Abstract
GATA4 is a particularly important cardiogenic transcription factor and serves as a potent driver of cardiogenesis. Recent progress in the field has made it clear that histone acetylation can influence gene expression through changing the structure of chromatin. Our previous research had revealed that hypo-acetylation could repress gata4 expression in cardiocytes, however the underlying mechanism by which this occurred was still unclear. To reveal the mechanism of histone acetylation involved in the regulation of gata4 transcription, we concentrated on P300, one of the important histone acetyltransferase associated with cardiogenesis. We found that P300 participated in gata4 expression through regulating histone acetylation in embryonic mouse hearts. RNAi-mediated downregulation of P300 modulated the global acetylation of H3 and the acetylation of H3K4, H3K9, and H3K27 in gata4 and Tbx5 promoters. Interestingly, there was an obvious inhibition of gata4 transcription, whereas Tbx5 was not influenced. Furthermore, SGC-CBP30, the selective inhibitor of the bromodomain in CBP/P300, downregulated gata4 transcription by repressing the acetylation of H3K4, H3K9, and H3K27 in the gata4 promoters. Taken together, our results identified that acetylation of H3K4, H3K9, and H3K27 mediated by P300 plays an important role in regulation of gata4 expression in cardiogenesis.
Keywords: gata4, P300, Acetylation, H3K4ac, H3K9ac, H3K27ac
Introduction
Embryonic cardiogenesis is a complex process controlled by an intricate regulatory network that involves several cardiac-specific genes, including cardiac transcription factors in the GATA, Mef2, Tbx, and Nkx2 families.1, 2 GATA4, a zinc finger protein is among these special transcription factors, and serves as a potent driver of cardiogenesis from the early stages of the embryonic heart to birth.3, 4 A link has been reported between several human congenital heart diseases and heterozygous mutations in the gata4 gene.2, 5 Additionally, GATA4 is essential for the formation of the proepicardium.6 Thus, its precise spatial and temporal expression is necessary for normal embryonic cardiogenesis. However, despite these important findings, the molecular basis underlying the time-dependent activation of gata4 gene expression during cardiogenesis remains largely unexplored.
It is well known that epigenetic modification, such as histone acetylation, can modulate gene expression patterns, further influencing the development of embryonic organs. Histone acetyltransferases (HATs) acetylate the N-terminal tails of the lysine residues on histones and facilitate the opening of chromatin spatial structures, resulting in the activation of transcription.7, 8, 9 The experimental hyper-acetylation of target gene histones results in the precocious differentiation of cardiac progenitor cells and cardiac developmental defects.10 Histone acetylation inhibitors can correct the over-expression of pathological cardiac genes that is due to alcohol exposure during pregnancy.11 The E1A-associated 300kD protein (P300) is one of the intrinsic HATs that can modulate embryonic development. In human neural differentiation, P300 can take part in the expression of neurodevelopmental genes through regulating H3K9ac.12 In the cardiac transcription network, P300 can facilitate the activation of target genes through the acetylation of the lysine residues of histones in the promoters of the target gene.13 Our previous research revealed that the hypo-acetylation induced by the P300 inhibitor represses gata4 expression in cardiocytes.14 These studies remind us that P300 may participate in embryonic gata4 expression.
Here, we sought to investigate the potential role of P300-mediated histone acetylation in gata4 transcription. We have demonstrated the time-course of gata4 in embryonic murine hearts using RT-qPCR. We also examined the enrichment of histone acetylation markers in gata4 promoters by taking advantage of chromatin immunoprecipitation coupled with quantitative PCR (ChIP-qPCR). Then we further confirmed our findings by selectively inhibiting the acetylation function of P300 and studying its role in gata4 transcription through P300 knockdown experiments in cardiac progenitor cells. Thus, we believe our study has proven that P300-mediated acetylation of histone H3K4, H3K9, and H3K27 served as an essential driver in the regulation of gata4 expression in cardiac progenitor cells, and have herein provided evidence for future investigations of epigenetic regulation in embryonic cardiogenesis.
Materials and methods
Collection of embryonic mouse hearts
Adult Kunming mice were obtained from the Laboratory Animal Center of Chongqing Medical University (Chongqing, China). The mice were housed in an environment with a stable temperature of 22.2 °C and had free access to normal water and food. Embryo day 0.5 (E0.5) was defined as the morning when conception plugs were found after cohabitation. The hearts of embryos between stages E 11.5 to postnatal day 1 (P1) were collected every day for further experiments.
Cell lines
Cardiac progenitor cells were kindly provided by the Molecular Oncology Laboratory of the University of Chicago Medical Center (Illinois, USA)15 and cultured in DMEM/F12 (Gibco, China) containing 10% fetal bovine serum (Gibco, Australia) and penicillin/streptomycin. The cells were incubated at 37 °C in a humidified atmosphere of 5% CO2. Lentivirus containing three target P300 siRNAs were obtained from HANBIO (Shanghai, China). The target sequence was as follows: 5′- GGAACTACCCTAC TTTGAAGG -3′. Transfection was performed according to the manufacturer's instructions. In the inhibitor experiment, SGC-CBP30 (Selleck, Shanghai, China) was added at a concentration of 2 μM for 6 h prior to RT-qPCR and Chromatin immunoprecipitation.
Flow cytometry
After 48 h of interference, cardiac progenitor cells were digested with 0.25% trypsin (Solarbio, Beijing, China) and made into single-cell suspensions in a 2M phosphate buffer solution. Cells were assayed with a flow cytometer (Becton Dickinson, USA). Data were collected and analyzed using FACSDiva software, version 6.1.2 (Becton Dickinson). The percentage of green fluorescence protein (GFP) represented the efficiency of lentiviral transfection.
RT-qPCR
We extracted total RNA using Trizol (Ambion, CA, USA), and reverse-transcribed cDNA using the PrimeScript RT reagent kit (Takara, Beijing, China), according to the manufacturer's instructions. Then, we amplified and quantified cDNA using the KAPA SYBR FAST qPCR KITS (Cape Town, South Africa) with a CFX96 Touch Real-Time PCR Detection System (Bio-Rad, CA, USA). The primer sequences used in our experiments were as follows: gata4 (F) 5′- CCCTCCCGCACGATTTCT-3′, (R) 5′- AGAGGCCCAACTCGCTCAA -3′; p300 (F) 5′- GCCCTGGATTAAGTTTGA-3′, (R) 5′- TGAGTAGGACCCTGATTTG -3′; tbx5 (F) 5′-CCAAAGACAGGTCTTGCGATTCG -3′, (R) 5′- TTCTCCTCCCTGCCTTGGTGAT -3′; gapdh (F) 5′- GGCATCTTGGGCTACAC -3′, (R) 5′- TACCAGGAAATGAGCTTGA -3′. Each transcript was quantified in triplicate and the expression levels of the target genes were normalized to the control gene (GAPDH) using the △△CT method.
Chromatin immunoprecipitation (ChIP)
ChIP assays were performed using the Chromatin Extraction kit (Abcam, Cambridge, England) and ChIP kit (Abcam, Cambridge, England), according to the manufacturer's instructions. The antibodies used in the assays were the following: anti-P300 antibody, anti-CBP antibody, anti-PCAF antibody, anti-GCN5 antibody, anti-Histone H3 acetyl antibody, anti-Histone H3 (acetyl K4) antibody, anti-Histone H3 (acetyl K9) antibody, and anti-Histone H3 (acetyl K27) antibody (Abcam, Cambridge, England). The level of immunoprecipitated DNA was measured by qRT-PCR using a KAPA SYBR FAST qPCR kit (Cape Town, South Africa). The primer sequences for ChIP-Q-PCR were; gata4 (F) 5′- CACTGACGCCGACTCCAAACTAA-3′, (R) 5′- CGACTGGGGTCCAATCAAAAGG -3′, and tbx5 (F) 5′-TCTAAGCCGTTCTGGAGCCC GACA-3′, (R) 5′-AGAGCCTCCCAGCGACTG CCCAC-3′. Then the ChIP DNA Ct values were normalized to the input (the total DNA collected after release) using the △△CT method.
Statistical analysis
All results were presented as mean ± SE for at least three independent experiments using SPSS 17.0 software (Chicago, Illinois, USA). Two-tailed Student's t test was used to evaluate statistical significance between two groups, and one-way ANOVA was used among multiple groups. A p-value <0.05 was considered to be statistically significant.
Results
Cardiac gata4 is expressed in a time-course manner in mouse embryos
In embryonic cardiogenesis, GATA4 serves as an essential transcription factor that can regulate the expression of structural and functional cardiac genes, which are expressed in a dynamic manner during the process.16 Because of GATA4's participation in the transcription pathway of these important cardiac genes, we set out to investigate the time-course of gata4 expression in embryonic cardiogenesis. We collected the hearts of normal Kunming mice at E11.5 (Embryonic day 11.5) to P1 (postnatal day 1). We then used RT-qPCR to detect gata4 mRNA in embryonic and newborn hearts. We found that there was a significant increase of gata4 mRNA at E14.5 compared to E11.5, and after that point levels remained relatively stable until birth (Fig. 1A). We thus believed that gata4 was expressed in a time-course manner in mouse embryos, and that E14.5 was a special time in the process.
Figure 1.
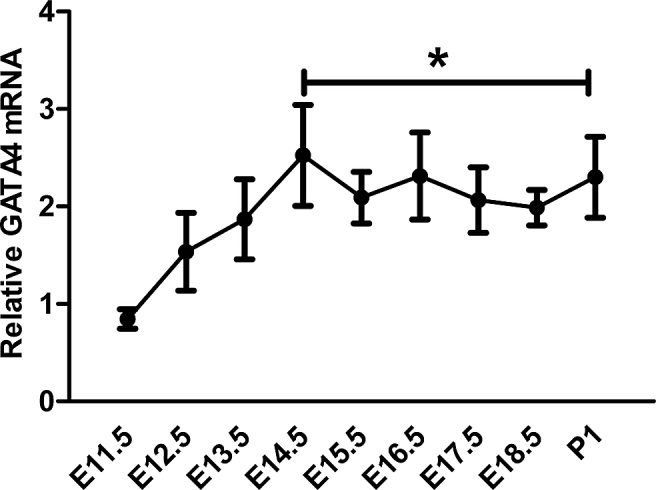
Dynamic expression of GATA4 in embryonic and newborn mouse hearts. RT-qPCR demonstrates a significant increase in GATA4 mRNA at E14.5 compared with E11.5, and GATA4 mRNA remain relatively stable until birth. The mRNA expression of GATA4 has been normalized to that of GAPDH. Data are represented as mean ± SD from three independent experiments. *P < 0.05, as compared with E11.5.
Histone acetylation regulates gata4 expression in the embryonic heart
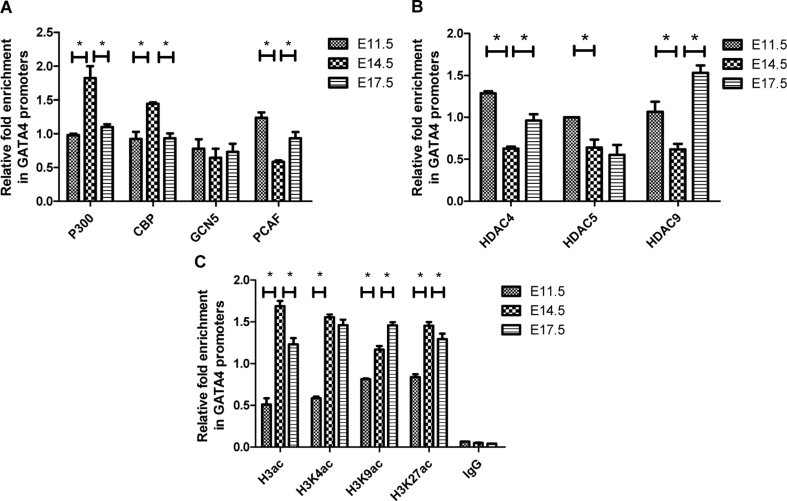
We have previously shown that histone hypoacetylation represses gata4 expression in cardiocytes.14 In this study, we wanted to investigate the molecular mechanism involved in the regulation of the gata4 expression time-course in embryonic mouse hearts. First, we performed ChIP analysis using antibodies against histone acetylases (P300, CBP, GCG5, and PCAF), and histone deacetylases (HDAC4, HDAC5, and HDAC9), followed by qPCR using primers targeting the gata4 promoters in different stages of the embryonic mouse hearts. As shown in Fig. 2A, we detected a significantly higher abundance of both P300 and CBP at E14.5 compared to E11.5. Simultaneously, we found that the level of GCN5 binding was stable along the course of embryonic development, and that there was a significant decrease in PCAF occupancy of the gata4 promoters at E14.5, indicating that P300 and CBP, but not GCN5 and PCAF, might participate in the physiological gata4 increase. As for the HDACs, our findings revealed that HDAC4, HDAC5, and HDAC9 bound less to gata4 promoters at E 14.5 when compared with E11.5 (Fig. 2B). These data suggested that there was a positive correlation between the higher expression of gata4 and the histone acetylation mediated by P300 and CBP, rather than GCG5 and PCAF, and also a negative correlation to HDAC4, HDAC5, and HDAC9.
Figure 2.
Histone acetylation near the GATA4 promoter in embryonic mouse hearts. ChIP-qPCR demonstrates that (A) Enrichment of p300 and CBP in the GATA4 promoter was highest at E14.5, while GCN5 was stable in the process and PCAF decreased at E14.5. (B) Enrichment of HDACs in the GATA4 promoter decreased significantly at E14.5. (C) Abundance of H3ac, H3K4ac, H3K9ac, and H3K27ac in the GATA4 promoter increased significantly at E14.5. In these graphs, relative enrichment represents the average fold enrichment of GATA4 promoter vs. input. Data are represented as mean ± SD from three independent experiments. *P < 0.05, as compared with neighboring groups.
After finding that histone acetylation regulated gata4 expression in the embryonic heart, we decided to concentrate on revealing how histone acetylation regulates gata4 in cardiac development. To explore the acetylation of different histone lysines in the gata4 promoters, ChIP was performed using antibodies against H3ac, H3K4ac, H3K9ac, and H3K27ac, followed by qPCR using primers targeting the gata4 promoters in E11.5, E14.5, and E17.5 embryonic mouse hearts. Our results showed that the global acetylation of H3 in gata4 promoters was highest at E14.5. Additionally, the acetylation of H3K4 increased significantly at E14.5 and remained stable until birth, a trend that was similarly seen in the expression of gata4 mRNA. We also observed sustainable growth of H3K9 acetylation. Meanwhile, acetylation of H3K27 exhibited a similar trend to that of global H3 acetylation in gata4 promoters (Fig. 2C). These data indicated that, in gata4 promoters, the acetylation of H3, H3K4, H3K9, and H3K27 could play a role in regulating its transcription.
Transcription of gata4 expression in cells is closely related to P300
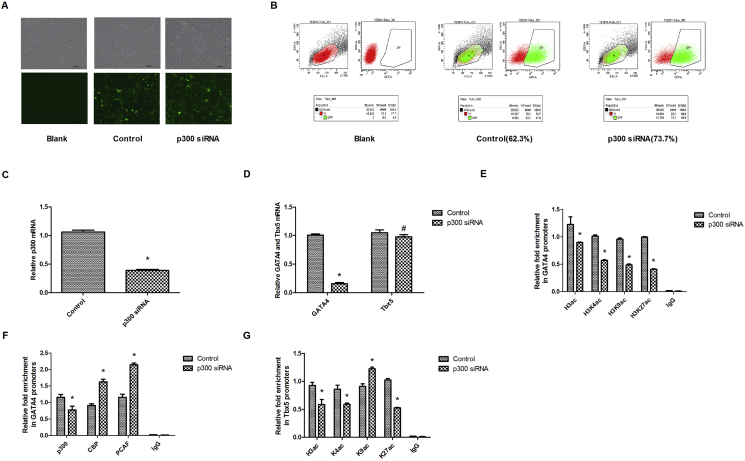
To verify the essential role that P300 plays in gata4 expression in cardiogenesis, we down-regulated P300 in cardiac progenitor cells in vitro. The cardiac progenitor cell lines were a gift from Dr. Mi Li (University of Chicago Medical Center, USA).15 We infected the cardiac progenitor cells with lentivirus containing P300 siRNAs for more than 48 h. A control group was established by infecting lentivirus containing blank vehicle. The expression of green fluorescent protein (GFP) served as a lentiviral marker, measured by a fluorescence microscope. As shown in Fig. 3A, cells in both the control group and the P300 siRNA group expressed GFP. Next, we used flow cytometry to detect the lentiviral infection efficiency. As shown in Fig. 3B, transfection efficiencies in the control group and the P300 siRNA group were 62.3% and 73.7%, respectively. Additionally, we detected the expression of P300 mRNA using RT-qPCR. As shown in Fig. 3C, P300 siRNA yielded an obvious inhibition of P300 expression, suggesting efficient interference of P300. We then assessed the relative mRNA levels of gata4 in cardiac progenitor cells. Tbx5 was chosen as control gene. Results from RT-qPCR demonstrated a significant decrease in the expression of gata4 in the P300 siRNA group, as compared to that in control group (Fig. 3D). At the same time, there was no obvious change in Tbx5 mRNA. This indicated that a down-regulation of P300 could result in decreased gata4 transcription.
Figure 3.
Downregulating p300 decreases GATA4 expression through histone hypo-acetylation. (A) Fluorescence microscopy was used to measure expression of green fluorescent protein (GFP) as a lentiviral marker (green). (B) FCM demonstrated that the transfection efficiencies of lentivirus in the control and P300 siRNA groups were 62.3% and 73.7%, respectively. (C–D) RT-qPCR analysis shows that p300 siRNA inhibited the expression of p300 by more than 50%. Interference of p300 resulted in an obvious decrease in GATA4 but no change in Tbx5. The mRNA expression of GATA4 has been normalized to that of GAPDH. (E–G) ChIP-qPCR revealed that the acetylation state of total H3, H3K4, H3K9, and H3K27 next to the GATA4 promoter was inhibited after p300 interference. Significant suppression of p300 in GATA4 promoter was detected in the p300 siRNA group, while more CBP and PCAF bound to the GATA4 promoter. Enrichment of H3ac, H3K4ac, and H3K27ac in Tbx5 promoters is suppressed due to the lack of p300, with the exception of H3K9ac. Relative enrichment represents the average fold enrichment of GATA4 or Tbx5 promoter vs. input. Data are represented as mean ± SD from three independent experiments. *P < 0.05, as compared with the control group. #P > 0.05, as compared with the control group.
P300 is a broad-spectrum histone acetylase. To narrow down which P300-mediated lysine acetylation takes part in gata4 transcription, we performed ChIP using antibodies against H3ac, H3K4ac, H3K9ac, and H3K27ac, followed by qPCR using primers targeting gata4 promoters in order to detect the acetylation state of histones in the gata4 promoters. As shown in Fig. 3E, the acetylation of global H3, H3K4, H3K9, and H3K27 in the gata4 promoters was inhibited after P300 interference. Simultaneously, the level of P300 binding in the gata4 promoters decreased after P300 mRNA was suppressed, while more CBP and PCAF bound to the gata4 promoters (Fig. 3F). This result suggested that suppression of P300 binding to the gata4 promoters influenced the transcription of gata4, rather than CBP and PCAF. On the other hand, enrichment of H3ac, H3K4ac, and H3K27ac in the Tbx5 promoters was suppressed due to the lack of P300, except for H3K9ac (Fig. 3G). Although P300 takes part in the H3K4ac and H3K27ac of histone in Tbx5 promoters, it cannot regulate Tbx5 transcription. We therefore concluded that this data could demonstrate that P300 has an essential role in gata4 transcription through modulating the acetylation of histone H3K4, H3K9, and H3K27 in gata4 promoters.
Bromodomain inhibitor CBP30 can decrease gata4 transcription through repression of P300 binding to gata4 promoters regions
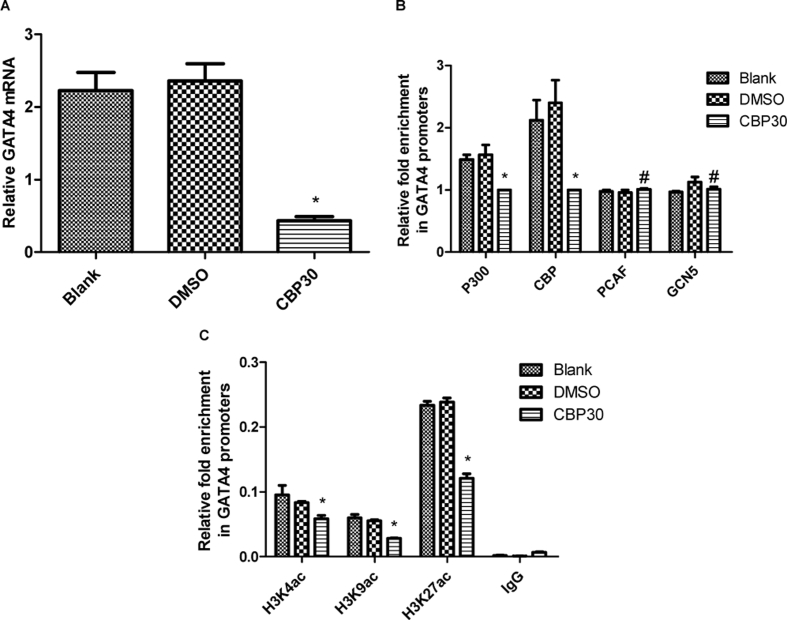
The P300 protein contains a bromodomain and a lysine acetyltransferase domain, which are involved in acetylation modification. After bromodomain recognizes acetyl-lysine in histones and non-histone proteins, the lysine acetyltransferase domain transfers an acetyl group from acetyl co-enzyme A to unmodified lysine residues.17, 18 To reinforce the P300-mediated histone acetylation that modulates gata4 transcription, we introduced CBP30, a selective CBP/P300 bromodomain inhibitor, to repress the acetyltransferase function of P300.19, 20 The cardiac progenitor cells were treated with 2 μM CBP30 while the same volume of DMSO was added to the culture medium of the control group. After 48 h, cells treated with CBP30 showed no obvious signs of toxicity (based on mortality and proliferation). Results from RT-qPCR demonstrated that after normalization to GAPDH, the level of gata4 mRNA was suppressed by CBP30 (Fig. 4A). We then performed ChIP-qPCR in order to detect the abundance of HATs to gata4 promoters. As shown in Fig. 4B, we observed a significant suppression of P300 and CBP binding to gata4 promoters following CBP30 treatment, whereas the enrichment of PCAF and GCN5 in gata4 promoters remained unchanged. This indicated that CBP30 could effectively and selectively repress enrichment of P300 and CBP in gata4 promoters, which is consistent with what Hammitzsch et al observed in human T cells.21 In order to identify the specific lysine residues in the gata4 promoters whose acetylation is important in gata4 transcription, we performed ChIP-qPCR to detect the acetylation state of H3K4, H3K9, and H3K27 in gata4 promoters. As shown in Fig. 4C, the enrichment of H3K4ac, H3K9ac, and H3K27ac in gata4 promoters was inhibited following CBP30 treatment. These data reinforced our hypothesis that P300-mediated acetylation of histone H3K4, H3K9, and H3K27 serves as an essential mechanism in the regulation of gata4 expression in cardiac progenitor cells.
Figure 4.
The p300 bromodomain inhibitor CBP30 decreases GATA4 transcription through histone acetylation. (A) RT-qPCR analysis shows that CBP30 suppressed GATA4 mRNA. The mRNA expression of GATA4 has been normalized to that of GAPDH. (B–C) ChIP-qPCR analysis indicates a significant suppression of p300 and CBP binding to GATA4 promoters in the CBP30 group as compared to the DMSO group, while the enrichment of PCAF and GCN5 was unchanged. The enrichment of H3K4ac, H3K9ac, and H3K27ac in the GATA4 promoters was inhibited after CBP30 treatment. Relative enrichment represents the average fold enrichment of the GATA4 promoter vs. input. Data are represented as mean ± SD from three independent experiments. *P < 0.05, as compared to the blank and DMSO groups. #P > 0.05, as compared to the blank and DMSO groups.
Discussion
Embryonic cardiogenesis is an intricate process that is modulated by several cardiac transcription factors, such as those in the GATA, Mef2, Tbx, and Nkx2 families. GATA4 is an important cardiac transcription factor and tends to regulate the expression of numerous cardiac structural and functional genes in cardiogenesis.22, 23, 24 The existence of gata4 mutations has been proven in plenty of patients with congenital heart disease.2, 25, 26 Deficiency of gata4 results in hearts defects such as common atrioventricular canal (CAVC), double outlet right ventricle (DORV), and hypoplastic ventricular myocardium.27 However, several genes and environmental factors regulate spatial and temporal expression of gata4.28 We found that gata4 was expressed in a time-course manner in embryonic mouse hearts, gradually increasing from embryonic age E11.5 to E14.5, and then remaining constant until birth. Although these findings highlight the potential importance of gata4 regulation, the molecular basis underlying gata4 gene expression during cardiogenesis remains largely unexplored.
Recent research has discovered that histone acetylation can activate gene expression and further influence the development of embryonic organs.29, 30, 31 HATs can facilitate transcription by acetylating the N-terminal tails of lysine residues of histones in promoters to open chromatin spatial structures.7, 8, 9 The four HATs reported to be closely involved in cardiogenesis are P300, CBP, GCN5, and PCAF. Our previous research revealed that the hypo-acetylation caused by inhibition of P300 could repress gata4 expression in cardiocytes.14 In this study, we found that it was the histone acetylation mediated by P300 and CBP that influenced gata4 expression, but not acetylation mediated by GCG5 and PCAF. Using P300 knockdown cardiac progenitor cells, we revealed that P300 is necessary for gata4 transcription, whereas a lack of P300 does not influence the other transcriptional factor Tbx5 mRNA expression.
Among different cell populations, the effects of acetylation of different lysine residues in histone could be quite distinct. H3K9ac plays a dual role in human embryonic stem cell pluripotency and neural differentiation.12 In retinal cells, inhibition of histone deacetylase 1 (hdac1) results in an increase of h3k9ac but not h3k27ac, and leads to rod photoreceptor differentiation.32 To determine the acetylation of which lysines takes part in gata4 transcription, we further demonstrated the enrichment of H3K4ac, H3K9ac, and H3K27ac in gata4 promoters. We found that acetylation of H3, H3K4, H3K9, and H3K27 in gata4 promoters may play a role in regulating its transcription. On the other hand, although P300 takes part in H3K4ac and H3K27ac of histone in Tbx5 promoters, it cannot regulate Tbx5 transcription.
SGC-CBP30 is a selective CBP/P300 bromodomain inhibitor, which can prevent bromodomain recognizing acetyl-lysine in histones, blocking the lysine acetyltransferase domain's transfer of an acetyl group from acetyl co-enzyme A to unmodified lysine residues.33 To explore the histone acetylation function of P300 that is responsible for modulation of gata4 transcription, we introduced SGC-CBP30 in order to repress the acetyltransferase activity of P300. We were able to reinforce our hypothesis that P300-mediated acetylation of histone H3K4, H3K9, and H3K27 serve as an essential mechanism in the regulation of gata4 expression in cardiac progenitor cells.
In conclusion, our observations reported that P300-mediated acetylation of H3K4, H3K9, and H3K27 regulated the expression of gata4 in cardiomyocytes. Our results represent a further advancement in the molecular dissection of the regulatory mechanisms controlling embryonic cardiogenesis. These findings will be to the benefit of the effort to reduce the incidence of congenital heart disease.
Conflicts of interest
The authors have no conflict of interests to declare.
Acknowledgement
The study was supported by research grants from the National Natural Science Foundation of China (Grant Number: 81300129).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2018.10.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zhou P., He A., Pu W.T. Regulation of gata4 transcriptional activity in cardiovascular development and disease. Curr Top Dev Biol. 2012;100:143–169. doi: 10.1016/B978-0-12-387786-4.00005-1. [DOI] [PubMed] [Google Scholar]
- 2.Xiong F., Li Q., Zhang C. Analyses of gata4, NKX2.5, and TFAP2B genes in subjects from southern China with sporadic congenital heart disease. Cardiovasc Pathol. 2013;22(2):141–145. doi: 10.1016/j.carpath.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Kuo C.T., Morrisey E.E., Anandappa R., Sigrist K., Lu M.M., Parmacek M.S. gata4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 1997;11:1048–1060. doi: 10.1101/gad.11.8.1048. [DOI] [PubMed] [Google Scholar]
- 4.Molkentin J.D., Lin Q., Duncan S.A., Olson E.N. Requirement of the transcription factor gata4 for heart tube formation and ventral morphogenesis. Genes Dev. 1997;11(8):1061–1072. doi: 10.1101/gad.11.8.1061. [DOI] [PubMed] [Google Scholar]
- 5.Xiang R., Fan L., Huang H. A novel mutation of gata4 (K319E) is responsible for familial atrial septal defect and pulmonary valve stenosis. Gene. 2014;534(2):320–323. [PubMed] [Google Scholar]
- 6.Watt A.J., Battle M.A., Li J., Duncan S.A. gata4 is essential for formation of the proepicardium and regulates cardiogenesis. Proc. Natl. Acad. Sci. U.S.A. 2004;101(34):12573–12578. doi: 10.1073/pnas.0400752101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clouaire T., Webb S., Bird A. Cfp1 is required for gene expression-dependent H3K4 trimethylation and H3K9 acetylation in embryonic stem cells. Genome Biol. 2014;15(9) doi: 10.1186/s13059-014-0451-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madhani H.D., Guillemette B., Drogaris P. H3 lysine 4 is acetylated at active gene promoters and is regulated by H3 lysine 4 methylation. PLoS Genet. 2011;7(3):e1001354. doi: 10.1371/journal.pgen.1001354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rollo C., Li Y., Jin X.L., O'Neill C. Histone 3 lysine 9 acetylation is a biomarker of the effects of culture on zygotes. Reproduction. 2017;154(4):375–385. doi: 10.1530/REP-17-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewandowski S.L., Janardhan H.P., Smee K.M. Histone deacetylase 3 modulates Tbx5 activity to regulate early cardiogenesis. Hum Mol Genet. 2014;23(14):3801–3809. doi: 10.1093/hmg/ddu093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng C., Zhu J., Sun H. Inhibition of histone H3K9 acetylation by anacardic acid can correct the over-expression of gata4 in the hearts of fetal mice exposed to alcohol during pregnancy. PloS One. 2014;9(8):e104135. doi: 10.1371/journal.pone.0104135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiao Y., Wang R., Yang X., Tang K., Jing N. Dual roles of histone H3 lysine 9 acetylation in human embryonic stem cell pluripotency and neural differentiation. J Biol Chem. 2015;290(4):2508–2520. doi: 10.1074/jbc.M114.603761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schübeler D., Schlesinger J., Schueler M. The cardiac transcription network modulated by gata4, Mef2a, Nkx2.5, srf, histone modifications, and MicroRNAs. PLoS Genet. 2011;7(2):e1001313. doi: 10.1371/journal.pgen.1001313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun H., Yang X., Zhu J. Inhibition of P300-HAT results in a reduced histone acetylation and down-regulation of gene expression in cardiac myocytes. Life Sci. 2010;87(23–26):707–714. doi: 10.1016/j.lfs.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Li M., Chen Y., Bi Y. Establishment and characterization of the reversibly immortalized mouse fetal heart progenitors. Int J Med Sci. 2013;10(8):1035–1046. doi: 10.7150/ijms.6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Afouda B.A., Lynch A.T., de Paiva Alves E., Hoppler S. Genome-wide transcriptomics analysis identifies sox7 and sox18 as specifically regulated by gata4 in cardiomyogenesis. Dev Biol. 2018;434(1):108–120. doi: 10.1016/j.ydbio.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meslamani J., Smith S.G., Sanchez R., Zhou M.-M. Structural features and inhibitors of bromodomains. Drug Discov Today Technol. 2016;19:3–15. doi: 10.1016/j.ddtec.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filippakopoulos P., Picaud S., Mangos M. Histone recognition and large-scale structural analysis of the human bromodomain family. Cell. 2012;149(1):214–231. doi: 10.1016/j.cell.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hay D.A., Fedorov O., Martin S. Discovery and optimization of small-molecule ligands for the CBP/P300 bromodomains. J Am Chem Soc. 2014;136(26):9308–9319. doi: 10.1021/ja412434f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrew R., Conery R.C.C., Adrianne Neiss. Bromodomain inhibition of the transcriptional coactivators CBP/EP300 as a therapeutic strategy to target the IRF4 network in multiple myeloma. Elife. 2016;5:e10483. doi: 10.7554/eLife.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammitzsch A., Tallant C., Fedorov O. CBP30, a selective CBP/P300 bromodomain inhibitor, suppresses human Th17 responses. Proc. Natl. Acad. Sci. U.S.A. 2015;112(34):10768–10773. doi: 10.1073/pnas.1501956112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malek Mohammadi M., Kattih B., Grund A. The transcription factor gata4 promotes myocardial regeneration in neonatal mice. EMBO Mol Med. 2017;9(2):265–279. doi: 10.15252/emmm.201606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Afouda B.A., Martin J., Liu F., Ciau-Uitz A., Patient R., Hoppler S. GATA transcription factors integrate Wnt signalling during heart development. Development. 2008;135(19):3185–3190. doi: 10.1242/dev.026443. [DOI] [PubMed] [Google Scholar]
- 24.Porrello E., Kinnunen S., Välimäki M. Nuclear receptor-like structure and interaction of congenital heart disease-associated factors gata4 and NKX2-5. PloS One. 2015;10(12):e0144145. doi: 10.1371/journal.pone.0144145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bose D., Vaigundan D., Shetty M., Krishnappa J., Kutty A.V. Identification of intronic-splice site mutations in gata4 gene in Indian patients with congenital heart disease. Mutat Res. 2017;803–805:26–34. doi: 10.1016/j.mrfmmm.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Baldwin S., Misra C., Sachan N. Congenital heart disease–causing gata4 mutation displays functional deficits in vivo. PLoS Genet. 2012;8(5):e1002690. doi: 10.1371/journal.pgen.1002690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pu W.T., Ishiwata T., Juraszek A.L., Ma Q., Izumo S. gata4 is a dosage-sensitive regulator of cardiac morphogenesis. Dev Biol. 2004;275(1):235–244. doi: 10.1016/j.ydbio.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Tang C., Deng Y., Duan H. The effect of maternal exposure to di-(2-ethylhexyl)-phthalate on fetal cardiac development in mice. J Appl Toxicol. 2018;38(6):834–842. doi: 10.1002/jat.3591. [DOI] [PubMed] [Google Scholar]
- 29.Padmanabhan J., Lu X., Deng Y. Histone acetyltransferase P300 mediates histone acetylation of PS1 and BACE1 in a cellular model of alzheimer's disease. PloS One. 2014;9(7):e103067. doi: 10.1371/journal.pone.0103067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y., Wang Y., Luo M. Novel curcumin analog C66 prevents diabetic nephropathy via JNK pathway with the involvement of P300/CBP-mediated histone acetylation. Biochim Biophys Acta. 2015;1852(1):34–46. doi: 10.1016/j.bbadis.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sengupta D., Kannan A., Kern M. Disruption of BRD4 at H3K27Ac-enriched enhancer region correlates with decreased c-Myc expression in Merkel cell carcinoma. Epigenetics. 2015;10(6):460–466. doi: 10.1080/15592294.2015.1034416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferreira R.C., Popova E.Y., James J., Briones M.R.S., Zhang S.S., Barnstable C.J. Histone deacetylase 1 is essential for rod photoreceptor differentiation by regulating acetylation at histone H3 lysine 9 and histone H4 lysine 12 in the mouse retina. J Biol Chem. 2017;292(6):2422–2440. doi: 10.1074/jbc.M116.756643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Carpizo V., Ruiz-Llorente S., Sarmentero J., Graña-Castro O., Pisano D.G., Barrero M.J. CREBBP/EP300 bromodomains are critical to sustain the GATA1/MYC regulatory axis in proliferation. Epigenet Chromatin. 2018;11(1) doi: 10.1186/s13072-018-0197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.