila melanogaster. Free Radic. Biol. Med. 2014;71:99–108. doi: 10.1016/j.freeradbiomed.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 63.Mattie M.D., Freedman J.H. Copper-inducible transcription: regulation by metal and oxidative stress-responsive pathways. Am. J. Physiol., Cell Physiol. 2004;286(2):293–301. doi: 10.1152/ajpcell.00293.2003. [DOI] [PubMed] [Google Scholar]
- 64.Gibellini L., Bianchini E., De Biasi S., Nasi M., Cossarizza A., Pinti M. Natural compounds modulating mitochondrial functions. Evid. Complement. Alternat. Med. 2015:1–13. doi: 10.1155/2015/527209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garcia-Garcia A., Zavala-Flores L., Rodriguez-Rocha H., Franco R. Thiol-redox signaling, dopaminergic cell death, and Parkinson’s disease. Antioxidants Redox Signal. 2012;17:1764–1784. doi: 10.1089/ars.2011.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ecker A., Gonzaga T.K.S.D.N., Seeger R.L., Santos M.M.D., Loreto J.S., Boligon A.A., Meinerz D.F., Lugokenski T.H., Rocha J.B.T., Barbosa N.V. High-sucrose diet induces diabetic-like phenotypes and oxidative stress in Drosophila melanogaster: protective role of Syzygiumcumini and Bauhinia forficate. Biomed. Pharmacother. 2017;89:605–616. doi: 10.1016/j.biopha.2017.02.076. [DOI] [PubMed] [Google Scholar]
- 67.Briffa M., Ghio S., Neuner J., Gauci A.J., Cacciottolo R., Marchal C., Caruana M., Cullin C., Vassallo N., Cauchi R.J. Extracts from two ubiquitous Mediterranean plants ameliorate cellular and animal models of neurodegenerative proteinopathies. Neurosci. Lett. 2017;638:12–20. doi: 10.1016/j.neulet.2016.11.058. [DOI] [PubMed] [Google Scholar]
- 68.Abolaji A.O., Kamdem J.P., Lugokenski T.H., Farombi E.O., Souza D.O. E.L. Da Silva Loreto, J.B.T. Rocha, Ovotoxicants 4-vinylcyclohexene 1,2-monoepoxide and 4-vinylcyclohexene diepoxide disrupt redox status and modify different electrophile sensitive target enzymes and genes in Drosophila melanogaster. Redox Biol. 2015;5:328–339. doi: 10.1016/j.redox.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Strausak D., Mercer J.F.B., Dieter H.H., Stremmelw S., Multhaup G. Copper in disorders with neurological symptoms: Alzheimer’s, menkes, and wilson diseases. Brain Res. Bull. 2001;55(2):175–185. doi: 10.1016/s0361-9230(01)00454-3. [DOI] [PubMed] [Google Scholar]
- 70.Nischwitz V., Achim B., Bernhard M. Speciation analysis of selected metals and determination of their total contents in paired serum and cerebrospinalfluid samples: an approach to investigate the permeability of the human blood- cerebrospinal fluid-barrier. Anal. Chim. Acta. 2008;627(2):258–269. doi: 10.1016/j.aca.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 71.Klaunig J.E., Kamendulis L.M., Hocevar B.A. Oxidative stress and oxidative damage in carcinogenesis. Toxicol. Pathol. 2010;38:96–109. doi: 10.1177/0192623309356453. [DOI] [PubMed] [Google Scholar]
- 72.Halliwell B. What nitrates tyrosine? Is nitrotyrosine specific as a biomarker of peroxynitrite formation in vivo. FEBS Lett. 1997;411:157–160. doi: 10.1016/s0014-5793(97)00469-9. [DOI] [PubMed] [Google Scholar]
- 73.Salsabili N., Mehrsai A.R., Jalaie S. Concentration of blood and seminal plasma elements and their relationships with semen parameters in men with spinal cord injury. Andrologia. 2009;41:24–28. doi: 10.1111/j.1439-0272.2008.00885.x. [DOI] [PubMed] [Google Scholar]
- 74.Cicchetti F., Drouin-Ouellet J., Gross R.E. Environmental toxins and Parkinson’s disease: what have we learned from pesticide-induced animal models? Trends Pharmacol. Sci. 2009;30(9):475–483. doi: 10.1016/j.tips.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 75.Craig L.A., Hong N.S., McDonald R.J. Revisiting the cholinergic hypothesis in the development of Alzheimer’s disease. Neurosci. Biobehav. Rev. 2011;35:1397–1409. doi: 10.1016/j.neubiorev.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 76.Drożak J., Bryła J., Metabolizmu Z.R. Dopamina–nietylkoneuroprzekaźnik (english- Dopamine: not just a neurotransmitter) PostepyHig. Med. Dosw. 2005;59:405–420. [PubMed] [Google Scholar]
- 77.Zhang S., Yin Y., Lu H., Guo A. Increased dopaminergic signaling impairs aversive olfactory memory retention in Drosophila. Biochem. Biophys. Res. Commun. 2008;370:82–86. doi: 10.1016/j.bbrc.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 78.Waddell S. Dopamine reveals neural circuit mechanisms of fly memory. Trends Neurosci. 2010;33:457–464. doi: 10.1016/j.tins.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Palop J.J., Mucke L. Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat. Neurosci. 2010;13:812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paris I., Dagnino-Subiabre A., Marcelain K., Bennett L.B., Caviedes P., Caviedes R., Olea-Azar C., Segura-Aguilar J. Copper neurotoxicity is dependent on dopamine-mediated copper uptake and one-electron reduction of aminochrome in a rat substantia nigra neuronal cell line. J. Neurochem. 2001;77:519–529. doi: 10.1046/j.1471-4159.2001.00243.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.